Abstract
Work in our laboratory has focused on the mechanisms by which cytokines can influence the brain and behavior in humans and non-human primates. Using administration of interferon (IFN)-alpha as a tool to unravel these mechanisms, we have expanded upon findings from the basic science literature implicating cytokine-induced changes in monoamine metabolism as a primary pathway to depression. More specifically, a role for serotonin metabolism has been supported by the clinical efficacy of serotonin reuptake inhibitors in blocking the development of IFN-alpha-induced depression, and the capacity of IFN-alpha to activate metabolic enzymes (indolamine 2,3 dioxygenase) and cytokine signaling pathways (p38 mitogen activated protein kinase) that can influence the synthesis and reuptake of serotonin. Our data also support a role for dopamine depletion as reflected by IFN-alpha-induced changes in behavior (psychomotor slowing and fatigue) and regional brain activity, which implicate the involvement of the basal ganglia, as well as the association of IFN-alpha-induced depressive-like behavior in rhesus monkeys with decreased cerebrospinal fluid concentrations of the dopamine metabolite, homovanillic acid. Neuroimaging data in IFN-alpha-treated patients also suggest that activation of neural circuits (dorsal anterior cingulate cortex) associated with anxiety and alarm may contribute to cytokine-induced behavioral changes. Taken together, these effects of cytokines on the brain and behavior appear to subserve competing evolutionary survival priorities that promote reduced activity to allow healing, and hypervigilance to protect against future attack. Depending on the relative balance between these behavioral accoutrements of an activated innate immune response, clinical presentations may be distinct and warrant individualized therapeutic approaches.
Keywords: Depression, Cytokine, Inflammation, Innate Immune System, Interferon-alpha, Serotonin, Dopamine, Basal Ganglia, Anterior Cingulate Cortex, Brain Imaging
Introduction
There has been increasing interest in the role of inflammation as a common mechanism of disease in a number of medical disorders including cardiovascular disease, diabetes and cancer. Indeed, epidemiological studies have found that biomarkers of inflammation can predict the development and progression of these illnesses, and basic science studies have identified a number of relevant mechanisms whereby inflammatory processes can contribute to their pathophysiology (Aggarwal et al., 2006; Bisoendial et al., 2007; Bouzakri and Zierath 2007; Pradhan and Ridker 2002; Ridker 2003). Data also indicate that activation of innate immune responses and the release of innate immune cytokines may contribute to the development of neuropsychiatric disorders including major depression (Dantzer et al., 2008; Raison et al., 2006). Such data provide insights into the high rate of co-morbid mood disorders in patients with medical illness and suggest novel targets for diagnosis and treatment of depression in both medically ill and medically healthy individuals. Nevertheless, much of the data documenting the potential role of innate immune system activation in depression is correlative in nature, and data addressing the mechanisms involved have been derived almost exclusively from laboratory animal studies (Dantzer et al., 2008; Raison et al., 2006).
To further evaluate relevant mechanisms by which cytokines might contribute to depression in humans, investigators have seized upon the unique opportunity of patients undergoing treatment with the innate immune cytokine, interferon (IFN)-alpha. IFN-alpha has been shown to induce a high rate of depression in humans, and therefore patients undergoing IFN-alpha therapy provide an unparalleled resource for translating findings from laboratory animals to humans and from bench to bedside and back (Capuron and Miller 2004). Work in our laboratory has been especially interested in the impact of IFN-alpha on monoamine systems, including serotonin and dopamine. Moreover, using neuroimaging approaches, we have examined relevant neural circuits that may be involved in cytokine-induced behavioral changes. Integration of our findings to date suggest that cytokine effects on the brain may subserve competing evolutionary survival priorities that involve shutting the organism down to facilitate reallocation of energy resources for fighting infection and healing wounds, while concurrently increasing vigilance to protect against future attack. Further understanding of the relative balance in the pathophysiologic pathways that underlie these behavioral accoutrements of an activated immune response may provide further insights into the varied presentations of cytokine-induced behavioral syndromes and will ultimately inform individualized approaches to their treatment.
Depression and Activation of the Innate Immune Response
Elaboration of the notion that an activated innate immune response may contribute to the development of major depression has been derived from a number of sources (Irwin and Miller 2007). Probably the earliest of findings came from observations that patients with major depression exhibit increased biomarkers of inflammation in the peripheral blood (Maes 1995). These early observations have been replicated and expanded by a number of investigators, and taken together, the data document that certain patients with major depression exhibit increases in innate immune cytokines and their soluble receptors in the peripheral blood and cerebrospinal fluid (CSF), as well as increases in peripheral blood acute phase proteins, chemokines and adhesion molecules (Irwin and Miller 2007; Raison et al., 2006). A meta-analysis and consensus reports suggest that increases in peripheral blood IL-6 and c-reactive protein (CRP) appear to be some of the most reliable findings in this regard (Mossner et al., 2007; Zorrilla et al., 2001). As noted above, data also indicates that administration of innate immune cytokines (including IFN-alpha) to both laboratory and humans can induce behavioral changes that significantly overlap with those used to make a diagnosis of major depression including depressed mood, anhedonia, fatigue, psychomotor slowing, decreased memory and concentration, impaired sleep, anorexia and anxiety (Capuron and Miller 2004; Dantzer et al., 2008).
Some of the more compelling data that inflammatory processes may contribute to depression are the findings that psychosocial stressors can activate the innate immune response in humans (Bierhaus et al., 2003). The contribution of psychosocial stress to depression is well-documented (Mundt et al., 2000), and findings that both acute and chronic psychosocial stress can activate innate immune signaling pathways such as nuclear factor kappa B (NF-kB) as well as innate immune cytokines such as IL-6 provide a critical mechanistic link between stress and depression (Bierhaus et al., 2003). It should be noted that aside from exhibiting increased baseline biomarkers of inflammation, depressed patients exhibit exaggerated stress-induced inflammatory responses (Pace et al., 2006). Early life stress may be especially relevant in this regard in that individuals with increased early life stress have been found to exhibit evidence of increased inflammation (Danese et al., 2007), and depressed patients with early life stress appear to be especially likely to exhibit increased innate immune responses at baseline and following stress (Danese et al., 2008; Pace et al., 2006).
Closing the circle of logic regarding the inflammation hypothesis of depression is a recent study indicating that antagonism of innate immune cytokines can improve depressed mood in patients with an inflammatory disorder. Indeed, in a double-blind, placebo-controlled trial in patients with psoriasis, the TNF-alpha antagonist, etanercept, was found to significantly decrease depressive symptoms independently of the effect of the drug on disease activity (Tyring et al., 2006). Although more studies are needed to substantiate the ability of cytokine antagonists to reverse depressive symptoms in clinical populations, these data in humans are consistent with findings in laboratory animals where, for example, “knocking out” the genes for TNF-alpha receptors in mice has been associated with an antidepressant phenotype and reduced development of anxiety-like behaviors during viral infection (Silverman et al., 2007; Simen et al., 2006).
Taken together, these data provide compelling evidence that inflammation may play a role in the development of depression in humans. However, aside from the treatment studies, much of the data is correlative in nature, and there is little information relevant to the mechanisms involved. Therefore, model systems are required to further elaborate the pathways by which cytokines influence behavior in humans and non-human primates. One such model system involves the use of IFN-alpha. Based on its antiviral and antineoplastic properties, IFN-alpha is used to treat both infectious diseases and cancer including hepatitis C and malignant melanoma (Raison et al., 2005b).
IFN-alpha and Cytokine-Induced Depression
IFN-alpha is notorious for inducing symptoms of depression in humans, with approximately 30–50% of patients developing the full complement of symptoms used to make a diagnosis of major depression as defined by standardized diagnostic criteria (Capuron and Miller 2004; Musselman et al., 2001). Symptoms are apparent in a number of relevant domains including depressive symptoms, anxious symptoms, cognitive symptoms, neurovegetative symptoms and somatic symptoms (see Table 1). Data from IFN-alpha-treated patients indicate that neurovegetative and somatic symptoms tend to occur early and persist, while mood, anxiety and cognitive symptoms tend to occur later during treatment (Capuron et al., 2002a; Capuron and Miller 2004). The appearance of neurovegetative symptoms (e.g. psychomotor slowing) has been found to predict the subsequent development of depressive symptoms as does the presence of depressive symptoms immediately prior to the initiation of IFN-alpha therapy (Capuron and Ravaud 1999; Capuron et al., 2001; Majer et al., 2008; Raison et al., 2005a). Baseline concentrations of the soluble receptors for TNF and IL-6 have also been found to predict the development of depression during IFN-alpha administration (Friebe et al., 2007), whereas increases in peripheral blood concentrations of both IFN-alpha as well as TNF-alpha and it soluble receptor (sTNF-R-II) are correlated with increases in depressive symptoms during IFN-alpha treatment (Raison et al., 2008).
Table 1.
