Abstract
Multidrug Resistance Protein 7 (MRP7, ABCC10) is an ATP-binding cassette transporter that is able to transport amphipathic anions and confer resistance to docetaxel and to a lesser extent vincristine and paclitaxel (Hopper-Borge et al., Cancer Res 14: 4927, 2004). While some detail on the resistance profile of MRP7 is known, the activities of the pump have not been completely determined. Here it is shown by the analysis of MRP7-transfected HEK293 cells that in addition to natural product agents, MRP7 is also able to confer resistance to nucleoside-based agents such as the anticancer agents Ara-C and gemcitabine, and the antiviral agents ddC and PMEA. Consistent with the operation of an efflux pump, expression of MRP7 reduced the accumulation of Ara-C and PMEA. In addition, MRP7 is also able to confer resistance to the microtubule stabilizing agent epothilone B. Ectopic expression of MRP7 in mouse embryo fibroblasts deficient in P-glycoprotein and Mrp1 revealed that MRP7 has a broad resistance profile for natural product agents. In this drug sensitive cellular background, MRP7 conferred high levels of resistance to docetaxel (46-fold), paclitaxel (116-fold), SN-38 (65-fold), daunorubicin (7.5-fold), etoposide (11-fold) and vincristine (56-fold). Buthionine sulfoximine did not attenuate MRP7-conferred resistance to docetaxel or AraC. These experiments indicate that the resistance capabilities of MRP7 include nucleoside-based agents and a range of natural product anti-cancer agents that includes non-taxane anti-microtubule agents that are not susceptible to P-glycoprotein mediated transport, and that unlike MRP1 and MRP2, MRP7-mediated drug transport does not involve glutathione.
Keywords: Transport, Resistance, Ara-C, PMEA, Epothilone B
INTRODUCTION
Cellular resistance to chemotherapeutic agents is a major obstacle to cancer treatment. ATP-binding cassette (ABC) transporters are involved in resistance by virtue of their ability to extrude drugs from the cell (1, 2). Many of the ABC transporters that function as drug efflux pumps are termed MRPs and reside in the C family of ABC transporters - one of the largest families of ABC transporters (3, 4). The MRP family is composed of nine members, eight of which have been characterized and established as having the facility for transporting amphipathic anions. Notable substrates of MRPs include conjugates of glutathione, glucuronic acid and sulfate, and members of this family are therefore considered to be transporters of the products of phase II of xenobiotic detoxification. MRPs are grouped according to whether they possess two or three transmembrane-spanning domains (5). With respect to drug transport the most extensively characterized MRPs are MRP1, MRP2 and MRP3 (three membrane-spanning domains) and MRP4, MRP5 and MRP8 (two membrane -spanning domains). This structural classification also appears to be relevant with respect to certain functional properties. MRPs in the former group are able to confer resistance to varying extents to natural product anti-cancer agents, whereas the shorter MRPs are distinguished by their ability to confer resistance to nucleoside-based agents. In addition, MRP4, MRP5 and MRP8 are able to transport cyclic nucleotides - a feature that is not shared by the larger MRPs (6, 7). Pharmacological studies on gene-disrupted mice have established that MRP1 and MRP4 are able to function in vivo as resistance factors for normal tissues (8–10), and that the latter pump, along with MRP2 and MRP3, are involved in various aspects of drug disposition (11). Studies on knock-out mice have also suggested physiological functions. Mrp1 has been implicated in dendritic cell function and inflammation by virtue of its ability to efflux leukotriene C4, Mrp3 and Mrp4 protect cholestatic liver from endogenous compounds such as bile acids, and Mrp4 has been implicated in PGE2-mediated inflammatory responses (12–16).
Recently our laboratory reported initial functional characterizations of MRP7, an MRP family member that possesses three membrane-spanning domains (7, 17). We found that although MRP7 is about equally related to C family ABC transporters involved in regulation of ion transport as it is to MRPs, it nevertheless possesses features that are characteristic of MRPs. MRP7 is competent in the transport of the canonical MRP transport substrate E217βG, but possesses little or no activity towards a range of other substrates handled by other MRPs, including glutathione and sulfate conjugates, bile acids and cyclic nucleotides (18). In addition, MRP7 is able to confer resistance to certain natural product agents, a property that is shared by other MRPs that have 3 membrane spanning domains (19). However, a distinctive feature of MRP7 is that it is able to confer sizable levels of resistance to docetaxel. In addition, it is able to confer lower levels of resistance to paclitaxel and vinca alkaloids. While these reports provided some detail on the functional characteristics of MRP7, the drug resistance properties of the pump have not been completely analyzed. Here, we use a combination of MRP7-transfected HEK293 and Pgp/Mrp1-deficient fibroblasts to more precisely define the MRP7 drug resistance profile. It is shown that unlike other large members of the MRP family, MRP7 is able to confer resistance to nucleoside-based agents. In addition, the pump’s resistance activity extends to a wider range of natural products agents than previously suspected. Notably, MRP7 is also able to confer resistance to epothilone B, a class of natural product agents that are not known to be susceptible to transport by other drug efflux pumps.
MATERIALS AND METHODS
Cell lines, plasmids and transfection
The generation of HEK293 clones stably transfected with MRP7 expression vector (HEK-MRP7-C17 and HEK-MRP7-C18) and parental vector-transfected control cells (HEK293-pcDNA) was previously described (18). Triple knock-out (TKO) Mrp1−/−, Mdr1a/b−/− fibroblasts (MEF3.8 cells) were kindly provided by Dr. Alfred Schinkel (20, 21). HEK293 cells, NIH/3T3 cells and MEF3.8 cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/mL penicillin, and 100 μg/mL streptomycin. The MEF 3.8 cells required the same medium with the addition 2.5 μg/mL puromycin to maintain MRP7 expression. The MDCKII cells were grown in low glucose DMEM supplemented with 10% fetal bovine serum, 2 mM glutamine, 100 units/mL penicillin and 100 μg/mL streptomycin. A vector for expression of MRP7 in Mrp1−/−, Mdr1a,b−/− mouse embryo fibroblasts was prepared by excising the cDNA insert encoding MRP7 from pcDNA 3.1 MRP7 (18) using SnaB and inserting it into the SnaB site of retroviral vector pBabe(puro) to create pBabe(puro)-MRP7. Phoenix retroviral packaging cells were transfected with pBabe(puro)-MRP7 or parental vector and harvested retroviral particles were used to transduce Mrp1−/−, Mdr1a,b−/− fibroblasts. After 48 hr, the cells were passaged and stable clones were selected in 2.5μg/mL puromycin. MRP7 overexpressing clones were identified by immunoblotting. A vector for expression of MRP7 in baculovirus was prepared by excising the MRP7 cDNA insert from pcDNA 3.1 MRP7 (18) and inserting into the Not1 and EcoR1 sites of PVL1392 (Pharmingen, San Diego, CA). MRP7 baculovirus was generated using BacVector 3000 (Invitrogen, Carslbad, CA).
