Abstract
Background
By inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase, statins not only reduce cholesterol biosynthesis but also decrease the formation of isoprenoids, which are important for mediating signaling through the Rho-associated coiled-coil containing protein kinase (ROCK) pathway. Increased ROCK activity has been implicated in endothelial dysfunction and vascular inflammation. We hypothesize that ezetimibe, which inhibits intestinal cholesterol absorption, may not exert similar cholesterol-independent or pleiotropic effects of statins and, when used with a lower dose of statin, have less effect on ROCK activity than a higher dose of statin.
Methods and Results
In a prospective, randomized, observer-blinded study, we treated 60 dyslipidemic subjects without cardiovascular disease with simvastatin 40 mg/d, simvastatin/ezetimibe 10/10 mg/d, or placebo tablets for 28 days (n=20 in each arm). We evaluated baseline demographics and lipid levels, ROCK activity, C-reactive protein, and flow-mediated dilation before and after treatment. Compared with the placebo group, both treatment regimens decreased low-density lipoprotein cholesterol by 38% and C-reactive protein by 38% to 40% after 28 days (P<0.01 for both compared with placebo). Although the low-density lipoprotein cholesterol and C-reactive protein reductions were comparable with either lipid-lowering regimen, only simvastatin 40 mg reduced ROCK activity and improved flow-mediated dilation (P<0.01 for both compared with baseline). Reduction in ROCK activity with simvastatin 40 mg remained significant even after controlling for changes in low-density lipoprotein cholesterol (P=0.01) and correlated with improvement in flow-mediated dilation (R2=−0.78, P<0.01). No correlation was found between changes in flow-mediated dilation and changes in low-density lipoprotein cholesterol or C-reactive protein.
Conclusion
These results indicate that high-dose statin monotherapy exerts greater effects on ROCK activity and endothelial function, but not on C-reactive protein, than low-dose statin plus ezetimibe. These findings provide additional evidence of statin benefits beyond cholesterol lowering.
Keywords: cholesterol, endothelium, inflammation, nitric oxide, vasodilation
Clinical trials with 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase inhibitors or statins have established these agents as the principal therapy for the primary and secondary prevention of atherosclerosis and cardiovascular disease.1–5 Although cholesterol lowering by statins is clearly linked to decreased atherosclerosis and a lower incidence of coronary heart disease in hypercholesterolemic subjects,1,5 several recent statin trials in subjects with low or normal cholesterol levels have suggested additional benefits beyond cholesterol lowering.3,4,6 Indeed, experimental studies have shown that high concentrations of statins enhance endothelial function,7 protect against stroke,8,9 and prevent the development of atherosclerosis,10 possibly by mechanisms involving inhibition of Rho-associated coiled-coil containing protein kinase (ROCK).11,12 These so-called pleiotropic effects of statins are thought to be responsible for the observed improvement in flow-mediated vasodilation,13,14 increased numbers of circulating endothelial progenitor cells,15 and perhaps the reduction in vascular inflammation.16
The difficulty in “proving” whether statin pleiotropy exists in humans is that statin therapy uniformly reduces cholesterol levels in humans. This often makes it difficult, if not impossible, to convincingly separate the cholesterol-lowering effects of statins from their pleiotropic effects. Currently, an inhibitor of intestinal cholesterol absorption, ezetimibe, is commercially available either alone or in conjunction with statins.17 Although ezetimibe alone reduces cholesterol by 15% to 20%, when used in conjunction with statins (ie, so-called dual therapy), it can enhance the cholesterol-lowering ability of the statins by an additional 20%.18 Because statin pleiotropy on endothelial function and inflammation appears to be dose related,13,19 we tested the effects of high-dose statin monotherapy with equivalent cholesterol-lowering efficacy of the same statin at a lower dose plus ezetimibe on ROCK activity, endothelial function, and inflammation. Here, we provide evidence that improvement in endothelial function is a cholesterol-independent or pleiotropic effect of statins that is mediated by inhibition of ROCK.
Methods
Study Design
A randomized, placebo-controlled, prospective trial with 3 parallel treatment arms was conducted at the National Cheng Kung University Hospital in Tainan, Taiwan. We prospectively screened 68 subjects with dyslipidemia but without documented cardiovascular disease. Subjects were excluded if they had established diabetes (3 subjects) or abnormal liver functions (3 subjects) or were unwilling to offer consent for being in the study (2 subjects) (see inclusion and exclusion criteria below). A central pharmacist at the National Cheng Kung University Hospital randomized the remaining 60 subjects to simvastatin 40 mg/d (n=20), simvastatin/ezetimibe 10/10 mg/d (n=20), or placebo tablets (n=20) for 28 days. Subjects who were already taking statins underwent a 2-week washout period before randomization. Clinicians, data collectors, outcomes assessors, and statisticians were blinded to treatment group. All subjects were evaluated 3 times during the study: a short initial screening visit, a visit for baseline data collection before randomization, and a final visit after 28 days of treatment. Patients were instructed to fast overnight for a minimum of 8 hours before the second and third visits. Patients also were advised to continue their current medications and lifestyle for the duration of the study. Three subjects declined to continue the study for nonmedical reasons after the initial screening visit. Four subjects did not return for the 28-day final visit. Therefore, the analysis was performed on the 53 patients who completed the entire study.
Outcomes Measurements
Primary Outcomes
The primary outcomes were the mean changes in leukocyte ROCK activity from baseline values in response to simvastatin 40 mg, simvastatin/ezetimibe 10/10 mg, or placebo at 28 days.
Secondary Outcomes
The secondary outcomes were the correlation between the mean changes in leukocyte ROCK activity with the mean changes in low-density lipoprotein cholesterol (LDL-C), high-sensitivity C-reactive protein (hsCRP), and flow-mediated dilation (FMD), as well as with any clinical characteristics.