Percentage of Patients Experiencing Moderate to Severe Intensity of the Listed Symptoms during IFN-alpha Therapy
| Percent | |
|---|---|
| Depressive Symptoms | |
| Depressed mood | 60 |
| Anhedonia | 30 |
| Suicidal Thoughts | 10 |
| Feelings of Guilt | 5 |
| Anxious Symptoms | |
| Tension/Irritability | 50 |
| Anxious Mood | 45 |
| Fear | 15 |
| Cognitive Symptoms | |
| Loss of Concentration | 30 |
| Memory Disturbances | 15 |
| Word–finding Problems | 15 |
| Episodes of Confusion | 10 |
| Indecisiveness | 10 |
| Neurovegetative Symptoms | |
| Fatigue/Loss of Energy | 80 |
| Abnormal Sleep | 45 |
| Psychomotor Retardation | 40 |
| Abnormal Appetite | 35 |
| Somatic Symptoms | |
| Pain | 55 |
| Gastrointestinal Symptoms | 50 |
Reprinted with permission from Capuron et. al., 2002, Neuropsychopharmacology, 26:648.
Administration of IFN-alpha to rhesus monkeys [using the same dose and non-pegylated preparation (Intron® A) of IFN-alpha used to treatment malignant melanoma in humans] has also been found to induce behavioral changes (Felger et al., 2007). Rhesus monkeys administered IFN-alpha exhibit increased anxiety-like behavior as well as “huddling”. Interestingly, huddling behavior is considered a depressive-like equivalent, and was described in the early 1970’s in the Harlow laboratory in rhesus monkeys chronically administered the monoamine depleting drug, reserpine (McKinney et al., 1971). Reserpine was developed for the treatment of hypertension, however, its psychotropic properties were immediately recognized including the development of depression in some hypertensive patients (Nutt 2006). The appearance of huddling behavior has also been observed in monkeys administered corticotropin releasing hormone (CRH) into the cerebral ventricles (Kalin 1985). Taken together, these data are consistent with the notion that IFN-alpha as well as other innate immune cytokines may interact with monoamine and neuroendocrine pathways to induce depressive and anxiety-like behaviors.
Monoamine Mechanisms of IFN-alpha-Induced Behavioral Change
Role of Serotonin
Somewhat surprisingly, some of the earliest data suggesting that monoamine pathways in general and serotonin in particular, may be involved in the development of depressive symptoms during IFN-alpha therapy came from studies on the treatment and prevention of IFN-alpha-induced behavioral changes (Hauser et al., 2000; Musselman et al., 2001). Work in our laboratory participated in these investigations and found that administration of the serotonin reuptake inhibitor, paroxetine, reduced the development of depression during IFN-alpha therapy by ~4 fold (Musselman et al., 2001). Indeed, in a double-blind, placebo-controlled trial of patients undergoing IFN-alpha treatment for non-metastatic malignant melanoma, 45% of patients who received placebo developed symptom criteria sufficient to make a diagnosis of major depression compared to only 11% of those who received paroxetine (Musselman et al., 2001). In addition, whereas 35% of the patients in the placebo group dropped out of IFN-alpha treatment secondary to behavioral toxicity, only 1 patient (5%) dropped out of treatment in the paroxetine group (Musselman et al., 2001). It should be noted that in this randomized clinical trial, antidepressant (or placebo) treatment was initiated 2 weeks prior to the initiation of IFN-alpha therapy based on findings from laboratory animal studies demonstrating that pretreatment of rats with antidepressants for 3 weeks was able to block the development of behavioral changes secondary to the administration of bacterial endotoxin (Yirmiya 1996). Taken together, these data demonstrated that increasing synaptic availability of serotonin significantly reduced the development of cytokine-induced depressive symptoms, suggesting that depletion of serotonin as a function of cytokine exposure is a primary mechanism by which cytokines influence behavior. In addition, the data indicated that proactive strategies targeted to the pathways by which cytokines change behavior can prevent cytokine-induced behavioral changes before they occur and thereby may be a useful clinical approach to medically ill patients at risk for depression in the context of an activated innate immune response. Patients at risk would include those undergoing surgery, radiation or chemotherapy, where the incumbent tissue damage and destruction can elicit a powerful but predictable inflammatory response. Of further relevance to identifying those who may be especially vulnerable to such cytokine influences on behavior are preliminary data from a recent collaborative study where polymorphisms in the IL-6 and serotonin transporter genes were found to predict the development of depression during IFN-alpha treatment for hepatitis C (Bull et al., 2008). While further implicating the role of serotonin in this process, these data contribute to a personalized approach to prevention that may be especially relevant in patients undergoing IFN-alpha treatment for hepatitis C. Indeed, in a recent double-blind, placebo-controlled trial of paroxetine in this patient population, patients with a low risk of developing IFN-alpha-induced depressive symptoms (as identified by low depressive symptoms at baseline) were found to receive no benefit from antidepressant pretreatment, whereas individuals at increased risk for depression (as identified by increased depressive symptoms at baseline) exhibited marked benefit from antidepressant therapy (Raison et al., 2007).
A second major area of research that has implicated serotonin pathways in the pathogenesis of cytokine (IFN-alpha)-induced depression is the effect of IFN-alpha on the enzyme, indolamine 2,3 dioxygenase (IDO), which breaks down tryptophan, the primary amino acid precursor of serotonin, into kynurenine (Figure 1) (Dantzer et al., 2008). Indeed, early studies indicated that the development of depressive symptoms during IFN-alpha therapy was associated with decreased peripheral blood concentrations of tryptophan (Capuron et al., 2002b; Maes et al., 2001). These studies were complemented by work in our laboratory that indicated that patients who developed major depression during IFN-alpha therapy (compared to those without depression) exhibited both prolonged accentuated decreases in plasma tryptophan as well as significantly increased plasma concentrations of kynurenine, supporting the notion that decreased peripheral blood concentrations of tryptophan were secondary to activation of IDO (Capuron et al., 2003a). Animal studies have recently further demonstrated a pivotal role for IDO in cytokine-induced behavioral changes. For example, inhibition of IDO activity by the IDO antagonist, 1-methyltryptophan, has been shown to block the development of depressive-like symptoms in mice following administration of lipopolysaccharide (LPS) (O’Connor et al., 2008). It should be noted, however, that although IDO may contribute to a reduction in tryptophan, it has yet to be established that IFN-alpha-induced changes in peripheral blood tryptophan translate into decreased availability of tryptophan and/or serotonin in the central nervous system (CNS). Moreover, there is increasing interest in the role of downstream IDO metabolites including kynurenine which can be further broken down into kynurenic acid (discussed below) and quinolinic acid, which is an endogenous N-methyl-D-aspartate receptor agonist that has been implicated in the neurotoxicity of several inflammatory brain diseases such as Human Immunodeficiency Virus (HIV)/Acquired Immune Deficiency Syndrome (AIDS) Dementia Complex and Alzheimer’s disease (Guillemin et al., 2005a; Guillemin et al., 2005b; Muller and Schwarz 2007; Wichers et al., 2005). Of note, human astrocytes, which provide trophic support to neurons, appear to be especially sensitive to the apoptotic effects of quinolinic acid (Guillemin et al., 2005b).
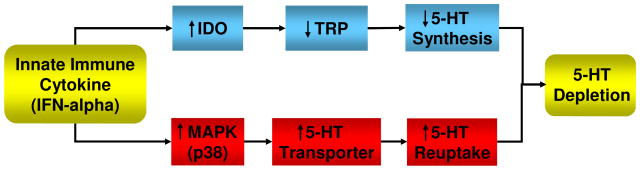
Figure 1. Potential Pathways Leading to Cytokine-Induced Changes in Serotonin Metabolism.
Two pathways by which cytokines may reduce serotonin (5-HT) availability in the synapse are depicted. Cytokine-induced activation of indolamine 2,3 dioxygenase (IDO) can lead to decreased tryptophan (TRP), the primary amino acid precursor of 5-HT, which in turn can contribute to decreased 5-HT synthesis. In addition, activation of p38 mitogen activated protein kinase (MAPK) can upregulate the expression and activity of the membrane transporter for 5-HT, leading to increased 5-HT reuptake.
Another major mechanism by which cytokines (IFN-alpha) may influence serotonin metabolism is through the induction of mitogen activated protein kinase (MAPK) signaling pathways including p38 (Figure 1). IFN-alpha as well as other innate immune cytokines are potent inducers of p38 MAPK, and activation of p38 MAPK has been shown to upregulate both the expression and function of the serotonin transporter (Zhu et al., 2006; Zhu et al., 2005). For example, treatment of rat brain synaptosome preparations or rat leukemia cell lines with IL-1 or TNF-alpha was found to increase serotonin reuptake by up to 100% in a dose dependent fashion (Zhu et al., 2006). These effects were reversed by a p38 antagonist. Interestingly, enhanced serotonin transporter function recently has been associated with depression in patients with seasonal affective disorder (Willeit et al., 2007). Work by our group has examined the relationship between p38 activation and serotonin availability in rhesus monkeys exposed to maternal abuse and rejection as infants (Sanchez et al., 2007). Significant correlations were found between activation of p38 in peripheral blood mononuclear cells (as determined by flow cytomety and intracellular staining for phosphorylated p38) and number of maternal rejections as infants as well as decreases in CSF concentrations of the serotonin metabolite, 5-hydroxyindoleacetic acid (5-HIAA) (Sanchez et al., 2007). Of note, decreased CSF 5-HIAA concentrations have been associated with increased anxiety-like behavior in these animals (Maestripieri et al., 2006). Taken with the potential effects of activation of IDO on serotonin metabolism, these data suggest that cytokines can inflict a double hit on serotonin availability through effects on the synthesis and reuptake of serotonin, both potentially contributing to reduced synaptic serotonin (Figure 1).
