Abstract
Hostility is associated with an increased risk for cardiovascular disease (CVD). Because central serotonin may modulate aggression, we might expect selective serotonin reuptake inhibitors (SSRIs) to be effective in reducing hostility. Such effects have never been examined in individuals scoring high on hostility who are otherwise free from major DSM-IV Axis I psychopathology. 159 participants (ages 30–50, 50 % female) scoring high on 2 measures of hostility and with no current major Axis I diagnosis were randomly assigned to 2 months of citalopram (40 mg, fixed flexible dose) or placebo. Adherence was assessed by electronic measurement and by drug exposure assessment. Treated subjects showed larger reductions in state anger (condition-by-time p = .01), hostile affect (p = 02), and, among women only, physical and verbal aggression (p = .005) relative to placebo controls. Treatment was also associated with relative increases in perceived social support (p = .04). Findings have implications for understanding the CNS correlates of hostility, its associations with other psychosocial risk factors for CVD, and, potentially, for the design of effective interventions.
Keywords: SSRIs, hostility, treatment, cardiovascular disease, social support
A large body of evidence demonstrates an elevated risk for incident cardiovascular (CVD) events among individuals scoring high on measures of hostility (Miller, Smith, Turner, Guijarro, & Hallet, 1996), especially among initially healthy individuals (Everson-Rose & Lewis, 2005). In these studies, hostility is defined as a personality trait involving behavioral tendencies (i.e., aggressiveness), cognitive biases (i.e., tendency to interpret situations in a suspicious and mistrustful manner), and/or emotional or motivational characteristics (i.e., experience of frequent and intense anger, (Smith, 1992). Such studies have rarely included measures tapping all three domains (Suls & Bunde, 2005).
Measures of hostility are also associated with other psychosocial traits implicated in cardiovascular health, such as impulsivity (Manuck, Flory, Ferrell, Mann, & Muldoon, 2000; Ramirez & Andreu, 2006), which is linked with smoking and alcohol use (Grano, Virtanen, Vahtera, Elovainio, & Kivimaki, 2004) and depression and low social support (Raynor, Pogue-Geile, Kamarck, McCaffery, & Manuck, 2002; Smith & Frohm, 1985) which are linked with CVD risk (Berkman, Leo-Summers, & Horwitz, 1992; Musselman, Evans, & Nemeroff, 1998).
A common neurobiological mechanism which could potentially explain these diverse associations involves deficits in central serotonergic function (R. B. Williams, 1994a, , 1994b). Accumulating evidence suggests that central serotonergic systems may regulate a number of behavioral processes that may contribute to CVD risk (Malone et al., 2003; Muldoon et al., 2006; Muldoon et al., 2004). Diminished central serotonergic function can be indexed by low concentrations of the serotonergic metabolite 5-HIAA in the cerebrospinal fluid (CSF), or by blunted prolactin responses to the acute infusion of a serotonergic agonist. Using these two measures, reduced central serotonergic function has been shown to be associated with increased aggression in primates (Botchin, Kaplan, Manuck, & Mann, 1993; Higley, King et al., 1996; Higley, Mehlman et al., 1996) and with measures of hostility among humans, especially among men (Cleare & Bond, 1997; Coccaro, 1997; Manuck et al., 1998). In addition to aggression, reduced central serotonergic function, as indexed by blunted prolactin responses to fenfluramine challenge, has also been shown to be associated with impulsivity (Manuck et al., 1998) and with current and past depressive episodes in human samples (Flory, Mann, Manuck, & Muldoon, 1998; Mann, McBride, Malone, DeMeo, & Kelip, 1995). Finally, concentrations of CSF 5-HIAA have been shown to be positively related to affiliation (e.g., grooming) (Mehlman et al., 1995) and social dominance (Higley, King et al., 1996) in nonhuman primates, and to measures of social competence in humans (Kruesi et al., 1990), implicating central serotonergic systems in behaviors that contribute to social support as well as to individual variability in negative affect.
Pharmacologic interventions that enhance central serotonergic activity, such as selective serotonin reuptake inhibitors (SSRIs) have been found to be effective in reducing aggression and hostile affect in psychiatric samples, such as those with personality disorders or clinically significant depression (Coccaro & Kavoussi, 1997; Fava et al., 1993; Salzman et al., 1995). Because other psychiatric symptoms (for example, anxiety and depression) are prevalent in such samples and may themselves be linked with irritability or aggression, it is difficult to know the extent to which reduced hostility in such studies may be simply a consequence of improvement in other aspects of one’s condition during treatment.
Unfortunately, only a small number of controlled studies have examined the effects of serotonergic interventions on hostility in nonpsychiatric samples. In a trial of healthy volunteers, Knutson et al. (Knutson et al., 1998) showed that a four-week course of paroxetine was associated with significant reductions on the Assaultiveness subscale of the Buss-Durkee Hostility Inventory (a measure of physical aggression) relative to placebo controls, and marginally significant reductions in the Irritability subscale as well. In a small (n =12) study of men with a history of conduct disorder but no current Axis I diagnosis, those assigned to 3 weeks of paroxetine showed significant decreases in aggressive responding on a standard laboratory task relative to those assigned to placebo (Cherek, Lane, Pietras, & Steinberg, 2002). Finally, in two double-blind crossover studies of unselected (Moskowitz, Pinard, Zuroff, Annable, & Young, 2001) and high hostile individuals (aan het Rot, Moskowitz, Pinard, & Young, 2006), a 12–15 day course of tryptophan supplementation was shown to decrease self-reports of quarrelsome behavior during social interactions. Because there are few empirically validated interventions for hostility (Gidron, Davidson, & Bata, 1999), these are promising leads with potential clinical relevance for understanding, and potentially intervening with, a group of individuals who demonstrate increased health risk. Unfortunately, none of these existing studies have employed measures of hostility that are most widely represented in the literature examining cardiovascular health, none of them have measured hostility as a multidimensional construct, and only one of them (aan het Rot, Moskowitz, Pinard, & Young, 2006), a study of tryptophan supplementation, selected appropriate candidates for intervention based upon initial hostility scores, a problem that could potentially reduce the magnitude of observed effects.
In addition to their impact on aggression or hostility, serotonergic interventions have also been examined with respect to their effects on depression, impulsivity, and sociability. The effects of SSRIs on ameliorating depressive symptomatology is well documented in clinically depressed samples (Nemeroff & Schatzberg, 2007). Only one study, to our knowledge, has examined the effects of serotonergic enhancement on impulsivity in a nonpsychiatric sample: In the study of men with a history of conduct disorder described earlier, paroxetine was shown to enhance delay of gratification, as assessed by a behavioral measure (Cherek, Lane, Pietras, & Steinberg, 2002). There are no published studies, to our knowledge, examining the effects of serotonergic manipulations on perceived social support, but there are a couple of findings suggesting that serotonergic interventions may increase prosocial behavior in the laboratory (Tse & Bond, 2002) or during daily life (aan het Rot, Moskowitz, Pinard, & Young, 2006) but see also (Moskowitz, Pinard, Zuroff, Annable, & Young, 2001).
In sum, previous evidence shows a higher risk for cardiovascular disease among hostile individuals, and a greater prevalence of other behavioral characteristics potentially linked with CVD in this group as well. Reduced central serotonergic function among hostile individuals may be one of the mechanisms explaining this increased disease risk; pharmacologic manipulations designed to enhance central serotonergic activity have been shown to have beneficial effects on hostility as well as a number of other relevant psychosocial traits.
This paper describes a placebo-controlled investigation examining the effects of a serotonergic agent on hostility among initially high hostile individuals with no current Axis I comorbidity. Citalopram was employed because of its high serotonin selectivity and relatively low affinity for other major neurotransmitter receptors (Scales & Doraiswamy, 1998). Unlike previous work in this area, our dependent measures in this study included measures of multiple dimensions of hostility, including those indices that have been most frequently shown to be associated with an elevated risk for CVD in previous reports. We hypothesize that a citalopram intervention will be associated with reduced hostility in this group, consistent with the possibility that reduced serotonergic function might explain, in part, the association between hostility and cardiovascular disease risk. We also propose that the intervention should have a salutary effect on other psychosocial characteristics (impulsivity, depression, and social support) that are associated with hostile traits or CVD risk.
Method
Participants
We recruited healthy middle aged adults (ages 30–50) with elevated scores on two standard measures of hostility (see below). This age range was designed to capture an adult population with risk factors for CVD that was not yet likely to be symptomatic or under treatment. Exclusionary criteria included history of CVD or other chronic medical conditions (e.g., diabetes), and current Axis I DSM-IV diagnosis (major depressive episode, dysthymia, bipolar disorder, alcohol or drug use disorder, panic disorder, agoraphobia, social phobia, OCD, PTSD, GAD, anorexia nervosa, bulimia nervosa, binge eating disorder, or psychotic disorder). Those with excessive alcohol use (> 14 drinks/week or > 2 binges per week), current use of street drugs by self report, or positive urine drug screens were also excluded. To reduce the potential for side effects, we excluded those on psychotropic medications, and to reduce expectancy effects, we excluded those who had taken SSRIs within the past 2 months.
Because of our interest in the impact of SSRIs on risk factors for CVD (not reported here), we excluded individuals currently prescribed medication for cholesterol or high blood pressure (or use of BP medications within the past year), and those on other medications with autonomic effects. Those with blood glucose over 140 or blood pressure > 160/100 were also excluded and were referred immediately for treatment. To reduce any potential teratogenic risk of the drug, we excluded pregnant women (positive pregnancy test), those who were planning to become pregnant, and premenopausal women unwilling to commit to use of a double barrier contraceptive method during the course of their participation in the study.
Procedures
Mass mailings with self-addressed return postcards were sent to 340,249 residents in the local metropolitan area (Allegheny County, Pennsylvania), targeting the age range of interest. 7,045 individuals called or returned postcards expressing interest in the study (2.1 % return rate). Telephone screening interviews were conducted with potential participants who had returned the postcard forms; 5,080 individuals were successfully contacted (72 % of those expressing interest). As part of this telephone interview, potential participants were administered a screening instrument which included 10 items from the Buss-Durkee Motor Aggression subscale ((Buss & Durkee, 1957), and 10 items from the three Barefoot subscales from the Cook Medley hostility scale (see below) (Barefoot, Dodge, Peterson, Dahlstrom, & Williams, 1989; Cook & Medley, 1954). Screening items were derived from the larger scales using factor loadings from a local normative sample selected for this age range (Muldoon et al., 2000), and cutpoint scores (4 or more item endorsements on each of these scales) were developed as inclusion criteria for this study, as these cutpoints selected those in the top tertile on each of the full scales from the normative sample.
