Abstract
Objectives
i) to assess the diagnostic specificity of MRI-defined hippocampal atrophy for Alzheimer's disease (AD) among individuals with a variety of pathologically confirmed conditions associated with dementia as well as changes attributable to typical aging, and, ii) to measure correlations among pre-mortem MRI measurements of hippocampal atrophy, mental status exam performance, and the pathologic stage of AD.
Methods
An un-selected series of 67 individuals participating in the Mayo Alzheimer's Disease Research Center/Alzheimer's Disease Patient Registry were identified who had undergone a standardized antemortem MRI study and also post-mortem examination. Hippocampal volumes were measured from antemortem MRI. Each post-mortem specimen was assigned a pathologic diagnosis, and in addition, the severity of AD pathology was staged using the method of Braak and Braak.
Results
Individuals with an isolated pathologic diagnosis of AD, hippocampal sclerosis, frontotemporal degeneration, and neurofibrillary tangle-only degeneration usually had substantial hippocampal atrophy while those with changes of typical aging did not. Among all 67 subjects, correlations (all p<0.001) were observed between hippocampal volume and Braak stage (r = −0.39), between hippocampal volume and MMSE score (r = 0.60), and between MMSE score and Braak stage (r = −0.41).
Conclusions
Hippocampal atrophy, while not specific for AD, was a fairly sensitive marker of the pathologic AD stage [particularly among subjects with isolated AD pathology (r = −0.63, p = 0.001)] and consequent cognitive status.
INTRODUCTION
The topographic distribution of Alzheimer type pathology, particularly neurofibrillary degeneration, is thought to progress in a stereotypical fashion. It first appears in medial temporal limbic areas, later extends to neocortical association areas, and involves primary neocortex only in the end-stage. Based on this, Braak and Braak1 have formulated a method for staging Alzheimer's disease (AD) pathology, and this topographic staging system has been incorporated into recent consensus recommendations for the post-mortem diagnosis of AD from the National Institute on Aging and the Reagan Institute working group2.
The fact that pathologic changes of AD begin in the medial temporal lobe has led investigators to employ imaging methods focused on this area of the brain for early diagnosis and characterization of AD. A widely accepted method has been magnetic resonance imaging (MRI)- based volume measurements of the hippocampus3–8. The hippocampus is one of the earliest medial temporal limbic structures involved in AD and its boundaries are precisely and reliably demarcated in all three orthogonal anatomic planes. This makes precise volume measurements of the hippocampal atrophy associated with AD possible. MRI measurements of hippocampal atrophy correlate with both functional measures of AD severity as well as performance on formal neuropsychological testing instruments, particularly those focused on memory9, 10. Both the clinical manifestations of AD as well as MRI measurements of hippocampal atrophy are presumed to depend on the underlying pathologic stage of the disease. There has been little autopsy verification, however, of the presumed relationships among pathologic stage, imaging assessment of pathologic stage, and clinical symptom severity. In addition, most studies of sensitivity and specificity for diagnostic imaging have relied on the clinical diagnoses as the gold standard against which imaging measurements are compared. Little documentation exists addressing the diagnostic specificity of imaging methods for AD compared to other dementing conditions using pathologic criteria as the gold standard.
We studied an un-selected series of individuals with two specific aims: i) To assess the diagnostic specificity of MRI-defined hippocampal atrophy for pathologically confirmed AD, and ii) To measure correlations among antemortem MRI measurements of hippocampal atrophy, mental status exam performance, and the pathologic stage of AD.
METHODS
Recruitment and Characterization of Subjects
Subjects studied were participants in the Mayo Rochester Alzheimer's Disease Research Center (ADRC) and Mayo Alzheimer's Disease Patient Registry (ADPR) 11. These are ongoing longitudinal studies of aging and dementia that provide a formal mechanism for identification, enrollment, and longitudinal clinical characterization of both community and referral subjects who are cognitively intact and subjects who are impaired. Both the ADRC and ADPR protocols include MRI studies, and autopsy permission is sought as well. Studies were performed with Mayo IRB approval and informed consent of the subject or an appropriate surrogate.
The 67 subjects studied represent an un-selected series of individuals participating in the ADRC/ADPR who had both undergone an antemortem MRI examination and also had an autopsy. Assessment of mental status exam performance was assessed with the Mini-Mental State Exam (MMSE). All participants were categorized by clinical diagnosis during consensus committee meetings consisting of a geriatrician, neurologists, neuropsychologists, psychometrists, and research nurses who had seen the patient. Although clinical diagnoses of specific dementing disorders were assigned, for purposes of this study we simply categorized each subject's antemortem clinical state as cognitively normal, demented, or mild cognitive impairment (MCI) 12, 13.
Patients with a suspected cognitive impairment were identified during general medical examinations by Mayo primary care physicians. A neurologist then performed a detailed neurologic examination and obtained a complete history from the patient and a collateral source. Neuropsychological tests were administered to assess memory, attention, language, visuospatial skills and problem solving. Patients were not excluded for the presence of ongoing medical problems such as diabetes, hypertension, or heart disease. The diagnosis of dementia was made according to the DSM –III-R criteria 14 at the consensus conference.
The diagnosis of MCI was a clinical judgment based on the following criteria: 1) memory complaint documented by the patient and collateral source; 2) normal general cognition as determined by measures of general intellectual function and mental status screening instruments; 3) normal activities of daily living; 4) not demented (DSM-III-R) 14; 5) memory impairment15; 6) Clinical Dementia Rating score of 0.512, 13, 16.
