Abstract
Background
Major depressive disorder (MDD) has been associated with both dysfunction of the central serotonergic system and abnormal responses to emotional stimuli. We used acute tryptophan depletion (ATD) to investigate the effect of temporarily reducing brain serotonin synthesis on neural and behavioural responses to emotional stimuli in remitted MDD subjects (rMDD) and healthy controls.
Methods
Twenty controls and 23 rMDD subjects who had been unmedicated and in remission for ≥3 months completed the study. Following tryptophan or sham depletion, participants performed an emotional-processing task during functional magnetic resonance imaging. In addition, resting-state regional blood-flow was measured using arterial spin labelling.
Results
Neither group exhibited significant mood-change following ATD. However, tryptophan depletion differentially affected the groups in terms of hemodynamic responses to emotional words in a number of structures implicated in the pathophysiology of MDD, including medial thalamus and caudate. These interactions were driven by increased responses to emotional words in the controls, with little effect in the patients under the ATD condition. Following ATD, habenula blood-flow increased significantly in the rMDD subjects relative to the controls, and increasing amygdala blood-flow was associated with more negative emotional bias score across both groups.
Conclusions
These data provide evidence for elevated habenula blood-flow and alterations in the neural processing of emotional stimuli following ATD in rMDD subjects, even in the absence of overt mood-change. However, further studies are required to determine whether these findings represent mechanisms of resilience or vulnerability to MDD.
Keywords: Depression, Serotonin, Acute Tryptophan Depletion, Functional Magnetic Resonance Imaging (fMRI), Emotional Processing, Affective Go/No-go (AGNG)
Introduction
It has long been hypothesised that dysfunction of the central serotonergic system plays a role in the pathogenesis of major depressive disorder (MDD). Aside from the efficacy of selective serotonin reuptake inhibitors (SSRIs) in treating depression, numerous studies have demonstrated that serotonin (5-HT) manipulations affect subjective emotional state(1). In particular, many medicated subjects with a history of MDD currently in remission (rMDD) experience a temporary return of depressive symptoms when administered an amino acid mixture selectively lacking in the precursor to 5-HT, tryptophan (acute tryptophan depletion: ATD)(2), though this effect does not occur commonly in unmedicated rMDD subjects who have been well for some months(3).
ATD has been utilized to investigate the role of 5-HT in a variety of cognitive processes(4). A consistent finding is that ATD impairs performance on tasks requiring the processing of affective stimuli in healthy volunteers. It has been reported that ATD impaired decision-making on gambling tasks(5, 6), attenuated motivation on a reaction-time task(7), impaired recognition of emotional expressions(8) and resulted in slower responses to positive words on an emotional inhibitory control paradigm, the Affective Go/No-go test (AGNG)(9).
A small number of studies used ATD in rMDD subjects while simultaneously acquiring neurophysiological measures, such as resting state regional cerebral blood-flow (rCBF) or glucose metabolism. These studies reported altered metabolism in a network of structures implicated in MDD, including orbitofrontal cortex (OFC), ventromedial prefrontal cortex (VMPFC), caudate, thalamus and habenula, in rMDD subjects following ATD(10-13). More recently, we reported changes in hemodynamic responses to emotional stimuli during the AGNG in this network following ATD in the healthy controls included in the present study(14). However, no study to date has investigated how neural responses to emotional stimuli differ between rMDD subjects and controls under conditions of 5-HT depletion. Such information is critically important in synthesizing cognitive, neurochemical and functional neuroanatomical accounts of MDD.
Therefore we used fMRI to investigate the effects of ATD on the neural correlates of emotional processing in unmedicated rMDD subjects and healthy controls. We included patients who were unmedicated and remitted to avoid the potential confounding effects of anti-depressant medication and depressive symptoms on neuronal and hemodynamic responses. We additionally used arterial spin labelling (ASL) to obtain measures of resting-state blood-flow in the same scanning session in order to exclude the possibility that any differences between the groups in the fMRI data might be related to altered neurovascular coupling following ATD. As an assessment of emotional processing we used the AGNG, on which currently depressed MDD subjects typically exhibit a bias towards negative information, termed the “mood-congruent processing bias”(15, 16). Previous studies suggested this bias was mediated by altered activity in the anterior cingulate cortex (ACC) and other prefrontal and subcortical structures(15), which form part of a “visceromotor” network(17). This network is thought to subserve the assessment of the emotional salience of sensory stimuli, and the organization of behavioral, autonomic and endocrine responses to such stimuli, and has been consistently implicated in the pathophysiology of MDD(18, 19).
Our analysis strategy for the task-related and resting-state analyses differed, though both focused on the visceromotor network. For the task-related analysis we employed a voxel-wise mapping approach, restricting our search for interactions to regions of interest (ROIs) based on a probabilistic atlas, and applied stringent correction for multiple comparisons. For the resting-state analysis we had less data, reducing the power of a voxel-wise analysis. Therefore, we calculated average blood-flow measurements across ROIs defined for each individual based on their own anatomical MRI scan.
We predicted that ATD would lead to a negative emotional bias on the AGNG in rMDD subjects, and hypothesised that this bias would be mediated by altered activity in the visceromotor network, i.e. OFC/VMPFC, together with anatomically-related areas of the striatum, thalamus and temporal lobe (amygdala; hippocampus; parahippocampal cortex; habenula; middle and superior temporal gyri (STG)). We additionally predicted that ATD would result in increased resting-state blood-flow in this network in rMDD subjects relative to controls, in particular in the habenula(13).
Methods and Materials
Full experimental methods are detailed in the Supplementary Online Materials (SOM). Twenty-three rMDD subjects and 20 healthy controls completed the study. The fMRI and behavioral data from the healthy controls were the subject of a previous report(14). Participants were right handed, aged 18-50 years, and in good physical health, as assessed by medical history, physical examination, laboratory testing and neuromorphological MRI scanning. Participants were psychiatrically evaluated using the structured clinical interview for DSM-IV(20) and a separate semi-structured interview with a psychiatrist. Inclusion criteria for the rMDD subjects required: meeting DSM-IV criteria for MDD in full remission(20) while unmedicated for at least 3 months; manifesting depression-onset prior to age 40 years; either having had at least two major depressive episodes (MDEs) or one MDE plus a history of MDD in at least one first-degree relative. Exclusion criteria for all subjects included: psychotropic drug exposure (including nicotine) within 3 months; major medical or neurological illness; illicit drug use or alcohol abuse within 1 year; lifetime history of alcohol or drug dependence; psychiatric disorders other than MDD (excepting a remote history of substance or alcohol abuse, as described above); current pregnancy or breast feeding; structural brain abnormalities on MRI; general MRI exclusions. Additional exclusion criteria for controls were: history of any psychiatric disorder (excepting a remote history of substance or alcohol abuse); history of mood or anxiety disorders in a first-degree relative. For females, testing in the week prior to menstruation or during the first 4 days of menses was avoided. Participants provided informed consent as approved by the NIMH IRB.
Participants attended two amino-acid challenge sessions separated by at least 1 week, in a randomized, double-blind, placebo-controlled, counterbalanced crossover design. On one occasion participants were orally administered a 31.5g amino-acid mixture containing the large neutral amino acids (LNAAs), but selectively lacking in L-tryptophan, via capsules (TRP-)(21). On the other occasion the mixture was the same except for the addition of 1.2g L-tryptophan (TRP+)(14). Five hours following amino-acid administration participants underwent MRI. Blood samples for assaying amino acid concentrations, Profile of Mood States (POMS: 22) scores, modified Hamilton Depression Rating Scale (HAM-D: 23) and Hamilton Anxiety Rating Scale (HAM-A: 24) ratings and vital signs were obtained immediately prior to the amino acid administration, immediately prior to scanning and immediately after scanning.
During fMRI, participants performed the AGNG, as described previously(14, 15). Whole-brain echo-planar images (EPI: TE=23msec; TR=2,000msec; flip=90°; field-of-view=22.0cm; 64*64 matrix; voxel-size=3.50*3.44*3.44mm) were acquired using a GE 3T Signa scanner and an 8-channel receiver coil array. During each block, participants were instructed to respond to one category of words (targets) while ignoring words of another category (distractors). Words were positive, negative or neutral, and each word category could either be a target or distractor. Structural scans were also acquired (spoiled-gradient recalled sequence; voxel size=1.2*0.86*0.86 mm).
To measure rCBF, whole-brain ASL images were acquired in the eyes-closed, at-rest state before and after the AGNG during each depletion session(25). Thirteen axial slices were acquired sequentially from superior to inferior using a single-shot 2D gradient-echo EPI sequence (TR=5.2sec, consisting of 3sec spin labelling, 1.4sec post-labelling delay and 0.8sec image acquisition; slice thickness=4mm, slice gap=1mm, TE=13.9ms, flip=90°; ramp-sampling; 64*64 matrix, in-plane resolution=3.75*3.75mm; total scan-time=4min 40sec).
