Abstract
OBJECTIVE
The Diabetes Control and Complications Trial (DCCT) demonstrated the powerful impact of glycemic control on the early manifestations of microvascular complications. Contemporary prospective data on the evolution of macrovascular and late microvascular complications of type 1 diabetes are limited. The Epidemiology of Diabetes Interventions and Complications (EDIC) study is a multicenter, longitudinal, observational study designed to use the well-characterized DCCT cohort of >1,400 patients to determine the long-term effects of prior separation of glycemic levels on micro- and macrovascular outcomes.
RESEARCH DESIGN AND METHODS
Using a standardized annual history and physical examination, 28 EDIC clinical centers that were DCCT clinics will follow the EDIC cohort for 10 years. Annual evaluation also includes resting electrocardiogram, Doppler ultrasound measurements of ankle/arm blood pressure, and screening for nephropathy. At regular intervals, a timed 4-h urine is collected, lipid profiles are obtained, and stereoscopic fundus photographs are taken. In addition, dual B-mode Doppler ultrasound scans of the common and internal carotid arteries will be performed at years 1 and 6 and at study end.
RESULTS
Written informed consent was obtained from 96% of the DCCT subjects. The participants, compared with nonparticipants, tended to have better glycemic control at the completion of the DCCT and were more likely to have their diabetes care provided by DCCT personnel. The EDIC baseline measurement stratified by sex delineates multiple cardiovascular disease risk factor differences such as age (older in men), waist-to-hip ratio (higher in men), HDL cholesterol (lower in men), hypertension (more prevalent in men), and maximum intimal-medial thickness of common and internal carotid arteries (thicker in men). Of the original conventional treatment group, 69% have changed to continuous subcutaneous insulin infusion or multiple daily injections. Although the mean HbA1c difference between the intensive and conventional treatment groups narrowed at EDIC years 1 and 2, HbA1c remained significantly lower in the intensive group. Of all expected clinic visits, 95% were completed, and the quality of EDIC data is very similar to that observed in the DCCT.
CONCLUSIONS
Although obvious problems exist in extended follow-up studies of completed clinical trials, these are balanced by the value of continued systematic observation of the DCCT cohort. In contrast to other epidemiologic studies, EDIC will provide 1) definitive data on type 1 as distinct from type 2 diabetes; 2) reliance on prospective rather than on cross-sectional analysis; 3) long-term follow-up in a large population; 4) consistent use of objective, reliable measures of outcomes and glycemia; and 5) observation of patients from before the onset of complications.
Morbidity and mortality in type 1 diabetic patients derive mainly from advanced microvascular, neuropathic, and macrovascular complications, with the major clinical impact beginning 15–20 years after the onset of diabetes (1,2). The Diabetes Control and Complications Trial (DCCT) demonstrated that therapy aimed at maintaining HbA1c levels as close to normal as feasible reduced the risks for the development and progression of early microvascular and neurologic complications of type 1 diabetes (3–5). While the reduction of the earlier stages of diabetic complications could reasonably be expected to slow the evolution to end-stage complications, such as loss of vision or renal failure, too few severe complications occurred during the DCCT to establish this conclusion. Similarly, although fewer intensively treated than conventionally treated patients in the DCCT experienced cardiovascular events (3,6), the numbers were too small to be conclusive and the differences were not statistically significant. Overall, relatively little is known about the development of cardiovascular disease in type 1 diabetes, although it is the major cause of mortality.
Currently available data on the evolution of long-term complications are limited by 1) failure to separate type 1 diabetes from type 2 diabetes in study populations; 2) reliance on cross-sectional studies that are prone to prevalence bias; 3) studies of small, selected populations with limited generalizability; and 4) relatively brief follow-up and significant attrition in prospective studies. Since most studies suggest that overt late-stage complications usually occur after 15–25 years’ duration of type 1 diabetes, further study of the DCCT cohort, with an average diabetes duration of 12 years at study end, would delineate the evolution of late-stage complications. In addition, the DCCT cohort offered the following advantages for a long-term follow-up study of advanced complications: 1) the early stages of these complications had been well characterized with reliable objective outcome measurements; 2) established and putative risk factors for cardiovascular complications had already been measured repeatedly; and 3) all of the subjects had been strongly advised to follow intensive treatment regimens after the conclusion of the DCCT. We therefore designed a protocol to examine the DCCT cohort in a prospective multi-center 10-year observational study. The Epidemiology of Diabetes Interventions and Complications (EDIC) study began in January 1994, shortly after the closeout of the DCCT, and after approval of the EDIC protocol by the Director of the National Institute of Diabetes, Digestive and Kidney Diseases (NIDDK). The EDIC study focuses on the interactions between established and putative risk factors for long-term microvascular, neurologic, and cardiovascular outcomes of type 1 diabetes, including prior diabetes treatment and the level of glycemic control during the DCCT.
Study objectives
The major study objectives include the following:
To describe the development and progression of cardiovascular (coronary, peripheral, and cerebral) disease in type 1 diabetes.
To study the effects and interactions of potential risk factors for cardiovascular disease in type 1 diabetes, including those established in nondiabetic and type 2 diabetic populations.
To examine the long-term effects of differences in prior diabetes treatment (conventional versus intensive) during the DCCT on the subsequent development and progression of cardiovascular disease.
To examine the development of abnormal lipid and lipoprotein levels over time, their relationship to metabolic and other variables, and their contribution, both independently and in conjunction with other risk factors, to the development of macrovascular diseases.
To relate early degrees of microalbuminuria, therapeutic interventions, and other established risk factors to the subsequent development of clinical nephropathy.
To study the rate of development of clinically significant neuropathy and its relationship to other complications and risk factors.
To examine the transition from background to more severe stages of retinopathy, such as proliferative diabetic retinopathy (PDR), and its relationship to established and putative risk factors, including previous treatment, ongoing level of glycemia, hypertension, and renal insufficiency.
To examine the long-term effects of differences in prior diabetes treatment during the DCCT on the development and progression of nephropathy, neuropathy, and retinopathy, refining estimates of the risks associated with varying levels of antecedent glycemic control.
To examine the effect(s) of putative genetic factors that may be identified in the future on the development and/or progression of all complications in type 1 diabetes and their interactions with other risk factors.
To observe the current health care provided to EDIC patients in the U.S. and Canada, including the implementation and maintenance of intensive therapy, and the associations between different types of medical care and health outcomes.
To study health-related quality of life (HRQOL) and the relationship between HRQOL and the development of clinically significant complications.
RESEARCH DESIGN AND METHODS
Subjects
At the completion of the DCCT, subjects were informed of the purpose, procedures, benefits, and risks of the EDIC. Written informed consent to participate in EDIC was obtained from 96% of DCCT subjects. All clinically relevant measurements obtained during follow-up would be provided to EDIC subjects and their physicians.
Organization
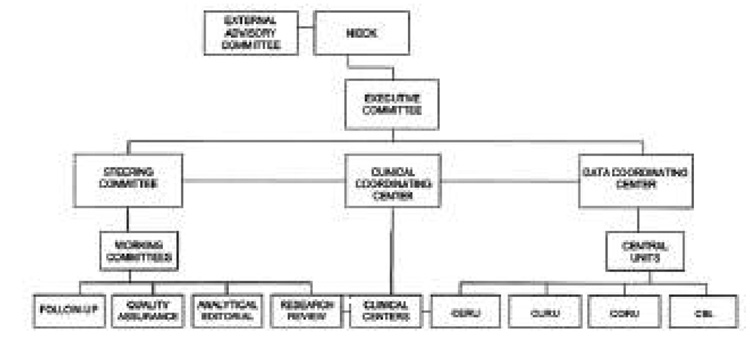
The organizational structure of the EDIC study is designed to coordinate the activities of the committees, laboratories, units, and review groups, and to ensure careful conduct of the study by uniform adherence to the Protocol and Manual of Operations (7) (Fig. 1). Of the 29 DCCT clinics, 28 opted to participate as EDIC clinical centers (1 of the 29 with relatively few patients was merged with its neighboring clinic). The organization of EDIC includes a clinical coordinating center, a data coordinating center, and four reading centers and laboratories (see ACKNOWLEDGMENTS for a listing of all EDIC participating centers, laboratories, and reading units). All study procedures and tests are performed in the EDIC clinical centers with standardized methods by trained and certified personnel. Analysis of samples and grading of eye photographs, carotid ultrasounds, and electrocardiograms (ECGs) are performed in the respective central laboratory or reading centers, using standardized quality-controlled methods.
Figure 1.
Organization chart for the EDIC study. CBL, central biochemical laboratory; CERU,-central ECG reading unit; CORU, central ophthalmologic reading unit; CURU, central ultrasound reading unit.
Procedures and methods
Each subject has a standardized annual history and physical examination on the anniversary of randomization into the DCCT (Table 1). This examination is performed within 4 months of the anniversary date and includes detailed evaluation of overall health status, diabetes management, occurrence of diabetic complications, development of new diseases since the previous annual visit, and all medications used. Measures of health satisfaction and quality of life are obtained every other year.
Table 1.