Cognitively normal subjects were recruited from the same pool of patients coming to Mayo primary care physicians for a general medical examination. Normal subjects were evaluated in the same way as cognitively impaired patients. Their status was reviewed at the consensus conference. Subjects categorized as cognitively normal had no active neurological or psychiatric disorders. Like the patients, some had ongoing medical problems, however the illnesses or their treatments did not interfere with cognitive function.
MRI Methods
All subjects were imaged at 1.5T (Signa, General Electric Medical Systems, Milwaukee, WI) using a standardized imaging protocol. Measurements of intracranial volume were derived from a T1-weighted sagittal sequence with 5 mm contiguous sections. Volume measurements of the hippocampi were derived from a T1-weighted 3D volumetric spoiled gradient recalled echo sequence with 124 contiguous partitions, 1.6 mm partition thickness, a 22 cm × 16.5 cm field of view, 192 views, and 45° flip angle.
All image processing steps in every subject were performed by one of us (YCX) who was blinded to all clinical and pathologic information. The image data were interpolated inplane to the equivalent of a 512 × 512 matrix and magnified two times. The voxel size of the fully processed image data was 0.316mm3. B1 field inhomogeneity correction was performed. After the boundaries of the hippocampi had been delineated on each anatomic slice, the number of voxels was calculated automatically with a summing region of interest function. These were multiplied by voxel volume to give a numeric value in mm3.
The borders of the right and left hippocampi were manually traced with a mouse driven cursor for each slice sequentially from posterior to anterior. Inplane hippocampal anatomic boundaries were defined to include the CA1 through CA4 sectors of the hippocampus proper, the dentate gyrus, and subiculum10. The posterior boundary of the hippocampus was determined by the oblique coronal anatomic section on which the crura of the fornices were identified in full profile. The intra-rater test re-test reproducibility of this method has been verified with a co-efficient of variation = 0.28%17. Intracranial volume was determined by tracing the margins of the inner table of the skull on contiguous images from the sagittal sequence.
Pathology Methods
At autopsy, the brains were removed using a standard protocol. One hemisphere was sectioned in 1-cm coronal slabs. Standard histologic sections were taken, including sections of the hippocampus with adjacent inferior temporal lobe. These paraffin embedded blocks of temporal lobe were sent to Mayo Clinic Jacksonville where the pathologic analyses were performed by DWD who was blinded to clinical, radiologic and pathologic information. The sections were stained with H & E and thioflavin S. Histopathologic features, such as cerebrovascular pathology and hippocampal sclerosis, were ascertained from the H&E section. Thioflavin-S sections were examined with fluorescence microscopy. The number of senile plaques was counted in three contiguous 100X fields that subtend 2 mm2 area in the entorhinal cortex. The density of plaques and tangles was also assessed in the temporal neocortical gyri, but was not recorded. Neurofibrillary tangles were counted in three 40x fields that subtend 0.125 mm2. Plaques and tangles were separately recorded in various sectors of Ammon’s horn (CA4, CA2/3, CA1 and the subiculum) as well as the entorhinal cortex. Tangles were recorded in layer II neuronal clusters in the entorhinal cortex. Sections were also studied with immunohistochemical methods. All cases were stained for tau, using either PHF-1 or CP13 (both monoclonal antibodies generously provided by Peter Davies, Albert Einstein College of Medicine, Bronx, NY), alpha-synuclein, using a polyclonal antibody that has been previously characterized18 and with a polyclonal antibody to ubiquitin that has also been previously characterized19. Immunostaining was done in baths to assure uniformity between cases and used an avidin-biotin peroxidase method (Vector Laboratories, Burlingame, CA). The density of Lewy bodies were counted in the entorhinal cortex using the alpha-synuclein stained sections and the presence of Lewy neurites in CA2/3 was also noted. The dentate fascia was examined for the presence of ubiquitin-positive inclusions that are characteristic of some frontotemporal degenerative disorders20.
The specimens were assigned one or more pathologic diagnostic categories. The pathologic diagnostic categories and criteria employed for the diagnoses were:
Typical aging changes: The presence of agyrophilic grains was considered a feature of typical aging. Limited neurofibrillary pathology (≤ Braak Stage IV) could be present. Senile plaques could be present up to 20 per 100X fields) if the plaques were diffuse amyloid deposits without cores or neuritic elements.
AD: The diagnosis was made according to pathologic criteria formulated by a working group of the Reagan Institute of the Alzheimer's Association and the National Institute on Aging2.
Diffuse Lewy body disease (DLBD): The diagnosis was established using standard criteria 21.
Hippocampal sclerosis (HpScl): The diagnosis was based upon selective neuronal loss and gliosis in CA1 and the subiculum22.
Frontotemporal degeneration (FTD): The diagnosis required neuronal loss and gliosis associated with laminar spongiosis in the temporal lobe and hippocampus and ubiquitin-positive, tau-negative inclusions in the dentate fascia23. The type of FTD defined by these criteria fits the description of the MND-type of FTD. It was a definition that permitted an accurate pathologic diagnosis with limited tissue sampling. Given that this study was confined to the temporal lobe and not other brain sections, we chose to use a restrictive definition of FTD, but one that provided for distinctive and diagnostic histopathology that could be readily detected in the temporal lobe. We acknowledge that some forms of FTD do not have ubiquitin-positive, tau-negative inclusions in the dentate fascia, but we did not feel confident in making this diagnosis without more pathologic information since such cases may have normal hippocampi.
Neurofibrillary tangle degeneration (NFT): Extensive neurofibrillary pathology was present in the medial temporal lobe without senile plaques or at most a few diffuse non-neuritic type plaques 24.
Cerebral infarction: The sections were evaluated for infarcts and hemorrhages and the lesions were histologically dated. Lesions that were acute and subacute (hours to days old) were not considered likely to have been associated with antemortem MRI findings 25.