Mood, behavioral and rCBF data were analyzed using repeated-measures analysis of variance in SPSS 16 (SPSS Inc). ROIs for assessing differences in blood-flow were defined on each individual's anatomical MRI after spatial-normalization to the Montreal Neurological Institute (MNI) template. We did not perform a voxel-wise analysis due to the reduced number of subjects included in the ASL analysis, limiting our power to detect effects following correction for multiple comparisons. Our primary ROIs consisted of: amygdala; anterior subgenual ACC; habenula; ventral striatum(10, 11, 13, 26, 27). To assess the specificity of differences found in the primary ROIs, blood-flow also was assessed post-hoc in secondary ROIs (see SOM). We analyzed the relationship between emotional bias scores and resting-state blood-flow using linear regression. Compound emotional bias scores were then computed from the RT and commission error biases, which correlated independently with right amygdala blood-flow following TRP-, using the unstandardized coefficients from a linear regression model that included both variables (compound emotional bias=(0.0015*RT emotional bias)+(0.052*commission error emotional bias)).
The blood oxygen-level dependent (BOLD)-fMRI data acquired during the AGNG task were analyzed in the context of the general linear model using Statistical Parametric Mapping (SPM5: http://www.fil.ion.ucl.ac.uk/spm). After discarding the first four images of each run, images were realigned to the fifth image, co-registered with each subject's own anatomical MRI scan, normalized to fit the MNI template and smoothed using an 8mm full-width half-maximum Gaussian kernel. Regressors representing each condition under TRP+ or TRP- were created and the resulting beta images were combined to create single-subject interaction maps of interest. These interaction maps were combined at the group level to identify areas in which rMDD subjects and controls differed in terms of BOLD responses to emotional words under conditions of tryptophan and sham depletion, using the summary-statistic approach to random-effects analyses. We did not calculate average percent signal change across ROIs in an analogous manner to that performed for the ASL analysis due to concerns regarding heterogeneity in task-related responses within the small structures comprising the visceromotor network. For differential BOLD responses located in our primary ROIs, corresponding to the affective cortico-striatal loop (OFC, VMPFC, striatum and thalamus)(28), the significance threshold was set at p<0.05 (small volume corrected: SVC). These ROIs were defined using the WFU Pickatlas toolbox with the AAL atlas(29). We additionally assessed BOLD responses in our ASL ROIs. For effects in our secondary ROIs (see SOM) the significance threshold was set at p<0.001 (uncorrected) with minimum cluster size of 10 voxels. Maxima located outside these regions were interpreted if they survived whole-brain correction for multiple comparisons at p<0.05.
Results
Demographic and clinical measures appear in Table 1. The groups did not differ on any demographic variable (p>0.1 for all). Plasma amino acid and mood-rating measures appear in Table 2. TRP- reduced the tryptophan:ΣLNAA ratio by >90%, but mood did not change significantly in either group relative to TRP+ (see also SOM).
Table 1.
Demographic and clinical characteristics. Figures represent mean (SD) unless stated otherwise.
| Controls | rMDD patients | |
|---|---|---|
| N (male) | 20 (7) | 23 (6) |
| Age | 30.5 (7.3) | 32.4 (9.8) |
| IQ | 122.9 (10.5) | 127.1 (10.5) |
| N with history of alcohol/substance abuse | 0 (0%) | 1 (4%) |
| Number of depressive episodes | - | 2.7 (2.0) |
| Number of first degree relatives with MDD | - | 1.0 (0.82) |
| Age of onset of first depressive episode | - | 20.1 (8.4) |
| Time since last treatment (months)1 | - | 24 (3-156) |
| N with at least 2 depressive episodes | - | 17 (74%) |
| N with hospitalization during depressive episode | - | 5 (22%) |
| N previously attempting suicide | - | 5 (22%) |
| Time in remission (months)1 | - | 18 (6-156) |
| N with any past SSRI treatment2 | - | 19 (83%) |
Median (range)
Of the remaining subjects one had been treated exclusively with trimipramine, one exclusively with bupropion and two were treatment-naive
Table 2.
Plasma amino acid concentrations and mood ratings prior to amino acid ingestion (T0), immediately prior to scanning (T5) and immediately after scanning (T7) in the rMDD patients and controls. Figures represent the mean (SD).
| Controls | rMDD patients | Statistical analysis | |||
|---|---|---|---|---|---|
| TRP+ | TRP- | TRP+ | TRP- | ||
| Tryptophan concentration (uM/l) | Treatment × time interaction: F(1,40)=214.1, p<0.001 | ||||
| T0 | 49.4 (15.9) | 50.8 (14.4) | 52.3 (9.7) | 55.0 (13.9) | |
| T5 | 91.7 (44.2) | 16.7 (11.2) | 105.4 (30.4) | 13.1 (5.6) | |
| T7 | 59.5 (23.0) | 21.8 (15.2) | 69.3 (29.9) | 16.2 (10.3) | |
| Tryptophan:ΣLNAA ratio | Treatment × time interaction: F(1,40)=50.7, p<0.001 | ||||
| T0 | 0.11 (0.06) | 0.11 (0.05) | 0.13 (0.05) | 0.13 (0.05) | |
| T5 | 0.07 (0.05) | 0.01 (0.01) | 0.08 (0.04) | 0.01 (0.01) | |
| T7 | 0.06 (0.04) | 0.02 (0.02) | 0.08 (0.04) | 0.02 (0.02) | |
| HAM-D | Main effect of group: F(1,41)=19.8, p<0.001 Main effect of time: F(2,82)=11.7, p<0.001 | ||||
| T0 | 0.6 (1.4) | 0.3 (0.7) | 1.2 (1.5) | 1.8 (1.7) | |
| T5 | 1.5 (2.0) | 1.5 (1.9) | 3.4 (3.8) | 2.4 (2.4) | |
| T7 | 1.0 (1.0) | 1.1 (1.5) | 3.5 (3.0) | 3.4 (3.6) | |
| HAM-A | Main effect of group: F(1,41)=16.6, p<0.001 Main effect of time: F(2,82)=8.0, p=0.001 | ||||
| T0 | 0.6 (0.9) | 0.7 (1.1) | 1.7 (1.8) | 2.1 (2.3) | |
| T5 | 1.5 (2.3) | 1.3 (1.8) | 2.6 (2.7) | 3.0 (3.5) | |
| T7 | 1.1 (1.3) | 1.3 (1.4) | 3.3 (2.9) | 4.0 (3.8) | |
| POMS tension | Main effect of group: F(1,40)=10.4, p=0.003 | ||||
| T0 | 2.2(2.3) | 1.8(1.6) | 3.1(3.1) | 4.5(4.5) | |
| T5 | 2.5(2.0) | 1.5(1.2) | 3.3(2.6) | 3.9(4.5) | |
| T7 | 1.6(1.8) | 1.9(1.3) | 4.1(5.2) | 5.0(5.4) | |
| POMS depression | Main effect of group: F(1,40)=6.0, p=0.018 Main effect of treatment: F(1,40)=4.8, p=0.034 | ||||
| T0 | 0.8(2.2) | 1.1(2.6) | 1.5(2.4) | 3.5(4.2) | |
| T5 | 0.3(0.7) | 0.4(1.2) | 0.9(1.9) | 1.7(4.1) | |
| T7 | 0.2(0.5) | 0.5(1.3) | 2.1(5.8) | 2.7(5.6) | |
| POMS anger | Main effect of group: F(1,40)=7.1, p=0.011 | ||||
| T0 | 0.6(1.1) | 0.8(1.9) | 1.0(1.4) | 2.3(3.5) | |
| T5 | 0.5(1.0) | 0.1(0.3) | 1.7(2.8) | 1.9(3.3) | |
| T7 | 0.5(1.1) | 0.3(0.8) | 1.5(3.4) | 2.8(6.8) | |
| POMS vigor | Main effect of group: F(1,40)=7.5, p=0.009 Main effect of time: F(2,80)=19.4, p<0.001 | ||||
| T0 | 19.6(6) | 19.6(5.8) | 16.8(7.4) | 14.6(7.4) | |
| T5 | 17.8(6) | 16.9(5.3) | 12.1(8.2) | 11.0(7.8) | |
| T7 | 17.5(6.6) | 17.1(5.9) | 13.4(8.9) | 9.9(9.2) | |
| POMS fatigue | Main effect of group: F(1,40)=6.2, p=0.017 Main effect of time: F(2,80)=8.9, p<0.001 | ||||
| T0 | 2.1(2.7) | 1.3(1.8) | 2.5(2.4) | 3.6(3.6) | |
| T5 | 3.3(4.4) | 2.6(3.1) | 5.0(5.4) | 5.3(5.9) | |
| T7 | 3.4(3.8) | 2.7(3.1) | 4.7(5.1) | 6.1(6.2) | |
| POMS confusion | Main effect of group: F(1,40)=12.1, p=0.001 | ||||
| T0 | 1.9(1.9) | 2.1(2.2) | 3.0(2.5) | 3.7(2.4) | |
| T5 | 2.4(1.4) | 2.4(1.7) | 3.9(2.4) | 4.3(3.4) | |
| T7 | 2.1(1.5) | 2.4(1.8) | 3.9(2.3) | 4.4(3.7) | |
Behavior on the Affective Go/No-go task (Table 3)
Table 3.
