Abstract
Agonists acting at α2-adrenergic and opioid receptors (α2ARs and ORs, respectively) inhibit pain transmission in the spinal cord. When co-administered, agonists activating these receptors interact in a synergistic manner. Although the existence of α2AR/OR synergy has been well characterized, its mechanism remains poorly understood.
The formation of hetero-oligomers has been proposed as a molecular basis for interactions between neuronal G-protein-coupled receptors. The relevance of hetero-oligomer formation to spinal analgesic synergy requires demonstration of the expression of both receptors within the same neuron as well as the localization of both receptors in the same neuronal compartment. We used immunohistochemistry to investigate the spatial relationship between α2ARs and ORs in the rat spinal cord to determine if co-expression could be demonstrated between these receptors. We observed extensive co-localization between α2A-adrenergic and delta-opioid receptors (DOP) on substance P (SP)-immunoreactive (ir) varicosities in the superficial dorsal horn of the spinal cord and in peripheral nerve terminals in the skin. α2AAR- and DOP-ir elements were co-localized in subcellular structures of 0.5 μm or less in diameter in isolated nerve terminals. Furthermore, co-incubation of isolated synaptosomes with α2AR and DOP agonists resulted in a greater-than-additive increase in the inhibition of K+-stimulated neuropeptide release.
These findings suggest that co-expression of the synergistic receptor pair α2AAR-DOP on primary afferent nociceptive fibers may represent an anatomical substrate for analgesic synergy, perhaps due to protein-protein interactions such as hetero-oligomerization.
Keywords: immunohistochemistry, large dense core vesicle, synaptosome, primary afferent, nociceptor, terminal
INTRODUCTION
Agonists acting at spinal α2-adrenergic and opioid receptors (α2ARs and ORs, respectively), share common signal transduction systems mediated through inhibitory G proteins, the activation of which inhibits pain transmission. In addition, agonists acting at α2ARs and ORs interact in a greater-than-additive (i.e. synergistic) manner when co-administered to the spinal cord (for review see Alguacil and Morales 2004). Synergistic interactions between analgesic agents acting at ORs and α2ARs have been observed repeatedly in several laboratories after spinal administration using both behavioral (Hylden and Wilcox 1983; Stevens et al. 1988; Monasky et al. 1990; Ossipov et al. 1990a; Ossipov et al. 1990b; Ossipov et al. 1990c; Roerig et al. 1992) and electrophysiological (Sullivan et al. 1987; Wilcox et al. 1987; Omote et al. 1990) methods. Synergistic interactions can result in greater than 100-fold increases in analgesic potency as well as increased maximum efficacy. Synergy is important in clinical pain management as it makes possible production of analgesia at lower doses for each drug, thus reducing undesired side effects and improving treatment outcomes (for review see Walker et al. 2002). Despite the widespread evaluation and characterization of these interactions, the mechanisms underlying analgesic synergy are unclear.
Within the α2ARs there are three primary subtypes, α2A-, α2B- and α2CAR (for reviews see Aantaa et al. 1995; MacDonald et al. 1997; Philipp et al. 2002). Similarly, there are three primary OR subtypes, delta- (DOP), mu- (MOP) and kappa- (KOP) (for reviews see Kieffer 1999; Law and Loh 1999). Pairs of receptors for which α2AR-OR synergistic interactions have been documented include DOP and α2AAR (Stone et al. 1997), MOP and α2AAR (Roerig et al. 1992; Stone et al. 1997), and DOP and α2CAR (Fairbanks et al. 2002; Guo et al. 2003).
It is now recognized that G-protein-coupled receptors (GPCRs) can form oligomeric complexes in addition to the traditionally envisaged monomeric species. The demonstration of functional oligomers in transfected cells has led to significant re-evaluation of the mechanisms thought to be involved in GPCR function in vivo. These associations can result in novel pharmacological properties distinct from either component receptor, including enhancement of ligand binding affinity, changes in functional coupling and altered receptor trafficking (for reviews see George et al. 2002; Bulenger et al. 2005). The generation of novel properties upon hetero-oligomer formation may represent a molecular mechanism for synergistic interactions between neuronal GPCRs that co-localize in vivo. Physical associations suggestive of hetero-oligomer formation have been demonstrated between α2ARs and ORs in transfected cells in vitro (Jordan et al. 2003; Rios et al. 2004; Zhang and Limbird 2004; Vilardaga et al. 2008). The potential relevance of these in vitro studies to spinal synergy requires the expression of both receptors within the same neuron as well as the localization of both receptors in the same neuronal compartment.
We examined the expression of DOP, MOP, α2AAR and α2CAR in the superficial dorsal horn of the spinal cord to determine the spatial relationships between these receptors. Co-expression in the spinal cord dorsal horn would provide anatomical evidence to support a role for oligomerization in the phenomena of α2AR/OR functional synergy. To determine if simultaneous activation of co-localized receptors results in functional synergy, we challenged depolarization-elicited CGRP release from spinal cord synaptosomes with agonists directed at DOP and α2AR.
METHODS
Animals
Male Sprague Dawley rats (150-250g; Harlan, WI) were housed in pairs in a temperature- and humidity-controlled environment and maintained on a 12 hr light/dark cycle with free access to food and water. All experiments were approved by either the University of Minnesota’s Institutional Animal Care and Use Committee or the McGill University Animal Care and Ethics Committees.
Antisera
The α2AAR antiserum (1:1000) was prepared in rabbit against a synthetic peptide corresponding to α2AAR436-450 (AFKKILCRGDRKRIV). This antiserum has been previously characterized and was determined to be specific for α2AAR based on the elimination of staining following pre-adsorption of the antisera with peptide (AFKKILCRGDRKRIV) and the ability of the antisera to label MDCK cells transfected with α2AAR but not those transfected with α2BAR or α2CAR (Stone et al. 1998). This antiserum recognizes a ∼50 kDa band by Western blot analysis (Figure 6).