Role of Dopamine
Data from our laboratory and others support the notion that IFN-alpha, as well as other innate immune cytokines, may also alter dopamine (DA) metabolism and the function of basal ganglia circuits, thereby contributing to cytokine-induced neurovegetative symptoms including anhedonia, psychomotor slowing, sleep disturbances and fatigue (Capuron and Miller 2004; Horikawa et al., 1999; Kamata et al., 2000; Kumai et al., 2000; Schaefer et al., 2003; Shuto et al., 1997; Sunami et al., 2000). DA in the basal ganglia is known to play an important role in the regulation of multiple behaviors including mood, motivation/reward (hedonia), motor activity, sleep/wake cycles (arousal) and cognition (Grace 2002; Roth and Elsworth 1995; Rye 2004; Salamone et al., 2005; Schultz 2007).
Data from patients undergoing IFN-alpha treatment for hepatitis C reveal significant IFN-alpha-induced motor slowing as assessed by a computerized neuropsychological test battery (Majer et al., 2008). IFN-alpha-induced motor slowing was in turn significantly correlated with the development of depression and fatigue. These findings are consistent with a previous study demonstrating a relationship between motor slowing following 5 days of IFN-alpha therapy and the development of depressive symptoms after 1 month of IFN-alpha treatment (Capuron et al., 2001). More specifically related to DA metabolism, studies in rhesus monkeys administered IFN-alpha have revealed that the development of huddling behavior (a behavioral equivalent of depression) in vulnerable animals is associated with significant decreases in CSF concentrations of the DA metabolite, homovanillic acid (HVA), compared to saline treatment. These results are consistent with experiments in mice that have shown that treatment with IFN-alpha for up to 5 days significantly decreases DA and its metabolite, 3,4-dihydroxyphenylacetic acid (DOPAC), in whole brain homogenates (Shuto et al., 1997). These DA changes were associated with reduced motor activity (consistent with the motor slowing and fatigue seen in IFN-alpha-treated patients). Studies by our group and others using positron emission tomography also have demonstrated that IFN-alpha increases resting state glucose metabolism in basal ganglia nuclei including the putamen and nucleus accumbens (Capuron et al., 2007; Juengling et al., 2000). IFN-alpha-induced changes in glucose metabolism were in turn correlated with the development of symptoms of fatigue (Capuron et al., 2007). These findings of increased basal ganglia resting-state glucose metabolism in IFN-alpha-treated patients may be indicative of reduced dopaminergic activity, as is seen in patients with Parkinson’s disease (PD). Increased glucose metabolism in the basal ganglia (similar to that following IFN-alpha administration) has been repeatedly demonstrated in PD patients (Eidelberg et al., 1994; Mentis et al., 2002; Spetsieris et al., 1995), where it is believed to reflect the degeneration of inhibitory neurocircuits related to the loss of dopaminergic neurons in the substantia nigra pars compacta (Wichmann and DeLong 2003a; Wichmann and DeLong 2003b). Disinhibition of dopaminergic inhibitory neurocircuits in turn leads to increased oscillatory burst activity in relevant basal ganglia nuclei (and thus increased metabolic activity) (Wichmann and DeLong 1999). Relevant to the role of diminished DA availability in basal ganglia hyperactivity, levodopa infusion has been shown to reduce glucose metabolism in the basal ganglia, notably in the putamen, and is associated with clinical improvement in PD patients (Feigin et al., 2001). Of note, IFN-alpha has been associated with development of Parkinson-like symptoms that were relieved by levodopa administration. Given the role of DA pathways in activating frontal cortex neurons (Alexander et al., 1986), altered basal ganglia and DA function may also contribute to the reduced metabolic activity that has been observed in the prefrontal cortex following IFN-alpha administration (Capuron et al., 2007; Juengling et al., 2000). Such decreases in frontal cortex activity also have been found in PD patients, especially those with depression (Mayberg et al., 1990).
Additional support regarding IFN-alpha’s effects on DA pathways and the basal ganglia are its potent induction of fatigue and anergia. Fatigue and anergia represent fundamental characteristics of diseases that affect the basal ganglia, including PD, multiple sclerosis, cortical stroke and AIDS (Chaudhuri and Behan 2000; Gray et al., 2001; Lou et al., 2001). In addition, it has been suggested that nucleus accumbens DA, aside from its role in reward circuitry, may contribute to anergia in patients with depression (Salamone et al., 2003; Salamone et al., 2005). Treatment with levodopa or other pharmacologic agents that increase DA release (e.g. amphetamines and other stimulants) have been shown to improve fatigue and energy in patients with basal ganglia disorders as well as in IFN-alpha-treated patients and patients with cancer (Lou et al., 2003; Schwartz et al., 2002). Taken together, these findings suggest that changes in basal ganglia activity during IFN-alpha therapy may be related to decreased DA neurotransmission and in turn may play a role in the pathophysiology of IFN-alpha-induced behavioral changes as well as cytokine-induced behavioral changes (fatigue, motor slowing, anhedonia and depression) in other patient populations including the medically ill.
There are a number of mechanisms by which IFN-alpha may alter DA metabolism and contribute to decreased DA neurotransmission (Figure 2). For example, intramuscular injection of IFN-alpha to rats has been shown to decrease CNS concentrations of tetrahydrobiopterin (BH4) (Kitagami et al., 2003). BH4 is an important enzyme co-factor for tyrosine hydroxlylase, which converts tyrosine to L-DOPA and is the rate limiting enzyme in the synthesis of DA. IFN-alpha effects on BH4 appear to be mediated by stimulation of nitric oxide (NO). Indeed, treatment with an inhibitor of NO synthesis was found to reverse IFN-alpha’s inhibitory effects on brain concentrations of both BH4 and DA (Kitagami et al., 2003). IL-6 (which we have shown is increased in the peripheral blood following acute IFN-alpha administration) also has been shown to reduce BH4 content in sympathetic neurons (Li et al., 2003). Of note, activation of an inflammatory response within the brain has been associated with increased NO production, suggesting that cytokine influences on BH4 via NO may be a common mechanism for innate immune cytokines and inflammation to reduce DA availability in the basal ganglia. Of relevance in this regard, IFN-alpha receptors have been identified throughout the brain especially in microglia (Yamada and Yamanaka 1995). Thus, IFN-alpha-induced activation of microglia may lead to the release of other innate immune cytokines, such as IL-6, which may contribute to alterations in DA and basal ganglia function through local inflammation and the production of NO.
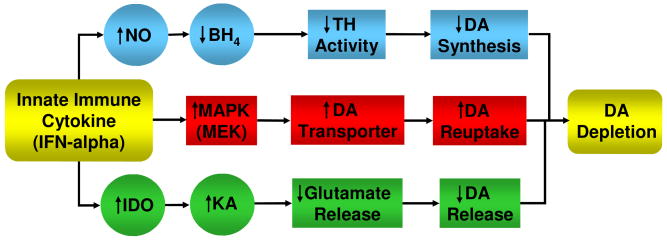
Figure 2. Potential Pathways Leading to Cytokine-Induced Changes in Dopamine Metabolism.
Three pathways by which cytokines can reduce dopamine (DA) availability in the synapse are depicted. Cytokine-induced activation of nitric oxide (NO) can lead to decreased tetrahydrobiopterin (BH4) which serves as a co-enzyme for tyrosine hydroxylase (TH), the rate limiting enzyme in DA synthesis. In addition, activation of mitogen activated protein kinase pathways (MAPK), including MAPK kinase (MEK), has been associated with upregulation of the activity of the DA transporter, which leads to increased DA reuptake. Finally, cytokine-induced activation of indolamine 2,3 dioxygenase (IDO) results in the breakdown of tryptophan into kynurenic acid (KA), which in turn has been associated with inhibition of the release of glutamate. Glutamate stimulates the release of DA, and therefore decreased glutamate release can lead to decreased DA release.
Another potential pathway which may lead to decreased synaptic availability of DA involves changes in kynurenic acid (KA), a tryptophan metabolite, which can affect DA release. As noted above, through activation of IDO, IFN-alpha treatment has been associated with increased plasma concentrations of kynurenine (especially in depressed patients), which can be metabolized to KA (Capuron et al., 2003a). Of relevance to DA, intrastriatal administration of KA has been shown to dramatically reduce extracellular DA in the rat striatum (Wu et al., 2007). This effect appears to be mediated by the inhibition of alpha7 nicotinic acetylcholine receptors (alpha7nAChRs) on glutamatergic afferents, which ultimately serve to inhibit striatal glutamate release (Wu et al., 2007). Glutamate in turn is believed to act locally to regulate tonic, impulse-independent, DAT-mediated DA release (Borland and Michael 2004; Grace 1991). Treatment with the alpha7nAChR agonist, galantamine, was able to reverse the effects of KA on extracellular DA levels in the striatum (Wu et al., 2007). Yet another pathway by which the innate immune response may influence DA metabolism is the capacity of IFN-alpha and other innate immune cytokines to activate MAPK signaling pathways. As noted above, MAPK pathways appear to play an important role in the regulation of the expression of monoamine transporters. For example, transient transfection of human (h)DAT-expressing HEK cells with constitutively active MAPK kinase (MEK) was found to increase the Vmax of the hDAT transporter while increasing hDAT surface expression (Moron et al., 2003). Moreover, inhibition of MAPK signaling was found to decrease DA uptake in a dose and time-dependent fashion in rat striatal synaptosome preparations and a human embryonic kidney (HEK) cell line (Moron et al., 2003). Of note, activation of p38 MAPK after treatment with either pharmacologic (anisomycin) or immunologic stimuli (IL-1 and TNF-α) has been shown to increase the expression and activity of both the serotonin as well as the norepinephrine transporter (Zhu et al., 2006; Zhu et al., 2005). Given the role of the norepinephrine transporter in the uptake of both norepinephrine (NE) and DA, increased DAT and NET expression and activity may contribute to reduced synaptic availability of DA (and NE), secondary to increased sequestration of neurotransmitter.