Immunoblot analysis
Monolayers were washed with ice cold phosphate buffered saline and incubated on ice for 20 minutes in RIPA buffer containing 10 μg/mL aprotinin, 5 μg/mL leupeptin and 1 mM phenylmethyl sulfonyl fluoride. Lysates were passed through a syringe and then centrifuged in a cold microfuge for 15 minutes at 15,000 rpm. Proteins were separated by SDS-PAGE on 4–12% Bis- Tris gels and proteins were transferred to nitrocellulose filters using a wet transfer system, as previously described (22, 23). MRP7 was detected using anti-MRP7 monoclonal antibody (1:10) in conjunction with horse radish peroxidase-labeled goat anti-mouse IgG (NEN, Boston, MA) used at a dilution of 1:2500. β-actin-HRP conjugated antibody was used at a dilution of 1:5000 (Abcam, Cambridge, MA) Membrane vesicles were prepared by the nitrogen cavitation method as described previously (24).
Drug sensitivity assays
Drug sensitivity was analyzed using a 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt/phenazine methosulfate (MTS/PMS) microtiter plate assay (CellTiter 96 Cell Proliferation Assay, Promega, Madison, WI). HEK-pcDNA3, HEK-MRP7-C17 and HEK293-MRP7-C18 were seeded in triplicate at 3000 cells per well in 96-well plates in DMEM containing 10% fetal bovine serum. Parental vector-transduced MEF3.8 cells (TKO-pBabe) and MRP7-transduced MEF3.8 cells (TKO-MRP7-7-21) were seeded at 1500 cells per well in 10% DMEM. All other cell lines were seeded at 3000 cells per well in 10% DMEM. The following day, drugs were added at various concentrations to the growth medium. Cellular proliferation assays were performed after 72 h of incubation in the presence of drug. Vincristine, vinblastine, paclitaxel, daunorubicin, cisplatin, 5-fluoro-2′-deoxyuridine, 5-fluoro-5′-deoxyuridine, 5-azacytidine, 5-fluorouracil, 6-thioguanine, 2′-chloro-2′ deoxythymidine, 2′,3′-dideoxycytidine, cytabarine, 6-mercaptopurine and buthionine sulfoximine were purchased from Sigma Chemical Company (St. Louis, MO). SN-38 was generously provided by Pharmacia Corporation (Kalamazoo, MI). Etoposide (Bristol Meyers Squibb, Princeton, NJ.), gemcitabine (Eli Lilly) and docetaxel (Aventis Pharmaceuticals, Bridgewater, NJ.) were obtained from the pharmacy of the Fox Chase Cancer Center. MAC231, MST997 and HTI286 were kindly provided by Wyeth Research. Epothilone B, epothilone A and phomopsin A were obtained from Calbiochem (La Jolla, CA.). PMEA (9-(-phosphonylmethoxynyl)adenine) was kindly provided by Gilead (Forest City, CA).
Drug accumulation assays
For PMEA accumulation, control HEK-pcDNA cells and HEK-MRP7-C18 cells were seeded in triplicate at 3 × 105 cells per well in 24-well dishes. The next day [3H] bis-pom PMEA (3.0 Ci/mmol, Moravek, Brea, CA) was added to a final concentration of 0.1 μM, and the cells were incubated at 37°C. At various time points the cells were washed with ice-cold PBS and trypsinized. An aliquot of cells was used to determine cell number, and the remaining cells were pelleted at 4°C and washed two times with ice-cold PBS. Radioactivity was measured by the use of a liquid scintillation counter (Packard Instrument Company, Inc, Downers Grove, IL, USA). For Ara-C accumulation, TKO-pBabe and TKO-MRP7-7-21 cells were seeded in triplicate in 24-well plates at 3 × 105 cells per well whereas HEK-pcDNA and HEK-MRP7-C18 cells were seeded in 24-well plates at 5 × 105 cells per well. After overnight incubation, [3H] Ara-C (24 Ci/mmol, Moravek) was added to a final concentration of 0.1 μM, and the cells were incubated at 37°C for various times. The cells were trypsinized, collected by centrifugation at 2,000 × rpm for 5 min, washed three times with ice-cold PBS and lysed in 10mM lysis buffer (1% Triton X-100, 0.2% SDS, pH 7.4). Lysates were placed in scintillation fluid and radioactivity was measured in a Packard TRI-CARB 1900CA liquid scintillation analyzer.
Generation of MRP7 monoclonal antibody
A cDNA fragment encoding amino acids 890–894 of MRP7 (17) was inserted downstream of the glutathione S- transferase coding sequence in the pGEX-2T prokaryotic expression vector (Pharmacia Biotech Inc., Piscataway NJ). The fusion protein was isolated by electroelution from a preparative SDS-PAGE gel. Immunization of 5 BALB/c mice, splenic fusion and enzyme linked immunoabsorbent assays were performed as described previously (25). For enzyme linked immunoadsorbent assays of hybridoma supernatants, lysates from insect cells infected with MRP7 baculovirus were used to coat 96-well dishes. Hybridoma preparation was accomplished by the Fox Chase Cancer Center hybridoma facility.
Measurement of cellular glutathione concentrations
Subconfluent cells grown in 25-cm2 flasks were harvested by trypsinization, pelleted, and washed with PBS. The cells were resuspended in 5% metaphosphoric acid and sonicated. The suspensions were then pelleted to remove cellular debris, and 200 μl of the supernatant was removed for determination of glutathione levels. Analysis of glutathione was accomplished using a BIOXYTECH GSH-400 kit (Oxis International, Foster City, CA.) according to the manufacturer’s instructions. To determine the effect of buthionine sulfoximine (BSO) on intracellular glutathione concentrations, the cells were grown overnight with 50 μM BSO before harvesting.
RESULTS
Having determined in a previous study involving natural product agents that MRP7 is able to confer resistance to microtubule active agents such as taxanes and vinca alkaloids (19), we sought to extend our characterization of the pump by defining its capabilities with respect to another important class of anticancer agents – nucleoside analogs. To investigate this, the sensitivities of two previously described clones of MRP7-transfected HEK293 cells and parental vector-transfected control cells were analyzed. A range of anticancer nucleoside analogs (gemcitabine, fluoropyrimidines, 5 Aza, Ara-C, and CDA) and nucleobase analogs (6-TG, 6-MP) were examined. As shown in Table 1, increased resistance was observed for gemcitabine and Ara-C, for which the MRP7-transfected cells exhibited 2.8 – 3.0 and 4.6 – 8.5 fold levels of resistance, respectively. Low levels of resistance towards 5-Aza (2.0-fold) and 5-FdUrd (1.6-fold) were observed for HEK-MRP7-C17 and HEK-MRP7-C18, respectively. In addition, resistance towards two antiviral agents, the nucleoside analog ddC and the nucleotide analog PMEA was analyzed. The MRP7-transfected cells exhibited resistance to each of these agents, with fold resistance levels of 4.9 – 7.4 and 2.8 towards ddC and PMEA, respectively. Representative dose response curves for nucleoside analogs are shown in Figure 1, A–C.