Interested individuals who passed all telephone screening criteria (n =584, or 12 % of those contacted by phone) were invited to participate in a laboratory screening session (Visit 1) during which they provided written informed consent followed by administration of a more detailed medical history interview (including anthropometric measures and self-reported smoking history), a portion of the Structured Clinical Interview for DSM-IV (SCID; (First, Spitzer, Gibbon, & Williams, 2002)) to rule out current Axis I diagnosis (see above), a clinic blood pressure screening and finger stick for blood glucose screen, a urine drug screen (to rule out current use of street drugs including cocaine, opiates, and amphetamine), a urine pregnancy test, and several additional questionnaires. The complete item set from the Buss-Durkee Motor Aggression subscale (43 items) and Cook Medley Barefoot subscales (27 items; see below) were administered at this point for re-screening purposes; those who scored below the median for their gender group, by local norms, on either one of these item sets were excluded from further participation.1
229 individuals (39 % of those invited to Visit 1) met all of the above criteria after inperson assessment during Visit 1 and were enrolled in the study. These participants who were interested and eligible after Visit 1 attended 5 additional pre-treatment visits over a 1–1/2 month period (range of 17–108 days). Additional risk factor information was assessed during Visit 2, Visit 3 involved a series of laboratory stressors (not reported here), and Visits 4–6 involved training and feedback for self-report field diary assessments (not reported here). Other questionnaires and interviews described below were also administered during these pre-treatment visits.
Following the pre-treatment period, subjects were scheduled for an appointment in the medication clinic (Visit 7). They were administered a second informed consent procedure by the study psychiatrist (RJH) during which the risks and side effects associated with the study drug were described in greater detail. A urine pregnancy test was re-administered to eligible participants and acceptable methods of contraception were discussed. Participants who remained eligible and interested were randomized to drug or placebo intervention, using a computer generated randomization list. Randomization was performed by the Investigational Drug Service in the School of Pharmacy at the University of Pittsburgh; investigators, research staff and participants were blinded as to condition assignment. At the end of Visit 7, participants were accompanied to the pharmacy facility where they were administered their drug/placebo bottles. Randomization was implemented by pharmacy workers using information received by the IDS. A MEMS dispenser unit was used, creating an electronic record of the time associated with each bottle closing event as a measure of adherence. Pill use was initiated immediately following this visit. 69 subjects (30 % of those enrolled in the study) were dropped during pre-treatment assessment or prior to randomization, leaving 160 randomized subjects.
The target sample size of 160 was based upon power analyses that drew upon published results in the existing literature; in this study, 81 subjects were randomized to drug and 79 were randomized to placebo.
Visit 7 and three additional treatment titration visits (Visit 8–10) were completed over a 1–1/2 month period (range of 39–61 days). During each of these visits, each approximately 30 mins in duration, participants met individually with a clinical research nurse. Pill counts were conducted, side effects were discussed, and the new drug dosage for the following visit was determined. A “fixed flexible” dosage range was prescribed: All subjects in the active intervention and placebo conditions were started on a 10 mg (one pill) dose. Those without significant side effects were administered an incremental increase in dose each visit for up to 40 mg (4 pills) by the end of Visit 10. A final medication review visit (Visit 11) was scheduled 2 weeks after the final dose was prescribed during which the fixed treatment period began.
During the five fixed treatment visits (Visit 11–15 over the next month, range of 9–104 days), we readministered each of the dependent measures that were collected during pre-treatment. Measures that were designed to assess initial eligibility for the study (SCID, Medical History Interview, urine drug screen) were not repeated. During this period, participants underwent three blood draws for assessment of citalopram exposure. Following Visit 15, participants underwent a two-week withdrawal period, during which their medication was gradually reduced and eliminated (or, in the placebo condition, pill dosage was gradually reduced). Visit 16 was a debriefing visit scheduled following the withdrawal period; participants met with investigators in the study (RJH, TWK) to discuss their reactions to the pills and to the study procedures. Consulting a confidential envelope provided on each participant by the Investigational Drug Service, the PI (TWK) debriefed the participants as to their condition assignment at this time. If they expressed interest, participants were referred for further psychological or pharmacologic treatment.
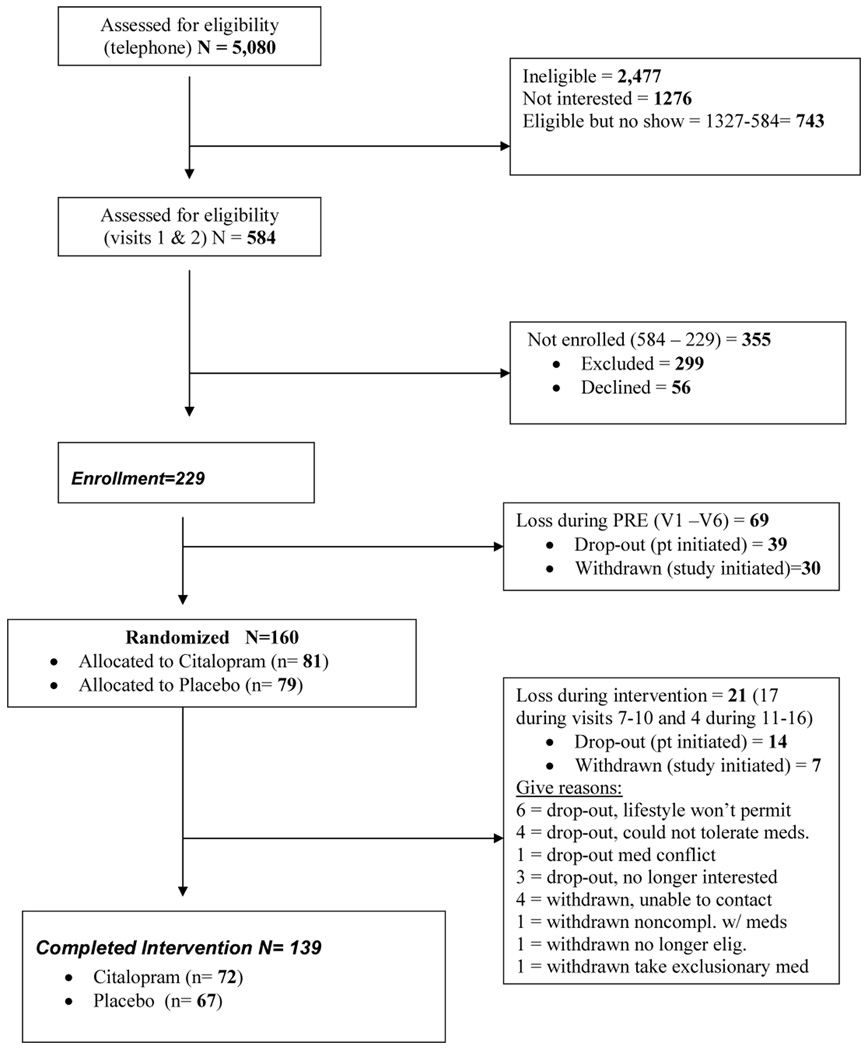
87 % (n = 139) of randomized participants completed the intervention and the endof-treatment assessments; 72 of these were in the drug condition, and 67 were in the placebo group. See Figure 1, Recruitment Flowchart. Data collection for this study spanned a 49-month period, from January 2002- March 2006. The study was conducted in compliance with the University of Pittsburgh Institutional Review Board.
Figure 1.
Recruitment flowchart.
Measures
Measures of Hostility
1. Cook-Medley Hostility Scale (1 scale score)
The Cook-Medley Hostility Scale (CMHS) (Cook & Medley, 1954) is a 50-item true-false questionnaire, derived from the MMPI, which has been shown to be associated with increased CVD risk and for all-cause premature mortality (Miller, Smith, Turner, Guijarro, & Hallet, 1996). Some evidence suggests that three sets of rationally derived subscales from the Cook-Medley may be especially predictive of mortality (Barefoot, Dodge, Peterson, Dahlstrom, & Williams, 1989) so these are sometimes used as a short form of the scale. In its full scale format, the CMHS has been shown to demonstrate reasonable psychometric properties, and to correlate most highly with “cognitive” measures of hostility, such as cynicism or mistrust (Smith, 1992). The 50-item CMHS scale was administered during Visits 5 (pre-treatment) and 14 (fixed treatment period).
2. Buss Durkee Hostility Inventory (BDHI) (8 subscale scores)
(Buss & Durkee, 1957). This 75-item measure has been widely employed in the literature on serotonergic function and hostility ((Knutson et al., 1998; Siever & Trestman, 1993). The BDHI includes 8 rationally derived subscales (Assault, Indirect Hostility, Irritability, Negativism, Resentment, Suspicion, Verbal Hostility, and Guilt); 2 higher order factors are commonly derived, but there is no clear consensus on the factor structure of this instrument (Bushman, Cooper, & Lemke, 1991).
Along with the CMHS Barefoot subscales, we used scale scores from the higher order Motor Aggression factor of the BDHI (Assault, Indirect Hostility, Irritability, and Verbal Hostility; (Buss & Durkee, 1957) as selection criteria for study eligibility. These scales are related to markers of serotonergic dysfunction (Cleare & Bond, 1997; Coccaro et al., 1989) and have been shown to be responsive to SSRI intervention (Knutson et al., 1998). Two studies have examined the relationship between BDHI scores and CVD, although findings have been inconsistent (Siegman, Dembroski, & Ringel, 1987) (Welin, Lappas, & Wilhelmsen, 2000). The full BDHI was administered during Visit 4 (pre-treatment) and 13 (fixed treatment period).
3. Buss Perry Aggression Questionnaire (BPAQ) (4 subscale scores)
(Buss & Perry, 1992). This is a 29-item revision of the BDHI, involving changes in response format and item content to improve clarity. The BPAQ contains 4 subscales, measuring physical aggression, verbal aggression, anger, and hostility. The 4-factor model appears to be a good fit, and these subscales show reasonable internal and re-test reliability (Buss & Perry, 1992; Harris, 1995). The BPAQ was administered during Visit 5 (pre-treatment) and 14 (fixed treatment period).
4. Spielberger State-Trait Anger Expression Inventory (STAXI) 5 subscale scores)
(Spielberger, 1988). This measure includes five subscales: State Anger (10 items), Trait Anger (10 items), Anger-in, Anger-out, and Anger Control (8 items each). These scales are internally consistent and are associated in the expected direction with existing instruments and with responses to hypothetical anger-provoking scenarios (Spielberger, 1988). Accumulating evidence links scores on these scales with CVD risk; findings with the Trait Anger scale tend to be the most consistent in this respect ((Bleil, McCaffery, Muldoon, Sutton-Tyrrell, & Manuck, 2004; J. E. Williams, Nieto, Sanford, Couper, & Tyroler, 2002; J. E. Williams et al., 2000).
With respect to the state-trait distinction, the Trait Anger scale assesses the appraisal of one’s typical mood (e.g., “I am quick tempered”) with items evaluated in terms of their frequency (ranging from 0= Almost Never to 3= Almost Always) whereas the State Anger scale assesses current mood (e.g., “I am furious”) with items evaluated with respect to their intensity (ranging from 0= “Not at all” to 3=”Very much so”). We revised the response options for the State Anger scale in the current study to reflect the reported frequency of anger during the past week (e.g., “I have felt furious, 1= Almost Never to 4= Almost Always). The State Anger scale was administered Visits 2–4 and on every visit during the treatment titration and fixed treatment periods (Visits 7–13)2. The other STAXI scales were administered once during pre-treatment and once during the fixed treatment period (Visits 6 and 13, respectively).
