Abstract
Specific therapies are not available for inflammatory muscle diseases. We and others have shown that the pro-inflammatory NF-κB pathway is highly activated in these conditions. Since NF-κB is an important therapeutic target, we decided to utilize an in vitro screening assay to identify potential inhibitors that block TNF-α induced NF-κB activation in a C2C12 muscle line that stably expresses an NF-κB luciferase reporter gene. We have tested multiple potential anti-inflammatory agents in undifferentiated myoblasts as well as differentiated myotubes and found different strengths of inhibitions in each cell type. Interestingly, we found that some drugs that are known to inhibit NF-κB in immune cells were not effective in muscle cells. Drug toxicity was assessed for using an MTT cell viability assay, and the validity of the luciferase assay was verified by immunostaining for NF-κB nuclear translocation in myoblasts. In conclusion, we have determined the optimal assay conditions for detecting potentially valuable NF-κB inhibitors for the first time in a muscle cell line that may have significant therapeutic potential for inflammatory muscle diseases.
Keywords: Inflammatory Myopathy, Duchenne Muscular Dystrophy, NF-κB inhibitor, High-Throughput Screening (HTS) Assay, Luciferase Assay, C2C12 muscle cells
INTRODUCTION
Corticosteroids have revolutionized treatment of several chronic inflammatory diseases including autoimmune and genetic muscle diseases. However, despite their therapeutic benefits, their use is limited due to significant side effects including steroid induced myopathy, fat deposition, adrenal deficiency, hyperglycemia and osteoporosis. The NF-κB signaling pathway is the primary activator of inflammation in inflammatory myopathies, and therefore is an attractive therapeutic target for these diseases [1, 2, 3].
The NF-κB family of proteins are dimeric transcription factors that regulate the expression of large a number of genes involved in inflammation, immune response and cell survival. In non-stimulated cells, NF-κB remains bound to the inhibitory protein I-κB and is sequestered in the cytoplasm. However, upon stimulation with cytokines such as TNF-α and infectious agents, the cytosolic kinase, IKK phosphorylates I-κB, at which point it becomes susceptible to ubiquitination and degradation. The activated NF-κB dimer is then able to translocate to the nucleus to bind NF-κB promoter elements and drive the expression of a large number of pro-inflammatory genes.
Transient activation of the NF-κB pathway is part of the normal regenerative process in muscle, but persistent over-activation has been shown to be detrimental, leading to large amounts of muscle wasting [4]. Studies by us and others have demonstrated that NF-κB is strongly upregulated in the biopsies of Duchenne Muscular Dystrophy (DMD) patients, localized to both myofibers and within infiltrating monocytes, a feature which also persists in inflammatory myopathy patients.
A problem with the use of anti-inflammatory drugs in patients suffering from inflammatory myopathies is that while the agents being administered have been shown to be effective in immune cells, they have not been shown to be effective at blocking inflammatory signaling in myocytes [5], which could result in suboptimal effectiveness in improving muscle pathology. Therefore, the purpose of this study was to design and evaluate an in vitro system to identify inhibitors that block cyotkine induced NF-κB activation in skeletal muscle cells; such inhibitors would likely be benecifical for inflammatory myopathy patients.
MATERIALS AND METHODS
Cell culture
Commercially available C2C12 stable cells that have an integrated chromosomal reporter consisting of six tandem NF-κB response elements coupled to luciferase were cultured with DMEM containing Penicillin (100U/ml) & Streptomycin (100μg/ml), Hygromycin (100μg/ml) and 10% Fetal Bovine Serum (ATCC). These myoblasts were differentiated to myotubes by reducing serum concentrations (replacing 10% FBS with 2% Horse Serum) and allowing them to remain in culture for a minimum of 48hours [6].
Drug Treatment
Myoblasts and myotubes were plated in either 96 well plates at a density of 1.0×104 cells/ml or in lab-tek chamber slides at a density of 2.2×103 cells/ml; all groups were plated in triplicate. Cells were allowed to adhere overnight, and then the media was replaced the following day with media containing compounds at 11 different ½ log incremental doses covering the range of 0.0001μM to 10μM. On the third day, NF-κB transcription was induced by directly adding 10ng/ml of TNF-α for 24 hours.
