Abstract
The membrane-bound rat growth hormone receptor (GH-R) and an alternatively spliced isoform, the soluble rat GH binding protein (GH-BP), are comprised of identical N-terminal GH binding domains, however, their C-terminal sequences differ. Immunological reagents are needed to distinguish between the two isoforms in order to understand their respective roles in mediating the actions of GH. Accordingly, a tetravalent multiple antigen peptide (MAP) dendrimer with four identical branches of a C-terminal peptide sequence of the rat GH-BP (GH-BP263-279) was synthesized and used as an immunogen in rabbits. Solid-phase peptide synthesis of four GH-BP263-279 segments onto a tetravalent Lys2-Lys-β-Ala-OH core peptide was carried out using N-(9-fluorenyl)methoxycarbonyl chemistry. The mass of the RP-HPLC purified synthetic product, 8398 Da, determined by ESI-MS, was identical to expected mass. Three anti-rat GH-BP263-279 MAP antisera, BETO-8039, BETO-8040 and BETO-8041, at dilutions of 10-3, recognized both the rat GH-BP263-279 MAP and recombinant mouse GH-BP with ED50s within a range of 5-10 fmol but did not cross-react with BSA in dot blot analyses. BETO-8041 antisera (10-3 dilution) recognized GH-BPs of rat serum and liver having Mrs ranging from 35-130 kDa but did not recognize full-length rat GH-Rs. The antisera also detected recombinant mouse GH-BPs. In summary, the tetravalent rat GH-BP263-279 MAP dendrimer served as an effective immunogenic antigen in eliciting high titer antisera specific for the C-termini of both rat and mouse GH-BPs. The antisera will facilitate studies aimed at improving our understanding of the biology of GH-BPs.
Keywords: Multiple antigen peptide dendrimer, antipeptide polyclonal antisera, growth hormone binding protein, growth hormone receptor, growth hormone
INTRODUCTION
The gene containing the growth hormone-receptor (GH-R) nucleotide sequence serves as a template for production of both a membrane-bound GH-R and an alternatively spliced soluble GH-binding protein (GH-BP) [1]. The existence of multiple GH-R gene products provides another layer of information that raises questions about our understanding of the molecular mechanisms of GH’s biological actions. Development of robust and reproducible immunological reagents for the quantitative and qualitative detection of GH-BPs and GH-Rs of cells and biological fluids will help us understand the roles they play in mediating the actions of GH. In this report, we have focused on the development of a high-affinity antipeptide immunological reagent for the specific detection of rat and mouse GH-BPs.
The amino acid sequence similarities and differences between the rat GH-R and rat GH-BP [2,3] can be seen in panel A of Figure 1. The rat GH-R and rat GH-BP have identical N-terminal signal peptides (residues 1-18) and identical GH-binding domains (residues 19-262). The unspliced rat GH-R has a 24 amino acid transmembrane domain and a 349 amino acid cytoplasmic domain that are absent in the rat GH-BP. Instead, the rat GH-BP contains a substituted 17-amino acid hydrophilic sequence comprised of residues 263-279 produced through alternative splicing of the rat GH-R gene.
Figure 1. Aligned amino acid sequences of the rat GH-R, rat GH-BP, mouse GH-R and mouse GH-BP.
Panel A: Alignment of the rat GH-R and its alternatively spliced isoform, the rat GH-BP. The isoforms have identical sequences for a stretch of the first 262 amino acids that includes the N-terminal signal peptide (residues 1-18) and the GH hormone binding domains (residues 19-262), although the rat GH-R has an extra 3 amino acids in its GH binding domain (residues 263-265). The rat GH-R has both a transmembrane domain (residues 266-289) and a cytoplasmic domain (residues 290-638) that are not present in the rat GH-BP. Instead, the rat GH-BP splice variant has a substituted C-terminal hydrophilic sequence (residues 263-279) shown in bold. The rat GH-BP263-279 sequence was used to generate polyclonal antipeptide antisera in this work. Panel B: Alignment of the mouse GH-R and its alternatively spliced isoform, the mouse GH-BP. The isoforms have identical sequences for a stretch of the first 270 amino acids that includes the N-terminal signal peptide (residues 1-24) and the GH hormone binding domains (residues 25-270), although the mouse GH-R has an extra 3 amino acids in its GH binding domain (residues 271-273). The mouse GH-R has both a transmembrane domain (residues 274-297) and a cytoplasmic domain (residues 298-650) that are not present in the mouse GH-BP. Instead, the mouse GH-BP splice variant has a sustituted C-terminal hydrophilic sequence (residues 271-297) shown in bold. Panel C: Alignment of the alternatively substituted C-terminal sequences of the rat GH-BP263-279 and of the mouse GH-BP271-297. Differences in the aligned sequences are depicted in plain lettering. The alternatively substituted C-terminal sequence of the mouse GH-BP has ten more amino acids than that of the rat GH-BP. Panel D: Alignment of the C-terminal sequences of the rat GH-R625-638 and of the mouse GH-R637-650. Sequences are identical and were previously used to generate the polyconal anti-mouse GH-R637-650 used in this work [6].
Similarly, the mouse GH-R and mouse GH-BP are products of a single gene and their aligned sequences are shown in panel B of Figure 1. They have identical N-terminal signal peptides (residues 1-24) and identical GH-binding domains (residues 25-270). The unspliced mouse GH-R has a transmembrane domain (24 amino acids) and a cytoplasmic domain (353 amino acids) not present in the mouse GH-BP. As a substitute, the mouse GH-BP has a 27-amino acid hydrophilic sequence comprised of residues 271-297 produced through alternative splicing of the mouse GH-R gene.
The rat and mouse GH-BPs have approximately 90% amino acid sequence homology [1]. The homology between the C-terminal amino acid sequences of the alternatively spliced GH-R isoforms of rat and mouse are shown in panel C of Figure 1. The aligned sequences differ at 2 of 15 loci and the mouse sequence has an additional 10 amino acids. The 17-amino acid C-terminal peptide sequence of the rat GH-BP was used to construct a tetravalent MAP dendrimer antigen for eliciting polyclonal antipeptide antibodies.
Methods for the development of monoclonal antibodies and polyclonal antisera as reagents for detection of GH-Rs and/or GH-BPs of rats, mice and rabbits have been reported. However, drawbacks relating to specificity of reagents and/or amount of effort in their production are associated with the reported approaches.
Monoclonal antibodies with specificities towards epitopes unique to either the GHR or GH-BP have been generated. Mice immunized with an affinity purified preparation of rabbit liver GHR produced four monoclonal antibodies to the GHR [4]. In another study a monoclonal antibody was raised to the rat GH-BP using a synthetic peptide comprising the C-terminal 17 amino acids as an immunogen [5]. However, a major drawback of generating monoclonal antibodies is that their production is labor intensive and costly.
The use of peptide sequences unique to the GH-R or to the GH-BP as antigens to elicit antibodies has also been described. In one report a synthetic peptide corresponding to amino acids 642–655 of the carboxyl-terminus of the mouse GH-R was coupled to keyhole limpet hemocyanin (KLH) and used as an immunogen [6]. In another study antibodies towards the rat GH-BP were generated in rabbits with an immunogen constructed of a 17-amino acid peptide similar to GH-BP carboxyl-terminus coupled to KLH [7]. Similarly, a 28-amino acid synthetic peptide corresponding to the carboxyl-terminal 27 amino acids of the mouse GH-BP was coupled to KLH and used as an immunogen in rabbits [8,9]. The use of synthetic peptides coupled to carrier proteins is a simple approach for the generation of sequence-specific antipeptide polyclonal antibodies. However, the coupling of carrier proteins (e.g., KLH, ovalbumin, bovine gamma-globulin, or bovine serum albumin) to a synthetic peptide of interest to increase its immunogenicity has a drawback. A subset of the antibodies in the polyclonal antisera will be directed towards the carrier protein. Hence, immunological reagents with varying degrees of non-specific cross-reactions in Westerns, dot blots, and immunoassays will confound interpretation of the results.
