Abstract
Introduction
Inactivation of the PTEN suppressor gene occurs in the majority of endometrial cancer cases. Somatic PTEN inactivation by deletion and/or mutation, the first detectible change of endometrial carcinogenesis, occurs at a high frequency in the endometrium of normal premenopausal women, though few of these progress to cancer. We hypothesized that the 50–60% reduced cancer risk of oral contraceptives (OCP) and intrauterine devices (IUD) occurs in part through their activity as negative selection factors for these subclinical mutated glands.
Methods
71 women with a history of oral contraceptive use and 80 with a history of IUD use were age matched with 191 and 119 controls, respectively. Endometrial biopsies were immunostained for PTEN and each scored for presence or absence of PTEN null glands (latent precancer).
Results
The frequency of latent precancers was significantly reduced in OCP (13%, OR 0.19, p<0.001) and IUD (18%, OR 0.42, p=0.015) exposed women compared to respective matched controls (43 and 34%). Presence or absence of endometritis did not significantly correlate with PTEN status within the IUD exposed group (p=0.24).
Conclusions
Normal appearing PTEN mutated endometrial glands, which are highly prevalent in the normal population, may be targets of endometrial cancer risk modulating exposures. Some exposures known to diminish endometrial cancer occurrences in epidemiologic outcome studies, including OCP and IUD use, are associated with a proportionate decline in the frequency of latent precancers. Involution of pre-existing endometrial latent precancers, as evaluated by PTEN analysis, may provide an accessible surrogate marker for long term endometrial cancer risk.
Keywords: endometrium, latent precancer, PTEN, oral contraceptive, intrauterine device
INTRODUCTION
The most common genetic defect in endometrial adenocarcinoma is biallelic inactivation of the tumor suppressor gene PTEN(1), seen in up to 83% of sporadic endometrioid endometrial cancers(2). PTEN mutations are the earliest detected changes of endometrial carcinogenesis, initially unaccompanied by any cytologic or architectural histologic modifications at the light microscopic level(3). Small numbers of isolated somatically mutated endometrial glands are seen in a high proportion, 43%, of normal appearing endometria of premenopausal women with natural menstrual cycles(3). Glands which have acquired PTEN mutations are typically few in number within any individual patient sample, appearing by PTEN immunohistochemistry as individual and clusters of non-staining glands offset by a background of PTEN-intact endometrial glands and stroma. Mutated cells persist, with over three quarters of latent PTEN-null clones being retained for at least one year, presumably because they participate in monthly regeneration of the endometrium after menstrual shedding (3). Only a small fraction of these latent precancers progress to adenocarcinoma, as the lifetime risk of carcinoma is only 2.6%(4). This “latent precancer” phase is subclinical, as it falls below the threshold of detection by routine diagnostic methods, and lacks a defined role in the clinical management of individual patients.
Direct observation of the effects of risk modifying factors upon latent precancers is now possible in the endometrium using PTEN inactivation within normal appearing tissues as a biomarker. A high incidence of sporadic PTEN mutation within endometrial tissues of the general population suggests that mutagenesis rates alone are not rate limiting. Rather, exposures which alter endometrial cancer risk may do so through their activity as positive or negative selection factors for these pre-existing mutant endometrial clones that spontaneously arise at a high frequency during monthly endometrial regeneration. Endometrial cancer is a disease very well suited to testing these ideas. Nongenetic risk modifiers are already well defined, endometrial biopsy is a common office procedure, PTEN mutation in tissues correlates tightly with loss of protein by routine immunohistochemistry, and sufficient residua of mutant clones remains after biopsy that the tissue sampling is nondestructive.