NF-κB Luciferase, MTT, & Immunostaining Assays
In the luciferase assay, cell contents were extracted 24hr after TNF-α treatment using reporter lysis buffer with one freeze thaw cycle, and 25μl of the lysate was transferred to Costar white plates for reading. Luciferase activity was measured using a single luciferase reporter assay system with a Berthold Centro LB 960 Luminometer, in which 50μl of luciferase reagent was automatically dispersed, shaken and read for 10 seconds. Raw luciferase values were normalized as a percentage of the TNF-α treated group, which represented 100% induction. For the MTT assay, cell viability was measured using a spectrophotometer based 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay, in which cells were incubated with reagent for 4 hours, media was removed, formazan crystals dissolved in MTT solvent (0.1N HCL in anhydrous isopropanol), and wells assayed for viability by spectrophotometer measuring absorbance at 570nm. Percent cell viability was calculated relative to untreated controls. For immunostaining, C2C12 myoblasts were plated on Lab-Tek chamber slides, exposed to IKK VII, Prednisolone or Celastrol, and then TNF-α, each for 24 hours. Lastly, cells were fixed with 6% paraformaldehyde (PFA) and stained for NF-κB p65 and counterstaind with DAPI to identify nuclei; the number of cells exhibiting NF-κB nuclear localization was qualitatively determined.
Statistical Analysis
Intra-assay and inter-assay variability were assessed by calculating CV and ICC values determined from measuring the variance in NF-κB inhibition luciferase activity of Prednisolone treated, TNF-α stimulated, C2C12 myoblasts that were plated in triplicate and run in three independent experiments. All other experiments were run in triplicate wells, and the resulting mean and standard errors were calculated and are represented.
Materials
The NF-κB C2C12 myoblast reporter cell line was purchased from Panomics (Fremont, CA). DMEM with 4.5g/L glucose and glutamine from Lonza BioWhittaker (Switzerland), Penicillin, Streptomycin, Hygromycin and Prednisolone from MP Biomedical (Irvine, CA), Fetal Bovine Serum and Horse Serum from ATCC (Manassas, VA), the Luciferase Reporter System and Reporter Lysis Buffer from Promega (Madison, WI), the MTT from Sigma Aldrich (St. Louis, Missouri), Celastrol, Withaferin, Bay11-7085, IKK VII and IKK II from EMD Chemicals (Darmstadt, Germany), Pirfenidone from Marnac Inc. (Dallas, Texas), FGF and TNF-α from R&D systems (Minneapolis, MN), EGCG from Nutriscience (Fairfield, Connecticut) and Thymosin ß4 from RegeneRx Biopharmaceuticals (Bethesda, MD).
RESULTS
A range of TNF-α (10ng/ml) treatment time points were initially tested in the NF-κB C2C12 myoblast reporter cell line to determine when the optimal induction of luciferase would occur. It was found that a minimum of 2.5 hours of TNF-α treatment was required in order to detect induction, which lead to a 5 fold increase in luciferase output (Supplementary Figure 1), whereas a TNF-α treatment time of 24 hours resulted in a 20 fold increase. Using this latter time point of treatment, we then tested Prednisolone’s ability to inhibit induction by pre-treating myoblasts with it for 24 hours; we noticed that significant inhibition was found at this time point. Having established that NF-κB elements could be strongly induced into the nucleus by TNF-α after 24 hours of treatment, and that Prednisolone, a known NF-κB inhibitor could prevent this translocation with 24 hours of pretreatment, we wanted to then assess the reproducibility of drug inhibition with this timing scheme. We pursued this by repeating the Prednisolone experiment, over three independent experiments ran in triplicate wells, and determined the intra (CV) and inter-variability (ICC) coefficients of the assay (Table 1). The average resulting CV value calculated was 14.4% and ICC value was 0.802, indicating that the assay regime selected is both robust and reproducible.