Each specimen was staged for severity of neurofibrillary pathology regardless of its pathologic diagnostic category using a modification of the method of Braak and Braak, which has been shown to have clinical validity in a previous study26. This involves matching the topographic distribution of neurofibrillary lesions to the best fitting published template 27. Each specimen was assigned to Braak Stages 0 through 6. Stage I, neurofibrillary degeneration (NFD) confined to the transentorhinal cortex (layer IV); II, NFD in entorhinal cortex (layer II); III, NFD in hippocampus (CA1 and subiculum); IV, NFD in temporal lobe association neocortex (mild); V, NFD in temporal lobe association neocortex (moderate to severe). Braak stage VI requires an assessment of the primary visual cortex, which was not available for this study, but in stage VI there are hippocampal changes (e.g., neurofibrillary tangles in the dentate fascia) that can be used as a guide to diagnosis of endstage disease.
Statistical Methods
MRI-based hippocampal volume measurements were normalized for individual variation in head size, age, and gender. The measured volume of the hippocampus ipsilateral to the pathologically assessed hemisphere in each individual was divided by the total intracranial volume of that individual to control for inter-subject variation in head size. We have previously determined age and gender specific normal percentiles for head-size normalized hippocampal volume in a group of 126 cognitively normal elderly controls10. Age and gender specific normal percentiles for each of the patients in the current study were calculated from this normal elderly sample. Each percentile was converted to a W score. The W score is the value from a standard normal distribution corresponding to the observed percentile in controls. For example, for a standard normal distribution, the 50th, 5th, and 2.5th percentiles are given by 0, −1.64, and −1.96 respectively. Thus, an individual with a head-size normalized hippocampal volume at the 5th percentile of normal, after adjustment for age and gender would receive a W score of −1.64. Similarly, an individual at the 50th percentile would receive a W score of 0. W scores provide a method to assess the degree to which head-size normalized hippocampal volume measurements of individual subjects deviate from that expected in normals after adjustment for age and gender.
Comparison of W scores among pathologic diagnostic groups was carried out using Kruksall-Wallis one-way ANOVA by ranks. Associations among hippocampal W score, Braak stage, and MMSE score were measured using Spearman rank correlations.
RESULTS
Single Pathologic Diagnoses
The subjects were divided into two major groups; those who received a single pathologic diagnosis (n=57, Table1), and those who received mixed pathologic diagnoses (n = 10, Table 2). Subjects in Table 1 can in turn be divided into three subgroups, those with i) typical aging changes (n = 25), ii) AD (n = 23), iii) pathologic diagnosis other than AD that were consistent with dementia (n = 9). Clinical diagnoses in the 25 subjects who were classified pathologically as having changes of typical aging were: cognitively normal control 10, MCI 9, and dementia 6. The six subjects clinically classified as demented but who did not have significant neurodegenerative pathology were: a 96 y/o woman, who was un-testable for MMSE; a 96 y/o man, MMSE = 20; a 90 y/o woman, MMSE = 22; an a 82 y/o man, MMSE = 21; an 83 y/o man, MMSE = 15; and an 81 y/o man, MMSE = 10.
Table 1.
Data by Group for Patients with a Single Pathologic Diagnosis
| Braak Stage | W Score | Duration from MR to Death (Yrs) |
Duration from final clinical evaluation to Death (Yrs) |
Age at Death (Yrs) |
MMSE** | Female Gender n (%) |
|
|---|---|---|---|---|---|---|---|
| Typical Aging (N=25) |
2.5 (0.0, 4.0) |
−0.5 (−2.1, 1.5) |
1.9 (0.1, 6.3) |
0.7 (0.04, 6.4) |
87.0 (74, 100) |
27.0 (10, 30) |
14 (56%) |
| AD (N=23) |
5.0 (2.0, 6.0) |
−2.1 (−2.9, 0.5) |
1.7 (0.2, 7.1) |
0.7 (0.02, 4.4) |
86.0 (68, 102) |
18.0 (2, 27) |
16 (70%) |
| HpScl (N=3) | 1.5 (0.0 2.0) |
−2.5 (−2.7, −1.9) |
1.3 (1.2, 5.1) |
1.1 (0.2, 1.4) |
94.0 (84, 94) |
10.0 (7, 16) |
1 (33%) |
| DLBD (N=3) | 2.0 (2.0, 3.0) |
0.5 (−1.3, 0.7) |
1.8 (0.2, 4.1) |
0.5 (0.2, 4.6) |
90.0 (77, 93) |
24.0 (24, 26) |
1 (33%) |
| FTD (N=2) | 0.0 (0.0, 0.0) |
−2.3 (−2.4, −2.2) |
4.1 (1.6, 6.7) |
1.3 (1.1, 1.6) |
59.5 (51, 68) |
11.0 | 1 (50%) |
| NFT (N=1) | 3.5 | −2.5 | 1.8 | 1.8 | 83.0 | 21.0 | 1 |
HpScl = hippocampal sclerosis, DLBD = diffuse Lewy body disease, FTD = fronto-temporal degeneration, NFT = neurofibrillary tangle-only degeneration, MMSE = mini-mental state exam.
One patient in each of the typical aging and FTD were untestable
Values in table represent median (range) except where specified as n (%).
Table 2.