Schedule of follow-up examinations
| EDIC year |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Examinations (outcomes) | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 |
| Cardiovascular (CABG, MI, angina, CHF, stroke, TIA) | ||||||||||
| Standardized history (including family) and physical exam | X | X | X | X | X | X | X | X | X | X |
| ECG | X | X | X | X | X | X | X | X | X | X |
| Duplex carotid ultrasonography central review | X | X | X | |||||||
| Peripheral vascular (foot ulcer, amputation, bypass graft) | ||||||||||
| Standardized history and physical exam | X | X | X | X | X | X | X | X | X | X |
| Ankle/arm index by Doppler | X | X | X | X | X | X | X | X | X | X |
| Lipoprotein levels (hypercholesterolemia, hypertriglyceridemia) | ||||||||||
| Total cholesterol | ||||||||||
| HDL cholesterol | Scheduling of visits is a function of randomization date (alternate years) | |||||||||
| Triglycerides | ||||||||||
| Calculated LDL cholesterol | ||||||||||
| Nephropathic (renal failure, transplant, dialysis, elevated serum creatinine) | ||||||||||
| Standardized history and physical exam | X | X | X | X | X | X | X | X | X | X |
| Serum creatinine | X | X | X | X | X | X | X | X | X | X |
| Glomerular filtration | ||||||||||
| Albumin excretion rate | Scheduling of visits is a function of randomization date (alternate years) | |||||||||
| 4-h standard creatinine clearance | ||||||||||
| Neuropathy | ||||||||||
| MNSI | X | X | X | X | X | X | X | X | X | X |
| 10-g filament examination | X | X | X | X | X | X | X | X | X | X |
| Retinopathic (photocoagulation, vitrectomy, blindness, vitreous hemorrhage) | ||||||||||
| Standardized history | X | X | X | X | X | X | X | X | X | X |
| Ophthalmological exam* | ||||||||||
| Visual acuity* | ||||||||||
| Fundus photographs* | Scheduling of visits is a function of randomization date | |||||||||
| Hypoglycemia (mortality/morbidity) | ||||||||||
| Standardized history | X | X | X | X | X | X | X | X | X | X |
| Metabolic (DKA, chronic glycemia) | ||||||||||
| Standardized history | X | X | X | X | X | X | X | X | X | X |
| HbA1c | X | X | X | X | X | X | X | X | X | X |
| Psychological | ||||||||||
| Quality of life questionnaire (DQOL) | X | X | X | X | X | X | ||||
| Health status questionnaire (SF-36) | X | X | X | X | X | X | ||||
| Health care delivery | ||||||||||
| Standardized questionnaire | X | X | X | X | X | X | X | X | X | X |
| Dietary | ||||||||||
| Food frequency recall questionnaire | In conjunction with lipids | |||||||||
Ophthalmological exam, visual acuity, and fundus photographs to be done on the patient’s 8th, 12th, and 16th anniversaries of randomization. CABG, coronary revascularization; CHF, congestive heart failure; DQOL, Diabetes Quality of Life; SF, short form; TIA, transient ischemic attack.
Annual evaluations also include resting ECGs and Doppler ultrasound measurement of ankle/arm blood pressure, as well as screening for peripheral neuropathy by both 10-g filament examination (8) and administration of the Michigan Neuropathy Screening Instrument (MNSI) (9). A timed 4-h urine is collected in alternate years for measurement of albumin excretion rate and creatinine clearance; lipid profiles are obtained in the years that renal studies are not performed; and a dietary recall questionnaire is given in conjunction with the lipid assessment. Dual B-mode Doppler ultrasound scans of the common and internal carotid arteries were carried out at entry into the EDIC study and are expected to be repeated at intervals of 5 years. Table 2 lists the specific methods used in EDIC.
Table 2.
Methods in EDIC
| Measurement | Method or assay |
|---|---|
| Glycosylated hemoglobin | High-performance ion-exchange liquid chromatography |
| Serum creatinine | Automated kinetic method with Jaffè reaction |
| Urine creatinine | Automated kinetic method with Jaffè reaction |
| Urine albumin | Solid-phase fluoroimmunoassay |
| Serum albumin | Thin-film adaption of a bromcresal colorimetric procedure |
| Serum cholesterol | Cholesterol oxidase, spectrophotometric |
| Serum triglyceride | Glycerol-blanked glycerol kinase/glycerol oxidase, spectrophotometric |
| Serum HDL cholesterol | Magnesium dextran precipitation |
| Calculated LDL cholesterol | Friedewald equation |
| 12-lead resting ECG | Central reading using revised Minnesota Code |
| Intimal-media wall thickness | High resolution β-mode ultrasound graded centrally with standardized protocol |
| Fundus photograph | Central reading of seven-field stereo photographs using final ETDRS grading scale for retinopathy and macular edema |
| Blood pressure | |
| Systolic | Sitting, right arm reading with sphygmomanometer |
| Diastolic | |
| Ankle-to-arm blood pressure ratio | Resting systolic blood pressure with a Doppler ultrasonic instrument |
| Food frequency | Harvard Food Frequency; self-administered |
| Current medication | EDIC Form 004; administered by study coordinator |
| Hypoglycemia | Interview at annual visit |
| Neuropathy | MNSI |
| HRQOL | HRQOL (DQOL, SF-36) |
DQOL, Diabetes Quality of Life; SF, short form.
DNA has been obtained from peripheral blood leukocytes in all subjects and stored as a long-term resource for potential analyses.
Quality control
Quality control procedures in the EDIC study include those in place internally in all the laboratories and reading centers as well as those implemented as part of EDIC data collection. The local clinic procedures that require training and certification include the performance of Doppler ankle-arm index, 10-g filament test, renal studies, ECG recording, carotid ultrasound, and fundus photographs. All blood and urine tests undergo repeated assessments for analytic precision by assays of split-duplicate samples in the central laboratory. Split-duplicate analysis is also used to monitor grading of the carotid ultrasound recordings, ECGs, and fundus photographs.
Analytic procedures
General principles
Defined incident events are coded for analysis based on the measurements and evaluations noted above. Previously documented or treated events in the DCCT are risk factors and not incident events. Deaths and major morbid events will be classified by a classification committee composed of a cardiologist and two diabetologists who are masked to previous treatment assignment in the DCCT and current diabetes treatment. The definitions of these events are as follows:
Cardiovascular disease: death secondary to cardiovascular disease or any sudden death judged not to be caused by hypoglycemia or other known reason, acute myocardial infarction (MI), silent MI appearing as a major new Q-wave abnormality on a routine ECG, initiation of thrombolytic therapy for suspected MI, coronary artery disease (CAD) requiring bypass surgery or angioplasty, or CAD confirmed by angiography or by a combination of angina and ischemia documented with noninvasive testing.
Hypercholesterolemia: calculated LDL cholesterol ≥160 mg/dl on two occasions 24 months apart or the use of lipid-lowering medication for previously documented hypercholesterolemia as defined in the DCCT (6).
Hypertriglyceridemia: serum triglyceride >400 mg/dl on two occasions 24 months apart or the use of lipid-lowering medication for previously documented hypertriglyceridemia as defined in the DCCT (6).
Cerebrovascular disease: stroke or transient ischemic attack confirmed by angiography or noninvasive testing.
Peripheral vascular disease (PVD): surgical amputation of a lower extremity necessitated by vascular disease, arterial vascular events requiring bypass or angioplasty, claudication with exercise testing or angiographic evidence of vascular disease, or an ankle-to-arm blood pressure ratio <0.8 or >1.4.
Lower-extremity ulcer: a traumatic or nontraumatic excavation or loss of subcutaneous tissue in the foot or leg that requires medical or surgical treatment by a health professional in an office or hospital setting irrespective of whether the etiology is neuropathic, ischemic, or both.
Hypertension: confirmed sitting systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg, or the use of antihypertensive medication for previously documented hypertension.
Microalbuminuria: urinary albumin excretion of ≥28 µg/min during the 4-h timed collection.
Albuminuria: urinary albumin excretion ≥208 µg/min during the 4-h timed collection.
Renal insufficiency: serum creatinine ≥2 mg/dl, glomerular filtration rate <70 ml · min−1 · 1.73 m−2, or the need for dialysis or renal transplantation.
Doubling of serum creatinine: doubling of centrally measured serum creatinine from DCCT baseline.
Advanced retinopathy: PDR according to the final Early Treatment of Diabetic Retinopathy Study (ETDRS) grading scale (10).
Blindness: loss of vision in one or both eyes, defined as visual acuity of 20/200 or worse.
Photocoagulation: focal or panretinal for macular edema or PDR.
Severe hypoglycemia: events that require assistance from another individual, including episodes of seizure and/or coma.
Diabetic ketoacidosis (DKA): an event characterized by hyperglycemia (>200 mg/dl) in the presence of ketonuria and acidemia requiring treatment at a health care facility.
Baseline
The baseline data for EDIC are defined as that collected during the first two visits (to provide the full complement of data, some of which was collected biannually) to an EDIC clinical center that occurred between 1 January 1994 and 31 December 1995. Those visits were scheduled as close as possible to the subject’s DCCT randomization anniversary.
Data management
Data management in the EDIC study follows the principles established in the DCCT, but specific procedures take advantage of new technical advances.
Data management and statistical analysis are conducted using SAS software (SAS Institute, Cary NC). Incoming data forms are keyed into SAS data files for the appropriate form types, then merged into the corresponding master files after new records are edited for possible data errors by SAS application programs.
