Abstract
Background and Purpose
Disruption of the corticospinal tract at various locations in the brain has been shown to predict worse spontaneous motor recovery after stroke. However, the anatomical specificity of previous findings was limited by the categorical classification of infarct locations. Here we used computational methods to more precisely determine the specific anatomical locations associated with impaired motor ability. More important, however, our study also used these techniques to evaluate whether infarct location could influence motor outcomes following Constraint-Induced Movement therapy (CI therapy), a specific and controlled form of physical therapy.
Methods
Quantitative voxel-based analyses were employed to determine whether infarct location could predict either initial motor ability or clinical improvement following CI therapy in chronic stroke patients.
Results
Although corona radiata infarcts were associated with worse in-laboratory motor ability at pre-treatment, infarct location did not predict improvement in either the laboratory or the life situation after CI therapy.
Conclusions
The extent of improvement from CI therapy does not depend on the location of neurological damage, despite there being a pre-treatment relationship between infarct location and in-laboratory motor ability. This dissociation could be explained by brain plasticity induced by CI therapy.
Keywords: infarct location, CI therapy, stroke rehabilitation, imaging, MRI, motor recovery
Introduction
Several studies have suggested that infarct location influences spontaneous recovery of motor function after stroke.1-5 Subcortical infarcts in the brainstem,2 posterior limb of the internal capsule,1,2 and corona radiata3 have been associated with worse spontaneous motor recovery in both acute1 and chronic2,3 stroke. Additionally, prolonged motor disability after stroke was found to be most severe in patients with infarcts involving both the thalamus and posterior limb of the internal capsule.4 Although categorical classification of infarct location limited the anatomical specificity of these studies, a common finding was that damage to the corticospinal tract (CST) appeared to impair spontaneous recovery of motor function after stroke.
Further support for the importance of the CST in motor recovery has been established in studies that employed alternate methods of measuring brain structure, excitability, or metabolism. Extensive Wallerian degeneration of the CST following cerebral infarction has been associated with sustained motor disability in stroke patients.6 Transcranial magnetic stimulation (TMS) and magnetoencephalography studies have suggested the importance of CST connectivity for motor recovery after stroke. Patients with worse spontaneous recovery have shown decreased cortical-muscular coherence7 and reduced motor evoked potentials after TMS of the cortex.2,8 Magnetic resonance spectroscopy has also demonstrated reduced capsular N-acetylaspartate signal (which is presumed to reflect axonal injury) in the posterior limb of the internal capsule that was strongly associated with motor deficit following ischemic stroke.9 Taken together, these studies provide compelling support for the importance of CST integrity in spontaneous recovery of function following stroke.
Despite the abundance of these findings, however, the influence of infarct location on specific motor rehabilitation outcomes remains to be determined. A neurorehabilitation technique termed Constraint-Induced Movement therapy (CI therapy) provides a good vehicle for examining this relationship. In contrast to standard physical therapy, CI therapy is a highly standardized and intensive motor intervention that has been been shown to substantially increase the amount of use and motor ability of an affected upper extremity after stroke.10-13
One of the mechanisms associated with improved motor ability through CI therapy is neuroplasticity.14,15 CI therapy has been shown to produce “functional” changes in brain metabolism, blood flow, and electrical excitability (summarized in 15). More recently, structural remodeling of sensorimotor cortices, more anterior motor areas, and hippocampus has been demonstrated following two weeks of CI therapy.14 Because these functions or structures can change, whereas the locations of cerebral infarctions can not, neuroplastic responses to CI therapy have the potential to moderate the limiting effects of infarct location on motor recovery.
We attempted to determine whether infarct location can predict motor improvement in chronic stroke patients given CI therapy. Specifically, we aimed to determine whether CI therapy could override the effects of infarct location, perhaps as a result of neuroplastic changes that CI therapy has been shown to produce. 14,15
Methods
Participants
Participants were adults with chronic post-stroke upper extremity hemiparesis recruited between 1997 and 2007 for several projects aimed at testing the efficacy of different forms of CI therapy. There were two main groups of patients: those that received a full CI therapy protocol (n = 44) and those that were randomly assigned to comparison groups that received attenuated versions of the therapy (see Therapy and Testing).11,14, 16-18 Those receiving full CI therapy had a mean age of 62.1 ± 12.2 years and median chronicity of 3.0 years; 23 were women, 22 exhibited right hemiparesis, and 41 were right-handed prior to stroke. The full sample of patients had a mean age of 63.0 ± 12.3 years and median chronicity of 2.3 years; 39 were women, 39 exhibited right hemiparesis, and 71 were right-handed prior to stroke. A breakdown of participant characteristics is provided in Table 1 (see online supplement for a more detailed description). Severity of initial motor impairment was independent of infarct volume, chronicity, age, side of motor deficit, or handedness.19 Our institution’s human subjects review board for research approved this study; all patients provided signed informed consent.