Table 1. Analysis of the intra (CV) and inter-variability (ICC) of the luciferase NF-κB inhibition assay in C2C12 myoblasts demonstrated that the assay shows little variability within both individual replicate plates and between repeated experiments. Lower CV values and higher ICC values are better scores, indicating less variability.
| * Prednisolone Concentration | Mean ± SD | CV | ICC |
|---|---|---|---|
| 0.1 μM | 51.68 ± 13.58 | 26.2% | 0.740 |
| 1.0 μM | 79.20 ± 9.22 | 11.6% | 0.776 |
| 10.0 μM | 85.41 ± 4.59 | 5.4% | 0.892 |
Three different concentrations of Prednisolone were tested in triplicate over the course of three independent experiments, and the mean and standard deviations of all values were calculated.
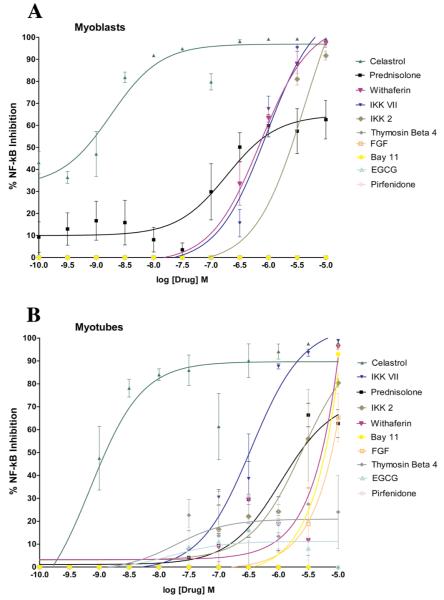
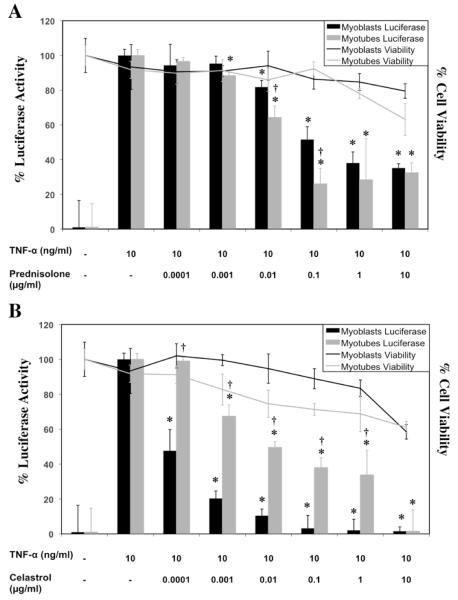
We pursued the testing of nine additional drugs that were screened at a range from 0.0001-10μM, and IC50 values were calculated for all drugs using Graphpad Prizm software by non-linear regression logarithmic dose response curve analysis (Figure 1, Table 2). Dose response data that also displays MTT toxicity data at each concentration for two of the drugs screened, Prednisolone and Celastrol, are highlighted in Figure 2. Overall, Celastrol was far more effective then Prednisolone at inhibiting NF-κB in both myoblasts and myotubes although it was also more toxic to myotubes (35%) at higher concentrations (10μg/ml) (Figure 2).
Figure 1.
C2C12 myoblasts (A) and myotubes (B) containing NF-κB response elements coupled to a luciferase reporter were plated for growth in triplicate groups and on the following day the media was changed to include ten different drugs at eleven different ½ log step increment doses. Twenty-four hours afterwards, 10ng/ml of TNF-α was added directly to the media and then 24 hours later, luciferase activity was recorded. The mean value for each parameter is shown, as well as the standard error amongst replicates.
Table 2. Ten drugs were tested for their ability to inhibit TNF-α induced NF-κB activation in a C2C12 luciferase reporter cell line. We found a broad range of drug ability to act as inhibitors, with notable differences in their efficacies between myoblasts and myotubes.
