Abstract
Background
Interruption of an NNRTI-regimen is often necessary, but must be performed with caution because NNRTIs have a low genetic barrier to resistance. Limited data exist to guide clinical practice on the best interruption strategy to use.
Methods
Patients in the drug-conservation arm of SMART who interrupted a fully suppressive NNRTI-regimen were evaluated. From 2003, SMART recommended interruption of an NNRTI by: a staggered-interruption, where the NNRTI was stopped before the NRTIs; or by replacing the NNRTI with another drug before interruption. Simultaneous-interruption of all ARVs was discouraged. Re-suppression rates four-to-eight months after re-initiating NNRTI-therapy were assessed, as was the detection of drug-resistance mutations within two months of the treatment interruption in a subset (N=141).
Results
Overall, 601/688 (87.4%) patients who re-started an NNRTI achieved viral re-suppression. The adjusted odds ratio (95% CI) for achieving re-suppression was 1.94 (1.02-3.69) for patients with a staggered-interruption and 3.64 (1.37-9.64) for those with a switched-interruption compared to patients with a simultaneous-interruption. At least one NNRTI-mutation was detected in the virus of 16.4% patients with simultaneous-interruption, 12.5% patients with staggered-interruption and 4.2% patients with switched-interruption. Fewer patients with detectable mutations (i.e. 69.2%) achieved HIV-RNA≤400 copies/mL compared to those in whom no mutations were detected (i.e. 86.7%), p=0.05.
Conclusions
In patients who interrupt a suppressive NNRTI-regimen, the choice of interruption-strategy may influence re-suppression rates when re-starting a similar regimen. NNRTI drug-resistance mutations were observed in a relatively high proportion of patients. These data provide additional support for a staggered- or switched-interruption strategy for NNRTI drugs.
Keywords: NNRTI-based therapy, treatment interruption strategies, viral re-suppression, genotypic resistance emergence
INTRODUCTION
Interruption of combination antiretroviral therapy is associated with an increased risk of all cause mortality, AIDS and other serious non-AIDS events (renal, hepatic and cardiovascular disease) compared to continuous therapy, as recently demonstrated in the SMART study1. Even so, there are still occasional circumstances where patients may want, or need, to interrupt some or all of the antiretrovirals (ARVs) in their regimen. For example, women who take ARVs to prevent vertical HIV-transmission may choose to discontinue therapy after delivery or may need to stop therapy if the drug supply is interrupted, particularly in resource limited settings.
ARVs have different plasma and intracellular half-lives2. The plasma elimination half-lives of most nucleoside reverse transcriptase inhibitors (NRTIs) vary from two to six hours, although the half-life of lamivudine is approximately seven hours, the half-life of emtricitabine approximately ten hours and the half-life of tenofovir approximately 15 hours. The intracellular half-lives of NRTIs are longer than their plasma half-lives; however, they are still generally less than nine hours. The NRTIs with the longest intracellular half-lives are lamivudine triphosphate (i.e. between 11 and 15 hours), emtricitabine triphosphate (i.e. approximately 39 hours) and tenofovir diphosphate (i.e. between 12 and 15 hours in activated lymphocytes and approximately 50 hours in resting lymphocytes)3. Protease inhibitors (PIs) also have plasma elimination half-lives that are less than eight hours, even when used with ritonavir boosting, except for darunavir-ritonavir which has a higher half-life of approximately 15 hours. The non-nucleoside reverse transcriptase inhibitors (NNRTIs), efavirenz and nevirapine, have much longer elimination half-lives than any other class of drugs, estimated to be between 35 and 45 hours.
Efavirenz clearance has been shown to be particularly slow in persons carrying a G→T substitution at position 516 of the cytochrome P450 (CYP) 2B6 gene, a polymorphism over-represented in persons of Black-African descent. After discontinuation, plasma efavirenz concentrations in patients with 516 GG, GT, and TT genotypes are predicted to exceed the 95% inhibitory concentration for wild-type virus (i.e. 46.7 ng/ml) for a median of 5.8 days (IQR: 4.4 to 8.3 days), 7.0 days (IQR: 5.0 to 8.0 days), and 14 days (IQR: 11.1 to 21.2 days), respectively4.
Given the prolonged half-life of current NNRTIs, there is a concern that simultaneous-interruption of all ARVs in a NNRTI-containing regimen may lead to a period in which plasma contains only the NNRTI. Monotherapy with an NNRTI is insufficient to maintain virological suppression, and consequently the risk of drug resistance increases5. The NNRTIs nevirapine and efavirenz have a low genetic barrier, since only one mutation is required to confer high-level drug resistance, so the consequences of resistance development for these drugs are particularly serious. This has led to the suggestion that the NNRTI component of a regimen could be stopped earlier than other ARVs in the regimen in order to avoid the potential for NNRTI monotherapy6. The emergence of drug resistance mutations may still be a problem in patients who interrupt treatment using these other strategies because they were predominantly defined on pharmacokinetic (PK) principles with little if any clinical data to support them7;8.
In this report we analyse data from a large trial of treatment interruptions (SMART) and examine the rates of viral re-suppression among patients who interrupt an NNRTI-containing regimen with HIV-RNA≤400 copies/mL and then re-start an NNRTI after the interruption. The detection of drug-resistance mutations two months after initiating a treatment interruption is also described for these patients.
PATIENTS AND METHODS
Study Population
From January 2002, 5472 patients were randomized to either CD4+ guided treatment-interruptions (N=2720, the drug conservation (DC) arm), or to continuous therapy (N=2752, the viral suppression (VS) arm) in SMART. Patients were >13 years, not pregnant or breastfeeding and had CD4+ cell counts >350 cells/mm3 at study entry. The trial was stopped prematurely on 11th January 2006 after an interim analysis showed that not only were more deaths and AIDS-events occurring in the DC-arm, but that serious non-AIDS events were also occurring more frequently1.
We included patients who were on an NNRTI-containing regimen, were randomized to the DC-arm of SMART and had HIV-RNA≤400 copies/mL at the time of their treatment interruption. These patients have already achieved viral suppression on an NNRTI-containing regimen and are likely to be those harbouring little resistance in their viral populations. Clinicians were permitted to interrupt a regimen based on their local practice standards but were advised to avoid interrupting all ARVs simultaneously (simultaneous-interruption). The protocol recommended two alternative strategies: a staggered-interruption (i.e. interrupting the NNRTI while maintaining NRTIs for an average period of seven days, although a longer interval of 10-21 days was recommended for Blacks and Hispanics), or they could replace the NNRTI with a boosted PI for a short period - given their shorter half-lives and higher genetic barrier -and then interrupt all drugs (switched-interruption). The recommendation to use other strategies rather than a simultaneous-interruption was implemented in 2003 when it was clear that resistance could be an issue. However, 20.2% of patients who interrupted an NNRTI in SMART still underwent a simultaneous-interruption. Patients with a simultaneous-interruption were compared to patients who interrupted an NNRTI using each of the other strategies because simultaneous-interruptions are medically discouraged9;10.