The disadvantages associated with using immunogenic carrier proteins for eliciting antipeptide antibodies have been overcome by advancements in development of highly immunogenic peptide dendrimers described by Tam and collaborators [10-15]. The subject has been extensively reviewed by Niederhafner and coworkers for peptide dendrimers [16] and glycopeptide dendrimers [17-19] and by Crespo and co-workers for peptide and amide-bond containing dendrimers [20]. The methodology uses a peptidyl core of radially branched lysine residues to which a peptide sequence of interest can be coupled using standard solid-phase chemistry. The high molar ratio and dense packing of multiple copies of the peptide epitope in the MAP system produces a strong immunogenic response. The MAP methodology continues to evolve as advances have been made in the construction of various artificial carriers of synthetic peptides [21]. New presentation strategies for the immunogen include oligomerization, dextran bead coupling and T-helper epitope conjugation [22] as well as construction of lipo-MAP peptides entrapped in liposomes [23]. Progress in the synthesis of MAPS with branched architectures [24] and in chemoselective peptide ligation [25,26] has also been made.
In this study we have used the MAP technology to generate antipeptide antisera towards the C-terminal 17-amino acid sequence (residues 263-279) of the rat GH-BP. The polyclonal rabbit anti-rat GH-BP263-279 MAP antisera will be useful in delineating the biological roles of GH-BPs.
MATERIALS AND METHODS
Materials
Two pregnant Fischer 344 rats (5-month old) and three New Zealand white rabbits were purchased from Harlan (Indianapolis, IN). Bovine growth hormone (bovine GH; L3836) was generously provided by Drs. C. H. Li and Harold Papkoff (UCSF Hormone Research Laboratory, University of California San Francisco). Culture media containing recombinant mouse GH-BP was generated as previously described [27]. Nitrocellulose sheets and Kaleidoscope molecular weight standards were purchased from Bio-Rad (Hercules, CA). Complete Freund’s Adjuvant, Incomplete Freunds’s Adjuvant, Protease Inhibitor Cocktail, non-fat dry milk, goat anti-rabbit IgG (H+L)-horseradish peroxidase (HRP), protein-A-HRP, bovine serum albumin (BSA), glycine, methanol, sodium chloride, sodium phosphate, potassium chloride, potassium phosphate, Tris-HCl, HEPES-HCl, Tween-20, magnesium chloride, pyrogallol-red, thimerosal, sodium molybdate, and sodium citrate were purchased from Sigma (St. Louis, MO). Serum separating tubes were acquired from Sarstedt (Newton, NC). SuperSignal® West Dura Extended Duration HRP chemiluminescent substrate kit, Micro BCA protein assay kit, and Restore™ Buffer were purchased from Pierce (Rockford, IL). MagicMark XP Western Protein Standards were bought from Invitrogen (Carlsbad, CA).
Synthesis, purification and ESI-MS of the tetravalent rat GH-BP263-279 MAP dendrimer
The Protein Core Facility of The University of Texas Health Science Center San Antonio chemically synthesized the tetravalent rat GH-BP263-279 MAP dendrimer as described [10,28] using an automated Multiple Peptide Synthesizer Model 396 MPS (Advanced ChemTech, Louisville, KY). Solid-phase peptide synthesis was carried out using standard N-(9-fluorenyl)methoxycarbonyl (Fmoc) chemistry [28,29]. Briefly, the tetravalent rat GH-BP263-279 MAP dendrimer synthesis was accomplished by activating Fmoc protected amino acids in a 0.32 M 1-hydroxybenzotriazole/N-methylpyrrolidone solution followed by their sequential addition onto each of the reactive amino ends of a four-branch MAP polystyrene resin having a tetravalent lysinyl dendrimer core (resin-β-Ala-Lys-[Lys(Fmoc)2]2) to form 17-mer arms consisting of the rat GH-BP263-279 sequence (263GPKFNSQHPHQEIDNHL279) [3] that upon cleavage from the resin formed a free tetravalent rat GH-BP263-279 MAP dendrimer containing a C-terminal ß-Ala as shown in Figure 2. After synthesis was complete the tetravalent rat GH-BP263-279 MAP dendrimer was cleaved from the resin by incubation for 1.5 hr in TFA/ triisopropylsilane /H2O 95:2.5:2.5. A total yield of 6 mg tetravalent rat GH-BP263-279 MAP dendrimer was obtained. The rat GH-BP263-279 sequence was selected because it is not found in the full-length rat GH-R (see panel A of Figure 1).
Figure 2. Structure of the synthetic tetravalent rat GH-BP263-279 MAP dendrimer.
The synthetic tetravalent rat GH-BP263-279 MAP dendrimer consists of an oligolysine core with two sequential levels of lysine residues onto which four copies of the rat GH-R625-638 peptide antigen is bound. GH-BP263-279 peptide = GPKFNSQHPHQEIDNHL.
Preparative RP-HPLC (C18) was used to separate components of the synthetic tetravalent rat GH-BP263-279 MAP dendrimer using a gradient mobile phase. Absorbance at 220 nm was used to monitor the column eluate for peptides. To form the gradient the initial mobile phase had a composition of 100% A (0.1% TFA) and 0% B (ACN). The final mobile phase had a composition of 50% A and 50% B. The mobile phase was flowed through the column at a rate of 1 mL/min for 30 min. Chromatographic fractions corresponding to the major peak of the separated synthetic products of tetravalent rat GH-BP263-279 MAP dendrimer were pooled and lyophilized.
Analytical RP-HPLC (C18) was used to assess the purity of the pooled tetravalent rat GH-BP263-279 MAP dendrimer fractions. Separation of an aliquot of the pooled fractions (25 μg) was accomplished using the following mobile phases at a flow rate of 1 mL /min: Mobile phase A was 0.1% TFA in water and mobile phase B was 0.1% TFA in ACN. Elution segment 1 was 10 min of isocratic 95% A/5% B. Elution segment 2 was a 25 min gradient from 95% A/5% B to 40% A/60% B. Elution segment 3 was a 5 min gradient from 40%A/60%B to 5%A/95%B. Elution segment 4 was 5 min of isocratic 5% A/95% B. Elution segment 5 was a 10 min gradient from 5% A/95% B to 95%A /5% B. Elution segment 6 was a 5 min of isocratic 95% A/5% B.
The mass of the synthetic tetravalent rat GH-BP263-279 MAP dendrimer was determined by ESI-MS. The sample was directly infused at 20 uL / min into a Finnigan LCQ Duo Ion-trap mass spectrometer in ESI positive mode and data were collected. The sample (0.01 μg/μL) was prepared by dissolving synthetic tetravalent rat GH-BP263-279 MAP dendrimer sample in 50/50 water/ACN containing 0.1% acetic acid.
Immunization protocol
Three male New Zealand white rabbits were housed at The University of Texas Health Science Center San Antonio Animal Facility and fed ad libidum. An IACUC-approved injection protocol (University of Texas at San Antonio and the University of Texas Health Science Center San Antonio) was followed. All injection volumes were 1 mL (0.2 mL/site). Primary subcutaneous injections were performed with 200 μg of tetravalent rat GH-BP263-279 MAP dendrimer solubilized in phosphate buffered saline (150 mM sodium chloride, 10 mM sodium phosphate, 2.5 mM potassium chloride, 2 mM potassium phosphate, pH. 7.4) at a concentration of 0.4 μg/μL and mixed with Complete Freund’s Adjuvant at a 1:1 (v/v) ratio. Secondary boosts were performed two weeks later followed by third and fourth boosts four weeks apart in which 200 μg of tetravalent rat GH-BP263-279 MAP dendrimer were injected subcutaneously in Incomplete Freunds’s Adjuvant at a 1:1 (v/v) ratio. Rabbits were ear-bled 15 days after each boost to check for antibody titer and specificity via antigen-antibody dot blots [30]. The three rabbits were terminally bled via heart puncture 12 days after the last boost. These rabbits yielded antisera designated as BETO-8039, BETO-8040, and BETO-8041. All blood was collected in serum separating tubes. Serum was separated from cells by centrifugation at 13,000 × g for 20 minutes at 5 °C.