5. Structured Interview for the Type A Behavior Pattern, (SI-TABP) (4 subscale scores)
The SI-TABP is a brief (15 min) interview designed to elicit and assess components of the Type A behavior pattern (Rosenman, 1978), including impatience, pressured speech, and hostility. Some evidence (Miller, Smith, Turner, Guijarro, & Hallet, 1996) suggests that hostility scores from this interview may be more strongly associated with health outcomes than those yielded by self-report questionnaires; unfortunately, scores from interviews and self-report measures of hostility are only moderately correlated (Barefoot & Lipkus, 1994).
For this study, responses to the SI-TABP were coded for hostility using the Hostility Facet Scoring System (Barefoot & Lipkus, 1994), yielding 4 sets of ratings: Hostile Content, Hostile Style, Hostile Intensity, and overall Potential for Hostility, each scored on a 5-point scale. Measures derived from this system have been shown to significantly distinguish CVD cases from controls (Dembroski, MacDougall, Costa, & Grandits, 1989).
Other Psychosocial Characteristics
We administered the Inventory of Interpersonal Problems Personality Disorder Scales (IIP-PD) as a screening tool during Visit 1 in order to characterize the sample with respect to the potential prevalence of personality disorders. The IIP-PD is a 47-item rating instrument comprised of 5 subscales (interpersonal sensitivity, interpersonal ambivalence, aggression, need for social approval, and lack of sociability); in several samples, item means across the first 3 subscales have been shown to distinguish patients with personality disorders, by clinical interview, from those without (Morse & Pilkonis, 2007; Pilkonis, Yookyung, Proietti, & Barkham, 1996).
We administered the Beck Depression Inventory—II (BDI) (Beck, Steer, & Brown, 1996) and the Center for Epidemiologic Studies Depression Scale (CES-D) (Radloff, 1977) during Visits 6 and 15 to assess the presence of depressive symptoms and the possibility that changes in depression might drive any observed changes in hostility in this sample. We administered the Interpersonal Support Evaluation List (ISEL) during Visits 4 and 13 to assess correlated changes in social support as a function of the intervention. The ISEL is a 40-item self-report questionnaire assessing the perceived availability of four major social support functions: appraisal or information support, belonging support, tangible support, and self-esteem support, as well as an overall measure of perceived social support (Cohen, Mermelstein, Kamarck, & Hoberman, 1985).
We administered the Barrett Impulsiveness Scale (BIS-11) during Visits 5 and 14 to assess treatment-related changes in impulsive traits. The BIS-11 is a 30-item scale assessing ‘motor,’ ‘cognitive,’ and ‘non-planning’ impulsiveness as well as an overall measure of impulsiveness (Barratt, 1985) The BIS has been shown to be associated with low central serotonergic function among men (Manuck et al., 1998).
Side Effects and Treatment Attribution Measure
At each of the treatment titration visits (Visits 8–11), participants were administered a 23-item questionnaire (Somatic Symptom Scale, SSS) inquiring about current physical symptoms potentially relevant to the use of citalopram (e.g., dizziness, nervousness, dry mouth or throat). For each symptom endorsed on the SSS questionnaire, participants were asked whether it was “not present,” “present but tolerable” or “present and causing significant distress or incapacity.” The number of symptoms endorsed by each participant in each of the latter two categories was averaged across sessions. Scores were log transformed to reduce skewness.
At two of the treatment titration visits (Visit 8 and Visit 10), participants were administered an additional 3–item questionnaire asking for 1) their guess about which condition to which they had been assigned (placebo vs. drug) (Treatment Assignment Questionnaire); 2) the level of confidence associated with this choice (scale of 1–5); and 3) the evidence influencing this choice (open-ended). The research nurse working with the participant in the treatment titration sessions was administered a parallel form of this instrument at each of these two visits.
Adherence Measure
A MEMS (Medication Event Monitoring System) cap (ARPEX/AARDEX Union City, CA ) was assigned to each participant at the start of the treatment titration period. This is a prescription drug bottle cap equipped with an electronic chip that is activated with each cap closing, used as a measure of medication adherence. Electronic event monitoring has been shown to produce more conservative estimates of adherence when compared with self-report (Burke, 2001). Participants were instructed to dispense the pills only from the bottle, to remove the cap only when taking the pills, and to replace the cap on the bottle after each medication dose. They were instructed not to open the bottle at other times. They were instructed to bring the cap and bottle with them to each of the intervention visits, at which point adherence data were retrieved electronically. Adherence was assessed as the proportion of days during treatment (Visits 7–15) during which the bottle cap was opened and closed one or more times, after correcting for bottle closings that occurred during the clinic visits. Because resulting scores were highly skewed with a bounded range (most scores near the top), octant scores were derived for this measure.
Drug Exposure Measure
Three blood draws were taken on three separate days during the post-testing assessment period, usually during Visits 11, 13, and 15. Citalopram levels were assayed in each by HPLC with UV detection, using a method developed in the Geriatric Psychopharmacology Laboratory at the University of Pittsburgh (Pollock et al., 1997). For control group subjects, only one blood sample assay was typically examined as a spot check. (One control subject, however, was shown to have evidence of blood citalopram on all 3 occasions of measurement. In conformance with the intention to treat approach, this subject was retained, as assigned, for the analyses).
A prior nonlinear mixed effects model describing citalopram pharmacokinetics was used to assess the oral drug clearance rate for each individual. The individual specific clearance estimates were determined using a Maximum a priori Bayesian (MAP) approach (Bies et al., 2004). For this model, MEMS data were used to drive dosage input (i.e., date and time of dosage self-administration along with knowledge of the dosage of citalopram received), and the intervals between most recent drug administration and each of the 3 blood draws along with the relevant blood citalopram values were used to model the clearance parameter estimate. Age, weight, sex, and race were used as covariates in the model. The prior model was validated using a modified posterior predictive check (Gellman, Carlin, Stern, & Rubin, 1995). The combination of nonlinear mixed effects modeling and MEMS dosage input information is one of the most robust methods for ascertaining overall drug exposure over a period of treatment (Vrijens et al., 2005).
For those in the drug intervention group, average daily drug exposure over the course of the study (area under the curve) was calculated as the cross-product of individual clearance rate and total drug administration. Total drug administration was estimated based upon the total number of events recorded by the MEMS and the dosage (ranging from 10–40 mg) associated with each.
Results
Demographic Characteristics, by Condition and Dropout Status
We compared those randomized to each of the two conditions (n = 81 drug intervention, n = 78 controls)3 with respect to their demographic characteristics. There were no significant condition differences with respect to age, sex, race, education or income (see Table 1). Among those randomized to the drug, 13 % (21/159) dropped out or were withdrawn prior to completing the study. Attritioners were marginally more likely to be non-white (p = .09 by Fisher’s Exact Test) than completers, but they did not differ with respect to age, sex, education, or income.
Table 1.
Demographic Characteristics of Sample, by Condition
| Treatment Condition |
Placebo Condition |
|
|---|---|---|
| n = 81 | n = 78 | |
| Age | 40.8 (6.08) | 39.8 (5.78) |
| Sex (% female) | 48 % | 53 % |
| Race (% non-white) | 19 % | 13 % |
| Education (% no BA)1 | 57 % | 56 % |
| Income (% < 35 K)2 | 29 % | 30 % |
Note. Age is displayed as M (std).
5 missing data points, 2 in Tx and 3 in Control.
14 missing data points, 6 in Tx and 8 in Control.
Baseline Measures of Hostility and Depression, by Sex and Condition
Table 2 presents the baseline scores from the 50-item Cook-Medley hostility scale (Visit 5 administration), the Buss-Durkee Motor Aggression scale (from Visit 4), the Buss Perry Aggression Questionnaire full scale score (Visit 5), and the BDI-II and the CES-D (Visit 6) by condition and sex. Men who were randomized to the drug had higher baseline levels of depression (BDI-II only) than those assigned to placebo control (t = 2.6, df = 77, p = .01), and women who were randomized to the drug scored higher on motor aggression (p = 2.99, df= 77, p = .004). There were no significant condition differences for any of these other scores.
Table 2.
Baseline Measures of Hostility and Depression, by Condition and Sex
| Treatment Condition |
Placebo Condition |
|
|---|---|---|
| n=42 men, 39 women | n=37 men, 41 women | |
| Cook-Medley Hostility Scale (range=0–50) | ||
| Men | 28.18 (8.30) | 27.08 (7.92)1 |
| Women | 24.20 (9.40) | 23.84 (7.94) |
| Buss-Durkee Hostility Inventory | ||
| Motor Aggression subscale (range=0–43) | ||
| Men | 27.59 (6.07)2 | 27.02 (6.13) |
| Women | 27.79 (5.60)3 | 24.10 (5.35) |
| Buss-Perry Aggression Questionnaire (range=29–145) | ||
| Men | 85.45 (16.89) | 84.97 (13.22)1 |
| Women | 84.10 (16.40) | 77.02 (17.97) |
| Beck Depression Inventory-II (range=0–21) | ||
| Men | 11.83 (8.33) | 7.49 (6.21) |
| Women | 10.23 (8.17) | 9.76 (8.21) |
| Center for Epidemiological Studies Depression Scale (range=0–20) | ||
| Men | 14.29 (8.54) | 13.49 (7.50) |
| Women | 15.64 (9.62) | 13.0 (7.12) |
Note. Scores are displayed as M (std).
n=36
n=41
n=38.
Baseline prevalence of Personality Disorders
A mean of 1.1 or greater on the Inventory of Interpersonal Problems-Personality Disorder (IIP-PD) screening measure (mean of first 3 subscales) has previously been shown to be an optimal cutpoint for discriminating those with a personality disorder from those without. In this sample, the mean score on this measure was 1.58 (sd= .57). 78 % of the sample scored at or above the cutpoint, suggesting a potentially high prevalence of pre-existing personality disorders in the sample.
Dose, Adherence, and Exposure Data
Among completers (n = 139), 90 % of those in the drug intervention group (65/72) and 88 % of those in the placebo control condition (59/67) successfully transitioned to the maximum pill dose (40 mg or 4 pills daily) by the end of the treatment titration period. The proportion of those taking the maximum pill dose did not differ by condition (Fisher’s exact test p = .79).
Among completers, the average rate of adherence (proportion of days in the study during which the MEMS bottle was opened one or more times, correcting for clinic visits) was 89 % (range= 39 %–100 %, with 82 % of the sample demonstrating adherence on 80 % or more of the days). There was a significant decline in rates of adherence during the fixed treatment period when compared with that during the treatment titration period (Time effect (F (1, 137) = 36.01, p < .001), with this decline being marginally larger in the drug intervention group compared to that among placebo subjects (Time by condition interaction F (1, 137) = 3.63, p = .06, in drug intervention group, 91 % vs. 83 %; in control group, 92 % vs. 87 %). There were no main effects of condition on rates of adherence (p = .48).