Data for Patients with Mixed Pathologic Diagnoses
| Braak Stage |
W Score | Duration from MR to Death (Yrs) |
Duration from final clinical evaluation to death (Yrs) |
Age at Death (Yrs) |
MMSE | Female Gender n (%) |
|
|---|---|---|---|---|---|---|---|
| AD plus DLBD (N=4) |
4.8 (4.5, 6.0) |
−2.1 (−2.6, −0.5) |
3.5 (0.9, 6.2) |
1.2 (0.3, 6.2) |
68.5 (66, 83) |
17.5 (8, 24) |
3 (75%) |
| AD plus HpScl (N=2) | 5.5 (5.0, 6.0) |
−2.4 (−2.7, −2.2) |
3.2 (1.4, 4.9) |
0.6 (0.0, 1.2) |
80.5 (78, 83) |
14.5 (3, 26) |
2 (100%) |
| AD plus infarction (N=1) | 4.5 | −1.7 | 1.1 | 1.3 | 98.0 | 25 | 1 |
| FTD plus HpScl (N=2) | 0.0 (0.0, 0.0) |
−2.5 (−3.0, −1.9) |
3.4 (2.2, 4.6) |
2.0 (0.7, 3.2) |
65.0 (54, 76) |
21.5 (16, 27) |
1 (50%) |
| HpScl plus DLBD (N=1) | 1.0 | −2.5 | 2.3 | 1.1 | 83.0 | 22.0 | 0 |
values in Table represent median (range) except where specified as n (%)
Twenty-three subjects were classified pathologically as AD and their clinical diagnoses were: dementia 18, MCI 2, and normal control 3. The three subjects clinically classified as cognitively normal, but who received a pathologic diagnosis of AD were; a 102 y/o woman, MMSE = 27, a 100 y/o woman, MMSE = 23; and a 83 y/o woman, MMSE = 26.
The remaining 9 subjects in Table 1 had pathologic diagnoses other than AD that were consistent with dementia. Three of these were pathologically classified as hippocampal sclerosis, two as frontotemporal degeneration, and one as neurofibrillary tangle-only degeneration; all of these were clinically labeled demented. Three subjects were pathologically classified as DLBD. Two were clinically demented. The third, a 93 y/o man MMSE = 24, received a clinical diagnosis of cognitively normal.
In general the pathologic diagnosis of isolated AD was associated with a high Braak stage, low MMSE score, and a low MRI hippocampal W score (i.e., significant hippocampal atrophy relative to controls). In contrast, those with a histologic diagnosis of typical aging had lower Braak scores, higher hippocampal W scores (i.e. little atrophy), and higher MMSE scores.
Among the subjects in the third subgroup from Table 1, isolated non-AD pathology associated with dementia, those with HpScl and FTD had low Braak scores, low hippocampal W scores, and low MMSE scores. The lone individual with NFT degeneration had a moderate Braak score, low hippocampal W score, and low MMSE. In contrast, while individuals with DLBD had low Braak scores as expected, two of the three had no hippocampal atrophy.
Mixed Pathologic Diagnoses
The 10 subjects with mixed pathology (Table 2) can be divided into two subgroups, i) those with mixed pathology in whom one of the pathologic diagnoses was AD (n=7) and ii) those with mixed pathology without AD (n=3). All subjects in Table 2 were clinically diagnosed as demented except one AD plus DLBD individual, MMSE = 24, who received a clinical diagnosis of MCI. The seven subjects with AD plus a second pathologic diagnosis all had high Braak scores and significant hippocampal atrophy. The three subjects with mixed pathology without AD all had low Braak scores, but still had significant hippocampal atrophy.
Correlation Between Hippocampal W Score and Pathologic Diagnosis
The median (range) hippocampal W scores by pathologic diagnostic subgroup were: i) typical aging pathology (n=25, Table1) – 0.5 (−2.1, 1.5), ii) AD (n=23, Table 1) −2.1 (−2.9, 0.5), iii) non-AD dementia pathology (n=9, Table 1) −2.2 (−2.7, 0.7), iv) mixed AD (n=7, Table 2) −2.2 (−2.7, 0.4), v) mixed non-AD dementia pathology (n=3, Table 2) −2.5 (−3.0, −1.9). The median hippocampal W scores were significantly smaller (more atrophic) in each of subgroups 2–4 above compared with the typical aging subgroup (p <0.01 vs. groups 2,4, and 5, and p = 0.02 vs. group 3). Pair-wise comparisons among subgroups 2–5 revealed no significant differences in hippocampal W score.
Correlation Among Braak Stage, Hippocampal W Score, and MMSE
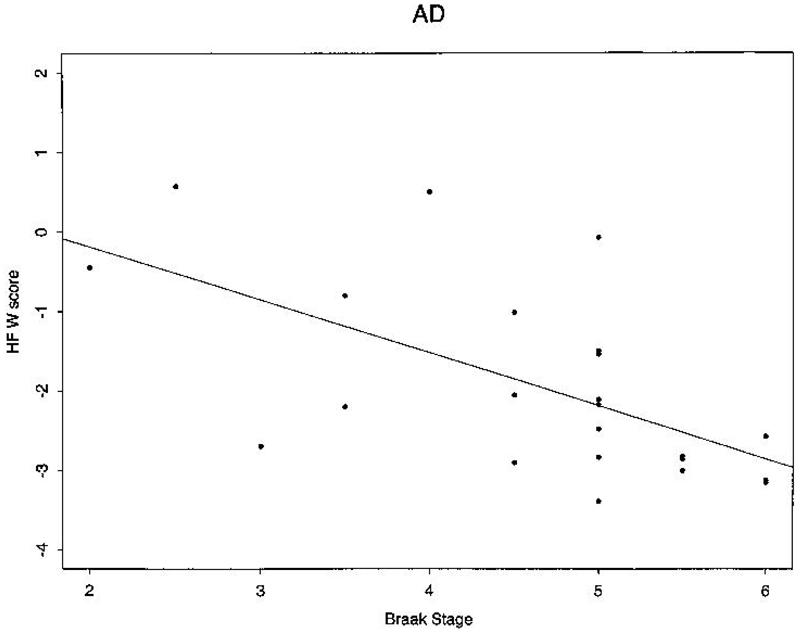
When all 67 subjects in Table 1 and Table 2, were considered together, significant correlations were present between hippocampal W score and Braak stage, hippocampal W score and MMSE, and MMSE and Braak Stage (Table 3). Of the five individual pathologic diagnostic subgroups, only the AD (n=23) and typical aging (n=25), subgroups had sufficient numbers of individuals to permit within-group statistical correlations between Braak staging, hippocampal volume, and MMSE. Among AD patients, Braak stage correlated inversely with both hippocampal W score (i.e. greater atrophy) and MMSE score (Fig 1). Hippocampal W score was also correlated with MMSE score. The only significant correlation present in typical aging patients was between hippocampal W score and MMSE.
