Abstract
The aberrant expression of membrane mucins such as Muc1 and Muc4 by tumor cells has been shown to engage signaling pathways that promote cellular properties associated with tumor progression. Our previous studies have shown that Muc4 interacts with and potentiates signaling by the ErbB2 (HER2) receptor tyrosine kinase through an epidermal growth factor–like domain in its extracellular region. Here, we show that expression of Muc4 in human A375 melanoma cells and MCF7 breast cancer cells confers resistance to apoptosis induced by a variety of stimuli, including chemotherapeutic agents, the absence of serum factors, and the loss of cellular adhesion. Mapping experiments revealed that the O-glycosylation and cytosolic domains of Muc4 are dispensable for its antiapoptotic activity, and are also dispensable for the potentiation of signaling by ErbB2. Knockdown of endogenous Muc4 in JIMT-1 breast cancer cells sensitizes cells to apoptotic stimuli, and this can be rescued by Muc4 forms lacking the O-glycosylation or cytosolic domains. Surprisingly, however, the molecular mechanisms underlying Muc4 antiapoptotic activity vary among cell lines. Although Muc4 in JIMT-1 cells engages ErbB2 to promote cell survival, its antiapoptotic mechanism in MCF7 and A375 cells seems to be independent of ErbB2. However, Muc4 expression in all cell lines culminates in the phosphorylation and inactivation of the proapoptotic protein Bad and the elevation of the prosurvival protein Bcl-xL. Our observations suggest that tumor cells can exploit the versatile antiapoptotic activities of Muc4 to acquire resistance to therapeutic agents, and augment cell survival after the loss of adhesion and microenvironment-derived survival factors.
Introduction
Mucins encompass a family of high molecular weight, heavily O-glycosylated proteins that are normally expressed by epithelial, endothelial, and other cell types to contribute to the lubrication of surfaces and to serve as a barrier to physical and biological assaults (1, 2). Mucins are often aberrantly expressed by human tumors and are thought to contribute to tumor progression by altering the surface properties of tumor cells. Several membrane mucins, members of the family that possess transmembrane and cytosolic domains, have additionally been shown to engage intracellular signaling pathways to elicit a variety of cellular responses (3, 4). The most well-characterized signaling membrane mucin is Muc1, the cytosolic domain of which has been shown to interact with a wide range of signaling proteins that promote cellular growth properties (2, 5).
Muc4 (called sialomucin complex or SMC in rat) is a non-covalently linked heterodimeric protein derived from a single gene. Proteolytic processing during biosynthesis creates its two subunits Muc4α and Muc4β. Muc4α contains the hallmark O-glycosylation domain characteristic of mucins, a tandem repeat rich in serine and threonine residues. Muc4β tightly but noncovalently associates with Muc4α and possesses a single transmembrane segment and a cytosolic domain of ~20 amino acids. Additionally, Muc4β contains other features in its extracellular region such as epidermal growth factor (EGF)-like and von Willebrand D domains that likely mediate Muc4 interactions with other membrane or extracellular proteins.
Muc4 is normally expressed on the apical surface of many epithelial tissues such as the female reproductive tract, breast, lung, and colon. However, aberrant Muc4 overexpression has been implicated in a variety of carcinomas such as breast (6), lung (7, 8), ovarian (9, 10), pancreatic (11, 12), and gall bladder (13). The high negative charge of sialyl moieties and sulfated glycosyl groups attached to the O-glycosylation domain, as well as the significant bulk of overexpressed mucin at the surface of the cell, are thought to contribute to numerous cellular activities that augment tumor progression. Specifically, the overexpression of Muc4 in tumor cells has been shown to interfere with cell-cell and cell-matrix interactions (14) and promote metastasis (15), to confer tumor cell resistance to therapeutic antibodies (16, 17), and to promote the ability of tumor cells to evade immune surveillance (18). Interestingly, expression of Muc4 suppresses tumor cell apoptosis in a xenograft model (19), suggesting that cellular survival signaling may also be a key Muc4 function in promoting tumor progression.
Although signaling by Muc4 remains to be fully explored, we have previously shown that Muc4 physically associates with the ErbB2 receptor tyrosine kinase in cells coexpressing the two proteins via one of the EGF-like domains located in its extracellular region (20). Moreover, Muc4 expression in cells potentiates growth factor signaling by ErbB2 by promoting the translocation of receptors from intracellular compartments to the cell surface (21). Several studies show that Muc4 and ErbB2 are coexpressed in some tumor types such as breast (6), non–small cell lung cancer (8), and pancreas (12), raising the possibility that Muc4 could elicit its antiapoptotic effects by engaging ErbB2. Growth factor receptors such as ErbB2 engage a variety of signaling cascades to elicit cellular responses such as proliferation and survival. Most notably, growth factor receptors commonly activate the lipid kinase PI3-kinase (22), which in turn activates the serine/threonione kinase Akt to mediate a variety of phosphorylation events that promote cellular survival (23). Mitogen-activated protein kinases such as extracellular signal-regulated kinase, p38, and c-Jun-NH2-kinase are also important targets of growth factor signaling that elicit diverse cellular responses, including cellular survival (24).
The delicate balance of members of the Bcl-2 family of proteins plays a central role in apoptosis by regulating mitochondrial permeability and cytochrome c release. The mitochondria-associated proteins Bcl-2 and Bcl-xL possess Bcl-2 homology domains (BH1 through BH4), and suppress cytochrome c release and cell death (25, 26). Levels of these proteins are often elevated in cells in response to a variety of stimuli that promote viability. On the other hand, BH3-only members of the Bcl family such as Bad translocate to mitochondria in response to apoptotic signals, and interact with and deactivate Bcl-2 and Bcl-xL (25, 27, 28). The proapoptotic activity of Bad may be suppressed in response to survival signaling cascades that lead to its phosphorylation on serine residues S75, S99, and S118 (S112, S136, and S155 in mouse). Bad phosphorylation in response to either PI3-kinase or mitogen-activated protein kinase (MAPK) pathway activation promotes its interaction with the scaffolding protein 14-3-3 (23), leading to its sequestration in the cytosol and inhibition of its proapoptotic activity.
In the studies outlined here, we use inducible and knockdown models of Muc4 expression in tumor cells to explore the role of Muc4 in promoting cellular survival in response to a variety of insults that mimic barriers to tumor progression to malignancy. Our results suggest that Muc4 promotes cellular survival through both ErbB2-dependent and ErbB2-independent mechanisms, depending on cellular context, and its O-glycosylation and intracellular domains seem to be dispensable for antiapoptosis. The multiple survival mechanisms engaged by Muc4 in turn could contribute to aggressive tumor properties such as chemotherapeutic resistance and metastasis.
Materials and Methods
Cell culture
A375-Rep8 and MCF7-Rep5 cell lines have been previously described (14, 15, 18–21). The A375-rMuc4β line was created by stably expressing the rat ASGP2 construct (29) in A375 cells using the tet-off system. ΔCyto was constructed using the QuikChange Site-Directed Mutagenesis kit (Stratagene) to mutate the second and third amino acids of rMuc4β immediately carboxy-terminal to the transmembrane domain: Cys to Arg and Gly to STOP. JIMT-1cells (30) were obtained from DSMZ and grown in 1:1 DMEM/F12, 10 µg/mL human insulin, 10% FCS, and 1% v/v penicillin/streptomycin.