Although decreased DA synthesis and/or increased DA reuptake are plausible mechanisms for decreased DA neurotransmission. An additional explanation for hypo-dopaminergic function during IFN-alpha treatment is that IFN-alpha is initially associated with increased dopaminergic activity, which subsequently leads to downregulation of DA neurotransmission. Indeed, studies have shown that IFN-alpha binds to mu opioid receptors (Wang et al., 2006), which are expressed on basal ganglia dopaminergic neurons and can cause presynaptic DA release (Di Chiara and Imperato 1988; Ho et al., 1992). Long-term IFN-alpha-induced DA release may lead to a compensatory reduction in the number and/or sensitivity of postsynaptic DA receptors (e.g. D2), as well as DA synthesis (Cooper 2003), ultimately leading to decreased dopaminergic tone.
IFN-alpha effects on DA and the basal ganglia may contribute to the development of IFN-alpha-induced depression (as well as fatigue – as discussed above). Considerable attention has been focused on the role of DA in the pathophysiology of depressive disorders (Dunlop and Nemeroff 2007). For example, rodent models of depression demonstrate altered mesolimbic DA system function, and certain antidepressants act by enhancing DA neurotransmission (Willner et al., 1992). Furthermore, several studies, including postmortem investigations, have shown that depressed patients, particularly those with psychomotor retardation, exhibit reduced concentrations of DA metabolites, primarily HVA, both in the CSF and in brain regions that mediate mood and motivation (Brown and Gershon 1993; Klimek et al., 2002; Mendels et al., 1972; Reddy et al., 1992; Roy et al., 1992). Several neuroimaging studies have also found evidence of reduced DA neurotransmission in depressed patients, including compensatory up-regulation of D2 receptors (D’Haenen H and Bossuyt 1994; Ebert et al., 1996; Martinot et al., 2001; Meyer et al., 2001; Shah et al., 1997; Tremblay et al., 2005). Interestingly, in one study, reduced DA neurotransmission, as indicated by increased binding of the DA D2/3 ligand 123IZBM in the striatum, was correlated with motor slowing in depressed patients (Shah et al., 1997).
Although much of our discussion has focused on IFN-alpha, other innate immune cytokines have been implicated in basal ganglia dysfunction. Relevant receptors for innate immune cytokines are expressed in abundance in the basal ganglia (Gray et al., 2001; Haas and Schauenstein 1997), and chronic infusion of LPS, a potent inducer of the inflammatory cytokine cascade, into the rat brain has been shown to induce a progressive and selective degeneration of nigral dopaminergic neurons through microglial activation (Gao et al., 2002). Interestingly, development of PD in animal models using N-methyl-4-phenyl-1, 2, 3, 6-tetrahydropyridine (MPTP) is in part dependent on expression of the inflammatory cytokine, TNF-alpha (Leng et al., 2005; Nagatsu and Sawada 2005). Similarly, basal ganglia dysfunction and decreased striatal DA following administration of polychlorinated biphenyls (PCBs) appears to be dependent on IL-6 (with PCB-induced neurotoxicity being markedly reduced in IL-6 knockout animals) (Goodwill et al., 2007). Finally, several infectious diseases including HIV/AIDS have been associated with basal ganglia alterations (Berger and Arendt 2000; von Giesen et al., 2005). Interestingly, in these diseases, both basal ganglia hypermetabolism (as seen during IFN-α administration) and hypometabolism have been observed, possibly related to the duration of pathogen exposure and/or the chronicity of the disease. For example, in HIV/AIDS, data has suggested that early stages of neurologic involvement are characterized by basal ganglia hypermetabolism followed by basal ganglia hypometabolism, potentially reflective of neurodegeneration (von Giesen et al., 2000). Of note, CSF concentrations of IFN-alpha have been correlated with HIV-related neurocognitive changes (Krivine et al., 1999; Rho et al., 1995).
IFN-alpha, Anxiety and Alarm
Although much attention has focused on the development of depressive and neurovegetative symptoms (e.g. fatigue and psychomotor slowing) during IFN-alpha administration, symptoms of anxiety and irritability are also common (Table 1). These findings have been expanded to suggest that a significant percentage of patients receiving IFN-alpha therapy exhibit hypomanic and, in some cases, manic features, which include marked irritability, inability to sleep and hyperactivity (Constant et al., 2005). Further relevant to the appearance of anxiety and hyperactivity during cytokine exposure is the appearance of increased anxiety-like behavior in rhesus monkeys receiving IFN-alpha, and increased locomotor activity in animals identified as dominant in socially-housed pairs (Felger et al., 2007). Increased anxiety has also been observed in humans 1–2 hours following administration of endotoxin (Reichenberg et al., 2001). Taken together, these data indicate that in addition to depression, humans and non-human primates exhibit behavioral features consistent with increased activity and arousal during exposure to IFN-alpha and other inflammatory stimuli, and for some selected individuals increased anxiety and irritability and increased locomotor activity may predominate the clinical presentation.
Recent neuroimaging data from patients receiving IFN-alpha for hepatitis C virus (HCV) infection may provide clues regarding the neural circuits that may underlie these behavioral changes. Using functional magnetic resonance imaging (fMRI) and a task of visuo-spatial attention, HCV patients receiving IFN-alpha were found to exhibit significantly greater activation in the dorsal anterior cingulate cortex (dACC) [Broadman’s Area (BA) 24], compared to non-IFN-alpha-treated HCV control individuals (Capuron et al., 2005). The dACC has been shown to play an important role in error detection and conflict monitoring (Carter et al., 1998). Activation of the dACC in IFN-alpha-treated subjects was highly correlated with the number of task-related errors made by these patients, whereas no correlation was found between dACC activation and task-related errors in the control group (Capuron et al., 2005). Of note, the error rate for the task was low in both groups and did not differ between groups. Increased activation of the dACC in the context of low error rates has been observed in individuals with high-trait anxiety (Paulus et al., 2004). In addition, increased activation of the dACC has been observed in subjects with neuroticism and obsessive compulsive disorder, both of which are associated with increases in anxiety and arousal (Eisenberger et al., 2005; Ursu et al., 2003). Interestingly, activation of the dACC has also been found during a fMRI task of social rejection (Eisenberger and Lieberman 2004). Indeed, activation of the dACC occurred during a ball toss game at a time corresponding to a point in the game when the subject was excluded (social rejection). dACC activation during social rejection was associated with emotional distress, and is consistent with the role of this brain region in the processing of social pain (Eisenberger and Lieberman 2004). Combined with its role in error detection and conflict monitoring, the dACC’s processing of social pain has been suggested to comprise a neural “alarm system”, which can both detect and respond to threatening environmental stimuli in the social domain (Eisenberger and Lieberman 2004). Based on the neuroimaging data from IFN-alpha-treated patients, it appears that one mechanism by which cytokines may lead to increased arousal, anxiety and alarm is through increased activation of neural circuits involving the dACC.
Evolutionary Significance of Cytokine-Induced Behavioral Change: Balancing Survival Priorities
When further examining the somewhat opposing behavioral manifestations of cytokine (IFN-alpha) exposure (e.g. fatigue/psychomotor slowing versus anxiety/irritability/arousal), consideration should be given to the evolutionarily-derived survival priorities with which a sick animal is faced. Previous investigators have emphasized that many of the behaviors associated with cytokines including depressed mood, anhedonia, fatigue, psychomotor slowing appear to subserve a shutting down of behavioral activity in the service of conserving energy resources (Dantzer and Kelley 2007; Hart 1991; Kluger et al., 1995; Maier and Watkins 1998). The metabolic demands of mounting a fever, fighting infection and healing wounds are vast and thus warrant shunting energy allocation away from non-essential motor activities and environmental exploration. In contrast, however, an animal that has been infected or wounded is vulnerable to attack and therefore must maintain increased vigilance in order to respond to intrusions from a predator. Such preservation of survival instincts has been demonstrated in lactating mice who maintain normal nest building and pup retrieval in cold ambient temperature despite having been injected with a behaviorally-relevant dose of LPS (Aubert et al., 1997). These data are consistent with the notion that in the context of cytokine exposure (which signals the presence of infection or injury), there is a reorganization of behavioral priorities, which places emphasis on the fundamental survival priorities of healing and protection from future attack. This theoretical framework, which involves the balancing of survival priorities, may help put into context the behavioral manifestations that are apparent in cytokine-exposed individuals and may help explain why some patients exhibit predominately neurovegetative symptoms while others may present with high levels of anxiety and irritability and in some cases hypomania or mania. Given the different pathophysiologic pathways that subserve cytokine-induced behavioral change, behavioral manifestations may develop along one of two lines (or both) depending upon the source or nature of the cytokine challenge as well as the genetics and environmental experience of the individual (Figure 3). Thus, the ultimate behavioral manifestations of cytokine exposure will represent an individualized balance of neurovegetative and alarm behaviors. Further understanding of the nature of the pathways that underlie these behaviors will help elucidate relevant treatment targets to alleviate symptom expression and thereby personalize the approach to cytokine-induced behavioral disturbances.