| Drug * | Myoblasts IC50 (M) † | Myoblasts % Cell Viability | Myotubes IC50 (M) | Myotubes % Cell Viability | |
|---|---|---|---|---|---|
| 1. | Celastrol | 8.91×10-10 | 83.9 | 1.17×10-9 | 72.1 |
| 2. | Prednisolone | 4.17×10-7 | 78.8 | 1.78×10-6 | 81.6 |
| 3. | Withaferin | 5.62×10-7 | 61.1 | 4.47×10-6 | 72.7 |
| 4. | IKK VII | 6.60×10-7 | 53.9 | 3.31×10-7 | 68.4 |
| 5. | IKK 2 | 2.04×10-6 | 74.1 | 1.91×10-6 | 102.6 |
| 6. | Bay 11-7085 | No Inhibition | n/a | 6.31×10-6 | 87.2 |
| 7. | FGF | No Inhibition | n/a | 8.51×10-6 | 131.1 |
EGCG, Thymosin β4, and Pirfenidone did not reach 50% inhibition in either myoblasts or myotubes. n/a = not avialable since no inhibition occured.
Graphpad Prizm software was used to plot logarithmic, non-linear regression curves for all data collection points (Supplementary Figure 2) and the dose of drug that reduced luciferase activity by 50% (IC50) was calculated. The MTT assay was used to determine the cell viability of all drugs at their respective IC50 concentrations; the mean value is shown above.
Figure 2.
C2C12 myoblasts and myotubes containing NF-κB response elements coupled to a luciferase reporter were plated for growth in triplicate groups and on the following day the media was changed to include (A) Prednisolone (B) Celastrol at multiple drug doses. Twenty-four hours afterwards, 10ng/ml of TNF-α was added directly to the media and then 24 hours later, luciferase activity and MTT measurements were recorded. The mean value for each parameter is shown, as well as the standard error amongst replicates. (*p-value <0.05 compared to TNF-α treated, †p-value <0.05 compared to myoblasts at same concentration)
We found that myoblasts and myotubes differed in their sensitivities to the same drug at the same concentration in both luciferase activity and cell viability (Table 2); most notably were Bay-11 7085 and FGF who exhibited NF-κB inhibition in myotubes but not myoblasts. Thymosin ß4, Pirfenidone and EGCG were the most inert of the drugs screened and did not inhibit NF-κB in either myoblasts or myotubes. The most toxic drug screened was IKK VII showing a reduction in myoblast cell viability to 53.9%. Celastrol was the most effective NF-κB inhibitor screened in both myoblasts and myotubes, with respective IC50 values of 8.91×10-10 and 1.17×10-9 M, and also displaying relatively minimal toxicities. We have noted a slight variability in the IC50 values of Prednisolone in Table 1 and Table 2, which are probably due to variation between experiments.
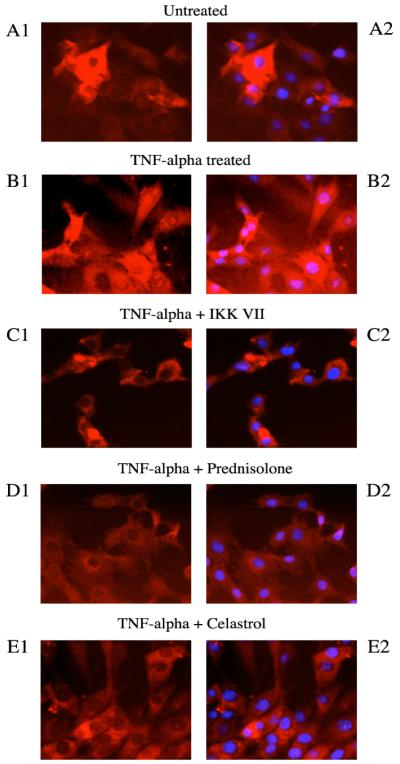
To reduce the assay time, we tested myoblasts with a 30min Prednisolone pretreatment followed by a 3hr TNF-α incubation (Figure 3). When comparing the inhibition values found at this shorter regime with those from the longer one (Figure 2), we determined that stronger inhibitions occurred with the shorter regime, with the largest increase observed at the 1μg/ml dose; Prednisolone reduced luciferase activity to 49.5% in the short compared with 38% in the long (a 23.3% increase). To validate the luciferase findings, C2C12 myoblasts were plated on Lab-Tek chamber slides, incubated with drug (1μg/ml) for 24 hours, then with TNF-α for 24 hours, and immunofluorescence staining for nuclear DAPI and NF-κB was performed. In untreated cells, NF-κB staining was observed primarily in the cytosol, whereas TNF-α treated cells showed nuclear translocation of NF-κB (Figure 4A, 4B). IKK VII, Prednisolone and Celastrol all appeared to block nuclear translocation (Figure 4C, 4D, 4E).