Table 1.
Drug sensitivity of MRP7-transfected HEK293 cells
| IC50a μM |
Fold resistanceb |
||||
|---|---|---|---|---|---|
| Drug | HEK-pcDNA3 | HEK-MRP7-C17 | HEK-MRP7-C18 | HEK-MRP7-C17 | HEK-MRP7-C18 |
| GEM | 2.6 ± 0.4 | 7.7 ± 0.9 | 7.2 ± 0.9 | 3.0c | 2.8 c |
| 5-FU | 9.6 ± 2.5 | 12 ± 1.7 | 12 ± 2.0 | 1.3 | 1.3 |
| 5-dFUrd | 0.011 ± 0.002 | 0.014 ± 0.002 | 0.018 ± 0.002 | 1.3 | 1.6 c |
| 5-FdUrd | 0.024 ± 0.007 | 0.020 ± 0.005 | 0.020 ± 0.003 | 0.8 | 0.8 |
| 5-AZ | 2.2 ± 0.6 | 4.3 ± 0.9 | 5.1 ± 1.5 | 2.0d | 2.3 |
| Ara-C | 0.20 ± 0.3 | 0.91 ± 0.4 | 1.7 ± 0.9 | 4.6 c | 8.5 d |
| CdA | 1.0 ± 0.2 | 1.4 ± 0.2 | 1.2 ± 0.3 | 1.4 | 1.2 |
| 6-TG | 1.2 ± 0.6 | 1.5 ± 0.8 | 1.6 ± 0.6 | 1.3 | 1.3 |
| 6-MP | 2.8 ± 0.7 | 1.6 ± 0.7 | 2.6 ± 0.7 | 0.6 | 0.9 |
| ddC | 76 ± 20 | 375 ± 150 | 562 ± 230 | 4.9 c | 7.4 c |
| PMEA | 24 ± 7.3 | 66 ± 11 | 67 ± 12 | 2.8 c | 2.8 c |
| MX | 0.41 ± 0.5 | 0.20 ± 0.4 | 0.25 ± 0.8 | 0.5 | 0.6 |
| EpoA (nM) | 5.4 ± 1.7 | 6.2 ± 2.2 | 4.4 ± 1.1 | 1.2 | 0.8 |
| EpoB (nM) | 0.99 ± 0.2 | 5.2 ± 1.9 | 6.7 ± 2.1 | 5.3 c | 6.8 c |
| PHOM (nM) | 1000 ± 300 | 1130 ± 370 | 1190 ± 480 | 1.1 | 1.2 |
| MAC321 (nM) | 63 ± 9.8 | 91 ± 10 | 105 ± 21 | 1.4 | 1.7 c |
| MST997 (nM) | 40 ± 8.4 | 48 ± 13 | 60 ± 10 | 1.2 | 1.5 c |
| HTI286 (nM) | 43 ± 18 | 77 ± 40 | 122 ± 54 | 1.8 | 2.8 c |
Drug sensitivities were analyzed by the use of a 3-day colorimetric assay in which cells were continuously exposed to the indicated agents. IC50 is the concentration that inhibited cell survival by 50% (means ± SE). N = 6 – 11 for Ara-C, GEM, 5-FU, 5-dFUrd, CdA, PMEA, MAC321, 5-FdUrd, 5-AZ, HTI286, ddC, and EpoB and PHOM; N = 3 – 5 for MX, and MST997, 6-TG, 6-MP, and EpoA.
IC50 of HEK-MRP7-C17 or HEK-MRP7-C18 divided by IC50 of control HEK-pcDNA3 cells.
Significantly different from the control transfectant as assessed by the nonparametric two tailed Wilcoxon test; P < .05.
P < .01.
GEM, gemcitabine; 5-FU, 5-fluorouracil; 5-FdURd, 5-fluoro-5′-deoxyuridine; 5-dFUrd, 5′-fluoro-2′-deoxyuridine; 5-AZ, 5-azacytidine; Ara-C, cytarabine; CdA, 2′-chloro-2′deoxyadenosine; 6-TG, 6-thioguanine; 6-MP, 6-mercaptopurine; ddC, 2′,3′-dideoxycytidine; PMEA, (9-(-phosphonylmethoxynyl)adenine); MX, mitoxantrone; EpoA, epothilone A; EpoB, epothilone B; PHOM, phomopsin A.
Fig. 1.
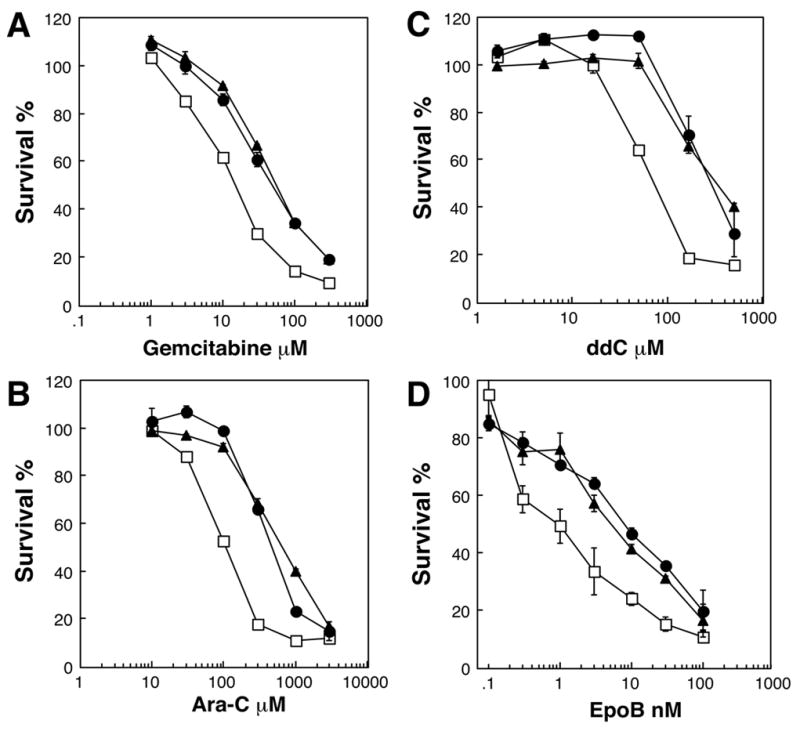
Sensitivity of control and MRP7 –transfected HEK293 cells to gemcitabine, Ara-C, ddC, and epothilone B. The drug sensitivities of parental vector-transfected HEK293 cells (HEK-pcDNA 3, (□), and MRP7-transfected HEK 293 cells (HEK-MRP7-C17, (▲) and HEK-MRP7-C18 (●)) were analyzed towards gemcitabine (A), Ara-C (B), ddC (C) and epothilone-B (D) using the MTS/PMS assay as described in “Materials and Methods”. Data points are means ± SDs of triplicate determinations. Representative experiments are shown.