Subjects
Participants were recruited from the ambulatory clinics at the National Cheng Kung University Hospital clinics in Tainan, Taiwan. Participating physicians referred patients for the initial screening visit in which the outline of the study was explained and the subject’s informed consent was obtained. Inclusion criteria included male and female subjects between 40 and 80 years of age with LDL-C >130 mg/dL and <2 traditional cardiovascular risk factors, which included hypertension, smoking, male >45 years of age or female >55 years of age, and family history of premature coronary artery disease. Subjects with diabetes were excluded from the study because of ethical considerations of withholding statins from this patient population. Other exclusion criteria included premenopausal women; current use of antibiotic, antiinflammatory, or immunosuppressive agents; hepatic dysfunction as determined by liver function >2 times the upper limits of normal; evidence of active inflammatory or neoplastic disease; and a history of coronary artery bypass surgery, percutaneous coronary interventions, or acute coronary syndrome within the past 3 months. No restriction existed with regard to socioeconomic status.
Treatment and Measurements
Subjects were randomized into 3 treatment groups receiving simvastatin 40 mg/d (n=20), simvastatin/ezetimibe 10/10 mg/d (n=20), or placebo (n=20). The drugs were purchased from Merck, Sharp & Dohme (Whitehouse Station, NJ) and Schering-Plough (Kenilworth, NJ). On the initial screening visit, 5 mL blood was taken from each subject to perform laboratory tests, including aspartate aminotransferase, alanine aminotransferase, and creatine phosphokinase. Three subjects in each group (15%) received statin therapy before the study, and all of these subjects underwent a 2-week washout period before the second visit to obtain baseline measurements. After randomization, all subjects received a bottle labeled with a number and instructions to take 1 pill each night. The second and third visits consisted of a blood draw of 20 mL (15 mL to isolate leukocytes for ROCK expression and activity and 5 mL to measure LDL-C and hsCRP levels) and measurement of FMD.20,21 During each visit, all subjects were questioned about compliance with their medications and whether they experienced any adverse effects such as muscle pain or weakness.
Laboratory Section
Leukocyte Rho-Kinase Assay
Leukocytes were isolated from 15 mL peripheral blood during the second and third visits following a validated and standardized protocol.22 The leukocytes were frozen and stored at −80°C until all samples were collected. The ROCK assays were performed on all leukocytes samples at the same time. The samples were analyzed by Western blotting for the phosphorylation of the myosin-binding subunit (MBS) of myosin light-chain phosphatase with an antibody that specifically recognizes phosphorylated Ser853 MBS.22 Interexperimental results were standardized to lysophosphatidic acid–induced MBS phosphorylation (positive control).
Measurement of FMD
After each participant had taken 10 minutes of bed rest in a quiet, temperature-controlled room, FMD was measured in response to reactive hyperemia in the left brachial artery. A high-resolution ultrasound machine (Sonos 2500, Hewlett-Packard Co, Andover, Mass) equipped with a 7.5-MHz linear-array probe was used for the study. Arterial diameter was measured at baseline and during reactive hyperemia. Reactive hyperemia was induced by inflation of a pneumatic cuff on the forearm to a pressure >250 mm Hg for 4.5 minutes. The brachial artery was scanned in longitudinal sections 2 to 5 cm above the elbow. The arterial diameter was measured at the end-diastolic phase from 1 media-adventitia interface to the other at the clearest section 6 times at baseline. Measurements were performed during ECG-gated diastolic phase to avoid vessel compliance interference, especially during the systolic phase. The average of 6 measurements was taken as the baseline value. Arterial diameter was then measured again 3 times every 30 seconds after reactive hyperemia for 1.5 minutes. The average of the 3 consecutive maximal diameters was considered the value after hyperemia. FMD was calculated as the percentage change in diameter compared with baseline. Percent of brachial artery changes after nitroglycerin-mediated dilatation was expressed as the percent increase in the diameter 3 minutes after administration of nitroglycerin (0.3 mg). Two investigators performed the measurements independently. The intraobserver and interobserver variations were 0.9% and 1.4%, respectively.
LDL-C and hsCRP
In a separate 5-mL sample of blood collected in orange tiger–topped tubes, the lipid panel and hsCRP were measured at the National Cheng Kung University Hospital Clinical Laboratory. Total cholesterol, high-density lipoprotein cholesterol, and triglycerides were measured by an autoanalyzer. LDL-C was calculated as described.23 If any patient had a triglyceride level >300 mg/dL, LDL-C was then measured directly with ultracentrifugation.
Sample Size and Power
The sample size calculation was based on our primary hypothesis that the mean changes in human ROCK activity in leukocytes correlate with the mean changes in the LDL-C from baseline to treatment. The sample size calculation was based on the differences in the mean between 2 groups with equal sample size, prespecified 5% type I error, and 90% power (Z1−β = 1.28). We performed a sample size calculation using Power Analysis Statistical Software (PASS 2000, license 18335841). A sample size of 20 subjects in the simvastatin 40 mg/d or simvastatin/ezetimibe 10/10 mg/d could achieve 90% power to detect a difference of 40% in leukocyte ROCK activity between the null hypothesis that both group differences in mean are 0.00 and the alternative hypothesis that the difference in mean between the 2 groups is 40% ROCK activity using a 2-sided test and a significance level of 0.01.24
Statistical Analysis
We analyzed this database using SPSS 13.0 version (SPSS Inc, Chicago, Ill). We performed an intention-to-treat analysis of all the patients randomized in our study. Values are expressed as mean±SD if they are normally distributed or as median with interquartile range if not normally distributed. The paired t test was used to access the difference in measured biochemical parameters before and after treatment course within each group. Two-way ANOVA, followed by Fisher protected least-significant-difference test, was used to compare mean values of continuous variables between 3 groups with post hoc analysis. For nonparametric analysis, we used the Mann-Whitney U test with post hoc analysis to evaluate the difference between 3 groups. Spearman rank correlation test was used to access the relation between the mean change value of ROCK activity, hsCRP, and measured FMD. Values of P<0.05 were considered significantly different.
The authors had full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
Results
Baseline Characteristics
Subjects in all treatment arms were matched for age, gender, race, cardiac risk factors, and medications (Table 1). Similar numbers of subjects were on statin treatment before study enrollment (n = 3 [15%] in each group) and underwent a 2-week washout period before randomization.