Behavior on the Affective Go/No-go test during scanning in the rMDD patients and controls. Figures represent the mean (SD).
| Measure | Target word valence | Distractor word valence | Controls | Patients | ||
|---|---|---|---|---|---|---|
| TRP+ | TRP- | TRP+ | TRP- | |||
| Reaction time (ms) | Happy | Sad | 601.8 (59.4) | 613.6 (58.7) | 645.3 (59.4) | 627.3 (60.6) |
| Happy | Neutral | 611.0 (69.7) | 633.5 (62.7) | 660.8 (59.9) | 658.1 (61.7) | |
| Sad | Happy | 611.8 (75.0) | 610.7 (67.6) | 654.5 (67.9) | 643.7 (60.1) | |
| Sad | Neutral | 615.1 (63.4) | 632.8 (66.0) | 653.0 (60.5) | 645.6 (61.6) | |
| Neutral | Happy | 684.2 (67.0) | 695.1 (59.9) | 714.7 (72.8) | 718.8 (80.2) | |
| Neutral | Sad | 673.7 (90.3) | 685.1 (63.2) | 698.4 (75.6) | 698.7 (86.6) | |
| Commission errors per block | Happy | Sad | 0.47 (0.65) | 0.41 (0.53) | 0.37 (0.56) | 0.57 (0.60) |
| Happy | Neutral | 0.60 (0.71) | 0.63 (0.88) | 0.55 (0.73) | 0.57 (0.82) | |
| Sad | Happy | 0.93 (1.30) | 0.49 (0.62) | 0.46 (0.60) | 0.43 (0.60) | |
| Sad | Neutral | 0.55 (0.64) | 0.71 (0.86) | 0.41 (0.53) | 0.45 (0.50) | |
| Neutral | Happy | 1.74 (1.23) | 1.50 (0.83) | 1.47 (1.07) | 1.78 (1.00) | |
| Neutral | Sad | 1.02 (0.88) | 1.36 (1.48) | 1.00 (1.14) | 1.01 (0.84) | |
| Omission errors per block | Happy | Sad | 0.83 (1.19) | 0.74 (0.96) | 0.60 (0.70) | 0.67 (0.87) |
| Happy | Neutral | 1.12 (1.48) | 1.33 (1.17) | 1.33 (1.24) | 0.97 (0.99) | |
| Sad | Happy | 0.68 (1.27) | 0.68 (1.09) | 0.67 (0.81) | 0.70 (0.83) | |
| Sad | Neutral | 0.87 (0.97) | 0.96 (1.17) | 0.95 (0.98) | 0.71 (0.90) | |
| Neutral | Happy | 1.12 (1.26) | 1.05 (1.25) | 1.18 (1.02) | 1.33 (1.24) | |
| Neutral | Sad | 1.31 (1.69) | 1.16 (1.33) | 1.06 (1.24) | 1.14 (1.21) | |
Participants responded more quickly to emotional targets than neutral targets (F(1,39)=115.5, p<0.001), but with similar speed to happy and sad targets (F<1), with no significant interactions with treatment or group.
Participants made similar numbers of inappropriate responses to emotional and neutral distractors (F<1), and more inappropriate responses to happy distractors than to sad distractors (F(1,39)=24.9, p<0.001), with no significant interactions with treatment or group.
Participants missed more neutral targets than emotional targets (F(1,39)=24.1, p<0.001), and missed more happy targets than sad targets (F(1,39)=6.3, p=0.017), with no significant interactions with treatment or group.
Since we had a strong a priori hypothesis that ATD would reinstate negative emotional biases in rMDD subjects, we assessed the presence of emotional biases using planned t-contrasts for each depletion condition in each group separately. Analysis of commission errors revealed that, as reported previously in this sample, controls exhibited a significant positive bias following TRP+, which was abolished following TRP-(14). However, surprisingly, the rMDD subjects exhibited significant positive biases following both TRP+ (p=0.045) and TRP- (p<0.001). Analysis of RT and omission error data did not reveal significant biases, although there was a trend towards a negative bias in terms of omission errors in the rMDD group following TRP+ (p=0.052).
BOLD responses during emotional processing
We list all activations satisfying our criteria for significance in Tables 4 and S1. Seventeen patients and seventeen controls were included in the fMRI analyses.
Table 4.
Regions where the effect of acute tryptophan on the relative effect of words of different emotional categories differed between the rMDD subjects and controls (diagnosis × treatment × emotional condition interactions)
| Region | Right/left | Talairach coordinates | Cluster size1 | Z value | ||
|---|---|---|---|---|---|---|
| Emotional-neutral targets | X | Y | Z | |||
| Thalamus | R | 18 | -25 | -2 | 148 | 4.07b# |
| Medial caudate | L | -4 | 12 | 7 | 32 | 3.78b# |
| Parietal operculum | L | -50 | -34 | 22 | 39 | 3.74a |
| Fusiform gyrus | L | -28 | -47 | -6 | 64 | 3.68a |
| Thalamus | R | 6 | -22 | -6 | 15 | 3.57b |
| Putamen | R | 22 | -6 | 6 | 13 | 3.46b |
| Thalamus | L | -16 | -22 | -6 | 10 | 3.42b |
| Posterior putamen | L | -30 | -27 | 3 | 25 | 3.38b |
| Anterior temporal cortex | L | -40 | 13 | -19 | 11 | 3.38b |
| Occipital cortex | R | 16 | -61 | 16 | 20 | 3.36b |
| Parahippocampal gyrus | R | 34 | -41 | -10 | 10 | 3.33a |
| Happy-sad targets | ||||||
| Precuneus | R | 8 | -51 | 38 | 14 | 3.37c |
| Emotional-neutral distractors | ||||||
| Dorsal anterior cingulate cortex | R | 2 | 25 | 32 | 240* | 4.08d |
| Cerebellum | R | 18 | -46 | -30 | 13 | 3.78b |
| Cerebellum | L | -10 | -68 | -30 | 19 | 3.61b |
| Dorsal medial prefrontal cortex | R | 6 | 16 | 49 | 14 | 3.42d |
| Sad-happy distractors | ||||||
| Occipital cortex | L | -10 | -95 | 7 | 13 | 3.47e |
Coordinates correspond to the stereotaxic array of Talairach and Tournoux (1988) and denote the distance in mm from the anterior commissure, with positive x = right of midline, positive y = anterior to the anterior commissure, and positive z = dorsal to a plane containing both the anterior and the posterior commissures.
Abbreviations: L – left; M – midline; R – right
cluster significant at p<0.05 (whole-brain corrected for multiple comparisons in terms of spatial extent)
voxel significant at p<0.05 (small volume corrected for multiple comparisons across a primary region of interest)
Corresponds to a threshold of p<0.001 (uncorrected)
Interaction characterised by increased BOLD response to emotional relative to neutral stimuli in the controls following tryptophan depletion, with no effect of tryptophan depletion in the patients
Interaction characterised by increased BOLD response to emotional relative to neutral stimuli in the controls following tryptophan depletion, and increased BOLD response to neutral relative to emotional stimuli following tryptophan depletion in the patients
Interaction characterised by decreased BOLD response to happy relative to sad stimuli in the controls following tryptophan depletion, and increased BOLD response to happy relative to sad stimuli following tryptophan depletion in the patients
Interaction characterised by increased BOLD response to neutral relative to emotional stimuli in the controls following tryptophan depletion, and increased BOLD response to emotional relative to neutral stimuli following tryptophan depletion in the patients
Interaction characterised by increased BOLD response to happy relative to sad stimuli in the controls following tryptophan depletion, and decreased BOLD response to happy relative to sad stimuli following tryptophan depletion in the patients;.
Italic font denotes that the maximum was contained within an a priori specified region of interest (primary or secondary).