Laboratory methods
Follow-up visits in SMART were scheduled to take place after one month, two months and every two months thereafter for the first year and every four months in the second and subsequent years of follow-up. At each visit, patients had their treatment history recorded and their CD4+ cell counts and plasma HIV-RNA levels measured. The resistance analysis was restricted to viruses from patients who had plasma HIV-RNA≥1000 copies/mL in the two months following a treatment interruption, but before resuming therapy. This time point was selected as one during which NNRTI mutations, if present, are most likely to be detected because NNRTI mutations are expected to emerge fairly rapidly during NNRTI monotherapy or during a treatment interruption11. In addition, we did not study resistance at a later time-point because, in the absence of therapy, the virus population is predicted to be dominated by wild type virus and consequently any mutations that arise during the interruption are less likely to be identified. A standard genotyping assay (TRUGENE™) was used. Sequence data were translated into amino acid substitutions (full or mixed) from a reference clade B strain (i.e. HXB2) for both reverse transcriptase (RT) and protease.
Statistical analysis
Baseline and pre-baseline characteristics were examined for patients who interrupted an NNRTI using each of the interruption strategies. For these comparisons, baseline was considered to be the time of the treatment interruption. Categorical variables were compared using Chi-squared and Fisher's exact tests. For continuous variables, we used either ANOVA models or Kruskal-Wallis tests depending on the distribution.
The proportion of patients with HIV-RNA≤400 copies/mL, four-to-eight months after re-starting therapy, was investigated using logistic regression analysis, irrespective of whether patients experienced a treatment change after resuming treatment (as an intention-to-treat analysis). In order to account for the fact that the patient may have changed regimens due to under-performance of the NNRTI, and consequently there may be misrepresentation of the overall impact of NNRTI-resistance, we also performed a switch=failure analysis. Here, a switch away from the NNRTI that was re-started was considered to constitute failure.
We then examined the number and type of resistance mutations (i.e. specific mutations and class of mutation) in patients with resistance data available. We used Fisher's exact tests to compare the occurrence of new mutations according to the NNRTI-interruption strategy, without adjustment for multiple comparisons. Unadjusted logistic regression analysis was also used. All statistical analyses were performed using STATA software (StataCorp. 2007. Stata Statistical Software: Version 9.2/SE, College Station, Texas, USA).
RESULTS
Patient characteristics
Of the 2720 patients who were randomised to the DC-arm of SMART there were 984 (36.2%) who discontinued an NNRTI and had HIV-RNA≤400 copies/mL at the start of their treatment interruption, referred to here as baseline. The 984 patients were primarily male (76%) and 27% had a CDC category stage C (Table 1).
Table 1.
Characteristics at the time of the treatment interruption, according to NNRTI interruption strategy
| Patients with HIV-RNA≤400 N=984 | Patients with interruption strategy recorded (N=717) |
Patients with resistance data available (N=141) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| Simultaneous-interruption N=145 | Staggered-interruption N=419 | Switched-interruption N=153 | P-valuea | Simultaneous-interruption N=61 | Staggered-interruption N=56 | Switched-interruption N=24 | P-valueb | ||
| Risk group | |||||||||
| Same sex | 460 (47%) | 67 (46%) | 182 (43%) | 79 (52%) | 0.05 | 31 (51%) | 22 (39%) | 11 (46%) | 0.70 |
| IVDU | 34 (3%) | 2 (1%) | 19 (5%) | 1 (1%) | 0 (0%) | 3 (5%) | 0 (0%) | ||
| Opposite sex | 339 (34%) | 47 (32%) | 163 (39%) | 56 (37%) | 17(28%) | 21 (38%) | 4 (17%) | ||
| Blood products | 11 (1%) | 3 (2%) | 7 (2%) | 1 (1%) | 1 (2%) | 1 (2%) | 0 (0%) | ||
| >1 risk factor | 111 (11%) | 20 (14%) | 34 (8%) | 13 (9%) | 10 (16%) | 6 (11%) | 8 (33%) | ||
| Other/Unknown | 29 (3%) | 6 (4%) | 14 (3%) | 3 (2%) | 2 (3%) | 3 (5%) | 1 (4%) | ||
| Gender N. Male, (%) | 743 (76%) | 110 (76%) | 311 (74%) | 123 (80%) | 0.31 | 47 (77%) | 41 (73%) | 21 (89%) | 0.95 |
| CDC category N. C, (%) | 268 (27%) | 32 (22%) | 113 (27%) | 42 (27%) | 0.47 | 11 (18%) | 21 (38%) | 7 (29%) | 0.04 |
| Race N. (%) | |||||||||
| Black | 231 (23%) | 38 (26%) | 74 (18%) | 26 (17%) | 0.17 | 19 (31%) | 26 (46%) | 9 (38%) | 0.08 |
| White | 622 (63%) | 93 (64%) | 293 (70%) | 105 (69%) | 36 (59%) | 21 (38%) | 11 (46%) | ||
| Other/unknown | 131 (13%) | 14 (10%) | 52 (12%) | 22 (14%) | 6 (10%) | 9 (16%) | 4 (17%) | ||
| Date of treatment | Mar 05 | Mar 05 | June 05 | July 05 | <0.001 | June 04 | April 05 | Sep 05 | <0.001 |
| interruption Med (IQR) | (Mar 04, Sept 05) | (July 04, Sept 05) | (Feb 05, Oct 05) | (Mar 05, Oct 05) | (Feb 04, Dec 04) | (Oct 04, Aug 05) | (May 05, Nov 05) | ||
| Age | 45 | 44 | 44 | 44 | 0.83 | 45 | 46 | 47 | 0.46 |
| Mean (95% CI) | (44, 45) | (43, 46) | (43, 45) | (42, 45) | (43, 48) | (44, 48) | (43, 51) | ||
| CD4+ (cells/mm3) Med (IQR) | 649 (491, 835) | 660 (492, 923) | 648 (490, 803) | 640 (481, 823) | 0.43 | 624 (471, 834) | 614 (462, 750) | 643 (522, 757) | 0.45 |
| CD4+ nadir (cells/mm3) | 230 | 229 | 229 | 223 | 0.84 | 208 | 177 | 209 | 0.13 |
| Med (IQR) | (140, 342) | (134, 366) | (150, 323) | (140, 340) | (101, 326) | (57, 277) | (71, 290) | ||
| HIV-RNA<50 cps/mL N.(%) | 607 (61.7%) | 75 (51.7%) | 276 (65.9%) | 113 (73.9%) | <0.001 | 27 (44.3%) | 35 (62.5%) | 13 (54.2%) | 0.06 |
P-values represent comparisons across all three treatment interruption strategies
P-values reflect a comparison of staggered- or switched-interruption versus simultaneous interruption, even though a more detailed breakdown has been provided.