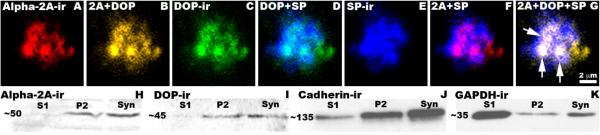
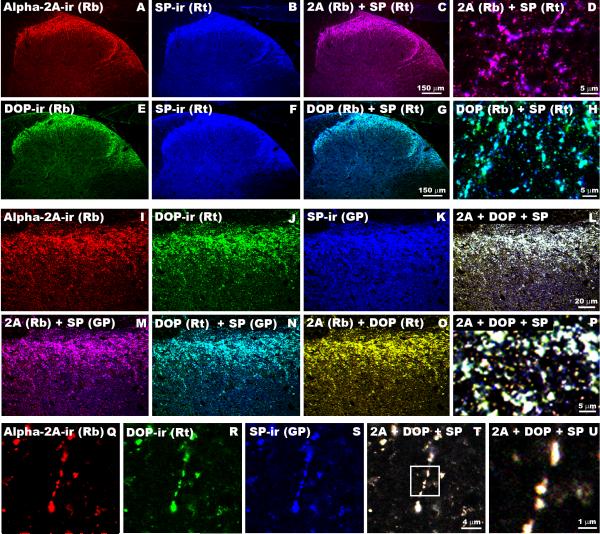
Figure 6. Labeling of α2AAR-ir, DOP-ir and SP-ir in Spinal Cord Synaptosome(s).
A-G: Nerve terminals were isolated from rat spinal cord and triple-labeled with rabbit-derived α2AAR (A, Red), rat-derived DOP (C, Green), and guinea pig-derived SP (E, Blue) antisera. Each of the possible digital pairings of these images is shown where B = α2AAR-ir + DOP-ir (co-localization = yellow); D = DOP-ir + SP-ir (co-localization = turquoise); F = α2AAR-ir + SP-ir (co-localization = fuchsia). Image G is the result of digital combination of Images A, C & E in which triple-labeled elements appear white. Arrows in G indicate several structural elements within the synaptosome that are triple-labeled. H-K: Enrichment of α2AAR-ir and DOP-ir in isolated nerve terminals. Subcellular fractions S1 (total protein), P2 (membrane fraction) and Syn (synaptosomes) were analyzed by Western blot. Immunoreactive bands for anti-α2AAR (∼50 kDa, L), anti-DOP (∼45 kDa, M) and the membrane marker pan-cadherin (∼135 kDa, N) were all enriched in the purified membrane and synaptosome fractions. The cytosolic marker GAPDH was most abundant in the S1 fraction (∼35 kDa, O).
The rabbit- and rat-derived DOP antisera were prepared against synthetic peptides and have been previously characterized. Rabbit anti-DOP3-17 (1:1000; LVPSARAELQSSPLV) was previously shown to exhibit staining identical to that of antisera raised against other regions of the receptor, including the rabbit anti-DOP30-46 (1:1000) and rat anti-DOP30-46 (1:300) antisera used in the current study (AGANASGSPGARSASSL; gift of Dr. Martin W. Wessendorf) (Arvidsson et al. 1995a). Staining with these antisera is eliminated following preadsorption with the corresponding peptides (Dado et al. 1993; Arvidsson et al. 1995a), is reduced following knock-down of DOP with antisense oligonucleotides (Lai et al. 1996) and is virtually eliminated by deletion of the DOP gene in mice (Zhu et al. 1999). The rabbit anti-DOP30-46 antiserum recognizes a ∼45 kDa band by Western blot analysis (Figure 6).
The rabbit- and guinea pig-derived MOP antisera were prepared against a synthetic peptide corresponding to MOP384-398 (QLENLEAETAPLP) of the predicted rat MOR1 gene and have been previously characterized (Arvidsson et al. 1995b). The rabbit-derived antiserum was determined to be specific for MOP based on the elimination of staining following pre-adsorption of the antiserum with the cognate peptide (QLENLEAETAPLP), the ability of the antiserum to label COS-7 cells transfected with MOP but not DOP or KOP and the recognition of an immunoreactive band by Western blot analysis of ∼60 kDa in rat brain membranes (Arvidsson et al. 1995b). The guinea pig-derived antiserum was generated against the same peptide and produces a staining pattern identical to the previously described rabbit-derived antiserum.
The α2CAR antisera were prepared in both guinea pig and rabbit against a synthetic peptide corresponding to α2CAR446-458 (HILFRRRRRGFRQ). These antisera have been previously characterized and were determined to be specific for α2CAR based on the elimination of staining following pre-adsorption of the antisera with the cognate peptide (HILFRRRRRGFRQ), the ability of the antisera to label MDCK cells transfected with α2CAR but not those transfected with α2AAR or α2BAR (Stone et al. 1998) and a reduction in immunoreactivity following knock-down of α2CAR with antisense oligonucleotides (Fairbanks et al. 2002). These antisera produce identical staining patterns and co-localize extensively.
SP antibodies raised in three different species and obtained from three different sources were used in these studies and produced similar results: rat anti-SP (1:1000; Accurate Chemical, NY); rabbit anti-SP (1:1000; gift of Dr. R. Ho to R. Elde); guinea pig anti-SP (1:500; Neuromics Antibodies, Inc. Minneapolis, MN). The rat anti-SP recognizes the COOH terminal of SP and was originally characterized by Cuello et al., (Cuello et al. 1979). This antibody is secreted from hybrid clone line NCI/34 derived from fusion of a mouse NSI/1-Ag 4-1 spleen cell and a spleen cell from a Wistar rat immunized with substance P (CRPKPQQFFGLM) conjugated to BSA. This antibody shows no cross-reactivity to leu- or met-enkephalin, somatostatin or beta-endorphin by radioimmunoassay. Immunofluorescence is blocked by pre-adsorption with synthetic substance P (220 μg/ml) (Cuello et al. 1979). The rabbit-derived SP-antiserum was obtained following immunization with a synthetic SP-thyroglobulin conjugate as originally reported by (Ho and DePalatis 1980). Immunoreactivity was blocked by pre-incubation of this antiserum with synthetic SP (10 μg/ml) but not by pre-treatment with met-enkephalin, neurophysin or somatostatin (Ho and DePalatis 1980). The polyclonal GP-derived antiserum was directed against residues 1-11 of rat SP and is blocked by pre-adsorption with 10 μg/ml of synthetic SP (data not shown). All three antibodies produce similar staining patterns and co-localize extensively.
The antibodies used as loading controls in Western blot analysis were both purchased from commercial sources. The rabbit polyclonal pan-cadherin antiserum (Cat# ab16505, Abcam) was generated against a synthetic peptide conjugated to KLH derived from within residues 850 to the C-terminus of human pan-cadherin. This antiserum recognizes a band of approximately 135 kDa in Western blot analysis that is blocked by pre-adsorption with the cognate peptide (Cat# ab17098, Abcam). A monoclonal antibody to the housekeeping gene glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was purchased from Millipore (Cat# MAB374, clone 6C5). This antibody was directed against the entire GAPDH protein isolated from rabbit muscle and recognizes a single band in rat spinal cord membranes by Western blot analysis (Figure 6).