Table 1.
Baseline Characteristics
| Characteristic | Placebo (n=20) | Simvastatin 40 mg (n=20) | Simvastatin/Ezetimibe 10/10 mg (n=20) |
|---|---|---|---|
| Age, y | 64.3±10 | 67.1±8.2 | 65.9±7.2 |
| Male, n (%) | 16 (80) | 16 (80) | 15 (75) |
| Hypertension, n (%) | 15 (75) | 18 (90) | 17 (85) |
| Diabetes mellitus, n (%) | 0 (0) | 0 (0) | 0 (0) |
| Smoker, n (%) | 2 (10) | 4 (20) | 3 (15) |
| Aspirin, n (%) | 17 (85) | 16 (80) | 16 (80) |
| β-Blocker, n (%) | 9 (45) | 9 (45) | 10 (50) |
| Prior statin use, n (%) | 3 (15) | 3 (15) | 3 (15) |
| Cholesterol, mg/dL | 222.1±18.5 | 220.0±22.2 | 228.4±23.1 |
| Triglycerides, mg/dL | 150.4±24.7 | 168.4±27.5 | 155.5±24.8 |
| LDL-C, mg/dL | 144.7±20.3 | 143.3±29.7 | 142.7±32.5 |
| HDL-C, mg/dL | 52.5±4.9 | 51.3±5.3 | 50.7±6.2 |
| hsCRP, mg/L | 2.58 (1.68–3.66) | 2.57 (1.49–3.67) | 2.54 (1.50–3.70) |
| Rho kinase activity, % | 163±23 | 162±20 | 158±18 |
HDL-C indicates high-density lipoprotein cholesterol. No differences were observed among the 3 treatment groups (all P>0.05). Data are presented as mean±SD or as median (interquartile range) unless otherwise indicated.
Effect of Lipid-Lowering Therapies on Lipid Levels
Subjects in all 3 groups had similar lipid profiles at randomization (Table 1). No changes were observed in any lipid parameter over the 28 days of the study in subjects randomized to placebo (Table 2). Compared with placebo, both the simvastatin 40 mg/d and simvastatin/ezetimibe 10/10 mg/d arms produced similar reductions in total cholesterol and LDL-C (P<0.01 for both versus placebo; P>0.05 versus each other). Furthermore, simvastatin 40 mg/d and simvastatin/ ezetimibe 10/10 mg/d reduced triglyceride levels to a similar extent relative to placebo (P=0.02). Neither simvastatin 40 mg/d nor simvastatin/ezetimibe 10/10 mg/d altered high-density lipoprotein cholesterol levels compared with placebo (P>0.05).
Table 2.
Effect of Treatment on Lipid Levels
| Day 0 | Day 28 | Change, % | P* | |
|---|---|---|---|---|
| Cholesterol, mg/dL | ||||
| Placebo | 222.1±18.5 | 230.2±20.1 | 3.6 | 0.88 |
| Simvastatin 40 mg | 220.0±22.2 | 158.8±17.2† | −27.8† | <0.001 |
| Simvastatin/ezetimibe 10/10 Mg | 228.4±23.1 | 170.1±18.7† | −25.5† | <0.001 |
| Triglycerides, mg/dL | ||||
| Placebo | 150.4±24.7 | 147.3±24.7 | −2.1 | 0.23 |
| Simvastatin 40 mg | 158.4±27.5 | 131.3±18.7‡ | −22.0‡ | 0.02 |
| Simvastatin/ezetimibe 10/10 mg | 155.5±24.8 | 121.8±17.3‡ | −21.7‡ | 0.02 |
| LDL-C, mg/dL | ||||
| Placebo | 144.7±20.3 | 147.9±21.5 | 2.2 | 0.34 |
| Simvastatin 40 mg | 143.3±29.7 | 88.1±14.3† | −38.5† | <0.001 |
| Simvastatin/ezetimibe 10/10 mg | 142.7±32.5 | 93.1±18.7† | −34.8† | <0.001 |
| HDL-C, mg/dL | ||||
| Placebo | 52.5±4.9 | 51.7±5.3 | −1.5 | 0.71 |
| Simvastatin 40 mg | 51.3±5.3 | 51.0±5.1 | −0.6 | 0.65 |
| Simvastatin/ezetimibe 10/10 mg | 50.7±6.2 | 52.8±6.2 | 4.1 | 0.62 |
HDL-C indicates high-density lipoprotein cholesterol. Values are expressed as mean±SD unless otherwise indicated.
Comparison of measured values before and after treatment within individual group.
P<0.01,
P≤0.05, difference between the simvastatin 40 mg and simvastatin/ezetimibe 10/10 mg group vs placebo group, respectively.
Effect of Lipid-Lowering Therapies on hsCRP
Baseline hsCRP did not differ between the 3 treatment groups (Table 1). No change was noted in hsCRP level over time in subjects randomized to placebo (P>0.05 compared with baseline) (Table 3). Both simvastatin 40 mg/d and simvastatin/ezetimibe 10/10 mg/d decreased hsCRP compared with placebo (P<0.01 for both compared with placebo).
Table 3.