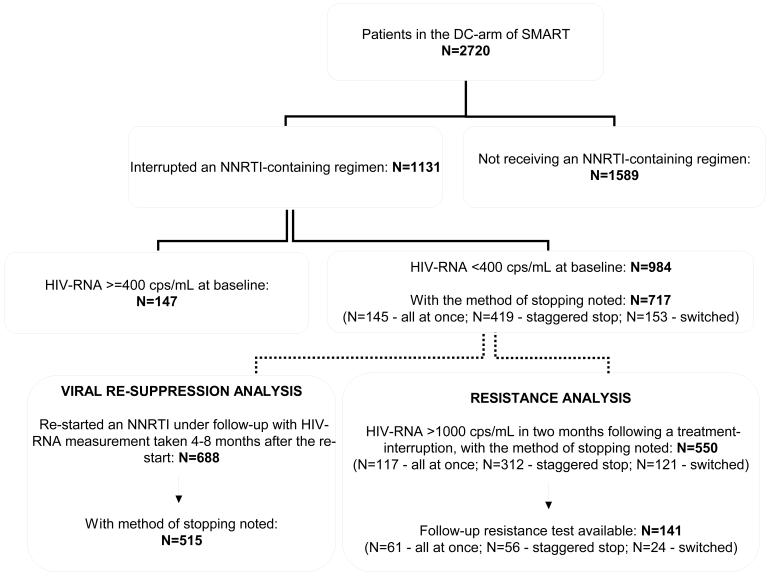
The analysis described in this report mainly focuses on patients who interrupted an NNRTI and have their interruption strategy recorded. From the 984 patients, 717 (72.9%) have their interruption strategy recorded (Figure 1). There were no differences in baseline characteristics between these patients and the 267 patients who do not have their interruption strategy recorded (data not shown).
Figure 1.
Overview of patients in the discontinuation arm of SMART
Baseline characteristics for the 717 patients are outlined in Table 1. Since interruption guidelines were amended after SMART began, patients who interrupted therapy earlier were more likely to undergo simultaneous-interruption compared to either staggered- or switched-interruption (p<0.001). Patients who underwent a simultaneous-interruption also had higher HIV-RNA levels immediately prior to the interruption compared to patients who interrupted treatment using alternative strategies, p<0.001.
Viral re-suppression after re-starting NNRTIs
There were 688 patients who re-started an NNRTI-containing regimen during follow-up and had at least one HIV-RNA measurement four-to-eight months after the re-start (Figure 1). Of these, 642 (93.3%) patients re-started the same NNRTI as they interrupted, 595 (86.5%) stayed on the NNRTI they re-started for ≥8 months following the re-start and 515 (74.9%) patients had their interruption strategy noted.
All 688 patients were initially included in these analyses, irrespective of whether their interruption strategy was known. In an intention-to-treat analysis, where treatment changes after resumption of therapy were not accounted for, the percentage of patients who achieved viral re-suppression (i.e. reached HIV-RNA≤400 copies/mL) according to the NNRTI that was re-started was 90.0% (404/449), 82.2% (194/236) and 100% (3/3) for patients who reinitiated efavirenz, nevirapine and delarvidine, respectively (p=0.012).
Although efavirenz, nevirapine and delarvidine were re-started in these patients, only 88.1% (356/404), 88.7% (172/194) and 100% (3/3), respectively, were using their NNRTI at the time of viral re-suppression. From the 87 patients who did not reach viral re-suppression in the eight months following resumption of therapy, there were 68.9% (31/45) who re-started efavirenz and 78.6% (33/42) who re-started nevirapine who were using their NNRTI at the time the re-suppression rate was determined. Overall, 89.3% (531/595) patients who remained on the same regimen reached viral re-suppression and 75.3% (70/93) patients who switched regimens achieved viral re-suppression, p<0.0001.
Physicians may capture a lack of viral response early in patients who resume a regimen that does not function effectively. As a result, patients with poor virological responses are likely to be those who experience treatment changes to their regimens. If, instead, we consider all patients who experience changes to their regimens as failures (i.e. they have a four-to-eight month HIV-RNA>400 copies/mL), the percentage of patients who achieved viral re-suppression according to the NNRTI that was reinitiated was 79.3% (356/449), 72.9% (172/236) and 100% (3/3) for patients who reinitiated efavirenz, nevirapine and delarvidine, respectively (p=0.11).
Next we focussed on the 515 patients who had their initial interruption strategy recorded. The median (IQR) time from NNRTI interruption to NNRTI re-initiation was 20.7 (11.7 to 40.8) weeks, 25.6 (12.4, 45.0) weeks and 20.3 (10.7, 43.0) weeks for patients who underwent a simultaneous-interruption, a staggered-interruption and a switched-interruption, respectively, p=0.46. Significant differences existed in the proportion of patients with HIV-RNA≤400 copies/mL four-to-eight months after re-starting therapy according to the interruption strategy (82.7% of patients with a simultaneous-interruption versus 90.2% of those with a staggered-interruption and 94.6% of those with a switched-interruption, p=0.02). In a multivariable logistic regression model, the odds ratio (OR) for achieving HIV-RNA re-suppression was: OR=1.94 (1.02 to 3.69) for patients with a staggered-interruption and OR=3.64 (1.37 to 9.64) for patients with a switched-interruption compared to patients with a simultaneous-interruption, indicating a benefit of either or both of these two strategies. Race, CD4+ cell counts at the time of the treatment interruption, hepatitis B co-infection and hepatitis C co-infection were not independent predictors of response.
Detection of drug resistance mutations
Out of the 717 patients who were fully virologically suppressed at baseline and have their treatment interruption strategy recorded, 141 (19.7%) have resistance data available in the two months following a treatment interruption (Figure 1): 61 underwent a simultaneous-interruption, 56 underwent a staggered-interruption and 24 underwent a switched-interruption. These patients were broadly reflective of all patients in SMART who interrupted an NNRTI. Patients with a resistance test were more likely to be Black (i.e. 38% versus 21%), p<0.0001, and they had lower CD4+ nadir counts (a median (IQR) of 200 (78, 299) versus 237 (149, 348), p=0.0004) compared to patients who did not have a resistance test available. In patients with resistance data, the only difference in baseline characteristics according to the NNRTI interruption strategy was that patients with simultaneous-interruption were more likely to have a CDC category C illness at baseline and that these patients were also more likely to have interrupted treatment at an earlier time-point (Table 1).
In the two months following a treatment-interruption: NRTI, NNRTI and/or PI-specific mutations were detected in the predominant virus of 23 (16%), 18 (13%) and 11 (8%) of 141 patients, respectively, according to the 2007 IAS-USA mutations lists12. No significant differences were observed in the proportion of class-specific mutations that were detected according to the method of stopping the NNRTI (Table 2).
Table 2.