Data forms are tracked through the data coordinating center by a check-off system that is reviewed at each step (log-in, keying, editing) to ensure that no forms are lost during paper handling. Lab result reports are distributed biweekly to all clinics. This system provides a mechanism for detecting errors and explaining irregularities in the study database.
Statistical analyses
Significance level
All significance tests for the comparisons (predominantly between the groups previously assigned to intensive and conventional therapy) will be two-sided. A Bonferroni adjustment (11) will be used to control for the multiple pairwise comparisons of treatment arms and the multiple primary outcomes. All results at P < 0.05 will be considered significant.
Intention to treat
All hypothesis testing with regard to possible persistent effects of intensive therapy during the DCCT will adhere to the intention-to-treat approach, i.e., all data will be analyzed according to the participants’ original DCCT treatment assignment regardless of subsequent treatment during EDIC. Epidemiologic analyses will use treatment assignment during the DCCT as one variable included in the study of risk factors and outcomes.
Stratification
Analyses may be stratified by retinopathy status at entry into the DCCT, duration of type 1 diabetes, HbA1c, age, sex, and other relevant factors. The original DCCT cohort was stratified into a primary prevention cohort and a secondary intervention cohort based on duration of diabetes and the presence of retinopathy (3). Where similar results are obtained for the two cohorts, a single pooled analysis for all EDIC patients combined may be presented.
For outcomes collected during the EDIC follow-up period (starting with year 1 EDIC time, 1994), analyses of cumulative incidence will use standard life-table methods for grouped time intervals (12). These include the modified actuarial life table with tests of differences between groups, using the log-rank test, and analyses adjusting for other covariates, using the proportional hazards regression model (12). Some outcome measures obtained in the DCCT and also in EDIC, e.g., retinopathy, will be assessed at time points relative to the original randomization into the DCCT (i.e., DCCT time). Therefore, at any fixed point in EDIC time, DCCT time will vary from subject to subject.
When outcomes collected in DCCT time are analyzed starting from the date of initiation of the EDIC, subjects will have staggered gaps of unequal length in their periods of observation. Likewise, observations collected in EDIC time and analyzed in DCCT time will also have unequal gaps between assessments. In such cases, analyses of cumulative incidence will be performed using methods for interval-censored observations that allow for unequal intervals between visits. These include the Turnbull estimate of the survival (cumulative incidence) function (13) and the generalizations of the log-rank test and the proportional hazards regression model (14) for such interval-censored data.
In addition, longitudinal analyses of point prevalence will be conducted. Most such analyses will be conducted in DCCT time using multivariate methods for the analysis of prevalence of quantitative, ordinal, or qualitative measures. These include the Wei-Lachin test for qualitative observations (15), the method of generalized estimating equations (16), and longitudinal mixed-effects growth-curve models (17).
The incidence of single or recurrent events, such as hypoglycemia, will be summarized as a crude rate. Such rates will be presented as the number of events per 100 patient-years based on the ratio of the observed number of events to the total patient-years of exposure. The standard error for such rates will be computed allowing for overdispersion (18). The risk ratio (relative risk) will be used to summarize the difference between groups, and tests will be based on the large-sample estimate of the variance of the log of relative risk (19). To account for the effects of covariates on the incidence rate, either the Poisson regression model (18) or the multiplicative intensity model (20) will be used. Analyses that assess the association between various outcomes and a time-dependent covariate, such as the HbA1c level over time, will use the appropriate regression models described above.
Power calculations
Estimates of the statistical power of intention-to-treat comparisons of cause-specific mortality between the two original DCCT treatment groups after an additional 10 years of follow-up in EDIC are given in Table A1 of APPENDIX 1. Estimates of 10-year mortality among patients randomized to conventional treatment in the DCCT were based on the weighted average of age-specific mortality rates reported in ETDRS (F. Ferris, personal communication). Since the ETDRS reported 5-year mortality, the estimate was constructed in two stages by applying the appropriate ETDRS mortality rates to the expected number of survivors at the end of the first 5-year period.
The estimated power of the EDIC to find a difference in the 10-year prevalence of combined nephropathy outcomes (death from kidney failure, kidney transplant, renal dialysis, candidacy for renal transplant or dialysis, and clinical proteinuria) is shown in Table A4 of APPENDIX 1. These estimates are based on two-sided comparisons of the original DCCT treatment groups at a significance level of 0.05. The 10-year prevalence of this outcome among subjects randomized to the DCCT conventional treatment group was also based on ETDRS data stratified by age and duration of type 1 diabetes.
RESULTS
Recruitment of DCCT subjects into the EDIC
In January 1994, the 1,425 surviving DCCT patients were invited to participate in the EDIC; 1,375 subjects (96%) agreed to participate, of whom 687 had been originally assigned to intensive treatment and 688 to conventional treatment. The major demographic and clinical characteristics of the active EDIC participants and of the 50 subjects who chose not to participate are shown in Table 3. Compared with participants, the nonparticipants tended to have worse glycemic control at the completion of the DCCT, fewer had been debriefed, and the majority were no longer under the care of former DCCT personnel (Table 3).
Table 3.
Characteristics of EDIC participants compared with nonparticipants
| Characteristic* | Participants | Nonparticipants | P value |
|---|---|---|---|
| n | 1,375 | 50 | — |
| Age (years) | 33.6 ± 7.0 | 31.0 ± 7.7 | 0.0155 |
| Sex (% female) | 48 | 45 | NS |
| Duration of type 1 diabetes (years) | 12.2 ± 4.8 | 11.6 ± 4.4 | NS |
| Treatment group during DCCT (% intensive) | 50 | 30 | 0.0048 |
| HbA1c at closeout of DCCT (%) | |||
| Intensive group | 7.4 ± 1.1 | 8.5 ± 1.6 | 0.0031 |
| Conventional group | 9.1 ± 1.5 | 9.6 ± 1.4 | 0.1123 |
| Debriefed at DCCT study’s end (%) | 99 | 74 | <0.0001 |
| Care transferred to non-DCCT personnel (%) | 48 | 79 | <0.0001 |
Data are means ± SD or %. P values for continuous variables are from Wilcoxon’s rank-sum test; P values for categorical variables are from the contingency-table χ2 test.
EDIC baseline
Table 4 presents various risk factors for cardiovascular disease separately for men and women at EDIC baseline. The men and women were similar with respect to age, duration of type 1 diabetes, HbA1c, and proportion who were currently smoking. As expected, the men and women differed on several risk factors. Hypertension, a low serum HDL cholesterol, and a high LDL cholesterol were more frequent in men. In Table 5, the baseline measurements of ankle-to-arm systolic blood pressure ratio (PVD) and of carotid artery intimal-medial thickness (atherosclerosis) are presented, stratified by sex and decade of age for EDIC patients. PVD, defined as ankle-to-arm systolic blood pressure ratio <0.8, although uncommon, was equally prevalent in men and women. There were no trends with age or duration of type 1 diabetes.
Table 4.
Risk factors measured during the first 2 years of the EDIC study, based on the most recent observation from each patient
| Men | Women | P value | |
|---|---|---|---|
| n (%) | 719 (52.4) | 653 (47.6) | — |
| Age (years) | 36.4 ± 6.6 | 35.4 ± 7.2 | 0.0068 |
| Duration of type 1 diabetes (years) | 14.3 ± 4.8 | 14.8 ± 5.0 | NS |
| BMI (kg/m2) | 26.6 ± 3.9 | 26.0 ± 4.2 | 0.0001 |
| Overweight (%) | 30.9 | 31.8 | NS |
| Waist-to-hip ratio | 0.88 ± 0.06 | 0.77 ± 0.07 | <0.0001 |
| Insulin dose (U · kg−1 · day−1) | 0.71 ± 0.25 | 0.69 ± 0.24 | NS |
| HbA1c (%) | 8.2 ± 1.3 | 8.3 ± 1.5 | NS |
| Total cholesterol (mg/dl) | 185.1 ± 35.6 | 188.1 ± 37.0 | NS |
| Triglyceride (mg/dl) | 96.8 ± 75.8 | 83.1 ± 73.3 | 0.0001 |
| HDL cholesterol (mg/dl) | 49.5 ± 12.0 | 59.2 ± 14.0 | <0.0001 |
| <35 mg/dl (%) | 8.2 | 1.6 | <0.0001 |
| LDL cholesterol (mg/dl) | 116.4 ± 30.8 | 112.1 ± 30.3 | 0.0083 |
| > 130 mg/dl (%) | 30.6 | 26.0 | NS |
| Hypertension (%) | 26.6 | 18.1 | 0.0002 |
| Current cigarette smoker (%) | 22.7 | 19.9 | NS |
| Exercise level | <0.001 | ||
| Strenuous | 10.3 | 2.9 | |
| Vigorous | 5.9 | 3.4 | |
| Moderate | 49.5 | 58.2 | |
| Sedentary | 34.3 | 35.4 | |
| Current alcohol use (%) | 47.4 | 32.2 | <0.001 |
| Urinary albumin excretion (mg/24 h) | 38.1 ± 118.4 | 41.8 ± 226.9 | NS |
| DQOL total score | 76.4 ± 9.4 | 75.3 ± 8.6 | 0.0184 |
Data are means ± SD or %. P values are for men versus women. Waist-to-hip ratio is based on natural waist circumference. Hypertension is percent diagnosed as hypertensive at any time during DCCT or EDIC and is defined as systolic blood pressure ≥140 mmHg and/or diastolic blood pressure ≥90 mmHg or use of anti-hypertensives. Alcohol use is percent reporting consumption of at least one alcoholic beverage per week. DQOL, Diabetes Quality of Life.