ROCK Activity, Endothelial Function, and Inflammation Before and After Treatment
| Day 0 | Day 28 | % change | P value† | |
|---|---|---|---|---|
| ROCK activity, % | ||||
| Placebo | 163±23 | 165±23 | 1.2 | 0.88 |
| Simvastatin 40 mg | 162±20 | 97±14* | −40.1* | <0.001 |
| Simvastatin/Ezetimibe 10/10 mg | 158±18 | 136±23 | −13.9 | 0.08 |
| FMD, % | ||||
| Placebo | 6.42±2.2 | 6.52±3.4 | 1.6 | 0.79 |
| Simvastatin 40 mg | 6.39±2.3 | 8.44±2.6* | 32.1* | <0.001 |
| Simvastatin/Ezetimibe 10/10 mg | 7.85±2.6 | 7.81±3.6 | −0.5 | 0.15 |
| NMD, % | ||||
| Placebo | 10.62±6.3 | 10.45±7.5 | −1.6 | 0.54 |
| Simvastatin 40 mg | 10.38±5.8 | 11.18±5.6 | 7.7 | 0.21 |
| Simvastatin/Ezetimibe 10/10 mg | 11.15±4.6 | 10.96±5.8 | −1.7 | 0.36 |
| hsCRP, mg/L | ||||
| Placebo | 2.58 (1.68–3.66) | 2.61 (1.66–3.69) | 0.8 | 0.71 |
| Simvastatin 40 mg | 2.57 (1.49–3.67) | 1.52 (1.05–3.0)* | −39.9* | <0.001 |
| Simvastatin/Ezetimibe 10/10 mg | 2.54 (1.50–3.70) | 1.62 (1.03–3.4)* | −34.0* | <0.001 |
Values are expressed as mean±SD or median with inter-quartile range. NMD indicates nitroglycerin-mediated dilation. Other abbreviations please see Table 1.
P< 0.01. Indicates difference between groups (either Simvastatin 40 mg or Simvastatin/Ezetimibe 10/10 mg) compared with placebo group, respectively.
Indicates comparison of measured values between before and after treatment course within individual group.
Effect of Lipid-Lowering Therapies on ROCK Activity
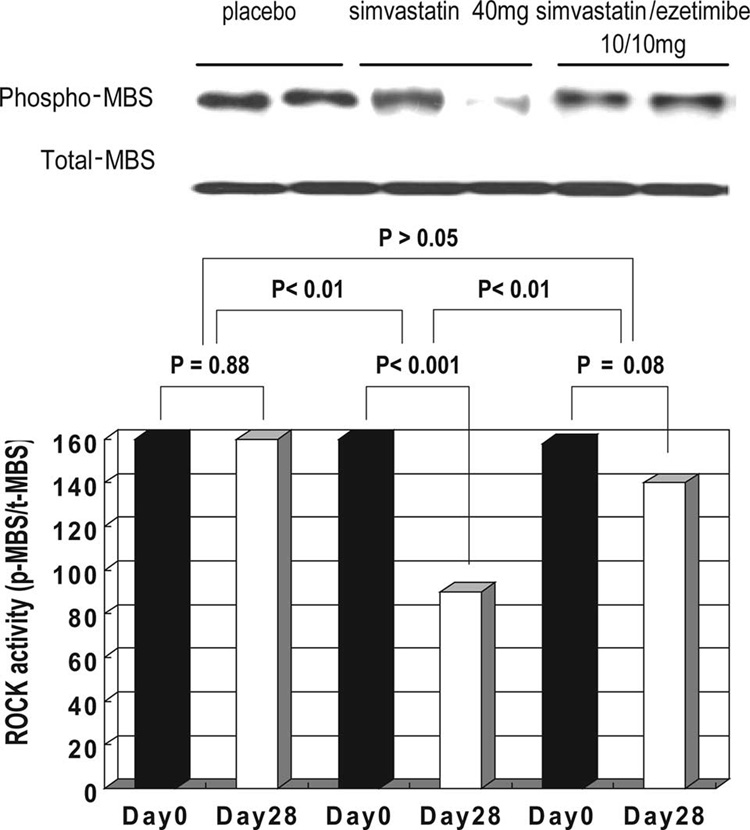
Baseline ROCK activity did not differ between the 3 treatment groups (Table 1). No change was found in ROCK activity over time in subjects randomized to placebo (P>0.05 versus baseline; Table 3) or to simvastatin/ezetimibe 10/10 mg/d (P>0.05 versus baseline; Figure 1). However, simvastatin 40 mg/d decreased ROCK activity before and after treatment (P<0.001), and this reduction in ROCK activity was significant compared with placebo (P<0.01; Figure 1)
Figure 1.
Effects of simvastatin (40 mg/d) and simvastatin/ezetimibe (10/10 mg/d) on leukocyte ROCK activity. Leukocyte ROCK activity was measured as percent staining of phosphorylated (p- and phospho-) MBS of myosin light-chain phosphatase (pThr853-MBS) relative to the staining of total (t) MBS.
Effect of Lipid-Lowering Therapies on Endothelial Function
Both baseline FMD and nitroglycerin-mediated dilatation were not different between the 3 treatment groups (Table 1). No change was found in FMD and nitroglycerin-mediated dilatation before and after treatment in subjects randomized to placebo or to simvastatin/ezetimibe 10/10 mg/d (P>0.05 versus baseline or placebo; Table 3). Compared with placebo, simvastatin 40 mg/d increased FMD (P<0.01) but not nitroglycerin-mediated dilatation (P>0.05) (Table 3).
Relationship Between Changes in LDL-C Levels and ROCK Activity
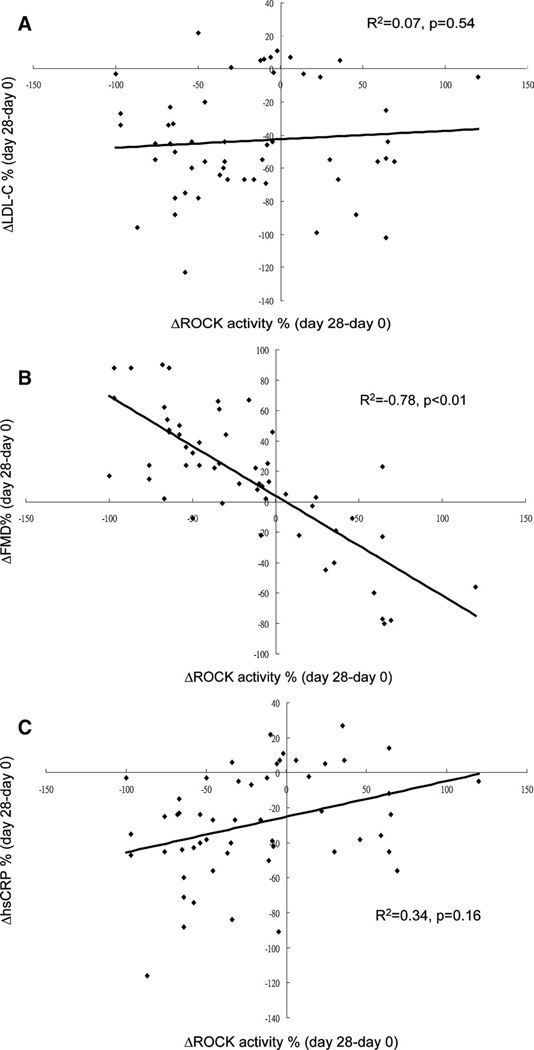
We evaluated the relationship between changes in LDL levels and ROCK inhibition. The effect of treatment on ROCK activity remained significant even after controlling for changes in LDL in subjects receiving simvastatin 40 mg/d (P<0.01) but not in subjects receiving simvastatin/ezetimibe 10/10 mg/d (P>0.05). Furthermore, no correlation was found between changes in LDL-C and changes in ROCK activity (P>0.05; Table 2).