Side Effects and Treatment Condition Attribution
Subjects endorsed an average of 2.5 symptoms per session on the Somatic Symptom Scale during the treatment titration phase of the study. There were no significant condition differences in the rates of symptom reporting (p = .4), there were no changes in the rates of symptom reporting over the course of the four treatment titration sessions (p = .8), and there were no significant time-by-condition effects in symptom reports (p = .7).
At visit 8 (2 weeks after the beginning of the drug intervention), participants’ ability to guess condition assignment was only marginally better than chance (58 % correct guesses (79/137) with 65 % correct (43/66) among those assigned to placebo and 51 % correct (36/71) among those assigned to drug intervention; chi square = 3.5, p = .06). By Visit 10 (4 weeks after the beginning of treatment), participants’ ability to guess condition assignment had increased to a higher than chance level among the same group (62 % correct guesses, with 56 % correct among placebo subjects and 68 % correct among the active treatment condition, chi sq, = 7.78, p = .01), presumably because of their experiences during the intervention. In contrast, the research nurses were unable to determine condition assignment at a level above chance at either of the 2 visits (p = .19, n = 132 for Visit 8, p = .21, n = 135 for Visit 10). Current symptom reports at visit 10 were significantly associated with an attribution of assignment to drug intervention among both the nurses and participants, even after statistical adjustment for actual condition assignment (all ps < .001; data available upon request).
Data Reduction for Hostility Measures
We administered 21 hostility subscales (all but the State Anger scale) on the same schedule, once during pre-treatment and once during the fixed treatment period. These were subject to data reduction, with the goal of deriving a smaller subset of theoretically meaningful hostility scores. Exploratory methods (principal components analysis with an eigenvalue criterion of 1 and a varimax solution) were used. Sample size was based upon the portion of the randomized sample with no missing data on any of these measures.
Internal reliabilities for all pre-treatment scale scores were adequate, save for those of the original BDHI subscales (mean alpha=.63, range .42–.72). Therefore, we subjected these to an initial data reduction prior to their inclusion in the omnibus model. Initial PCA yielded a 3 factor solution for the BDHI, with 3 subscales loading (above .50) on the first factor (resentment, suspicion, and guilt), 3 loading on the second factor (assault, verbal, negativism), and 2 loading on the third factor (indirect, irritability) after rotation. The 4 SI-TABP scores were also subject to an initial data reduction; in this case, the first principal component accounted for 69 % of the variance, so item scores were summed across the four ratings and entered as a single factor.
The new pre-treatment BDHI factors (3 scores) were included along with the CMHI (one score), BPAQ (4 scores), STAXI (4 scores), and SI-TABP (one score) subscales as input to an omnibus PCA, once again, yielding a 3 factor solution. After varimax rotation, all of the pre-treatment input measures loaded above .50 on one and only one of the 3 factors, with the exception of the SI-TABP rating score (associated with modest loadings on each of the 3 factors) and the STAXI anger-out scale (which loaded above .50 on two of the three rotated factors). As such, we eliminated these two subscales when calculating factor scale scores. When comparable measures for the post-testing period were used, the same pattern of results was obtained, with the same subscales loading above the .50 threshold on their respective factors.
For each of the two time points, the four subscales loading on the first factor (BPAQ hostility scale, BDHI factor 1, CMHI, and STAXI anger-in scale) appear to represent measures of Hostile Cognition or attitudinal hostility, the 4 subscales loading uniquely on a second factor (BPAQ Anger scale, BDHI factor 3, STAXI Trait Anger scale, and STAXI Anger control) appear to represent measures that focus on Hostile Affect or anger experience, and the three subscales loading uniquely on a third factor (BPAQ Verbal Aggression, BPAQ Physical Aggresssion and BDHI factor 2) appear to represent measures of Hostile Behavior. When examined in this manner, these scales produce measures consistent with a three factor Affect-Behavior-Cognition (ABC) model of hostility which has previously been reported (Martin, Watson, & Wan, 2000) (see Table 3).
Table 3.
Hostility Factor Scores (Varimax Rotated Factor Pattern), Baseline Measures (n = 156)
| Hostile Cognition | Hostile Affect | Hostile Behavior | |
|---|---|---|---|
| Buss Perry Hostility subscale | .80 | (.27) | (.12) |
| Buss Durkee factor 11 | .83 | (.11) | (.13) |
| Cook Medley Hostility scale | .79 | (.06) | (.32) |
| Spielberger Anger-in subscale | .78 | (.02) | (−.22) |
| Buss Perry Anger subscale | (.16) | .75 | (.19) |
| Buss Durkee factor 32 | (.36) | .68 | (−.03) |
| Spielberger Trait Anger subscale | (.33) | .68 | (.37) |
| Spielberger Anger control subscale | (.16) | −.72 | (−.24) |
| Buss Perry Verbal Aggression subscale | (.07) | (.25) | .68 |
| Buss Perry Physical Aggression subscale | (.29) | (.10) | .73 |
| Buss Durkee factor 23 | (.12) | (.18) | .87 |
| Spielberger Anger Out4 | (−.02) | (.61) | (.51) |
| Structured Interview Hostility4, 5 | (−.23) | (.16) | (.38) |
Sum of Resentment, Suspicion, and Guilt subscales
Sum of Indirect and Irritability subscales
Sum of Assault, Verbal, and Negativism subscales
These two scores were not included in the final hostility factor measures.
Sum of four hostility ratings for Structured Interview (Hostile Content, Hostile Style, Hostile Intensity, Potential for Hostility)
Unit weighted measures were derived for each of these 3 factors by averaging the standard (z) scores associated with each of the highly loading component subscales. Fixed treatment period factor scores were normalized to pre-treatment using pre-treatment subscale means and standard deviations.
There were no significant differences between attritioners and nonattritioners with respect to pre-treatment measures of hostility (all p values > .30). There was a marginally significant condition effect in the pre-treatment Hostile Affect score (t (154) = 1.68, p = .096), however, with slightly higher scores in the citalopram condition compared to controls.
Major Results
Maximum likelihood methods (SAS Proc Mixed) were used to estimate the effects of condition assignment (placebo vs. drug), time (pre-treatment vs. fixed treatment) and their interaction on each of the three hostility factor scores. When repeated measures models are used, such methods can handle missing data points for the dependent variable, at pre-test or post-test, without the use of listwise deletion, allowing for a fuller utilization of existing data. Analyses were run using all randomized participants (n = 159, intention to treat model) and, again, using only those who completed the intervention (n =139). Because the results of these two approaches did not substantially differ, we present only the intention to treat model results here.
Hostility Factor Scores
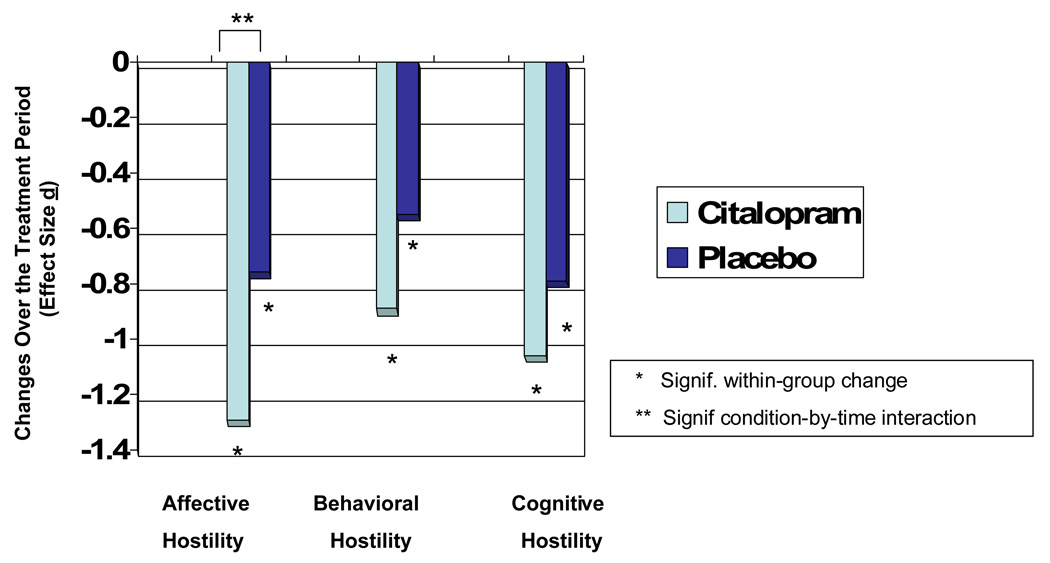
Both the citalopram and the control conditions were associated with significant reductions in Hostile Affect, Behavior, and Cognition (significant main effects for time for each, with p < .001 in each case). Citalopram intervention, however, was associated with significant and specific effects only on Hostile Affect (time by condition interaction t (1, 133) = 2.29, p = .02) and not on Hostile Behavior (p = .13) or cognition (time by condition interaction effects (p = .26). Adjusting for pre-treatment Hostile Affect did not alter the magnitude of this observed effect (time by condition interaction t (1, 133) = 2.34, p = .02). The effect size for the change in Hostile Affect in the drug intervention group was substantial in this study (d = 1.29), and it far exceeded that associated with either of the other two hostility factor scores (d =.89 for Hostile Behavior, d = .75 for Hostile Cognition, see Figure 2). Because of the large placebo effects, however, the correlation between condition assignment and change in hostility (that is, the effect size associated with specific treatment effects) was only modest (phi coefficient = −.17 for Hostile Affect, −.13 for Hostile Behavior, and −.09 for Hostile Cognition).
Figure 2.
Treatment effects on hostility factor scores, by condition
Individual Hostility Scale Scores
For exploratory purposes, and for comparison with the previous literature, we examined individual scale components of the factors we developed here, to determine whether there were any specific measures that may have uniquely contributed to the observed treatment effect. Three component scales that contributed to the Hostile Affect factor were associated with significant condition-by-time interaction effects (BDHI Irritability t (1, 134)= −2.11, p = .04 , BDHI Indirect t (1, 134) = −2.09, p = .04, and BPAQ Anger scale t (1, 136) = −3.12, p = .002), along with one of the component measures that contributed to the Hostile Behavior factor (BDHI Assaultiveness scale, t (1, 134) = −2.3, p = .02. Two of these measures (BDHI Irritability and BDHI Assaultiveness) have been previously shown to be associated with significant SSRI intervention effects (Knutson et al., 1998). On these measures, changes in hostility in the drug intervention group were somewhat larger in the present sample than that shown previously (for Assaultiveness, d = .67 in the present sample vs. .60 in Knutson et al., for Irritability, d = .99 in the present sample vs. .30 in Knutson et al.) although the magnitude of the specific treatment effects were roughly equivalent (phi= .24 in previous sample vs. .19 in the present sample for Assaultiveness; .17 in previous sample vs. .18 in present sample for Irritability). None of the component scales associated with Hostile Cognition was associated with significant condition-by-time interaction effects in this sample.