Several studies have already used PTEN immunohistochemistry to sequentially examine the fate of latent endometrial precancers in small numbers of individual patients over time (Table 1). Turnover can be described as emergence (null glands appear in the second sample), persistence (null glands present both samples), or regression (null glands present in first sample are lacking in the second). Patients without null glands in any sample are non informative. Premenopausal naturally cycling women most frequently show a pattern of clonal emergence (37%) or persistence (53%) over time, only rarely (10%) demonstrating regression of pre-existing clones(3). In contrast, regression of preexisting clones is the predominant effect of progestin therapy, whether it be administered by oral (75% regression)(5) or intrauterine (93% regression)(6) routes. These data suggest that PTEN mutant clones arise during the reproductive years when regenerative activity is highest in the endometrium, and that hormonal factors are capable of selectively eliminating them.
Table 1.
Summary of previously published results of latent precancer dynamics over time in resampled individual women under differing hormonal conditions. Patterns of latent precancer emergence, persistence, and regression (columns) differ significantly between women who are naturally cycling, treated with oral progestins, or treated with intrauterine progestins (rows). (Fishers exact p<0.001).
| Hormonal Environment |
Emergence | Persistence | Regression | Total Informative |
Reference |
|---|---|---|---|---|---|
| Natural cycling |
7 (36.8%) | 10 (52.6%) | 2 (10.5%) | 19 | (3) |
| Oral progestin administration |
2 (16.7%) | 1 (8.3%) | 9 (75.0%) | 12 | (5) |
| Intrauterine progestins (Mirena device) |
1 (7.1%) | 0 (0%) | 13 (92.9%) | 14 | (6) |
Both hormonal and non-hormonal factors are capable of reducing endometrial cancer risk, with an effect that lingers for years following exposure. Use of hormonally inert mechanical intrauterine devices (IUD) for contraception confers a long term cancer relative risk 0.6 times that of matched controls(7). Women who use progestin containing low dose combined oral contraceptives (OCP) also have a 0.5 fold endometrial cancer risk relative to controls(8;9). The latent precancer model presented above suggests the hypothesis that these exposures are associated with destruction of pre-existing latent precancers, and we here test this indirectly by comparing the prevalence of latent precancers between populations of exposed and unexposed women.
We here report upon a cross-sectional study based on a retrospective clinical abstraction of medical record data, histologic review and immunohistochemistry for PTEN to study the effects of endometrial cancer risk-reducing exposures on latent precancer rates. Cases were selected by history of IUD or oral contraceptive use, and controls matched by age. All underwent laboratory testing for latent precancers in their endometrial tissues, and latent precancer rates between groups were compared.
MATERIALS ND METHODS
Case Selection
Pathology reports and electronic clinical medical records at Brigham and Women's Hospital in Boston, MA, were screened, with IRB approval, for a history of either intrauterine device or oral contraceptive use, and available endometrial biopsies (either curettage, or biopsy) retrieved for PTEN immunohistochemistry. Matched control patient groups were separately assembled for the IUD and OCP cases, to tailor age matching, and specifically exclude prior IUD or OCP use, respectively. Patients with a prior history of endometrial neoplasm (endometrial intraepithelial neoplasia (EIN), adenocarcinoma) were excluded. Because the menstrual cycle is suspended or abnormal in patients actively taking oral contraceptives or with an IUD in place, it was not possible to “match” menstrual cycle phases between control and exposed groups. Histologic sections were reviewed, and accepted into the study if benign endometrium was present and blocks available.
Indications for biopsy were retrieved by review (by KB) of clinical information provided on the pathology requisitions (blinded to PTEN status), and classified into one of four general classes as follows: 1)extrinsic, endometrial biopsy performed as part of workup of known non-endometrial disease (ex. Uterine fibroids, known non-endometrial pathology such as cervical disease); 2)intrinsic: endometrial biopsy performed because of known endometrial diagnosis documented prior to biopsy (ex. prior endometritis), or symptoms (bleeding) directly referable to the endometrium itself; 3)screen: endometrial sampling performed incidental to endometrial unrelated procedure (ex. tubal ligation), in reflex to a nonspecific screening test (endometrial cells on pap or thick endometrium on ultrasound), or in response to nonspecific symptoms or signs (infertility, pelvic pain); or 4)unknown: no indication for biopsy provided by the clinician.