Tissue Preparation (Spinal Cord)
Animals were anesthetized with an intramuscular injection of a mixture of 75 mg/kg ketamine, 5 mg/kg xylazine and 1 mg/kg acepromazine and perfused transcardially with 180 mL of oxygenated ice-cold Tyrodes solution (116 mM NaCl, 5 mM KCl, 2 mM MgCl2·6H2O, 406 μM MgSO4·7H2O, 2.9 mM glucose, 26 mM NaHCO2). This was followed by 500 mL fixative (4% (w/v) paraformaldehyde, 0.2% (v/v) saturated picric acid solution in 0.1 M phosphate-buffered saline (PBS; 150 mM KH2PO4, 170 mM NaHPO4·7H2O, pH 6.9) and thereafter by 400 mL of 10% sucrose solution (10% (w/v) sucrose in PBS). Spinal cords were dissected and stored overnight in 10% sucrose at 4°C. Tissue sections were prepared using a cryostat at a thickness of 10 or 14 μm, thaw-mounted onto gelatin-coated slides and stored at -20°C.
Immunofluorescence Histochemistry (Spinal Cord)
Cryostat sections were pre-incubated for one hour at room temperature (RT) in diluent containing 1% normal donkey serum, 0.3% Triton X-100, 0.01% sodium azide and 1% bovine serum albumin in PBS. Sections were incubated overnight at 4°C in a humid chamber with primary antisera, rinsed 3 × 10 min with PBS, incubated with fluorescently-tagged species-specific secondary antisera (Jackson Immunoresearch, West Grove, PA) for one hour at RT, rinsed 3 × 10 min with PBS and cover-slipped using either a mixture of glycerol and PBS containing 0.1% p-phenylenediamine or dehydrated, cleared in xylene and mounted with DPX (Fluka). Double- and triple-labeling procedures were adapted from previous studies (Wessendorf and Elde 1985).
Adsorption Controls
To control for cross-reactivity between DOP and α2AAR antisera, immunohistochemistry was performed as above with the exception that the primary antibodies were pre-incubated with 10 μg/mL of either peptide α2AAR436-450 (AFKKILCRGDRKRIV) or peptide DOP130-46 (AGANASGSPGARSASSL) (Arvidsson et al. 1995a; Stone et al. 1998). To ensure that secondary antibodies did not cross-react, control experiments were performed in which one or the other primary antisera were omitted and no staining was observed in either of these cases.
Tissue Preparation (Skin)
Animals were anesthetized with 0.4 mL/kg Equithesin (6.5 mg chloral hydrate and 3 mg sodium pentobarbital in a volume of 0.3 mL, i.p., per 100 g body weight) and perfused transcardially with a mixture of 4% paraformaldehyde and 15% (v/v) saturated solution of picric acid in 0.1 M phosphate buffer (PB), pH 7.4, for 30 min. Rat lower lip skin was dissected and post-fixed for 1 hour in the above fixative. The tissue was then incubated in a 30% sucrose solution in PB for at least 12 hours before further processing.
Immunofluorescence Histochemistry (Skin)
Tissue sections were trimmed and embedded in an Optical Cutting Temperature (OCT) compound (Tissue-Tek, Sakura Finetek, USA, Torrance, CA, USA). Fifty μm sections were cut on a cryostat (Leica) and collected in PBS containing 0.2% Triton-X 100 (PBS+T). Sections were treated with 50% ethanol for 30 min and then 0.3% hydrogen peroxide (H2O2) in PBS for 10 min at RT. Non-specific staining was blocked by incubating sections in 10% normal goat serum and 10% normal donkey serum (NDS) in PBS+T for one hour. Sections were incubated overnight at 4°C in primary antisera, washed with PBS+T, and then incubated for 2 hours with fluorescently-tagged species-specific secondary antisera. These included the highly cross-adsorbed Alexa Fluor 488-conjugated goat anti-rabbit IgG (1:400; Molecular Probes, Eugene, OR) and the rhodamine Red-X-conjugated donkey anti-guinea pig IgG (1:500; Jackson Immunoresearch, West Grove, PA). Sections were mounted on gelatin-coated slides and coverslipped with Aquapolymount (Polysciences, Warrington, PA).
Synaptosome Preparation
This preparation is described in greater detail elsewhere (Goracke-Postle et al. 2006). Briefly, spinal cords were collected, homogenized and centrifuged at 800 x g for 10 min at 4°C. The supernatant (S1) was then centrifuged at 15,000 x g for 20 min at 4°C. The resultant pellet (P2) was resuspended and the synaptosomes were further purified by sucrose gradient as previously described (Fried et al. 1989). This preparation has been validated using both functional and anatomical criteria (Goracke-Postle et al. 2006; Goracke-Postle et al. 2007).
Synaptosome Immunofluorescence
The synaptosomes were aliquoted into 4-well Nunc Lab-Tek II CC2 Chamber Slides and incubated at 37°C for 30 min. Slides were then exposed to fixative (see above) for 15 min and washed 3 × 5 min in PBS and incubated in diluent (see above) for 15 minutes. Slides were then incubated with primary antisera for 30 min at RT in a humid chamber, rinsed 3 × 5 min with PBS, incubated with fluorescently-tagged species-specific secondary antisera (Jackson Immunoresearch, West Grove, PA) for 30 minutes at RT and rinsed 3 × 5 min with PBS. The barriers separating the individual wells were removed and the slides were cover-slipped with Vectashield mounting medium (Vector Laboratories, Burlingame, CA).
Western Blot Analysis
Proteins from the S1, P2 and synaptosomal fraction obtained from mouse spinal cord were denatured in SDS sample buffer and 20 μg of protein was loaded onto a 10% SDS-PAGE gel and transferred to a PVDF membrane. Membranes were blocked with 5% non-fat dry milk in TBST (Tris-buffered saline with 0.01% Tween, pH 7.4) for 1 hour at RT followed by incubation with either rabbit anti-DOP (1:1000) or anti-α2AAR (1:1000) for 3 hours at RT. After washing 3 × 10 min with TBST, blots were incubated with a peroxidase-conjugated mouse-anti-rabbit IgG antibody (1:10,000, Jackson Immunoresearch, West Grove, PA) and washed 3 × 10 min. Membranes were revealed with SuperSignal West Femto Chemiluminescent Substrate (Pierce) and visualized with a digital imaging system equipped with a cooled CCD camera (ChemiGenius, SYNGENE). Membranes were then stripped with Restore Western Blot Stripping Buffer (Pierce) and probed with either a mouse-anti-GAPDH (1:20,000, Chemicon) or a rabbit anti-cadherin antibody (1:20,000, Abcam) for 60 minutes at RT. After washing 3 × 10 min with TBST, blots were incubated with a peroxidase-conjugated mouse-anti-rabbit IgG or goat-anti-mouse secondary antibody (1:10,000, Jackson Immunoresearch, West Grove, PA). Following the final 3 × 10 min washes in TBST, membranes were revealed with enhanced chemiluminescence (Pierce) and exposed to light-sensitive film (Clonex Corporation).