Rabbit anti-mouse GH-R antisera
The previously described rabbit anti-mouse GH-R antisera (GHR-2) [6] was used in this study to detect the rat GH-R. The polyclonal antisera GHR-2 was produced by coupling KLH to a synthetic peptide that corresponds to the C-terminal 14 amino acids of the unspliced mouse GH-R, mouse GH-R637-650, which are identical to the C-terminal 14 amino acids of the unspliced rat GH-R, GH-R625-638, as shown in panel D of Figure 1. Therefore, the GHR-2 antibody detects both the mouse and rat GH-Rs. The GHR-2 antisera does not cross-react with the rat GH-BP because the C-terminal 14 amino acids of the rat GH-R are spliced out of the rat GH-BP and substituted with an alternate sequence of amino acids. Likewise, the GHR-2 antisera does not cross-react with the mouse GH-BP.
Construction of bovine GH-affinity column and purification of recombinant mouse GH-BP
Culture media containing recombinant mouse GH-BP [27] was used as the source of GH-BP. A bovine GH-affinity column was constructed as described in the following steps. First, 15 mL of Reacti-Gel GF-2000 were rinsed over a sintered glass funel with 100 mL of NaHCO3 Buffer (0.1 M NaHCO3, pH. 9.0) then the step was repeated. Next, 50 mg bovine GH (2 mg/mL NaHCO3 Buffer) were incubated with the Reacti-Gel matrix in 50 mL plastic conical tubes for 16 hr at 5 °C on a rotating mixer. Afterwards the supernatant containing uncoupled bovine GH was decanted and the bovine GH-Reacti-Gel matrix was rinsed twice with 50 mL of NaHCO3 Buffer. The gel was then washed twice with 50 mL of cold (5 °C) NaHCO3 Buffer containing 0.5 M NaCl. The sites on the resin remaining uncoupled were then blocked by incubating it at 5 °C in Blocking Buffer (1.0 M Tris-HCl, pH 9.0) for 10 minutes then repeating the step with fresh buffer.
After construction of the bovine GH-Reacti-Gel affinity matrix it was resuspended in Binding Buffer [0.01 M HEPES-HCl, 0.5 M sodium chloride, 0.01% (v/v) Tween-20, pH 8.0] and combined with culture media containing recombinant mouse GH-BP at a ratio of 1:1 (v/v) in 50 mL plastic conical tubes then incubated for 16 hr at 5 °C on a rotating mixer. The supernatant was decanted and the bovine GH-Reacti-Gel matrix containing bound recombinant mouse GH-BP was resuspended in Binding Buffer and packed ito a glass column (13 cm × 1 cm) to a final bed volume of 10.2 mL. Chromatography was performed using a BioLogic Workstation™ (Bio Rad) to deliver mobile phase flow rates of 0.5 mL/min. Absorbance at 280 nm was used to monitor the column eluate for proteins. The affinity column was first washed with 10 bed volumes of Binding Buffer then with 5 bed volumes of Binding Buffer containing 1 M NaCl. Recombinant mouse GH-BP was eluted with 3 bed volumes of Low Magnesium Buffer [0.01 M HEPES-HC1, 0.2 M magnesium chloride, 0.01% (v/v) Tween-20, pH 8.0] then with a High Magnesium Buffer [0.01 M HEPES-HC1, 2 M magnesium chloride, 0.01% (v/v) Tween-20, pH 8.0]. Column eluates were collected in 2 mL fractions. Aliquots of fractions were assayed for purity by precipitating proteins with Pyrogallol-Red Molybdate Reagent [31] followed by analytical SDS-PAGE [32] separation of precipitated proteins. Immunoreactivity of separated proteins towards rabbit anti-mouse GH-BP antisera [8], which detects the mouse GH-BP but does not detect the rat GH-BP, was assessed by Western blot analysis [33].
Preparation of rat liver extracts
Livers were obtained from pregnant Fischer 344 rats and homogenized in EBC Buffer [50 mM Tris-HCl, 150 mM sodium chloride, 0.5% (v/v) NP-40, pH 8.0] containing 1 mL of Protease Inhibitor Cocktail (Sigma) per 20 g of tissue. The homogenate was then centrifuged at 5 °C for 10 minutes at 10,000 × g and the supernatant was collected. The protein concentration of the supernatant was determined using the Micro BCA Protein Assay Kit with BSA as a standard [34].
Collection and preparation of rat serum samples
Blood was collected from pregnant Fischer 344 rats in serum separating tubes. Serum was separated from cells by centrifuging the tubes at 12,000 × g for 15 minutes at 5 °C. The serum supernatant was then diluted 1:20 (v/v) in Laemmli’s SDS Sample Buffer [32] and stored at -20 °C until used in Western blot analyses.
Sensitivity and specificity of various polyclonal antisera to tetrameric rat GH-BP263-279 MAP dendrimer assessed by dot blotting
Dot blot analyses [30] using three antisera (BETO-8039, BETO-8040 and BETO-8041) from rabbits immunized with the tetravalent rat GH-BP263-279 MAP dendrimer were carried out to monitor their sensitivity and specificity. Peptides and proteins (tetrameric rat GH-BP263-279 MAP dendrimer, recombinant mouse GH-BP and BSA) were dot-blotted onto nitrocellulose membranes in amounts ranging from 2-20 pmol in a 96-well format using the Bio-Dot® Microfiltration Apparatus (Bio-Rad). Blots were then incubated twice for 10 min per incubation in Tween-TBS [0.05% (v/v) Tween-20, 10 mM Tris-HCl, 150 mM NaCI, pH 7.6]. The blots were then incubated twice for 10 min per incubation in TBS (10 mM Tris-HCl, 150 mM NaCI, pH 7.6). Thereafter, non-specific protein binding sites on the nitrocellulose blots were blocked by incubating blots in Blocking Solution [TBS supplemented with 3% (w/v) nonfat dry milk, 0.1% (w/v) BSA, 0.01% (w/v) thimerosal] for 1 hour at 25 °C. Blots were then incubated for 16 hr at 5 °C with three rabbit antisera (BETO-8039, BETO-8040 or BETO-8041) at 1:1000 dilutions. Next, blots were incubated twice for 10 min per incubation in Tween-TBS then incubated twice for 10 min per incubation in TBS. Blots were then incubated with a 1:1000 dilution of goat anti-rabbit IgG (H+L)-horseradish peroxidase (HRP) in Blocking Solution for 1 hour at 25 °C. Next, blots were again incubated twice for 10 min per incubation in Tween-TBS then incubated twice for 10 min per incubation in TBS. Afterwards, blots were incubated in 1:1 (v/v) ratio of SuperSignal® West Dura Luminol/Enhancer Solution and SuperSignal® West Dura Stable Peroxide Solution for 5 min at 25 °C. Subsequently, the membranes were blotted until semi-dry then placed in a Kodak Image Station 2000R for 5 min at 25 °C to capture the chemiluminescent signals of immunoreactive spots.