Table 3.
Correlation Among Braak Stage, Hippocampal Volume, and MMSE
| Pathologic Diagnostic Group | Variables Correlated | |||
|---|---|---|---|---|
| Bilateral Hippocampal W* vs Braak |
Unilateral Hippocampal W vs Braak |
Bilateral Hippocampal W vs MMSE |
Braak vs MMSE | |
| All Subjects (N=67) | −0.39 (<0.01) | −0.36 (<0.01) | 0.60 (<0.01) | −0.41 (<0.01) |
| Typical Aging (N=25) | (NS) | (NS) | 0.48 (0.02) | (NS) |
| AD (N=23) | −0.63 (<0.01) | −0.42 (0.05) | 0.47 (0.02) | −0.67 (<0.01) |
Values in the body of the table represent r (p value), where r = Spearmans correlation coefficient.
"Unilateral Hippocampal W" refers to correlations performed with only the hippocampal volume ipsilateral the autopsy specimen.
"Bilateral Hippocampal W" refers to correlation performed with both right and left hippocampal volume measurements.
Subject groups in the table are those with 1) all 67 subjects from Table 1 and Table 2 combined 2) typical aging changes (in Table 1), and, 3) AD only (in Table 1).
Figure 1.
Braak's stage vs normalized bilateral hippocampal volume in isolated AD pathology subjects.
DISCUSSION
Our first aim was to assess whether antemortem MRI defined hippocampal atrophy was specific for AD, using the pathologic diagnosis as the gold standard. Although the number of patients with single pathologic diagnoses consistent with dementia other than AD was small, it is clear that hippocampal atrophy is not specific for the pathology of AD. This was confirmed by finding no difference in median hippocampal W score between subjects with AD (n = 23, Table 1) and subjects with non-AD dementia pathology (n = 9, Table 1), or between those with AD and those with mixed non-AD dementia pathology (n = 3, Table 2). Hippocampal sclerosis, frontotemporal degeneration, and neurofibrillary tangle-only degeneration all resulted in significant MRI-defined hippocampal atrophy in the absence of any concurrent AD pathology. This result is in agreement with other published series, which have found hippocampal atrophy in non-AD dementing disorders; however, for the most part prior publications have classified subjects on clinical grounds without pathologic confirmation28–35.
The term hippocampal sclerosis in this study should not be inferred to be a specific etiologic entity. The pattern of selective neuronal loss in the vulnerable regions of the hippocampus might have been due to vascular/ischemic disease, degenerative disease, or even epilepsy. In patients with epilepsy due to mesial temporal sclerosis, hippocampi are both atrophic and have increased signal on T2-weighted MRI36, 37. Conversely, elevated T2 signal is not a feature of the hippocampal atrophy associated with AD 38. This raises the intriguing possibility that the absence of elevated T2 signal might differentiate hippocampal atrophy due to AD from atrophy due to hippocampal sclerosis in elderly demented subjects. With only three pathologically confirmed cases of isolated hippocampal sclerosis, only one of whom had antemortem Fluid Attenuated Inversion Recovery (FLAIR) imaging, we do not have sufficient case material to prove or disprove this possibility.
Two of the three patient with isolated DLBD pathology (Table 1) had no evidence of hippocampal atrophy. In a recent study, hippocampal areas were measured in autopsy specimens using stereologic techniques and correlated with the pathologic diagnostic designations of pure AD, pure DLBD mixed AD–DLBD, and normal aging39. The authors found that hippocampal atrophy was profound in the AD group, not present in normal aging or DLBD groups and was intermediate in severity in mixed AD–DLBD. They concluded that among the patients they examined, hippocampal atrophy was specific for AD and that in patients with mixed AD–DLBD pathology the hippocampal atrophy was likely due to AD not DLBD. With only three cases of isolated DLBD pathology, we do not have sufficient case material to address this thoroughly. However, two of the three DLBD cases had hippocampal W scores >0, (i.e. larger than the average in normal subjects matched for age and gender) so it seems unlikely that hippocampal atrophy is either a prominent or a consistent feature of DLBD.