Next, the activity of MRP7 towards several microtubule active agents that are currently in development was analyzed (Table 1). These agents included the macrolides epothilones A and B (26), the macrocyclic heptapeptide phomopsin (27), the docetaxel analogs MAC-321 and MST-997 (28, 29), and HTI-286, an analog of the tripeptide hemiasterlin (30). Notably, the MRP7-transfected cells exhibited resistance towards epothilone B (5.3 – 6.8-fold; Figure 1D). In addition, low levels of resistance (1.5 – 2.8-fold) were observed for HEK-MRP7-C18 towards MAC-321, MST-997 and HTI-286.
To confirm that MRP7 affects sensitivity to nucleoside analogs by reducing cellular accumulation, the cellular kinetics of radiolabeled PMEA and Ara-C was analyzed. As shown in Figure 2, HEK-MRP7-C18 exhibited reduced accumulation for each of these agents. At 30 minutes, accumulation of PMEA was reduced 34% as compared to the control cells, and this deficit was maintained at the 60-minute time point (Figure 2A). At 90 minutes the accumulation of Ara-C was reduced 43% in MRP7-transfected cells (Figure 2B).
Fig. 2.
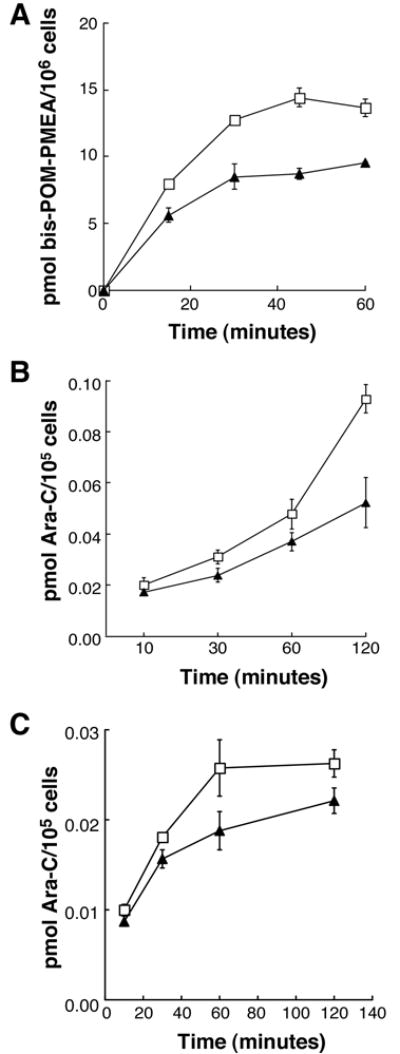
Accumulation of nucleoside analog-based agents in MRP7-transfected HEK293 cells and MEF3.8 cells. (A) Accumulation of PMEA and Ara-C in control HEK-pcDNA 3.1 (□) and MRP7-transfected HEK-MRP7-C18 (▲) cells. (B) Accumulation of Ara-C in control TKO-pBabe (Δ) and MRP7-transduced TKO-MRP7-7-21 (◆) cells. Cells were incubated in the presence of 0.1 μM [3H]bis-POM-PMEA or 0.1 μM [3H] Ara-C and accumulation was measured at various time points as described in “Materials and Methods”. Data points are means ± SDs.
To more precisely characterize the resistance activity of MRP7, the pump was expressed in mouse embryonic fibroblasts that are genetically deficient in Mdr1a/1b and Mrp1. The absence of these two pumps renders the cells drug sensitive to the substrates of the latter two pumps (21, 31), and thus provides a cellular background that affords a clearer picture of the activity of ectopically expressed resistance factors. Figure 3A shows the ectopic expression of MRP7 in Mdr1a/1b, Mrp1-deficient MEF3.8 mouse embryo fibroblasts. In this cellular background MRP7 exhibited robust activity towards almost all of the natural product agents tested. Notably, resistance was observed towards agents for which activity was not apparent in the HEK293 cellular background, such as SN-38, daunorubicin and etoposide (Table 2; for comparison, our previously reported results using MRP7-transfected HEK293 cells are provided in parentheses in the last column). Particularly high resistance levels were exhibited by TKO-MRP7-7-21 for paclitaxel, docetaxel, SN-38 (the active metabolite of irinotecan) and vincristine (116, 46, 65 and 56-fold resistance, respectively). TKO-MRP7-7-21 was also resistant to etoposide (11-fold) and daunorubicin (7.5-fold). By contrast with these agents, the levels of resistance observed for epothilone B, gemcitabine, 5-FU, Ara-C and CDDP were roughly comparable to the levels observed for MRP7-transfected HEK293 cells (Table 2). Analysis of a second MEF3.8 transfectant, which expressed lower levels of MRP7 compared to TKO-MRP7-7-21, revealed resistance levels of 14-fold to paclitaxel, 12-fold to docetaxel, 23-fold to vincristine, 29-fold to SN-38 and 6.4-fold to epothilone B (data not shown). As expected, when cellular kinetics was analyzed in TKO-MRP7-7-21 cells using radiolabeled Ara-C as a probe, reduced accumulation was observed (Figure 2C).
Fig. 3.