The total chemiluminescent densities (total pixels × average pixel density) of spots were compiled from data obtained via image capture. The chemiluminescent densities of the spots for each blot were normalized to give a % total spot density (highest total spot density = 100% and the lowest total spot density = 0%). Data were plotted and fitted to the four-parameter logistic equation using nonlinear curve-fitting to derive Hillslopes and ED50s for each dose-response curve using the GraphPad Prism™ (GraphPad Software Inc.) statistical suite. Hillslopes of dose-response curves were tested for parallelism using an F test. If the Hill Slopes of given dose-response curves were parallel, then statistical differences between their ED50s were assessed using an unpaired t test.
Sensitivity and specificity of polyclonal antisera BETO-8041 to tetrameric rat GH-BP263-279 MAP dendrimer assessed by Western blotting
Western blotting methodology [33] was also used to assess the specificity of the anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera. Recombinant mouse GH-BP (100 ng), rat serum (1 μL) and rat liver extract (100 μg) were separated on 10% SDS-polyacrylamide gels under reducing conditions [32]. Proteins were electrophoretically transferred onto nitrocellulose sheets using 100 V for 1 hr at a temperature of 5 °C in Transfer Buffer [25 mM Tris-HCl, 200 mM glycine and 20% (v/v) methanol, pH. 8.3] [33]. The nitrocellulose blots were then incubated for 16 hr at 5 °C in sera diluted in Binding Buffer containing a 10-3 dilution of anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera (BETO-8041) or a 10-4 dilution of anti-mouse GH-R antisera (GHR-2). Four negative controls were used in these experiments. In the first negative control, a 10-3 dilution of pre-immune rabbit serum was used as a replacement for the primary antisera. In the second negative control, the primary antisera was eliminated from the immunostaining protocol. In the third negative control, blots were immunoprobed with a 10-3 dilution of anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera (BETO-8041) which had been pre-absorbed for two hours on ice with 10 μg of antigen (tetrameric rat GH-BP263-279 MAP dendrimer). In the fourth negative control, nitrocellulose blots were incubated with a 10-4 dilution of anti-mouse GH-R antisera (GHR-2) which had been pre-absorbed for two hours on ice with 10 μg of its antigen (C-terminal 14 amino acid peptide of the mouse GH-R, GH-R637-650) [6]. To minimize the immunoreactivity of heavy and light immunoglobulin chains contained in rat serum samples, enzymatic visualization of immunoprobed Westerns was carried out using Protein-A-HRP at a final dilution of 1 μg/mL. Chemiluminescent detection was performed as described for dot blot analyses. Stripping of blots for subsequent re-probing was carried out by incubating developed blots in Restore™ Buffer (Pierce) for 30 min at 37 °C. To verify that the stripping of the probed blots was complete, they were incubated with Protein-A-HRP followed by HRP chemiluminescent substrate then re-imaged. Absence of a chemiluminescent signal indicated that antibodies had been stripped from the nitrocellulose blot and could then undergo a subsequent immunoprobing.
RESULTS
Synthesis, purification and ESI-MS analysis of tetrameric rat GH-BP263-279 MAP dendrimer
Automated solid-phase Fmoc peptide synthesis produced a total yield of 6 mg of tetrameric rat GH-BP263-279 MAP dendrimer product. Preparative RP-HPLC separation of the tetrameric rat GH-BP263-279 MAP dendrimer product is shown in Figure 3 panel A. Fractions constituting the major peak (shaded area) eluting between 38.7% ACN and 40.3% ACN were pooled. An analytical RP-HPLC separation of the pooled fractions, shown in Figure 3 panel B, was then performed. The chromatograph shows a single peptide peak eluting at 33% ACN/0.1% TFA in a range between 32% ACN/0.1% TFA and 34% ACN/0.1% TFA. The ESI-MS spectrum of the purified synthetic tetrameric rat GH-BP263-279 MAP dendrimer is shown in Figure 4. The spectrum displays signals corresponding to [M + 9H]9+, [M + 10H]10+ and [M + 11H]11+ with m/z values of 8399 Da, 8399 Da and 8397 Da, respectively. The average molecular weight of these ions matches the calculated molecular weight of 8398 Da for the tetrameric rat GH-BP263-279 MAP dendrimer.
Figure 3. RP-HPLC purification and analysis of synthetic tetravalent rat GH-R625-638 MAP dendrimer.
Panel A: Preparative RP-HPLC (C18) chromatograph showing separation of synthetic tetravalent rat GH-BP263-279 MAP dendrimer products. Column fractions were monitored using absorbance at 220 nm for detection of peptides. Eluent: mobile phase gradient over 30 min from 100% A (0.1% TFA) and 0% B (ACN) to 50% A and 50% B. Fractions 23-24 were pooled and lyophilized. Panel B: Analytical RP-HPLC chromatograph showing purity of tetravalent rat GH-BP263-279 MAP dendrimer pooled fractions. Absorbance at 220 nm was used to monitor the column eluate for peptides. Mobile phase A: 0.1% TFA/H2O; mobile phase B: 0.1% TFA/ACN. Elution: Segment 1, 10 min of isocratic 95% A/5% B; segment 2, 25 min gradient from 95% A/5% B to 40% A/60% B; segment 3, 5 min gradient from 40% A/60% B to 5% A/95% B; segment 4, 5 min of isocratic 5% A/95% B; segment 5, 10 min gradient from 5% A/95% B to 95%A /5% B; segment 6, 5 min of isocratic 95% A/5% B; flow rate:1 mL/min. A single GH-BP263-279 MAP peak eluted at 33% B in a range between 32% B and 34% B.
Figure 4. ESI-MS of RP-HPLC purified tetravalent rat GH-BP263-279 MAP dendrimer product.
Positive ion mode ESI-MS of tetravalent rat GH-BP263-279 MAP dendrimer provided an average observed mass of 8398 Da from signals corresponding to [M + 9H]9+, [M + 10H]10+ and [M + 11H]11+. The observed molecular weight matched the calculated molecular mass of 8398 Da for the tetravalent rat GH-BP263-279 MAP dendrimer.
Sensitivity and specificity of various polyclonal rabbit anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera assessed by dot blot analyses
Reactivities of three rabbit anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera are shown in the dot blots of Figure 5. Panels A, B, and C show the reactivities of antisera BETO-8039, BETO-8040 and BETO-8041, respectively, towards the tetrameric rat GH-BP263-279 MAP dendrimer (●-●), recombinant mouse GH-BP (○-○) and BSA (x-x) which were dot blotted in amounts ranging from 2-20 pmol. All three antisera at dilutions of 1:1000 recognized the tetrameric rat GH-BP263-279 MAP dendrimer and the recombinant mouse GH-BP but they did not react with BSA, demonstrating specificity of the antisera for the C-terminal epitope of the rat/mouse GH-BP. Table 1 shows the Hillslopes and ED50 values for detection of the tetrameric rat GH-BP263-279 MAP dendrimer and the recombinant mouse GH-BP by each antisera. Regarding each antisera, the dose-response curves of tetrameric rat GH-BP263-279 MAP dendrimer and of recombinant mouse GH-BP were parallel because their Hillslopes were not statistically different from each other in an F-test. Parallelism of the dose-response curves for tetrameric rat GH-BP263-279 MAP dendrimer and of recombinant mouse GH-BP allowed statistical comparison of their ED50s which were within a range of 5-10 pmol. The ED50s of tetrameric rat GH-BP263-279 MAP dendrimer and recombinant mouse GH-BP were statistically different from each other regardless of the antisera used. The tetrameric rat GH-BP263-279 MAP dendrimer was detected at a lower ED50 dose (5.56 fmol) compared to the recombinant mouse GH-BP (9.72 fmol) by BETO-8039. Similarly, the BETO-8040 antisera detected the tetrameric rat GH-BP263-279 MAP dendrimer at a lower ED50 dose (5.58 fmol) than recombinant mouse GH-BP (8.95 fmol). In contrast, BETO-8041 antisera detected recombinant mouse GH-BP at a lower ED50 dose (7.67 fmol) compared to the tetrameric rat GH-BP263-279 MAP dendrimer (9.22 fmol).