Confocal Microscopy
Images of spinal cords and synaptosomes were collected using a BioRad MRC 1000 confocal microscope (Bio-Rad Microscience Division, Cambridge, MA). A 60X, 1.4 NA objective and zoom values ranging from 1 to 5 were used for high magnification. Illumination was supplied by a Kr/Ar-ion laser with emission lines at 488, 568, and 647 nm. The skin samples were examined using a Zeiss LSM 510 confocal scanning laser microscope with argon and helium neon lasers. Micrograghs used in plates were minimally processed (adjusted for sharpness, contrast and brightness) and assembled using Photoshop (Adobe). Semi-quantitative analysis of the images in Figure 1, Column IV was performed in Photoshop (Adobe) by counting yellow (co-localized) pixels and dividing by the total number of red, green and yellow pixels. Similar results were obtained using the colocalization RGB plugin in ImageJ; data are reported for the photoshop method.
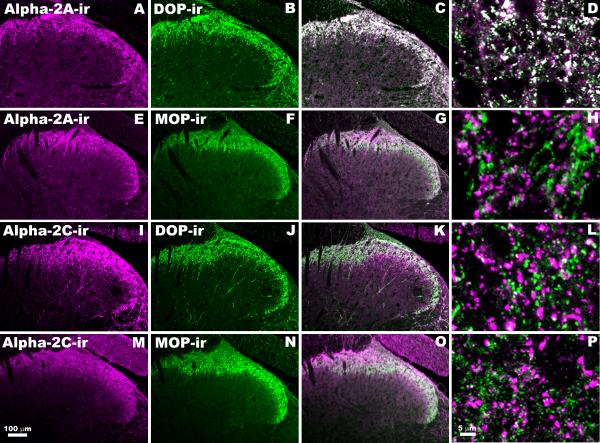
Figure 1. Relationship between α2AAR-ir, α2CAR-ir, DOP-ir and MOP-ir in Rat Spinal Cord Dorsal Horn.
Single confocal optical sections of lumbar rat spinal cord were double-labeled with combinations of guinea pig, rat or rabbit-derived antisera. The 1st column depicts immunoreactivity of antisera directed against α2AAR (A,E) and α2CAR (I,M). The 2nd column represents the same sections double-labeled with either DOP (B,J) or MOP (G,O) antisera. In the 3rd column the results of digitally merging images from the first two columns are shown (C,G,K,O). The 4th column contains higher magnification images of these combinations (D,H,L,P). In merged images, the appearance of white indicates probable co-localization. Antisera combinations were as follows: A-D: Rabbit-derived anti-α2AAR (A) with rat-derived anti-DOP (B), highly co-localized (C,D). E-H: Rabbit-derived anti-α2AAR (E) with guinea pig-derived anti-MOP (F), rarely co-localized (G,H). I-L: Guinea pig-derived anti-α2CAR (I) with rabbit-derived anti-DOP (J), rarely co-localized (K,L). M-P: Rabbit-derived anti-α2CAR (M) with guinea pig-derived anti-MOP (N), rarely co-localized (O,P). The lack of overlap between α2CAR and MOP was further supported by identical results obtained using rabbit-derived MOP paired with guinea pig-derived α2CAR (data not shown).
Neuropeptide Release Assay
Synaptosomes were prepared as described above, oxygenated for 1 min and allowed to seal during an incubation period of 30 min at 37°C. Samples were centrifuged for 5 min at 21,380 x g and supernatant was removed. After an initial wash in HEPES, a 10 min baseline sample was collected. The synaptosomes were then exposed to either vehicle or test compound (deltorphin II, clonidine or combination) for 10 min and subsequently stimulated with 60mM K+ (10 min). Samples were centrifuged again for 5 min at 21,380 x g. All supernatants were collected for analysis of immunoreactive calcitonin gene-related peptide (iCGRP) content by a commercial radioimmunoassay (RIA) kit (Phoenix Pharmaceuticals, Burlingame, CA). Data are presented as mean +/- SEM and expressed as the % inhibition of neuropeptide release according to the equation: % Inhibition = [(High K+ + Drug) -(High K+)]/[High K+] x 100.
RESULTS
Relationship between α2AAR-ir, α2CAR-ir, DOP-ir and MOP-ir in the Dorsal Horn of Rat Spinal Cord
The anatomical relationships between α2AAR-ir, α2CAR-ir, DOP-ir and MOP-ir were investigated in rat lumbar spinal cord. We observed extensive co-localization between α2AAR-ir and DOP-ir. In contrast, co-localization was minimal between α2AAR-ir and MOP-ir, α2CAR-ir and DOP-ir or α2CAR-ir and MOP-ir (Figure 1). Semi-quantitative assessment of co-localization at high magnification ranged from 58% (D, α2AAR-ir + DOP-ir) to 8.2% (H, α2AAR-ir + MOP-ir), 6.1% (L, α2CAR-ir + DOP-ir) and 6.7% (P, α2CAR-ir + MOP-ir).