Structured Interview Ratings
We examined intervention effects on the SI-TABP ratings, which were not included in the factor score calculations described above. When we examined each of the four SI-TABP hostility ratings within the intention to treat sample, none of the relevant condition-by-time interaction effects were significant, although two of these (Hostile Intensity and Potential for Hostility) were marginally so (p = .05 and p = .08, respectively). Hostile Intensity ratings showed significant declines in the drug group (b = −.2713, t = −2.25, p = .02) but not in placebo (b = .07, p = .58), as predicted. Potential for Hostility ratings, on the other hand, were associated with increases in the placebo group (b = .2215, t = 1.96, p = .05) but no change among those assigned to the drug condition ( b = −.05, p = .63).
Spielberger State Anger Scale Scores
Unlike all of the other measures of hostility, the STAXI State Anger scale was administered at multiple points in time during the pre-treatment, treatment titration, and fixed treatment phases of the study. We used growth curve analyses of these data (Proc Mixed) which permitted us to make full use of the information provided at the multiple time points. Data for the pre-treatment (Visits 2 through 7), treatment titration (Visits 7 through 11), and fixed treatment periods (Visits 11 through 13) were run separately, since we expected different trends for each.
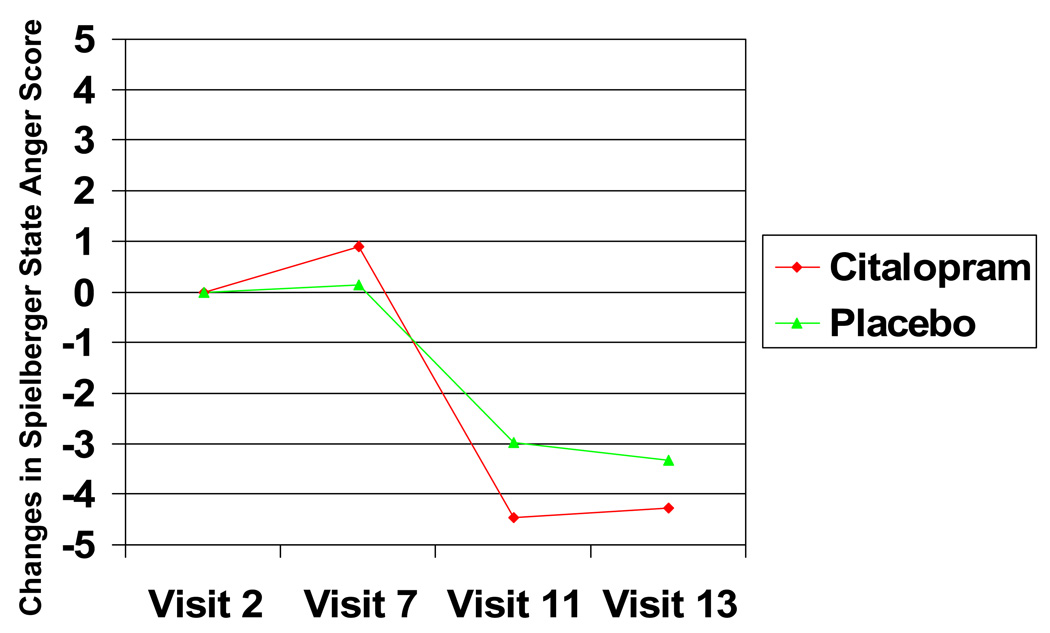
During the pre-treatment phase, State Anger was stable over time (Time effect p = .81), and was not associated with eventual condition assignment ( p = .22). The condition-by-time interaction effect on State Anger was also nonsignificant during pre-treatment (p = .30; estimated State Anger scores at end of pre-treatment were 20.6 for drug condition and 19.0 for placebo). During the treatment titration period, there was a significant Time effect on State Anger (F 1, 579) = 23.99, p < .0001), modified by a significant Time-by-Condition interaction effect (F (1, 579) = 6.53, p = .01; main effect of condition was marginal, p = .07). As hypothesized, the linear change in State Anger over the course of the intervention was greater in the drug group than among controls (see Figure 3; estimated State Anger scores at end of treatment titration were 14.7 for drug condition and 15.7 for placebo). This was a large effect size (d = 1.39) within the drug group and a small (phi= .11) specific treatment effect. Adjusting for pre-treatment (Visit 7) State Anger did not substantially alter the magnitude of this observed effect (time by condition interaction (F (1, 573) = 5.46, p = .02)).
Figure 3.
Treatment effects on Spielberger State Anger scores (scale range= 10–40), by condition
There was no detectable change in State Anger during the fixed treatment period (Time effect nonsignificant during this phase, p = .45) nor were there significant condition or time-by-condition effects (p s = .27, .39, respectively; estimated State Anger scores at the end of the fixed treatment period were 15.4 for drug condition and 15.7 for placebo). Drug related changes in State Anger that were observed at the end of treatment titration were maintained at the end of the fixed treatment period. When we analyzed the data from Visit 7 (end of pre-treatment) to Visit 13 (2 data points), we observed a significant effect of condition at Visit 7 (F (1,157) = 4.46, p = .04), but continued to observe a significant Time effect on State Anger (F (1, 125) = 21.17, p =.0001), modified by a significant Time-by-Condition interaction (F 1, 125)= 4.26, p = .04).
Other Psychosocial Measures
We examined the effects of the intervention on three other psychosocial characteristics: impulsivity (as measured by the Barratt Impulsiveness scale), depressive symptoms (as measured by the BDI and by the CES-D), and perceived social support (as measured by the Interpersonal Support Evaluation List; ISEL). Although impulsivity showed significant declines from pre-treatment to fixed treatment (significant effects of time, F (1, 136) = 15.89, ,p = .0001 for overall score), there were no significant condition effects or condition-by-time interactions (p = .85, .15, respectively for overall score). Similarly, on the CES-D, there were significant declines in depressive symptomatology from pre-treatment to fixed treatment (Time effect F (1, 137)= 12.13, p = .0007), but there was no significant effect of condition (p = .17) or condition by time interaction (p = .35). The drug group endorsed more symptoms than the placebo condition on the BDI at pre-treatment (Condition effect ( F (1, 157) = 4.45, p = .04, mean of 11.06 for Drug, mean of 8.7 for Placebo), but, once again, Condition did not modify the effects of time on BDI scores (Time effect F (1, 137) = 6.56, p = .01; Time by Condition p = .26).
Perceived social support was associated with a different pattern of results than the measures described above. There were no main effects of time (p = .80) or condition (p = .51) on total ISEL scale scores. There was a clear treatment-related effect however: a significant Time by Condition interaction (F (1, 134) = 4.2, p = .04), such that those in the drug intervention condition showed a significant increase in perceived social support over the course of the study (t (134) = 3.18, p = .008; effect size = .49) and those in the placebo control group did not (t (134) = .25, p = .80; effect size= .04, specific treatment effect size r = .17).
Follow-up analyses showed significant time by condition effects for only one of the four ISEL component subscales: Self esteem support (F (1, 134) = 7.42, p = .007), with increases occurring in the drug intervention group (p < .0001), but not among controls, (p = .6). Belonging support was associated with a marginally significant condition by time effect p = .08, with increases in treatment and controls of p = .004, and p = .6, respectively). Tangible and Appraisal support were associated with no treatment-related changes (condition by time p = .13 and .57, respectively).
Effects of the drug intervention on hostility and social support appeared to be largely independent of one another: When the change in ISEL scores between pre-treatment and fixed treatment was entered as a covariate term, the Time by Condition effect on Hostile Affect remained significant (F (1, 133) = 3.93, p = .0496, and co-varying for change in Hostile Affect did not substantially alter the Time by Condition interaction effect on ISEL scores (F (1, 133) = 2.05, p = .04).
Ancillary Analyses: Moderating effects
Of Adherence
We examined the effects of adherence as a moderator of intervention outcomes. For these analyses, we restricted our analyses to the sample of participants that completed the intervention. When we examined the drug condition separately, there were no significant effects of adherence on treatment outcome. When we examined both conditions together, there were no significant moderating (i.e., time by condition by adherence) effects of adherence on outcomes for pre-post measures of hostility or for any of the other psychosocial variables. There were also no significant adherence-related differences in the effects of the intervention on State Anger (slope of change during visits 7 through 11).
Of Drug Exposure
We examined the effects of degree of drug exposure on intervention outcomes within the active drug group. There were no significant associations between degree of drug exposure (average ug/L/day) and pre-post changes in hostility or the degree of change (visits 7–11) in State Anger. With respect to the other psychosocial variables, only changes in BDI scores were associated with degree of drug exposure, such that those in the drug intervention group with greater average serum drug concentrations over the course of the intervention showed larger pre-post reductions in BDI scores (r = −.25, n = 72, p = .04).
Of Gender
We examined the moderating effects of gender as a main effect and as a moderator of intervention outcomes. At pre-treatment, there were significant gender differences associated with Hostile Affect by t-test ( t (154) =2.24, p = .03, with men showing higher standard scores, .10 vs. −.10), but there were no pre-existing differences in Hostile Cognition or Behavior (p = .07, p = .13, respectively). There were significant gender differences in perceived social support (ISEL) at pre-treatment (t (155) = 3.41, p = .0008, with women showing higher scores, 89.5 vs. 80.2), but there were no pre-existing gender differences in depressive symptoms (BDI or CES-D) or impulsivity (BIS).
With respect to gender differences in intervention response, women responded more favorably to the drug intervention than did men, but this difference was only significant for pre-post measures of Hostile Behavior (Time by Condition by Gender F (1, 131) = 4.69, p = .03; Time by Condition effect significant for women (F (1, 69) = 8.53, p = .005, but not for men p = .63), and not for Hostile Affect (p = .18 for 3-way interaction) or Cognition (p = .15). There were significant changes over time within the drug intervention group for each of these measures for both men and women; these moderating effects, then, pertain specifically to gender differences in the relative magnitude of condition differences over time.
There were no gender differences in the effects of treatment (visits 7–11) on current measures of state anger. There were no significant moderating effects of gender on treatment outcomes for any of the other psychosocial variables.
Discussion
We showed that a two-month citalopram intervention reduces self-ratings of hostility in a sample of individuals free from major Axis I disorders but with initially elevated hostility scores. Active drug effects were significantly larger than placebo effects for measures of state anger and hostile affect and, among women, for reports of hostile behavior (verbal and physical) as well. Interview behavior indicative of hostility was also reduced by citalopram, although these effects were only marginally greater than those associated with placebo. Hostile cognitions or attitudes were not differentially affected by the use of the drug.