Drug resistance detection according to the method of interrupting an NNRTI
| Patients who interrupted efavirenz | Patients who interrupted nevirapine | ||||||
|---|---|---|---|---|---|---|---|
| All patients who stopped an NNRTI* N=141 | Simultaneous-interruption N=29 | Staggered-interruption N=45 | Switched-interruption N=13 | Simultaneous-interruption N=31 | Staggered-interruption N=11 | Switched-interruption N=11 | |
| Follow-up resistance mutations (N, %): | |||||||
| NRTI mutations | 23 (16.3%) | 6 (20.7%) | 9 (20.0%) | 1 (7.7%) | 2 (6.5%) | 2 (18.2%) | 2 (18.2%) |
| NNRTI mutations | 18 (12.8%) | 5 (17.2%) | 6 (13.3%) | 0 (0.0%) | 5 (16.1%) | 1 (9.1%) | 1 (9.1%) |
| PI mutations | 11 (7.8%) | 2 (6.9%) | 4 (8.9%) | 1 (7.7%) | 0 (0.0%) | 2 (18.2%) | 1 (9.1%) |
| Follow-up resistance mutations (N, %): | 39 (27.7%) | 9 (31.0%) | 15 (33.3%) | 1 (7.7%) | 6 (19.3%) | 4 (36.4%) | 3 (17.3%) |
| NRTI mutations (N, %): | |||||||
| 41L | 6 (4.3%) | 0 (0.0%) | 4 (8.9%) | 0 (0.0%) | 2 (6.5%) | 0 (0.0%) | 0 (0.0%) |
| 62V | 2 (1.4%) | 1 (3.5%) | 0 (0.0%) | 0 (0.0%) | 1 (3.2%) | 0 (0.0%) | 0 (0.0%) |
| 65R | 1 (0.7%) | 1 (3.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 67N | 6 (4.3%) | 2 (6.9%) | 2 (4.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) |
| 69 insertion | 1 (0.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (3.2%) | 0 (0.0%) | 0 (0.0%) |
| 70R | 8 (5.7%) | 1 (3.5%) | 3 (6.7%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | 2 (18.2%) |
| 74V | 2 (1.4%) | 1 (3.5%) | 0 (0.0%) | 0 (0.0%) | 1 (3.2%) | 0 (0.0%) | 0 (0.0%) |
| 75I | 1 (0.7%) | 1 (3.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 116Y | 1 (0.7%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 151M | 1 (0.7%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 184I/V | 13 (9.2%) | 2 (6.9%) | 4 (8.9%) | 1 (7.7%) | 2 (6.5%) | 1 (9.1%) | 2 (18.2%) |
| 210W | 5 (3.6%) | 0 (0.0%) | 3 (6.7%) | 0 (0.0%) | 1 (3.2%) | 1 (9.1%) | 0 (0.0%) |
| 215Y/F | 9 (6.4%) | 1 (3.5%) | 4 (8.9%) | 0 (0.0%) | 1 (3.2%) | 1 (9.1%) | 2 (18.2%) |
| 219Q/E | 5 (3.6%) | 1 (3.5%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | 1 (9.1%) |
| NNRTI mutations (N, %): | |||||||
| 103N | 5 (3.6%) | 2 (6.9%) | 3 (6.7%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 108I | 5 (3.6%) | 0 (0.0%) | 3 (6.7%) | 0 (0.0%) | 1 (3.2%) | 0 (0.0%) | 1 (9.1%) |
| 181C/I | 2 (1.4%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (6.5%) | 0 (0.0%) | 0 (0.0%) |
| 188C/H/L | 4 (2.8%) | 3 (10.3%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 190S/A | 4 (2.8%) | 1 (3.5%) | 0 (0.0%) | 0 (0.0%) | 2 (6.5%) | 1 (9.1%) | 0 (0.0%) |
| PI mutations (N, %): | |||||||
| 30N | 3 (2.1%) | 1 (3.5%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | 0 (0.0%) |
| 46I/L | 4 (2.8%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 1 (9.1%) | 1 (9.1%) |
| 54M/L | 1 (0.7%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 82A/F/L/T/S | 1 (0.7%) | 0 (0.0%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 84V | 2 (1.4%) | 1 (3.5%) | 1 (2.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 90M | 3 (2.1%) | 1 (3.5%) | 0 (0.0%) | 1 (7.7%) | 1 (3.2%) | 0 (0.0%) | 0 (0.0%) |
NB: In one patient who interrupted a delarvidine containing regimen the following mutations were detected: RT: 67N, 70R, 184I/V, 219Q/E, PRO: 50L/V
Any IAS-USA mutation that is not listed in the above table was not seen in the predominant virus of patients in our comparisons (i.e. NRTI: 77L, 115F; NNRTI: 100I, 106A/M, 225H, 236L; and PI: 32I, 33F, 47V/A, 48V, 76V, 88S).
Although not significant, NNRTI mutations were less likely to be detected in patients who had a staggered- or switched-interruption compared to those with a simultaneous-interruption (Odds ratio for the detection of an NNRTI mutation: OR (95% CI): 0.57 (0.21 to 1.53) for staggered- or switched-interruption versus simultaneous-interruption, p=0.29), and NRTI mutations were more likely to be detected in the predominant virus of patients who underwent a staggered- or switched-interruption compared to those with a simultaneous-interruption (OR for the detection of an NRTI mutation (95% CI): 1.23 (0.49 to 3.05) for staggered- or switched-interruption versus simultaneous-interruption, p=0.66). Other factors, such as CD4+ cell counts and HIV-RNA levels at the time of the treatment interruption, hepatitis co-infection, race, the NNRTI restarted and the mode of infection were not predictors of resistance in this population. Mutations that are selected by patients who interrupted each NNRTI are described separately in table 2.
We explored the detection of specific resistance mutations and investigated how many drug classes were compromised by the presence of resistance in these patients. No significant differences were seen in these comparisons, but the number of mutations that were detected overall was small so we only have limited power to illustrate any differences if they truly exist (Table 2).
Viral re-suppression according to mutations detected in the resistance test
Out of the 141 patients with resistance data available, there were 101 (71.6%) who restarted an NNRTI according to protocol recommendations and have HIV-RNA data available four-to-eight months after resuming therapy (42 patients with simultaneous-interruption, 40 patients with staggered-interruption and 19 patients with switched-interruption): 83 (82.2%) of these patients reached an HIV-RNA≤400 copies/mL a maximum of eight months after the re-start. The median (IQR) HIV-RNA at the time of the restart was 4.55 (4.04 to 5.00) log10 copies/mL for patients with a simultaneous-interruption, 4.61 (4.35 to 5.08) log10 copies/mL for patients with a staggered-interruption and 4.88 (4.53 to 5.04) log10 copies/mL for patients with a switched-interruption.
Among patients who re-started an NNRTI, significantly fewer patients who had a mutation detected (18 of 26 (69.2%)) achieved viral re-suppression compared to those who did not have any mutations in their resistance tests (65 of 75 (86.7%)), p=0.05. The proportion of patients who reached virological re-suppression after re-starting therapy did not differ significantly according to the class of resistance mutations that were present in the follow up resistance test. Six out of nine (66.7%) patients with a virus containing NRTI mutations only, seven out of nine (77.8%) patients with a virus containing NNRTI mutations, five out of eight (62.5%) patients with a virus containing PI mutations and 65 of 75 (86.7%) patients with a virus containing no IAS-USA mutations, achieved viral load re-suppression four-to-eight months after re-starting therapy.