Co-localization of α2AR-ir and DOP-ir in the Dorsal Horn of Rat Spinal Cord
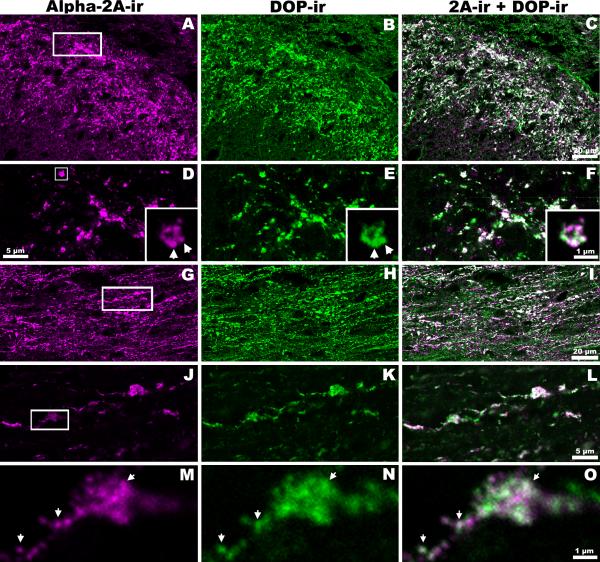
The anatomical relationship between α2AAR-ir and DOP-ir was further investigated. In rat lumbar spinal cord, extensive co-localization (white) between α2AAR-ir and DOP-ir was observed in the superficial dorsal horn (Figure 2). This level of co-localization was apparent in confocal images of coronal (Figure 2, A-F) and horizontal (Figure 2, G-O) spinal cord sections. Sections were double-labeled with rabbit-derived anti-α2AAR (Figure 2, 1st column) and rat-derived anti-DOP (Figure 2, 2nd column) and each image represents a single optical section. These pairs of unmerged images illustrate the similarity in staining patterns observed with the different antisera. The results of digital merging revealed extensive co-localization (Figure 2, 3rd column). High magnification microscopy detected α2AAR-ir and DOP-ir co-localization within the same neuronal processes 0.25 μm or less in diameter.
Figure 2. Close Association of DOP-ir and α2AAR-ir in Rat Spinal Cord Dorsal Horn.
Single confocal optical sections of rat spinal cord dorsal horn double-labeled with rabbit-derived α2AAR and rat-derived DOP antisera. The 1st column depicts α2AAR-ir (A,D,G,J,M) and the 2nd column represents DOP-ir (B,E,H,K,N) in the same sections. Pairs of unmerged images illustrate the similarity in expression patterns of α2A AR and DOP. When the images in the 1st and 2nd columns are digitally merged (C,F,I,L,O), the large proportion of white suggests extensive co-localization. A-F: Coronal sections of rat dorsal horn at low (A-C) and higher (D-F) magnification. The insets in D-F are enlargements of the boxed area marked in D. G-I: A horizontal section at moderate magnification. J-L: Higher magnification images of the boxed area shown in G. M-O: Enlargements of the boxed region in J. Images in D-F (insets) and M-O demonstrate close associations of α2AAR-ir and DOP-ir in the same sub-cellular regions 0.25 μm or less in diameter (arrows: possible vesicles or clusters of vesicles).
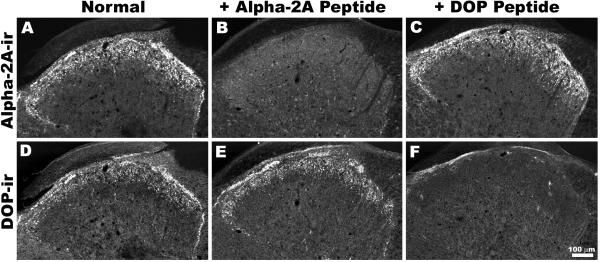
Adjacent sections of rat spinal cord were double-labeled for α2AAR-ir and DOP-ir in the presence or absence of each receptor’s cognate peptide (Figure 3). Each cognate peptide binds with high affinity to its corresponding antiserum, thereby inhibiting binding to the tissue and reducing the degree of immunoreactivity observed for that target while leaving the binding of other antiserum unaffected. In the presence of the α2AAR cognate peptide, α2AAR-ir was absent (Figure 3B) whereas DOP-ir (Figure 3E) was unaffected. Similarly, pre-incubation with the cognate peptide for the DOP antiserum blocked DOP-ir (Figure 3F) but not α2AAR-ir (Figure 3C). In parallel experiments, tissue was exposed to one or the other primary antisera and both secondary antibodies or one primary and both secondary antibodies. In these experiments, only immunoreactivity corresponding to the anticipated pair of primary and secondary antibodies was observed (data not shown). Taken together, these studies indicate that antisera used in this study do not cross-react.
Figure 3. Cross-reactivity Controls for DOP-ir and α2AAR-ir Co-localization.
Single confocal optical sections show the results of double labeling in the presence or absence of cognate peptide absorption controls in three adjacent sections. Under normal conditions, α2AAR-ir (A) and DOP-ir (D) exhibit similar patterns of expression. In the presence of its cognate peptide, labeling for α2AAR is absent (B) whereas DOP-ir (E) is unaffected. Similarly, pre-incubation with the cognate peptide for the DOP antisera blocks DOP-ir (F), but not α2AAR-ir (C).
Co-localization of α2AAR-ir, DOP-ir and SP-ir in the Dorsal Horn of Rat Spinal Cord
Previous reports have demonstrated that both α2AAR and DOP are expressed in the peptidergic population of primary afferent sensory neurons (Dado et al. 1993; Arvidsson et al. 1995a; Stone et al. 1998; Zhang et al. 1998). Therefore, we double- or triple-labeled spinal cord sections with antibodies directed against α2AAR, DOP and the neuropeptide substance P (SP) (Figure 4). Co-localization of α2AAR-ir and SP-ir was observed with the rabbit-derived α2AAR and both rat- and guinea pig-derived SP antibodies obtained from independent sources (Figure 4A-D, M). Similarly, rabbit-derived anti-DOP labeling co-localized with both the rat- and guinea pig-derived SP antibodies (Figure 4E-H & data not shown). The independent co-localization of α2AAR-ir and DOP-ir with several SP antibodies strongly suggests that the co-localization observed between α2AAR-ir and DOP-ir is not artifactual. Furthermore, DOP-ir and SP-ir co-localization was observed with rabbit- and rat-derived anti-DOP antisera that target different regions of the receptor (Figure 4E-H, N).
Figure 4. Triple Labeling of α2AAR-ir, DOP-ir and SP-ir in Rat Spinal Cord Dorsal Horn.
A-D: Representative section of rat spinal cord double-labeled with rabbit-derived α2AAR (A, Red) and rat-derived SP (B, Blue) antibodies. When images A & B are digitally merged (C, D), instances of co-localization appears as fushia. E-H: Representative section of rat spinal cord double-labeled with rabbit-derived DOP (E, Green) and rat-derived SP (F, Blue) antisera. When images E & F are digitally merged (G, H), instances of co-localization appears as turquoise. I-P: A single confocal optical section of rat spinal cord triple-labeled with rabbit-derived α2AAR (I, Red), rat-derived DOP (J, Green), and guinea pig-derived SP (K, Blue) antisera. Each of the possible digital pairings of these images is shown where M = α2AAR-ir + SP-ir (co-localization = fuchsia); N = DOP-ir + SP-ir (co-localization = turquoise) and O = α2AAR-ir + DOP-ir (co-localization = yellow). Image L is the result of digital combination of Images I, J & K in which triple-labeled elements appear white. Enlargement of an area from L is shown in P. Q-U: Example of a triple-labeled single fiber for α2AAR-ir (Q, Red), DOP-ir (R, Green) and SP-ir (S, Blue). Image T is the result of digital combination of Images Q, R & S in which triple-labeled elements appear white. Image U is an enlargement the area indicated in T. The close association of all three markers along a single fiber suggests that α2AAR and DOP may be associated with SP-containing pre-synaptic vesicles.