Table 1.
Participant Characteristics
| Quantity | Range | |
|---|---|---|
| All patients (n = 81) | ||
| Age | 63.0 ± 12.3 * | 35 – 88 y |
| Chronicity | 2.3 † | 10 mo – 20 y |
| Female | 39 | |
| Right-sided hemiparesis | 39 | |
| Right-handed | 71 | |
| Infarct Characteristics | ||
| Volume | 1.9 cm3 † | .02 – 157.1 cm3 |
| Cortex involved | 41 | |
| Multiple infarcts within single hemisphere | 18 | |
| Bilateral Infarcts | 28 | |
| CI therapy patients (n = 44) | ||
| Age | 62.1 ± 12.2 * | 35 – 88 y |
| Chronicity | 3.0 † | 10 mo – 20 y |
| Female | 23 | |
| Right-sided hemiparesis | 22 | |
| Right-handed | 41 | |
| Infarct Characteristics | ||
| Volume | 1.3 cm3 † | .10 - 157.1 cm3 |
| Cortex involved | 18 | |
| Multiple infarcts within single hemisphere | 11 | |
| Bilateral Infarcts | 9 | |
Mean ± standard deviation
Median (positively skewed distribution)
Procedures
Therapy and Testing
Patients received T1 weighted MRI scans within one week prior to receiving therapy. Patients randomized to full CI therapy received all three components of the therapy: 1) intensive in-laboratory training of the more impaired arm on functional tasks for 10 consecutive weekdays, 2) restraint of the less-impaired arm for a target 90% of waking hours, and 3) a number of behavioral techniques termed the ‘transfer package’ that lasted an additional 0.5 hours on each treatment day in the laboratory. 13 The transfer package, designed to facilitate transfer of therapeutic gains in the laboratory to real world activities, included daily monitoring of life situation use of the more affected arm in several ways and problem-solving with a therapist to overcome perceived barriers to using the extremity. Though details of the treatment protocol varied somewhat among different groups of patients receiving full CI therapy11, 16-18 (e.g., some patients received sling restraint of the entire upper extremity rather than the more recently implemented protective safety mitt13), clinical outcomes were equivalent. Groups of patients receiving the attenuated therapies were denied either in-laboratory motor training or the transfer package and showed significantly decreased improvements in real world arm use. Therefore, to assess the effect of infarction location on CI therapy outcome, only patients who had received full CI therapy were included in the analysis (n = 44). In contrast, the effect of infarction location on pre-treatment motor functions was assessed in the entire patient group (n = 81).
All patients performed the Wolf Motor Function Test (WMFT) and rated the amount and quality of their arm use on the Arm Use scale of the Motor Activity Log (MAL) before and after the therapy course to assess baseline performance and treatment efficacy. The WMFT is a laboratory motor function test that measures performance time as patients perform a series of tasks as rapidly and as well as they can. It is a highly standardized, valid, and reliable objective measure of in-laboratory motor ability.20, 21 Performance time on the WMFT was recorded as a log2 transformation of the mean time in seconds to more accurately portray patient progress.19 The MAL is a scripted, structured, therapist-administered interview of how well and how often the patient uses the impaired arm spontaneously in the life situation for 30 frequently performed activities of daily living (e.g. brushing teeth, washing hands);10 it is a highly reliable and valid measure of real-world arm use22, 23 and correlates well with objective measures of arm use in the life situation.24 The WMFT and the MAL measure different aspects of motor function and scores on the two tests frequently diverge substantially; therefore, analyses of WMFT and MAL data were performed separately.
Infarct Location Analysis
Infarcts were initially identified and manually traced under supervision of a neurologist (V.W.M.). To reduce subjectivity, they were then refined by trained observers using a semi-automated intensity thresholding technique available through MRIcro (version 1.4) software. The threshold was selected to approximate the intensity of the cerebrospinal fluid of each image, thus restricting the lesion tracing to regions that were presumed to be necrotic. Images were equated for deficit side by flipping left to right the brains of patients with right arm hemiparesis. Each patient’s T1 scan was normalized to a standard brain template provided by the International Consortium for Brain Mapping (http://www.loni.ucla.edu/ICBM/Downloads/Downloads_ICBMtemplate.shtml) using the Statistical Parametric Mapping toolbox (SPM5, http://www.fil.ion.ucl.ac.uk/spm); infarcts were masked during this process to guard against distortions in the normalization. Deformation parameters from this normalization were then applied to each patient’s infarct tracing, resulting in infarcts that were mapped to the standard brain template. An infarct overlap map was generated by overlaying all patients’ infarcts onto the template brain (online figure 1). To prevent analysis of brain voxels with an insufficient number of observations from which to draw accurate statistical conclusions, brain voxels that had an infarct overlap of less than 5 (i.e., fewer than 5 patients had infarcts occupying the particular brain voxel) were excluded from analysis.