For the OCP group, endometrial biopsies obtained between December 2004 and June 2006 from premenopausal women under the age of 45 with any history of oral contraceptive use were identified by medical record review. Women with an ongoing pregnancy at time of endometrial sampling, or a history of prior non-OCP exogenous reproductive hormone use (progestin injections, high dose Megace, estrogen only, tamoxifen, raloxifene, contraceptive patches or slow release implants) were excluded. Controls from the same population and biopsy interval were selected by age and the above exclusions.
For the IUD group, premenopausal women having a record of IUD use concurrent with, or prior to, an endometrial biopsy obtained between November 1996 and October 2004 were identified by medical record review. Women with hormone-impregnated IUDs (such as Mirena device) or reproductive hormone use at the time of biopsy were excluded. Controls were age matched from those women in the same population without a history of IUD use.
Histologic review
Hematoxylin and eosin stained diagnostic slides from the diagnostic files were reviewed. Women whose biopsies showed a recent or active gestation (implantation site with or without placental villi) were excluded. Patients with a prior or concurrent endometrial neoplasm (EIN or carcinoma), and those lacking native endometrial functionalis (specimens with polyps only, for example) were excluded. A representative tissue block containing native endometrial functionalis was selected for laboratory analysis. In the IUD cases, a diagnosis of chronic endometritis was made if plasma cells were identified in the endometrial compartment.
PTEN immunohistochemistry and scoring
Paraffin sections of selected blocks were immuno stained for PTEN expression using published protocols(3). In brief, paraffin sections were rehydrated and underwent microwave antigen retrieval before adding primary anti-PTEN antibody 6H2.1 (Cascade Biosciences, Winchester, MA, Cat.#ABM-2052) at 1:300 dilution overnight at 4°C. Slides were washed, incubated with appropriate secondary biotinylated immunoglobulin (Vectastain ABC kit, Vector Laboratories, Inc., Burlingame, CA) and signal detected by sequential addition of avidin peroxidase and 3,3′-diaminobenzidine. Epithelial staining of independent replicate experiments was scored by two pathologists (GM, MCL) blinded to the patient group using endometrial stroma as an internal positive control, and discordant interpretations resolved by consensus review of all materials, and where necessary, a third immunohistochemical run. All tissue fragments were examined, and a biopsy was scored as PTEN null (latent precancer present) when signal was absent in the nuclear and cytoplasmic compartments of all cells in at least one gland.
Typically, PTEN defective glands are sharply offset at high contrast from endometrial stroma, which serves as an internal positive control for the stain. Affected glands are always flanked by expressing glands as well, offering a second internal positive control for interpretation of PTEN loss.
Statistical Analysis
Data were analyzed by Systat v.12 (Systat Software Inc, San Jose, CA) by the study statistician (DN). Patient characteristics were calculated by descriptive statistics and tabulation. Significance of an association between presence or absence of PTEN null glands and patient group were calculated using Fisher's exact test or pearsons chi square test.