Table 5.
New measurements in the EDIC protocol
| Systolic blood pressure ratio of resting ankle to arm |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Prevalence of abnormal ankle-to-arm ratio (percent in any four ratios) |
Maximum intimal-medial thickness of common and internal carotid artery |
|||||||||||
| Age decade | n | Right | Left | Percent <0.8 | P (0.8) | Percent <1.4 | Percent either | n | Common (mm) | Internal (mm) | ||
| Women* | 20–29 | 154 | 1.08 ± 0.11 | 1.08 ± 0.13 | 2.6 | 0.9864 | 0.0 | 2.6 | 172 | 0.616 ± 0.073 | 0.583 ± 0.092 | |
| 30–39 | 289 | 1.11 ± 0.12 | 1.10 ± 0.13 | 2.8 | 0.1307 | 5.9 | 8.3 | 278 | 0.657 ± 0.081 | 0.632 ± 0.147 | ||
| 40–49 | 202 | 1.09 ± 0.12 | 1.07 ± 0.11 | 3.5 | 0.7093 | 1.5 | 5.0 | 178 | 0.696 ± 0.079 | 0.719 ± 0.226 | ||
| Men* | 20–29 | 117 | 1.07 ± 0.11 | 1.08 ± 0.10 | 2.6 | 2.6 | 5.1 | 125 | 0.636 ± 0.059 | 0.629 ± 0.083 | ||
| 30–39 | 351 | 1.11 ± 0.12 | 1.10 ± 0.12 | 1.1 | 3.7 | 4.8 | 350 | 0.684 ± 0.083 | 0.684 ± 0.114 | |||
| 40–49 | 241 | 1.13 ± 0.13 | 1.12 ± 0.14 | 4.1 | 3.7 | 7.9 | 211 | 0.745 ± 0.104 | 0.806 ± 0.261 | |||
Data are n, means ± SD, or %. Dorsalis pedis and posterior tibral pressures were combined using an algorithm of Hiatt et al. (51). P values are for men vs. women.
P value for trend in percent <0.8: women, 0.6171; men, 0.1513. P < 0.0001 for both common and internal intimal-medial thickness; all are from Wilcoxon’s rank-sum test after linear adjustment for covariance with age.
The average maximums of the carotid artery wall thickness for the common and internal carotid arteries were different between men and women (P < 0.0001). Adjusting for height reduced, but did not eliminate, the difference (P = 0.0007). A test of trend for maximum wall thickness over decades of age was significant in all strata (P ≤ 0.0001). Attained duration of diabetes was associated with wall thickness in both carotid arteries in men (r = 0.13 and 0.12, P < 0.01) but only in the internal carotid artery in women (r = 0.12, P < 0.01).
Table 6 describes diabetes management in the EDIC cohort in the first 24 months after DCCT closeout. After the completion of DCCT data collection, conventionally treated subjects were offered, and strongly encouraged to accept, DCCT clinic help in implementing intensive therapy. Following this, an orderly transfer of diabetes care, either to personnel in the center (former DCCT or non-DCCT physicians) or to other care providers was effected for all subjects. This transition took place between June and December of 1993. During the first 2 years after DCCT closeout, 69% of the original conventional group were using either multiple daily injections (MDI) or continuous subcutaneous insulin injection (CSII) therapy while 95% of those originally assigned to the intensive treatment group continued using MDI or CSII. Similar proportions of the two original DCCT treatment groups were using human insulin preparations, with the previous intensive group continuing to use more daily insulin. A higher proportion of the previous intensive group was performing ≥4 self-monitoring of blood glucose tests per day. Rates of severe hypoglycemia and DKA were comparable in the two groups, but a larger fraction of the DCCT intensive treatment cohort was classified as overweight (Table 5).
Table 6.
Diabetes management of EDIC cohort during the first 2 years of EDIC
| DCCT treatment group assignment |
|||
|---|---|---|---|
| Intensive | Conventional | P value | |
| n | 687 | 688 | — |
| Insulin delivery during EDIC | <0.0001 | ||
| CSII | 37.0 | 12.6 | |
| MDI | 57.6 | 56.9 | |
| One or two injections/day | 5.3 | 30.3 | |
| Unknown | 0.1 | 0.3 | — |
| Human insulin (% of subjects using) | 91.1 | 90.8 | NS |
| Insulin dose (U · kg−1 · day−1) | 0.75 ± 0.28 | 0.67 ± 0.20 | <0.0001 |
| Self-monitored blood glucose ≥4/day (%) | 46.4 | 36.4 | 0.0002 |
| Hypoglycemia (rate per 100 patient-years) | |||
| Coma/seizure | 6.3 | 7.1 | NS |
| Requiring assistance | 25.4 | 25.7 | NS |
| DKA (rate per 100 patient-years) | 2.68 | 2.37 | NS |
| Overweight (%) | |||
| Men | 32.5 | 29.7 | NS |
| Women | 38.4 | 25.2 | 0.0005 |
Data are means ± SD. P values are from the contingency-table χ2 test for categorical variables, Wilcoxon’s rank-sum test for continuous variables, and from a Wald test of the log-relative adjusted for overdispersion of event rates. Overweight is defined for men as BMI (kg/m2) >27.8 from the second National Health and Nutrition Examination Survey (NHANES II) of 1976 to 1980 (50) and for women as BMI (kg/m2) >27.3.
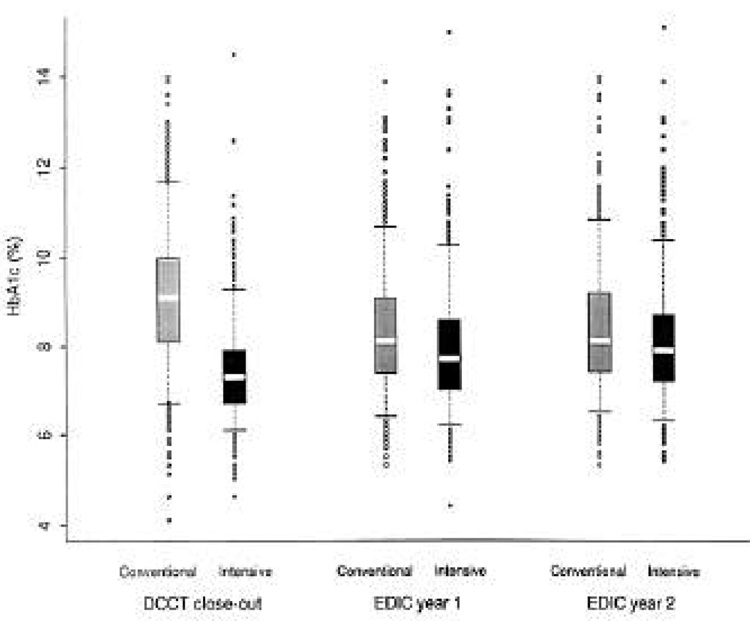
Figure 2 presents the distribution of HbA1c for the intensive and conventional treatment groups at DCCT closeout and at EDIC years 1 and 2. Although the difference between the treatment groups narrowed, HbA1c remained significantly lower in the intensive group (P < 0.0001) at each time point.
Figure 2.
Distribution of HbA1c (intensive vs. conventional treatment group) at DCCT closeout and EDIC years 1 and 2. For each distribution, the median is shown by the white area with 25th and 75th percentiles shown by the boxes and 5th and 95th pecentiles shown by the bars.
Data completeness and timeliness
Of all expected clinic visits, 95% occurred, and no decline occurred between years 1 and 2. Typically, there was a 1-month interval between the collection of each sample in the clinic and the feedback report to the clinic.
Data quality
The precision of analysis of HbA1c and lipids and renal function, ECGs, carotid ultrasound, and fundus photographs during the initial 2-year follow-up ranged from 0.88 (ECG) to 0.99 (HbA1c, albumin excretion rate, serum cholesterol, and serum LDL cholesterol). The precision of these measurements is very similar to that observed over the 9 years of the DCCT
CONCLUSIONS
The DCCT recruited 1,441 subjects with type 1 diabetes between 1983 and 1989 to a randomized clinical trial designed to examine the effects of intensive treatment compared with conventional treatment on the development and progression of early microvascular, neurologic, and other complications (3–6). The adherence of the subjects to the complex protocol was extraordinary, with <3% loss to follow-up and <3% non-study-mandated deviation from assigned treatment over the 10 years of the study After the closeout of the DCCT, these subjects have continued to demonstrate their remarkable stability as a research cohort, and 96% of them are now enrolled in the EDIC study.