This was the largest placebo-controlled study of SSRI treatment for hostility, and the only study of this type which has examined such effects in a sample free of major Axis I psychopathology selected for high hostile traits. The exclusion of concurrent major Axis I diagnoses in our participants ensured that any effects observed could not be attributed simply to the well-known effects of the drug on reducing depressive or anxious symptoms. At the same time, selection for high hostile traits contributed to the clinical significance of the study and potentially enhanced the power to detect effects. Another strength of the design involved the use of verified adherence measures; our data suggest that treatment adherence was adequate in both conditions. Perhaps as a result of these sampling procedures, design features, and the duration of treatment, the effect sizes associated with hostility changes in the drug intervention group were larger in this study than in previous comparable research.
In contrast to the large body of evidence linking use of SSRI’s with relief of depressive symptoms, we showed no effects of citalopram on depressive symptomatology in this study. Once again, subject selection criteria may account for these differences: In the present sample, we excluded those with a current diagnosis of major depressive disorder. Because mean levels of depressive symptoms at pre-treatment were low, there was a potential floor effect on these measures, with little room for change with treatment. Of note, however, we did demonstrate an effect of drug exposure (a measure that took into consideration individual differences in drug clearance and adherence) on changes in depressive symptoms in the drug intervention group.
We showed specific treatment effects on Hostile Affect, no specific effects on Hostile Cognition, and specific effects on Hostile Behavior that were intermediate in magnitude. It is possible that hostile affect or behavior are more strongly or specifically associated with central serotonergic systems than hostile cognition. Existing evidence linking increased central serotonergic function with behavioral constraint (Depue & Spoont, 1986; Spoont, 1992) would appear to be most relevant to the normalization of affective and behavioral rather than to cognitive dimensions of hostility.
There appeared to be substantial placebo effects across all three domains of hostility in this study, as illustrated by the significant effects of time on each of these measures in the placebo condition. Perhaps because of its lack of clear behavioral or experiential referent, Hostile Cognition appeared to be somewhat most susceptible to such placebo effects. We observed significant gender differences in this study, with the effects of citalopram on behavioral hostility being larger for women than for men. We might speculate that men and women may differ in the extent to which affective factors may drive verbal and physical aggression, such that the two may more closely “travel together” for women, when compared with men. More research is needed examining potential gender differences in responsiveness to interventions of this sort.
Citalopram led to significant increases in perceived social support as well as decreases in hostility in this study. These effects appeared to be accounted for by changes in perception of one’s standing relative to one’s peers (self-esteem and belonging support), rather than by changes in the perception of availability of help from others per se (tangible and appraisal support). Such findings are consistent with the evidence linking serotonergic function with increased dominance in animals ((Higley, King et al., 1996; Raleigh, McGuire, Brammer, Pollack, & Yuwiler, 1991) and with the evidence suggesting that tryptophan administration may enhance self-ratings of dominance in interpersonal interactions among human volunteers (aan het Rot, Moskowitz, Pinard, & Young, 2006). Interestingly, treatment-related changes in hostility and perceived social support were largely independent in this current study; these may represent independent pathways by which serotonergic function may affect social behavior.
There were a number of limitations associated with this study. First, because the study design required compliant treatment seekers, we may have reduced the representation of individuals with a more impulsive or antisocial aggressive style. If this is the case, it is possible that the effects of the intervention on behavioral hostility per se may not have been accurately portrayed (understated or overstated) in this study. Second, the numerous visits associated with this study with the associated intensive self-observation and contact with research staff may have exacerbated placebo effects. Because pharmacological treatment in the natural environment, especially those associated with an internist’s prescription, may involve a less intensive response burden, it is possible that such conditions would produce effects that are stronger relative to placebo. Being more conservative thereby, the current design may have understated the magnitude of effects. Because there are differences in the neuropharmacologic effects of different SSRIs, the extent to which the effects observed here may generalize to other drugs in the same class cannot be determined from this study. Similarly, the extent to which our specific findings (e.g., reductions in hostile affect with SSRIs) may generalize to more representative samples (i.e., unselected with respect to hostility ratings) cannot be determined based upon these results. It should be acknowledged that the measures assessed here involve self-reports, which, while widely employed, are only indirect indices of observable behavior. Finally, it is important to acknowledge that the potential effects of such an intervention on CVD outcomes or in CVD patients cannot be determined from this study.
Whereas we excluded those with current anxiety disorders and depression, we did not screen for Intermittent Explosive Disorder in this study, a DSM-IV disorder of impulse control. The prevalence of this disorder is not known in the current sample, but it is likely elevated relative to community norms. Similarly, our screening instrument (IIP-PD) suggested a high potential prevalence of personality disorders in this sample. Future studies should consider the treatment implications of comorbid diagnostic heterogeneity among high hostile individuals. Of note, we do not believe that the potentially high prevalence of these disorders in our sample detracts from our efforts to obtain a diagnostically “clean” sample, as these conditions (in contrast to anxiety, mood and other Axis I disorders) are conceptually intertwined with hostility, and can be seen as a logical consequence of the characteristics that were selected for here.
Although there were a number of limitations of this study, the design was also associated with a number of strengths. The exclusion of individuals with major current Axis I disorders has already been cited. Another strength involves the use of hostility measures that have been previously linked with increased CVD risk. Interestingly, the specific measures of hostility most frequently linked with disease risk in the literature (for example, the CMHI, the SI-TABP, and The STAXI Trait Anger scale) were, in fact, not associated with significant drug intervention effects here.
On the other hand, it may be most useful to attend to factor scores rather than to individual scale score results in interpreting findings from the present study. Another strength of the current investigation involves its capacity to examine hostility as a multidimensional construct. Marginal treatment effects shown across a number of correlated scales may have been amplified by the aggregation methods used here, providing more robust and interpretable results than might be apparent in examining individual scales. Because most of the previous studies in this area have involved single scales, the existing literature does not enable us to determine the extent to which affective, behavioral, or cognitive components of hostility are differentially associated with elevated risk. Future research examining the disease risks linked with hostility, and interventions for reducing it, should employ multiple methods of assessment, in a manner that will allow us to more clearly characterize the components of this construct that are most pathogenic (Suls & Bunde, 2005).
The results in this study, that citalopram has specific treatment effects on hostile affect and perceived social support, has several important implications. First, these findings have implications for understanding some of the neurobiological mechanisms by which hostility and other psychosocial measures that have been found to be linked with cardiovascular disease may be interrelated. We have previously shown that shared genetic factors may account for the interrelationship between some measures of hostility, depression, and social support, for example (Raynor, Pogue-Geile, Kamarck, McCaffery, & Manuck, 2002); the present findings are consistent with the possibility that central serotonergic function may be one of the candidate pathways accounting for such shared genetic effects. Such a speculation would need to be tempered by the recognition that the effects on hostility and social support were relatively uncorrelated in the present study, perhaps representing effects for which there may be differential susceptibility across individuals.
Second, in addition to their implications for understanding the relationships between psychosocial factors implicated in CHD, these findings may also have implications for understanding the mechanisms accounting for the observed associations between hostility and disease per se. There is some evidence that central serotonergic functioning may be implicated in a number of behavioral and biological risk factors for coronary heart disease (Muldoon et al., 2006; Muldoon et al., 2004). Recent correlational findings showing an enhanced prognosis associated with the use of serotonergic drugs among patients with cardiovascular disease (Glassman et al., 2002; Rasmussen et al., 2003; Taylor et al., 2005) are consistent with the notion that this neurotransmitter system may be important in disease pathogenesis. The central and peripheral physiologic effects associated with serotonin availability are quite diverse (including, for example, effects on peripheral platelet function (Menys, Smith, Lewins, Farmer, & Noble, 1996; Pollock, Laghrissi-Thode, & Wagner, 2000). The present findings are consistent with the possibility that the pathogenic effects of hostility may be linked to disease risk by virtue of a common association with central serotonergic function, by whatever mechanisms these effects may be conferred.
Finally, this study also represents a possible contribution to the literature on the treatment effects of serotonergic drugs, suggesting that the impact of such interventions on hostility may be relatively independent of their well-known effects on depression, and that such interventions may potentially have application beyond those with major Axis I disorders. Importantly, such findings bear replication before their specific implications for treatment can be confirmed, and the risk-benefit ratio of such interventions should be considered, in light of potential side effects of SSRIs, the population in question (Friedman & Leon, 2007), and alternative treatment options available. More intervention research is needed in at-risk and in cardiac samples examining the effects of SSRIs on longer term health prognosis. Such studies should examine the relative effectiveness and cost-effectiveness of pharmacologic and behavioral treatments for hostility (cf (Gidron, Davidson, & Bata, 1999) in these samples, and the differential mechanisms that may be implicated in each.
Acknowledgments
This research was supported by the National Heart, Lung, and Blood Institute (HL040962) and by the Pittsburgh Mind-Body Center (HL076852 [University of Pittsburgh], HL076858 [Carnegie Mellon University]). Some of these data were presented previously at the Annual Meeting of the Society of Behavioral Medicine, March, 2007, Washington, DC. One of the co-authors of this paper, Bruce Pollock, is a faculty member of the Lundbeck Institute, has served as a consultant for Takeda and Lundbeck, and has served on the advisory board for Forest Laboratories, which produces citalopram.
Appendix
CONSORT Checklist
| TITLE AND ABSTRACT | How participants were allocated to intervention | 1–2 |
| INTRODUCTION | Scientific background and rationale | 3–6 |
| METHODS | ||
| Participants | Eligibility criteria, settings and locations | 7 |
| Interventions | Details of interventions, how and when | 9–11 |
| Objectives | Specific objectives and hypotheses | 6 |
| Outcomes | Clearly defined outcome measures, methods | 11–17, 18–20 |
| Sample size | How sample size determined, stopping rules | 10 |
| Randomization | Method used to generate random allocation | |
| Sequence Generation | 9 | |
| Allocation concealment | 9–11 | |
| Blinding | Including success of blinding | 9–11, 17 |
| Statistical methods | 16, 19–21 | |
| RESULTS | ||
| Participant flow | 7–11, 45, 50 | |
| Recruitment | Dates defining periods of recruitment, follow-up | 11 |
| Baseline data | Demographic, clinical characteristics | 8,17–18, 46–47 |
| Numbers analyzed | Number of participants, intention to treat | 10–11, 21, 45, 50 |
| Outcomes and estimation | Results and effect sizes | 21–27 |
| Ancillary analyses | Reporting other analyses performed | 24–27 |
| Adverse events | Report side effects in each intervention group | 18–19 |
| DISCUSSION | ||
| Interpretation | 27–33 | |
| Generalizability | 27–31 | |
| Overall evidence | 31–33 | |
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at a. http://www.apa.org/journals/ccp
The Visit 1 re-screening procedure was not initiated until 9 months after data collection was begun, therefore, the first 36 participants were not re-screened (25 of these were randomized into active or placebo treatment). All of the subjects were administered complete versions of the Cook Medley and Buss Durkee scales during Visits 4 and 5. We derived re-screening scores from these complete scales to examine whether any of those subjects who were not re-screened might have been excluded had we begun the rescreening procedures earlier. 7 of the relevant 25 subjects (28 %) scored below the respective gender-specific cut scores for hostility on one or both scales during Visits 4 and 5. Among all of the subjects who were re-screened (n = 193), 68 (35 %) scored below criteria on the hostility re-screening measures during Visit 1 and were excluded from the study. All of the participants in this study met initial hostility criteria during the telephone intake interview.