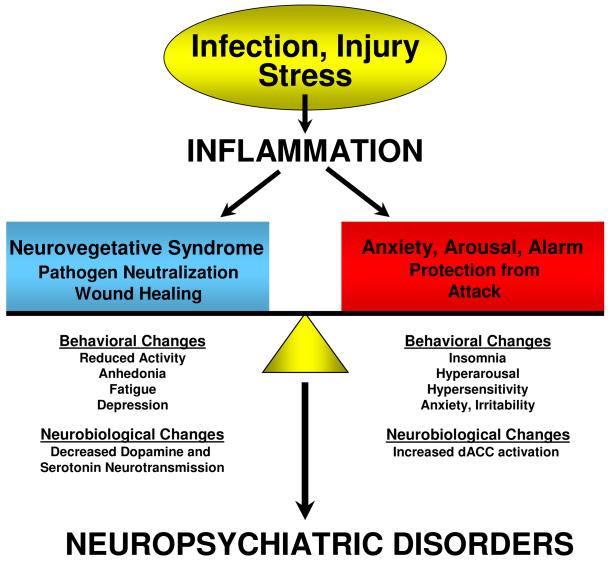
Figure 3. The Contribution of the Neurovegetative Syndrome and Anxiety, Arousal and Alarm to Cytokine-Induced Neuropsychiatric Disorders.
Cytokine-induced inflammatory responses lead to a host of behavioral changes that can be grouped into 1) a “neurovegetative syndrome” that subserves shutting the organism down to facilitate fighting infection and would healing and 2) an “anxiety, arousal and alarm state” that subserves protection against future attack. These behavioral responses are mediated by the impact of cytokines on relevant neurobiological pathways and represent a reorganization of behavior to address competing survival priorities, the balance of which will determine the clinical manifestations of related cytokine-induced neuropsychiatric disorders.
Consideration also must be given to what factors account for the transition from the normally adaptive behavioral responses to innate immune system activation to the development of psychopathology. Clearly, individual variables based on past experience and genetics have been shown to interact and contribute to the manifestation of psychiatric disease in the context of chronic stress (Caspi et al., 2003), which as noted previously can activate innate immune responses. On the immune side, however, less information is available. It remains to be determined what factors may lead to an immunologic signal capable of inducing enduring behavioral changes that bespeak psychopathology. Obvious contributors include chronic or excessive immune activation secondary to autoimmune, inflammatory or infectious diseases and/or chronic stress, trauma and various medical interventions (e.g. surgery, radiation and chemotherapy). Relevant polymorphisms in immune genes, such as IL-6, which as noted above have been associated with the development IFN-alpha induced depression (Bull et al., 2008), are also likely involved. Another factor may include exposure to infections during development. Indeed, early (neonatal) exposure to LPS has been shown to result in increased adult responsiveness of the hypothalamic-pituitary-adrenal axis (Shanks et al., 1995), a neuroendocrine alteration which was also associated with the development of depression during IFN-alpha administration (Capuron et al., 2003b). Future investigation is warranted to further elucidate these potential vulnerability factors, such that individuals at risk for immune-based behavioral changes can be identified and offered intervention strategies for prevention and treatment.
Conclusions
Using IFN-alpha treatment as a method to study the mechanisms by which cytokines influence the brain and behavior has led to further insights into the impact of cytokines on monoamine metabolism and regional brain activity in humans and non-human primates. The data indicate that cytokine-induced alterations in serotonin metabolism and DA in the basal ganglia play important roles in the development of depression and fatigue, and alterations in neural circuits involving the dACC may contribute to a heightened sense of arousal and alarm as well as increased anxiety and irritability. These behavioral changes appear to subserve competing survival priorities including the need to neutralize pathogens and heal wounds in addition to the need for protection against future attack. Further understanding of how IFN-alpha changes behavior in patients with infectious diseases and cancer will facilitate a more focused approach to the study of pathways by which cytokines influence behavior in other medical disorders as well as in patients undergoing medical treatments (and stress) that activate inflammatory responses. Such work will also help identify novel treatment strategies to improve the quality of life of both medically ill and medically healthy individuals with cytokine-induced neuropsychiatric disorders and prevent these problems before they occur.
Acknowledgments
The author would like to thank the many individuals who made this work possible including Charles Raison, Thaddeus Pace, Bobbi Woolwine, Lucile Capuron, Carmine Pariante, Giuseppe Pagnoni, Gregory Berns, David Rye, Dominique Musselman, David Lawson, Xiaohong Wang, Bradley Pearce, Mar Sanchez, Marni Silverman, Jennifer Felger, Andrea Liatis, Anlys Olivera, Monica Cowles, Oyetunde Alagbe, Matthias Majer, Gerald Vogt, Fang Hu, Daniel Drake, Carla Hernandez and Breanne Massung. This review was supported in part by grants MH 069124, HL 073921, MH 067990, MH 075102, MH 070553, MH 020018, MH 069056, MH 58922, the Emory University General Research Center (NCRR M01-RR00039), the DANA Foundation, the Centers for Disease Control and Prevention, Schering-Plough, and GlaxoSmithKline.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G. Inflammation and cancer: how hot is the link? Biochem Pharmacol. 2006;72:1605–21. doi: 10.1016/j.bcp.2006.06.029. [DOI] [PubMed] [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–81. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Dantzer R, Gheusi G. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav Immun. 1997;11:107–18. doi: 10.1006/brbi.1997.0485. [DOI] [PubMed] [Google Scholar]
- Berger JR, Arendt G. HIV dementia: the role of the basal ganglia and dopaminergic systems. J Psychopharmacol. 2000;14:214–21. doi: 10.1177/026988110001400304. [DOI] [PubMed] [Google Scholar]
- Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP. A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci U S A. 2003;100:1920–5. doi: 10.1073/pnas.0438019100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisoendial RJ, Kastelein JJ, Stroes ES. C-reactive protein and atherogenesis: from fatty streak to clinical event. Atherosclerosis. 2007;195:e10–8. doi: 10.1016/j.atherosclerosis.2007.04.053. [DOI] [PubMed] [Google Scholar]
- Borland LM, Michael AC. Voltammetric study of the control of striatal dopamine release by glutamate. J Neurochem. 2004;91:220–9. doi: 10.1111/j.1471-4159.2004.02708.x. [DOI] [PubMed] [Google Scholar]
- Bouzakri K, Zierath JR. MAP4K4 gene silencing in human skeletal muscle prevents tumor necrosis factor-alpha-induced insulin resistance. J Biol Chem. 2007;282:7783–9. doi: 10.1074/jbc.M608602200. [DOI] [PubMed] [Google Scholar]
- Brown AS, Gershon S. Dopamine and depression. J Neural Transm Gen Sect. 1993;91:75–109. doi: 10.1007/BF01245227. [DOI] [PubMed] [Google Scholar]
- Bull SJ, Huezo-Diaz P, Binder EB, Cubells JF, Ranjith G, Maddock C, Miyazaki C, Alexander N, Hotopf M, Cleare AJ, Norris S, Cassidy E, Aitchison KJ, Miller AH, Pariante CM. Functional polymorphisms in the interleukin-6 and serotonin transporter genes, and depression and fatigue induced by interferon-alpha and ribavirin treatment. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH. Neurobehavioral effects of interferon-alpha in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology. 2002a;26:643–52. doi: 10.1016/S0893-133X(01)00407-9. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Cytokines and psychopathology: lessons from interferon-alpha. Biol Psychiatry. 2004;56:819–24. doi: 10.1016/j.biopsych.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH. Interferon-alpha-induced changes in tryptophan metabolism. relationship to depression and paroxetine treatment. Biol Psychiatry. 2003a;54:906–14. doi: 10.1016/s0006-3223(03)00173-2. [DOI] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH. Anterior cingulate activation and error processing during interferon-alpha treatment. Biol Psychiatry. 2005;58:190–6. doi: 10.1016/j.biopsych.2005.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine B, Berns GS, Nemeroff CB, Miller AH. Basal ganglia hypermetabolism and symptoms of fatigue during interferon-alpha therapy. Neuropsychopharmacology. 2007;32:2384–92. doi: 10.1038/sj.npp.1301362. [DOI] [PubMed] [Google Scholar]
- Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH. Association of exaggerated HPA axis response to the initial injection of interferon-alpha with development of depression during interferon-alpha therapy. Am J Psychiatry. 2003b;160:1342–5. doi: 10.1176/appi.ajp.160.7.1342. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A. Prediction of the depressive effects of interferon alfa therapy by the patient’s initial affective state. N Engl J Med. 1999;340:1370. doi: 10.1056/NEJM199904293401716. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Dantzer R. Timing and specificity of the cognitive changes induced by interleukin-2 and interferon-alpha treatments in cancer patients. Psychosom Med. 2001;63:376–86. doi: 10.1097/00006842-200105000-00007. [DOI] [PubMed] [Google Scholar]