Knockdown experiments
ErbB2 was knocked down using Dharmacon On-Target plus SMART pool ErbB2-directed oligonucleotides and siControl nontargeting control. Oligonucleotides were transfected using DharmaFect 1 (Dharmacon) according to the manufacturer’s instructions. Muc4 was stably knocked down in JIMT-1 cells by retroviral-mediated introduction of the short-hairpin RNA interference oligonucleotide sequences 5′-gatccccACGCAAGCATCGGACTTCAttcaagagaTGAAGTCCGATGCTTGCGTttttta-3′ and 5′-agcttaaaaaACGCAAGCATCGGACTTCAtctcttgaaTGAAGTCCGATGCTTGCGTggg-3′. Sequences for control scrambled shRNA oligonucleotides were 5′-gatccccGGAAACGAGGTTGAATACAttcaagagaTGTATTCAACCTCGTTTCCttttta-3′ and 5′-agcttaaaaaGGAAACGAGGTTGAATACAtctcttgaaTGTATTCAACCTCGTTTCCggg-3′. Uppercase letters for Muc4-RNAi are directed toward identical sequences present in all known Muc4 splice variants. Annealed oligonucleotides were ligated into the BglII/HindIII sites of the pSuper.retro.neo+gfp vector (OligoEngine, Inc.). 293GPG packaging cells (31) were transfected and virus produced as previously described (32). JIMT-1 cells infected with Muc4 or control knockdown viruses were selected using 400 µg/mL G418 (Cellgro).
Immunoblotting experiments
Growth factor stimulation and immunoblotting experiments were carried out according to our previously described procedures (21). 4557W, LY294002, and U0126 were from Calbiochem, and were added to cells 30 min before assay, or for the duration of anoikis or serum-free growth experiments. Most primary antibodies were from commercial sources. Muc4 antibody 1G8 (hybridoma cell line #2D10, clone HL1718) was provided by Kermit Carraway, University of Miami, Miami, FL.
Apoptosis assays
For anoikis assays, cells were first grown in serum-starved media (containing 0.1% fetal bovine serum) without or with tetracycline for 48 h to induce Muc4 expression, and then trypsinized and replated in ultra low attachment 60-mm flat-bottomed plates (Corning) in complete media containing 1% methyl cellulose (Sigma) for 72 h. Suspended cells were then collected by centrifugation, washed in PBS, and either lysed in 2× sample buffer for immunoblot analysis or fixed in 4% paraformaldehyde for 1h, then switched to 70% ethanol for 1 h to overnight, followed by terminal deoxynucleotidyl-transferase–mediated dUTP nick-end labeling (TUNEL; Roche Applied Sciences). Cells were then incubated for 30 min in propidium iodide solution (Sigma), EDTA, RNase, and spermine (Sigma), and analyzed using a Becton Dickinson fluorescence-activated cell sorting (FACS) scan for FITC fluorescence and propidium iodide staining. Twenty-five thousand to 30,000 cells per sample were analyzed by Cell Quest software. The Forest meta-analysis plots (Fig. 2A and Fig 4B) were carried out using GraphPad Prism software. Odds ratios were calculated using the FITC-positive and nonpositive populations for both Muc4-On and Muc4-Off cells.
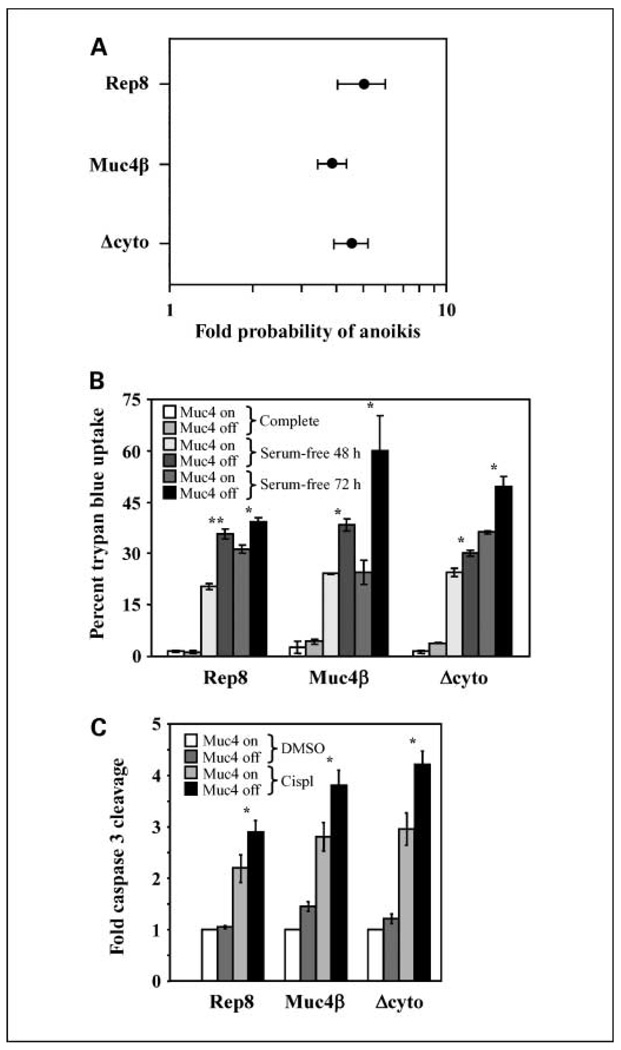
Figure 2.
The cytosolic and O-glycosylation domains are dispensable for Muc4 antiapoptotic activity. A, A375 cells inducibly expressing Rep8, Muc4β, or Δcyto were suspended for 72 h, and anoikis was measured by TUNEL followed by FACS. The fold increase in the probability that the cells will undergo apoptosis in the absence of Muc4 relative to Muc4 expression was calculated and plotted for each of the three constructs. In the Forest meta-analysis plot, 1 represents no effect and 10 represents a 10-fold higher probability as calculated by odds ratio, with a P value of <0.001. Error bars, the 95% confidence interval for the population; points, the center of the confidence interval. B, inducible cell lines were grown in serum-free media for 48 or 72 h, as indicated, and apoptosis was measured by trypan blue uptake. C, cells with Muc4 forms turned on or off were treated with DMSO vehicle or with 5.5 µg/mL cisplatin (cispl ) for 18 h, cleaved caspase 3 levels were determined by immunoblotting and bands were quantified digitally. The fold enhancement of cleaved caspase levels over levels observed in the presence of Muc4 but the absence of drug is plotted for each cell line. *, P < 0.05; **, P < 0.001.