The generalizability of the findings in this study cohort to the population of type 1 diabetes is germane to the rationale for EDIC. A collaborative study between the DCCT and the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) compared the DCCT cohort to a population-based type 1 diabetes cohort (22,23). The EDIC cohort has a narrower age range (age at entry to EDIC is ~17–50 years vs. 16–78 in WESDR in 1994) and is healthier, with relatively few subjects having clinically significant diabetes complications. Comparisons of the conventionally treated DCCT subjects at baseline with the respective WESDR group revealed older age and older age at diagnosis, lower HbA1c, and more frequent insulin injections and monitoring in the DCCT cohort, but few other substantive differences between the populations. Moreover, the 4-year progression of retinopathy and its association with baseline HbA1c were similar for the two cohorts, except for a lower rate of progression in the DCCT secondary intervention cohort than in its WESDR counterpart, perhaps because of lower HbA1c in the DCCT (23). Thus, the entire EDIC cohort is reasonably representative of the type 1 diabetic population, at least with respect to retinopathy.
CAD
With respect to potential risk factors for macrovascular disease, the DCCT excluded patients with hypertension, nondiabetic hyperlipidemia, and known CAD (3). The EDIC cohort provides an opportunity to examine a population of type 1 diabetic patients without obvious CAD risk factors at baseline, other than their diabetes, that has had careful prospective measurement of many of the CAD risk factors established in type 2 diabetic and nondiabetic populations. The randomized interventions during the DCCT might influence the development of CAD either directly, by altering glycemia, or indirectly, by altering lipid levels (6) or by changing the development of nephropathy (5). The DCCT did not show an effect of intensive treatment on blood pressure (6). In addition, other effects of intensive therapy, such as increased weight gain, which has persisted in the EDIC cohort previously treated with intensive therapy, might alter the risk for CAD. Therefore, randomized treatment assignment and HbA1c during the DCCT will be included as a covariate in analyses of CAD outcomes.
At the end of the DCCT in 1993, the entire cohort had a mean age of 33 years and mean durations of diabetes of 9 and 15 years in the primary prevention and secondary intervention cohorts, respectively. Consistent with their young age, the exclusion of patients with preexisting macrovascular risk factors, and the low incidence of nephropathy during the DCCT, only a small number of CAD events had occurred by the study’s end (6). However, CAD events are likely to increase in frequency during the 10-year follow-up of the EDIC study. By study’s end, the mean age of the EDIC population will approach 43 years, and mean duration of diabetes will be 19 and 25 years in the primary prevention and secondary intervention cohorts, respectively. Based on estimates derived from previous studies (24–31), the prevalence of CAD as manifested clinically and/or as detected by ECG or exercise tolerance tests is likely to be 20–40% in the DCCT secondary intervention cohort. Because age appears to be more important than duration of diabetes for the risk of CAD, only a modest downward adjustment in expected prevalence (to 15–30%) is required for the DCCT primary prevention cohort. Although clinical event rates may be further lowered by aggressive treatment with antihypertensive and hypolipidemic agents during the course of EDIC, the use of carotid ultrasound to determine intimal-medial wall thickness will enhance the sensitivity for detecting atherosclerosis. In a recent study of type 1 diabetes, patients with a 10-year history of better glycemic control exhibited significantly less arterial disease, as measured by carotid ultrasound, than poorly controlled patients (32).
PVD
PVD is also a major cause of morbidity loss of productivity and hospital expense and contributes to mortality in type 1 diabetes (33). The development of PVD and its relationship to potential risk factors in type 1 diabetes have not been determined definitively Studies such as the Pittsburgh Epidemiology of Diabetes Complications Study provide data on which to base the expected prevalence of PVD in type 1 diabetes (24). As in CAD, attained age appears to be a more important predictor of PVD than duration of diabetes. For patients 18–29 years of age, PVD prevalence defined by an ankle-to-arm blood pressure ratio <0.8 is 2–4% for diabetes duration ranging from 5–9 years to 25–29 years, compared with 18% for patients aged >30 years with similar duration. A prevalence of 16% was found in a sample of type 1 diabetic patients from a Seattle, Washington, registry with a mean age of 34 years and a mean duration of diabetes of 17 years. The prevalence of PVD increased from ~12 to 40% between the ages of 34 and 45 (32). A threefold greater prevalence of PVD in women than in men has been observed, with smoking and hypertension (24) and retinopathy (34) identified as potential risk factors.
Based on these data, we estimate that the cumulative prevalence of PVD detected by ankle-to-arm blood pressure ratios at the end of EDIC will be 32% in the primary prevention cohort and 44% in the secondary intervention group of the DCCT. To ascertain accurately and objectively the development of PVD, we have implemented measurement of the ankle-to-arm blood pressure ratio, a sensitive and specific method that is relatively easy to apply and standardize in the context of a multi-center study.
Cerebrovascular disease
The relatively low frequency of stroke compounded by the previous lack of widely available sensitive noninvasive diagnostic methods has made the study of cerebrovascular disease in type 1 diabetes problematic. Although it is assumed that cerebrovascular disease is more common in the diabetic population than in the nondiabetic population, there are few reports of its prevalence (35,36).
The 30-year-old data from the Joslin Clinic suggest that cerebrovascular disease accounts for 6.8% of deaths in diabetic patients with diabetes onset at age <20 years (37). Cerebrovascular disease also accounted for 7% of deaths among youth-onset Danish diabetic patients, a 50% higher rate than the level in similarly aged nondiabetic subjects (36). Neither study included sufficient data on vascular disease risk factors or chronic glycemia to permit analysis. More current data from the ETDRS indicate an increasing frequency of strokes with increasing age in their type 1 diabetic population (F. Ferris, personal communication). By extrapolation of the ETDRS data, the prevalence of stroke in the age range of the EDIC cohort at the end of 10-year follow-up will be ~6–8%.
This relatively low anticipated prevalence of cerebrovascular disease events may yield too few cases to analyze meaningfully However, noninvasive measurement of the carotid artery wall thickness provides a relatively accurate and specific means of quantifying carotid atherosclerosis. High-resolution B-mode ultrasonography can be performed with standardized methods at multiple centers with a high degree of reproducibility and acceptable center-to-center variability (38). The quality of the data obtained in the EDIC cohort at baseline suggests that this method will provide a useful and reproducible measurement of carotid artery wall thickness as it changes over time (39).
Diabetic nephropathy
Because of the relatively brief duration of type 1 diabetes in the DCCT cohort and the exclusion of patients with proteinuria, the patient cohort recruited experienced only a small number of advanced renal events (clinical grade proteinuria, n = 55, and/or renal insufficiency, n = 2) by study’s end. Whether the demonstrated decrease in development of microalbuminuria and clinical albuminuria with intensive therapy translates into a decrease in more advanced renal disease is a clinically important question that will be answered in the EDIC study Previous observational studies have shown that the yearly incidence of clinical nephropathy (i.e., >500 mg proteinuria per day) begins to rise at 10 years’ duration of type 1 diabetes and reaches a peak between 11 and 15 years. In the ETDRS, renal insufficiency requiring dialysis or transplantation developed within 5 years in 12.9% of subjects with duration of type 1 diabetes between 11 and 15 years at entry and in 8.7% of subjects with a duration between 15 and 20 years (F. Ferris, personal communication). Thus, 20–25% of EDIC patients who had diabetes of >11 years’ duration would be predicted to develop renal insufficiency over a 10-year follow-up during the EDIC study. The implementation of preventive and therapeutic modalities, such as treatment of hypertension or the use of ACE inhibitors, will be tracked and can be adjusted for by multivariate analysis.
Advanced diabetic retinopathy
The EDIC will study the development of more advanced retinopathy in the DCCT cohort. Longitudinal and cross-sectional studies suggest a progressive increase in diabetic retinopathy from background to preproliferative to proliferative stages (22,40,41). The baseline degree of retinopathy and, in particular, the number of microaneurysms and retinal hemorrhages are high-risk factors for later development of PDR (22). Therefore, the demonstration by the DCCT of a beneficial effect of intensive insulin treatment on progression from no retinopathy to background retinopathy and ultimately to preproliferative and severe retinopathy suggests that glycemic control should have a similar protective effect in PDR with high-risk characteristics. Long-term study of the EDIC population should address this question directly. Older retrospective studies indicate that the period of most rapid development of PDR begins at 10–13 years of duration (41). At closeout, the DCCT cohort had a mean duration of 12 years. From the prospective WESDR study results, we can expect ~20% of the EDIC cohort to develop PDR (and 4–5% to reach high-risk characteristics) in each 5-year segment of the study. A higher proportion of these events would be expected to occur in the original DCCT secondary intervention group than in the primary prevention group. Other factors have been demonstrated to contribute to the risk of retinopathy progression, including higher diastolic blood pressure (42), renal insufficiency and microalbuminuria (43), and duration of diabetes (22). Certain HLA haplotypes have been reported to be associated with higher risk (44,45). Of particular interest, the DCCT has now demonstrated concordance for severity of retinopathy within multiplex families with type 1 diabetes (46). How these risk factors interact with glycemic control will be addressed in the EDIC study.
Value of extended follow-up of a clinical trial
The potential scientific gains of conducting extended observational follow-up of subjects from completed randomized clinical trials have been summarized (47,48). These include the following: 1) additional beneficial or adverse effects of a treatment regimen on more slowly developing, but possibly more serious, consequences may be discovered (an example is the demonstration by a 9-year extended follow-up that nicotinic acid reduced coronary heart disease mortality and all-cause mortality after the original 6-year randomized clinical trial had demonstrated a decrease in MIs but not in mortality [49]); 2) particular subgroups with differential treatment benefits may be identified, generating new hypotheses and new randomized clinical trials; and 3) acquisition of up-to-date natural history data in the context of community treatment can help in the design of future intervention trials.