DISCUSSION
The SMART study contains the largest database of patients who were virologically suppressed on an NNRTI-containing regimen and interrupted their ARVs according to one of three pre-defined strategies (one of which was actively discouraged). Although SMART was a randomized clinical trial, this manuscript represents an analysis of observational data since selection of the interruption strategy was at the discretion of the treating clinician. Although re-suppression rates were high in this study (i.e. 89.7% of patients achieved viral re-suppression), we have shown that interruption from an NNRTI containing regimen does not always result in complete viral re-suppression once therapy is resumed. We have also provided some evidence that, if a patient is interrupting an NNRTI-containing regimen, it is particularly important not to interrupt all ARVs simultaneously, but to undergo a staggered- or switched-interruption instead. There also appears to be some evidence that a staggered-interruption may be worse than a switched-interruption; an observation that is biologically plausible. However, since these comparisons were performed on a small number of patients, further evidence is required to state this conclusively.
Drug resistant HIV has been shown to emerge during periods of increased HIV replication when drug concentrations are present but suboptimal6;13-16. Although the absolute number of mutations detected in our study was small, NNRTI mutations were observed in a relatively high proportion of patients two months after undergoing a treatment-interruption. Even though we cannot exclude the possibility that these mutations were present before therapy interruption, these results emphasize the need for treatment-interruptions to be performed with caution (and only when essential), in order to avoid the emergence of NNRTI-specific mutations that compromise future treatment options. Among patients who re-started therapy, significantly fewer patients who had a mutation in their resistance test achieved viral re-suppression (69.2%) compared to those who did not have any mutations in their resistance test (86.7%), p=0.05, re-emphasising the need to interrupt NNRTI-based regimens wisely.
Overall, the absolute number of mutations that were detected in the reverse transcriptase and protease genes was small for patients who interrupted an NNRTI using each interruption strategy. High rates of resistance to NNRTIs were seen in the TRIVACAN trial, where resistance was examined among patients undergoing a treatment interruption in Sub-Saharan Africa, although the method of interrupting the NNRTI was not studied in detail17. The ISS-PART study demonstrated a low risk of mutation emergence for patients interrupting an NNRTI containing regimen, but a higher risk among patients who interrupt a regimen including an unboosted PI18. NNRTI resistance emergence has been associated with NNRTI drug levels prior to the treatment-interruption, with drug concentrations close to the lower limit of the therapeutic range associated with more resistance emergence19.
In our study, NNRTI-mutations were less likely to arise after a staggered- or switched-interruption compared to patients with a simultaneous-interruption, but the number of patients in whom resistance mutations were detected was too small for any of the differences to reach statistical significance. Although the trend was not significant, a staggered or switched-interruption was still associated with nearly half the odds of detecting an NNRTI mutation in the predominant virus population compared to a simultaneous-interruption.
Patients who withdraw consent to continue in trials or become lost to follow up are likely to remain off treatment until they return to clinical care. These data give us some insight into the risk of mutation-development (and hence the prevalence of resistance) in patients who drop out of ARV programmes worldwide. They also indicate the likelihood that these patients will experience virological suppression if a similar regimen is restarted at a later stage.
This analysis only looks at the risk of resistance emergence after a first treatment-interruption in SMART. Repeat interruptions of the same regimen could increase the risk of resistance14. Some patients show little evidence of resistance during a first interruption, but an elevated risk after a second or third interruption of the same regimen14. Conversely, Arnedo-Valero et al showed that the number of mutations did not increase with the number of interruptions20. In SMART, patients could re-initiate the same regimen after their treatment-interruption, but since the study was terminated prematurely, it was not possible to examine the relationship between mutation emergence and further interruption-cycles in more detail.
It is not known whether the mutations observed in this study are attributable to the occurrence of new mutations or to the re-appearance of previously existing mutations. Since all of these patients had an HIV-RNA≤400 copies/mL at baseline it was not possible to perform genotypic resistance testing. Our assumptions that patients with an HIV-RNA≤400 copies/mL at baseline had no NNRTI mutations in their virus population may not be correct21.
This study used population sequencing, rather than more sensitive methods for identifying mutants that are present as minority viral strains, for assessing drug resistance. If ultra-sensitive genotyping analyses (e.g., allele specific PCT, clonal or single genome sequencing) had been used, more NNRTI mutations (i.e. low frequency drug resistant variants) may have been detected22. Patients may appear to have wild-type strain at the time of their resistance test even if mutations in proviral DNA are present13. It is, therefore, possible that our data under-estimate the incidence of resistance mutations as a result of the treatment interruption13.
To conclude, the method of interrupting an NNRTI and the NNRTI re-started may impact on the chances of viral re-suppression on an NNRTI-containing regimen after a treatment-interruption. NNRTI mutations were observed in a relatively high proportion of patients two months after a treatment-interruption. While no significant differences were observed between stopping strategies in the number, type, or class of mutation that emerged, there was a consistent trend for more mutations to emerge when a simultaneous-interruption strategy was used. The simultaneous-interruption of all ARVs in a suppressive NNRTI/NRTI containing regimen may negatively impact on the response to the future use of ARVs due to the emergence of resistance mutations. These data provide further evidence for negative consequences of interruption of an NNRTI-containing regimen and further support the recommendation to avoid interruption entirely. Where such interruptions are essential, these data suggest that a staggered- or switched-approach when interrupting an NNRTI may reduce the risk of resistance emerging.
Acknowledgements
Support provided by: NIAID, NIH grants U01AI042170 and U01AI46362. Clinical Trials.gov identifier: NCT00027352.
Investigators in the SMART Study Group SMART was initiated by the Terry Beirn Community Programs for Clinical Research on AIDS (CPCRA) and implemented in collaboration with international coordinating centers in Copenhagen (Copenhagen HIV Programme), London (Medical Research Council, Clinical Trials Unit), Sydney (National Centre in HIV Epidemiology and Clinical Research) and Washington (CPCRA). Participating staff are listed below.
Copenhagen International Coordinating Center JD Lundgren, KB Jensen, DC Gey, L Borup, M Pearson, PO Jansson, BG Jensen, J Tverland, H Juncker-Benzon, Z Fox, AN Phillips.
London Internatioal Coordinating Center JH Darbyshire, AG Babiker, AJ Palfreeman, SL Fleck, W Dodds, E King, B Cordwell, F van Hooff, Y Collaco-Moraes.
Sydney International Coordinating Center DA Cooper, S Emery, FM Drummond, SA Connor, CS Satchell, S Gunn, S Oka, MA Delfino, K Merlin, C McGinley.
Washington International Coordinating Center F Gordin, E Finley, D Dietz, C Chesson, M Vjecha, B Standridge.