Pre-treatment and post-treatment administration of the State Anger scale was not initiated until 9 months after data collection was begun.
By request, all of the pre-treatment data were destroyed for one of the attritioners; all subsequent analyses are based upon the remainder of the sample (n = 159).
Contributor Information
Thomas W. Kamarck, University of Pittsburgh
Roger F. Haskett, University of Pittsburgh
Matthew Muldoon, University of Pittsburgh.
Janine D. Flory, Queens College, Flushing, NY
Barbara Anderson, University of Pittsburgh.
Rob Bies, University of Pittsburgh.
Bruce Pollock, University of Pittsburgh.
Stephen B. Manuck, University of Pittsburgh
References
- aan het Rot M, Moskowitz DS, Pinard G, Young SN. Social behaviour and mood in everyday life: The effects of tryptophan in quarrelsome individuals. Journal of Psychiatry and Neuroscience. 2006;31:253–262. [PMC free article] [PubMed] [Google Scholar]
- Barefoot JC, Dodge KA, Peterson BL, Dahlstrom WG, Williams RB. The Cook-Medley hostility scale: Item content and ability to predict survival. Psychosomatic Medicine. 1989;51:46–57. doi: 10.1097/00006842-198901000-00005. [DOI] [PubMed] [Google Scholar]
- Barefoot JC, Lipkus IM. The assessment of anger and hostility. In: Smith TW, editor. Anger, Hostility, and the Heart. Hillsdale, N.J: Erlbaum; 1994. pp. 43–66. [Google Scholar]
- Barratt ES. Impulsiveness subtraits: Arousal and information processing. In: Spence JT, Izard CE, editors. Motivation, Emotion, and Personality. Amsterdam: Elsevier Science Publishers; 1985. pp. 137–146. [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory. 2nd edition. San Antonio, TX: Psychological Corporation; 1996. ed. [Google Scholar]
- Berkman LF, Leo-Summers L, Horwitz RI. Emotional support and survival after myocardial infaction: A prospective, population-based study of the elderly. Annals of Internal Medicine. 1992;117:1003–1009. doi: 10.7326/0003-4819-117-12-1003. [DOI] [PubMed] [Google Scholar]
- Bies RR, Feng Y, Lotrich FE, Kirshner MA, Roose S, Kupfer DJ, et al. Utility of sparse concentration sampling for citalopram in elderly clinical trial subjects. Journal of Clinical Pharmacology. 2004;44:1352–1359. doi: 10.1177/0091270004269647. [DOI] [PubMed] [Google Scholar]
- Bleil ME, McCaffery JM, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Anger-related personality traits and carotid artery atherosclerosis in untreated hypertensive men. Psychosomatic Medicine. 2004;66:633–639. doi: 10.1097/01.psy.0000138128.68838.50. [DOI] [PubMed] [Google Scholar]
- Botchin MB, Kaplan JR, Manuck SB, Mann JJ. Low versus high prolactin responders to fenfluramine challenge: Marker of behavioral differences in adult male cynomolgus macaques. Neuropsychopharmacology. 1993;9:93–99. doi: 10.1038/npp.1993.47. [DOI] [PubMed] [Google Scholar]
- Burke LE. Electronic measurement. In: Burke LE, Ockene IS, editors. Compliance in healthcare and research. Armonk, N.Y: Futura Publishing Company; 2001. pp. 117–138. [Google Scholar]
- Bushman BJ, Cooper HM, Lemke KM. Meta-analysis of factor analyses: An illustration using the Buss-Durkee Hostility Inventory. Personality and Social Psychology Bulletin. 1991;17:344–349. [Google Scholar]
- Buss AH, Durkee A. An inventory for assessing different kinds of hostility. Journal of Consulting Psychology. 1957;21:343–349. doi: 10.1037/h0046900. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The Aggression Questionnaire. Journal of Personality and Social Psychology. 1992;63:452–459. doi: 10.1037//0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Cherek DR, Lane SC, Pietras CJ, Steinberg JL. Effects of chronic paroxetine administration on measures of aggressive and impuslive responses of adult males with a history of conduct disorder. Psychopharmacology. 2002;159:266–274. doi: 10.1007/s002130100915. [DOI] [PubMed] [Google Scholar]
- Cleare AJ, Bond AJ. Does central serotonergic function correlate inversely with aggression? A study using D-fenfluramine in healthy subjects. Psychiatry Research. 1997;69:89–95. doi: 10.1016/s0165-1781(96)03052-1. [DOI] [PubMed] [Google Scholar]
- Coccaro EF. Central serotonin activity and aggression: Inverse relationship with prolactin response to d-Fenfluramine, but not CSF 5-HIAA concentration, in human subjects. American Journal of Psychiatry. 1997;154:1430–1435. doi: 10.1176/ajp.154.10.1430. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Kavoussi RJ. Fluoxetine and impulsive aggressive behavior in personality-disordered subjects. Archives of General Psychiatry. 1997;54:1081–1088. doi: 10.1001/archpsyc.1997.01830240035005. [DOI] [PubMed] [Google Scholar]
- Coccaro EF, Siver LJ, Kalar HM, Maurer G, Cochrane K, Cooper TB, et al. Serotonergic studies in patients with affective and personality disorders. Archives of General Psychiatry. 1989;46:587–599. doi: 10.1001/archpsyc.1989.01810070013002. [DOI] [PubMed] [Google Scholar]
- Cohen S, Mermelstein R, Kamarck TW, Hoberman HM. Measuring the functional components of social support. In: Sarason IG, Sarason BR, editors. Social support: Theory, research and apoplications. Boston, MA: Martinus Nijhoff; 1985. pp. 73–94. [Google Scholar]
- Cook WW, Medley DM. Proposed hostility and pharisaic virtue scales for the MMPI. The Journal of Applied Psychology. 1954;38:414–418. [Google Scholar]
- Dembroski TM, MacDougall JM, Costa PT, Grandits GAPM. Components of hostility as predictors of sudden death and myocardial infarction in the multiple risk factor intervention trial. Psychosomatic Medicine. 1989;51:514–522. doi: 10.1097/00006842-198909000-00003. [DOI] [PubMed] [Google Scholar]
- Depue RA, Spoont MR. Conceptualizing a serotonin trait: A behavioral dimension of constraint. Annals of the New York Academy of Sciences. 1986;487:47–62. doi: 10.1111/j.1749-6632.1986.tb27885.x. [DOI] [PubMed] [Google Scholar]
- Everson-Rose SA, Lewis TT. Psychosocial factors and cardiovascular diseases. Annual Review of Public Heatlh. 2005;26:469–500. doi: 10.1146/annurev.publhealth.26.021304.144542. [DOI] [PubMed] [Google Scholar]
- Fava M, Rosenbaum JR, Pava JA, McCarthy MK, Steingard RJ, Bouffides E. Anger attacks in unipolar depression, Part 1: Clinical correlates and response to fluoxetine treatment. American Journal of Psychiatry. 1993;150:1158–1163. doi: 10.1176/ajp.150.8.1158. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured clinical interview for DSM-IV-TR Axis I Disorders, Research Version, Non-patient edition (SCID-I/NP) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Flory JD, Mann JJ, Manuck SB, Muldoon MF. Recovery from major depression is not associated with normalization of serotonergic function. Biological Psychiatry. 1998;43:320–326. doi: 10.1016/s0006-3223(97)00480-0. [DOI] [PubMed] [Google Scholar]
- Friedman RA, Leon AC. Expanding the black box--depression, antidepressants, and the risk of suicide. New England Journal of Medicine. 2007;356:2343–2346. doi: 10.1056/NEJMp078015. [DOI] [PubMed] [Google Scholar]
- Gellman A, Carlin JB, Stern HS, Rubin DB. Bayesian data analysis. Boca Raton, FL: Chapman & Hall; 1995. [Google Scholar]
- Gidron Y, Davidson K, Bata I. The short-term effects of a hostility-reduction intervention on male coronary heart disease patients. Health Psychology. 1999;18:416–420. doi: 10.1037//0278-6133.18.4.416. [DOI] [PubMed] [Google Scholar]
- Glassman AH, O'Connor CM, Califf RM, Swedberg K, Schwartz P, Bigger JT, et al. Sertraline treatment of major depression in patients with acute MI or unstable angina. JAMA. 2002;288:701–709. doi: 10.1001/jama.288.6.701. [DOI] [PubMed] [Google Scholar]
- Grano N, Virtanen M, Vahtera J, Elovainio M, Kivimaki M. Impulsivity as a predictor of smoking and alcohol consumption. Personality and Individual Differences. 2004;37:1693–1700. [Google Scholar]
- Harris JA. Confirmatory factor-analysis of the Aggression Questionnaire. Behaviour Research and Therapy. 1995;33:991–993. doi: 10.1016/0005-7967(95)00038-y. [DOI] [PubMed] [Google Scholar]
- Higley JD, King ST, Hasert MF, Champoux M, Suomi SJ, Linnoila DM. Stability of interindividual differences in serotonin function and its relationship to severe aggression and competent social behavior in Rhesus Macaque females. Neuropsychopharmacology. 1996;14:67–76. doi: 10.1016/S0893-133X(96)80060-1. [DOI] [PubMed] [Google Scholar]
- Higley JD, Mehlman PT, Higley SB, Fernald B, Vickers J, Lindell SG, et al. Excessive mortality in young free-ranging male nonhuman primates with low cerebrospinal fluid 5-hydroxyindoleacetic acid concentrations. Archives of General Psychiatry. 1996;53:436–441. doi: 10.1001/archpsyc.1996.01830060083011. [DOI] [PubMed] [Google Scholar]
- Knutson B, Wolkowitz OM, Cole SW, Chan T, Moore EA, Johnson rC, et al. Selective alteration of personality and social behavior by serotonergic intervension. American Journal of Psychiatry. 1998;155:373–379. doi: 10.1176/ajp.155.3.373. [DOI] [PubMed] [Google Scholar]
- Kruesi MJ, Rapoport JL, Hamburger S, Hibbs E, Potter WZ, Lenane M, et al. Cerebrospinal fluid monoamine metabolites, aggression, and impulsivity in disrutpive behavior disorders of children and adolescents. Archives of General Psychiatry. 1990;47:419–426. doi: 10.1001/archpsyc.1990.01810170019003. [DOI] [PubMed] [Google Scholar]
- Malone KM, Waternaux C, Haas GL, Cooper TB, Li S, Mann JJ. Cigarette smoking, suicidal behavior, and serotonin function in major psychiatric disorders. American Journal of Psychiatry. 2003;160:773–779. doi: 10.1176/appi.ajp.160.4.773. [DOI] [PubMed] [Google Scholar]