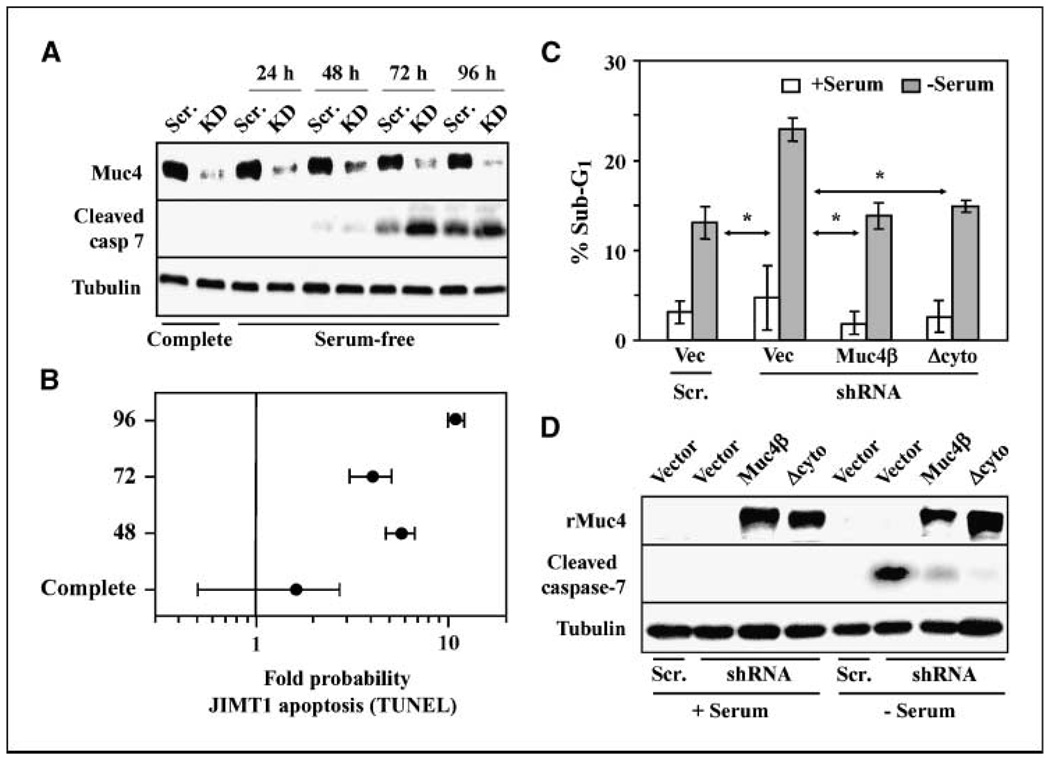
Figure 4.
Endogenous Muc4 promotes the viability of JIMT-1 breast tumor cells. A, JIMT-1 cells stably transduced with retrovirus expressing a Muc4-directed shRNA construct (KD) or a scrambled control (Scr.) were grown in complete or serum-free media for the indicated times. Lysates were immunoblotted with antibodies to Muc4, cleaved caspase 7 (cleaved casp 7),and tubulin. B, JIMT-1 cells transduced with scrambled or Muc4-directed shRNA constructs were grown in serum-free media for the indicated times, and the extent of apoptosis was determined by TUNEL followed by FACS. The fold increase in the probability that the cells will undergo apoptosis when Muc4 is knocked down relative to Muc4 expression was calculated and plotted for three time points. C, wild-type JIMT-1 cells were transiently cotransfected with either Muc4-directed shRNA or scrambled vectors along with Muc4β, Δcyto, or control (vec) plasmids, as indicated. Cells grown in the presence and absence of serum were assayed for apoptosis by sub-G1 content. *, P < 0.05 relative to shRNA+vector with no serum. D, whole cell lysates from cells depicted in C were blotted with the indicated antibodies.
For serum deprivation assays, cells were first grown to 60% to 70% confluence and Muc4 expression was induced for 48 h in serum-starved media for inducible cells. Media was exchanged for serum-free media without or with tetracycline, cells were incubated for various times, and both floating and adherent cell populations were collected as described above. Cells were processed for FACS analysis, lysed in sample buffer for Western blot analysis, or stained with 1% trypan blue and counted. Four hundred to 500 cells per sample were counted using a hemacytometer, and assays were carried out in triplicate and repeated thrice. For cisplatin-induced apoptosis assays, cells were grown to 60% to 70% confluence and Muc4 expression induced for 48 h. Cells were then treated with DMSO (Sigma) vehicle or 5.5 µg/mL cisplatin (Calbiochem) for 18 h, and lysates were analyzed by immunoblotting or processed for FACS analysis.
For the knockdown/rescue experiment, wild-type JIMT-1cells were grown to 50% confluency and triply cotransfected in duplicate with 2 sets of plasmids. One set included either pSuper-RNAi-Scramble or pSuper-hMuc4 shRNA to knockdown endogenous human Muc4. The second set involved TetActivator plasmid along with empty vector control, pTre-Muc4β, or pTre-Δcyto. Transfection proceeded for 48 h, and media was refreshed to complete JIMT1 media or serum-free media for an additional 72 h. Cells were collected for flow cytometry as described above. A small aliquot of cells was taken from each sample for Western blot analysis. Cells were stained with propidium iodide only, and 40,000 to 50,000 cells were collected and analyzed using ModFit cell cycle analysis software for sub-G1 DNA content.
Results
Rat Muc4 expression confers resistance to apoptosis induced by a variety of insults
To examine the role of Muc4 in apoptotic processes, we assessed the ability of inducibly expressed rat Muc4 to influence the survival of human A375 melanoma cells under conditions of cellular suspension, lack of serum, or treatment with the commonly used chemotherapeutic agent cisplatin. A375-Rep8 cells express full-length rat Muc4 containing eight repeats in its O-glycosylation domain under tetracycline “tetoff ” control (14). Removal of tetracycline from the growth media potently induces Rep8 expression (e.g., see Fig. 1C), causing the disruption of cell-cell and cell-matrix interactions (14) and promoting the metastasis of these cells in a xenograft model (15).
Figure 1.
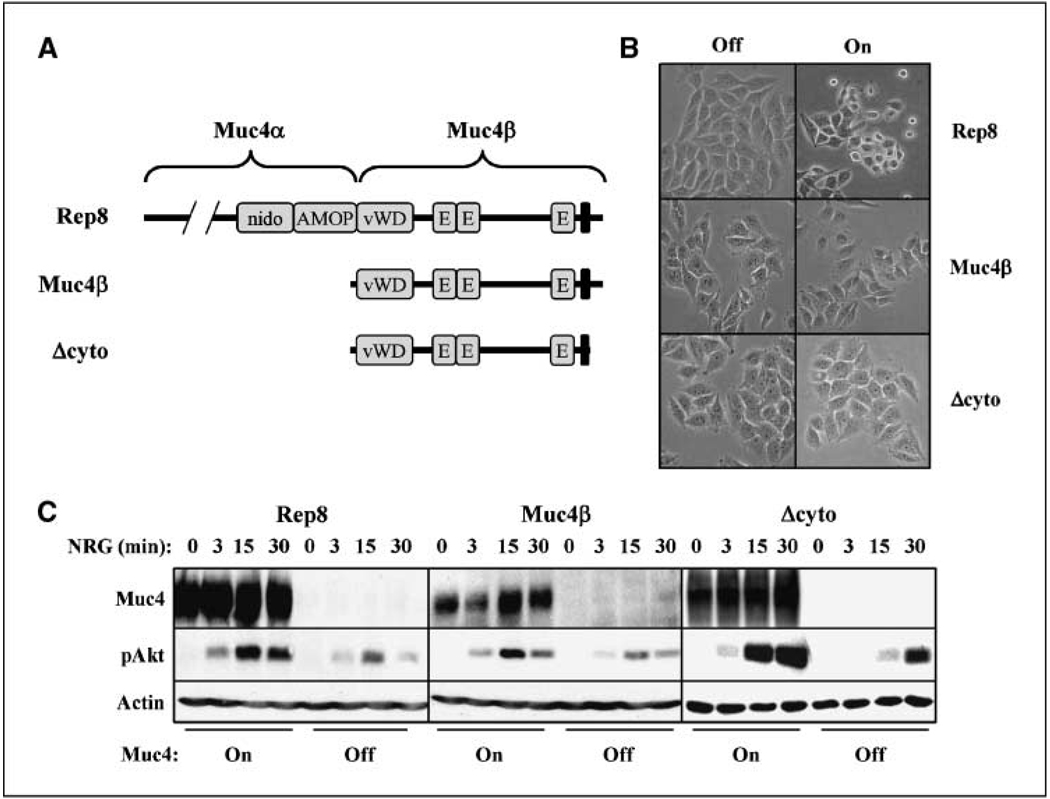
The cytosolic and O-glycosylation domains are dispensable for Muc4-potentiated signaling by ErbB receptors. A, the domain structures of the Muc4 forms used are depicted. The illustrated domains in rat Rep8 are as follows: amino terminal O-glycosylation, nidogen, AMOP, von Willebrand D, three EGF-like domains, a transmembrane segment, and a short cytoplasmic tail. B, A375 cells inducibly expressing rat full-length Muc4 (Rep8),Muc4β, or Δcyto were treated with and without tetracycline for 72 h to induce expression, and were photographed. C, cell lines were treated without and with tetracycline for 48 h, and then treated with NRG1 for the indicated times. Cell lysates were blotted with antibodies to Muc4, phospho-Akt, and actin.