INSIGHT Network Coordinating Center JD Neaton, G Bartsch, A DuChene, M George, B Grund, M Harrison, E Krum, G Larson, C Miller, R Nelson, J Neuhaus, MP Roediger, T Schultz.
ECG Reading Center R Prineas, C Campbell, Z-M Zhang.
Endpoint Review Committee G Perez (co-chair), A Lifson (co-chair), D Duprez, J Hoy, C Lahart, D Perlman, R Price, R Prineas, F Rhame, J Sampson, J Worley.
NIAID Data and Safety Monitoring Board M Rein (chair), R DerSimonian (executive secretary), BA Brody, ES Daar, NN Dubler, TR Fleming, DJ Freeman, JP Kahn, KM Kim, G Medoff, JF Modlin, R Moellering Jr, BE Murray, B Pick, ML Robb, DO Scharfstein, J Sugarman, A Tsiatis, C Tuazon, L Zoloth.
NIH, NIAID: K Klingman, S Lehrman.
SMART Clinical Site Investigators by Country (SMART enrollment)
Argentina (147): J Lazovski, WH Belloso, MH Losso, JA Benetucci, S Aquilia, V Bittar, EP Bogdanowicz, PE Cahn, AD Casiró, I Cassetti, JM Contarelli, JA Corral, A Crinejo, L Daciuk, DO David, G Guaragna, MT Ishida, A Krolewiecki, HE Laplume, MB Lasala, L Lourtau, SH Lupo, A Maranzana, F Masciottra, M Michaan, L Ruggieri, E Salazar, M Sánchez, C Somenzini.
Australia (170): JF Hoy, GD Rogers, AM Allworth, JStC Anderson, J Armishaw, K Barnes, A Carr, A Chiam, JCP Chuah, MC Curry, RL Dever, WA Donohue, NC Doong, DE Dwyer, J Dyer, B Eu, VW Ferguson, MAH French, RJ Garsia, J Gold, JH Hudson, S Jeganathan, P Konecny, J Leung, CL McCormack, M McMurchie, N Medland, RJ Moore, MB Moussa, D Orth, M Piper, T Read, JJ Roney, N Roth, DR Shaw, J Silvers, DJ Smith, AC Street, RJ Vale, NA Wendt, H Wood, DW Youds, J Zillman.
Austria (16): A Rieger, V Tozeau, A Aichelburg, N Vetter.
Belgium (95): N Clumeck, S Dewit, A de Roo, K Kabeya, P Leonard, L Lynen, M Moutschen, E O'Doherty.
Brazil (292): LC Pereira Jr, TNL Souza, M Schechter, R Zajdenverg, MMTB Almeida, F Araujo, F Bahia, C Brites, MM Caseiro, J Casseb, A Etzel, GG Falco, ECJ Filho, SR Flint, CR Gonzales, JVR Madruga, LN Passos, T Reuter, LC Sidi, ALC Toscano.
Canada (102): D Zarowny, E Cherban, J Cohen, B Conway, C Dufour, M Ellis, A Foster, D Haase, H Haldane, M Houde, C Kato, M Klein, B Lessard, A Martel, C Martel, N McFarland, E Paradis, A Piche, R Sandre, W Schlech, S Schmidt, F Smaill, B Thompson, S Trottier, S Vezina, S Walmsley.
Chile (49): MJ Wolff Reyes, R Northland..
Denmark (19): L Ostergaard, C Pedersen, H Nielsen, L Hergens, IR Loftheim, KB Jensen.
Estonia (5): M Raukas, K Zilmer.
Finland (21): J Justinen, M Ristola.
France (272): PM Girard, R Landman, S Abel, S Abgrall, K Amat, L Auperin, R Barruet, A Benalycherif, N Benammar, M Bensalem, M Bentata, JM Besnier, M Blanc, O Bouchaud, A Cabié, P Chavannet, JM Chennebault, S Dargere, X de la Tribonniere, T Debord, N Decaux, J Delgado, M Dupon, J Durant, V Frixon-Marin, C Genet, L Gérard, J Gilquin, B Hoen, V Jeantils, H Kouadio, P Leclercq, J-D Lelièvre, Y Levy, CP Michon, P Nau, J Pacanowski, C Piketty, I Poizot-Martin, I Raymond, D Salmon, JL Schmit, MA Serini, A Simon, S Tassi, F Touam, R Verdon, P Weinbreck, L Weiss, Y Yazdanpanah, P Yeni.
Germany (215): G Fätkenheuer, S Staszewski, F Bergmann, S Bitsch, JR Bogner, N Brockmeyer, S Esser, FD Goebel, M Hartmann, H Klinker, C Lehmann, T Lennemann, A Plettenberg, A Potthof, J Rockstroh, B Ross, A Stoehr, JC Wasmuth, K Wiedemeyer, R Winzer.
Greece (95): A Hatzakis, G Touloumi, A Antoniadou, GL Daikos, A Dimitrakaki, P Gargalianos-Kakolyris, M Giannaris, A Karafoulidou, A Katsambas, O Katsarou, AN Kontos, T Kordossis, MK Lazanas, P Panagopoulos, G Panos, V Paparizos, V Papastamopoulos, G Petrikkos, H Sambatakou, A Skoutelis, N Tsogas, G Xylomenos.
Ireland (2): CJ Bergin, B Mooka.
Israel (13): S Pollack, MG Mamorksy, N Agmon-Levin, R Karplus, E Kedem, S Maayan, E Shahar, Z Sthoeger, D Turner, I Yust.
Italy (88): G Tambussi, V Rusconi, C Abeli, M Bechi, A Biglino, S Bonora, L Butini, G Carosi, S Casari, A Corpolongo, M De Gioanni, G Di Perri, M Di Pietro, G D'Offizi, R Esposito, F Mazzotta, M Montroni, G Nardini, S Nozza, T Quirino, E Raise.
Japan (15): M Honda, M Ishisaka.
Lithuania (4): S Caplinskas, V Uzdaviniene.
Luxembourg (3): JC Schmit, T Staub.
Morocco (42): H Himmich, K Marhoum El Filali.
New Zealand (7): GD Mills, T Blackmore, JA Masters, J Morgan, A Pithie.
Norway (17): J Brunn, V Ormasssen.
Peru (57): A La Rosa, O Guerra, M Espichan, L Gutierrez, F Mendo, R Salazar.
Poland (54): B Knytz, A Horban, E Bakowska, M Beniowski, J Gasiorowski, J Kwiatkowski.
Portugal (73): F Antunes, RS Castro, M Doroana, A Horta, K Mansinho, AC Miranda, IV Pinto, E Valadas, J Vera.
Russia (17): A Rakhmanova, E Vinogradova, A Yakovlev, N Zakharova.
South Africa (26): R Wood, C Orrel.
Spain (100): J Gatell, JA Arnaiz, R Carrillo, B Clotet, D Dalmau, A González, Q Jordano, A Jou, H Knobel, M Larrousse, R Mata, JS Moreno, E Oretaga, JN Pena, F Pulido, R Rubio, J Sanz, P Viciana.