Figure 5. Dot blots demonstrating sensitivity and specificity of three antisera raised against the tetrameric rat GH-BP263-279 MAP dendrimer.
Dot blots spotted with tetrameric rat GH-BP263-279 MAP dendrimer (•), recombinant mouse GH-BP (∘), and BSA (x) in amounts ranging from 2 to 20 fmol were immunostained with either antisera BETO-8039 (Panel A), antisera BETO-8040 (Panel B), or antisera BETO 8041 (Panel C). Reactivities of the primary antisera towards spotted peptides and proteins were ascertained by overlaying the blots with a horseradish perxidase-coupled goat anti-rabbit IgG and thereafter using a substrate (Supersignal™ West Dura Extended Duration HRP) that is enzymatically converted into a chemiluminescent product. The luminescence of each spot was detected and quantified using a Kodak Image Station 2000R. The Total Spot Density for each dot blot is a normalized value (highest total spot density = 100% and the lowest total spot density = 0%). Data were plotted and fitted to the four-parameter logistic model using nonlinear curve-fitting to derive ED50s and Hill Slopes for each dose-response curve using the GraphPad Prism™ statistical suite.
Table 1.
Specificity and sensitivity of several antisera developed towards the tetrameric rat GH-BP263-279 MAP dendrimer.
| Antisera | Ligand | Hillslope | Log ED50 | ED50 (moles) |
|---|---|---|---|---|
| BETO-8039 | Tetrameric rat GH-BP263-279 MAP dendrimer | 2.87 ± 1.24 | -11.25 ± 0.07** | 5.56 ± 0.90 × 10-12 |
| Recombinant mouse GH-BP | 2.93 ± 0.75 | -11.01 ± 0.04 | 9.72 ± 0.84 × 10-12 | |
|
| ||||
| BETO-8040 | Tetrameric rat GH-BP263-279 MAP dendrimer | 2.93 ± 0.72 | -11.25 ± 0.03* | 5.58 ± 0.34 × 10-12 |
| Recombinant mouse GH-BP | 3.58 ± 0.72 | -11.05 ± 0.01 | 8.95 ± 0.29 × 10-12 | |
|
| ||||
| BETO-8041 | Tetrameric rat GH-BP263-279 MAP dendrimer | 5.55 ± 1.78 | -11.04 ± 0.02** | 9.22 ± 0.46 × 10-12 |
| Recombinant mouse GH-BP | 4.34 ± 0.16 | -11.12 ± 0.00 | 7.67 ± 0.05 × 10-12 | |
Numbers indicate values ±SE.
P < 0.001
P < 0.05 when comparing tetrameric rat GH-BP263-279 MAP dendrimer to recombinant mouse GH-BP using unpaired t-test.
Sensitivity and specificity of anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera BETO-8041assessed by Western blot analyses
To further assess anti-rat GH-BP263-279 MAP titer and specificity, samples containing recombinant mouse GH-BPs, rat serum GH-BPs, and rat tissue GH-BPs were separated by SDS-PAGE, transferred to nitrocellulose and probed with anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera (BETO-8041), as shown in Figure 6. When the anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera was used at a dilution of 10-2 (Panel A) it readily detected 100 ng recombinant mouse GH-BP (Lane 1), GH-BPs in 1 μL of rat serum (Lane 2), and GH-BPs in 100 μg of rat liver extract (Lane 3). The 37 kDa recombinant mouse GH-BP immunoreactive band (Lane 1) corresponds to that previously described [27]. The 75 kDa recombinant mouse GH-BP immunoreactive band (Lane 1), absent in our initial purified recombinant mouse GH-BP preparation, was apparent after storage. It may be a disulfide-linked dimer which is stable to reducing agents as observed for a mercaptoethanol-stable dimeric isoform of human GH [35]. The rat serum GH-BPs (Lane 2) had apparent molecular weights of 44 kDa and 46 kDa, consistent with those reported for glycosylated serum GH-BPs [5,36,37]. The rat liver GH-BPs (Lane 3) had apparent molecular weights of 44 kDa and 120 kDa, similar to those reported for rat serum GH-BPs [5]. When anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera was used at a dilution of 10-3 (Panel B) it again visualized 100 ng recombinant mouse GH-BP (Lane 1), GH-BPs in 1 μL of rat serum (Lane 2), and GH-BPs in 100 μg of rat liver extract (Lane 3). When anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera was used at a dilution of 10-4, it easily detected 100 ng of purified recombinant mouse GH-BP, however, the GH-BPs in 1 μL of rat serum were not detected and the GH-BPs in 100 μg of rat liver extract were only slightly visible.
Figure 6. Western blots demonstrating sensitivity and specificity of antisera towards the tetrameric rat GH-BP263-279 MAP dendrimer.
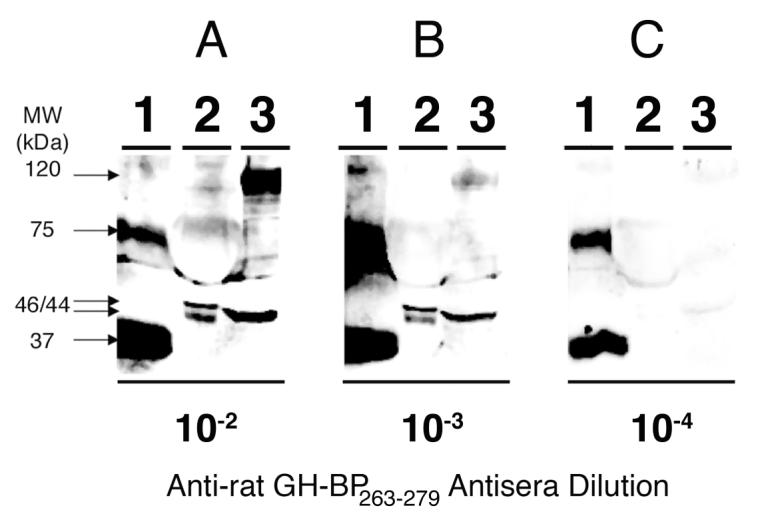
Samples of purified recombinant mouse GH-BP (100 ng) in Lane 1, rat serum (1 μL) in Lane 2 and rat liver extract (100 μg) in Lane 3 were loaded onto 10 % polyacrylamide gels and transferred onto nitrocellulose membranes. Blots were subsequently immunoprobed with rabbit anti-termeric rat GH-BP263-279 dendrimer antisera (BETO-8041) at dilutions of 10-2 (Panel A), 10-3 (Panel B) and 10-4 (Panel C). Antigen-antibody complexes were visualized by overlaying the blots with a horseradish perxidase-coupled goat anti-rabbit IgG and thereafter using a substrate (Supersignal™ West Dura Extended Duration HRP) that is enzymatically converted into a chemiluminescent product. Images of the chemiluminescent blots were acquired with a Kodak Image Station 2000R.