Using several methods of apoptosis detection, we observed that Rep8 expression in these cells markedly stimulates their survival under adverse conditions. For example, Rep8 expression inhibited detachment-induced anoikis by 5-fold, as assessed by TUNEL labeling followed by FACS analysis (Supplementary Fig. S1A), and similarly stimulated the serum-free survival of cells as assessed by quantification of the sub-G1 population by FACS after propidium iodide staining (Supplementary Fig. S1B). In addition, Rep8 expression suppressed cisplatin-induced cleavage of caspase-3 (Supplementary Fig. S1C), a marker characteristic of apoptosis. Table 1 summarizes our analysis of Rep8 antiapoptotic activity toward A375 cells. Although the extent of insult-induced apoptosis varied somewhat with the analysis method, in every case, Rep8 expression markedly interfered with apoptosis. Very similar antiapoptotic effects of Muc4 were observed with MCF7-Rep5 cells, a human breast cancer cell line that inducibly expresses full-length Muc4 containing five repeats in its O-glycosylation domain (data not shown). Ectopic expression of human Muc4 in MCF10A-immortalized breast epithelial cells similarly promoted cell survival.1 Together, these observations suggest that Muc4 expression by solid tumors could promote progression by allowing cells to surpass key apoptotic barriers to malignancy.
Table 1.
Summary of the effects of Muc4 expression on apoptosis induced by serum-free conditions for 48 h, suspension for 72 h, and 5.5 µg/mL cisplatin treatment for 18 h, as assessed by quantitative cleaved caspase 3 immunoblotting, sub-G1 analysis by FACS, TUNEL/FACS analysis, and trypan blue exclusion
| Serum-free | Suspension | Cisplatin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Caspase* | Sub-G1 | TUNEL | Trypan* | Caspase | Sub-G1* | TUNEL* | Trypan | Caspase † | Sub-G1* | TUNEL* | Trypan | |
| +Rep8 | 1.0 (0) | 1.1% | ND | 20.0 (0.79) | 1.0 | 3.0 | 0.8 | ND | 1.0 (0.24) | 11.8% | 27% | ND |
| −Rep8 | 2.2 (0.21) | 7.1% | ND | 35.5% (1.44) | 1.3 | 13.5 | 4.0 | ND | 1.3 (0.2) | 20.2% | 43% | ND |
NOTE: SEs are in parentheses.
Abbreviation: ND, not determined.
P < 0.001 of 3 to 5 independent experiments.
P < 0.05.
The glycosylation and cytosolic domains are dispensable for antiapoptotic activity
To begin to understand the mechanisms by which Muc4 expression promotes tumor cell survival, we examined the roles of two of its key functional domains. The cytosolic domain of Muc1 has been shown to engage a variety of cytosolic signaling pathways, raising the possibility that the Muc4 intracellular domain contributes to its antiapoptotic signaling (2–5). On the other hand, the O-glycosylation domain of the yeast mucin Mbs2 modulates its signaling through the HOG1 MAPK pathway to promote filamentous growth in response to stress conditions (33). To determine their roles in Muc4 antiapoptotic activity, we inducibly expressed rat Muc4 forms lacking these domains in A375 cells. The deletion constructs used were Muc4β, lacking the entire α subunit of the mucin (29), and Δcyto, a derivative of Muc4β lacking 15 of the 17 intracellular domain amino acids (Fig. 1A). We observed that only Rep8 was capable of disrupting A375 cell adherence upon induction of expression (Fig. 1B), suggesting that the O-glycosylation domain is required for Muc4 antiadhesive activity. However, all three forms mediated the potentiation of ErbB receptor signaling, reflected in the elevated extent and duration of Akt phosphorylation after treatment of cells with the ErbB2-ErbB3 activating growth factor NRG1β (Fig. 1C). These observations are consistent with the presence in all three forms of the EGF-like domain 1, which our previous studies have shown is necessary for Muc4β interaction with ErbB2 (20) and facilitates receptor trafficking to the cell surface (21).
We similarly observed that all three Muc4 forms protected cells from apoptosis. Figure 2A shows that lack of expression of any of the Muc4 forms in A375 cells elevated the probability that suspended cells will undergo apoptosis by 4- to 5-fold, as assessed by Forest meta-analysis of TUNEL/FACS data. Likewise, expression of all three Muc4 forms reproducibly protected cells from apoptosis upon 48 and 72 hours of exposure to serum-free media (Fig. 2B), and protected cells from exposure to 5.5 µg/mL cisplatin (Fig. 2C). Collectively, these observations indicate that the O-glycosylation and cytosolic domains are dispensable for the ErbB potentiation and antiapoptotic activities of Muc4.