Emotional relative to neutral targets
In our primary ROIs, significant group*treatment* word-type interactions were identified in the thalamus (p=0.011, SVC) and caudate (p=0.021, SVC), with a trend result in the putamen (p=0.090, SVC) (Figure 1, Table 4). Post-hoc analysis revealed that controls showed a greater BOLD response to neutral relative to emotional targets following TRP+, with the opposite pattern of response following TRP- (Figure 1b). In the rMDD subjects a different pattern of BOLD response was present, with either no difference between the TRP+ and TRP- conditions, or a greater BOLD response to emotional relative to neutral targets following TRP+ (Figure 1c). In our secondary ROIs, similar interactions were identified in the anterior temporal cortex and parahippocampal gyrus (PHG). The interaction in the anterior temporal cortex remained significant after applying corrections for multiple comparisons across our ASL STG ROI (p=0.037, SVC). Significant group*word-type interactions were also identified in our secondary ROIs: VLPFC, middle temporal gyrus and PHG (Table S1).
Figure 1. Differential effect of tryptophan depletion on neural responses to emotional relative to neutral target words in the two groups.
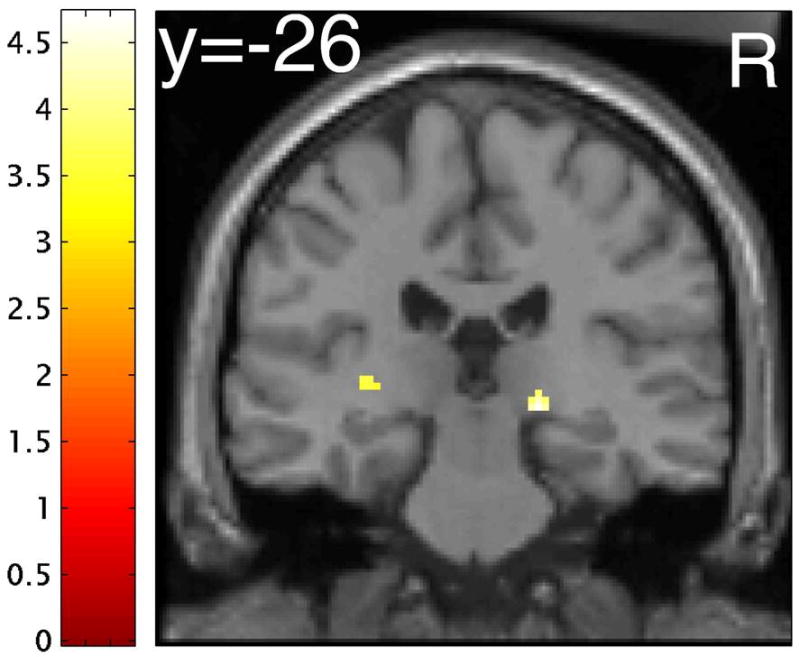
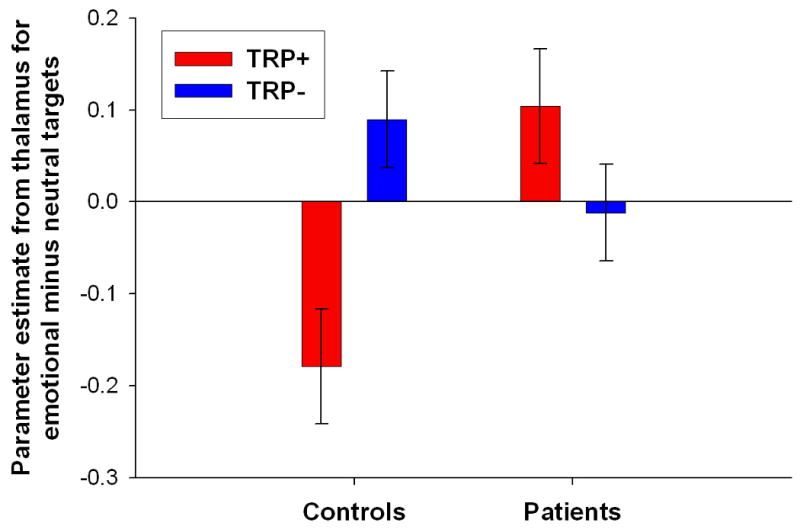
(A) Word type × treatment × group interaction in the right thalamus ([x=18, y=-25, z=-2], peak Z-score=4.07). Effects were significant at p<0.001 (uncorrected), minimum cluster size 10 voxels. Color bars indicate t-values and images are thresholded at p<0.001 (uncorrected). Right is on the right. The activation on the left is in the posterior putamen. (B) Plot of parameter estimates for the emotional minus neutral target words contrast under tryptophan (TRP-) and sham (TRP+) depletion conditions for the peak voxel in the thalamus. Controls exhibited greater response to emotional relative to neutral target stimuli following TRP-, while major depressive disorder patients in remission (rMDD) exhibited greater response to emotional relative to neutral stimuli following TRP+. Error bars represent 1 SEM.
Happy relative to sad targets
A significant group* valence interaction was identified in the STG secondary ROI (Table S1).
Emotional relative to neutral distractors
A significant group*treatment* word-type interaction was identified in the dorsal ACC (dACC), which survived whole-brain correction for multiple comparisons at the cluster-level (p=0.020; Figure 2, Table 4). Post-hoc analysis revealed that the controls showed a greater BOLD response to neutral relative to emotional distractors following TRP-, with no difference between emotional and neutral distractors following TRP+ (Figure 2b). However, the rMDD subjects showed a greater BOLD response to neutral relative to emotional distractors following TRP+, with no difference between emotional and neutral distractors following TRP- (Figure 2c).
Figure 2. Differential effect of tryptophan depletion on neural responses to emotional relative to neutral distractor words in the two groups.
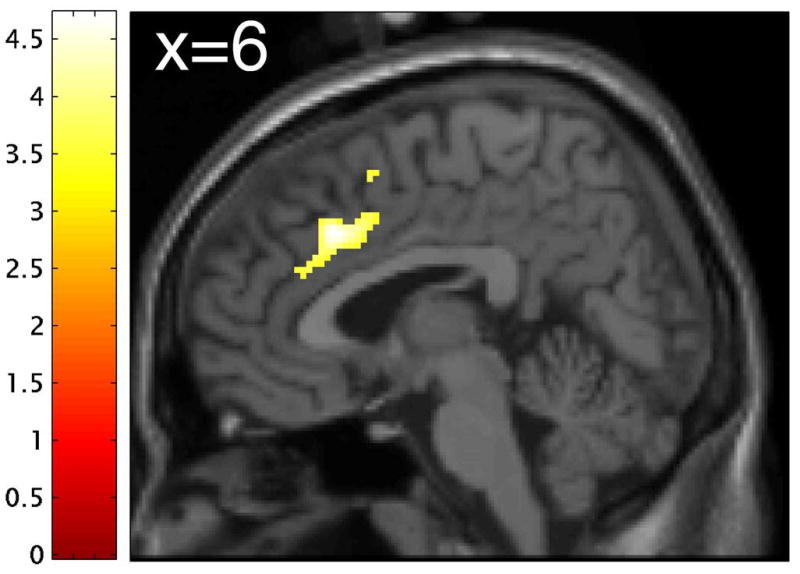
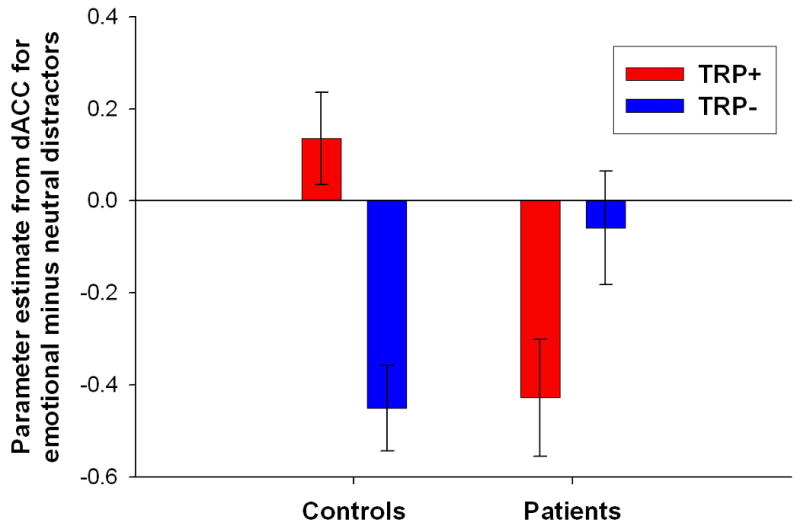
(A) Word type × treatment × group interaction in the dorsal anterior cingulate (dACC) ([x=2, y=25, z=32], peak Z-score=4.08). Effects were significant at p<0.001 (uncorrected), minimum cluster size 10 voxels. Color bars indicate t-values and images are thresholded at p<0.001 (uncorrected). (B) Plot of parameter estimates for emotional relative to neutral distractor words under tryptophan (TRP-) and sham (TRP+) depletion conditions for the peak voxel in the dACC. Controls exhibited greater response to neutral relative to emotional target stimuli following TRP-, while major depressive disorder patients in remission (rMDD) exhibited greater response to neutral relative to emotional stimuli following TRP+. Error bars represent 1 SEM.