- Capuron L, Ravaud A, Neveu PJ, Miller AH, Maes M, Dantzer R. Association between decreased serum tryptophan concentrations and depressive symptoms in cancer patients undergoing cytokine therapy. Mol Psychiatry. 2002b;7:468–73. doi: 10.1038/sj.mp.4000995. [DOI] [PubMed] [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–9. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Caspi A, Sugden K, Moffitt TE, Taylor A, Craig IW, Harrington H, McClay J, Mill J, Martin J, Braithwaite A, Poulton R. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science. 2003;301:386–9. doi: 10.1126/science.1083968. [DOI] [PubMed] [Google Scholar]
- Chaudhuri A, Behan PO. Fatigue and basal ganglia. J Neurol Sci. 2000;179:34–42. doi: 10.1016/s0022-510x(00)00411-1. [DOI] [PubMed] [Google Scholar]
- Constant A, Castera L, Dantzer R, Couzigou P, de Ledinghen V, Demotes-Mainard J, Henry C. Mood alterations during interferon-alfa therapy in patients with chronic hepatitis C: evidence for an overlap between manic/hypomanic and depressive symptoms. J Clin Psychiatry. 2005;66:1050–7. doi: 10.4088/jcp.v66n0814. [DOI] [PubMed] [Google Scholar]
- Cooper J, Bloom FE, Roth RH. The Biochemical Basis of Neuropharmacology. 8. New York: Oxford University Press; 2003. [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–15. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danese A, Pariante CM, Caspi A, Taylor A, Poulton R. Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci U S A. 2007;104:1319–24. doi: 10.1073/pnas.0610362104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Kelley KW. Twenty years of research on cytokine-induced sickness behavior. Brain Behav Immun. 2007;21:153–60. doi: 10.1016/j.bbi.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Haenen HA, Bossuyt A. Dopamine D2 receptors in depression measured with single photon emission computed tomography. Biol Psychiatry. 1994;35:128–32. doi: 10.1016/0006-3223(94)91202-5. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Opposite effects of mu and kappa opiate agonists on dopamine release in the nucleus accumbens and in the dorsal caudate of freely moving rats. J Pharmacol Exp Ther. 1988;244:1067–80. [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327–37. doi: 10.1001/archpsyc.64.3.327. [DOI] [PubMed] [Google Scholar]
- Ebert D, Feistel H, Loew T, Pirner A. Dopamine and depression--striatal dopamine D2 receptor SPECT before and after antidepressant therapy. Psychopharmacology (Berl) 1996;126:91–4. doi: 10.1007/BF02246416. [DOI] [PubMed] [Google Scholar]
- Eidelberg D, Moeller JR, Dhawan V, Spetsieris P, Takikawa S, Ishikawa T, Chaly T, Robeson W, Margouleff D, Przedborski S, et al. The metabolic topography of parkinsonism. J Cereb Blood Flow Metab. 1994;14:783–801. doi: 10.1038/jcbfm.1994.99. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD. Why rejection hurts: a common neural alarm system for physical and social pain. Trends Cogn Sci. 2004;8:294–300. doi: 10.1016/j.tics.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Eisenberger NI, Lieberman MD, Satpute AB. Personality from a controlled processing perspective: an fMRI study of neuroticism, extraversion, and self-consciousness. Cogn Affect Behav Neurosci. 2005;5:169–81. doi: 10.3758/cabn.5.2.169. [DOI] [PubMed] [Google Scholar]
- Feigin A, Fukuda M, Dhawan V, Przedborski S, Jackson-Lewis V, Mentis MJ, Moeller JR, Eidelberg D. Metabolic correlates of levodopa response in Parkinson’s disease. Neurology. 2001;57:2083–8. doi: 10.1212/wnl.57.11.2083. [DOI] [PubMed] [Google Scholar]
- Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH. Effects of interferon-alpha on rhesus monkeys: a nonhuman primate model of cytokine-induced depression. Biol Psychiatry. 2007;62:1324–33. doi: 10.1016/j.biopsych.2007.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friebe A, Schwarz MJ, Schmid-Wendtner M, Volkenandt M, Schmidt F, Horn M, Janssen G, Schaefer M. Pretreatment levels of sTNF-R1 and sIL-6R are associated with a higher vulnerability for IFN-alpha-induced depressive symptoms in patients with malignant melanoma. J Immunother (1997) 2007;30:333–7. doi: 10.1097/01.cji.0000211346.19330.c9. [DOI] [PubMed] [Google Scholar]
- Gao HM, Jiang J, Wilson B, Zhang W, Hong JS, Liu B. Microglial activation-mediated delayed and progressive degeneration of rat nigral dopaminergic neurons: relevance to Parkinson’s disease. J Neurochem. 2002;81:1285–97. doi: 10.1046/j.1471-4159.2002.00928.x. [DOI] [PubMed] [Google Scholar]
- Goodwill MH, Lawrence DA, Seegal RF. Polychlorinated biphenyls induce proinflammatory cytokine release and dopaminergic dysfunction: protection in interleukin-6 knockout mice. J Neuroimmunol. 2007;183:125–32. doi: 10.1016/j.jneuroim.2006.11.030. [DOI] [PubMed] [Google Scholar]
- Grace AA. Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: a hypothesis for the etiology of schizophrenia. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- Grace AA. Dopamine. In: Charney KLDDS, Coyle JT, Nemeroff CB, editors. Neuropsychopharmacology: The Fifth Generation of Progress. Philadelphia: Lippincott Williams and Wilkins; 2002. pp. 119–132. [Google Scholar]
- Gray F, Adle-Biassette H, Chretien F, Lorin de la Grandmaison G, Force G, Keohane C. Neuropathology and neurodegeneration in human immunodeficiency virus infection. Pathogenesis of HIV-induced lesions of the brain, correlations with HIV-associated disorders and modifications according to treatments. Clin Neuropathol. 2001;20:146–55. [PubMed] [Google Scholar]
- Guillemin GJ, Brew BJ, Noonan CE, Takikawa O, Cullen KM. Indoleamine 2,3 dioxygenase and quinolinic acid Immunoreactivity in Alzheimer’s disease hippocampus. Neuropathol Appl Neurobiol. 2005a;31:395–404. doi: 10.1111/j.1365-2990.2005.00655.x. [DOI] [PubMed] [Google Scholar]
- Guillemin GJ, Wang L, Brew BJ. Quinolinic acid selectively induces apoptosis of human astrocytes: potential role in AIDS dementia complex. J Neuroinflammation. 2005b;2:16. doi: 10.1186/1742-2094-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haas HS, Schauenstein K. Neuroimmunomodulation via limbic structures--the neuroanatomy of psychoimmunology. Prog Neurobiol. 1997;51:195–222. doi: 10.1016/s0301-0082(96)00055-x. [DOI] [PubMed] [Google Scholar]
- Hart BL. The behavior of sick animals. Vet Clin North Am Small Anim Pract. 1991;21:225–37. doi: 10.1016/s0195-5616(91)50028-0. [DOI] [PubMed] [Google Scholar]
- Hauser P, Soler R, Reed S, Kane R, Gulati M, Khosla J, Kling MA, Valentine AD, Meyers CA. Prophylactic treatment of depression induced by interferon-alpha. Psychosomatics. 2000;41:439–41. doi: 10.1176/appi.psy.41.5.439. [DOI] [PubMed] [Google Scholar]
- Ho BT, Huo YY, Lu JG, Tansey LW, Levin VA. Opioid-dopaminergic mechanisms in the potentiation of d-amphetamine discrimination by interferon-alpha. Pharmacol Biochem Behav. 1992;42:57–60. doi: 10.1016/0091-3057(92)90446-m. [DOI] [PubMed] [Google Scholar]
- Horikawa N, Yamazaki T, Sagawa M, Nagata T. A case of akathisia during interferon-alpha therapy for chronic hepatitis type C. Gen Hosp Psychiatry. 1999;21:134–5. doi: 10.1016/s0163-8343(98)00082-6. [DOI] [PubMed] [Google Scholar]
- Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–83. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K. Prefrontal cortical hypometabolism during low-dose interferon alpha treatment. Psychopharmacology (Berl) 2000;152:383–9. doi: 10.1007/s002130000549. [DOI] [PubMed] [Google Scholar]
- Kalin NH. Behavioral effects of ovine corticotropin-releasing factor administered to rhesus monkeys. Fed Proc. 1985;44:249–53. [PubMed] [Google Scholar]
- Kamata M, Higuchi H, Yoshimoto M, Yoshida K, Shimizu T. Effect of single intracerebroventricular injection of alpha-interferon on monoamine concentrations in the rat brain. Eur Neuropsychopharmacol. 2000;10:129–32. doi: 10.1016/s0924-977x(99)00067-x. [DOI] [PubMed] [Google Scholar]
- Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, Ohta T. Mechanism of systemically injected interferon-alpha impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res. 2003;978:104–14. doi: 10.1016/s0006-8993(03)02776-8. [DOI] [PubMed] [Google Scholar]