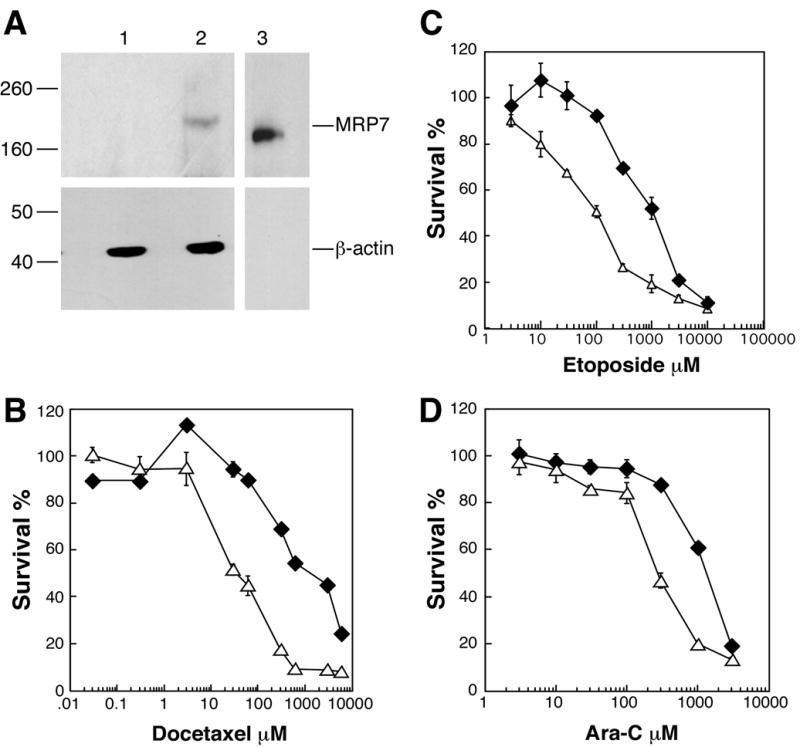
Immunoblot detection of MRP7 in MEF3.8 cells and drug sensitivity analysis. (A), Immunoblot detection of MRP7 expressed in MEF3.8 cells. Total cellular lysates were prepared from MRP7-transduced (TKO-MRP7-7-21) and parental vector-transduced MEF3.8 (TKO-pBabe) cells, and proteins (90μg) were separated by SDS-PAGE. Lane 1, TKO-pBabe.; lane 2, TKO-MRP7-21; lane 3, membrane vesicles prepared from MRP7 baculovirus-infected Sf9 cells (positive control). MRP7 was detected using anti-MRP7 monoclonal antibody. MRP7 expressed in glycosylation-impaired insect cells runs slightly faster than in MEF3.8 cells. Molecular weight markers are shown to the left. (B–C), Sensitivity of TKO-pBabe (Δ) and TKO-MRP7-21 cells (◆) to docetaxel, etoposide and Ara-C. Drug sensitivity was analyzed using the MTS/PMS assay as described in “Materials and Methods”. Data points are means ± SDs of triplicate determinations. Representative experiments are shown.
Table 2.
Drug sensitivity of MRP7-transfected Mrp1−/−, Mdr1a,b−/− MEF3.8 (TKO) cells
| IC50a nM |
Fold resistanceb |
|||
|---|---|---|---|---|
| Drugc | TKO-pBabe | TKO-MRP7-7–21 | TKO-MRP7-7–21 | HEK293-MRP7d |
| PAC | 7.0 ± 2.2 | 811 ± 380 | 116e | (3.3–3.4) |
| DOC | 23 ± 14 | 1050 ± 490 | 46e | (8.7–12.7) |
| SN-38 | 16 ± 4.7 | 1040 ± 400 | 65e | (0.24–0.25) |
| DNR | 10 ± 1.4 | 75 ± 8.6 | 7.5e | (1.0–1.1) |
| ETOP (μM) | 173 ± 61 | 1920 ± 840 | 11e | (1.1–1.0) |
| VCR | 1.5 ± 0.3 | 84 ± 31 | 56e | (3.3–3.4) |
| EpoB | 9.3 ± 3.3 | 31 ± 14 | 3.3f | 5.3–6.8 |
| GEM | 14 ± 2.2 | 32 ± 5.0 | 2.3 | 2.8–3.0 |
| 5-FU (μM) | 901 ± 180 | 1440 ± 110 | 1.6e | 1.3 |
| Ara-C | 239 ± 54 | 1230 ± 310 | 5.1e | 4.6–8.5 |
| CDDP ( μM) | 9.8 ± 4.4 | 18 ± 4.2 | 1.8e | (1.1–1.4) |
Drug sensitivities were analyzed by the use of a 3-day colorimetric assay in which cells were continuously exposed to the indicated agents. IC50 is the concentration that inhibited cell survival by 50% (means ± SE). N = 8 for ETOP, EpoB; N =7 for DOC, SN-38, DNR, VCR, CDDP; N =6 for PAC, 5-FU, Ara-C; N =5 for GEM.
IC50 of TKO-7-21 divided by IC50 of TKO-pBabe.
PAC, paclitaxel; DOC, docetaxel; DNR, daunorubucin; ETOP, etoposide; VCR, vincristine; EpoB, epothilone B; GEM, gemcitabine; 5-FU, 5-fluorouracil; Ara-C, cytarabine; CDDP, cisplatin
Values in parentheses from Hopper-Borge et al, Cancer Res. 2004; other values from Table 1.
Significantly different from the control transfectant as assessed by the nonparametric two-tailed Wilcoxon test. P< .05.
P<.01.
Glutathione has been implicated in the transport of natural product agents by MRP1 and MRP2 (32–34). To evaluate this feature of MRP7 the ability of buthionine sulfoximine (BSO), an agent that depresses cellular glutathione levels by inhibiting the rate limiting step in glutathione synthesis, to inhibit resistance was assessed. As shown in Supplemental Table 1, treatment of MRP7-transfected HEK293 cells with 50 μM buthionine sulfoximine did not affect resistance towards either docetaxel or Ara-C. Under these conditions, cellular glutathione levels were depressed by ~ 84%, a marked reduction (in the presence of BSO glutathione levels were 2.06 ± 0.04 nmol/106 cells and 1.73 ± 0.28 nmol/106, as compared to 12.4 ± 0.39 nmol/106 cells and 11.4 ± 0.16 nmol/106 cells in the absence, respectively, for HEK-pcDNA3 and HEK-MRP7-C18 cells). Activity towards docetaxel is a prominent feature of the MRP7 resistance profile. To determine whether other MRP family members that confer resistance to natural product drugs could also transport docetaxel, MRP1, MRP2 and MRP3-transfected cells were tested. Resistance activity towards docetaxel was not detected for these efflux pumps (Supplemental Table 2).
DISCUSSION
The present analysis of MRP7 activity provides surprising detail on the resistance profile of the pump. In a previous study, we focused our analysis on natural product anti-cancer agents because resistance towards at least some agents of this family is characteristic of MRP family members that possess three membrane spanning domains. However, the relatively low degree of similarity between MRP7 and other MRP family members raised the possibility that its activity might not be restricted to natural product agents. By investigating this conjecture, we determined that the MRP7 resistance profile includes nucleoside-based agents, a class of compounds which are not known to be components of the resistance profiles of other large members of the MRP family, but instead are agents towards which the small members, such as MRP4, MRP5 and MRP8 have activity (35–38). The nucleoside-based agents towards which MRP7 is able to confer resistance included anticancer agents such as Ara-C, a mainstay in the treatment of acute myelogenous leukemia, and gemcitabine, an agent with utility in pancreatic and lung cancers. This is the first example of an MRP family member that is able to confer resistance to either of these widely employed agents. In addition, MRP7 is also able to confer resistance to antiviral agents such as ddC, similar to MRP8 and PMEA, as observed with MRP4, MRP5 and MRP8. It is likely that MRP7, which we have established as being competent in the transport of amphipathic anions (18), effluxes the negatively charged monophosphate metabolites of Ara-C, gemcitabine and ddC, as opposed to the uncharged parent nucleosides. This mechanism of resistance would be in accord with the ability, for example, of MRP8 to transport the monophosphate of 5-FU but not the uncharged parent nucleoside analog (38). By contrast with the former nucleoside analogs, PMEA is a charged nucleotide analog which is likely to be a direct substrate of MRP7.