Happy relative to sad distractors
No significant interactions with group were identified within our ROIs.
Regional Cerebral Blood-Flow
ASL data were available for 10 controls and 11 rMDD subjects. We describe in the text only effects in our primary ROIs (see Table S2 for main effects of depletion condition and group*treatment interactions in secondary ROIs).
Analysis of left habenula data revealed significantly higher blood-flow in rMDD subjects following TRP- relative to TRP+, but not in controls (post-hoc: rMDD TRP- vs TRP+ p=0.011; controls TRP- vs TRP+ p=0.6; Table 5 and Figure 3).
Table 5.
Effects of tryptophan depletion on regional blood-flow measured using arterial spin labelling for primary regions-of-interest. Figures represent mean (SD) values for the change in globally-normalized blood flow following TRP-, as a percentage of the TRP+ value. Positive values represent an increase in regional blood-flow following TRP-.
| Region | Controls (N=10) |
Patients (N=11) |
Main effect of treatment | Diagnosis × treatment interaction |
|---|---|---|---|---|
| L amygdala | -9.2 (19.4) | 0.7 (13.0) | F(1,19)=1.2 p=0.29 | F(1,19)=1.9, p=0.18 |
| R amygdala | 4.3 (28.2) | 0.0 (23.8) | F<1 | F<1 |
| L subgenual cingulate | -8.3 (13.0) | -1.4 (14.3) | F(1,19)=2.4 p=0.14 | F(1,19)=1.3, p=0.27 |
| R subgenual cingulate | 10.8 (24.2) | -2.3 (6.5) | F(1,19)=1.0 p=0.33 | F(1,19)=3.0, p=0.098 |
| L habenula | -2.3 (13.3) | 20.8 (22.0) | F(1,19)=4.3 p=0.050 | F(1,19)=8.3, p=0.010* |
| R habenula | 4.0 (28.1) | 9.2 (16.8) | F(1,19)=1.9 p=0.19 | F<1 |
| L ventral striatum | -9.0 (15.8) | 3.9 (17.3) | F<1 | F(1,19)=3.1, p=0.093 |
| R ventral striatum | 2.3 (24.4) | 4.0 (31.0) | F<1 | F<1 |
Abbreviations: L=left; R=right; PFC = prefrontal cortex
indicates statistically significant interaction; L=left; R=right; PFC = prefrontal cortex. For post-hoc analysis of statistically significant interactions see text.
Figure 3.
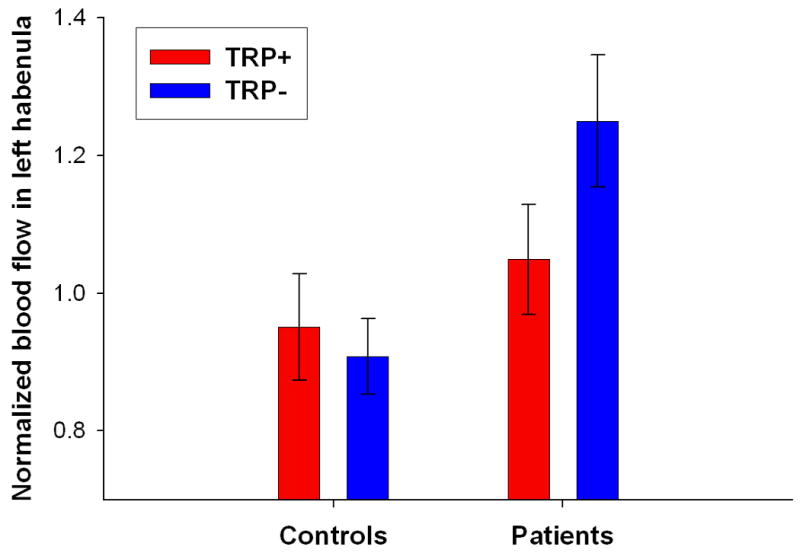
Differential effect of tryptophan depletion on normalized resting-state blood flow in the left habenula in the two groups. Tryptophan depletion significantly increased blood flow in the left habenlua in major depressive disorder patients in remission (rMDD), but had no effect in the controls. Error bars represent 1 SEM.
Across all subjects, higher right amygdala resting-state blood-flow values were associated with more negative emotional bias scores on the AGNG following TRP-. Right amygdala blood-flow correlated significantly with affective bias as indexed both by latency (r=-0.59, p=0.005) and commission errors (r=-0.54, p=0.012). Including both measures of affective bias, which were themselves uncorrelated (r=0.05, p=0.8), in the same regression model accounted for over one-half of the variance in right amygdala blood-flow following TRP- (F(2,18)=14.1, p=0.0002, adjusted r2=0.57) (Figure 4), but not following TRP+ (r=-0.19 for latency, r=0.065 for commission errors). There was also a significant correlation between the change in compound emotional bias score from TRP+ to TRP- and right amygdala blood-flow following TRP- (r=-0.46, p=0.037).
Figure 4.
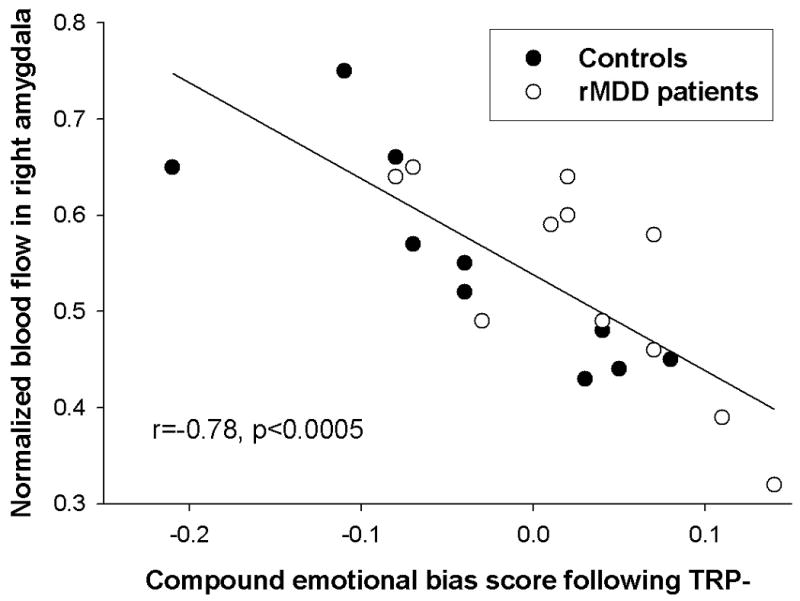
Relationship between normalized resting-state blood flow and emotional bias score on the Affective Go/No-go task (AGNG) following tryptophan depletion (TRP-). In both major depressive disorder patients in remission (rMDD - empty circles) and controls (filled circles), higher resting-state blood flow in the right amygdala was associated with a more negative emotional bias on the AGNG following TRP- (controls: r=-0.84, p=0.003; rMDD: r=-0.79, p=0.004).
Discussion
This is the first study to assess the effects of ATD on neural responses to emotional stimuli in rMDD subjects and healthy controls. Neither group exhibited reliable mood-change following ATD. However, ATD robustly increased hemodynamic responses to emotional words in the thalamus and caudate in the controls, but not in the rMDD subjects. We also demonstrate for the first time that attentional bias towards negative stimuli is strongly associated with resting-state blood-flow in the right amygdala following ATD. Finally, we were able to confirm a previous report of increased blood-flow in the habenula following ATD in rMDD subjects(13), and show that this effect does not occur in healthy controls.
Consistent with previous reports that unmedicated rMDD subjects were resistant to mood-change following ATD(3), the HAM-D scores of the rMDD group did not increase following ATD; neither did they exhibit negative emotional biases. Therefore our predictions that ATD would lead to a worsening of mood and negative emotional biases in rMDD subjects could not be confirmed. Previous studies reported that although a large proportion of currently-medicated rMDD subjects exhibit a return of depressive symptoms following ATD(2), unmedicated patients prove less vulnerable(30, 31). Moreover, a meta-analysis suggested that patients unmedicated and remitted for ≥6 months do not show reliable mood-change following ATD(3); notably, nearly all of our rMDD sample had been in remission and medication-free for ≥6 months. The lack of effect of ATD on depressive symptomology in the rMDD group in our study may thus be explained by the extended period during which most patients had been unmedicated and remitted(3).