Association Between FMD, LDL-C Levels, and ROCK Activity
We further analyzed whether any correlation existed between changes in FMD, LDL-C levels, and ROCK activity in the 2 lipid-lowering treatment arms. No correlation was found between changes in ROCK activity and changes in LDL-C or hsCRP in both lipid-lowering treatment groups (Figure 2A and 2C). However, a strong association was observed between changes in FMD in subjects receiving simvastatin 40 mg/d and simvastatin/ezetimibe 10/10 mg/d with changes in ROCK activity (Figure 2B).
Figure 2.
Correlation between changes in ROCK activity and changes in LDL-C (A) , FMD (B), and hsCRP (C). Scatterplots depict the correlation in the change from day 28 to baseline for both parameters.
Discussion
The results of this study demonstrate that a higher dose of simvastatin alone inhibits ROCK activity and increases FMD to a greater extent than the combination of a lower dose of simvastatin and ezetimibe despite comparable lipid-lowering effects. Indeed, only simvastatin (40 mg/d) achieved a significant improvement in endothelial function and inhibition of ROCK activity compared with simvastatin/ezetimibe (10/10 mg/d). The inhibition of ROCK activity was statistically significant even after controlling for changes in LDL-C, further supporting the hypothesis that inhibition of ROCK is a lipid-independent effect of statin therapy.
Statins have been shown to improve cardiovascular outcomes in numerous primary and secondary prevention trials.25 However, emerging evidence suggests that the benefits of statin therapy may extend beyond their lipid-lowering effects.26 These so-called “pleiotropic” effects of statin derive partly from clinical trials suggesting that the improvement in outcomes is related as much to the antiinflammatory actions of statins as to their LDL-C–lowering effects.16,26 Further evidence supporting the potential lipid-independent actions of statin therapy derives from studies that have compared the actions of statins with ezetimibe, an agent that reduces cholesterol absorption without inhibiting HMG-CoA reductase.13,14,27 In these studies, only statins, not ezetimibe, were shown to improve endothelial function and to reduce platelet reactivity and proinflammatory cytokines production despite an equivalent LDL-C reduction by both therapies.
Inhibition of the Rho/ROCK signal transduction pathway has been implicated as a potential mechanism underlying the pleiotropic benefits of statin therapy.28 By inhibiting mevalonate synthesis, statins prevent the formation of isoprenoid intermediates that are required for the intracellular trafficking and function of small GTPases such as Rho, Ras, and Rac.29 Increased Rho/ROCK activity has been implicated in atherogenesis,30 and deletion of ROCK1 in macrophages leads to decreased atherosclerosis in LDLr−/− mice.12 Furthermore, direct inhibition of Rho or its downstream effector, ROCK, augments endothelial nitric oxide synthesis,7,31 decreases vascular smooth muscle cell contraction and proliferation,32,33 decreases cytokine formation and leukocyte recruitment,34 and reduces thrombogenicity of the vessel wall.35,36
We found that simvastatin 40 mg inhibited leukocyte ROCK activity by ≈46% compared with placebo. Interestingly, short-term administration of the ROCK inhibitor fasudil to human subjects with atherosclerosis inhibited leukocyte ROCK activity by 56% compared with placebo; this correlated with an improvement in FMD without changes in lipid levels.37 Indeed, fasudil has been shown to reduce myocardial ischemia in 2 multicenter studies of patients with stable atherosclerosis.38,39 Thus, it is likely that some of the cardiovascular benefits of simvastatin may be due to its cholesterol-independent inhibitory effects on ROCK.
The cholesterol-dependent and -independent actions of statins are difficult to separate in humans, in part because statins uniformly reduce LDL-C in human subjects and because the mechanism for both actions is the same (ie, inhibition of HMG-CoA reductase). However, using ezetimibe, which lowers LDL-C by a different mechanism, one could begin to determine whether the pleiotropic effects of statins actually exist. Indeed, a recent study in whites with diabetes or coronary artery disease showed that simvastatin 80 mg and simvastatin/ezetimibe 10/10 mg produced comparable lipid-lowering effects and changes in FMD and CRP, suggesting that lipid lowering may be more important than the pleiotropic effects of statins.40 This finding is in contrast to the findings of our study, which showed that simvastatin 40 mg improved endothelial function but simvastatin/ezetimibe 10/10 mg did not, although both treatments produced comparable lowering of lipid levels and hsCRP. However, several important differences exist between our study and the study by Settergren et al.40 The patients in the study by Settergren et al were at higher risk for cardiovascular disease; therefore, no placebo group was available for comparison. Without an active placebo arm, it is possible that the reduction in LDL and improvement in FMD in both treatment groups could have occurred by chance (ie, an association rather than a correlation), which is likely given the small sample size in their study. Furthermore, it is possible that whites and Asians may respond differently to statins and ezetimibe in terms of lowering LDL, improving endothelial function, and reducing CRP. Nevertheless, other studies also showed that ezetimibe, either alone or in combination with statins, is less effective in improving endothelial function than statin monotherapy.13,14
The lack of additional benefits of ezetimibe beyond LDL-C and hsCRP lowering may explain in part the unexpected findings of the Effect of Ezetimibe Plus Simvastatin Versus Simvastatin Alone on Atherosclerosis in the Carotid Artery (ENHANCE) study in which ezetimibe, when added to a statin, did not alter the progression of carotid artery intima-media thickening despite a further reduction in LDL-C and inflammatory biomarkers such as hsCRP compared with statins alone in patients with familial hypercholesterolemia.41 Interestingly, ezetimibe alone does not lower hsCRP but, in conjunction with a statin, enhances the CRP-lowering capability of a given statin,42,43 suggesting that the CRP-lowering effect of ezetimibe may perhaps be a “cosmetic” effect that is not associated with any antiinflammatory actions. In this respect, perhaps CRP is not the best measure of vascular inflammation. Indeed, compared with statins, the LDL-lowering benefits of ezetimibe alone are not associated with an improvement in endothelial function.14
Conclusions
We found that a higher dose of simvastatin inhibits ROCK activity and improves endothelial function to a greater extent than the combination of a lower dose of simvastatin and ezetimibe despite comparable lipid-lowering efficacy. The effect of simvastatin on ROCK activity was statistically significant even after controlling for changes in LDL-C, supporting the concept that inhibition of ROCK may contribute to some of the lipid-independent or pleiotropic effects of statin therapy. It remains to be determined, however, whether these lipid-independent effects of statins contribute to the outcome benefits of statin therapy.