Figure 1.
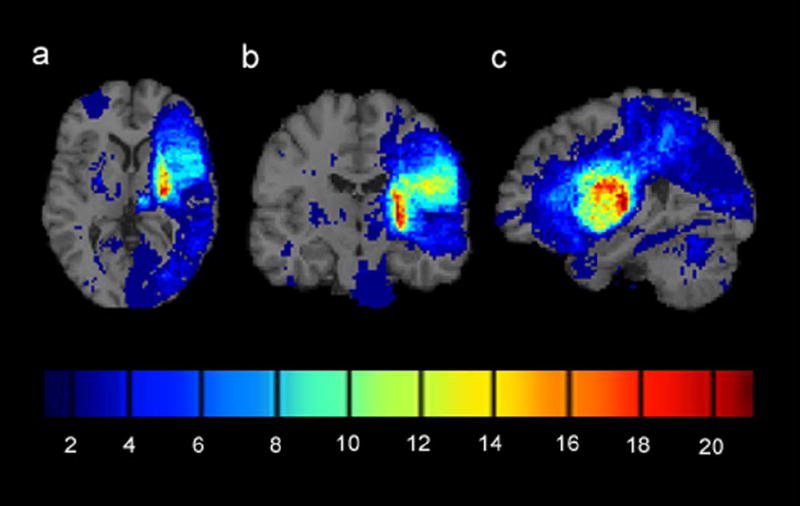
Infarct overlap map for 81 chronic stroke patients with mild to moderate upper extremity hemiparesis. Brains of patients with right arm hemiparesis were flipped left to right to equate for deficit side. (a) Axial cross-section through the basal ganglia. (b) Coronal cross-section through the primary motor area. (c) Sagittal cross-section through the medial temporal lobe. The colorbar indicates the number of patients with infarcts at each voxel. Infarcts were most frequently observed in the posterior limb of the internal capsule contralateral to the side of motor deficit (colored red).
Determination of effects of infarct location on motor scores at pre-treatment
Two-sample, two-tailed t tests were performed at each voxel comparing test scores of patients who had infarcts at that particular voxel location to those of patients without infarcts at that voxel. The result was a 3D map of t values (and associated probability values) that encompassed all voxel locations. Given that 14,241 voxels were tested in the analysis, it was necessary to adjust for family-wise error (FWE) by employing a multiple comparison correction procedure. Infarct location data are spatially correlated (i.e., if a patient’s infarct occupies a particular voxel, it is highly likely that it also occupies neighboring voxels); therefore, commonly used multiple comparison procedures (e.g. Bonferroni correction) are not appropriate. Permutation analysis is a relatively conservative procedure for multiple comparison correction of spatially correlated data25 and has been shown to be superior to other methods of multiple comparison correction for voxel-based lesion-symptom mapping.26 The theory behind permutation analysis is that if the null hypothesis were true (i.e., motor scores at pre-treatment are independent of infarct location), motor scores could be randomly shuffled across patients with no change in results. Therefore, a null distribution for this data set was created by performing 5000 shuffles of the data: randomly assigning motor scores to different patients, and calculating probability values for each shuffle at each voxel using two-sample t tests comparing patients with an infarction at that voxel to those without one. To correct for multiple comparisons, only the smallest probability value from each shuffle was stored in the null distribution (i.e., of the 14,241 probability values across the brain, only a single probability value - the smallest of the 14,241 probability values produced in any given shuffle - was entered into the null distribution). Thus, the null distribution is a distribution of minimum probability values that could be anticipated under the null hypothesis, and therefore the most conservative distribution with respect to rejecting the null. Voxels from our data set that yielded a probability value within the smallest 5% of this empirically derived null distribution were deemed significant. Code specific to this analysis was developed using Matlab 7.5 software and can be provided upon request.
Determination of effects of infarct location on motor outcomes following CI therapy
As noted, for the analyses relating treatment change scores on the WMFT and the MAL to infarct location, only data from patients who received the full CI therapy intervention were included (n = 44). A separate infarct overlap map was constructed for this subset of patients, and voxels with an infarct overlap of less than 5 cases were again excluded from analysis. Two-sample t tests were performed at each of the remaining voxels (12,079) comparing the WMFT and MAL change scores for patients who suffered an infarct at a particular voxel location to those who did not. Permutation analysis was implemented to correct for FWE.
Comparison of findings to those obtained using older techniques
We assessed the validity of our permutation analysis method by comparing our findings to those obtained using a more common qualitative infarct overlap analysis technique (e.g.,27). Pre-treatment test scores were partitioned into thirds. Separate infarct overlap images were generated for patients falling within the top third (n = 27) and for those falling within the bottom third (n = 27) on both the WMFT and the MAL. Qualitative differences between infarct overlap maps for the bottom and top tertiles were then cross-referenced with our voxel-wise t score maps at pre-treatment.