Figure 3.
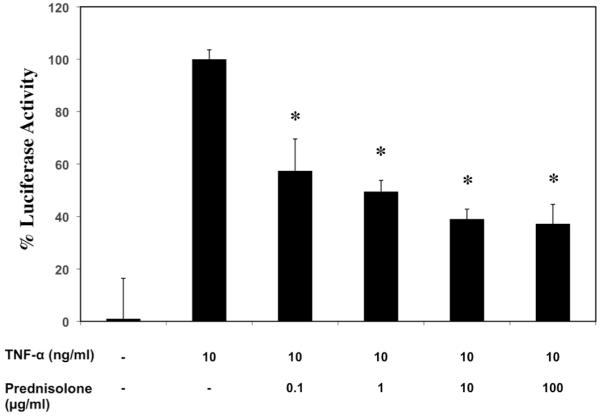
C2C12 myoblasts were pretreated with Prednisolone at multiple concentrations in triplicate for 30minutes prior to direct addition of 10ng/ml TNF-α to the media, then three hours later mean luciferase measurements were recorded, then means and standard errors calculated. (*p-value <0.05 compared to TNF-α treated)
Figure 4.
C2C12 myoblasts were plated on Lab-Tek chamber slides and exposed to drug treatment on Day 2 and TNF-α treatment on Day 3, as described above. On Day 4, cells were fixed with 6% PFA and stained for NF-κB (red, shown in both left and right panels) and the nuclear stain DAPI (blue, shown in right side panels only). (A1-A2) In untreated cells, NF-κB resides primarily in the cytosol, where it is sequestered by I-κB. (B1-B2) TNF-α treated cells signal for I-κB degradation and NF-κB nuclear translocation. (C1-C2, D1-D2, E1-E2) Pretreatment with IKK VII, Prednisolone and Celastrol prior to TNF-α addition all reduced the amount of nuclear translocation.
DISCUSSION
The NF-kB pathway can be activated by multiple pro-inflammatory molecules such as IL-1 and LPS, but the in vitro system we used specifically monitors only TNF-α induced NF-κB activation. It is important to acknowledge that it is possible that exploratory agents tested using this particular assay could ultimately inhibit luciferase readout by blocking the TNF-α and/or NF-κB pathways. Therefore, the type of NF-κB inhibition that is desired to screen for is also dependent on the choice of pro-inflammatory molecule used for its induction.
Prednisolone, one of the most frequently prescribed anti-inflammatory drugs, is unsurprisingly also a potent inhibitor of the NF-κB signaling pathway in immune cells [7]. We have now demonstrated that it is also effective at inhibiting the NF-κB pathway in myoblasts and myotubes (Figure 1). It ranked amongst the most potent agents that we tested, being the second strongest NF-κB inhibitor in myoblasts and third strongest in myotubes (Table 2). This result could partially explain why Prednisolone is substantially more effective than other anti-inflammatory drugs that have been used to treat inflammatory myopathies. The 417nM concentration that we have found in our in vitro system is within the the therpareutic range of Prednisolone [8, 9].
Celastrol is a quinone methide triterpene derived from the medicinal plant Tripterygium wilfordii and was by far the most potent inhibitor in our drug screen, being three orders of magnitude more potent than Prednisolone in both myoblasts and myotubes. It has previously been shown to be an inhibitor of TNF-α induced NF-κB activation in epithelial, lung and kidney cell lines, having maximal effects at a concentration of 5μM [10]. In another study that gauged its potency at inhibiting LPS-induced NF-κB in a macrophage cell line, it was found to have an IC50 value of 270nM [11]. Taken together, it seems that while Celastrol is a blocker of NF-κB in multiple cell types, it could be most effective in myocytes since it was shown here to have IC50 values at ∼1nM. It must be noted that since different measures of NF-κB blockade and TNF-α concentrations were utilized, further validation studies would be needed to support the above statement. Celastrol is currently being used to treat a number of autoimmune disease including Arthritis, Asthma, and systemic Lupus. In an in vivo model of Arthritis, Celastrol was found to reduce paw swelling a similar amount as Prednisolone at a dose of 10mg/kg [12]; while this does not mirror the potencies seen here, this is also is not a disease of muscle. It would be worthwhile to test its ability to treat inflammatory myopathies, although the dosing will be an important factor because while it was the most potent of the drugs that we screened, it was also somewhat toxic to myotubes.