The present study also revealed that the range of MRP7 towards natural product agents is much broader than had been previously inferred from our prior analysis of MRP7-transfected HEK293 cells (17). Confirming our previous reported findings, we show that expression of MRP7 in the context of Pgp/Mrp1 null fibroblasts confers resistance to taxanes and vinca alkaloids. However in this genetically deficient context, MRP7 also protected cells from anthracyclines (daunorubicin), camptothecins (SN-38) and epipodophyllotoxins (etoposide). The ability to detect increased activity of MRP7 towards the former agents, and emergence of activity towards the latter agents when the pump is expressed in Pgp/Mrp1 deficient fibroblasts compared to HEK293 cells (Table 2), is consistent with the phenotype of MEF3.8 cells. This cell line is reported to be 11–51, 2–12, 4–6, 5–9 and 19–41-fold more sensitive to paclitaxel, docetaxel, SN-38, daunorubucin, etoposide and vincristine, respectively, compared to wild-type fibroblasts (21). The lack of significant enhancement of MRP7 activity for nucleoside analogs such as Ara-C and the alkylating agent cisplatin in Pgp/Mrp1 deficient cells compared to HEK293 cells is also consistent with the absence of sensitization of MEF3.8 cells towards these two agents (0.8–1.5 and 0.6–1.3-fold, respectively). That MRP7 activity (e.g. docetaxel) is enhanced in MEF3.8 cells towards agents for which this cell line is sensitized, whereas its activity (e.g. Ara-C) is not increased towards agents for which MEF3.8 cells are not sensitized, tends to support the validity of the MRP7 phenotype we describe here.
Our previous study disclosed that MRP7 is able to confer resistance to two classes of agents that target microtubules (vinca alkaloids and taxanes). It was therefore of interest to determine if MRP7 might also confer resistance to newer anti-microtubule agents. A notable feature of MRP7 that emerged from this line of investigation is that it is able to confer resistance to epothilone B. Of the non-taxane microtubule-stabilizing agents that are in clinical development, epothilones are the most advanced (39–41). Epothilones, which have broad antitumor activity, are considered to be particularly attractive agents because they are not susceptible in vitro or in vivo to transport by P-glycoprotein or any previously tested drug efflux pumps (39). In accord with this situation, the absence of enhanced levels of MRP7-conferred resistance towards epothilone B in the MEF3.8 system as compared to HEK293 cells is expected, in that MEFs that are deficient in P-glycoprotein and MRP1 should not be rendered sensitive to this agent. The finding that MRP7-transfected HEK293 cells are resistant to epothilone B indicates that there is at least one pump capable of effluxing this agent. By contrast with epothilone B, we did not detect resistance towards epothilone A, which differs from the former compound by the absence of a methyl group at C12. In addition, MRP7-transfected HEK293 cells exhibited either relatively modest or no resistance to the taxane analogs MAC-321 and MST-997, and the microtubule destabilizing agents phomoposin A and HTI-286.
Another feature of MRP7 that was investigated in this study is the involvement of glutathione in MRP7-mediated resistance. The finding that BSO had no effect on resistance to docetaxel or Ara-C suggests that transport of anticancer agents by MRP7 does not require glutathione. This finding, in combination with the observations that glutathione levels are not significantly decreased in MRP7-transfected HEK293 cells (19) or MRP7-transfected MEF3.8 cells (present study), suggests that glutathione is not a substrate of MRP7. In this regard, MRP7 is distinct from MRP1 and MRP2, both of which require glutathione for transport of natural product agents and are competent in the transport this tripeptide (3). Instead, MRP7 is similar to MRP3 which appears to transport etoposide in a glutathione-independent fashion (42). We also show in the present study that in apparent contrast to MRP7, all three of the latter pumps are unable to confer resistance to docetaxel. However, with respect to MRP3, in contrast to studies using MRP3-transfected HEK293 cells, ovarian cancer 2008 cells, and P-glycoprotein and Mrp1-deficient MEF cells (42–44), a recent report found that when expressed in a breast cancer cell line MRP3 is able to confer resistance to paclitaxel (45). This suggests that the activity of MRP3 may depend on cell context. Therefore, with regard to the activity of MRP3 towards docetaxel, further studies are warranted using additional cell lines to the HEK293 cells used here.
In order to better understand the potential impact of MRP7 on the sensitivity of normal tissues and tumors detailed information will be needed on the protein expression pattern of the pump. Our laboratory and others have detected MRP7 transcript in a variety of tissues including pancreas, liver, placenta, kidney, brain, ovary, spleen, heart, skeletal muscle, testis, intestine, prostate, and white blood cells, as well as in various fetal tissues (17, 46) (Supplemental Figure 1). Transcript has also been detected in several adenocarcinomas, including tumors such as breast, ovary and lung (46, 47). This is of potential interest in that the latter tumors are treated with taxanes. In addition, MRP7 may be induced by anti-cancer agents towards which the pump confers resistance. MRP7 transcript and protein were reported to be induced by vincristine exposure in two salivary gland adenocarcinoma cell lines that are cross resistant to docetaxel (48), and transcript was reported to be increased in MCF7 cells by exposure to doxorubicin (46). In addition, it was recently reported that MRP7 is induced by paclitaxel in a non-small cell lung cancer cell line (49). MRP7 antibodies and an Mrp7 gene-disrupted mouse we have generated (E.H-B. and G.D.K, unpublished) should be valuable tools for understanding the potential impact of MRP7 on drug sensitivity.
Supplementary Material
Acknowledgments
This work was supported by National Cancer Institute grants CA11457 and CA073728 to G.D.K. This work was also supported by National Cancer Institute grant KO1CA120091 awarded to E.H-B.