Results
Clinical Outcomes
Full CI therapy produced significant improvements on the WMFT (t(43) = 4.5, P < .0001) and the MAL (t(43) = 14.6, P < .0001), indicating that the therapy was effective at improving both in-laboratory and real-world arm use (table 2). The magnitude of treatment change is consistent with studies published previously from this laboratory10,11,14,16-18 and reflects a large clinical effect (d’ = -.68 & 2.2, respectively, large values according to conventions of the meta-analysis literature28). One study29 has found that the minimum clinically important difference for the MAL is 1.0 (difference here was 1.7).
Table 2.
Motor scores for the more impaired arm before and after CI therapy (Mean ± SD)
| Pre-treatment motor scores | Treatment change | d’ * | |
|---|---|---|---|
| All patients (n = 81) | |||
| Motor Activity Log | .9 ± .69 | ||
| Quality of Movement scale (0-5 points) | |||
| Wolf Motor Function Test | 1.28 ± 1.00 | ||
| Log2 Performance Time (s) | |||
| CI therapy patients (n = 44) | |||
| Motor Activity Log | .9 ± .64 | 1.7 ± .79 | 2.2 |
| Quality of Movement scale (0-5 points) | |||
| Wolf Motor Function Test | 1.35 ± .96 | -.28 ± .41 | -.68 |
| Log2 Performance Time (s) *† | |||
Cohen’s d’ is a within subjects measure of effect size; a value of .57 is considered large according to conventions of the meta-analysis literature.28
A log2 transformation was applied to the mean Performance Time scores to correct for skewness of the distribution of scores. A decrease in performance time represents an improvement.
Effects of infarct location on motor scores at pre-treatment
Infarction of the centrum semiovale contralateral to the more-affected arm, specifically the portion of the inferior corona radiata deep to primary motor cortex that intersects the crossing fibers of the corpus callosum, was significantly associated with worse performance on the WMFT at pre-treatment (PFWE = .004, online figure 2b). Effect sizes at each voxel, as calculated using Cohen’s d (the mean difference in motor scores between infarcted and noninfarcted subjects divided by the pooled standard deviation of the motor scores), exceeded 2.0 (online figure 2c). No significant relationship was observed between infarct location and pre-treatment performance on the MAL (PFWE = .219).
Figure 2.
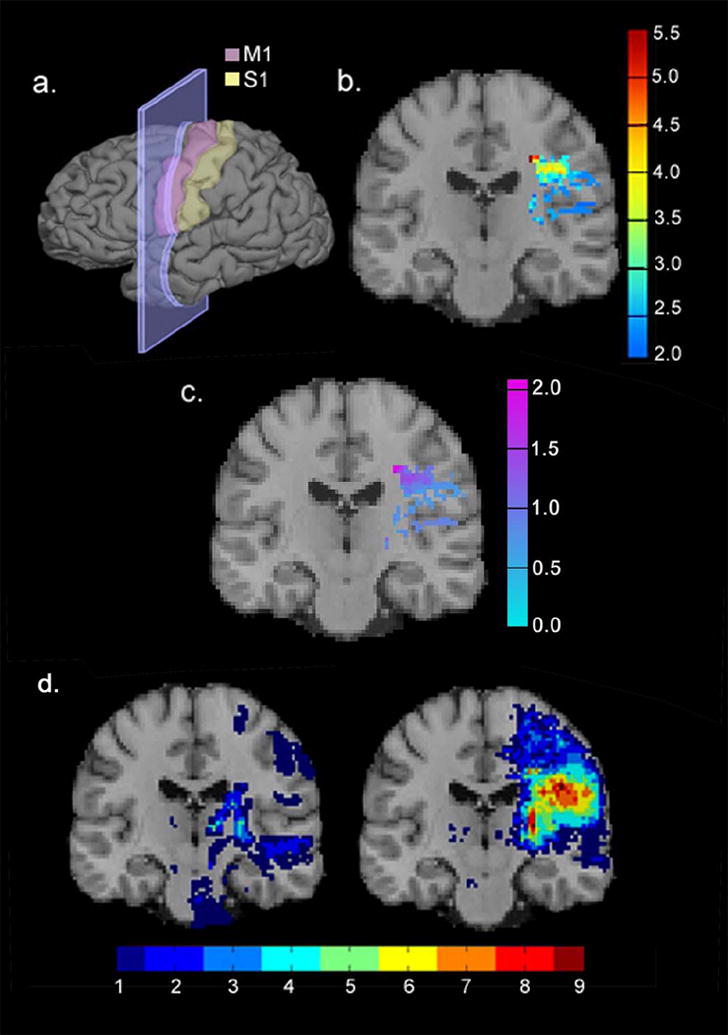
Infarct location predicted performance on a laboratory-based measure of pre-treatment motor ability. (a) Results are displayed in coronal cross-sections through the primary motor area (shown in pink). (b) A map of t values from the quantitative voxel-based analysis: red-colored voxels indicate infarct locations that predicted poorer pre-treatment performance on the WMFT after correcting for multiple comparisons. (c) Map of Cohen’s d values indicating effect size for the quantitative voxel-based analysis. (d) Infarct overlap maps for the third of patients with the strongest performances (left), and weakest performances (right) on the WMFT before treatment.