The antiapoptotic activity of Muc4 is independent of ErbB2 in A375 and MCF7 cells
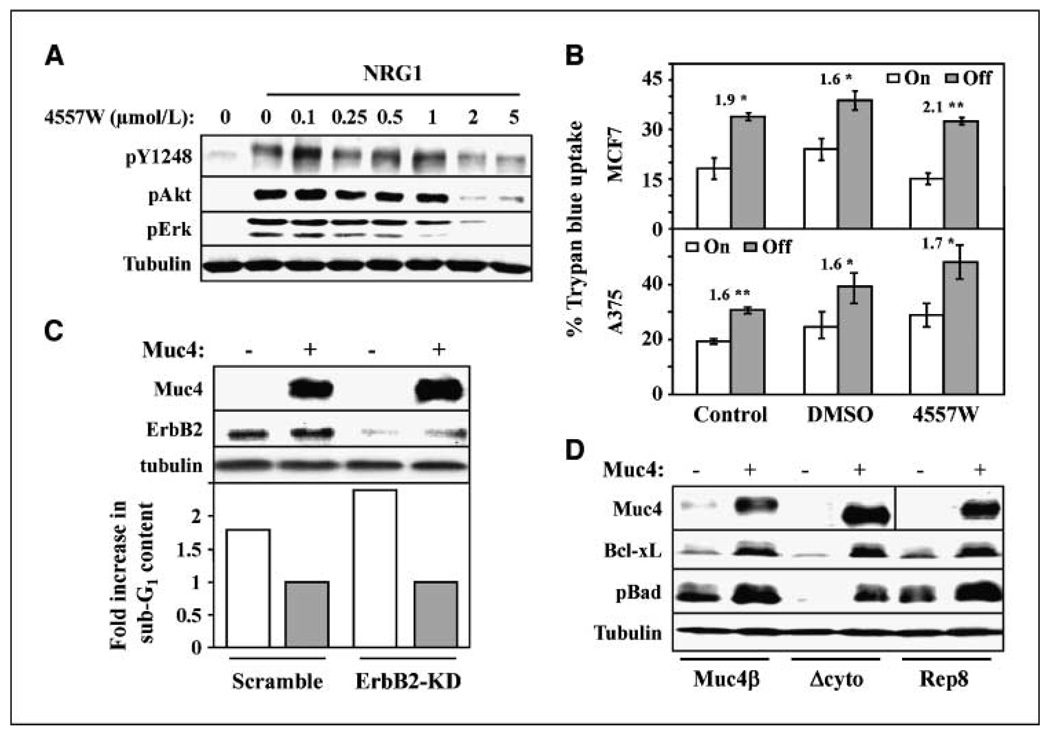
Because the Muc4 constructs retain the EGF-like domain responsible for Muc4 interaction with ErbB2, our deletion mutagenesis studies are consistent with the possibility that Muc4 signals through ErbB2 to promote cellular survival. However, our previous studies indicate that Muc4 expression in the absence of growth factor addition in A375-Rep8 and MCF7-Rep5 cells does not significantly elevate ErbB2, ErbB3, or Akt phosphorylation (see also Fig. 1C; ref.21). To test the necessity of ErbB2, we assessed the effect of ErbB2 pharmacologic inhibition and knockdown on the antiapoptotic activity of Muc4. The ErbB2-directed small molecule inhibitor 4557W potently suppressed NRG1-stimulated phosphorylation of Akt and ErbB2 Y1248 in MCF7-Rep5 (Fig. 3A) and A375-Rep8 cells (data not shown) but had no effect on the Muc4-induced survival of either cell line (Fig. 3B). siRNA-mediated knockdown of ErbB2 also did not effect Muc4-induced cellular survival in MCF7-Rep5 cells (Fig. 3C), or A375-Rep8, A375-Muc4β, or A375-Δcyto cells (data not shown). These observations are consistent with the interpretation that Muc4 does not use ErbB2 signaling in promoting the survival of these cell types. However, Muc4 expression elevated Bcl-xL expression levels and Bad phosphorylation (Fig. 3D) in A375-Rep8 cells, suggesting that the signaling pathways engaged by Muc4 funnel through Bc12 family members.
Figure 3.
ErbB2 is dispensable for antiapoptotic activity in MCF7 and A375 cells. A, MCF7-Rep5 cells were serum starved overnight and treated without and with NRG1 for 10 min, as indicated, and with increasing concentrations of 4557W. Cell lysates were immunoblotted with the indicated antibodies. B, MCF7-Rep5 cells (top) or A375-Rep8 cells growing in serum-free media for 48 h were left untreated or were treated with DMSO control or 2 µmol/L 4557W, as indicated, in the presence or absence of Muc4 expression. The percentage of viable cells was determined by trypan blue exclusion. The fold increased apoptosis in the absence relative to the presence of Muc4 expression is indicated. *, P < 0.05; **, P < 0.001. C, MCF7-Rep5 cells were transfected with scrambled or ErbB2-directed knockdown oligonucleotides, and were simultaneously treated with and without tetracycline for 48 h to induce Muc4 expression. Lysates were blotted with the indicated antibodies (top). Apoptosis for the indicated conditions was determined by sub-G1 analysis, and the fold increase in apoptosis in the absence of Muc4 relative to its presence is plotted (bottom). D, lysates from A375-Rep8, Muc4β, and Δcyto cells grown in the absence of serum for 72 h were blotted with antibodies to Muc4, Bcl-xL, phosphor-Bad, and tubulin.
Endogenous Muc4 promotes the viability of JIMT-1 human breast cancer cells
To address the role of endogenous tumor cell-expressed Muc4, we examined the effect of Muc4 knockdown on the survival of JIMT-1 breast cancer cells grown in serum-free media. We created JIMT-1 derivatives stably transduced with retroviral vector expressing Muc4-directed shRNA or scrambled control. Muc4 knockdown cells exhibited significantly lower levels of Muc4 protein, and significantly elevated levels of cleaved caspase-7 upon prolonged growth in serum-free media (Fig. 4A). Muc4 knockdown cells also exhibited a 4- to 10-fold elevated probability of undergoing apoptosis at several time points after serum withdrawal (Fig. 4B), indicating that these cells are at least partially dependent on Muc4 expression for their survival under stressful conditions. To examine the role of Muc4 domains in JIMT-1 cell survival, we assessed the ability of the rat Muc4 deletion mutants to rescue knockdown-induced apoptosis. In these experiments, wild-type JIMT-1 cells were transiently transfected with scrambled or Muc4-directed shRNA to suppress endogenous human Muc4, and simultaneously cotransfected with vector, rat Muc4β, or rat Δcyto as a replacement. Cells were then grown in the absence or presence of serum for 72 h, and apoptosis was measured by flow cytometery. In these experiments, both Muc4β and Δcyto were able to fully rescue the loss of endogenous Muc4 as assessed by sub-G1 DNA content (Fig. 4C) and by cleaved caspase-7 immunoblotting (Fig. 4D), indicating that the O-glycosylation and intracellular domains are dispensable for Muc4 survival signaling in these cells as well.