While there are potential problems in extended follow-up studies, EDIC may have limited, if not eliminated, them (47,48). First, the design and data to be collected are subject to bias if they are based on knowledge of results from the randomized trial itself. However, the EDIC protocol was largely developed before unmasking of the DCCT results and was, therefore, uninfluenced by them. Second, data acquired with extended follow-up may lack completeness due to subject unwillingness to undergo sufficiently frequent or rigorous examination, diminished resources, and/or long-term subject attrition. The experience of EDIC thus far suggests that subject adherence has not declined, and the study is sufficiently supported by available resources to anticipate nearly complete data collection. Third, future differences in outcomes may be blurred by treatment crossovers, by introduction of new treatments, or by withdrawal from treatments because treatment is not systematically regulated during the post-trial follow-up. The former intensive treatment group has largely continued on that regimen, albeit with a modest increase in average HbA1c. The former conventional treatment group was appropriately encouraged to cross over to intensive treatment based on the results of the DCCT. It is, therefore, possible that the narrowed differences between the two groups with regard to treatments and in the resultant mean HbA1c levels may partly obscure differences in long-term outcomes over time. Multivariate analyses can help to identify such confounding (4). Estimates of the statistical power to detect differences in outcomes during extended follow-up by continuation of intention-to-treat analysis are subject to uncertainty with regard to future dropout rates, introduction of new therapies that will influence measured outcomes, or inadequacy of the natural history data that were available for the power calculations. None of these potential difficulties are likely to be limiting in EDIC. Subject dropout can be strongly influenced by the strong bonds that developed with the subjects during the DCCT (5). Moreover, our subjects realize that EDIC is a unique study and do not seem inclined to join other studies. It may be difficult to ascertain accurately the rigor with which each of two previous randomized treatments is actually used by subjects during a follow-up study. In EDIC, HbA1c measurements are a reasonable surrogate for the diabetes treatment regimens themselves. Since glycemic differences were the dominant factor that generated treatment-group differences in outcomes during the DCCT, monitoring of HbA1c will permit analysis of glycemia as a risk factor. The EDIC investigators and the NIDDK have concluded that the potential weaknesses of extended follow-up studies are greatly outweighed by the significant value of continued systematic observation of the DCCT cohort and that the EDIC is a valuable natural history study of a cohort previously enrolled in a controlled clinical trial.
In conclusion, the EDIC study successfully enrolled 96% of the surviving DCCT cohort in a long-term study that will focus on late-occurring more severe micro- and macrovascular complications of diabetes. EDIC has achieved a high degree of baseline data collection using reliable quality-controlled measurements. The cohort has been comprehensively characterized at baseline in terms of the presence or absence of micro- and macrovascular complications of type 1 diabetes, the major recognized risk factors for these complications, preceding and current levels of chronic glycemia, and treatment. The study is adequately powered to examine hypotheses related to progression of cardiovascular disease, nephropathy, neuropathy, and retinopathy.
Acknowledgments
The EDIC research group is sponsored by the Division of Diabetes, Endocrinology and Metabolic Diseases of the National Institute of Diabetes and Digestive and Kidney Diseases, the National Institutes of Health, through research contracts and by the General Clinical Research Center Program, National Center for Research Resources, National Institutes of Health.
Participants in EDIC include the following: Study chairs: S. Genuth, D. Nathan; Albert Einstein College of Medicine: H. Shamoon, H. Duffy; Case Western Reserve University: B. Dahms, L. Mayer; Cornell University Medical Center: D. Brillion, M. Lackaye; Henry Ford Health System: F. Whitehouse, D. Kruger; International Diabetes Center: R. Bergenstal, M. Johnson; Joslin Diabetes Center: A. Jacobson, J. Doyle, M. Waters; Massachusetts General Hospital: D. Nathan, S. Crowell, J. Godine, C. McKitrick; Mayo Foundation: J. Service, G. Ziegler; Medical University of South Carolina: J. Colwell, D. Wood, R. Maytield; Northwestern University: M. Molitch, B. Schaefer; University of California, San Diego: O. Kolterman, G. Lorenzi; University of Iowa: W. Sivitz, M. Bayless; University of Maryland School of Medicine: D. Counts, A. Kowarski (past), D. Ostrowski; University of Michigan: D. Greene, C. Martin; University of Minnesota: J. Bantle, B. Rogness; University of Missouri: D. Goldstein, A. Smith; University of New Mexico: D. Schade, C. Johannes; University of Pennsylvania: S. Schwartz, B.J. Maschak-Carey; University of Pittsburgh: T. Orchard, N. Silvers; University of South Florida: J. Malone, A. Mangione; University of Tennessee: A. Kitabchi, M.B. Murphy; University of Texas Southwestern University Medical Center: P. Raskin, S. Strowig; University of Toronto: B. Zinman, A. Barnie; University of Washington: J. Palmer, J. Ginsberg; University of Western Ontario: J. Dupre, J. Harth; Vanderbilt University: R. Lorenz, J. Lipps; Washington University, St. Louis: N, White, J. Santiago (deceased), L. Levandoski; Yale University School of Medicine: W. Tamborlane, P. Gatcomb; Clinical coordinating center (Case Western Reserve University): B. Dahms, P. Corcoran, J. Quin; Data coordinating center (The George Washington University, Biostatistics Center): J. Lachin, P. Cleary, D. Kenny, L. Diminick, D. Lamas; National Institute of Diabetes and Digestive and Kidney Disease program office: C. Cowie, R. Eastman; Central fundus photograph reading center (University of Wisconsin): M. Davis, L. Hubbard, P. Geithman, J. Brickbauer, L. Kastorff, M. Neider; Central biochemistry laboratory (University of Minnesota): M. Steffes, J. Bucksa, B. Chavers; Central carotid ultrasound unit (New England Medical Center): D. O’Leary; Central electrocardiogram reading unit (University of Minnesota): R. Crow, C. O’Donnell; External advisory committee: G. Weir (Chair), C. Clark, R. D’Agostino, M. Espeland, B. Klein, H. Jacobson, T. Manolio, L. Rand, D. Singer, M. Stern; Writing team: H. Shamoon (Chair), P. Cleary, A. Barnie, S. Genuth, C. Maffin, W. Tamborlane, J. Wesche; and Editor, EDIC publications: D. Nathan.
Abbreviations
- CAD
coronary artery disease
- CSII
continuous subcutaneous insulin infusion
- DCCT
Diabetes Control and Complications Trial
- DKA
diabetic ketoacidosis
- ECG
electrocardiogram
- EDIC
Epidemiology of Diabetes Intervention and Complications
- ETDRS
Early Treatment of Diabetic Retinopathy Study
- HRQOL
health-related quality of life
- MDI
multiple daily injections
- MI
myocardial infarction
- MNSI
Michigan Neuropathy Screening Instrument
- NIDDK
National Institute of Diabetes, Digestive and Kidney Diseases
- PDR
proliferative diabetic retinopathy
- PVD
peripheral vascular disease
- WESDR
Wisconsin Epidemiologic Study of Diabetic Retinopathy
APPENDIX 1
Estimated power of mortality comparisons in the EDIC patient population
Table A1–Table A3 provide estimates of the statistical power of intent-to-treat comparisons of mortality between the two randomized treatment groups of the DCCT after an additional 10 years of follow-up in the EDIC study. These estimates are based on a simple test of proportions after 10 years of observation. Survival analysis may provide a more powerful test of treatment differences, but the increase in power is difficult to estimate without making untestable assumptions about the future shapes of the respective hazard functions.
The 10-year mortality among patients randomized to conventional treatment in the DCCT was estimated as the weighted average of the age-specific mortality rates reported by the ETDRS, shown in APPENDIX 2. Since the ETDRS reported 5-year mortality, the estimate was constructed in two stages by applying the appropriate ETDRS mortality rates to the expected number of survivors at the end of the first 5-year period.
Alternative hypotheses are expressed as the anticipated reduction in the risk of death associated with assignment to intensive therapy during the DCCT. It is assumed that all tests will be two-sided at the 0.05 significance level.
Estimated power of intent-to-treat comparisons of combined nephropathic outcomes in the EDIC
Table A4 describes the estimated power of the EDIC to find a difference in the combined 10-year prevalence of death from kidney failure, kidney transplant, renal dialysis, awaiting renal transplant or dialysis, and clinical proteinuria. Once again, they are based on two-sided comparisons of the original DCCT treatment groups at a significance level of 0.05. ETDRS data stratified by age and duration of type 1 diabetes were used to estimate the 10-year prevalence of this outcome among patients randomized to the conventional group of the DCCT.
APPENDIX 2
Data on 5-year mortality in the ETDRS and the ages of EDIC participants at DCCT closeout can be found in Table A5 and Table A6.
Table A1.