- Klimek V, Schenck JE, Han H, Stockmeier CA, Ordway GA. Dopaminergic abnormalities in amygdaloid nuclei in major depression: a postmortem study. Biol Psychiatry. 2002;52:740–8. doi: 10.1016/s0006-3223(02)01383-5. [DOI] [PubMed] [Google Scholar]
- Kluger MJ, Kozak W, Leon LR, Soszynski D, Conn CA. Cytokines and fever. Neuroimmunomodulation. 1995;2:216–23. doi: 10.1159/000097199. [DOI] [PubMed] [Google Scholar]
- Krivine A, Force G, Servan J, Cabee A, Rozenberg F, Dighiero L, Marguet F, Lebon P. Measuring HIV-1 RNA and interferon-alpha in the cerebrospinal fluid of AIDS patients: insights into the pathogenesis of AIDS Dementia Complex. J Neurovirol. 1999;5:500–6. doi: 10.3109/13550289909045379. [DOI] [PubMed] [Google Scholar]
- Kumai T, Tateishi T, Tanaka M, Watanabe M, Shimizu H, Kobayashi S. Effect of interferon-alpha on tyrosine hydroxylase and catecholamine levels in the brain of rats. Life Sci. 2000;67:663–9. doi: 10.1016/s0024-3205(00)00660-3. [DOI] [PubMed] [Google Scholar]
- Leng A, Mura A, Feldon J, Ferger B. Tumor necrosis factor-alpha receptor ablation in a chronic MPTP mouse model of Parkinson’s disease. Neurosci Lett. 2005;375:107–11. doi: 10.1016/j.neulet.2004.10.077. [DOI] [PubMed] [Google Scholar]
- Li W, Knowlton D, Woodward WR, Habecker BA. Regulation of noradrenergic function by inflammatory cytokines and depolarization. J Neurochem. 2003;86:774–83. doi: 10.1046/j.1471-4159.2003.01890.x. [DOI] [PubMed] [Google Scholar]
- Lou JS, Kearns G, Benice T, Oken B, Sexton G, Nutt J. Levodopa improves physical fatigue in Parkinson’s disease: a double-blind, placebo-controlled, crossover study. Mov Disord. 2003;18:1108–14. doi: 10.1002/mds.10505. [DOI] [PubMed] [Google Scholar]
- Lou JS, Kearns G, Oken B, Sexton G, Nutt J. Exacerbated physical fatigue and mental fatigue in Parkinson’s disease. Mov Disord. 2001;16:190–6. doi: 10.1002/mds.1042. [DOI] [PubMed] [Google Scholar]
- Maes M. Evidence for an immune response in major depression: a review and hypothesis. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:11–38. doi: 10.1016/0278-5846(94)00101-m. [DOI] [PubMed] [Google Scholar]
- Maes M, Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Meltzer H. Treatment with interferon-alpha (IFN alpha) of hepatitis C patients induces lower serum dipeptidyl peptidase IV activity, which is related to IFN alpha-induced depressive and anxiety symptoms and immune activation. Mol Psychiatry. 2001;6:475–80. doi: 10.1038/sj.mp.4000872. [DOI] [PubMed] [Google Scholar]
- Maestripieri D, McCormack K, Lindell SG, Higley JD, Sanchez MM. Influence of parenting style on the offspring’s behaviour and CSF monoamine metabolite levels in crossfostered and noncrossfostered female rhesus macaques. Behav Brain Res. 2006;175:90–5. doi: 10.1016/j.bbr.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev. 1998;105:83–107. doi: 10.1037/0033-295x.105.1.83. [DOI] [PubMed] [Google Scholar]
- Majer M, Welberg LA, Capuron L, Pagnoni G, Raison CL, Miller AH. IFN-alpha-induced motor slowing is associated with increased depression and fatigue in patients with chronic hepatitis C. Brain Behav Immun. 2008;22:870–80. doi: 10.1016/j.bbi.2007.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinot M, Bragulat V, Artiges E, Dolle F, Hinnen F, Jouvent R, Martinot J. Decreased presynaptic dopamine function in the left caudate of depressed patients with affective flattening and psychomotor retardation. Am J Psychiatry. 2001;158:314–6. doi: 10.1176/appi.ajp.158.2.314. [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Starkstein SE, Sadzot B, Preziosi T, Andrezejewski PL, Dannals RF, Wagner HN, Jr, Robinson RG. Selective hypometabolism in the inferior frontal lobe in depressed patients with Parkinson’s disease. Ann Neurol. 1990;28:57–64. doi: 10.1002/ana.410280111. [DOI] [PubMed] [Google Scholar]
- McKinney WT, Jr, Eising RG, Moran EC, Suomi SJ, Harlow HF. Effects of reserpine on the social behavior of rhesus monkeys. Dis Nerv Syst. 1971;32:735–41. [PubMed] [Google Scholar]
- Mendels J, Frazer A, Fitzgerald RG, Ramsey TA, Stokes JW. Biogenic amine metabolites in cerebrospinal fluid of depressed and manic patients. Science. 1972;175:1380–2. doi: 10.1126/science.175.4028.1380. [DOI] [PubMed] [Google Scholar]
- Mentis MJ, McIntosh AR, Perrine K, Dhawan V, Berlin B, Feigin A, Edwards C, Mattis P, Eidelberg D. Relationships among the metabolic patterns that correlate with mnemonic, visuospatial, and mood symptoms in Parkinson’s disease. Am J Psychiatry. 2002;159:746–54. doi: 10.1176/appi.ajp.159.5.746. [DOI] [PubMed] [Google Scholar]
- Meyer JH, Kruger S, Wilson AA, Christensen BK, Goulding VS, Schaffer A, Minifie C, Houle S, Hussey D, Kennedy SH. Lower dopamine transporter binding potential in striatum during depression. Neuroreport. 2001;12:4121–5. doi: 10.1097/00001756-200112210-00052. [DOI] [PubMed] [Google Scholar]
- Moron JA, Zakharova I, Ferrer JV, Merrill GA, Hope B, Lafer EM, Lin ZC, Wang JB, Javitch JA, Galli A, Shippenberg TS. Mitogen-activated protein kinase regulates dopamine transporter surface expression and dopamine transport capacity. J Neurosci. 2003;23:8480–8. doi: 10.1523/JNEUROSCI.23-24-08480.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mossner R, Mikova O, Koutsilieri E, Saoud M, Ehlis AC, Muller N, Fallgatter AJ, Riederer P. Consensus paper of the WFSBP Task Force on Biological Markers: biological markers in depression. World J Biol Psychiatry. 2007;8:141–74. doi: 10.1080/15622970701263303. [DOI] [PubMed] [Google Scholar]
- Muller N, Schwarz MJ. The immune-mediated alteration of serotonin and glutamate: towards an integrated view of depression. Mol Psychiatry. 2007;12:988–1000. doi: 10.1038/sj.mp.4002006. [DOI] [PubMed] [Google Scholar]
- Mundt C, Reck C, Backenstrass M, Kronmuller K, Fiedler P. Reconfirming the role of life events for the timing of depressive episodes. A two-year prospective follow-up study. J Affect Disord. 2000;59:23–30. doi: 10.1016/s0165-0327(99)00127-5. [DOI] [PubMed] [Google Scholar]
- Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- Nagatsu T, Sawada M. Inflammatory process in Parkinson’s disease: role for cytokines. Curr Pharm Des. 2005;11:999–1016. doi: 10.2174/1381612053381620. [DOI] [PubMed] [Google Scholar]
- Nutt DJ. The role of dopamine and norepinephrine in depression and antidepressant treatment. J Clin Psychiatry. 2006;67(Suppl 6):3–8. [PubMed] [Google Scholar]
- O’Connor JC, Lawson MA, Andre C, Moreau M, Lestage J, Castanon N, Kelley KW, Dantzer R. Lipopolysaccharide-induced depressive-like behavior is mediated by indoleamine 2,3-dioxygenase activation in mice. Mol Psychiatry. 2008 doi: 10.1038/sj.mp.4002148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM. Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry. 2006;163:1630–3. doi: 10.1176/ajp.2006.163.9.1630. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Feinstein JS, Simmons A, Stein MB. Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biol Psychiatry. 2004;55:1179–87. doi: 10.1016/j.biopsych.2004.02.023. [DOI] [PubMed] [Google Scholar]
- Pradhan AD, Ridker PM. Do atherosclerosis and type 2 diabetes share a common inflammatory basis? Eur Heart J. 2002;23:831–4. doi: 10.1053/euhj.2001.3052. [DOI] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Broadwell SD, Capuron L, Woolwine BJ, Jacobson IM, Nemeroff CB, Miller AH. Depression during pegylated interferon-alpha plus ribavirin therapy: prevalence and prediction. J Clin Psychiatry. 2005a;66:41–8. doi: 10.4088/jcp.v66n0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Borisov AS, Woolwine BJ, Massung B, Vogt G, Miller AH. Interferon-alpha effects on diurnal hypothalamic-pituitary-adrenal axis activity: relationship with proinflammatory cytokines and behavior. Mol Psychiatry. 2008 doi: 10.1038/mp.2008.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Capuron L, Miller AH. Cytokines sing the blues: inflammation and the pathogenesis of depression. Trends Immunol. 2006;27:24–31. doi: 10.1016/j.it.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Demetrashvili M, Capuron L, Miller AH. Neuropsychiatric adverse effects of interferon-alpha: recognition and management. CNS Drugs. 2005b;19:105–23. doi: 10.2165/00023210-200519020-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raison CL, Woolwine BJ, Demetrashvili MF, Borisov AS, Weinreib R, Staab JP, Zajecka JM, Bruno CJ, Henderson MA, Reinus JF, Evans DL, Asnis GM, Miller AH. Paroxetine for prevention of depressive symptoms induced by interferon-alpha and ribavirin for hepatitis C. Aliment Pharmacol Ther. 2007;25:1163–74. doi: 10.1111/j.1365-2036.2007.03316.x. [DOI] [PubMed] [Google Scholar]