Our second aim was to measure the correlations among pre-mortem MRI hippocampal volume, mental status exam performance, and Braak stage. Given the 3-way correlations among hippocampal volume, Braak stage, and MMSE in the 23 pure AD patients (Table 1 and Table 3) we can conclude that in AD, hippocampal volume measurements provide a quantitative marker of the pathologic substrate that produces the observed cognitive deficit. When all 67 subjects were considered together, a significant negative correlation was seen between Braak stage and hippocampal W score; that is, the more advanced the pathologic stage, the more atrophic the hippocampus. Both greater hippocampal atrophy and more advanced Braak stage were correlated with worse MMSE performance. No widely accepted pathologic staging method analogous to Braak scoring for AD exists for degenerative conditions such as FTD or HpScl. We can therefore not directly infer the same cause and effect relationship between pathologic stage, MRI measure, and cognitive status in these non-AD degenerative conditions. However, it does seem likely that similar relationships hold. That is, greater pathologic severity produces greater atrophy on MR as well as greater cognitive deficit. This concept has in principle been validated recently for non-AD dementias defined on clinical grounds using rates of global brain atrophy as the MRI measure 40.
There are several limitations inherent in these data that should be acknowledged. The median time from MRI and the last clinical assessment to autopsy was about 1 year, and therefore, on average, the MRI/clinical assessment underestimates the extent of pathology at the time of autopsy. By definition, an autopsy series represents a convenience sample in which it is difficult to control for intergroup differences in age, gender distribution, and the time from the final antemortem cognitive assessment and MRI to death. Another limitation is the small patient numbers in some of the pathologic groups. Given the infrequency of pathologic conditions such as HpScl, NFT degeneration, and FTD, this is unavoidable.
We limited the scope of this study to the correlations between antemortem MRI-defined hippocampal volume, pathologic diagnosis and staging, and antemortem cognitive status testing, and did not formally evaluate clinical-pathologic diagnostic correlation. This was done intentionally in the interest of brevity and clarity of focus. In general patients who received pathologic diagnoses consistent with a dementing condition were clinically labeled demented, and those who received a pathologic diagnosis of typical aging changes were not clinically demented. However, there were exceptions. Some patients were clinically impaired, but did not display significant neurodegenerative pathology, while others harbored histologically significant but clinically silent AD pathology. Some of the clinical-pathologic diagnostic mismatches were in individuals who were greater than 90 years old, and in some cases over 100 years old. In this age range the clinical categorization of dementia is more difficult. However, the mere presence of some clinical-pathologic diagnostic mismatches does raise questions about the specificity of pathologic diagnosis as a predictor of ante-mortem clinical status, and vice versa. Isolated clinical-pathologic mismatches have been a consistent finding in other autopsy studies41–44.
Another group recently reported strong correlations between post-mortem imaging measures of hippocampal volume and histologic measures of hippocampal area, as well as neuron counts in pathologically confirmed AD cases45. We found a significant correlation between antemortem hippocampal W score and Braak stage in AD patients. This correlation was of greater magnitude (−0.63) and significance when the W scores for both hippocampi were correlated with the Braak stage, than if only a unilateral W score was correlated ( Table 3). As atrophy due to AD is a global phenomenon that generally does not preferentially affect one hemisphere, the stronger correlation when both hippocampi are included was likely due to greater measurement precision from a larger sample. Including the opposite hippocampal measurement in the correlation is justified by the fact that Braak staging, while limited to one of the temporal lobes in this case, involves an assessment of brain areas other than the ipsilateral hippocampus. While the hippocampal volume is a reasonably good measure of disease severity in the medial temporal lobe in AD, it does not directly measure the severity of pathology elsewhere in the brain. We speculate that hippocampal MRI measurements might be a useful marker of pathologic stage in the early stages of AD when the disease is largely confined to medial temporal limbic areas, while MRI measures of atrophy in neocortex might be a better marker of advanced Braak stage. Such MRI measurements may be useful in situations where it is advantageous to classify patients on the basis of pathologic disease severity, including clinical assessment, clinical observational studies, or therapeutic trials.
Acknowledgments
Brenda Maxwell – Typing
Anthony Corral - Immunohistochemistry
Grant Support: NIH, NIA, AG11378, AG06786, AG16574, NS 29059, The Dana Foundation, The Alzheimer's Association.
REFERENCES