Effects of infarct location on motor outcomes following CI therapy
Infarct location was not significantly related to CI therapy treatment outcome as measured by either the WMFT or the MAL (PFWE = .741 and PFWE = .894, respectively).
Comparison of findings to those obtained using older techniques
The infarct overlap image for the 27 patients who performed most poorly on the WMFT was compared to the infarct overlap image for the 27 patients with the strongest performances on the WMFT. Among patients performing the worst on the WMFT, 12 (44%) had infarcts in the centrum semiovale. In contrast, infarcts to this region were virtually absent in patients with the strongest performances on the WMFT (online figure 2d). The striking difference between these two maps is consistent with quantitative findings that infarcts in the centrum semiovale are associated with poorer pre-treatment motor ability. As noted, no relationship was observed between infarct location and pre-treatment performance on the MAL using our quantitative measure. Similarly, no qualitative differences were clearly observed between patients scoring in the upper versus lower third on the MAL (online figure 3). Thus, what one observes qualitatively is reflected in our quantitative measures assessing the effects of infarct location on pre-treatment motor ability.
Figure 3.
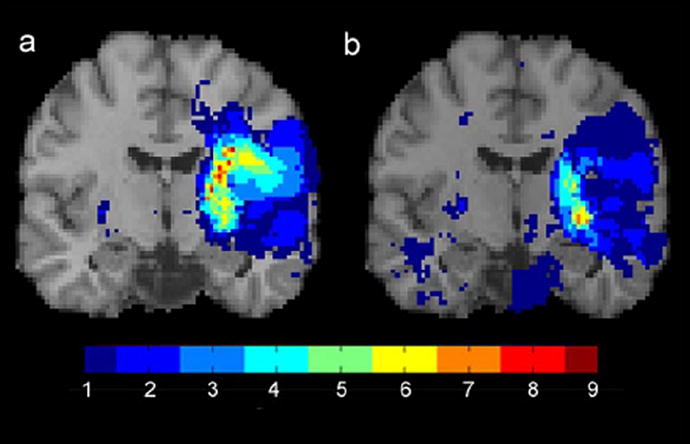
Infarct overlap maps for the patients scoring in the upper third (a) versus lower third (b) on the MAL. In contrast to the pattern for WMFT scores, the location of the infarcts in patients with MAL scores in the upper versus lower thirds are similar. Results are displayed in coronal cross-section through the precentral sulcus.
Discussion
Infarcts in the inferior corona radiata resulted in poorer in-laboratory motor function in a group of 81 chronic stroke patients with mild to moderate upper extremity hemiparesis. This finding is generally consistent with findings from smaller studies utilizing less sophisticated categorical methods of classifying infarct location:1-4 disruption of the CST is associated with poorer motor ability. Unlike earlier studies, however, we identified only a single specific location within the CST in which the infarction was strongly associated with poorer motor outcomes, whereas infarcts in other regions of the CST, such as the posterior limb of the internal capsule, did not show this relationship. This location was at the intersection of the corona radiata and fibers that traverse the corpus callosum. One might speculate that decreased motor ability after corona radiata infarcts results from the combination of damage to the CST and disrupted inter-hemispheric communication,30,31 perhaps altering the role of the healthy hemisphere in plastic reorganization of function. Although the role of the undamaged hemisphere in stroke recovery remains unclear, physiological changes have been observed in the undamaged hemisphere following stroke,32-35 and disruption of the intact hemisphere in chronic stroke can exacerbate motor impairment.36,37
Infarcts to inferior portions of the CST (e.g., the posterior limb of the internal capsule) were not significantly associated with extent of motor deficit. This discrepancy with other findings1,2 may be explained by methodological differences. The relationship between motor deficit and infarct location in the acute phase of stroke1 may differ from that seen in chronic stroke because the motor system has more time to reorganize after chronic stroke. In addition, previous studies employed different motor measures (with little emphasis on speed), smaller sample sizes, and the use of gross categorical classification of infarct location and motor ability.
It is important to note that although infarct location predicted in-laboratory motor ability (WMFT score), it did not predict the amount and quality of arm use in the life situation (MAL score). This finding demonstrates a dissociation between motor impairment and its associated dysfunction. The former appears to result directly from damage to specific brain tissue, whereas the latter may be modulated by a variety of learning and motivational factors13 in addition to physical disruption of the motor system.