In fact, the rMDD group demonstrated a robust positive emotional bias on the AGNG, as measured by their rate of inappropriate responding to positive relative to negative distractors, though this result was complicated by a trend towards a negative bias for omission errors following TRP+. It has been hypothesized that one effect of 5-HT depletion is to negatively bias the processing of emotional information by impacting on the neural mechanisms subserving these processes(32). Consistent with this hypothesis, resting-state blood-flow in the right amygdala following ATD was associated with emotional bias on the AGNG, as measured by latency and inappropriate responding, accounting for over one-half of the variance in right amygdala blood-flow, though this effect was similar in rMDD subjects and controls. These data appear compatible with previous findings demonstrating increased resting-state amygdala rCBF and negative emotional biases in acutely depressed patients(16, 33-35), and suggest that serotonergic modulation of amygdala activity may play a crucial role in mediating resistance to distracting negative information. However, we did not identify significant correlations between behavioral emotional biases and differential amygdala BOLD responses to emotional words across subjects, likely due to the lack of amygdala responses elicited by the verbal stimuli used in the AGNG(15). These data underscore the utility of collecting resting-state ASL data, which provide a good measure of baseline blood-flow, in addition to task-related BOLD responses in pharmacological fMRI studies.
The areas in which ATD differentially affected BOLD responses to emotional relative to neutral stimuli between rMDD subjects and controls receive abundant serotonergic projections from the dorsal/median raphe nuclei (DRN/MRN)(36, 37), and form part of the “affective” cortico-striatal loop central to the extended visceromotor network(18, 28, 38), which participates in the processing of emotional information(39). The only study to measure regional glucose metabolism in unmedicated rMDD subjects following ATD also reported abnormalities in these regions(11). Similar structures were identified in a previous study reporting blunted neural responses to emotional stimuli in medicated, acutely-depressed patients using the AGNG(15). Both depressed and remitted unmedicated MDD subjects show pervasive decreases in 5-HT1A receptor binding in these regions(40, 41), and physiological responses to 5-HT1A receptor agonist challenge are blunted in MDD subjects versus controls(42). Within the context of such a deficit, the further reduction in serotonergic transmission caused by ATD may have contributed to the differences that we observed in neurophysiological responses to emotional stimuli between the groups. Alternatively or additionally, the differential neurophysiological responses to ATD between groups may reflect a greater ability of the controls as compared to the rMDD subjects to recruit other neurotransmitter systems to compensate for the effects of lowered 5-HT transmission.
The group*treatment*word-type interactions in this study were generally driven by increases in responses to emotional targets in controls following TRP-, for example in the thalamus and caudate, with small effects in rMDD subjects. However, the interaction in the dACC, which was apparent in the analysis of distractors, was qualitatively different and in the opposite direction. Strikingly, the rMDD subjects exhibited increased responses to emotional distractors (relative to neutral distractors) following TRP- relative to TRP+, with the opposite pattern in controls. This dACC region has been implicated in the processing of conflict and behavioral adjustment(43, 44), consistent with an effect driven by distractors. However, it is unclear whether these differences in neural responses following TRP- represent resilience or vulnerability in the rMDD subjects. On the one hand, the relatively increased dACC responses to emotional distractors following 5-HT depletion in the rMDD group might reflect a mechanism by which conflict arising from the processing of extraneous emotional information is controlled; notably, the rMDD subjects also displayed a robust positive emotional bias following TRP-. On the other hand, the relatively blunted responses to emotional target words in the visceromotor network following TRP- in the rMDD group are redolent of previous findings using the AGNG in currently depressed patients(15). Discriminating between these explanations requires studies employing longitudinal designs that additionally include rMDD subjects vulnerable to ATD-induced mood-change.
Consistent with a previous report, we observed increased blood-flow in the habenula following ATD in rMDD subjects(13). We were able to establish this finding using relatively high-resolution blood-flow measures. The previous report of increased habenula activity during ATD relied on low-resolution PET measures that precluded resolution of the habenula from the adjacent thalamus. In our study the habenula volume was comparable to that of one ASL image voxel(45), and the habenula ROI was accurately positioned in each subject's anatomical MRI scan. Finally, we extended the report of Morris and colleagues(13), which did not include assessments in a control group, by demonstrating that the increase in blood-flow under ATD is specific to rMDD subjects.
The habenula plays a central role in regulating the function of multiple monoaminergic neurotransmitter systems, sharing extensive reciprocal projections with the DRN/MRN(46-48). The habenula also projects to the ventral tegmental area and substantia nigra, in which dopamine neuronal firing activity is profoundly inhibited during habenula stimulation(49, 50). These data are consistent with recent findings that habenula responses occur following negative feedback in humans(51), since the omission of a predicted reward is associated with reduced firing of dopamine neurons(52). It thus has been hypothesized that elevated habenular activity causes depressive symptoms by inhibiting tonic dopamine neuronal firing even in the absence of reward omission(53, 54). Supporting this contention, three rodent models of depression(55), and congenitally helpless rats (bred to display helplessness following chronic stress: 56), exhibited elevated habenula metabolism. Our data suggest that 5-HT depletion-induced habenula hyperactivity represents a specific vulnerability in depressive patients, and support a role for this structure in the pathogenesis of MDD. However, this interpretation is speculative and requires testing in studies that additionally include rMDD subjects who do show ATD-induced mood-change.
Some limitations of our study merit comment. Fewer than 20 participants in each group could be included in the analysis of task-related hemodynamic responses, and only half this number in the analysis of resting-state blood-flow. Furthermore, while the TRP+ mixture increased the absolute concentration of plasma tryptophan, the tryptophan:ΣLNAA ratio, the best peripheral index of tryptophan availability to the brain for serotonin synthesis(57), was reduced following TRP+, which may have resulted in a small reduction in 5-HT synthesis. However, the reduction in tryptophan:ΣLNAA ratio following TRP- was approximately 90%, suggesting that despite the relatively reduced amino acid load employed relative to other studies (31g), our intervention robustly reduced tryptophan availability to the brain for serotonin synthesis, consistent with previous studies(58). Notably, the administration of amino acids via capsules constituted a strength of our methods. This route ensured that the total load of LNAAs was ingested, and achieved a depletion comparable to that reported using 100g amino acids administered in drink form(59), far greater than the depletion reported by studies administering similar quantities of amino acids in drink form (so-called ‘low-dose’ ATD)(60, 61).
With respect to the ASL technique, T1 relaxation data were not measured in individual subjects, and instead the ASL signal changes were converted to blood-flow values using an assumed T1 of gray matter(25). However, the use of globally-normalized values in statistical analyses avoided possible errors due to incorrect quantitation of blood-flow for voxels containing either gray matter alone or both gray and white matter. Consequently, the ASL method provided valid and reliable measures of the difference in globally-normalized, rCBF between conditions, analogous to the validity and reliability of measuring globally-normalized, regional tissue radioactivity measured using the PET-[O-15]water technique.
Finally, while our results were unconfounded by mood-change in the rMDD group, we did not include patients vulnerable to ATD-induced mood-change in this study, limiting our inference to a comparison with healthy volunteers. Furthermore, our resilient group may not be representative of other groups of rMDD subjects who did show mood-change following ATD(62). Future studies investigating ATD in rMDD subjects should include groups of patients who do show mood-change in order to directly compare between resilient and non-resilient individuals.
In summary, ATD differentially modulated responses to emotional stimuli in rMDD subjects and controls in the thalamus, caudate and dACC and increased blood-flow in rMDD patients in the habenula, without affecting subjective emotional state or behavior. However, further studies are required to determine whether these findings represent mechanisms of resilience or vulnerability to MDD.
Supplementary Material
Acknowledgments
We thank Judy Starling for preparation of the amino acid mixtures, Mike Franklin for analysis of the plasma amino acids, Harvey Iwamoto for programming the AGNG, Rebecca Elliott and Judy Rubinsztein for providing the original word lists for the AGNG, Jeanette Black and Renee Hill for technologist support, the nurses of ward 5 South-West for their clinical support and Karl Friston for guidance regarding fMRI analysis. We thank Joan Williams, Michele Drevets and Paul Carlson for help with recruitment and clinical support. Finally, we would like to thank the volunteers who participated.
This research was supported by the Intramural Research Program of the NIMH. JPR was supported by the NIH-Cambridge Health Science Scholars Program. JPR and BJS have received compensation for consultancy work from Cambridge Cognition Ltd., who now own the behavioral version of the AGNG.
Footnotes
Financial Disclosures: The other authors have no conflicts of interest to declare.