IKK VII and IKK 2 are known inhibitors of IKK, which is the enzyme that phosphorylates I-κB and allows NF-κB activation [13]. They have shown to be potent at inhibiting I-κB degradation, as well as ICAM and VCAM in an endothelial cell line (IC50 = 1-100μM) and we found that they had similar potencies in our assay.
Pirfenidone, EGCG, Thymosin ß4 and Fibroglast growth factor (FGF) were all mostly ineffective at inhibiting NF-κB in myocytes. Pirfenidone is an anti-fibrotic agent that has previously been shown to have anti-inflammatory capabilities in lung and immune cells [14, 15]. It has also been shown to improve cardiac function through the reduction of fibrosis in the mdx model at a dose of 1.2mg/kg [16], however was not effective at inhibiting NF-κB in our assay. Epigallocatechin gallate (EGCG) is the most abundant polyphenol in green tea and has been shown to have anti-oxidant and anti-inflammatory properties in multiple cell types. In contrast to our results in myocytes, it has previously been shown to be an effective NF-κB inhibitor (IC50 = 12 μM) in a macrophage reporter cell line [17]. In vivo, a dose of 5mg/kg has been shown to improve mdx mouse muscle histology, force contractions and creatine kinase leakage [18]. It has been hypothesized that the EGCGs ability to quench free radicals in mdx muscle as well as subdue the invasion of inflammatory cells are this agents primary mechanisms of action. Thymosin ß4 is a small actin-sequestering protein that reduces inflammation and promotes healing in multiple tissues including muscle [19]. FGF has recently been shown to increase p65 nuclear translocation in a mammary cell line [20], and we found that it was completely ineffective at blocking NF-κB activation in myoblasts and only mildly effective in myotubes.
Withaferin is a steroidal lactone purified from Withania somnifera and has previously been shown to inhibit LPS induced NF-κB in primary human peripheral blood and mononuclear cells, with a maximal effective dose at 1.0mg/ml [21]. We found that Withaferin was effective at inhibiting NF-κB in both myoblasts and myotubes in the μg range, but doses in the mg range would certainly reach toxicity in myocytes.
BAY 11-7085 was demonstrated to selectively and irreversibly inhibit the TNF-α induced phosphorylation of I-κB (IC50 = 10 μM) in an endothelial cell line, resulting in a decreased expression of NF-κB and of adhesion molecules [22]. Additionally, it has been show to inhibit TNF-α induced surface expression of the endothelial-leukocyte cell adhesion molecules E-selectin, VCAM-1, and ICAM-1. We found a very similar potency of this drug in our study in myoblasts (IC50 = 8.5 μM), while no inhibition was found in myotubes.
Since we found moderately similar results for Prednisolone in NF-κB inhibition using the shorter treatment scheme (Figure 3), those conditions would be acceptable for utilization in high throughput screening. While time can be saved using the shorter assay regime, it is unlikely to identify inhibitors that indirectly block NF-κB activation. This is not the case with Prednisolone, as with other glucocorticoids, which are known to enter the nucleus and exert their effects rapidly. Ideally, both regimes could be used to assess the speed at which a potential drug candidate can inhibit NF-κB inflammatory signaling.
In summary, we have verified a robust and reproducible assay with the optimal conditions for the screening of effective, non-toxic NF-κB inhibitors for muscle diseases. By using this relatively fast approach, the pursuit of finding optimal therapies for the inflammatory myopathies will be improved.