The specificity and cross-reativity of the anti- tetrameric rat GH-BP263-279 MAP dendrimer antisera was assessed using the immunoprobed Western blots of Figure 6. Blots depicted in Figure 6 were stripped of bound antibodies then re-probed and the results are shown in Figure 7. Panel A shows that upon re-probing blots with anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera at a dilution of 10-3, strong immunoreactive bands appeared for the recombinant mouse GH-BPs (Lane 1), rat serum GH-BPs (Lane 2), and rat liver GH-BPs (Lane 3). Panel B shows that when re-probing blots with a 10-3 dilution of anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera that had been pre-absorbed with 10 μg of tetrameric rat GH-BP263-279 MAP dendrimer, detection of mouse GH-BP was drastically minimized (Lane 1) while GH-BPs in rat serum (Lane 2) and liver (Lane 3) were undetectable, demonstrating the antiseum’s specificity. Panel C shows that re-probing blots with pre-immune rabbit serum did not immunostain the mouse GH-BPs (Lane 1), the rat serum GH-BPs (Lane 2) nor the rat liver GH-BPs (lane 3), indicating that the immunoreactive bands visualized with the anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera are not due to antibodies present in serum from the pre-immunized rabbits. Panel D shows that re-probing blots with rabbit antisera specific for the C-terminal portion of the rat GH-R (rat GH-R625-638) at a dilution of 10-4, the mouse GH-BPs were not immunoreactive (Lane 1) nor were the GH-BPs of either rat serum (Lane 2) or of rat liver extract (Lane 3). However, immunoreactive GH-R bands were observed in the rat liver extract with apparent molecular weights of 27 kDa, 94 kDa and 96 kDa, consistent with previous reports [2,37-40]. The 27 kDa band corresponds to a proteolytically-cleaved product of the full-length GH-R [41]. Panel E shows that re-probing blots with rabbit anti-rat GH-R625-638 antisera at a dilution of 10-4 that had been pre-absorbed with 10 μg of synthetic peptide rat GH-R625-638, eliminated rat GH-R immunoreactive bands in the rat liver extract (Lane 3), demonstrating the specificity of the anti-rat GH-R625-638 antiseum for the rat GH-R. The immuno-stained bands detected with the antisera developed towards the tetrameric rat GH-BP263-279 MAP dendrimer shown in Panel A do not correspond to the anti-rat GH-R625-638 immuno-stained bands of Panel D, demonstrating that the anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera does not detect the rat GH-R.
Figure 7. Western blots showing lack of superimposition of immunoreactive bands detected by anti-tertameric rat GH-BP263-279 MAP dendrimer with those detected by anti-GH-R antisera.
Blots that had been immnoprobed in Figure 3 were stripped then re-probed. Lanes 1-3 of each blot contained purified recombinant mouse GH-BP (100 ng), rat serum (1 μL) and rat liver extract (100 μg), respectively. Panel A shows a blot that was re-probed with rabbit anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera (BETO-8041) at dilution of 10-3. Panel B shows a blot that was re-probed with rabbit anti-tetrameric rat GH-BP263-279 MAP dendrimer antisera (BETO-8041) at dilution of 10-3 that had been pre-absorbed with 10 μg of tetrameric rat GH-BP263-279 MAP dendrimer. Panel C shows a blot that was re-probed with pre-immune rabbit serum at a dilution of 10-3. Panel D shows a blot that was re-probed with rabbit anti-rat GH-R625-638 antisera (GHR-2) specific for the C-terminal portion of the rat GH-R at a dilution of 10-4. Panel E shows a blot that was re-probed with rabbit anti-rat GH-R625-638 antisera at a dilution of 10-4 that had been pre-absorbed with 10 μg of synthetic peptide corresponding to the C-terminal fourteen amino acids (625-638) of the rat GH-R. Antigen-antibody complexes were visualized by overlaying the blots with a horseradish perxidase-coupled goat anti-rabbit IgG and thereafter using a substrate (Supersignal™ West Dura Extended Duration HRP) that is enzymatically converted into a chemiluminescent product. Images of the chemiluminescent blots were acquired with a Kodak Image Station 2000R.
DISCUSSION
The GH-R and the GH-BP are important in the regulation of reproduction [42], metabolism [43], pregnancy [44], adiposity [45,46], lung development [47], retinal development [48], B-cell development [49] and longevity [50]. Although it has been demonstrated that the GH-BP regulates the bioactivity of GH, the biological functions of the GH-BP are unknown [51,52]. Multiple studies have shown that a correlation exists between GH-BP levels and clinical/biological parameters such as body composition, age, gender, pregnancy, diabetes, cirrhosis and gonadal dysfunction, however, the cause-effect relationships are unclear [51,52]. Furthermore, although we have some knowledge regarding the structure and regulation of the GH-R/GH-BP [9,53-55], the membrane-associated GH-BP [56-58] and their interactions with GH [59], the relationships between them are unclear.
To help us better understand the physiological regulation and biological actions of the GH-BPs we have developed polyclonal antisera specific for GH-BPs of rat and mouse by immunizing rabbits with a synthetic tetrameric rat GH-BP263-279 MAP dendrimer. The tetrameric rat GH-BP263-279 MAP dendrimer was immunogenic, without the need to couple the peptide to a carrier protein. Although it has been argued that carboxy-terminal epitopes are not good immunogens as they may assume an unusual structure that would not mimic the structure of the cognate protein [60], the antisera recognized low pmol amounts of recombinant mouse GH-BP. Our laboratory is using the antisera help us understanding the role of GH-BPs in the brain. In a preliminary study employing the antisera we were able to detect the rat GH-BP in a rat hippocampal cell line [61]. Studies in progress are aimed at understanding endocytosis and cellular trafficking of the rat GH-BP in a rat hippocampal cell line. In other studies we are employing the antisera to measure the regulation of GB-BP levels during the lifespan of the rat. We are also planning to use the antisera to study the role of GH-BPs in neuronal stem cell differentiation. Future applications will also use the antisera for quantification of GH-BPs in biological fluids of animals through the use of enzyme linked immunoassays and radioimmunoassays.
ACKNOWLEDGEMENTS
The authors would like to thank The University of Texas Health Science Center San Antonio Animal Facility for their help and support in producing the antisera herein and caring for our animals. We thank Dr. Stephan B. Bach and Conor Mullens of the Mass Spectrometry Facility in the Department of Chemistry at The University of Texas at San Antonio for the ESI-MS analysis.
This work was supported by the National Institutes of Health (GM08194, GM60655) and the Sloan Foundation.