Muc4 antiapoptotic activity is dependent on ErbB2 signaling in JIMT-1 cells
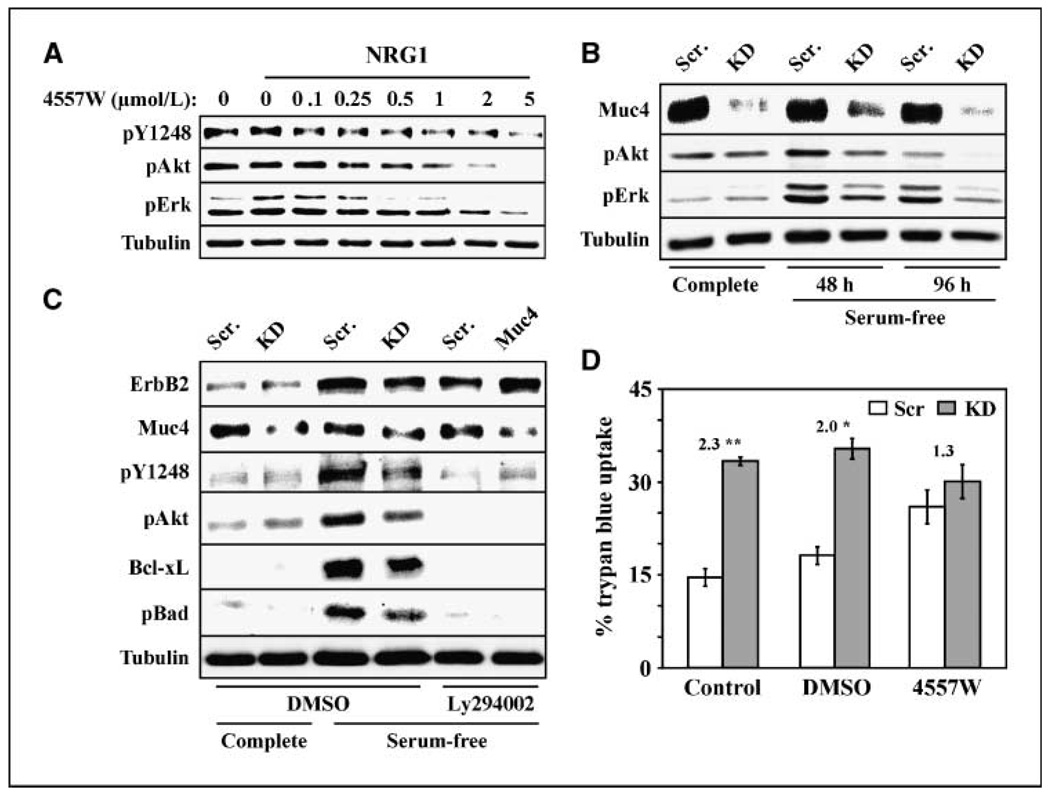
JIMT-1cells express abundant, constitutively active ErbB2 receptor, and it has been suggested that endogenous Muc4 physically shields surface ErbB2 from the ErbB2-directed therapeutic antibody trastuzumab (17). To determine whether Muc4 engages ErbB2 to promote survival in JIMT-1 cells, we first examined signaling pathways downstream of ErbB2 and Muc4. ErbB2, Erk, and Akt are all constitutively phosphorylated in JIMT-1 cells grown in serum-free media, such that addition of NRG1 growth factor does not significantly elevate their activities (Fig. 5A). The ErbB2 inhibitor 4557W markedly inhibited the phosphorylation of all three proteins (Fig. 5A), strongly suggesting that Erk and Akt activities are dependent on active ErbB2 in these cells. Muc4 knockdown also suppressed ErbB2, Akt, and Erk phosphorylation in JIMT-1 cells (Fig. 5B and C), indicating that Muc4 expression augments ErbB2 signaling to Erk and Akt. Notably, Muc4 knockdown also suppressed Bad phosphorylation and Bcl-xL levels, and inhibition of PI3-kinase activity with the small molecule inhibitor Ly294002 inhibited these events (Fig. 5C). Finally, ErbB2 inhibition by 4557W suppressed Muc4-induced survival when cells were grown in serum-free media (Fig. 5D). Taken together, these observations suggest that in JIMT-1 cells Muc4 engages ErbB2 signaling through PI3-kinase to regulate Bcl-2 family proteins and promote cellular survival under stressful conditions. To our knowledge, this is the first demonstration that tumor cells can be dependent on the Muc4-ErbB2 axis for their survival.
Figure 5.
Muc4-induced survival is mediated by ErbB2 signaling in JIMT-1 cells. A, JIMT-1-Scr cells grown in serum-free media overnight were treated without and with NRG1 for 10 min, as indicated, and with increasing concentrations (0–5 µmol/L) of 4557W ErbB inhibitor. Lysates were blotted with phospho-ErbB2 (pY1248),phospho-Akt, phospho-Erk, and tubulin antibodies. B, lysates from JIMT-1-Scr and JIMT-1-KD cells grown in complete or serum-free media for the indicated times were blotted with the indicated antibodies. C, JIMT-1-Scr and JIMT-1-KD cells grown in complete or serum-free media for 72 h were treated with DMSO control or 20 µmol/L Ly294002. Lysates were blotted with the indicated antibodies. D, JIMT-1-Scr and JIMT-1-KD cells grown in serum-free media for 72 h were left untreated or treated with DMSO or 4 µmol/L 4557W ErbB inhibitor, as indicated, and the percentage cells exhibiting trypan blue uptake was determined. The fold increase in apoptosis in the absence relative to the presence of Muc4 expression is indicated. *, P < 0.05; **, P < 0.001.
Discussion
Aberrant Muc4 expression has been observed in primary tumors of several carcinoma types, and often correlates with tumor malignancy. In invasive ductal carcinoma of the pancreas, a tissue that does not normally express Muc4, expression correlates with a significantly worsened prognosis compared with patients whose tumors express low levels or no Muc4 (11, 12). Similarly, high Muc4 expression correlates with a significantly worsened prognosis in patients with cholangiohepatic or cholangiohepatic carcinomas (34). Rakha and colleagues (6) reported that 95% of over 1,400 breast cancer cases exhibited Muc4 expression, suggesting that expression may be particularly prevalent in this tumor type. Moreover, a recent study of peritoneal effusions from ovarian carcinoma patients indicated that Muc4 was expressed in 141 of 142 patients (10), and its presence in effusions may hold some promise for earlier detection (9). These observations provide support for the notion that Muc4 could actively contribute the progression of carcinomas.
Our observations suggest that the potent antiapoptotic activity of Muc4 could allow tumor cells to surmount key barriers to tumor progression. Previous xenograft studies have shown that Muc4 expression promotes tumor cell dissemination and metastasis (15), and inhibits the apoptosis of primary tumor cells in vivo (19). To metastasize, tumor cells must survive in the circulatory or lymphatic systems in the absence of adhesion-induced survival signaling or stromal-derived survival factors. Moreover, primary tumor cells often have limited access to diffusible stromal or circulating survival factors. Here, we show for the first time that Muc4 expression inhibits anoikis, and promotes cellular survival in the absence of exogenous factors derived from serum. These observations suggest that the viability of single metastasizing cells in circulation, as well as the survival of primary tumor cells under adverse conditions, may be supported by the expression of Muc4.
We also observed that Muc4 expression suppresses cisplatin-induced apoptosis, thus promoting resistance to chemotherapeutics. These observations are consistent with a previous report suggesting that Muc4-expressing cells exhibit higher IC50s for cancer therapeutics such as Taxol, doxorubicin, and vinblastine than nonexpressing cells (35). Previous reports have also suggested that Muc4 expression contributes to tumor cell resistance to ErbB2-directed antibody therapies such as trastuzumab (16, 17) but involve mechanisms related to steric interference with the drug rather than antiapoptotic signaling. Taken together, however, these observations very strongly suggest that tumor cells can augment their malignancy by exploiting Muc4 expression to establish resistance to a variety of therapeutics. In the future, it will be important to determine whether Muc4 expression levels serve as predictors of patient response to therapy.