All-cause mortality
| Assumed treatment effect |
|||||||
|---|---|---|---|---|---|---|---|
| n | 20% | 25% | 30% | 35% | 40% | 45% | 50% |
| 350 | 0.173 | 0.249 | 0.344 | 0.453 | 0.570 | 0.684 | 0.786 |
| 400 | 0.191 | 0.278 | 0.384 | 0.504 | 0.627 | 0.741 | 0.837 |
| 450 | 0.209 | 0.307 | 0.423 | 0.552 | 0.678 | 0.790 | 0.877 |
| 500 | 0.227 | 0.335 | 0.461 | 0.596 | 0.724 | 0.831 | 0.908 |
| 550 | 0.246 | 0.362 | 0.498 | 0.637 | 0.764 | 0.864 | 0.932 |
| 600 | 0.264 | 0.389 | 0.532 | 0.675 | 0.799 | 0.892 | 0.950 |
| 650 | 0.281 | 0.416 | 0.565 | 0.710 | 0.830 | 0.914 | 0.964 |
The assumed combined 10-year mortality in the DCCT conventional group is 11.8%.
Table A2.
Mortality due to CAD
| Assumed treatment effect |
|||||||
|---|---|---|---|---|---|---|---|
| n | 20% | 25% | 30% | 35% | 40% | 45% | 50% |
| 350 | 0.138 | 0.192 | 0.262 | 0.346 | 0.441 | 0.544 | 0.647 |
| 400 | 0.150 | 0.213 | 0.292 | 0.386 | 0.491 | 0.600 | 0.705 |
| 450 | 0.163 | 0.234 | 0.322 | 0.426 | 0.538 | 0.651 | 0.755 |
| 500 | 0.176 | 0.255 | 0.352 | 0.464 | 0.582 | 0.697 | 0.798 |
| 550 | 0.189 | 0.275 | 0.381 | 0.500 | 0.623 | 0.738 | 0.834 |
| 600 | 0.202 | 0.296 | 0.409 | 0.535 | 0.661 | 0.774 | 0.865 |
| 650 | 0.215 | 0.316 | 0.437 | 0.568 | 0.695 | 0.806 | 0.890 |
The assumed combined 10-year mortality in the DCCT conventional group is 8.7%.
Table A3.
Mortality due to stroke
| Assumed treatment effect |
|||||||
|---|---|---|---|---|---|---|---|
| n | 20% | 25% | 30% | 35% | 40% | 45% | 50% |
| 350 | 0.060 | 0.066 | 0.074 | 0.083 | 0.095 | 0.109 | 0.126 |
| 400 | 0.061 | 0.068 | 0.077 | 0.088 | 0.102 | 0.118 | 0.137 |
| 450 | 0.063 | 0.070 | 0.080 | 0.093 | 0.108 | 0.127 | 0.148 |
| 500 | 0.064 | 0.073 | 0.084 | 0.098 | 0.115 | 0.135 | 0.160 |
| 550 | 0.065 | 0.075 | 0.087 | 0.103 | 0.122 | 0.144 | 0.171 |
| 600 | 0.067 | 0.077 | 0.091 | 0.108 | 0.128 | 0.153 | 0.182 |
| 650 | 0.068 | 0.080 | 0.094 | 0.113 | 0.135 | 0.162 | 0.194 |
The assumed combined 10-year mortality in the DCCT conventional group is 1.1%.
Table A4.
Combined renal outcomes
| Assumed treatment effect |
|||||||
|---|---|---|---|---|---|---|---|
| n | 20% | 25% | 30% | 35% | 40% | 45% | 50% |
| 350 | 0.245 | 0.360 | 0.495 | 0.633 | 0.760 | 0.860 | 0.929 |
| 400 | 0.273 | 0.403 | 0.548 | 0.692 | 0.813 | 0.902 | 0.956 |
| 450 | 0.301 | 0.443 | 0.598 | 0.742 | 0.856 | 0.932 | 0.973 |
| 500 | 0.328 | 0.482 | 0.643 | 0.786 | 0.890 | 0.953 | 0.984 |
| 550 | 0.355 | 0.520 | 0.685 | 0.823 | 0.917 | 0.968 | 0.991 |
| 600 | 0.382 | 0.555 | 0.723 | 0.854 | 0.937 | 0.979 | 0.994 |
| 650 | 0.408 | 0.598 | 0.756 | 0.881 | 0.953 | 0.986 | 0.997 |
The assumed 10-year prevalence in the DCCT conventional group is 17.4%. Adapted from the EDIC Data Coordinating Center Manual of Operations (7).
Table A5.
The 5-year mortality in the ETDRS
| Age at entry (years) |
|||||
|---|---|---|---|---|---|
| 18–29 | 30–34 | 35–39 | 40–44 | 45+ | |
| n | 609 | 262 | 209 | 132 | 232 |
| Cause of death | |||||
| All causes | 2.0 | 4.6 | 7.2 | 6.1 | 13.4 |
| CAD | 0.8 | 3.1 | 5.3 | 4.6 | 11.2 |
| Stroke | 0.2 | 0.8 | 0.5 | 0.8 | 0.4 |
Data are (%). Adapted from the EDIC Data Coordinating Center Manual of Operations (7).
Table A6.
Ages of EDIC participants at DCCT closeout
| Age Range (years) | All patients | Conventional group |
|---|---|---|
| 17–24 | 172 | 95 |
| 25–29 | 244 | 122 |
| 30–34 | 348 | 185 |
| 35–39 | 340 | 173 |
| 40–44 | 249 | 123 |
| 45+ | 74 | 32 |
Data are n.
Footnotes
A complete listing of the EDIC Research Group appears in ACKNOWLEDGMENTS.
A table elsewhere in this issue shows conventional and Système International (SI) units and conversion factors for many substances.
References
- 1.Jacobs J, Sena M, Fox N. The cost of hospitalization for the late complications of diabetes in the United States. Diabet Med. 1991;8:S23–S29. doi: 10.1111/j.1464-5491.1991.tb02151.x. [DOI] [PubMed] [Google Scholar]
- 2.Andersen AR, Christiansen JS, Andersen JK, Kreiner S, Deckert T. Diabetic nephropathy in type 1 (insulin-dependent) diabetes: an epidemiological study. Diabetologia. 1983;25:496–501. doi: 10.1007/BF00284458. [DOI] [PubMed] [Google Scholar]
- 3.DCCT Research Group. The effect of intensive diabetes treatment on the development and progression of long-term complications in insulin-dependent diabetes mellitus: the Diabetes Control and Complications Trial. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 4.DCCT Research Group. Prevention of neuropathy: the effect of intensive diabetes therapy on the development and progression of neuropathy in the DCCT. Ann Intern Med. 1995;122:564–568. doi: 10.7326/0003-4819-122-8-199504150-00001. [DOI] [PubMed] [Google Scholar]
- 5.DCCT Research Group. Effect of intensive therapy on the development of diabetic nephropathy in the DCCT. Kidney Int. 1995;47:1703–1720. doi: 10.1038/ki.1995.236. [DOI] [PubMed] [Google Scholar]
- 6.DCCT Research Group. The effect of intensive diabetes management on macrovascular events and risk factors in the DCCT. Am J Cardiol. 1995;75:894–903. doi: 10.1016/s0002-9149(99)80683-3. [DOI] [PubMed] [Google Scholar]
- 7.EDIC Data Coordinating Center. Epidemiology of Diabetes Interventions and Complications Protocol and Manual of Operations. Washington, DC: Biostatistics Center, The George Washington University; 1995. [Google Scholar]
- 8.Stevens MJ, Edwards ME, Foster AVM, Watkins PJ. Selective neuropathy and pressured vascular response in the diabetic charcot foot. Diabetologia. 1992;35:148–154. doi: 10.1007/BF00402547. [DOI] [PubMed] [Google Scholar]
- 9.Feldman EL, Stevens MJ, Thomas PK, Brown MB, Canal N, Greene DA. A practical two-step quantitative clinical and electrophysiological assessment for the diagnosis and staging of diabetic neuropathy. Diabetes Care. 1994;17:1281–1289. doi: 10.2337/diacare.17.11.1281. [DOI] [PubMed] [Google Scholar]
- 10.ETDRS Research Group. Grading diabetic retinopathy from stereotopic color fundus photographs: an extension of the modified Airlie House Classification: ETDRS report No. 100. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 11.Abt K. Problems of repeated significance testing. Controlled Clin Trials. 1981;1:377–381. doi: 10.1016/0197-2456(81)90042-8. [DOI] [PubMed] [Google Scholar]
- 12.Cox DR. Regression models and life tables. J R Stat Soc Series B. 1972;34:187–220. [Google Scholar]
- 13.Turnbull BW. The empirical distribution function with arbitrarily grouped, censored, and truncated data. J R Stat Soc Series B. 1976;38:290–295. [Google Scholar]
- 14.Finkelstein DM. A proportional hazards model for interval-censored failure time data. Biometrics. 1986;42:845–854. [PubMed] [Google Scholar]
- 15.Lachin JM, Wei LJ. Estimators and tests in the analysis of nonindependent 2×2 tables with partially missing observations. Biometrics. 1988;44:513–528. [PubMed] [Google Scholar]
- 16.Zeger SL, Liang K. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- 17.Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics. 1982;38:963–974. [PubMed] [Google Scholar]
- 18.McCullogh P, Nelder JA. Generalized Linear Models. 2nd ed. New York: Chapman & Hall; 1982. [Google Scholar]
- 19.Fleming TR, Harrington DP. Counting Processes and Survival Analyses. New York: John Wiley; 1991. [Google Scholar]
- 20.Breslow NE, Day NE. Statistical Methods in Cancer Research, Volume II: The Design and Analysis of Cohort Studies Lyon, France, International Agency for Research in Cancer. London: Oxford University Press; 1987. [PubMed] [Google Scholar]