- 1.Braak H, Braak E. Neuropathological staging of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 2.Consensus recommendations for the postmortem diagnosis of Alzheimer's disease. The national Institute on Aging, and Reagan Institute Working Group on Diagnostic Criteria for the Neuropathological Assessment of Alzheimer's Disease. Neurobiol Aging. 1997;18:S1–S2. [PubMed] [Google Scholar]
- 3.de Leon MJ, Golomb J, Convit A, et al. Measurement of medial temporal lobe atrophy in diagnosis of Alzheimer's disease. The Lancet. 1993;341:125. doi: 10.1016/0140-6736(93)92610-6. [DOI] [PubMed] [Google Scholar]
- 4.Fox NC, Warrington EK, Freeborough PA, et al. Presymptomatic hippocampal atrophy in Alzheimer's disease. A longitudinal MRI study. Brain. 1996;119:2001–2007. doi: 10.1093/brain/119.6.2001. [DOI] [PubMed] [Google Scholar]
- 5.Jack CR, Jr, Petersen RC, O'Brien PC, et al. MR-based hippocampal volumetry in the diagnosis of Alzheimer's disease. Neurology. 1992;42:183–188. doi: 10.1212/wnl.42.1.183. [DOI] [PubMed] [Google Scholar]
- 6.Jobst KA, Smith AD, Szatmari M, et al. Rapidly progressing atrophy of medial temporal lobe in Alzheimer's disease. Lancet. 1994;343:829–380. doi: 10.1016/s0140-6736(94)92028-1. [DOI] [PubMed] [Google Scholar]
- 7.Juottonen K, Laakso P, Insausti R, et al. Volumes of the entorhinal and perirhinal cortices in Alzheimer's disease. Neurobiology of Aging. 1998;19:15–22. doi: 10.1016/s0197-4580(98)00007-4. [DOI] [PubMed] [Google Scholar]
- 8.Laakso MP, Soininen H, Partanen K, et al. Volumes of hippocampus, amygdala and frontal lobes in the MRI-based diagnosis of early Alzheimer's disease: correlation with memory functions. J of Neural Transmission. 1995;9:73–86. doi: 10.1007/BF02252964. [DOI] [PubMed] [Google Scholar]
- 9.Petersen RC, Jack CR, Jr, Xu YC, Waring SC, O'Brien PC, Smith GE, Ivnik RJ, et al. Memory and MRI-based hippocampal volumes in aging and Alzheimer's disease. Neurology. 2000;54:581–587. doi: 10.1212/wnl.54.3.581. [DOI] [PubMed] [Google Scholar]
- 10.Jack CR, Jr, Petersen RC, Xu YC, et al. Medial temporal atrophy on MRI in normal aging and very mild Alzheimer's disease. Neurology. 1997;49:786–794. doi: 10.1212/wnl.49.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen RC, Kokmen E, Tangalos EG, et al. Mayo Clinic Alzheimer's Disease Patient Registry. Aging. 1990;2:408–415. doi: 10.1007/BF03323961. [DOI] [PubMed] [Google Scholar]
- 12.Petersen RC, Smith GE, Ivnik RJ, Tangalos EG, et al. Apolipoprotein E status as a predictor of the development of Alzheimer's disease in memory impaired individuals. JAMA. 1995;273:1274–1278. [PubMed] [Google Scholar]
- 13.Smith GE, Petersen RC, Parisi JE, et al. Definition, course, and outcome of mild cognitive impairment. Aging, Cognition, and Neuropsychology. 1996;3:141–147. [Google Scholar]
- 14.American Psychiatric Association, editor. Diagnostic and Statistical Manual of Mental Disorders. Revised Third Edition. Washington, D C: 1987. [Google Scholar]
- 15.Ivnik RJ, Malec JF, Smith GE. Mayo's older Americans normative studies: WAIS-R, WMS-R, and AVLT norms for ages 56 through 97. The Clinical Neuropsychologist. 1992;6:1–103. [Google Scholar]
- 16.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 17.Jack CR, Jr, Petersen RC, Xu Y, et al. The rate of medial temporal lobe atrophy in typical aging and Alzheimer's disease. Neurology. 1998;51:993–999. doi: 10.1212/wnl.51.4.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwinn-Hardy K, Mehta N, Farrer M, Maraganore D, Muenter M, Hardy J, Dickson D. Distinctive neuropathology revealed by alpha-synuclein antibodies in hereditary Parkinsonism and dementia linked to chromosome 4p. Acata Neuropathol. 2000;99:663–672. doi: 10.1007/s004010051177. [DOI] [PubMed] [Google Scholar]
- 19.Dickson D, Wertkin A, Kress Y, Ksiezak-Reding H, Yen S-H. Ubiquitin-immunoreactive structures in normal brains: distribution and developmental aspects. Lab Invest. 1990;63:87–99. [PubMed] [Google Scholar]
- 20.Dickson D, Yen S-H. In: Ubiquitin, the cytoskeleton and neurodegenerative diseases, in Heat Shock or Stress Proteins and The Nervous System. Mayer RJ, Brown IR, editors. London: Academic Press; 1994. pp. 235–262. [Google Scholar]
- 21.McKeith IG, Galasko D, Kosaka K, et al. Clinical and pathological diagnosis of dementia with Lewy bodies (DLB): Report of the Consortium on Dementia with Lewy Bodies International Workshop. Neurology. 1996;47:1113–1124. doi: 10.1212/wnl.47.5.1113. [DOI] [PubMed] [Google Scholar]
- 22.Dickson DW, Davies P, Bevona C, Van Hoeven KH, et al. Hippocampal sclerosis: a common pathological feature of dementia in very old (greater than 80 years of age) humans. Acta Neuropathol. 1994;88:212–221. doi: 10.1007/BF00293396. [DOI] [PubMed] [Google Scholar]
- 23.Brun A, England B, Gustafson L, Passant U, et al. Clinical and neuropathological criteria for frontotemporal dementia: The Lund and Manchester groups. J Neurol Neurosurg Psychiatry. 1994;57:416–418. doi: 10.1136/jnnp.57.4.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jellinger KA, Bancher C. Senile dementia with tangles (tangle predominant form of senile dementia) Brain Pathol. 1998;8:367–376. doi: 10.1111/j.1750-3639.1998.tb00160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vinters HV, Ellis WG, Zabrow C, Saias BW, Jagust WJ, Mack WJ, Chui H. Neuropathologic substrates of ischemic vascular dementia. J Neuropathol Exp Neurol. 2000;59:931–579. doi: 10.1093/jnen/59.11.931. [DOI] [PubMed] [Google Scholar]