RESULTS
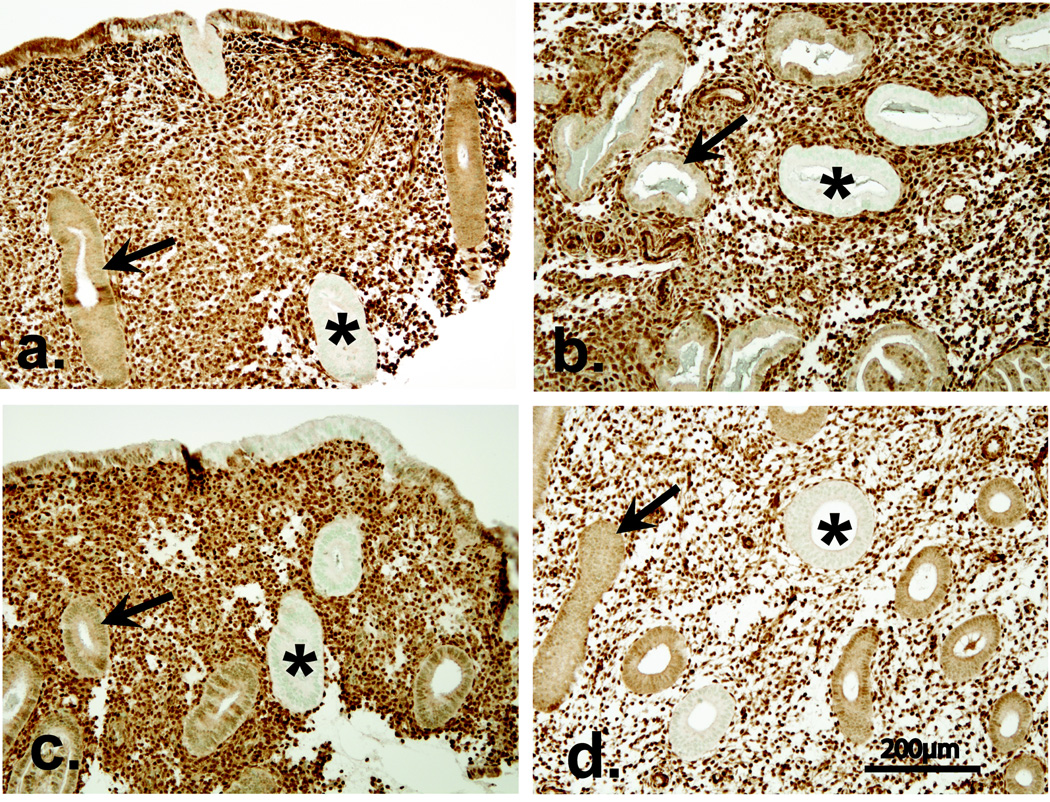
Representative examples of latent precancers as detected by PTEN immunohistochemistry are shown in Figure 1 for all compared groups. The distribution of null glands was usually in small clusters (Figure 1), representing either one gland cut in multiple planes or contiguous but separate glands regenerated from a common mutated clone in the basal layer.
Figure 1.
Latent endometrial precancers detected by PTEN immunohistochemistry in study groups. PTEN inactivated glands (asterisk, pale glands) are admixed with PTEN expressing glands (arrow, brown glands) within otherwise histologically normal appearing endometrium of an OCP exposed (Panel A), OCP non-exposed control (Panel B), IUD exposed (Panel C) and IUD non-exposed control (Panel D) patient. PTEN immunohistochemistry using monoclonal antibody 6h2.1 and a brown colorimetric detection system.
Clinical indications for endometrial sampling (extrinsic, intrinsic, screen, unknown) did vary between respective experimental and control groups patient groups (Fishers exact p<0.001 for IUD vs IUD control, p=0.004 for OCP vs OCP control). Biopsy indication did not, however, correlate with PTEN status (all 461 patients, Pearson Chi-Square p=0.72) (Table 2). The PTEN-null rate amongst the control samples did not vary significantly by menstrual cycle histotype (chi-square p=0.107).
Table 2.
Biopsy indication differed between respective control and exposure groups (Fishers exact p<0.001 IUD, p=0.004 OCP), but did not predict PTEN status (p=0.72 for all groups).
| Biopsy Indication |
Total | Clinical Biopsy Indications, by Group n(%) |
PTEN null rates, n (%) |
|||
|---|---|---|---|---|---|---|
| OCP | OCP control |
IUD | IUD control |
All Groups | ||
|
Extrinsic: non-endometrial disease |
43 | 17/71 (26.9%) |
14/191 (7.3%) |
2/80 (2.5%) |
10/119 (8.4%) |
13/43 (30.2%) |
|
Intrinsic: endometrial symptoms or prior diagnosis |
257 | 29/71 (40.8%) |
98/191 (51.3%) |
64/80 (80.0%) |
66/119 (55.5%) |
86/257 (33.5%) |
|
Screen: biopsy part of general workup or reflex to nonspecific screening result |
106 | 19/71 (26.8%) |
52/191 (27.2%) |
14/80 (17.5%) |
21/119 (17.6%) |
29/106 (27.4%) |
|
Unknown indication unknown |
55 | 6/71 (8.5%) |
27/191 (14.1%) |
0/80 (0.0%) |
22/119 (18.5%) |
17/55 (30.9%) |
| Total | 461 | 71 | 191 | 80 | 119 |
145/461 (31.5%) |
OCP Results
137 women with endometrial biopsies and a history of oral contraceptive use were identified in the medical record. Of these, 27 were ineligible because the date of OCP documentation was after the endometrial biopsy. An additional 39 were ineligible because of inadequate tissue (13), duplicate biopsies in a single patient (6), concurrent pregnancy (6), EIN in the biopsy (1), use of disqualifying non-OCP hormones (8), or failed PTEN staining (5). This left eligible interpretable PTEN results in 71 women with OCP use, and these were compared to 191 age matched controls.