Switzerland (91): B Hirschel, R Spycher, M Battegay, E Bernasconi, S Bottone, M Cavassini, A Christen, C Franc, HJ Furrer, A Gayet-Ageron, D Genné, S Hochstrasser, L Magenta, C Moens, N Müller, R Nüesch.
Thailand (159): P Phanuphak, K Ruxrungtham, W Pumpradit, P Chetchotisakd, S Dangthongdee, S Kiertiburanakul, V Klinbuayaem, P Mootsikapun, S Nonenoy, B Piyavong, W Prasithsirikul, P Raksakulkarn.
United Kingdom (214): BG Gazzard, JG Ainsworth, J Anderson, BJ Angus, TJ Barber, MG Brook, CD Care, DR Chadwick, M Chikohora, DR Churchill, D Cornforth, DH Dockrell, PJ Easterbrook, PA Fox, R Fox, PA Gomez, MM Gompels, GM Harris, S Herman, AGA Jackson, SPR Jebakumar, MA Johnson, GR Kinghorn, KA Kuldanek, N Larbalestier, C Leen, M Lumsden, T Maher, J Mantell, R Maw, S McKernan, L McLean, S Morris, L Muromba, CM Orkin, AJ Palfreeman, BS Peters, TEA Peto, SD Portsmouth, S Rajamanoharan, A Ronan, A Schwenk, MA Slinn, CJ Stroud, RC Thomas, MH Wansbrough-Jones, HJ Whiles, DJ White, E Williams, IG Williams, M Youle.
United States (2989): DI Abrams, EA Acosta, S Adams, A Adamski, L Andrews, D Antoniskis, DR Aragon, R Arduino, R Artz, J Bailowitz, BJ Barnett, C Baroni, M Barron, JD Baxter, D Beers, M Beilke, D Bemenderfer, A Bernard, CL Besch, MT Bessesen, JT Bethel, S Blue, JD Blum, S Boarden, RK Bolan, JB Borgman, I Brar, BK Braxton, UF Bredeek, R Brennan, DE Britt, J Brockelman, S Brown, V Bruzzese, D Bulgin-Coleman, DE Bullock, V Cafaro, B Campbell, S Caras, J Carroll, KK Casey, F Chiang, G Childress, RB Cindrich, C Clark, M Climo, C Cohen, J Coley, DV Condoluci, R Contreras, J Corser, J Cozzolino, LR Crane, L Daley, D Dandridge, V D'Antuono, JG Darcourt Rizo Patron, JA DeHovitz, E DeJesus, J DesJardin, M Diaz-Linares, C Dietrich, P Dodson, E Dolce, K Elliott, D Erickson, M Estes, LL Faber, J Falbo, MJ Farrough, CF Farthing, P Ferrell-Gonzalez, H Flynn, C Frank, M Frank, KF Freeman, N French, G Friedland, N Fujita, L Gahagan, K Genther, I Gilson, MB Goetz, E Goodwin, F Graziano, CK Guity, P Gulick, ER Gunderson, CM Hale, K Hannah, H Henderson, K Hennessey, WK Henry, DT Higgins, SL Hodder, HW Horowitz, M Howe-Pittman, J Hubbard, R Hudson, H Hunter, C Hutelmyer, MT Insignares, L Jackson, L Jenny, M John, DL Johnson, G Johnson, J Johnson, L Johnson, J Kaatz, J Kaczmarski, S Kagan, C Kantor, T Kempner, K Kieckhaus, N Kimmel, BM Klaus, N Klimas, JR Koeppe, J Koirala, J Kopka, JR Kostman, MJ Kozal, A Kumar, A Labriola, H Lampiris, C Lamprecht, KM Lattanzi, J Lee, J Leggett, C Long, A Loquere, K Loveless, CJ Lucasti, R Luskin-Hawk, M MacVeigh, LH Makohon, S Mannheimer, NP Markowitz, C Marks, N Martinez, C Martorell, E McFeaters, B McGee, DM McIntyre, J McKee, E McManus, LG Melecio, D Melton, S Mercado, E Merrifield, JA Mieras, M Mogyoros, FM Moran, K Murphy, D Mushatt, S Mutic, I Nadeem, R Nahass, D Nixon, S O'Brien, A Ognjan, M O'Hearn, K O'Keefe, PC Okhuysen, E Oldfield, D Olson, R Orenstein, R Ortiz, J Osterberger, W Owen, F Parpart, V Pastore-Lange, S Paul, A Pavlatos, DD Pearce, R Pelz, G Perez, S Peterson, G Pierone Jr, D Pitrak, SL Powers, HC Pujet, JW Raaum, J Ravishankar, J Reeder, N Regevik, NA Reilly, C Reyelt, J Riddell IV, D Rimland, ML Robinson, AE Rodriguez, MC Rodriguez-Barradas, V Rodriguez Derouen, R Roland, C Rosmarin, WL Rossen, JR Rouff, JH Sampson, M Sands, C Savini, S Schrader, MM Schulte, C Scott, R Scott, H Seedhom, M Sension, A Sheble-Hall, A Sheridan, J Shuter, LN Slater, R Slotten, D Slowinski, M Smith, S Snap, C Somboonwit, DM States, M Stewart, G Stringer, J Sullivan, KK Summers, K Swanson, IB Sweeton, S Szabo, EM Tedaldi, EE Telzak, Z Temesgen, D Thomas, MA Thompson, S Thompson, C Ting Hong Bong, C Tobin, J Uy, A Vaccaro, LM Vasco, I Vecino, GK Verlinghieri, F Visnegarwala, BH Wade, V Watson, SE Weis, JA Weise, S Weissman, AM Wilkin, L Williams, JH Witter, L Wojtusic, TJ Wright, V Yeh, B Young, C Zeana, J Zeh.
Uruguay (3): E Savio, M Vacarezza.