References
- 1.Booij L, Van der Does AJ, Riedel WJ. Monoamine depletion in psychiatric and healthy populations: review. Mol Psychiatry. 2003 Nov;8(12):951–73. doi: 10.1038/sj.mp.4001423. [DOI] [PubMed] [Google Scholar]
- 2.Booij L, Van der Does W, Benkelfat C, Bremner JD, Cowen PJ, Fava M, et al. Predictors of mood response to acute tryptophan depletion. A reanalysis. Neuropsychopharmacology. 2002 Nov;27(5):852–61. doi: 10.1016/S0893-133X(02)00361-5. [DOI] [PubMed] [Google Scholar]
- 3.Ruhe HG, Mason NS, Schene AH. Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: a meta-analysis of monoamine depletion studies. Mol Psychiatry. 2007 Apr;12(4):331–59. doi: 10.1038/sj.mp.4001949. [DOI] [PubMed] [Google Scholar]
- 4.Delgado PL, Charney DS, Price LH, Aghajanian GK, Landis H, Heninger GR. Serotonin function and the mechanism of antidepressant action. Reversal of antidepressant-induced remission by rapid depletion of plasma tryptophan. Arch Gen Psychiatry. 1990 May;47(5):411–8. doi: 10.1001/archpsyc.1990.01810170011002. [DOI] [PubMed] [Google Scholar]
- 5.Rogers RD, Everitt BJ, Baldacchino A, Blackshaw AJ, Swainson R, Wynne K, et al. Dissociable deficits in the decision-making cognition of chronic amphetamine abusers, opiate abusers, patients with focal damage to prefrontal cortex, and tryptophan-depleted normal volunteers: evidence for monoaminergic mechanisms. Neuropsychopharmacology. 1999;20(4):322–39. doi: 10.1016/S0893-133X(98)00091-8. [DOI] [PubMed] [Google Scholar]
- 6.Rogers RD, Tunbridge EM, Bhagwagar Z, Drevets WC, Sahakian BJ, Carter CS. Tryptophan depletion alters the decision-making of healthy volunteers through altered processing of reward cues. Neuropsychopharmacology. 2003 Jan;28(1):153–62. doi: 10.1038/sj.npp.1300001. [DOI] [PubMed] [Google Scholar]
- 7.Cools R, Blackwell A, Clark L, Menzies L, Cox S, Robbins TW. Tryptophan depletion disrupts the motivational guidance of goal-directed behavior as a function of trait impulsivity. Neuropsychopharmacology. 2005 Jul;30(7):1362–73. doi: 10.1038/sj.npp.1300704. [DOI] [PubMed] [Google Scholar]
- 8.Harmer CJ, Rogers RD, Tunbridge E, Cowen PJ, Goodwin GM. Tryptophan depletion decreases the recognition of fear in female volunteers. Psychopharmacology (Berl) 2003 Apr 4; doi: 10.1007/s00213-003-1401-6. [DOI] [PubMed] [Google Scholar]
- 9.Murphy FC, Smith KA, Cowen PJ, Robbins TW, Sahakian BJ. The effects of tryptophan depletion on cognitive and affective processing in healthy volunteers. Psychopharmacology (Berl) 2002 Aug;163(1):42–53. doi: 10.1007/s00213-002-1128-9. [DOI] [PubMed] [Google Scholar]
- 10.Smith KA, Morris JS, Friston KJ, Cowen PJ, Dolan RJ. Brain mechanisms associated with depressive relapse and associated cognitive impairment following acute tryptophan depletion. Br J Psychiatry. 1999 Jun;174:525–9. doi: 10.1192/bjp.174.6.525. [DOI] [PubMed] [Google Scholar]
- 11.Neumeister A, Nugent AC, Waldeck T, Geraci M, Schwarz M, Bonne O, et al. Neural and behavioral responses to tryptophan depletion in unmedicated patients with remitted major depressive disorder and controls. Arch Gen Psychiatry. 2004 Aug;61(8):765–73. doi: 10.1001/archpsyc.61.8.765. [DOI] [PubMed] [Google Scholar]
- 12.Bremner JD, Innis RB, Salomon RM, Staib LH, Ng CK, Miller HL, et al. Positron emission tomography measurement of cerebral metabolic correlates of tryptophan depletion-induced depressive relapse. Arch Gen Psychiatry. 1997 Apr;54(4):364–74. doi: 10.1001/archpsyc.1997.01830160092012. [DOI] [PubMed] [Google Scholar]
- 13.Morris JS, Smith KA, Cowen PJ, Friston KJ, Dolan RJ. Covariation of activity in habenula and dorsal raphe nuclei following tryptophan depletion. Neuroimage. 1999 Aug;10(2):163–72. doi: 10.1006/nimg.1999.0455. [DOI] [PubMed] [Google Scholar]
- 14.Roiser JP, Levy J, Fromm SJ, Wang H, Hasler G, Sahakian BJ, et al. The effect of acute tryptophan depletion on the neural correlates of emotional processing in healthy volunteers. Neuropsychopharmacology. 2008 Jul;33(8):1992–2006. doi: 10.1038/sj.npp.1301581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Elliott R, Rubinsztein JS, Sahakian BJ, Dolan RJ. The neural basis of mood-congruent processing biases in depression. Arch Gen Psychiatry. 2002 Jul;59(7):597–604. doi: 10.1001/archpsyc.59.7.597. [DOI] [PubMed] [Google Scholar]
- 16.Murphy FC, Sahakian BJ, Rubinsztein JS, Michael A, Rogers RD, Robbins TW, et al. Emotional bias and inhibitory control processes in mania and depression. Psychological Medicine. 1999;29(6):1307–21. doi: 10.1017/s0033291799001233. [DOI] [PubMed] [Google Scholar]
- 17.Ongur D, Price JL. The organization of networks within the orbital and medial prefrontal cortex of rats, monkeys and humans. Cereb Cortex. 2000 Mar;10(3):206–19. doi: 10.1093/cercor/10.3.206. [DOI] [PubMed] [Google Scholar]
- 18.Ongur D, Ferry AT, Price JL. Architectonic subdivision of the human orbital and medial prefrontal cortex. J Comp Neurol. 2003 Jun 2;460(3):425–49. doi: 10.1002/cne.10609. [DOI] [PubMed] [Google Scholar]
- 19.Drevets WC, Furey ML. Brain Structural and Functional Abnormalities in Mood Disorders: Implications for Neurocircuitry Models of Depression. Brain Structure and Function. doi: 10.1007/s00429-008-0189-x. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spitzer MB, Gibbon M, Williams JBW. Stuctured Clinical Interview for DSM-IV-TR, Research Version, Patient Edition (SCID-I/P) New York: Biometrics Research, New York State Biometrics Institute; 2002. [Google Scholar]
- 21.Neumeister A, Konstantinidis A, Stastny J, Schwarz MJ, Vitouch O, Willeit M, et al. Association between serotonin transporter gene promoter polymorphism (5HTTLPR) and behavioral responses to tryptophan depletion in healthy women with and without family history of depression. Arch Gen Psychiatry. 2002 Jul;59(7):613–20. doi: 10.1001/archpsyc.59.7.613. [DOI] [PubMed] [Google Scholar]
- 22.McNair DM, Lorr M, Droppleman LF. Manual: Profile of mood states (POMS) San Diego: Educational & Industrial Testing Service; 1971. [Google Scholar]
- 23.Hamilton M. A rating scale for depression. Journal of Neurology, Neurosurgery, and Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology. 1959;32:50–5. doi: 10.1111/j.2044-8341.1959.tb00467.x. [DOI] [PubMed] [Google Scholar]
- 25.Talagala SL, Ye FQ, Ledden PJ, Chesnick S. Whole-brain 3D perfusion MRI at 3.0 T using CASL with a separate labeling coil. Magn Reson Med. 2004 Jul;52(1):131–40. doi: 10.1002/mrm.20124. [DOI] [PubMed] [Google Scholar]
- 26.Drevets WC, Videen TO, Price JL, Preskorn SH, Carmichael ST, Raichle ME. A functional anatomical study of unipolar depression. J Neurosci. 1992 Sep;12(9):3628–41. doi: 10.1523/JNEUROSCI.12-09-03628.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997 Apr 24;386(6627):824–7. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 28.Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- 29.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage. 2003 Jul;19(3):1233–9. doi: 10.1016/s1053-8119(03)00169-1. [DOI] [PubMed] [Google Scholar]
- 30.Haynes PL, McQuaid JR, Kelsoe J, Rapaport M, Gillin JC. Affective state and EEG sleep profile in response to rapid tryptophan depletion in recently recovered nonmedicated depressed individuals. J Affect Disord. 2004 Dec;83(23):253–62. doi: 10.1016/j.jad.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 31.O'Reardon JP, Chopra MP, Bergan A, Gallop R, DeRubeis RJ, Crits-Christoph P. Response to tryptophan depletion in major depression treated with either cognitive therapy or selective serotonin reuptake inhibitor antidepressants. Biol Psychiatry. 2004 May 1;55(9):957–9. doi: 10.1016/j.biopsych.2003.12.020. [DOI] [PubMed] [Google Scholar]