Abbreviations
- Fmoc
N-(9-fluorenyl)methoxycarbonyl
- GH
growth hormone
- GH-R
growth hormone receptor
- GH-BP
growth hormone binding protein
- MAP
multiple antigen peptide
- TBS
Tris buffered saline
REFERENCES
- 1.Edens A, Talamantes F. Alternative processing of growth hormone receptor transcripts. Endocr. Rev. 1998;19:559–582. doi: 10.1210/edrv.19.5.0347. [DOI] [PubMed] [Google Scholar]
- 2.Smith WC, Kuniyoshi J, Talamantes F. Mouse serum growth hormone (GH) binding protein has GH receptor extracellular and substituted transmembrane domains. Mol. Endocrinol. 1989;3:984–990. doi: 10.1210/mend-3-6-984. [DOI] [PubMed] [Google Scholar]
- 3.Baumbach WR, Horner DL, Logan JS. The growth hormone-binding protein in rat serum is an alternatively spliced form of the rat growth hormone receptor. Genes Dev. 1989;3:1199–1205. doi: 10.1101/gad.3.8.1199. [DOI] [PubMed] [Google Scholar]
- 4.Barnard R, Bundesen PG, Rylatt DB, Waters MJ. Monoclonal antibodies to the rabbit liver growth hormone receptor: production and characterization. Endocrinology. 1984;115:1805–1813. doi: 10.1210/endo-115-5-1805. [DOI] [PubMed] [Google Scholar]
- 5.Sadeghi H, Wang BS, Lumanglas AL, Logan JS, Baumbach WR. Identification of the origin of the growth hormone-binding protein in rat serum. Mol. Endocrinol. 1990;4:1799–1805. doi: 10.1210/mend-4-12-1799. [DOI] [PubMed] [Google Scholar]
- 6.Camarillo IG, Thordarson G, Ilkbahar YN, Talamantes F. Development of a homologous radioimmunoassay for mouse growth hormone receptor. Endocrinology. 1998;139:3585–3589. doi: 10.1210/endo.139.8.6160. [DOI] [PubMed] [Google Scholar]
- 7.Frick GP, Goodman HM. Characterization of the short isoform of the growth hormone receptor synthesized by rat adipocytes. Endocrinology. 1992;131:3083–3090. doi: 10.1210/endo.131.6.1446642. [DOI] [PubMed] [Google Scholar]
- 8.Cramer SD, Barnard R, Engbers C, Thordarson G, Talamantes F. A mouse growth hormone-binding protein RIA: concentrations in maternal serum during pregnancy. Endocrinology. 1992;130:1074–1076. doi: 10.1210/endo.130.2.1733707. [DOI] [PubMed] [Google Scholar]
- 9.Sotelo AI, Bartke A, Kopchick JJ, Knapp JR, Turyn D. Growth hormone (GH) receptors, binding proteins and IGF-I concentrations in the serum of transgenic mice expressing bovine GH agonist or antagonist. J. Endocrinol. 1998;158:53–59. doi: 10.1677/joe.0.1580053. [DOI] [PubMed] [Google Scholar]
- 10.Tam JP. Synthetic peptide vaccine design: synthesis and properties of a high-density multiple antigenic peptide system. Proc. Natl. Acad. Sci. U. S. A. 1988;85:5409–5413. doi: 10.1073/pnas.85.15.5409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Defoort JP, Nardelli B, Huang W, Tam JP. A rational design of synthetic peptide vaccine with a built-in adjuvant. A modular approach for unambiguity. Int. J. Pept. Protein Res. 1992;40:214–221. doi: 10.1111/j.1399-3011.1992.tb00294.x. [DOI] [PubMed] [Google Scholar]
- 12.Nardelli B, Tam JP. The MAP system. A flexible and unambiguous vaccine design of branched peptides. Pharm. Biotechnol. 1995;6:803–819. [PubMed] [Google Scholar]
- 13.Tam JP. Recent advances in multiple antigen peptides. J. Immunol. Methods. 1996;196:17–32. doi: 10.1016/0022-1759(96)00066-x. [DOI] [PubMed] [Google Scholar]
- 14.Tam JP, Spetzler JC. Multiple antigen peptide system. Methods Enzymol. 1997;289:612–637. doi: 10.1016/s0076-6879(97)89067-2. [DOI] [PubMed] [Google Scholar]
- 15.Sadler K, Tam JP. Peptide dendrimers: applications and synthesis. J. Biotechnol. 2002;90:195–229. doi: 10.1016/s1389-0352(01)00061-7. [DOI] [PubMed] [Google Scholar]
- 16.Niederhafner P, Sebestik J, Jezek J. Peptide dendrimers. J. Pept. Sci. 2005;11:757–788. doi: 10.1002/psc.721. [DOI] [PubMed] [Google Scholar]
- 17.Niederhafner P, Sebestik J, Jezek J. Glycopeptide dendrimers. Part I. J. Pept. Sci. 2008;14:2–43. doi: 10.1002/psc.931. [DOI] [PubMed] [Google Scholar]
- 18.Niederhafner P, Sebestik J, Jezek J. Glycopeptide dendrimers. Part II. J. Pept. Sci. 2008;14:44–65. doi: 10.1002/psc.945. [DOI] [PubMed] [Google Scholar]
- 19.Niederhafner P, Reinis M, Sebestik J, Jezek J. Glycopeptide dendrimers, part III: a review. Use of glycopeptide dendrimers in immunotherapy and diagnosis of cancer and viral diseases. J. Pept. Sci. 2008;14:556–587. doi: 10.1002/psc.1011. [DOI] [PubMed] [Google Scholar]
- 20.Crespo L, Sanclimens G, Pons M, Giralt E, Royo M, Albericio F. Peptide and amide bond-containing dendrimers. Chem. Rev. 2005;105:1663–1681. doi: 10.1021/cr030449l. [DOI] [PubMed] [Google Scholar]
- 21.Sakarellos-Daitsiotis M, Krikorian D, Panou-Pomonis E, Sakarellos C. Artificial carriers: a strategy for constructing antigenic/immunogenic conjugates. Curr. Top. Med. Chem. 2006;6:1715–1735. doi: 10.2174/156802606778194190. [DOI] [PubMed] [Google Scholar]
- 22.Cruz LJ, Iglesias E, Aguilar JC, Gonzalez LJ, Reyes O, Albericio F, Andreu D. A comparative study of different presentation strategies for an HIV peptide immunogen. Bioconjug. Chem. 2004;15:112–120. doi: 10.1021/bc034119j. [DOI] [PubMed] [Google Scholar]
- 23.Haro I, Perez S, Garcia M, Chan WC, Ercilla G. Liposome entrapment and immunogenic studies of a synthetic lipophilic multiple antigenic peptide bearing VP1 and VP3 domains of the hepatitis A virus: a robust method for vaccine design. FEBS Lett. 2003;540:133–140. doi: 10.1016/s0014-5793(03)00249-7. [DOI] [PubMed] [Google Scholar]
- 24.Papas S, Strongylis C, Tsikaris V. Synthetic approaches for total chemical synthesis of proteins and protein-like macromolecules of branched architecture. Curr. Org. Chem. 2006;10:1727–1744. [Google Scholar]
- 25.Tam JP, Yu Q, Miao Z. Orthogonal ligation strategies for peptide and protein. Biopolymers. 1999;51:311–332. doi: 10.1002/(SICI)1097-0282(1999)51:5<311::AID-BIP2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 26.Tam JP, Xu J, Eom KD. Methods and strategies of peptide ligation. Biopolymers. 2001;60:194–205. doi: 10.1002/1097-0282(2001)60:3<194::AID-BIP10031>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 27.Thordarson G, Wu K, Talamantes F. Purification and characterization of recombinant mouse growth hormone binding protein produced in the baculovirus expression system. Protein Expr. Purif. 1996;7:74–80. doi: 10.1006/prep.1996.0011. [DOI] [PubMed] [Google Scholar]
- 28.Fields GB, editor. Solid-phase peptide synthesis. Academic Press; San Diego: 1997. [Google Scholar]
- 29.Fields GB, Noble RL. Solid phase peptide synthesis utilizing 9-fluorenylmethoxycarbonyl amino acids. Int. J Pept. Protein Res. 1990;35:161–214. doi: 10.1111/j.1399-3011.1990.tb00939.x. [DOI] [PubMed] [Google Scholar]
- 30.Hawkes R, Niday E, Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal. Biochem. 1982;119:142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- 31.Aguilar RM, Bustamante JJ, Hernandez PG, Martinez AO, Haro LS. Precipitation of dilute chromatographic samples (ng/ml) containing interfering substances for SDS-PAGE. Anal. Biochem. 1999;267:344–350. doi: 10.1006/abio.1998.3018. [DOI] [PubMed] [Google Scholar]