Although corona radiata infarcts were associated with poorer pre-treatment motor ability, infarct location did not predict clinical improvement from CI therapy. Similarly, Dawes and colleagues found that despite a correlation between the extent of infarction to the CST and walking performance, CST involvement did not enhance prediction of outcomes from four weeks of treadmill training.5 Perhaps this dissociation between the effect of infarct location on initial motor ability versus treatment outcome can be explained by enhanced brain plasticity promoted by efficacious rehabilitation. CI therapy has been associated with changes in brain structure14 and function.15 These plastic changes could improve motor function by compensating in part for the damage-induced motor deficit. The lack of relationship between infarct location and CI therapy outcome suggests that CI therapy-induced plasticity may operate effectively irrespective of the pathways in the motor network that are damaged. Moreover, CI therapy has been used to treat the motor deficit associated with a number of different neurological conditions including traumatic brain injury,38 multiple sclerosis,39 cerebral palsy,40 and juvenile hemispherectomy40 with similar clinical outcomes. Taken together, these findings speak to the robustness of CI therapy for treating motor dysfunction of varying brain pathologies.
Despite the potential contribution of this study to understanding neuroplasticity of the motor system, it should be noted that the results and methods applied here have several limitations. Voxel-based techniques that allow identifying lesion effects on function with greater precision than earlier qualitative techniques require large sample sizes. Due to excluding brain voxels with an infarct overlap of fewer than 5 cases, our analyses were restricted to examining the middle cerebral artery territory (online figure 1) and thus were not able to test the effects of less common infarct locations (e.g., cerebellum, brainstem) on motor outcomes due to an insufficient number of cases with infarcts in those areas. Voxel-based techniques also do not readily enable examination of infarct effects according to a “functional network” model (e.g., perhaps combined damage to different parts of the motor network can yield substantially greater deficits than single infarcts to these areas4). This study also recruited patients with mild to moderate upper extremity hemiparesis, all of which had some preserved hand function, perhaps limiting the generalizability of our findings.
Despite these limitations, the results from this study have significant implications. This research has demonstrated that in patients with mild to moderate motor impairment, motor recovery produced by CI therapy is independent of the location of neurological damage, despite a significant relationship between infarct location and one aspect of motor ability at pre-treatment. Before treatment, patients with damage to the corona radiata performed worse on a laboratory motor function test, yet these patients benefited as much from CI therapy as those without infarctions in this area. The finding that disparate damage to the central nervous system results in relatively equivalent outcomes provides further evidence for CI therapy’s broad applicability.
Supplementary Material
Acknowledgments
This work was supported by grant HD34273 from the National Institutes of Health and approved by the University of Alabama at Birmingham Institutional Review Board.
Footnotes
Conflicts of Interest None of the authors has financial relationships relevant to this article.
References
- 1.Shelton FN, Reding MJ. Effect of lesion location on upper limb motor recovery after stroke. Stroke. 2001;32:107–112. doi: 10.1161/01.str.32.1.107. [DOI] [PubMed] [Google Scholar]
- 2.Pennisi G, Alagona G, Rapisarda G, Nicoletti F, Constanzo E, Ferri R, Malaguarnera M, Bella R. Transcranial magnetic stimulation after pure motor stroke. Clin Neurophysiol. 2002;113:1536–1543. doi: 10.1016/s1388-2457(02)00255-9. [DOI] [PubMed] [Google Scholar]
- 3.Kwon YH, Lee MY, Park JW, Kang JH, Yang DS, Kim Y, Ahn SH, Jang SH. Differences of cortical activation pattern between cortical and corona radiata infarct. Neurosci Lett. 2007;417:138–142. doi: 10.1016/j.neulet.2007.01.084. [DOI] [PubMed] [Google Scholar]
- 4.Fries W, Danek A, Scheidtmann K, Hamburger C. Motor recovery following capsular stroke. Brain. 1993;116:369–382. doi: 10.1093/brain/116.2.369. [DOI] [PubMed] [Google Scholar]