- 22.Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. Arch Ophthalmol. 1989;107:237–243. doi: 10.1001/archopht.1989.01070010243030. [DOI] [PubMed] [Google Scholar]
- 23.DCCT Research Group. Klein R, Moss S. A comparison of the study populations in the Diabetes Control and Complications Trial (DCCT) and the Wisconsin Epidemiologic Study of Diabetic Retinopathy (WESDR) Arch Intern Med. 1995;155:745–754. [PubMed] [Google Scholar]
- 24.Orchard TJ, Dorman JS, Maser RE, Becker DJ, Drash AL, Ellis D, LaPorte RE, Kuller LH. Prevalence of complications in IDDM by sex and duration: Pittsburgh Epidemiology of Diabetes Complications Study II. Diabetes. 1990;39:1116–1124. doi: 10.2337/diab.39.9.1116. [DOI] [PubMed] [Google Scholar]
- 25.Krolewski AS, Kosinski EJ, Warram JH, Leland OS, Busick EJ, Asmal AC, Rand LI, Christlieb AR, Bradley RF, Kahn CR. Magnitude and determinants of coronary artery disease in juvenile-onset, insulin-dependent diabetes mellitus. Am J Cardiol. 1987;59:750–755. doi: 10.1016/0002-9149(87)91086-1. [DOI] [PubMed] [Google Scholar]
- 26.Jensen T, Borch-Johnsen K, Kofoed-Enevoldsen A, Deckert T. Coronary heart disease in young type I (insulin-dependent) diabetic patients with and without diabetic nephropathy: incidence and risk factors. Diabetologia. 1987;30:144–148. doi: 10.1007/BF00274218. [DOI] [PubMed] [Google Scholar]
- 27.Deckert T, Feldt-Rasmussen B, Borch-Johnsen K, Jensen T, Kofoed-Enevoldsen A. Albuminuria reflects widespread vascular damage: the Steno hypothesis. Diabetologia. 1989;32:219–226. doi: 10.1007/BF00285287. [DOI] [PubMed] [Google Scholar]
- 28.Fisher BM, Gillen G, Lindop GBM, Dargie HJ, Frier BM. Cardiac function and coronary arteriography in asymptomatic type 1 (insulin-dependent) diabetic patients: evidence for a specific diabetic heart disease. Diabetologia. 1986;29:706–712. doi: 10.1007/BF00870280. [DOI] [PubMed] [Google Scholar]
- 29.Koistinen MJ. Prevalence of asymptomatic myocardial ischaemia in diabetic subjects. BMJ. 1990;301:92–95. doi: 10.1136/bmj.301.6743.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gerson MC, Khoury JC, Hertzberg VS, Fischer EE, Scott RS. Prediction of coronary artery disease in a population of insulin-requiring diabetic patients: results of an 8-year follow-up study. Am Heart J. 1988;116:820–826. doi: 10.1016/0002-8703(88)90343-2. [DOI] [PubMed] [Google Scholar]
- 31.Persson G. Exercise tests in male diabetics. Acta Med Scand Suppl. 1977;305:7–23. [PubMed] [Google Scholar]
- 32.Jensen-Urstad KJ, Reichard PG, Rosfors JS, Lindblad LEL, Jensen-Urstand MT. Early atherosclerosis is retarded by improved long-term blood glucose control in patients with IDDM. Diabetes. 1996;45:1253–1258. doi: 10.2337/diab.45.9.1253. [DOI] [PubMed] [Google Scholar]
- 33.Palumbo PJ, Melton LJ. Peripheral vascular disease and diabetes. In: Harris MI, Cowie CC, Stern MP, Boyko EJ, Reiber GE, Bennett PH, editors. Diabetes in America. 2nd ed. 1995. (NIH publ. no. 95–1468) [Google Scholar]
- 34.Riccardi G, Vaccaro O, Riveliese A, Romano G, Cambri V, Rubba P, Pauciullo P, Greco G, Iovine L, Mancini M. Association between retinopathy and impaired peripheral arterial circulation in insulin dependent diabetic patients. Arterioscleorsis. 1988;8:509–514. doi: 10.1161/01.atv.8.5.509. [DOI] [PubMed] [Google Scholar]
- 35.Carter SA. Clinical measurement of systolic pressures in limbs with arterial occlusive disease. JAMA. 1969;207:1869–1874. [PubMed] [Google Scholar]
- 36.Deckert T, Poulsen JE, Larsen M. Prognosis of diabetics with diabetes onset before the age of 31. Diabetologia. 1978;14:63–70. doi: 10.1007/BF01228130. [DOI] [PubMed] [Google Scholar]
- 37.Entmacher PS, Root HF, Marks HH. Longevity of diabetic patients in recent years. Diabetes. 1964;13:373–377. doi: 10.2337/diab.13.4.373. [DOI] [PubMed] [Google Scholar]
- 38.Riley WA, Bames RW, Bond MG, Evans G, Chambless LE, Heiss G. High-resolution B-mode ultrasound reading methods in the Atherosclerosis Risk in Communities (ARIC) cohort. J Neuroimag. 1991;1:168–172. [PubMed] [Google Scholar]
- 39.Zinman B, Cleary P, O’Leary D, Orchard T for the EDIC Study Group. The effect of intensive diabetes treatment on carotid artery wall thickness in the Epidemiology of Diabetes Interventions and Complications (Abstract) Diabetes. 1996;45 Suppl. 2:188A. [Google Scholar]
- 40.Rand LI, Krolewski AS, Aiello LM, Warram JH, Barker RS, Maki T. Multiple factors in the prediction of risk of proliferative diabetic retinopathy. N Engl J Med. 1985;313:1433–1438. doi: 10.1056/NEJM198512053132302. [DOI] [PubMed] [Google Scholar]
- 41.Krolewski AS, Warram JH, Rand LI, Christlieb AR, Busick EJ, Kahn CR. Risk of proliferative diabetic retinopathy in juvenile-onset type I diabetes: a 40-yr follow-up study. Diabetes Care. 1986;9:443–452. doi: 10.2337/diacare.9.5.443. [DOI] [PubMed] [Google Scholar]
- 42.Janka HU, Warram JH, Rand LI, Krowlewski AS. Risk factors for progression of background retinopathy in long-standing IDDM. Diabetes. 1989;38:460–464. doi: 10.2337/diab.38.4.460. [DOI] [PubMed] [Google Scholar]
- 43.Winocour PH, Durrington PN, Ishola M, Anderson DC, Cohen H. Influence of proteinuria on vascular disease, blood pressure, and lipoproteins in insulin-dependent diabetes mellitus. BMJ. 1987;294:1648–1651. doi: 10.1136/bmj.294.6588.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dornan TL, Ting A, McPherson CK, Peckar CO, Mann JI, Turner RC, Morris PJ. Genetic susceptibility to the development of retinopathy in insulin-dependent diabetics. Diabetes. 1982;31:226–231. doi: 10.2337/diab.31.3.226. [DOI] [PubMed] [Google Scholar]
- 45.Cruickshanks KJ, Vadheim CM, Moss SE, Roth M-P, Riley WJ, Maclaren NK, Lang-field D, Sparkes RS, Klein R, Rotter JI. Genetic marker associations with proliferative retinopathy in persons diagnosed with diabetes before 30 yr of age. Diabetes. 1992;41:879–885. doi: 10.2337/diab.41.7.879. [DOI] [PubMed] [Google Scholar]
- 46.The Diabetes Control and Complications Trial Research Group. Clustering of long-term complications in families with diabetes in the Diabetes Control and Complications Trial. Diabetes. 1997;46:1829–1839. [PubMed] [Google Scholar]
- 47.Holme I. Extension of clinical trials: 10 1/2 year follow-up of the Multiple Risk Factor Intervention Trial. Circulation. 1990;82:1857–1858. doi: 10.1161/01.cir.82.5.1857. [DOI] [PubMed] [Google Scholar]
- 48.Kannel WB. Coronary Artery Surgery Study revisited: limitation of the intent-to-treat principle. Circulation. 1990;82:1859–1862. doi: 10.1161/01.cir.82.5.1859. [DOI] [PubMed] [Google Scholar]
- 49.Canner PL, Berge KG, Wenger NK, Stamler J, Freedman L, Prineas RJ, Friedewald W for the Coronary Drug Research Group. Fifteen year mortality in coronary drug project patients: long term benefit with niacin. J Am Coll Cardiol. 1986;8:1244–1245. doi: 10.1016/s0735-1097(86)80293-5. [DOI] [PubMed] [Google Scholar]
- 50.Najjar MF, Rowland M. Anthropometric reference data and prevalence of overweight, United States, 1976–1980. United Health Stat. 1987;11 No. 230. [PubMed] [Google Scholar]
- 51.Hiatt WR, Marshall JA, Baxter J, Sandoval R, Hildebrandt W, Kahn LR, Hamman RF. Diagnostic methods for peripheral arterial disease in the San Luis Valley Diabetes Study. J Clin Epidemiol. 1990;43:597–606. doi: 10.1016/0895-4356(90)90164-k. [DOI] [PubMed] [Google Scholar]