- Reddy PL, Khanna S, Subhash MN, Channabasavanna SM, Rao BS. CSF amine metabolites in depression. Biol Psychiatry. 1992;31:112–8. doi: 10.1016/0006-3223(92)90198-9. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–52. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Rho MB, Wesselingh S, Glass JD, McArthur JC, Choi S, Griffin J, Tyor WR. A potential role for interferon-alpha in the pathogenesis of HIV-associated dementia. Brain Behav Immun. 1995;9:366–77. doi: 10.1006/brbi.1995.1034. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Clinical application of C-reactive protein for cardiovascular disease detection and prevention. Circulation. 2003;107:363–9. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Roth RH, Elsworth JD. Biochemical Pharmacology of Midbrain Dopamine Neurons. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: The Fourth Generation of Progress. Philadelphia: Lippincott Williams and Wilkins; 1995. [Google Scholar]
- Roy A, Karoum F, Pollack S. Marked reduction in indexes of dopamine metabolism among patients with depression who attempt suicide. Arch Gen Psychiatry. 1992;49:447–50. doi: 10.1001/archpsyc.1992.01820060027004. [DOI] [PubMed] [Google Scholar]
- Rye DB. The two faces of Eve: dopamine’s modulation of wakefulness and sleep. Neurology. 2004;63:S2–7. doi: 10.1212/wnl.63.8_suppl_3.s2. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote S, Weber SM. Nucleus accumbens dopamine and the regulation of effort in food-seeking behavior: implications for studies of natural motivation, psychiatry, and drug abuse. J Pharmacol Exp Ther. 2003;305:1–8. doi: 10.1124/jpet.102.035063. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Mingote SM, Weber SM. Beyond the reward hypothesis: alternative functions of nucleus accumbens dopamine. Curr Opin Pharmacol. 2005;5:34–41. doi: 10.1016/j.coph.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Sanchez MM, Alagbe O, Felger JC, Zhang J, Graff AE, Grand AP, Maestripieri D, Miller AH. Activated p38 MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry. 2007;12:895–7. doi: 10.1038/sj.mp.4002025. [DOI] [PubMed] [Google Scholar]
- Schaefer M, Schwaiger M, Pich M, Lieb K, Heinz A. Neurotransmitter changes by interferon-alpha and therapeutic implications. Pharmacopsychiatry. 2003;36(Suppl 3):S203–6. doi: 10.1055/s-2003-45131. [DOI] [PubMed] [Google Scholar]
- Schultz W. Multiple dopamine functions at different time courses. Annu Rev Neurosci. 2007;30:259–88. doi: 10.1146/annurev.neuro.28.061604.135722. [DOI] [PubMed] [Google Scholar]
- Schwartz AL, Thompson JA, Masood N. Interferon-induced fatigue in patients with melanoma: a pilot study of exercise and methylphenidate. Oncol Nurs Forum. 2002;29:E85–90. doi: 10.1188/02.ONF.E85-E90. [DOI] [PubMed] [Google Scholar]
- Shah PJ, Ogilvie AD, Goodwin GM, Ebmeier KP. Clinical and psychometric correlates of dopamine D2 binding in depression. Psychol Med. 1997;27:1247–56. doi: 10.1017/s0033291797005382. [DOI] [PubMed] [Google Scholar]
- Shanks N, Larocque S, Meaney MJ. Neonatal endotoxin exposure alters the development of the hypothalamic-pituitary-adrenal axis: early illness and later responsivity to stress. J Neurosci. 1995;15:376–84. doi: 10.1523/JNEUROSCI.15-01-00376.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R. Repeated interferon-alpha administration inhibits dopaminergic neural activity in the mouse brain. Brain Res. 1997;747:348–51. doi: 10.1016/s0006-8993(96)01371-6. [DOI] [PubMed] [Google Scholar]
- Silverman MN, Macdougall MG, Hu F, Pace TW, Raison CL, Miller AH. Endogenous glucocorticoids protect against TNF-alpha-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry. 2007;12:408–17. doi: 10.1038/sj.mp.4001921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simen BB, Duman CH, Simen AA, Duman RS. TNFalpha signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry. 2006;59:775–85. doi: 10.1016/j.biopsych.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Spetsieris PG, Moeller JR, Dhawan V, Ishikawa T, Eidelberg D. Visualizing the evolution of abnormal metabolic networks in the brain using PET. Comput Med Imaging Graph. 1995;19:295–306. doi: 10.1016/0895-6111(95)00011-e. [DOI] [PubMed] [Google Scholar]
- Sunami M, Nishikawa T, Yorogi A, Shimoda M. Intravenous administration of levodopa ameliorated a refractory akathisia case induced by interferon-alpha. Clin Neuropharmacol. 2000;23:59–61. doi: 10.1097/00002826-200001000-00012. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Arch Gen Psychiatry. 2005;62:1228–36. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35. doi: 10.1016/S0140-6736(05)67763-X. [DOI] [PubMed] [Google Scholar]
- Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS. Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci. 2003;14:347–53. doi: 10.1111/1467-9280.24411. [DOI] [PubMed] [Google Scholar]
- von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G. Potential time course of human immunodeficiency virus type 1-associated minor motor deficits: electrophysiologic and positron emission tomography findings. Arch Neurol. 2000;57:1601–7. doi: 10.1001/archneur.57.11.1601. [DOI] [PubMed] [Google Scholar]
- von Giesen HJ, Haslinger BA, Rohe S, Koller H, Arendt G. HIV Dementia Scale and psychomotor slowing--the best methods in screening for neuro-AIDS. J Neuropsychiatry Clin Neurosci. 2005;17:185–91. doi: 10.1176/jnp.17.2.185. [DOI] [PubMed] [Google Scholar]
- Wang JY, Zeng XY, Fan GX, Yuan YK, Tang JS. mu- but not delta- and kappa-opioid receptor mediates the nucleus submedius interferon-alpha-evoked antinociception in the rat. Neurosci Lett. 2006;397:254–8. doi: 10.1016/j.neulet.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Wichers MC, Koek GH, Robaeys G, Verkerk R, Scharpe S, Maes M. IDO and interferon-alpha-induced depressive symptoms: a shift in hypothesis from tryptophan depletion to neurotoxicity. Mol Psychiatry. 2005;10:538–44. doi: 10.1038/sj.mp.4001600. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Oscillations in the basal ganglia. Nature. 1999;400:621–2. doi: 10.1038/23148. [DOI] [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Functional neuroanatomy of the basal ganglia in Parkinson’s disease. Adv Neurol. 2003a;91:9–18. [PubMed] [Google Scholar]
- Wichmann T, DeLong MR. Pathophysiology of Parkinson’s disease: the MPTP primate model of the human disorder. Ann N Y Acad Sci. 2003b;991:199–213. doi: 10.1111/j.1749-6632.2003.tb07477.x. [DOI] [PubMed] [Google Scholar]
- Willeit M, Sitte HH, Thierry N, Michalek K, Praschak-Rieder N, Zill P, Winkler D, Brannath W, Fischer MB, Bondy B, Kasper S, Singer EA. Enhanced Serotonin Transporter Function during Depression in Seasonal Affective Disorder. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301560. [DOI] [PubMed] [Google Scholar]
- Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- Wu HQ, Rassoulpour A, Schwarcz R. Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm. 2007;114:33–41. doi: 10.1007/s00702-006-0562-y. [DOI] [PubMed] [Google Scholar]
- Yamada T, Yamanaka I. Microglial localization of alpha-interferon receptor in human brain tissues. Neurosci Lett. 1995;189:73–6. doi: 10.1016/0304-3940(95)11452-3. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Endotoxin produces a depressive-like episode in rats. Brain Res. 1996;711:163–74. doi: 10.1016/0006-8993(95)01415-2. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Blakely RD, Hewlett WA. The proinflammatory cytokines interleukin-1beta and tumor necrosis factor-alpha activate serotonin transporters. Neuropsychopharmacology. 2006;31:2121–31. doi: 10.1038/sj.npp.1301029. [DOI] [PubMed] [Google Scholar]
- Zhu CB, Carneiro AM, Dostmann WR, Hewlett WA, Blakely RD. p38 MAPK activation elevates serotonin transport activity via a trafficking-independent, protein phosphatase 2A-dependent process. J Biol Chem. 2005;280:15649–58. doi: 10.1074/jbc.M410858200. [DOI] [PubMed] [Google Scholar]
- Zorrilla EP, Luborsky L, McKay JR, Rosenthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K. The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun. 2001;15:199–226. doi: 10.1006/brbi.2000.0597. [DOI] [PubMed] [Google Scholar]