References
- 1.Kruh GD. Introduction to resistance to anticancer agents. Oncogene. 2003;22(47):7262–4. doi: 10.1038/sj.onc.1206932. [DOI] [PubMed] [Google Scholar]
- 2.Gottesman MM, Fojo T, Bates SE. Multidrug resistance in cancer: role of ATP-dependent transporters. Nat Rev Cancer. 2002;2(1):48–58. doi: 10.1038/nrc706. [DOI] [PubMed] [Google Scholar]
- 3.Kruh GD, Belinsky MG. The MRP family of drug efflux pumps. Oncogene. 2003;22(47):7537–52. doi: 10.1038/sj.onc.1206953. [DOI] [PubMed] [Google Scholar]
- 4.Deeley RG, Westlake C, Cole SP. Transmembrane transport of endo- and xenobiotics by mammalian ATP-binding cassette multidrug resistance proteins. Physiol Rev. 2006;86(3):849–99. doi: 10.1152/physrev.00035.2005. [DOI] [PubMed] [Google Scholar]
- 5.Belinsky MG, Bain LJ, Balsara BB, Testa JR, Kruh GD. Characterization of MOAT-C and MOAT-D, new members of the MRP/cMOAT subfamily of transporter proteins. J Natl Cancer Inst. 1998;90(22):1735–41. doi: 10.1093/jnci/90.22.1735. [DOI] [PubMed] [Google Scholar]
- 6.Borst P, de Wolf C, van de Wetering K. Multidrug resistance-associated proteins 3, 4, and 5. Pflugers Arch. 2006 doi: 10.1007/s00424-006-0054-9. [DOI] [PubMed] [Google Scholar]
- 7.Kruh GD, Guo Y, Hopper-Borge E, Belinsky MG, Chen ZS. ABCC10, ABCC11, and ABCC12. Pflugers Arch. 2006 doi: 10.1007/s00424-006-0114-1. [DOI] [PubMed] [Google Scholar]
- 8.Johnson DR, Finch RA, Lin ZP, Zeiss CJ, Sartorelli AC. The pharmacological phenotype of combined multidrug-resistance mdr1a/1b- and mrp1-deficient mice. Cancer research. 2001;61(4):1469–76. [PubMed] [Google Scholar]
- 9.Wijnholds J, Evers R, van Leusden MR, et al. Increased sensitivity to anticancer drugs and decreased inflammatory response in mice lacking the multidrug resistance-associated protein. Nat Med. 1997;3(11):1275–9. doi: 10.1038/nm1197-1275. [DOI] [PubMed] [Google Scholar]
- 10.Belinsky MG, Guo P, Lee K, et al. Multidrug resistance protein 4 protects bone marrow, thymus, spleen, and intestine from nucleotide analogue-induced damage. Cancer research. 2007;67(1):262–8. doi: 10.1158/0008-5472.CAN-06-2680. [DOI] [PubMed] [Google Scholar]
- 11.Kruh GD, Belinsky MG, Gallo JM, Lee K. Physiological and pharmacological functions of Mrp2, Mrp3 and Mrp4 as determined from recent studies on gene-disrupted mice. Cancer Metastasis Rev. 2007;26(1):5–14. doi: 10.1007/s10555-007-9039-1. [DOI] [PubMed] [Google Scholar]
- 12.Robbiani DF, Finch RA, Jager D, Muller WA, Sartorelli AC, Randolph GJ. The leukotriene C(4) transporter MRP1 regulates CCL19 (MIP-3beta, ELC)- dependent mobilization of dendritic cells to lymph nodes. Cell. 2000;103(5):757–68. doi: 10.1016/s0092-8674(00)00179-3. [DOI] [PubMed] [Google Scholar]
- 13.Belinsky MG, Dawson PA, Shchaveleva I, et al. Analysis of the in vivo functions of Mrp3. Mol Pharmacol. 2005;68(1):160–8. doi: 10.1124/mol.104.010587. [DOI] [PubMed] [Google Scholar]
- 14.Mennone A, Soroka CJ, Cai SY, et al. Mrp4−/− mice have an impaired cytoprotective response in obstructive cholestasis. Hepatology. 2006;43(5):1013–21. doi: 10.1002/hep.21158. [DOI] [PubMed] [Google Scholar]
- 15.Zelcer N, Wetering KV, Waart RD, et al. Mice lacking Mrp3 (Abcc3) have normal bile salt transport, but altered hepatic transport of endogenous glucuronides. J Hepatol. 2005 doi: 10.1016/j.jhep.2005.07.022. [DOI] [PubMed] [Google Scholar]
- 16.Lin ZP, Zhu YL, Johnson DR, et al. Disruption of cAMP and prostaglandin E2 transport by multidrug resistance protein 4 deficiency alters cAMP-mediated signaling and nociceptive response. Mol Pharmacol. 2008;73(1):243–51. doi: 10.1124/mol.107.039594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hopper E, Belinsky MG, Zeng H, Tosolini A, Testa JR, Kruh GD. Analysis of the structure and expression pattern of MRP7 (ABCC10), a new member of the MRP subfamily. Cancer Lett. 2001;162(2):181–91. doi: 10.1016/s0304-3835(00)00646-7. [DOI] [PubMed] [Google Scholar]
- 18.Chen Z-S, Hopper-Borge E, Belinsky MG, Shchaveleva I, Kotova E, Kruh GD. Characterization of the transport properties of human multidrug resistance protein 7 (MRP7, ABCC10) Mol Pharmacol. 2003;63(2):351–8. doi: 10.1124/mol.63.2.351. [DOI] [PubMed] [Google Scholar]
- 19.Hopper-Borge E, Chen Z-S, Shchaveleva I, Belinsky MG, Kruh GD. Analysis of the drug resistance profile of MRP7 (ABCC10): resistance to docetaxel. Cancer research. 2004;64(14):4927–30. doi: 10.1158/0008-5472.CAN-03-3111. [DOI] [PubMed] [Google Scholar]
- 20.Evers R, Kool M, van Deemter L, et al. Drug export activity of the human canalicular multispecific organic anion transporter in polarized kidney MDCK cells expressing cMOAT (MRP2) cDNA. J Clin Invest. 1998;101(7):1310–9. doi: 10.1172/JCI119886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allen JD, Brinkhuis RF, van Deemter L, Wijnholds J, Schinkel AH. Extensive contribution of the multidrug transporters P-glycoprotein and Mrp1 to basal drug resistance. Cancer research. 2000;60(20):5761–6. [PubMed] [Google Scholar]
- 22.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227(259):680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 23.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76(9):4350–4. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cornwell MM, Gottesman MM, Pastan IH. Increased vinblastine binding to membrane vesicles from multidrug- resistant KB cells. J Biol Chem. 1986;261(17):7921–8. [PubMed] [Google Scholar]
- 25.Bizub-Bender D, Kulkosky J, Skalka AM. Monoclonal antibodies against HIV type 1 integrase: clues to molecular structure. AIDS Res Hum Retroviruses. 1994;10(9):1105–15. doi: 10.1089/aid.1994.10.1105. [DOI] [PubMed] [Google Scholar]
- 26.Altmann KH. Epothilone B and its analogs - a new family of anticancer agents. Mini reviews in medicinal chemistry. 2003;3(2):149–58. doi: 10.2174/1389557033405269. [DOI] [PubMed] [Google Scholar]