Triple-labeled sections of spinal cord are depicted in Figure 4I-U. Images resulting from each antiserum alone (Figure 4I-K) and all possible digitally merged pairs (Figure 4M-O) are shown separately to illustrate the extensive overlap between the antigens. Upon merging the digital images of sections labeled with all three antisera, triple-labeled elements appear white (Figure 4 L,P). Apparent single fibers were identified that showed α2AAR-ir, DOP-ir and SP-ir (Figure 4Q-T). An enlargement of one such fiber demonstrates co-localization of all three markers along the fiber (Figure 4U).
Co-localization of α2AAR-ir and DOP-ir with SP-ir in Rat Peripheral Nerve Terminals
Coincident co-localization of SP-ir was observed with α2AAR-ir and DOP-ir in epidermis (Figure 5) and dermis (data not shown) of skin obtained from the rat lip. Although direct co-localization of α2AAR-ir and DOP-ir was not possible in peripheral tissues due to technical considerations (the rat-derived DOP antiserum needed for direct α2AAR/DOP co-staining is incompatible with peripheral rat tissues), the coincident co-localization of each of the rabbit-derived receptor antisera with SP strongly suggests that α2AAR and DOP co-localize in peripheral nerve terminals.
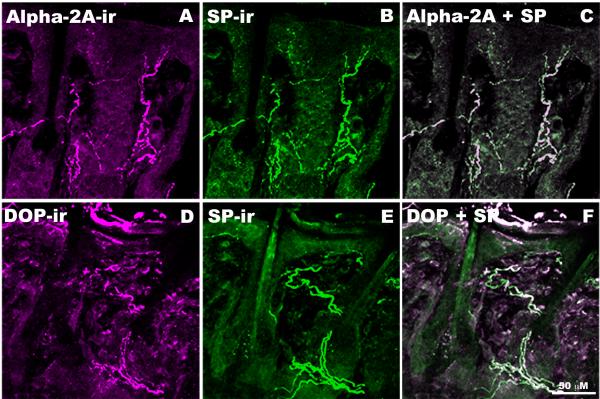
Figure 5. Co-localization of α2AAR and DOP with SP in rat skin.
A-C: Representative images of rat lower lip skin double-labeled with rabbit-derived α2AAR (A, Magenta) and rat-derived SP (B, Green) antisera. When images A & B are digitally merged (C), instances of co-localization appears as white. D-F: Representative section of rat spinal cord double-labeled with rabbit-derived DOP (D, Magenta) and guinea pig-derived SP (E, Green) antisera. When images D & E are digitally merged (F), instances of co-localization appears as white. The extensive co-localization observed between both α2AAR and DOP with SP suggests that α2AAR and DOP co-localize on SP-containing fibers in the periphery.
Localization of α2AAR-ir and DOP-ir to Isolated Spinal Cord Synaptosomes
To determine if α2AAR and DOP are co-localized in nerve terminals, isolated nerve terminals (synaptosomes) obtained from whole rat spinal cords were examined. Co-localization of α2AAR-ir, DOP-ir and SP-ir was observed within synaptosomes isolated from rat spinal cord (Figure 6, A-G). At the light level, this triple labeling of structural elements within the synaptosome preparation appeared to be localized in substructures less than 0.5 μm in diameter, consistent with localization on pre-synaptic vesicles.
To confirm the localization of DOP and α2AAR to pre-synaptic nerve terminals, we analyzed the composition of the S1, P2 and synaptosomal fractions obtained during synaptosome preparation by Western blot analysis. According to the subcellular fractionation procedure used, S1 should contain total cell extract minus large structures such as nuclei and cellular debris, P2 is a crude membrane preparation including synaptosomes, mitochondria and other organelles, and the synaptosomal fraction is enriched in isolated nerve terminals. We observed a ∼50 kDa immunoreactive band when probing with the rabbit anti-α2AAR (Figure 6L) and a ∼45 kDa immunoreactive band when probing with the rabbit anti-DOP (Figure 6M); both bands increase in intensity as the fractions become enriched in nerve terminals.
Although the same amount of protein from each fraction was loaded in each gel, we could not use a typical loading control strategy since the S1, P2 and synaptosomes fractions have different cytosolic to membrane protein ratios. We therefore used a membrane protein marker, pan-cadherin (Figure 6N), and a cytosolic protein marker, GAPDH (Figure 6O), to demonstrate the simultaneous enrichment in membrane proteins and depletion in cytosolic proteins as the purification progressed. Consistent with our observations with α2AAR and DOP, the intensity of the immunoreactive pan-cadherin band increased as the samples became enriched in synaptosomes. On the other hand, the GAPDH immunoreactive band intensity decreased from S1 to P2, indicating that cytosolic proteins were lost at this step. The slight increase in GAPDH intensity between P2 and the synaptosome fraction is expected because membrane-bound synaptosomes containing some cytoplasm are segregated from the total membrane fraction, resulting in a relative increase in cytosolic proteins.
The synaptosome preparation performed here is known to produce a fraction enriched in nerve endings packed with synaptic vesicles containing numerous neurotransmitters, including SP (Fried et al., 1989; Gray and Whittaker, 1962). Our observation that α2AAR and DOP are both enriched in synaptosomes, together with the previously demonstrated enrichment of SP, further supports the hypothesis that the receptors may be localized in SP-expressing pre-synaptic nerve terminals in spinal cord dorsal horn.