Supplementary Material
Supplementary Figure 1 - C2C12 myoblasts were treated with 10ng/ml of TNF-α that was added directly to media and luciferase output was quantified at multiple later time points in triplicate and recorded as the mean fold change of treated to untreated signal, and standard errors were calculated. (*p-value <0.05 compared to 5 minute timepoint)
ACKNOWLEDGEMENTS
Andreas R. Baudy is a pre-doctoral student in the Molecular Medicine Program of the Institute for Biomedical Sciences at the George Washington University. This work is from a dissertation to be presented to the above program in partial fulfillment of the requirements for the Ph.D. degree.
GRANTS
Dr. Nagaraju is supported by National Institute of Health (RO1-AR050478 and 5U54HD053177), Foundation to Eradicate Dystrophy, Muscular Dystrophy Association, The Myositis Association and US Department of Defense (W81XWH-05-1-0616).
Dr. Hoffman is supported by National Institute of Health (RO1-5U54HD053177) and the US Department of Defense (W81XWH-05-1-0616).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1).Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-kappaB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60:993–7. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- 2).Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, Chang J, Dwivedi S, Mitsak M, Chen YW, Plotz P, Rosen A, Hoffman E, Raben N. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheumatol. 2005;52:1824–35. doi: 10.1002/art.21103. [DOI] [PubMed] [Google Scholar]
- 3).Chen YW, Nagaraju K, Bakay M, McIntyre O, Rawat R, Shi R, Hoffman EP. Early onset of inflammation and later involvement of TGFbeta in Duchenne muscular dystrophy. Neurology. 2005;65:826–34. doi: 10.1212/01.wnl.0000173836.09176.c4. [DOI] [PubMed] [Google Scholar]
- 4).Cai D, Frantz JD, Tawa NE, Jr, Melendez PA, Oh BC, Lidov HG, Hasselgren PO, Frontera WR, Lee J, Glass DJ, Shoelson SE. IKKBeta/NF-kappaB activation causes severe muscle wasting in mice. Cell. 2004;119(2):285–98. doi: 10.1016/j.cell.2004.09.027. [DOI] [PubMed] [Google Scholar]
- 5).Nagaraju K. Immunological capabilities of skeletal muscle cells. Acta Physiol Scand. 2001;171:215–23. doi: 10.1046/j.1365-201x.2001.00823.x. [DOI] [PubMed] [Google Scholar]
- 6).Sarabia V, Ramlal T, Klip A. Glucose uptake in human and animal muscle cells in culture. Biochem Cell Biol. 1990;68:536–42. doi: 10.1139/o90-076. [DOI] [PubMed] [Google Scholar]
- 7).Auphan N, DiDonato JA, Rosette C, Helmberg A, Karin M. Immunosuppression by glucocorticoids: Inhibition of nf-kappa B activity through induction of I kappa B synthesis. Science. 1995 Oct 13;270:286–90. doi: 10.1126/science.270.5234.286. [DOI] [PubMed] [Google Scholar]
- 8).Manzur AY, Kuntzer T, Pike M, Swan A. Glucocorticoid corticosteroids for Duchenne muscular dystrophy. Cochrane Database Syst Rev. 2008;1 doi: 10.1002/14651858.CD003725.pub3. CD003725. [DOI] [PubMed] [Google Scholar]
- 9).Seshadri R, Feldman BM, Ilowite N, Cawkwell G, Pachman LM. The role of aggressive corticosteroid therapy in patients with juvenille dematomyositis: a propensity score analysis. Arthritis Rheumatol. 2008;59:989–95. doi: 10.1002/art.23829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10).Sethi G, Ahn KS, Pandey MK, Aggarwal BB. Celastrol, a novel triterpene, potentiates TNF-induced apoptosis and suppresses invasion of tumor cells by inhibiting NF-kappaB-regulated gene products and TAK1-mediated NF-kappaB activation. Blood. 2007;109:2727–35. doi: 10.1182/blood-2006-10-050807. [DOI] [PubMed] [Google Scholar]