Mean ages in the OCP and control groups were 35.5 and 35.8 years, respectively(t-test p=0.71). Oral contraceptives used include the following: Alesse (1),Aviane (5), Desogen (8), Levlen (1), Levora (1), Loestrin (9), Lo-Ovral (6), Low-Ogestrel (1), Microgestin (1), Mircette (2), Necon (11), Nordette (1), Ortho-cyclin (8), Ortho-novum (3), Ovcon (1), Triphasil (3), Yasmin (4), Zovia (5). The average interval from first documented oral contraceptive use to biopsy was 3.2 years (median 2.29, range 0(on OCP for unspecified duration at point of biopsy) to 13.78, standard deviation 3.1). 8.6% (3/35) of cases below the median exposure interval contained PTEN-null glands, compared to 16.7% (6/36) of those above, a non-significant difference (Fishers exact p=0.48).
Table 3 shows PTEN results for the OCP exposed (“OCP”, 12.7% null) and matched controls (“OCP Control,“ 43% null) patients, which is significantly different (Fishers exact p<0.001).
Table 3.
PTEN null rates of OCP and IUD exposed patients compared to age matched unexposed controls.
| OCP | OCP control | IUD | IUD control | ||
|---|---|---|---|---|---|
| Latent Precancers by PTEN stain |
PTEN null, n(%) |
9 (13%) | 82 (43%) | 14 (18%) | 40 (34%) |
| Total, n(%) | 71(100%) | 191(100%) | 80(100%) | 119 (100%) | |
| Fishers Exact, p |
p<0.001 | p=0.015 | |||
| Odds Ratio (95% CI) |
0.19 (0.09–0.41) | 0.42 (0.21–0.84) | |||
IUD Results
146 women with endometrial biopsies and a history of IUD use were identified in the medical record. Of these, 52 were ineligible because of presence of EIN in the biopsy (2), inadequate tissue (22), failed PTEN immunohistochemistry (5), disqualifying hormone use (7), progesterone impregnation of IUD (2), or unavailable slides or blocks (14). This left 80 eligible IUD exposed biopsies, which were successfully matched with 119 controls. Mean ages in the IUD and their matched control groups was 41.1 and 39.9 years, respectively (t-test p=0.230). The duration of IUD exposure could not be accurately reconstructed in most cases, as the IUD placement date was not documented in the available medical records. 35% of IUD cases had the IUD in place at the time of endometrial biopsy, with the remainder having a history of previous IUD use. Current compared to prior IUD use did not have an effect on the PTEN null rate, which was 19% (4/28) and 14.3% (4/28), respectively (Fishers exact p=0.760).
Table 3 shows PTEN results for the IUD exposed (“IUD”, 18%) and matched controls (“IUD control”, 34%), which were significantly different (Fishers Exact p=0.015). The thickness of the section prohibited interpretation in one case leaving a total of 79 cases evaluable for the presence of chronic endometritis. A total of 48% (38/79) of the IUD exposed endometria contained identifiable plasma cells consistent with chronic endometritis. Within the IUD exposed, and IUD control patient groups, presence or absence of endometritis was not significantly associated with PTEN status (Fishers exact IUD p=0.242, control, p=0.331).