We have shown that the Muc4 O-glycosylation domain is dispensable for its antiapoptotic activity, suggesting that splice variants lacking this domain are able to support antiapoptotic processes associated with tumor progression. Indeed, studies with the yeast mucin Mbs2 suggest that its O-glycosylation domain suppresses signaling through the HOG1 MAPK pathway (33), pointing to a potential advantage for tumor cells to express mucin forms lacking this domain. The documented Muc4/X and Muc4/Y splice variants lack the O-glycosylation domain, and although in-depth studies of their expression in tumors have not yet been carried out, their overall structure is analogous to the Muc1/X and Muc1/Y splice variants commonly found in tumors but not normal or nonmalignant disease tissues (36, 37). On the other hand, we and others (14) have shown that the presence of the O-glycosylation domain is necessary for the efficient disruption of cell-cell and cell-substrate contacts. These observations imply that the presence of this domain could facilitate early events in tumor initiation, such as the breakdown of tight junctions, adherens junctions, and other contacts (38), but may be dispensable at later stages of tumor development. Thus, the expression of Muc4 splice variants in human tumors of various stages and grades is also a question that must be addressed to fully understand Muc4 contribution to cancer.
An unexpected finding that emerged from our studies is that Muc4 can engage ErbB2-dependent and ErbB2-independent pathways to elicit its antiapoptotic effects, depending on cell context. In ErbB2-overexpressing JIMT-1 cells, Muc4 expression promotes ErbB2 phosphorylation and signaling through the PI3-kinase and Erk pathways. These observations raise the possibility that the development of therapeutic strategies to disrupt Muc4-ErbB2 interaction could benefit patients whose tumors overexpress the two proteins, particularly because these patients’ tumors are predicted to be resistant to the conventional ErbB2-directed therapy trastuzumab.
However, Muc4-induced ErbB2 receptor activation does not seem to be universal because our previous observations indicate that Muc4 expression does not stimulate ErbB2 or its downstream pathways in several ErbB2-expressing cell lines (21). Moreover, our inhibitor and knockdown studies virtually rule out the possibility of ErbB2 involvement. In these cell types, Muc4 potentiates the response of ErbB receptors to exogenously added growth factor by trafficking intracellular receptor populations to the cell surface to facilitate their activation (21). With this in mind, we propose that Muc4 physically associates with ErbB2 and ErbB3 receptors to mediate the relocalization of intracellular receptors to the surface of JIMT-1 cells, thus exposing these receptors to autocrine growth factor ligands and potentiating receptor activity and signaling.
It seems that Muc4 can use multiple mechanisms to engage pathways leading to tumor cell progression. These observations raise questions as to the mechanisms by which Muc4 inhibits apoptosis in ErbB2-independent cell types. One possibility is that such as Muc1, Muc4 directly engages cytosolic signaling proteins through its intracellular domain. The intracellular domain of Muc1 is phosphorylated by several kinases, interacts with numerous intracellular signaling proteins, and may be proteolytically released from the membrane and translocated to the nucleus (5). In contrast, the intracellular domain of Muc4 is significantly more modest in size and does not harbor any motifs predicted to be phosphorylated by kinases or to mediate interactions with signaling proteins. Consistent with this, we have observed that the Muc4 cytoplasmic domain is dispensable for its antiapoptotic activity in all cell lines that we have examined. Our observations suggest that the extracellular region of the Muc4β subunit associates with another cell surface protein(s) in some cell types to engage cellular survival signaling pathways. Future studies will be aimed at identifying Muc4 interacting components, and identifying signaling pathways that couple Muc4 expression to augmented survival.
Supplementary Material
Acknowledgments
Grant support: NIH grants GM068994 (K.L. Carraway) and CA118384 (C. Sweeney), T32 RR07038 (H.C. Workman), and statistical support was made possible by UL1 RR024146 from the National Center for Research Resources, a component of the NIH. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of NCRR or NIH.
We thank Andrew Manford for construction of the pcDNA3.1-Lrig1 leader sequence vector, Melanie Funes for the inducible Muc4β cell line, Richard Mulligan for the 293GPG packaging line, and Kermit Carraway (University of Miami) for cell lines, antibodies and critical reading of the manuscript.
Footnotes
Unpublished observations.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Note: Supplementary data for this article are available at Cancer Research Online (http://cancerres.aacrjournals.org/).
References
- 1.Chaturvedi P, Singh AP, Batra SK. Structure, evolution, and biology of the MUC4 mucin. FASEB J. 2008;22:966–981. doi: 10.1096/fj.07-9673rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hattrup CL, Gendler SJ. Structure and function of the cell surface (tethered) mucins. Annu Rev Physiol. 2008;70:431–457. doi: 10.1146/annurev.physiol.70.113006.100659. [DOI] [PubMed] [Google Scholar]
- 3.Carraway KL, III, Funes M, Workman HC, Sweeney C. Contribution of membrane mucins to tumor progression through modulation of cellular growth signaling pathways. Curr Top Dev Biol. 2007;78:1–22. doi: 10.1016/S0070-2153(06)78001-2. [DOI] [PubMed] [Google Scholar]
- 4.Cullen PJ. Signaling mucins: the new kids on the MAPK block. Crit Rev Eukaryot Gene Expr. 2007;17:241–257. doi: 10.1615/critreveukargeneexpr.v17.i3.50. [DOI] [PubMed] [Google Scholar]
- 5.Singh PK, Hollingsworth MA. Cell surface-associated mucins in signal transduction. Trends Cell Biol. 2006;16:467–476. doi: 10.1016/j.tcb.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 6.Rakha EA, Boyce RW, Abd El-Rehim D, et al. Expression of mucins (MUC1, MUC2, MUC3, MUC4, MUC5AC and MUC6) and their prognostic significance in human breast cancer. Mod Pathol. 2005;18:1295–1304. doi: 10.1038/modpathol.3800445. [DOI] [PubMed] [Google Scholar]
- 7.Kwon KY, Ro JY, Singhal N, et al. MUC4 expression in non-small cell lung carcinomas: relationship to tumor histology and patient survival. Arch Pathol Lab Med. 2007;131:593–598. doi: 10.5858/2007-131-593-MEINCL. [DOI] [PubMed] [Google Scholar]
- 8.Karg A, Dinc ZA, Basok O, Ucvet A. MUC4 expression and its relation to ErbB2 expression, apoptosis, proliferation, differentiation, and tumor stage in non-small cell lung cancer (NSCLC) Pathol Res Pract. 2006;202:577–583. doi: 10.1016/j.prp.2006.04.002. [DOI] [PubMed] [Google Scholar]