- 32.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 33.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith PK, Krohn RI, Hermanson GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal. Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 35.Grigorian AL, Bustamante JJ, Hernandez P, Martinez AO, Haro LS. Extraordinarily stable disulfide-linked homodimer of human growth hormone. Protein Sci. 2005;14:902–913. doi: 10.1110/ps.041048805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smith WC, Talamantes F. Gestational profile and affinity cross-linking of the mouse serum growth hormone-binding protein. Endocrinology. 1988;123:1489–1494. doi: 10.1210/endo-123-3-1489. [DOI] [PubMed] [Google Scholar]
- 37.Frick GP, Tai LR, Baumbach WR, Goodman HM. Tissue distribution, turnover, and glycosylation of the long and short growth hormone receptor isoforms in rat tissues. Endocrinology. 1998;139:2824–2830. doi: 10.1210/endo.139.6.6047. [DOI] [PubMed] [Google Scholar]
- 38.Spencer SA, Hammonds RG, Henzel WJ, Rodriguez H, Waters MJ, Wood WI. Rabbit liver growth hormone receptor and serum binding protein. Purification, characterization, and sequence. J. Biol. Chem. 1988;263:7862–7867. [PubMed] [Google Scholar]
- 39.Smith WC, Linzer DI, Talamantes F. Detection of two growth hormone receptor mRNAs and primary translation products in the mouse. Proc. Natl. Acad. Sci. U. S. A. 1988;85:9576–9579. doi: 10.1073/pnas.85.24.9576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leung DW, Spencer SA, Cachianes G, Hammonds RG, Collins C, Henzel WJ, Barnard R, Waters MJ, Wood WI. Growth hormone receptor and serum binding protein: purification, cloning and expression. Nature. 1987;330:537–543. doi: 10.1038/330537a0. [DOI] [PubMed] [Google Scholar]
- 41.Cowan JW, Wang X, Guan R, He K, Jiang J, Baumann G, Black RA, Wolfe MS, Frank SJ. Growth hormone receptor is a target for presenilin-dependent gamma-secretase cleavage. J. Biol. Chem. 2005;280:19331–19342. doi: 10.1074/jbc.M500621200. [DOI] [PubMed] [Google Scholar]
- 42.Blumenfeld Z, Amit T. The role of growth hormone (GH), GH-receptor and GH-binding protein in reproduction and ovulation induction. J. Pediatr. Endocrinol. Metab. 1996;9:145–162. [PubMed] [Google Scholar]
- 43.List EO, Coschigano KT, Kopchick JJ. Growth hormone receptor/binding protein (GHR/BP) knockout mice: a 3-year update. Mol. Genet. Metab. 2001;73:1–10. doi: 10.1006/mgme.2001.3164. [DOI] [PubMed] [Google Scholar]
- 44.Barnard R, Waters MJ. The serum growth hormone binding protein: pregnant with possibilities. J. Endocrinol. 1997;153:1–14. doi: 10.1677/joe.0.1530001. [DOI] [PubMed] [Google Scholar]
- 45.Berryman DE, List EO, Coschigano KT, Behar K, Kim JK, Kopchick JJ. Comparing adiposity profiles in three mouse models with altered GH signaling. Growth Horm. IGF Res. 2004;14:309–318. doi: 10.1016/j.ghir.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Kelder B, Berryman DE, Clark R, Li A, List EO, Kopchick JJ. CIDE-A gene expression is decreased in white adipose tissue of growth hormone receptor/binding protein gene disrupted mice and with high-fat feeding of normal mice. Growth Horm. IGF Res. 2007;17:346–351. doi: 10.1016/j.ghir.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 47.Beyea JA, Sawicki G, Olson DM, List E, Kopchick JJ, Harvey S. Growth hormone (GH) receptor knockout mice reveal actions of GH in lung development. Proteomics. 2006;6:341–348. doi: 10.1002/pmic.200500168. [DOI] [PubMed] [Google Scholar]
- 48.Baudet ML, Hassanali Z, Sawicki G, List EO, Kopchick JJ, Harvey S. Growth hormone action in the developing neural retina: a proteomic analysis. Proteomics. 2008;8:389–401. doi: 10.1002/pmic.200700952. [DOI] [PubMed] [Google Scholar]
- 49.Luna M, Rodriguez-Mendez AJ, Berumen L, Carranza M, Riesgo-Escovar J, Baudet ML, Harvey S, Aramburo C. Immune growth hormone (GH): Localization of GH and GH mRNA in the bursa of Fabricius. Dev. Comp. Immunol. 2008;32:1313–1325. doi: 10.1016/j.dci.2008.04.008. [DOI] [PubMed] [Google Scholar]
- 50.Coschigano KT, Holland AN, Riders ME, List EO, Flyvbjerg A, Kopchick JJ. Deletion, but not antagonism, of the mouse growth hormone receptor results in severely decreased body weights, insulin, and insulin-like growth factor I levels and increased life span. Endocrinology. 2003;144:3799–3810. doi: 10.1210/en.2003-0374. [DOI] [PubMed] [Google Scholar]
- 51.Baumann G. Growth hormone binding protein. The soluble growth hormone receptor. Minerva Endocrinol. 2002;27:265–276. [PubMed] [Google Scholar]
- 52.Fisker S. Physiology and pathophysiology of growth hormone-binding protein: methodological and clinical aspects. Growth Horm. IGF Res. 2006;16:1–28. doi: 10.1016/j.ghir.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Talamantes F. The structure and regulation of expression of the mouse growth hormone receptor and binding protein. Proc. Soc. Exp. Biol. Med. 1994;206:254–256. doi: 10.3181/00379727-206-43754. [DOI] [PubMed] [Google Scholar]
- 54.Southard JN, Barrett BA, Bikbulatova L, Ilkbahar Y, Wu K, Talamantes F. Growth hormone (GH) receptor and GH-binding protein messenger ribonucleic acids with alternative 5′-untranslated regions are differentially expressed in mouse liver and placenta. Endocrinology. 1995;136:2913–2921. doi: 10.1210/endo.136.7.7789316. [DOI] [PubMed] [Google Scholar]
- 55.Moffat JG, Edens A, Talamantes F. Structure and expression of the mouse growth hormone receptor/growth hormone binding protein gene. J. Mol. Endocrinol. 1999;23:33–44. doi: 10.1677/jme.0.0230033. [DOI] [PubMed] [Google Scholar]
- 56.Gonzalez L, Curto LM, Miquet JG, Bartke A, Turyn D, Sotelo AI. Differential regulation of membrane associated-growth hormone binding protein (MA-GH-BP) and growth hormone receptor (GHR) expression by growth hormone (GH) in mouse liver. Growth Horm. IGF Res. 2007;17:104–112. doi: 10.1016/j.ghir.2006.12.002. [DOI] [PubMed] [Google Scholar]
- 57.Cerio RJ, Xing F, Fatula RJ, Keith DE, Yang X, Talamantes F, Southard JN, Southard JN. Structurally distinct membrane-associated and soluble forms of GH-binding protein in the mouse. J. Endocrinol. 2002;172:321–331. doi: 10.1677/joe.0.1720321. [DOI] [PubMed] [Google Scholar]
- 58.Gonzalez L, Sotelo AI, Bartke A, Turyn D. Growth hormone (GH) and estradiol regulation of membrane-associated GH binding protein and GH receptors in GH releasing hormone transgenic mice. Growth Horm. IGF Res. 2001;11:34–40. doi: 10.1054/ghir.2000.0187. [DOI] [PubMed] [Google Scholar]
- 59.Turyn D, Dominici FP, Sotelo AI, Bartke A. Specific interactions of growth hormone (GH) with GH-receptors and GH-binding proteins in vivo in genetically GH-deficient Ames dwarf mice. Growth Horm IGF Res. 1998;8:389–396. doi: 10.1016/s1096-6374(98)80309-2. [DOI] [PubMed] [Google Scholar]
- 60.Briand JP, Barin C, Van Regenmortel MH, Muller S. Application and limitations of the multiple antigen peptide (MAP) system in the production and evaluation of anti-peptide and anti-protein antibodies. J. Immunol. Methods. 1992;156:255–265. doi: 10.1016/0022-1759(92)90033-p. [DOI] [PubMed] [Google Scholar]
- 61.Aguilar RM, Munoz J, Martinez AO, Haro LS. Characterization of a cell line to determine the biological roles of growth hormone in neuronal cells, 87th Annual Meeting of The Endocrine Society; San Diego, CA. 2005.p. 658. [Google Scholar]