- 5.Dawes H, Enzinger C, Johansen-Berg H, Bogdanovic M, Guy C, Collett J, Izadi H, Stagg C, Wade D, Matthews PM. Walking performance and its recovery in chronic stroke in relation to extent of lesion overlap with the descending motor tract. Exp Brain Res. 2008;186:325–333. doi: 10.1007/s00221-007-1237-0. [DOI] [PubMed] [Google Scholar]
- 6.Feydy A, Carlier R, Roby-Brami A, Bussel B, Cazalis F, Pierot L, Burnod Y, Maier MA. Longitudinal study of motor recovery after stroke: recruitment and focusing of brain activation. Stroke. 2002;33:1610–1617. doi: 10.1161/01.str.0000017100.68294.52. [DOI] [PubMed] [Google Scholar]
- 7.Braun C, Staudt M, Schmitt C, Preissl H, Birbaumer N, Gerloff C. Crossed cortico-spinal motor control after capsular stroke. Eur J Neurosci. 2007;25:2935–2945. doi: 10.1111/j.1460-9568.2007.05526.x. [DOI] [PubMed] [Google Scholar]
- 8.Binkofski F, Seitz RJ, Arnold S, Classen J, Benecke R, Freund HJ. Thalamic metabolism and corticospinal tract integrity determine motor recovery in stroke. Ann Neurol. 1996;39:460–470. doi: 10.1002/ana.410390408. [DOI] [PubMed] [Google Scholar]
- 9.Pendelbury ST, Blamire AM, Lee MA, Styles P, Matthews PM. Axonal injury in the internal capsule correlates with motor impairment after stroke. Stroke. 1999;30:956–962. doi: 10.1161/01.str.30.5.956. [DOI] [PubMed] [Google Scholar]
- 10.Taub E, Miller NE, Novack TA, Cook EW, III, Fleming WC, Nepomuceno CS, Connell JS, Crago JE. Technique to improve chronic motor deficit after stroke. Arch Phys Med Rehabil. 1993;74:347–354. [PubMed] [Google Scholar]
- 11.Taub E, Uswatte G, King DK, Morris D, Crago JE, Chatterjee A. A placebo controlled trial of constraint-induced movement therapy for upper extremity after stroke. Stroke. 2006;37:1045–1049. doi: 10.1161/01.STR.0000206463.66461.97. [DOI] [PubMed] [Google Scholar]
- 12.Wolf SL, Winstein CJ, Miller JP, Taub E, Uswatte G, Morris D, Giuliani C, Light KE, Nichols-Larsen D. Effect of constraint-induced movement therapy on upper extremity function 3 to 9 months after stroke: the excite randomized clinical trial. JAMA. 2006;296:2095–2104. doi: 10.1001/jama.296.17.2095. [DOI] [PubMed] [Google Scholar]
- 13.Morris DM, Taub E, Mark VW. Constraint-induced movement therapy: characterizing the intervention protocol. Eura Medicophys. 2006;42:257–268. [PubMed] [Google Scholar]
- 14.Gauthier LV, Taub E, Perkins C, Ortmann M, Mark VW, Uswatte G. Remodeling the brain: plastic structural brain changes produced by different motor therapies after stroke. Stroke. 2008;39:1520–1525. doi: 10.1161/STROKEAHA.107.502229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mark VW, Taub E, Morris DM. Neuroplasticity and constraint-induced movement therapy. Eura Medicophys. 2006;42:269–284. [PubMed] [Google Scholar]
- 16.Taub E, Lum PS, Hardin P, Mark VW, Uswatte G. Automated Delivery of CI Therapy With Reduced Effort by Therapists. Stroke. 2005;36:1301–1304. doi: 10.1161/01.STR.0000166043.27545.e8. [DOI] [PubMed] [Google Scholar]
- 17.Uswatte G, Taub E, Morris D, Barman J, Crago J. Contribution of the shaping and restraint components of constraint-induced movement therapy to treatment outcome. NeuroRehabilitation. 2006;21:147–156. [PubMed] [Google Scholar]
- 18.Taub E, Uswatte G, Pidikiti R. Constraint-induced movement therapy: a new family of techniques with broad application to physical rehabilitation-a clinical review. J Rehabil Res Dev. 1999;36:237–251. [PubMed] [Google Scholar]
- 19.Mark V, Taub E, Perkins C, Gauthier L, Uswatte G. MRI infarction load and CI therapy outcomes for chronic post-stroke hemiparesis. Restor Neurol Neurosci. 2008;26:13–33. [PubMed] [Google Scholar]
- 20.Wolf SL, Catlin PA, Ellis M, Archer AL, Morgan B, Piacentino A. Assessing Wolf Motor Function Test as outcome measure for research in patients after stroke. Stroke. 2001;32:1635–1639. doi: 10.1161/01.str.32.7.1635. [DOI] [PubMed] [Google Scholar]
- 21.Morris DM, Uswatte G, Crago JE, Cook EW, III, Taub E. The reliability of the Wolf Motor Function Test for assessing upper extremity function after stroke. Arch Phys Med Rehabil. 2001;82:750–755. doi: 10.1053/apmr.2001.23183. [DOI] [PubMed] [Google Scholar]
- 22.Van der Lee JH, Beckerman H, Knol DL, de Vet HC, Bouter LM. Clinimetric properties of the Motor Activity Log for the assessment of arm use in hemiparetic patients. Stroke. 2004;35:1–5. doi: 10.1161/01.STR.0000126900.24964.7e. [DOI] [PubMed] [Google Scholar]