Reference List
- (1).El-Sadr WM, Lundgren JD, Neaton JD, Gordin F, Abrams D, Arduino RC, et al. CD4+ count-guided interruption of antiretroviral treatment. New England Journal of Medicine. 2006;355(22):2283–2296. doi: 10.1056/NEJMoa062360. [DOI] [PubMed] [Google Scholar]
- (2).Arduino R. CD4 cell count-guided treatment interruption: Be smart and wait for more evidence. Clinical Infectious Diseases. 2005;40(5):735–737. doi: 10.1086/427885. [DOI] [PubMed] [Google Scholar]
- (3).Robbins BL, Srinivas RV, Kim C, Bischofberger N, Fridland A. Anti-human immunodeficiency virus activity and cellular metabolism of a potential prodrug of the acyclic nucleoside phosphonate 9-R-(2-phosphonomethoxypropyl)adenine (PMPA), Bis(isopropyloxymethylcarbonyl)PMPA. Antimicrob Agents Chemother. 1998;42(3):612–617. doi: 10.1128/aac.42.3.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Ribaudo HJ, Haas DW, Tierney C, Kim RB, Wilkinson GR, Gulick RM, et al. Pharmacogenetics of plasma efavirenz exposure after treatment discontinuation: an Adult AIDS Clinical Trials Group Study. Clin Infect Dis. 2006;42(3):401–407. doi: 10.1086/499364. [DOI] [PubMed] [Google Scholar]
- (5).Taylor S, Boffito M, Khoo S, Smit E, Back D. Stopping antiretroviral therapy. AIDS. 2007;21(13):1673–1682. doi: 10.1097/QAD.0b013e3281c61394. [DOI] [PubMed] [Google Scholar]
- (6).Schweighardt B, Ortiz GM, Grant RM, Wellons M, Miralles GD, Kostrikis LG, et al. Emergence of drug-resistant HIV-1 variants in patients undergoing structured treatment interruptions. AIDS. 2002;16(17):2342–2344. doi: 10.1097/00002030-200211220-00018. [DOI] [PubMed] [Google Scholar]
- (7).Dargere S, Parienti JJ, Verdon R. Treatment resistance after sequential interruption of a non-nucleoside reverse transcriptase inhibitor-based regimen. AIDS. 2007;21(7):879–880. doi: 10.1097/QAD.0b013e3280b077b6. [DOI] [PubMed] [Google Scholar]
- (8).Oyugi JH, Byakika-Tusiime J, Ragland K, Laeyendecker O, Mugerwa R, Kityo C, et al. Treatment interruptions predict resistance in HIV-positive individuals purchasing fixed-dose combination antiretroviral therapy in Kampala, Uganda. AIDS. 2007;21(8):965–971. doi: 10.1097/QAD.0b013e32802e6bfa. [DOI] [PubMed] [Google Scholar]
- (9).U.S.Department of Health and Human Services Public Health Service Task Force Recommendations for Use of Antiretroviral Drugs in Pregnant HIV-Infected Women for Maternal Health and Interventions to Reduce Perinatal HIV Transmission in the United States. Clinical Guidelines Portal. 2007
- (10).Hawkins D, Blott M, Clayden P, de Ruiter A, Foster G, Gilling-Smith C, et al. Guidelines for the management of HIV infection in pregnant women and the prevention of mother-to-child transmission of HIV. HIV Medicine. 2005;6:107–148. doi: 10.1111/j.1468-1293.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- (11).Palmer S, Boltz V, Maldarelli F, Kearney M, Halvas EK, Rock D, et al. Selection and persistence of non-nucleoside reverse transcriptase inhibitor-resistant HIV-1 in patients starting and stopping non-nucleoside therapy. AIDS. 2006;20(5):701–710. doi: 10.1097/01.aids.0000216370.69066.7f. [DOI] [PubMed] [Google Scholar]
- (12).Johnson VA, Brun-Vezinet F, Clotet B, Gunthard HF, Kuritzkes DR, Pillay D, et al. Update of the Drug Resistance Mutations in HIV-1: 2007. Topics in HIV Medicine. 2007;15(4):119–125. [PubMed] [Google Scholar]
- (13).Daniel N, Schneider V, Pialoux G, Krivine A, Grabar S, Nguyen TH, et al. Emergence of HIV-1 mutated strains after interruption of highly active antiretroviral therapy in chronically infected patients. AIDS. 2003;17(14):2126–2129. doi: 10.1097/00002030-200309260-00017. [DOI] [PubMed] [Google Scholar]
- (14).Martinez-Picado J, Morales-Lopetegi K, Wrin T, Prado J, Frost S, Petropoulos CJ, et al. Selection of drug-resistant HIV-1 mutants in response to repeated structured treatment interruptions. AIDS. 2002;16:895–899. doi: 10.1097/00002030-200204120-00009. [DOI] [PubMed] [Google Scholar]
- (15).Metzner KJ, Bonhoeffer S, Fischer M, Karanicolas R, Allers K, Joos B, et al. Emergence of minor populations of human immunodeficiency virus type 1 carrying the M184V and L90M mutations in subjects undergoing structured treatment interruptions. Journal of Infectious Diseases. 2003;188(10):1433–1443. doi: 10.1086/379215. [DOI] [PubMed] [Google Scholar]
- (16).Ruiz L, Paredes R, Gomez G, Romeu J, Domingo P, Perez-Alvarez N, et al. Antiretroviral therapy interruption guided by CD4 cell counts and plasma HIV-1 RNA levels in chronically HIV-1-infected patients. AIDS. 2007;21(2):169–178. doi: 10.1097/QAD.0b013e328011033a. [DOI] [PubMed] [Google Scholar]
- (17).Danel C, Moh R, Chaix ML, Gabillard D, Kouadio B, Toni T, et al. A 2-Months-off/4-Months-on HAART Is Clinically Not Inferior to Continuous Therapy but Leads to Unacceptable Resistance Rates in African Adults with >350 CD4/mm3 at First Interruption: Final Results of the Trivacan Trial. 15th Conference on Retroviruses and Opportunistic Infections; 2008. Session 127:778. [Google Scholar]
- (18).Palmisano L, Giuliano M, Bucciardini R, Fragola V, Andreotti M, Galluzzo C, et al. Determinants of virologic and immunologic outcomes in chronically HIV-infected subjects undergoing repeated treatment interruptions: the Istituto Superiore di Sanita-Pulsed Antiretroviral Therapy (ISS-PART) study. J Acquir Immune Defic Syndr. 2007;46(1):39–47. [PubMed] [Google Scholar]
- (19).Darwich L, Bellido R, Blanco A, Ruiz L, Esteva A, Cabrera C, et al. Non-nucleoside reverse transcriptase inhibitor (NNRTI) plasma concentrations determine different NNRTI resistance pattern in virological responders during treatment interruptions. Antiviral therapy. 2007;12(S73) Abstract number 64. [Google Scholar]
- (20).Arnedo-Valero M, Garcia F, Gil C, Guila T, Fumero E, Castro P, et al. Risk of selecting de novo drug-resistance mutations during structured treatment interruptions in patients with chronic HIV infection. Clinical Infectious Diseases. 2005;41(6):883–890. doi: 10.1086/432881. [DOI] [PubMed] [Google Scholar]
- (21).Metzner KJ, Allers K, Rauch P, Harrer T. Rapid selection of drug-resistant HIV-1 during the first months of suppressive ART in treatment-naive patients. AIDS. 2007;21(6):703–711. doi: 10.1097/QAD.0b013e3280121ac6. [DOI] [PubMed] [Google Scholar]
- (22).Simen BB, Huppler Hullsiek K, Novak RM, MacArthur RD, Baxter JD, Huang C, et al. Prevalence of low abundant drug resistant variants by ultra-deep sequencing in chronically HIV-infected antiretroviral naive patients and the impact on virologic outcomes. Antiviral therapy. 2007;12(S149) Abstract number 134. [Google Scholar]