CLINICAL PERSPECTIVE
Although cholesterol lowering by 3-hydroxy-3-methylglutaryl coenzyme A reductase inhibitors or statins is clearly linked to decreased cardiovascular disease, several recent statin trials suggest additional benefits beyond cholesterol lowering, possibly by mechanisms involving inhibition of Rho-associated coiled-coil containing protein kinase (ROCK). These so-called pleiotropic effects of statins are thought to be responsible for the observed improvement in flow-mediated vasodilation, the increased numbers of circulating endothelial progenitor cells, and perhaps the reduction in vascular inflammation. We hypothesized that ezetimibe, which inhibits intestinal cholesterol absorption, may not exert cholesterol-independent or pleiotropic effects similar to those of statins and, when used with a lower dose of statin, may have less effect on ROCK activity than a higher dose of statin. In a prospective randomized study of 60 dyslipidemic subjects without cardiovascular disease treated with simvastatin 40 mg/d, simvastatin/ezetimibe 10/10 mg/d, or placebo tablets for 28 days (n=20 in each arm), we found similar low-density lipoprotein lowering and reduction of high-sensitivity C-reactive protein between the 2 treatment groups. However, only simvastatin 40 mg reduced ROCK activity and improved flow-mediated dilation. Reduction in ROCK activity with simvastatin 40 mg remained significant even after controlling for changes in low-density lipoprotein cholesterol and correlated with an improvement in flow-mediated dilation. No correlation was found between changes in flow-mediated dilation and changes in low-density lipoprotein cholesterol or C-reactive protein. These results indicate that high-dose statin monotherapy exerts greater effects on ROCK activity and endothelial function, but not CRP, than low-dose statin plus ezetimibe. These findings provide additional evidence of statin benefits beyond cholesterol lowering.
Acknowledgments
Sources of Funding
This work was supported in part by grants from the National Institutes of Health (HL052233 to Dr Liao) and National Health Research Institute of Taiwan (Dr Liu).
Footnotes
Clinical trial registration information—URL: http://www.clinicaltrial.gov. Unique identifier: NCT00560170.
Disclosures
Dr Liao is a consultant for Merck, Pfizer, AstraZeneca, Boehringer Ingelheim, and Asahi-Kasei Pharmaceuticals and receives research support from Pfizer, Boehringer Ingelheim, and Asahi-Kasei Pharmaceuticals. Dr Liao is also on the speakers’ bureau for Merck, Pfizer, AstraZeneca, and Boehringer Ingelheim.
References
- 1.Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–1389. [PubMed] [Google Scholar]
- 2.Prevention of cardiovascular events and death with pravastatin in patients with coronary heart disease and a broad range of initial cholesterol levels: the Long-Term Intervention With Pravastatin in Ischaemic Disease (LIPID) Study Group. N Engl J Med. 1998;339:1349–1357. doi: 10.1056/NEJM199811053391902. [DOI] [PubMed] [Google Scholar]
- 3.MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- 4.Sacks FM, Pfeffer MA, Moye LA, Rouleau JL, Rutherford JD, Cole TG, Brown L, Warnica JW, Arnold JM, Wun CC, Davis BR, Braunwald E. The effect of pravastatin on coronary events after myocardial infarction in patients with average cholesterol levels: Cholesterol and Recurrent Events Trial Investigators. N Engl J Med. 1996;335:1001–1009. doi: 10.1056/NEJM199610033351401. [DOI] [PubMed] [Google Scholar]
- 5.Shepherd J, Cobbe SM, Ford I, Isles CG, Lorimer AR, MacFarlane PW, McKillop JH, Packard CJ. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia: West of Scotland Coronary Prevention Study Group. N Engl J Med. 1995;333:1301–1307. doi: 10.1056/NEJM199511163332001. [DOI] [PubMed] [Google Scholar]
- 6.Cannon CP, Braunwald E, McCabe CH, Rader DJ, Rouleau JL, Belder R, Joyal SV, Hill KA, Pfeffer MA, Skene AM. Intensive versus moderate lipid lowering with statins after acute coronary syndromes. N Engl J Med. 2004;350:1495–1504. doi: 10.1056/NEJMoa040583. [DOI] [PubMed] [Google Scholar]
- 7.Laufs U, La Fata V, Plutzky J, Liao JK. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 8.Endres M, Laufs U, Huang Z, Nakamura T, Huang P, Moskowitz MA, Liao JK. Stroke protection by 3-hydroxy-3-methylglutaryl (HMG)-CoA reductase inhibitors mediated by endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 1998;95:8880–8885. doi: 10.1073/pnas.95.15.8880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Laufs U, Endres M, Stagliano N, Amin-Hanjani S, Chui DS, Yang SX, Simoncini T, Yamada M, Rabkin E, Allen PG, Huang PL, Bohm M, Schoen FJ, Moskowitz MA, Liao JK. Neuroprotection mediated by changes in the endothelial actin cytoskeleton. J Clin Invest. 2000;106:15–24. doi: 10.1172/JCI9639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kleemann R, Princen HM, Emeis JJ, Jukema JW, Fontijn RD, Horrevoets AJ, Kooistra T, Havekes LM. Rosuvastatin reduces atherosclerosis development beyond and independent of its plasma cholesterol-lowering effect in APOE*3-Leiden transgenic mice: evidence for antiinflammatory effects of rosuvastatin. Circulation. 2003;108:1368–1374. doi: 10.1161/01.CIR.0000086460.55494.AF. [DOI] [PubMed] [Google Scholar]