- 32.Harmer CJ. Serotonin and emotional processing: Does it help explain antidepressant drug action? Neuropharmacology. 2008 Jun 27; doi: 10.1016/j.neuropharm.2008.06.036. [DOI] [PubMed] [Google Scholar]
- 33.Drevets WC, Raichle ME. Neuroanatomical circuits in depression: implications for treatment mechanisms. Psychopharmacol Bull. 1992;28(3):261–74. [PubMed] [Google Scholar]
- 34.Drevets WC. Neuroimaging studies of mood disorders. Biol Psychiatry. 2000 Oct 15;48(8):813–29. doi: 10.1016/s0006-3223(00)01020-9. [DOI] [PubMed] [Google Scholar]
- 35.Erickson K, Drevets WC, Clark L, Cannon DM, Bain EE, Zarate CA, Jr, et al. Mood-congruent bias in affective go/no-go performance of unmedicated patients with major depressive disorder. Am J Psychiatry. 2005 Nov;162(11):2171–3. doi: 10.1176/appi.ajp.162.11.2171. [DOI] [PubMed] [Google Scholar]
- 36.Jacobs BL, Azmitia EC. Structure and function of the brain serotonin system. Physiol Rev. 1992 Jan;72(1):165–229. doi: 10.1152/physrev.1992.72.1.165. [DOI] [PubMed] [Google Scholar]
- 37.Varnas K, Halldin C, Hall H. Autoradiographic distribution of serotonin transporters and receptor subtypes in human brain. Hum Brain Mapp. 2004 Jul;22(3):246–60. doi: 10.1002/hbm.20035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Middleton FA, Strick PL. Basal ganglia output and cognition: evidence from anatomical, behavioral, and clinical studies. Brain Cogn. 2000 Mar;42(2):183–200. doi: 10.1006/brcg.1999.1099. [DOI] [PubMed] [Google Scholar]
- 39.Van der Werf YD, Witter MP, Groenewegen HJ. The intralaminar and midline nuclei of the thalamus. Anatomical and functional evidence for participation in processes of arousal and awareness. Brain Res Brain Res Rev. 2002 Sep;39(23):107–40. doi: 10.1016/s0165-0173(02)00181-9. [DOI] [PubMed] [Google Scholar]
- 40.Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004 Apr;9(4):386–92. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 41.Hirvonen J, Karlsson H, Kajander J, Lepola A, Markkula J, Rasi-Hakala H, et al. Decreased brain serotonin 5-HT1A receptor availability in medication-naive patients with major depressive disorder: an in-vivo imaging study using PET and [carbonyl-C]WAY-100635. Int J Neuropsychopharmacol. 2007 Oct 31;:1–12. doi: 10.1017/S1461145707008140. [DOI] [PubMed] [Google Scholar]
- 42.Cowen PJ. Psychopharmacology of 5-HT(1A) receptors. Nucl Med Biol. 2000 Jul;27(5):437–9. doi: 10.1016/s0969-8051(00)00108-6. [DOI] [PubMed] [Google Scholar]
- 43.di Pellegrino G, Ciaramelli E, Ladavas E. The regulation of cognitive control following rostral anterior cingulate cortex lesion in humans. J Cogn Neurosci. 2007 Feb;19(2):275–86. doi: 10.1162/jocn.2007.19.2.275. [DOI] [PubMed] [Google Scholar]
- 44.Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004 Oct 15;306(5695):443–7. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- 45.Mai JK, Assheuer J, Paxinos G. Atlas of the human brain. 2nd. San Diego: Academic Press; 2003. [Google Scholar]
- 46.Stern WC, Johnson A, Bronzino JD, Morgane PJ. Neuropharmacology of the afferent projections from the lateral habenula and substantia nigra to the anterior raphe in the rat. Neuropharmacology. 1981 Oct;20(10):979–89. doi: 10.1016/0028-3908(81)90029-0. [DOI] [PubMed] [Google Scholar]
- 47.Sutherland RJ. The dorsal diencephalic conduction system: a review of the anatomy and functions of the habenular complex. Neurosci Biobehav Rev. 1982 Spring;6(1):1–13. doi: 10.1016/0149-7634(82)90003-3. [DOI] [PubMed] [Google Scholar]
- 48.Sakai K, Salvert D, Touret M, Jouvet M. Afferent connections of the nucleus raphe dorsalis in the cat as visualized by the horseradish peroxidase technique. Brain Res. 1977 Nov 25;137(1):11–35. doi: 10.1016/0006-8993(77)91010-1. [DOI] [PubMed] [Google Scholar]
- 49.Christoph GR, Leonzio RJ, Wilcox KS. Stimulation of the lateral habenula inhibits dopamine-containing neurons in the substantia nigra and ventral tegmental area of the rat. J Neurosci. 1986 Mar;6(3):613–9. doi: 10.1523/JNEUROSCI.06-03-00613.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ji H, Shepard PD. Lateral habenula stimulation inhibits rat midbrain dopamine neurons through a GABA(A) receptor-mediated mechanism. J Neurosci. 2007 Jun 27;27(26):6923–30. doi: 10.1523/JNEUROSCI.0958-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ullsperger M, von Cramon DY. Error monitoring using external feedback: specific roles of the habenular complex, the reward system, and the cingulate motor area revealed by functional magnetic resonance imaging. J Neurosci. 2003 May 15;23(10):4308–14. doi: 10.1523/JNEUROSCI.23-10-04308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schultz W, Dayan P, Montague PR. A neural substrate of prediction and reward. Science. 1997 Mar 14;275(5306):1593–9. doi: 10.1126/science.275.5306.1593. [DOI] [PubMed] [Google Scholar]
- 53.Sartorius A, Henn FA. Deep brain stimulation of the lateral habenula in treatment resistant major depression. Med Hypotheses. 2007;69(6):1305–8. doi: 10.1016/j.mehy.2007.03.021. [DOI] [PubMed] [Google Scholar]
- 54.Hikosaka O, Sesack SR, Lecourtier L, Shepard PD. Habenula: crossroad between the basal ganglia and the limbic system. J Neurosci. 2008 Nov 12;28(46):11825–9. doi: 10.1523/JNEUROSCI.3463-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Caldecott-Hazard S, Mazziotta J, Phelps M. Cerebral correlates of depressed behavior in rats, visualized using 14C-2-deoxyglucose autoradiography. J Neurosci. 1988 Jun;8(6):1951–61. doi: 10.1523/JNEUROSCI.08-06-01951.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shumake J, Edwards E, Gonzalez-Lima F. Opposite metabolic changes in the habenula and ventral tegmental area of a genetic model of helpless behavior. Brain Res. 2003 Feb 14;963(12):274–81. doi: 10.1016/s0006-8993(02)04048-9. [DOI] [PubMed] [Google Scholar]
- 57.Fernstrom JD, Wurtman RJ. Brain serotonin content: physiological regulation by plasma neutral amino acids. Science. 1972 Oct 27;178(59):414–6. doi: 10.1126/science.178.4059.414. [DOI] [PubMed] [Google Scholar]
- 58.Wolfe BE, Metzger ED, Jimerson DC. Comparison of the effects of amino acid mixture and placebo on plasma tryptophan to large neutral amino acid ratio. Life Sci. 1995 Mar 17;56(17):1395–400. doi: 10.1016/0024-3205(95)00103-4. [DOI] [PubMed] [Google Scholar]
- 59.Merens W, Booij L, Haffmans PM, Van der Does AJ. The effects of experimentally lowered serotonin function on emotional information processing and memory in remitted depressed patients. J Psychopharmacol. 2008 Feb 28; doi: 10.1177/0269881107081531. [DOI] [PubMed] [Google Scholar]
- 60.Booij L, Van der Does AJ, Haffmans PM, Riedel WJ, Fekkes D, Blom MJ. The effects of high-dose and low-dose tryptophan depletion on mood and cognitive functions of remitted depressed patients. J Psychopharmacol. 2005 May;19(3):267–75. doi: 10.1177/0269881105051538. [DOI] [PubMed] [Google Scholar]
- 61.Hayward G, Goodwin GM, Cowen PJ, Harmer CJ. Low-dose tryptophan depletion in recovered depressed patients induces changes in cognitive processing without depressive symptoms. Biol Psychiatry. 2005 Mar 1;57(5):517–24. doi: 10.1016/j.biopsych.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 62.Smith KA, Fairburn CG, Cowen PJ. Relapse of depression after rapid depletion of tryptophan. Lancet. 1997;349(9056):915–9. doi: 10.1016/s0140-6736(96)07044-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