- Mann JJ, McBride A, Malone KM, DeMeo M, Kelip J. Blunted serotonergic responsivity in depressed inpatients. Neuropsychopharmacology. 1995;13:53–64. doi: 10.1016/0893-133X(95)00016-7. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, Ferrell RE, Mann JJ, Muldoon MF. A regulatory polymorphism of the monoamine oxidase-A gene may be associated with variability in aggression, impulsivity, and central nervous system serotonergic responsivity. Psychiatry Research. 2000;95:9–23. doi: 10.1016/s0165-1781(00)00162-1. [DOI] [PubMed] [Google Scholar]
- Manuck SB, Flory JD, McCaffery JM, Matthews KA, Mann JJ, Muldoon MF. Aggression, impulsivity and central nervous system serotonergic responsivity in a nonpatient sample. Neuropsychopharmacology. 1998;19:287–299. doi: 10.1016/S0893-133X(98)00015-3. [DOI] [PubMed] [Google Scholar]
- Martin R, Watson D, Wan CK. A three-factor model of trait anger: Dimensions of affect, behavior, and cognition. Journal of Personality. 2000;68:869–897. doi: 10.1111/1467-6494.00119. [DOI] [PubMed] [Google Scholar]
- Mehlman PT, Higley JD, Faucher I, Lilly AA, Taub DM, Vickers J, et al. Correlation of CSF 5-HIAA concentration with sociality and the timing of emigration in free-ranging primates. American Journal of Psychiatry. 1995;152:907–913. doi: 10.1176/ajp.152.6.907. [DOI] [PubMed] [Google Scholar]
- Menys VC, Smith C, Lewins P, Farmer R, Noble M. Platelet 5-hydrosytrptamine is decreased in a preliminary group of depressed patients receiving the 5-hydroxytrptamine re-uptake inhibiting drug fluoxetine. Clinical Science. 1996;91:87–92. doi: 10.1042/cs0910087. [DOI] [PubMed] [Google Scholar]
- Miller TQ, Smith TW, Turner CW, Guijarro ML, Hallet AJ. A meta-analytic review of research on hostility and physical health. Psychological Bulletin. 1996;119 doi: 10.1037/0033-2909.119.2.322. 322-248. [DOI] [PubMed] [Google Scholar]
- Morse JQ, Pilkonis PA. Screening for personality disorders. Journal of Personality Disorders. 2007;21:179–198. doi: 10.1521/pedi.2007.21.2.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz DS, Pinard G, Zuroff DC, Annable L, Young SN. The effect of tryptophan on social interaction in everyday life: A placebo-controlled study. Neuropsychopharmacology. 2001;25:277–289. doi: 10.1016/S0893-133X(01)00219-6. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, et al. Effects of lovastatin on cognitive function and psychological well-being. American Journal of Medicine. 2000;108:538–547. doi: 10.1016/s0002-9343(00)00353-3. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Mackey RH, Korytkowski MT, Flory JD, Pollock BG, Manuck SB. The metabolic syndrome is associated with reduced central serotonergic responsivity in healthy community volunteers. Journal of Clinical Endocrinology and Metabolism. 2006;91:718–721. doi: 10.1210/jc.2005-1654. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Mackey RH, Williams KV, Korytkowski MT, Flory JD, Manuck SB. Low central nervous system serotonergic responsivity is associated with the metabolic syndrome and physical inactivity. Journal of Clinical Endocrinology and Metabolism. 2004;89:266–271. doi: 10.1210/jc.2003-031295. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Evans DL, Nemeroff CB. The relationship of depression to cardiovascular disease: Epidemiology, biology, and treatment. Archives of General Psychiatry. 1998;55:580–592. doi: 10.1001/archpsyc.55.7.580. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Schatzberg AF. Pharmacological treatments for unipolar depression. In: Nathan PE, Gorman JM, editors. A guide to treatments that work. 3rd ed. New York: Oxford University Press; 2007. pp. 271–307. [Google Scholar]
- Pilkonis P, Yookyung K, Proietti JM, Barkham M. Scales for personality disorders developed from the Inventory of Interpersonal Problems. Journal of Personality Disorders. 1996;10:355–369. [Google Scholar]
- Pollock BG, Laghrissi-Thode F, Wagner WR. Evaluation of platelet activation in depressed patients with ischemic heart disease after paroxetine or nortriptyline treatment. Journal of Clinical Psychopharmacology. 2000;20:137–140. doi: 10.1097/00004714-200004000-00004. [DOI] [PubMed] [Google Scholar]
- Pollock BG, Mulsant BH, Sweet RA, Burgio LD, Kirshner MA, Shuster K. An open pilot study of citalopram for behavioral disturbances of dementia: Plasma levels and real-time observations. American Journal of Geriatric Psychiatry. 1997;5:70–78. [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Raleigh MJ, McGuire MT, Brammer GL, Pollack DB, Yuwiler A. Serotonergic mechanisms promote dominance acquisition in adult male vervet monkeys. Brain Research. 1991;559:181–190. doi: 10.1016/0006-8993(91)90001-c. [DOI] [PubMed] [Google Scholar]
- Ramirez JM, Andreu JM. Aggression, and some related psychological constructs (anger, hostility, and impulsivity) Some comments from a research project. Neuroscience and Biobehavioral Reviews. 2006;30:276–291. doi: 10.1016/j.neubiorev.2005.04.015. [DOI] [PubMed] [Google Scholar]
- Rasmussen A, Lunde M, Poulsen DL, Sorensen K, Qvitzau S, Bech P. A double-blind, placebo-controlled study of sertraline in the prevention of depression in stroke patients. Psychosomatics. 2003;44:216–221. doi: 10.1176/appi.psy.44.3.216. [DOI] [PubMed] [Google Scholar]
- Raynor DA, Pogue-Geile MF, Kamarck TW, McCaffery JM, Manuck SB. Covariation of psychosocial characteristics associated with cardiovascular disease: genetic and environmental influences. Psychosomatic Medicine. 2002;64:191–203. doi: 10.1097/00006842-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Rosenman RH. The interview method of assessment of the coronary-prone behavior pattern. In: Dembroski TM, Shields JL, Haynes SG, Feinlieb M, editors. Coronary-prone behavior. New York: Springer-Verlag; 1978. [Google Scholar]
- Salzman C, Wolfson AN, Schatzberg A, Looper J, Henke R, Albanese M, et al. Effect of fluoxetine on anger in symptomatic volunteers with borderline personality disorder. Journal of Clinical Psychopharmacology. 1995;15:23–29. doi: 10.1097/00004714-199502000-00005. [DOI] [PubMed] [Google Scholar]
- Scales AC, Doraiswamy PM. Focus on citalopram: A selective serotonin reuptake inhibitor for the treatment of depression. Forumlary. 1998;33:725–743. [Google Scholar]
- Siegman AW, Dembroski TM, Ringel N. Components of hostility and the severity of coronary artery disease. Psychosomatic Medicine. 1987;49:127–135. doi: 10.1097/00006842-198703000-00003. [DOI] [PubMed] [Google Scholar]
- Siever L, Trestman RL. The serotonin system and aggressive personality disorder. International Clinical Psychopharmacology. 1993;8:33–39. doi: 10.1097/00004850-199311002-00005. [DOI] [PubMed] [Google Scholar]
- Smith TW. Current status of a psychosomatic hypothesis. Health Psychology. 1992;11:139–150. doi: 10.1037//0278-6133.11.3.139. [DOI] [PubMed] [Google Scholar]
- Smith TW, Frohm KD. What's so unhealthy about hostility? Construct validity and psychosocial correlates of the Cook and Medley Ho scale. Health Psychology. 1985;4:503–520. doi: 10.1037//0278-6133.4.6.503. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Manual for the State-Trait Anger Expression Inventory (STAXI) Odessa, FL: Psychological Assessment Resources; 1988. [Google Scholar]
- Spoont MR. Modulatory role of serotonin in neural information processing: Implications for human psychopathology. Psychological Bulletin. 1992;112:330–350. doi: 10.1037/0033-2909.112.2.330. [DOI] [PubMed] [Google Scholar]
- Suls J, Bunde J. Anger, anxiety, and depression as risk factors for cardiovascular disease: The problems and implications of overlapping affective dispositions. Psychological Bulletin. 2005;131:260–300. doi: 10.1037/0033-2909.131.2.260. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Youngblood ME, Catellier D, Veith RC, Carney RM, Burg MM, et al. Effects of antidepressant medication on morbidity and mortality in depressed patients after myocardial infarction. Archives of General Psychiatry. 2005;62:792–798. doi: 10.1001/archpsyc.62.7.792. [DOI] [PubMed] [Google Scholar]
- Tse WS, Bond AJ. Serotonergic intervension affects both social dominance and affiliative behaviour. Psychopharmacology. 2002;161:324–330. doi: 10.1007/s00213-002-1049-7. [DOI] [PubMed] [Google Scholar]
- Vrijens B, Tousset E, Rode R, Bertz R, Mayer S, Urquhart J, et al. Successful projection of the time course of drug concentration in plasma during a 1-year period from electronically compiled dosing-time data used as input to individually parameterized pharmacokinetic models. Journal of Clinical Pharmacology. 2005;45:461–467. doi: 10.1177/0091270004274433. [DOI] [PubMed] [Google Scholar]
- Welin C, Lappas G, Wilhelmsen L. Indpendent importance of psychosocial factors for prognosis after myocardial infarction. Journal of Internal Medicine. 2000;247(247):629–639. doi: 10.1046/j.1365-2796.2000.00694.x. [DOI] [PubMed] [Google Scholar]
- Williams JE, Nieto FJ, Sanford CP, Couper DJ, Tyroler HA. The association between trait anger and incident stroke risk: The atherosclerosis risk in communitites (ARIC) study. Stroke. 2002;33:19–20. doi: 10.1161/hs0102.101625. [DOI] [PubMed] [Google Scholar]
- Williams JE, Paton CC, Siegler IC, Eigenbrodt ML, Nieto FJ, Tyroler HA. Anger proneness predicts coronary heart disease risk: prospective analysis from the atherosclerosis risk in communities (ARIC) study. Circulation. 2000;101:2034–2039. doi: 10.1161/01.cir.101.17.2034. [DOI] [PubMed] [Google Scholar]
- Williams RB. Basic biological mechanisms. In: Siegman AW, Smith TW, editors. Anger, hostility, and the heart. Hillsdale, New Jersey: Hove and London; 1994a. pp. 117–126. [Google Scholar]
- Williams RB. Neuorbiology, cellular and molecular biology, and psychosomatic medicine. Psychosomatic Medicine. 1994b;56:308–315. doi: 10.1097/00006842-199407000-00006. [DOI] [PubMed] [Google Scholar]