- 11.Noma K, Rikitake Y, Oyama N, Yan G, Alcaide P, Liu PY, Wang H, Ahl D, Sawada N, Okamoto R, Hiroi Y, Shimizu K, Luscinskas FW, Sun J, Liao JK. ROCK1 mediates leukocyte recruitment and neointima formation following vascular injury. J Clin Invest. 2008;118:1632–1644. doi: 10.1172/JCI29226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang HW, Liu PY, Oyama N, Rikitake Y, Kitamoto S, Gitlin J, Liao JK, Boisvert WA. Deficiency of ROCK1 in bone marrow-derived cells protects against atherosclerosis in LDLR−/− mice. FASEB J. 2008;22:3561–3570. doi: 10.1096/fj.08-108829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fichtlscherer S, Schmidt-Lucke C, Bojunga S, Rossig L, Heeschen C, Dimmeler S, Zeiher AM. Differential effects of short-term lipid lowering with ezetimibe and statins on endothelial function in patients with CAD: clinical evidence for “pleiotropic” functions of statin therapy. Eur Heart J. 2006;27:1182–1190. doi: 10.1093/eurheartj/ehi881. [DOI] [PubMed] [Google Scholar]
- 14.Landmesser U, Bahlmann F, Mueller M, Spiekermann S, Kirchhoff N, Schulz S, Manes C, Fischer D, de Groot K, Fliser D, Fauler G, Marz W, Drexler H. Simvastatin versus ezetimibe: pleiotropic and lipid-lowering effects on endothelial function in humans. Circulation. 2005;111:2356–2363. doi: 10.1161/01.CIR.0000164260.82417.3F. [DOI] [PubMed] [Google Scholar]
- 15.Dimmeler S, Aicher A, Vasa M, Mildner-Rihm C, Adler K, Tiemann M, Rutten H, Fichtlscherer S, Martin H, Zeiher AM. HMG-CoA reductase inhibitors (statins) increase endothelial progenitor cells via the PI 3-kinase/Akt pathway. J Clin Invest. 2001;108:391–397. doi: 10.1172/JCI13152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ridker PM, Cannon CP, Morrow D, Rifai N, Rose LM, McCabe CH, Pfeffer MA, Braunwald E. C-reactive protein levels and outcomes after statin therapy. N Engl J Med. 2005;352:20–28. doi: 10.1056/NEJMoa042378. [DOI] [PubMed] [Google Scholar]
- 17.Sudhop T, Lutjohann D, Kodal A, Igel M, Tribble DL, Shah S, Perevozskaya I, von Bergmann K. Inhibition of intestinal cholesterol absorption by ezetimibe in humans. Circulation. 2002;106:1943–1948. doi: 10.1161/01.cir.0000034044.95911.dc. [DOI] [PubMed] [Google Scholar]
- 18.Gagne C, Gaudet D, Bruckert E. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation. 2002;105:2469–2475. doi: 10.1161/01.cir.0000018744.58460.62. [DOI] [PubMed] [Google Scholar]
- 19.Betteridge DJ, Gibson JM, Sager PT. Comparison of effectiveness of rosuvastatin versus atorvastatin on the achievement of combined C-reactive protein (<2 mg/L) and low-density lipoprotein cholesterol (<70 mg/dl) targets in patients with type 2 diabetes mellitus (from the ANDROMEDA study) Am J Cardiol. 2007;100:1245–1248. doi: 10.1016/j.amjcard.2007.05.044. [DOI] [PubMed] [Google Scholar]
- 20.Corretti MC, Plotnick GD, Vogel RA. Technical aspects of evaluating brachial artery vasodilatation using high-frequency ultrasound. Am J Physiol. 1995;268:H1397–H1404. doi: 10.1152/ajpheart.1995.268.4.H1397. [DOI] [PubMed] [Google Scholar]
- 21.Vogel RA. Measurement of endothelial function by brachial artery flow-mediated vasodilation. Am J Cardiol. 2001;88:31E–34E. doi: 10.1016/s0002-9149(01)01764-7. [DOI] [PubMed] [Google Scholar]
- 22.Liu PY, Liao JK. A method for measuring Rho kinase activity in tissues and cells. Methods Enzymol. 2008;439:181–189. doi: 10.1016/S0076-6879(07)00414-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 24.Liu PY, Chen JH, Lin LJ, Liao JK. Increased Rho kinase activity in a Taiwanese population with metabolic syndrome. J Am Coll Cardiol. 2007;49:1619–1624. doi: 10.1016/j.jacc.2006.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang CY, Liu PY, Liao JK. Pleiotropic effects of statin therapy:molecular mechanisms and clinical results. Trends Mol Med. 2008;14:37–44. doi: 10.1016/j.molmed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liao JK. Clinical implications for statin pleiotropy. Curr Opin Lipidol. 2005;16:624–629. doi: 10.1097/01.mol.0000191913.16321.60. [DOI] [PubMed] [Google Scholar]
- 27.Piorkowski M, Fischer S, Stellbaum C, Jaster M, Martus P, Morguet AJ, Schultheiss HP, Rauch U. Treatment with ezetimibe plus low-dose atorvastatin compared with higher-dose atorvastatin alone: is sufficient cholesterol-lowering enough to inhibit platelets? Am Coll Cardiol. 2007;49:1035–1042. doi: 10.1016/j.jacc.2006.10.064. [DOI] [PubMed] [Google Scholar]