- 23.Uswatte G, Taub E, Morris D, Light K, Thompson PA. The Motor Activity Log-28: assessing daily use of the hemiparetic arm after stroke. Neurology. 2006;67:1189–1194. doi: 10.1212/01.wnl.0000238164.90657.c2. [DOI] [PubMed] [Google Scholar]
- 24.Uswatte G, Giuliani C, Winstein C, Zeringue A, Hobbs L, Wolf SL. Validity of accelerometry for monitoring real-world arm activity in patients with subacute stroke: evidence from the extremity constraint-induced therapy evaluation trial. Arch Phys Med Rehabil. 2006;87:1340–1345. doi: 10.1016/j.apmr.2006.06.006. [DOI] [PubMed] [Google Scholar]
- 25.Nichols T, Holmes A. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 2001;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kimberg DY, Branch Coslett H, Schwartz MF. Power in voxel-based lesion-symptom mapping. J Cogn Neurosci. 2007;19:1067–1080. doi: 10.1162/jocn.2007.19.7.1067. [DOI] [PubMed] [Google Scholar]
- 27.Damasio H, Tranel D, Grabowski T, Adolphs R, Damasio A. Neural systems behind word and concept retrieval. Cognition. 2004;92:179–229. doi: 10.1016/j.cognition.2002.07.001. [DOI] [PubMed] [Google Scholar]
- 28.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. chap. 2. Hillsdale, New Jersey: Lawrence Erlbaum Associates, Inc.; 1988. pp. 48–52. [Google Scholar]
- 29.Lang CE, Edwards DF, Birkenmeier RL, Dromerick AW. Estimating minimal clinically important differences of upper extremity measures early after stroke. Arch Phys Med Rehabil. 2008;89:1693–1700. doi: 10.1016/j.apmr.2008.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boroojerdi B, Diefenbach K, Ferbert A. Transcallosal inhibition in cortical and subcortical cerebral vascular lesions. J Neurol Sci. 1996;144:160–170. doi: 10.1016/s0022-510x(96)00222-5. [DOI] [PubMed] [Google Scholar]
- 31.Liepert J, Hamzei F, Weiller C. Lesion-induced and training-induced brain reorganization. Restor Neurol Neurosci. 2004;22:269–277. [PubMed] [Google Scholar]
- 32.Foltys H, Krings T, Meister IG, Sparinga R, Boroojerdia B, Thron A, Töpper R. Motor representation in patients rapidly recovering after stroke: a functional magnetic resonance imaging and transcranial magnetic stimulation study. Clin Neurophysiol. 2003;114:2404–2415. doi: 10.1016/s1388-2457(03)00263-3. [DOI] [PubMed] [Google Scholar]
- 33.Seitz RJ, Höflich P, Binkofski F, Tellmann L, Herzog H, Freund HJ. Role of the premotor cortex in recovery from middle cerebral artery infarction. Arch Neurol. 1998;55:1082–1088. doi: 10.1001/archneur.55.8.1081. [DOI] [PubMed] [Google Scholar]
- 34.Luft AR, Waller S, Forrester L, Smith GV, Whitall J, Macko RF, Schulz JB, Hanley DF. Lesion location alters brain activation in chronically impaired stroke survivors. Neuroimage. 2004;21:924–935. doi: 10.1016/j.neuroimage.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 35.Tombari D, Loubinoux I, Pariente J, Gerdelat A, Albucher JF, Tardy J, Cassol E, Chollet F. A longitudinal fMRI study: in recovering and then in clinically stable subcortical stroke patients. Neuroimage. 2004;23:827–839. doi: 10.1016/j.neuroimage.2004.07.058. [DOI] [PubMed] [Google Scholar]
- 36.Ago T, Kitazono T, Ooboshi H, Takada J, Yoshiura T, Mihara F, Ibayashi S, Iida M. Deterioration of pre-existing hemiparesis brought about by subsequent ipsilateral lacunar infarction. J Neurol Neurosurg Psychiatry. 2003;74:1152–1153. doi: 10.1136/jnnp.74.8.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johansen-Berg H, Rushworth MFS, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM. The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA. 2002;99:14518–14523. doi: 10.1073/pnas.222536799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shaw SE, Morris DM, Uswatte G, McKay S, Meythaler JM, Taub E. Constraint-induced movement therapy for recovery of upper-limb function following traumatic brain injury. J Rehabil Res Dev. 2005;42:769–778. doi: 10.1682/jrrd.2005.06.0094. [DOI] [PubMed] [Google Scholar]
- 39.Mark VW, Taub E, Bashir K, Uswatte G, Delgado A, Bowman MH, Bryson CC, McKay S, Cutter GR. Constraint-induced movement therapy can improve hemiparetic progressive multiple sclerosis. Preliminary findings. Mult Scler. 2008;14:992–994. doi: 10.1177/1352458508090223. [DOI] [PubMed] [Google Scholar]
- 40.Taub E, Griffin A, Nick J, Gammons K, Uswatte G, Law CR. Pediatric CI therapy for stroke-induced hemiparesis in young children. Dev Neurorehabil. 2007;10:3–18. doi: 10.1080/13638490601151836. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