Greater-Than-Additive Inhibition of Neuropeptide Release by α2AAR and DOP agonists in Spinal Cord Synaptosomes
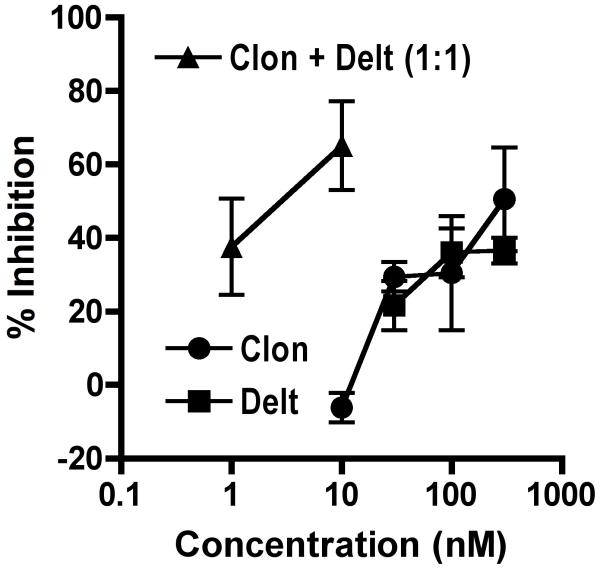
We tested our synaptosome preparation for evidence of functional α2AAR and DOP by evaluating the ability of agonists acting at these receptors to inhibit K+-stimulated release of the neuropeptide CGRP. Upon stimulation of spinal cord synaptosomes with 60 mM K+, immunoreactive CGRP (iCGRP) was increased from 127 pg/ml to 161 pg/ml. This increase was inhibited in a concentration-dependent manner by the non-α2AR subtype selective agonist clonidine and by the DOP agonist deltorphin II (Figure 7). In order to determine if an interaction exists between the receptors in the synaptosome preparation, samples were incubated with both drugs in combination and the resultant inhibition of K+-stimulated release was determined. The combination treatment resulted in significant enhancement in both potency and efficacy, suggesting a synergistic interaction exists between the receptors on isolated spinal nerve terminals.
Figure 7. Inhibition of neuropeptide release by α2AR and DOR agonists in spinal cord synaptosomes.
Synaptosomes were exposed to vehicle, the α2AR agonist clonidine (circles), the DOP agonist deltorphin II (squares) or the combination of clonidine + deltorphin II (triangles) and stimulated with 60 mM K+. Clonidine and deltorphin II inhibited calcitonin gene-related peptide (CGRP) release in a concentration-dependent manner. Co-incubation with both agonists together resulted in enhanced effectiveness over either agonist alone. Error bars represent ±SEM for each concentration (n = 3 replicates/concentration).
DISCUSSION
The current study revealed extensive overlap between α2AAR-ir and DOP-ir on SP-expressing primary afferent fibers in the dorsal horn of the rat spinal cord, in rat skin obtained from the lip and in isolated nerve terminals (synaptosomes) prepared from whole spinal cord. In contrast, co-localization was not observed between any of the other α2AR/OR receptor subtype pairs: α2AAR/MOP, α2CAR/MOP or α2CAR/DOP.
Simultaneous activation of α2AAR and DOP resulted in a greater-than-additive interaction in a functional assay measuring inhibition of K+-stimulated neuropeptide release in synaptosomes. These data indicate that the synergistic interaction observed in vivo between spinally administered α2AAR and DOP agonists may be mediated at the level of co-localized receptor pairs within single nerve terminals and has significant implications regarding the mechanism(s) underlying that synergy. Although inter-receptor synergy is not a guaranteed consequence of co-localization, the synergy observed here in isolated synaptic terminals strongly argues for co-localization at the level of primary afferent terminals in a manner not dependent on the specificity of receptor-directed antisera; synergy is unlikely to have occurred if the receptors were localized in separate terminals.
DOP and α2AAR Co-localization in Primary Afferent Nerve Terminals
The extensive overlap between α2AAR-ir and DOP-ir in SP-containing fibers and nerve terminals is consistent with previous reports localizing DOP-ir and α2AAR-ir to peptidergic primary afferent fibers (Dado et al. 1993; Arvidsson et al. 1995a; Stone et al. 1998; Zhang et al. 1998). The presence of SP suggests that these are likely to be C- or A-delta fibers (Lawson et al. 1993). Spinal α2AAR-ir and DOP-ir have both been shown to be decreased following dorsal rhizotomy, suggesting that a major source of these receptors in the spinal cord is the central terminals of primary afferent fibers (Dado et al. 1993; Stone et al. 1998). Similarly, autoradiographic labeling showed that a majority of DOP receptor binding is lost after unilateral dorsal rhizotomy (Besse et al. 1990). The observation in the current study that both DOP-ir and α2AAR-ir co-localize with SP-ir in peripheral nerve terminals in skin strongly suggests that SP-expressing, dorsal root ganglion neurons express DOP and α2AAR and traffic both receptors to both central and peripheral nerve terminals. The examples of co-localization obtained using double- or triple-labeled immunofluorescence histochemistry presented here most likely represent co-localization rather than superposition because the estimated thickness of the high magnification optical sections (<1.0 μm) minimizes the possibility of superposition of terminals with dimensions of 1-2 μm. However, light microscopy lacks the resolution required to a) confirm co-localization within the same neuronal structures and b) to identify the nature of DOP-ir and α2AAR-ir structures (e.g. axons vs. nerve terminals). Further studies examining the anatomical relationship between DOP-ir and α2AAR-ir at the ultrastructural level within spinal cord are necessary to confirm and extend the present data.
The immunohistochemical demonstrations of co-localization of both receptors and SP-ir in isolated nerve terminals (Figure 6) is augmented by the enrichment of both receptor immunoreactivities shown in the synaptosomal fraction using Western blot and by the supra-additive interaction observed between agonists targeting the two receptors. Although the synaptosomes in this study were isolated from whole spinal cord, the co-localization of α2AAR-ir and DOP-ir with SP-ir in both superficial dorsal horn and in the synaptosome preparation indicates that a subset of the isolated nerve terminals are derived from SP-ir terminals. In addition, the inhibition of CGRP-ir by α2AR and DOP agonists in the synaptosome preparation suggests that their site of action is the terminals of primary afferent fibers because dorsal root ganglia neurons are thought to be the only source of CGRP within the spinal cord (Chung et al. 1988).
The following observations support the hypothesis that DOP/α2AAR co-localization is not due to cross-reactivity between anti-DOP and α2AAR antisera: 1) α2AAR-ir and DOP-ir co-existed with SP-ir in the presence and absence of antisera to the other receptor. In the case of α2AAR/SP, co-localization was observed using the rabbit-derived anti-α2AAR antiserum and both rat- and guinea pig-derived SP antibodies. In the case of DOP/SP, two DOP antisera, each raised in a different species against a different region of the receptor, co-localized with two different SP antibodies (rabbit-DOP with rat- and guinea pig-SP, rat-DOP with rabbit- and guinea pig-SP); 2) co-localization was abolished by the omission of either the DOP or the α2AAR antisera; 3) co-localization was abolished by pre-adsorption of either the DOP or the α2AAR antisera with the respective cognate peptides.