DISCUSSION
These data show that women with a history of non-medicated intrauterine device or oral contraceptive use have a lower rate of latent endometrial precancers detected by PTEN immunohistochemistry when compared to controls. Latent precancer differences between groups cannot be explained by varying clinical indications for biopsy, as biopsy indication itself did not significantly correlate with presence or absence of latent precancers. The magnitude of latent precancer decline across the group of exposed women is proportionate to the extent of diminished endometrial cancer risk as previously determined by epidemiologic clinical outcome studies. Selective ablation of latent precancers by these hormonal and non-hormonal interventions may be one mechanism for the lowered cancer risk they confer.
Combined progestin-estrogen oral contraceptive use decreases endometrial cancer risk approximately 50% overall(8). The effect is duration dependent, however, with likelihood declining from 0.5 at 3–10 years to 0.2 after more than 10 years of use(9). Our patients with an OR for latent precancers of 0.19 had an average interval between first recorded OCP prescription at our institution and biopsy of 3.2 years.
The anti-carcinogenic effects of (inert, non-hormonally impregnated) intrauterine device use are well established, if somewhat less known, than those of oral contraceptives. A recent metaanalysis of 10 studies calculated a pooled adjusted odds ratio of endometrial cancer of 0.6 (95% confidence interval 0.4–0.7) for women who had ever used an intrauterine device. This compares favorably to an odds ratio for latent precancers in our study of 0.42 for IUD use. Others have shown previously that the protective effect does not vary significantly by duration or type of IUD, or age at use(7), so we did not stratify our results by those variables. Mediators of the cancer protective effects of IUDs are unknown, but direct or indirect sequelae of secondary inflammation may be relevant. IUD placement is accompanied by macrophage(10) and lymphocytic infiltrates, and increased concentrations of the cytokines interleukin-6, and tumor necrosis factor-alpha(11). Patients with IUDs are also susceptible to secondary bacterial infection and resultant chronic endometritis. In our study, within the IUD group we did not see an association between chronic endometritis and occurrence of latent precancers.
A precise timeline, and long term stability, of latent precancer destruction is not directly known from this case-control study, as sequential sampling was not performed, and exposures varied widely over a period of months to years between patients. A response time of several months can be inferred from prior studies showing loss of latent precancers after an average of 5.7 months of oral(5), or 1.6 months(6) of intrauterine, progestin. Based upon these estimates, loss of latent precancers should be detectible by direct examination of endometrial tissue following 6 months to 1 year of risk reducing exposure.
The significance of loss of PTEN function depends upon the context in which it is seen. In abnormal endometrium with histopathologic changes of unopposed estrogens, hyperplasia, or EIN, elevated cancer risk is already evident from the pathologic diagnosis itself. Preselection for these high risk endometrial histologies overwhelms any additive increased risk predictive value of PTEN(12). We have now evaluated a different endpoint, risk reduction below that of the general population, in a series of endometrial tissues with unremarkable histology. In that setting, risk reducing exposures are significantly associated with a decrease in the prevalence of PTEN deficient endometrial glands, and this is not discernible by direct tissue examination without specific assessment of this biomarker. The term “latent precancer” emphasizes that these normal tissues do not yet have the full characteristics of a clinically evident premalignant lesion (they lack increased cancer risk) but are nonetheless consistent with an early stage of carcinogenesis, and when destroyed may correspondingly decrease cancer risk.
Although depletion of latent precancers may explain a large component of cancer risk reduction below a population baseline, risk elevation cannot be explained by increased frequency of latent precancers. For women with 5 or more years of unopposed estrogen exposure, endometrial cancer risk increases tenfold(13). A proportionate increase in latent precancer rates from a baseline of 43% to 430% is impossible. Rather, risk elevating exposure promotes progression of pre-existing latent precancers to overt histologic lesions such as EIN and carcinoma. By the time patients develop a diagnosable EIN lesion, approximately 39% already have a concurrent endometrial adenocarcinoma(14) and there is an 46-fold increased future cancer risk(14). Theoretically, many endometrial cancer cases might be prevented by those interventions which selectively destroy latent precancer cells. Correspondingly, the efficacy of such interventions might be measured in short order by direct observation of changes in the latent precancer prevalence before and after therapy.