- 27.Hamel E. Natural products which interact with tubulin in the vinca domain: maytansine, rhizoxin, phomopsin A, dolastatins 10 and 15 and halichondrin B. Pharmacol Ther. 1992;55(1):31–51. doi: 10.1016/0163-7258(92)90028-x. [DOI] [PubMed] [Google Scholar]
- 28.Sampath D, Discafani CM, Loganzo F, et al. MAC-321, a novel taxane with greater efficacy than paclitaxel and docetaxel in vitro and in vivo. Molecular cancer therapeutics. 2003;2(9):873–84. [PubMed] [Google Scholar]
- 29.Sampath D, Greenberger LM, Beyer C, et al. Preclinical pharmacologic evaluation of MST-997, an orally active taxane with superior in vitro and in vivo efficacy in paclitaxel- and docetaxel-resistant tumor models. Clin Cancer Res. 2006;12(11 Pt 1):3459–69. doi: 10.1158/1078-0432.CCR-05-2349. [DOI] [PubMed] [Google Scholar]
- 30.Loganzo F, Discafani CM, Annable T, et al. HTI-286, a synthetic analogue of the tripeptide hemiasterlin, is a potent antimicrotubule agent that circumvents P-glycoprotein-mediated resistance in vitro and in vivo. Cancer research. 2003;63(8):1838–45. [PubMed] [Google Scholar]
- 31.Lin ZP, Johnson DR, Finch RA, Belinsky MG, Kruh GD, Sartorelli AC. Comparative study of the importance of multidrug resistance-associated protein 1 and P-glycoprotein to drug sensitivity in immortalized mouse embryonic fibroblasts. Molecular cancer therapeutics. 2002;1(12):1105–14. [PubMed] [Google Scholar]
- 32.Versantvoort CH, Broxterman HJ, Bagrij T, Scheper RJ, Twentyman PR. Regulation by glutathione of drug transport in multidrug-resistant human lung tumour cell lines overexpressing multidrug resistance-associated protein. Br J Cancer. 1995;72(1):82–9. doi: 10.1038/bjc.1995.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Loe DW, Almquist KC, Deeley RG, Cole SP. Multidrug resistance protein (MRP)-mediated transport of leukotriene C4 and chemotherapeutic agents in membrane vesicles. Demonstration of glutathione-dependent vincristine transport. J Biol Chem. 1996;271(16):9675–82. doi: 10.1074/jbc.271.16.9675. [DOI] [PubMed] [Google Scholar]
- 34.Evers R, de Haas M, Sparidans R, et al. Vinblastine and sulfinpyrazone export by the multidrug resistance protein MRP2 is associated with glutathione export. Br J Cancer. 2000;83(3):375–83. doi: 10.1054/bjoc.2000.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee K, Klein-Szanto AJ, Kruh GD. Analysis of the MRP4 drug resistance profile in transfected NIH3T3 cells. J Natl Cancer Inst. 2000;92(23):1934–40. doi: 10.1093/jnci/92.23.1934. [DOI] [PubMed] [Google Scholar]
- 36.Schuetz JD, Connelly MC, Sun D, et al. MRP4: A previously unidentified factor in resistance to nucleoside- based antiviral drugs. Nat Med. 1999;5(9):1048–51. doi: 10.1038/12487. [DOI] [PubMed] [Google Scholar]
- 37.Wijnholds J, Mol CA, van Deemter L, et al. Multidrug-resistance protein 5 is a multispecific organic anion transporter able to transport nucleotide analogs. Proc Natl Acad Sci U S A. 2000;97(13):7476–81. doi: 10.1073/pnas.120159197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo Y, Kotova E, Chen Z-S, et al. MRP8, ATP-binding cassette C11 (ABCC11), is a cyclic nucleotide efflux pump and a resistance factor for fluoropyrimidines, 2′,3′-dideoxycytidine and 9′-(2′-phosphonylmethoxyethyl)adenine. J Biol Chem. 2003;278(32):29509–14. doi: 10.1074/jbc.M304059200. [DOI] [PubMed] [Google Scholar]
- 39.Goodin S, Kane MP, Rubin EH. Epothilones: mechanism of action and biologic activity. J Clin Oncol. 2004;22(10):2015–25. doi: 10.1200/JCO.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 40.Fojo T, Menefee M. Mechanisms of multidrug resistance: the potential role of microtubule-stabilizing agents. Ann Oncol. 2007;18 (Suppl 5):v3–8. doi: 10.1093/annonc/mdm172. [DOI] [PubMed] [Google Scholar]
- 41.Lee JJ, Swain SM. The epothilones: translating from the laboratory to the clinic. Clin Cancer Res. 2008;14(6):1618–24. doi: 10.1158/1078-0432.CCR-07-2201. [DOI] [PubMed] [Google Scholar]
- 42.Zelcer N, Saeki T, Reid G, Beijnen JH, Borst P. Characterization of drug transport by the human multidrug resistance protein 3 (ABCC3) J Biol Chem. 2001;276(49):46400–7. doi: 10.1074/jbc.M107041200. [DOI] [PubMed] [Google Scholar]
- 43.Kool M, van der Linden M, de Haas M, et al. MRP3, an organic anion transporter able to transport anti-cancer drugs. Proc Natl Acad Sci U S A. 1999;96(12):6914–9. doi: 10.1073/pnas.96.12.6914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zeng H, Bain LJ, Belinsky MG, Kruh GD. Expression of multidrug resistance protein-3 (multispecific organic anion transporter-D) in human embryonic kidney 293 cells confers resistance to anticancer agents. Cancer research. 1999;59:5964–7. [PubMed] [Google Scholar]
- 45.O’Brien C, Cavet G, Pandita A, et al. Functional genomics identifies ABCC3 as a mediator of taxane resistance in HER2-amplified breast cancer. Cancer research. 2008;68(13):5380–9. doi: 10.1158/0008-5472.CAN-08-0234. [DOI] [PubMed] [Google Scholar]
- 46.Takayanagi S, Kataoka T, Ohara O, Oishi M, Kuo MT, Ishikawa T. Human ATP-binding cassette transporter ABCC10: expression profile and p53-dependent upregulation. J Exp Ther Oncol. 2004;4(3):239–46. [PubMed] [Google Scholar]
- 47.Dabrowska M, Sirotnak F. Regulation of transcription of the human MRP7 gene. Characteristics of the basal promoter and identification of tumor-derived transcripts encoding additional 5′ end heterogeneity. Gene. 2004;341:129–39. doi: 10.1016/j.gene.2004.06.022. [DOI] [PubMed] [Google Scholar]
- 48.Naramoto H, Uematsu T, Uchihashi T, et al. Multidrug resistance-associated protein 7 expression is involved in cross-resistance to docetaxel in salivary gland adenocarcinoma cell lines. Int J Oncol. 2007;30(2):393–401. [PubMed] [Google Scholar]
- 49.Oguri T, Ozasa H, Uemura T, et al. MRP7/ABCC10 expression is a predictive biomarker for the resistance to paclitaxel in non-small cell lung cancer. Molecular cancer therapeutics. 2008;7(5):1150–5. doi: 10.1158/1535-7163.MCT-07-2088. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