- 28.Rikitake Y, Liao JK. ROCKs as therapeutic targets in cardiovascular diseases. Expert Rev Cardiovasc Ther. 2005;3:441–451. doi: 10.1586/14779072.3.3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao JK. Isoprenoids as mediators of the biological effects of statins. J Clin Invest. 2002;110:285–288. doi: 10.1172/JCI16421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mallat Z, Gojova A, Sauzeau V, Brun V, Silvestre JS, Esposito B, Merval R, Groux H, Loirand G, Tedgui A. Rho-associated protein kinase contributes to early atherosclerotic lesion formation in mice. Circ Res. 2003;93:884–888. doi: 10.1161/01.RES.0000099062.55042.9A. [DOI] [PubMed] [Google Scholar]
- 31.Takemoto M, Sun J, Hiroki J, Shimokawa H, Liao JK. Rho-kinase mediates hypoxia-induced downregulation of endothelial nitric oxide synthase. Circulation. 2002;106:57–62. doi: 10.1161/01.cir.0000020682.73694.ab. [DOI] [PubMed] [Google Scholar]
- 32.Sawada N, Itoh H, Ueyama K, Yamashita J, Doi K, Chun TH, Inoue M, Masatsugu K, Saito T, Fukunaga Y, Sakaguchi S, Arai H, Ohno N, Komeda M, Nakao K. Inhibition of rho-associated kinase results in suppression of neointimal formation of balloon-injured arteries. Circulation. 2000;101:2030–2033. doi: 10.1161/01.cir.101.17.2030. [DOI] [PubMed] [Google Scholar]
- 33.Seasholtz TM, Majumdar M, Kaplan DD, Brown JH. Rho and Rho kinase mediate thrombin-stimulated vascular smooth muscle cell DNA synthesis and migration. Circ Res. 1999;84:1186–1193. doi: 10.1161/01.res.84.10.1186. [DOI] [PubMed] [Google Scholar]
- 34.Wojciak-Stothard B, Williams L, Ridley AJ. Monocyte adhesion and spreading on human endothelial cells is dependent on Rho-regulated receptor clustering. J Cell Biol. 1999;145:1293–1307. doi: 10.1083/jcb.145.6.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bourcier T, Libby P. HMG CoA reductase inhibitors reduce plasminogen activator inhibitor-1 expression by human vascular smooth muscle and endothelial cells. Arterioscler Thromb Vasc Biol. 2000;20:556–562. doi: 10.1161/01.atv.20.2.556. [DOI] [PubMed] [Google Scholar]
- 36.Essig M, Nguyen G, Prie D, Escoubet B, Sraer JD, Friedlander G. 3-Hydroxy-3-methylglutaryl coenzyme A reductase inhibitors increase fibrinolytic activity in rat aortic endothelial cells: role of geranylgeranylation and Rho proteins. Circ Res. 1998;83:683–690. doi: 10.1161/01.res.83.7.683. [DOI] [PubMed] [Google Scholar]
- 37.Nohria A, Grunert ME, Rikitake Y, Noma K, Prsic A, Ganz P, Liao JK, Creager MA. Rho kinase inhibition improves endothelial function in human subjects with coronary artery disease. Circ Res. 2006;99:1426–1432. doi: 10.1161/01.RES.0000251668.39526.c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimokawa H, Hiramori K, Iinuma H, Hosoda S, Kishida H, Osada H, Katagiri T, Yamauchi K, Yui Y, Minamino T, Nakashima M, Kato K. Anti-anginal effect of fasudil, a Rho-kinase inhibitor, in patients with stable effort angina: a multicenter study. J Cardiovasc Pharmacol. 2002;40:751–761. doi: 10.1097/00005344-200211000-00013. [DOI] [PubMed] [Google Scholar]
- 39.Vicari RM, Chaitman B, Keefe D, Smith WB, Chrysant SG, Tonkon MJ, Bittar N, Weiss RJ, Morales-Ballejo H, Thadani U. Efficacy and safety of fasudil in patients with stable angina: a double-blind, placebo-controlled, phase 2 trial. J Am Coll Cardiol. 2005;46:1803–1811. doi: 10.1016/j.jacc.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 40.Settergren M, Bohm F, Ryden L, Pernow J. Cholesterol lowering is more important than pleiotropic effects of statins for endothelial function in patients with dysglycaemia and coronary artery disease. Eur Heart J. 2008;29:1753–1760. doi: 10.1093/eurheartj/ehn166. [DOI] [PubMed] [Google Scholar]
- 41.for the ENHANCE Investigators. Kastelein JJP, Akdim F, Stroes ESG, Zwinderman AH, Bots ML, Stalenhoef AFH, Visseren FLJ, Sijbrands EJG, Trip MD, Stein EA, Gaudet D, Duivenvoorden R, Veltri EP, Marais AD, de Groot E. Simvastatin with or without ezetimibe in familial hypercholesterolemia. N Engl J Med. 2008;358:1431–1443. doi: 10.1056/NEJMoa0800742. [DOI] [PubMed] [Google Scholar]
- 42.Ballantyne CM, Houri J, Notarbartolo A, Melani L, Lipka LJ, Suresh R, Sun S, LeBeaut AP, Sager PT, Veltri EP. Effect of ezetimibe coadministered with atorvastatin in 628 patients with primary hypercholesterolemia: a prospective, randomized, double-blind trial. Circulation. 2003;107:2409–2415. doi: 10.1161/01.CIR.0000068312.21969.C8. [DOI] [PubMed] [Google Scholar]
- 43.Sager PT, Melani L, Lipka L, Strony J, Yang B, Suresh R, Veltri E. Effect of coadministration of ezetimibe and simvastatin on high-sensitivity C-reactive protein. Am J Cardiol. 2003;92:1414–1418. doi: 10.1016/j.amjcard.2003.08.048. [DOI] [PubMed] [Google Scholar]