We have previously validated endometrial PTEN immunohistochemistry as a means of detecting mutation and/or deletion of the PTEN gene(2;3;15;16), by immuno directed microdissection of PTEN protein null and expressing glands and comparison of the genotype of isolated DNA. All PTEN expressing glands identified by immunohistochemistry have a wild-type (normal) genotype, whereas 84% of non-expressing samples have either a mutation or loss of at least one 10q23 heterozygous marker in the region of the PTEN locus(3). Crucial to realizing such a high concordance between mutation and loss of protein by immunohistochemistry is use of appropriate antibodies (17) and reference to positive endometrial stromal staining internal as a positive control for each tissue block. The current study involving hundreds of patients was possible because this previously validated approach is technically simple, inexpensive to perform, and amenable to high throughput application.
The preclinical interval of carcinogenesis provides a window into mechanisms of risk modulation within populations of women exposed to known cancer promoting or preventing agents. Our data support a model incorporating elements of the “butterfly effect,” in which long term outcomes of an extremely sensitive element are irreversibly altered by a small magnitude intervention at an early susceptible moment(18). In our model of endometrial cancer risk modulation, the target is the highly prevalent latent precancer, normal endometrial tissues are the site of action, and resultant secondary effects cascade through years of latency. These considerations are limited to a normal population of women with unremarkable endometrial histology by routine methods (H&E staining). Patients with a histological premalignant lesion (EIN, or atypical endometrial hyperplasia) are at such elevated risk for concurrent (37%)(19) or future (45-fold increase)(20) carcinoma, that more aggressive therapies are indicated.
Acknowledgements
We would like to thank Nicolas Monte for help in performing the immunostains.
Financial Support: This work was supported by NIH grant RO1-CA100833 (G. Mutter), and a NHRI Physician Scientist Award, Taiwan (M.Lin). Dr. Viswanathan receives support from the NIH (Grant K07 CA117979-01)
REFERENCES
- 1.Mutter GL. PTEN, a protean tumor suppressor. Am J Pathol. 2001;158:1895–1898. doi: 10.1016/S0002-9440(10)64656-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Baak JPA, Lees J, et al. Altered PTEN expression as a diagnostic marker for the earliest endometrial precancers. Journal of the National Cancer Institute. 2000;92:924–930. doi: 10.1093/jnci/92.11.924. [DOI] [PubMed] [Google Scholar]
- 3.Mutter GL, Ince TA, Baak JPA, Kust G, Zhou X, Eng C. Molecular identification of latent precancers in histologically normal endometrium. Cancer Research. 2001;61:4311–4314. [PubMed] [Google Scholar]
- 4.Ries LAG, Eisner MP, Kosary CL, Hankey BF, Miller BA, Clegg L, Mariotto A, Feuer EJ, Edwards BK. SEER Cancer Statistics Review, 1975–2002. Bethesda, MD: National Cancer Institute; 2005. Available Online at http://seer.cancer.gov/csr/1975_2002/ [Google Scholar]
- 5.Zheng W, Baker HE, Mutter GL. Involution of PTEN-Null Endometrial Glands with Progestin Therapy. Gynecologic Oncology. 2004;92:1008–1013. doi: 10.1016/j.ygyno.2003.11.026. [DOI] [PubMed] [Google Scholar]