- 11).Jin HZ, Hwang BY, Kim HS, Lee JH, Kim YH, Lee JJ. Antiinflammatory consitutents of Celastrus orbiculatus inhibit the NF-kappaB activation and NO production. J Nat Prod. 2002;65:89–91. doi: 10.1021/np010428r. [DOI] [PubMed] [Google Scholar]
- 12).Li H, Zhang YY, Tan HW, Jia YF, Li D. Therapeutic effect of tripterine on adjuvant arthritis in rats. J Enthnopharmacol. 2008;118:479–84. doi: 10.1016/j.jep.2008.05.028. [DOI] [PubMed] [Google Scholar]
- 13).Waelchli R, Bollbuck B, Bruns C, Buhl T, Eder J, Feifel R, Hersperger R, Janser P, Revesz L, Zerwes HG, Schlapbach A. Design and preparation of 2-benzamido-pyrmidines as inhibitors of IKK. Bioorg Med Chem Lett. 2005;16:108–12. doi: 10.1016/j.bmcl.2005.09.035. [DOI] [PubMed] [Google Scholar]
- 14).Iyer SN, Hyde DM, Giri SN. Anti-inflammatory effect of pirfenidone in the bleomycin-hamster model of lung inflammation. Inflammation. 2000;24:477–91. doi: 10.1023/a:1007068313370. [DOI] [PubMed] [Google Scholar]
- 15).Nakazato H, Oku H, Yamane S, Tsuruta Y, Suzuki R. A novel anti-fibrotic agent pirfenidone suppresses tumor necrosis factor-alpha at the translational level. Eur J Pharmacol. 2002;446:177–85. doi: 10.1016/s0014-2999(02)01758-2. [DOI] [PubMed] [Google Scholar]
- 16).Van Erp C, Irwin NG, Hoey AJ. Long-term administration of pirfenidone improves cardiac function in mdx mice. Muscle Nerve. 2006;34:327–34. doi: 10.1002/mus.20590. [DOI] [PubMed] [Google Scholar]
- 17).Lin RW, Chen CH, Wang YH, Ho ML, Hung SH, Chen IS, Wang GJ. (-)-Epigallocatchin gallate inhibition of osteoclastic differentiation via NF-kappaB. Biochem Biophys Res Commun. 2009;379:1003–7. doi: 10.1016/j.bbrc.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 18).Nakae Y, Hirasaka K, Goto J, Nikawa T, Shono M, Yoshida M, Stoward PJ. Subcutaneous injection, from birth, of epigallocatechin-3-gallate, a component of green tea, limits the onset of muscular dystrophy in mdx mice: a quantitative histological, immunohistochemical and electrophysiological study. Histochem Cell Biol. 2008;129:489–501. doi: 10.1007/s00418-008-0390-2. [DOI] [PubMed] [Google Scholar]
- 19).Huff T, Müller CS, Otto AM, Netzker R, Hannappel E. beta-Thymosins, small acidic peptides with multiple functions. Int J Biochem Cell Biol. 2001;33:205–20. doi: 10.1016/s1357-2725(00)00087-x. [DOI] [PubMed] [Google Scholar]
- 20).Lungu G, Covaleda L, Mendes O, Martini-Stoica H, Stoica G. FGF-1-induced matrix metalloproteinase-9 expression in breast cancer cells is mediated by increased activities of NF-kappaB and activating protein-1. Mol Carcinog. 2008;47:424–35. doi: 10.1002/mc.20398. [DOI] [PubMed] [Google Scholar]
- 21).Singh D, Aggarwal A, Maurya R, Naik S. Withania somnifera inhibits NF-kappab and AP-1 transcription factors in human peripheral blood and synovial fluid mononuclear cells. Phytother Res. 2007;21:905–13. doi: 10.1002/ptr.2180. [DOI] [PubMed] [Google Scholar]
- 22).Pierce JW, Schoenleber R, Jesmok G, Best J, Moore SA, Collins T, Gerritsen ME. Novel inhibitors of cyotkine-induced IkappaBalpha phosphorylation and endothelial cell adhesion molecule expression show anti-inflammatory effects in vivo. J Biol Chem. 1997;272:21096–103. doi: 10.1074/jbc.272.34.21096. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1 - C2C12 myoblasts were treated with 10ng/ml of TNF-α that was added directly to media and luciferase output was quantified at multiple later time points in triplicate and recorded as the mean fold change of treated to untreated signal, and standard errors were calculated. (*p-value <0.05 compared to 5 minute timepoint)