- 9.Giuntoli RL, II, Rodriguez GC, Whitaker RS, Dodge R, Voynow JA. Mucin gene expression in ovarian cancers. Cancer Res. 1998;58:5546–5550. [PubMed] [Google Scholar]
- 10.Davidson B, Baekelandt M, Shih I. MUC4 is upregulated in ovarian carcinoma effusions and differentiates carcinoma cells from mesothelial cells. Diagn Cytopathol. 2007;35:756–760. doi: 10.1002/dc.20771. [DOI] [PubMed] [Google Scholar]
- 11.Saitou M, Goto M, Horinouchi M, et al. MUC4 expression is a novel prognostic factor in patients with invasive ductal carcinoma of the pancreas. J Clin Pathol. 2005;58:845–852. doi: 10.1136/jcp.2004.023572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagata K, Horinouchi M, Saitou M, et al. Mucin expression profile in pancreatic cancer and the precursor lesions. J Hepatobiliary Pancreat Surg. 2007;14:243–254. doi: 10.1007/s00534-006-1169-2. [DOI] [PubMed] [Google Scholar]
- 13.Miyahara N, Shoda J, Ishige K, et al. MUC4 interacts with ErbB2 in human gallbladder carcinoma: potential pathobiological implications. Eur J Cancer. 2008;44:1048–1056. doi: 10.1016/j.ejca.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 14.Komatsu M, Carraway CA, Fregien NL, Carraway KL. Reversible disruption of cell-matrix and cell-cell interactions by overexpression of sialomucin complex. J Biol Chem. 1997;272:33245–33254. doi: 10.1074/jbc.272.52.33245. [DOI] [PubMed] [Google Scholar]
- 15.Komatsu M, Tatum L, Altman NH, Carothers Carraway CA, Carraway KL. Potentiation of metastasis by cell surface sialomucin complex (rat MUC4), a multifunctional anti-adhesive glycoprotein. Int J Cancer. 2000;87:480–486. doi: 10.1002/1097-0215(20000815)87:4<480::aid-ijc4>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Price-Schiavi SA, Jepson S, Li P, et al. Rat Muc4 (sialomucin complex) reduces binding of anti-ErbB2 antibodies to tumor cell surfaces, a potential mechanism for herceptin resistance. Int J Cancer. 2002;99:783–791. doi: 10.1002/ijc.10410. [DOI] [PubMed] [Google Scholar]
- 17.Nagy P, Friedlander E, Tanner M, et al. Decreased accessibility and lack of activation of ErbB2 in JIMT-1, a herceptin-resistant, MUC4-expressing breast cancer cell line. Cancer Res. 2005;65:473–482. [PubMed] [Google Scholar]
- 18.Komatsu M, Yee L, Carraway KL. Overexpression of sialomucin complex, a rat homologue of MUC4, inhibits tumor killing by lymphokine-activated killer cells. Cancer Res. 1999;59:2229–2236. [PubMed] [Google Scholar]
- 19.Komatsu M, Jepson S, Arango ME, Carothers Carraway CA, Carraway KL. Muc4/sialomucin complex, an intramembrane modulator of ErbB2/HER2/Neu, potentiates primary tumor growth and suppresses apoptosis in a xenotransplanted tumor. Oncogene. 2001;20:461–470. doi: 10.1038/sj.onc.1204106. [DOI] [PubMed] [Google Scholar]
- 20.Carraway KL, III, Rossi EA, Komatsu M, et al. An intramembrane modulator of the ErbB2 receptor tyrosine kinase that potentiates neuregulin signaling. J Biol Chem. 1999;274:5263–5266. doi: 10.1074/jbc.274.9.5263. [DOI] [PubMed] [Google Scholar]
- 21.Funes M, Miller JK, Lai C, Carraway KL, III, Sweeney C. The mucin Muc4 potentiates neuregulin signaling by increasing the cell-surface populations of ErbB2 and ErbB3. J Biol Chem. 2006;281:19310–19319. doi: 10.1074/jbc.M603225200. [DOI] [PubMed] [Google Scholar]
- 22.Cantley LC. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 23.Downward J. How BAD phosphorylation is good for survival. Nat Cell Biol. 1999;1:E33–E35. doi: 10.1038/10026. [DOI] [PubMed] [Google Scholar]
- 24.Junttila MR, Li SP, Westermarck J. Phosphatase-mediated crosstalk between MAPK signaling pathways in the regulation of cell survival. FASEB J. 2008;22:954–965. doi: 10.1096/fj.06-7859rev. [DOI] [PubMed] [Google Scholar]
- 25.Thomadaki H, Scorilas A. BCL2 family of apoptosis-related genes: functions and clinical implications in cancer. Crit Rev Clin Lab Sci. 2006;43:1–67. doi: 10.1080/10408360500295626. [DOI] [PubMed] [Google Scholar]
- 26.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 27.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–291. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 28.Zha J, Harada H, Osipov K, Jockel J, Waksman G, Korsmeyer SJ. BH3 domain of BAD is required for heterodimerization with BCL-XL and pro-apoptotic activity. J Biol Chem. 1997;272:24101–24104. doi: 10.1074/jbc.272.39.24101. [DOI] [PubMed] [Google Scholar]
- 29.Rossi EA, McNeer RR, Price-Schiavi SA, et al. Sialomucin complex, a heterodimeric glycoprotein complex. Expression as a soluble, secretable form in lactating mammary gland and colon. J Biol Chem. 1996:33476–33485. doi: 10.1074/jbc.271.52.33476. [DOI] [PubMed] [Google Scholar]
- 30.Tanner M, Kapanen AI, Junttila T, et al. Characterization of a novel cell line established from a patient with Herceptin-resistant breast cancer. Mol Cancer Ther. 2004;3:1585–1592. [PubMed] [Google Scholar]
- 31.Ory DS, Neugeboren BA, Mulligan RC. A stable human-derived packaging cell line for production of high titer retrovirus/vesicular stomatitis virus G pseudotypes. Proc Natl Acad Sci U S A. 1996;93:11400–11406. doi: 10.1073/pnas.93.21.11400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yen L, Cao Z, Wu X, et al. Loss of Nrdp1enha nces ErbB2/ErbB3-dependent breast tumor cell growth. Cancer Res. 2006;66:11279–11286. doi: 10.1158/0008-5472.CAN-06-2319. [DOI] [PubMed] [Google Scholar]
- 33.Cullen PJ, Sabbagh WJ, Graham E, et al. A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Genes Dev. 2004;18:1695–1708. doi: 10.1101/gad.1178604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shibahara H, Tamada S, Goto M, et al. Pathologic features of mucin-producing bile duct tumors: two histopathologic categories as counterparts of pancreatic intraductal papillary-mucinous neoplasms. Am J Surg Pathol. 2004;28:327–338. doi: 10.1097/00000478-200403000-00005. [DOI] [PubMed] [Google Scholar]
- 35.Hu YP, Haq B, Carraway KL, Savaraj N, Lampidis TJ. Multidrug resistance correlates with overexpression of Muc4 but inversely with P-glycoprotein and multidrug resistance related protein in transfected human melanoma cells. Biochem Pharmacol. 2003;65:1419–1425. doi: 10.1016/s0006-2952(03)00086-8. [DOI] [PubMed] [Google Scholar]
- 36.Baruch A, Hartmann M, Zrihan-Licht S, et al. Preferential expression of novel MUC1tumor antigen isoforms in human epithelial tumors and their tumor-potentiating function. Int J Cancer. 1997;71:741–749. doi: 10.1002/(sici)1097-0215(19970529)71:5<741::aid-ijc9>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 37.Passebosc-Faure K, Li G, Lambert C, et al. Evaluation of a panel of molecular markers for the diagnosis of malignant serous effusions. Clin Cancer Res. 2005;11:6862–6867. doi: 10.1158/1078-0432.CCR-05-0043. [DOI] [PubMed] [Google Scholar]
- 38.Carraway KL, Ramsauer VP, Carraway CA. Glycoprotein contributions to mammary gland and mammary tumor structure and function: roles of adherens junctions, ErbBs and membrane MUCs. J Cell Biochem. 2005;96:914–926. doi: 10.1002/jcb.20612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.