- 26.Grober E, Dickson D, Sliwinski MJ, et al. Memory and mental status correlates of modified Braak staging. Neurobiology of Aging. 1999;20:573–579. doi: 10.1016/s0197-4580(99)00063-9. [DOI] [PubMed] [Google Scholar]
- 27.Braak H, Braak E, Bohl J. Staging of Alzheimer-related cortical destruction. Eur Neurol. 1993;33:403–408. doi: 10.1159/000116984. [DOI] [PubMed] [Google Scholar]
- 28.Maunoury C, Michot J-L, Caillet H, et al. Specificity of temporal amygdala atrophy in Alzheimer's disease: quantitative assessment with magnetic resonance imaging. Dementia. 1996;7:10–14. doi: 10.1159/000106846. [DOI] [PubMed] [Google Scholar]
- 29.Laakso MP, Partanen K, Riekkinen P, et al. Hippocampal volumes in Alzheimer's disease, Parkinson's disease with and without dementia, and in vascular dementia: An MRI study. Neurology. 1996;46:678–681. doi: 10.1212/wnl.46.3.678. [DOI] [PubMed] [Google Scholar]
- 30.Smith AD, Jobst KA. Use of structural imaging to study the progression of Alzheimer's disease. British Medical Bulletin. 1996;52:575–586. doi: 10.1093/oxfordjournals.bmb.a011568. [DOI] [PubMed] [Google Scholar]
- 31.Davis PC, Gray L, Albert M, et al. The consortium to establish a registry for Alzheimer's disease (CERAD). Part III. Reliability of a standardized MRI evaluation of Alzheimer's disease. Neurology. 1992;42:1676–1680. doi: 10.1212/wnl.42.9.1676. [DOI] [PubMed] [Google Scholar]
- 32.Riekkinen P, Kejonen K, Laakso MP, Soininen H, Partanen K, Riekkinen M. Hippocampal atrophy is related to impaired memory, but not frontal functions in non-demented Parkinson's disease patients. NeuroReport. 1998;9:1507–1511. doi: 10.1097/00001756-199805110-00048. [DOI] [PubMed] [Google Scholar]
- 33.Nagy Z, Hindley NJ, Braak H, Braak E, Yilmazer-Hanke DM, Schults C, Barnetson L, et al. Relationship between clinical and radiological diagnostic criteria for Alzheimer's disease and the extent of neuropathology as reflected by stages: a prospective study. Dementia Geriatr Cogn Disord. 1999;10:109–114. doi: 10.1159/000017110. [DOI] [PubMed] [Google Scholar]
- 34.Nagy Z, Hindley NJ, Braak H, Braak E, Yilmazer-Hanke DM, Schults C, Barnetson L, et al. The progression of Alzheimer's disease from limbic regions to the neocortex: clinical, radiological and pathological relationships. Dement Geriatr Cogn Disord. 1999;10:115–120. doi: 10.1159/000017111. [DOI] [PubMed] [Google Scholar]
- 35.Holodny AI, Waxman R, George AE, Rusinek H, Kalmin AJ, de Leon M. MR differential diagnosis of normal-pressure hydrocephalus and Alzheimer disease: significance of perihippocampal fissures. AJNR. 1998;19:813–819. [PMC free article] [PubMed] [Google Scholar]
- 36.Jack CRJ, Rydberg CH, Krecke KN, Trenerry MR, Parisi JE, Rydberg JN, Cascino GD, et al. Mesial Temporal Sclerosis: Diagnosis with fluid-attenuated inversion-recovery versus spin-echo MR Imaging. Radiology. 1996;199:367–373. doi: 10.1148/radiology.199.2.8668780. [DOI] [PubMed] [Google Scholar]
- 37.Jack CR, Jr, Sharbrough FW, Twomey CK, Cascino GD, Hirschorn KA, Marsh WR, Zinsmeister AR, et al. Temporal Lobe Seizures: Lateralization with MR volume measurements of hippocampal formation. Radiology. 1990;175:423–429. doi: 10.1148/radiology.175.2.2183282. [DOI] [PubMed] [Google Scholar]
- 38.Campeau NG, Petersen RC, Felmlee JP, et al. Hippocampal transverse relaxation time (T2) measurements in patients with Alzheimer's disease and in elderly nondemented controls. Radiology. 1997;205:197–201. doi: 10.1148/radiology.205.1.9314985. [DOI] [PubMed] [Google Scholar]
- 39.Lippa C, Johnson R, Smith T. The medial temporal lobe in dementia with Lewy bodies: a comparative study with Alzheimer's disease. Ann Neurol. 1998;43:102–106. doi: 10.1002/ana.410430117. [DOI] [PubMed] [Google Scholar]
- 40.O'Brien J, Paling S, Barber R, Williams E, Ballard C, McKeith I, et al. Progressive brain atrophy on serial MRI in dementia with Lewy bodies, AD, and vascular dementia. Neurology. 2001;56:1386–1388. doi: 10.1212/wnl.56.10.1386. [DOI] [PubMed] [Google Scholar]
- 41.Price JL, Davis PB, Morris JC, White DL. The distribution of tangles, plaques, and related immunohistochemical markers in healthy aging and Alzheimer's disease. Neurobiology of Aging. 1991;12:295–312. doi: 10.1016/0197-4580(91)90006-6. [DOI] [PubMed] [Google Scholar]
- 42.Davis D, Schmitt F, Wekstein D, Markesbery W. Alzheimer neuropathologic alterations in aged cognitively normal subjects. J Neuropathol Exp Neurol. 1999;58:376–388. doi: 10.1097/00005072-199904000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Morris J, Storandt M, MeKeel D, Rubin EH, Price JL, Grant EA, Berg L. Cerebral amyloid deposition and diffuse plaques in "normal" aging: evidence for presymptomatic and very mild Alzheimer's disease. Neurology. 1996;46:707–719. doi: 10.1212/wnl.46.3.707. [DOI] [PubMed] [Google Scholar]
- 44.Green M, Kaye J, Ball M. The Oregon Brain Aging Study. Neurology. 2000;54:105–113. doi: 10.1212/wnl.54.1.105. [DOI] [PubMed] [Google Scholar]
- 45.Bobinski M, deLeon MJ, Wegiel J, Desanti S, et al. The histological validation of post mortem magnetic resonance imaging-determined hippocampal volume in Alzheimer's disease. Neuroscience. 2000;95:721–725. doi: 10.1016/s0306-4522(99)00476-5. [DOI] [PubMed] [Google Scholar]