- 6.Orbo A, Rise CE, Mutter GL. Regression of latent endometrial precancers by progestin infiltrated intrauterine device. Cancer Research. 2006;66:5613–5617. doi: 10.1158/0008-5472.CAN-05-4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Curtis KM, Marchbanks PA, Peterson HB. Neoplasia with use of intrauterine devices. Contraception. 2007;75:S60–S69. doi: 10.1016/j.contraception.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 8.Grimes DA, Economy KE. Primary prevention of gynecologic cancers. American Journal of Obstetrics and Gynecology. 1995;172:227–235. doi: 10.1016/0002-9378(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 9.Weiderpass E, Adami HO, Baron JA, Magnusson C, Lindgren A, Persson I. Use of oral contraceptives and endometrial cancer risk (Sweden) Cancer Causes Control. 1999;10:277–284. doi: 10.1023/a:1008945721786. [DOI] [PubMed] [Google Scholar]
- 10.Dechaud H, Maudelonde T, Daures JP, Rossi JF, Hedon B. Evaluation of endometrial inflammation by quantification of macrophages, T lymphocytes, and interleukin-1 and -6 in human endometrium. J.Assist.Reprod.Genet. 1998;15:612–618. doi: 10.1023/A:1020337528607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Archer DF, DeSoto KR, Baker JM. Interleukin-6 and tumor necrosis factor-alpha concentrations in the intrauterine cavity of postmenopausal women using an intrauterine delivery system releasing progesterone. A possible mechanism of action of the intrauterine device. Contraception. 1999;59:175–179. doi: 10.1016/s0010-7824(99)00014-1. [DOI] [PubMed] [Google Scholar]
- 12.Lacey JV, Jr, Mutter GL, Ronnett BM, Ioffe OB, Duggan MA, Rush BB, et al. PTEN expression in endometrial biopsies as a marker of progression to endometrial carcinoma. Cancer Research. 2008;68:6014–6020. doi: 10.1158/0008-5472.CAN-08-1154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antunes CM, Strolley PD, Rosenshein NB, Davies JL, Tonascia JA, Brown C, et al. Endometrial cancer and estrogen use. Report of a large case-control study. New England Journal of Medicine. 1979;300:9–13. doi: 10.1056/NEJM197901043000103. [DOI] [PubMed] [Google Scholar]
- 14.Baak JP, Mutter GL. EIN and WHO94. Journal of Clinical Pathology. 2005;58:1–6. doi: 10.1136/jcp.2004.021071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gimm O, Perren A, Weng LP, Marsh DJ, Yeh JJ, Ziebold U, et al. Differential Nuclear and Cytoplasmic Expression of PTEN in Normal Thyroid Tissue, and Benign and Malignant Epithelial Thyroid Tumors. American Journal of Pathology. 2000;156:1693–1700. doi: 10.1016/s0002-9440(10)65040-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perren A, Weng L, Boag A, Ziebold U, Thakore K, Dahia P, et al. Immunocytochemical evidence of loss of PTEN expression in primary ductal adenocarcinomas of the breast. American Journal of Pathology. 1999;155:1253–1260. doi: 10.1016/S0002-9440(10)65227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pallares J, Bussaglia E, Martinez-Guitarte JL, Dolcet X, Llobet D, Rue M, et al. Immunohistochemical analysis of PTEN in endometrial carcinoma: a tissue microarray study with a comparison of four commercial antibodies in correlation with molecular abnormalities. Mod.Pathol. 2005;18:719–727. doi: 10.1038/modpathol.3800347. [DOI] [PubMed] [Google Scholar]
- 18.Hilborn RC. Sea gulls, butterflies, and grasshoppers: A brief history of the butterfly effect in nonlinear dynamics. American Journal of Physics. 2004;72:425–427. [Google Scholar]
- 19.Mutter GL, Kauderer J, Baak JPA, Alberts DA. Biopsy histomorphometry predicts uterine myoinvasion by endometrial carcinoma: A Gynecologic Oncology Group Study. Human Pathology. 2008;39:866–874. doi: 10.1016/j.humpath.2007.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Baak JP, Mutter GL, Robboy S, van Diest PJ, Uyterlinde AM, Orbo A, et al. The molecular genetics and morphometry-based endometrial intraepithelial neoplasia classification system predicts disease progression in endometrial hyperplasia more accurately than the 1994 World Health Organization classification system. Cancer. 2005;103(11):2304–2312. doi: 10.1002/cncr.21058. [DOI] [PMC free article] [PubMed] [Google Scholar]