Functional studies further support the localization patterns of α2AAR and DOP observed in the current study. Activation of both α2ARs and ORs has been shown to inhibit release of excitatory neurotransmitters (Jessell and Iversen 1977; Kuraishi et al. 1985; Kamisaki et al. 1993; Takano et al. 1993; Zachariou and Goldstein 1996; Li and Eisenach 2001) and to reduce excitatory neurotransmission from primary afferent fibers onto neurons in the superficial dorsal horn (Glaum et al. 1994; Kohno et al. 1999; Kawasaki et al. 2003; Sonohata et al. 2004; Kondo et al. 2005). Spinal analgesic synergy between DOP and α2AAR agonists has been previously documented (Stone et al. 1997). In the current study, we demonstrate inhibition of CGRP release from spinal cord synaptosomes by α2AR and DOP agonists, consistent with prior reports, and report a greater-than-additive interaction between these agonists. This greater-than-additive interaction in synaptosomes, where no neuronal circuitry remains intact, further supports co-localization at the terminal level because co-activation of receptors localized in separate CGRP-positive synaptosomes could only result in an additive interaction. These data also indicate that synergistic interactions observed in vivo between α2AAR and DOP likely occur at the level of co-localized receptor pairs within single nerve terminals.
Lack of Co-localization between α2AAR/MOP, α2CAR/MOP or α2CAR/DOP in Rat Spinal Cord
Synergy between MOP and α2AR agonists has been widely reported (Sullivan et al. 1987; Wilcox et al. 1987; Ossipov et al. 1989; Ossipov et al. 1990a; Ossipov et al. 1990b; Ossipov et al. 1990c; Sullivan et al. 1992; Ossipov et al. 1997; Stone et al. 1997; Fairbanks et al. 2000a; Fairbanks et al. 2000b; Fairbanks et al. 2002; Guo et al. 2003). The present study did not detect co-localization of MOP-ir with either α2AAR-ir or α2CAR-ir. One possible interpretation of this finding is that synergy between non-co-localized receptor pairs relies on multicellular mechanisms that have not yet been elucidated. An alternative possibility is that the antisera used in this study do not recognize their target receptors in all possible cellular environments or in all forms.
The lack of co-expression between MOP-ir and either α2AAR-ir or DOP-ir (or SP, data not shown) is potentially surprising. First, functional studies have shown that incubation with morphine results in receptor-mediated inhibition of neuropeptide release in vitro in slices of spinal trigeminal nucleus (Jessell and Iversen 1977) and in vivo following intrathecal administration (Yaksh et al. 1980). This inhibition is thought to result from activation of both MOP and DOP (Kondo et al. 2005). Second, anatomical studies clearly localize MOP to the cell bodies and/or central terminals of primary afferent neurons by mRNA (Mansour et al. 1995), autoradiography (Besse et al. 1990) and immunohistochemisty (Arvidsson et al. 1995b). However, careful examination of the relationship between MOP-ir and SP-ir in the spinal cord revealed that co-localization is only occasionally detected (Ding et al. 1995). In fact, the percentage of SP-ir terminals in trigeminal and cervical spinal cord that were also MOP-ir was only 12% and 6%, respectively (Aicher et al. 2000). In contrast, SP-ir terminals are often (>50%) contacted by MOP-ir dendrites (Aicher et al. 2000). The apparent contradiction inherent in the aforementioned studies may be explained by the existence of MOP splice variants. Specifically, in the superficial laminae of the rat spinal cord, immunoreactivity produced by antisera generated against the MOP-1C splice variant differed from that of MOP-1 despite being derived from the same gene (Abbadie et al. 2000); whereas MOP-1 rarely co-localized with SP-ir or CGRP-ir, MOP-1C-ir was often co-localized with CGRP. In the current study, we used an antibody directed against MOP-1 and not MOP-1C. It is therefore possible that DOP and α2AAR will be found in future studies to co-exist with MOP-1C, and this co-localization may provide another anatomical substrate for synergistic receptor interactions.
In addition to MOP, other antisera used in this study may not recognize their target receptors in all possible cellular environments or in all forms. The presence of mRNA encoding α2AAR in spinal cord neurons stands in contrast to the absence of immunohistochemical staining (Shi et al. 1999) and different DOP antibodies differentially label somatodendritic versus axonal compartments (Cahill et al. 2001a). The absence of co-localization between any of the other α2AR/OP receptor subtype pairs (α2AAR/MOP, α2CAR/MOP and α2CAR/DOP) may therefore be attributable to technical limitations of the antisera used rather than an absence of co-localization. Furthermore, the use of fluorescence for the detection of immunoreactivity may result in under- or over-representation of fiber subtypes.
Regardless, the possibility that our antisera did not recognize all forms of the receptors under evaluation does not detract from the significance of the positive co-localization results; the triple labeling reported in this study is likely to represent expression of both DOP and α2AAR within the same SP-expressing neuronal processes.
Implications of DOP and α2AAR Co-localization
The current results suggest an anatomical substrate for the formation of α2AAR and DOP hetero-oligomers in vivo Such associations between GPCRs have been shown to result in novel pharmacological properties distinct from either component receptor, including enhancement of ligand binding affinity, changes in functional coupling and altered receptor trafficking (for reviews, see (George et al. 2002; Bulenger et al. 2005)). Thus, the generation of novel properties upon α2AAR and DOP hetero-oligomer formation may represent a molecular mechanism for the synergistic interactions previously observed between these receptors in vivo, and that we now report in vitro Physical associations suggestive of hetero-oligomer formation have been demonstrated between α2AARs and both DOP and MOP in transfected cells in vitro (Jordan et al. 2003; Rios et al. 2004; Zhang and Limbird 2004; Vilardaga et al. 2008). The relevance of these in vitro studies to spinal α2AAR-DOP synergy requires the expression of both receptors within the same subcellular structures in native tissues as well as the demonstration of a functional interaction that can be attributed to that cellular localization. The combination of the previous in vitro studies and the current findings provide a structural and functional framework for the existence of α2AAR and DOP hetero-oligomers in vivo
DOP-ir is associated with large, dense-core vesicles (LDCVs) in axon terminals and ultrastructural evidence exists that these vesicles contain SP (Cheng et al. 1995; Zhang et al. 1998; Bao et al. 2003; Guan et al. 2005). It is thought that DOPs are trafficked to axon terminals in the membranes of these vesicles, where they may be inserted into the plasma membrane in a stimulus-dependent manner (Bao et al. 2003; Guan et al. 2005). DOP availability for binding and activation by extracellular ligands in the terminal is regulated, at least in part, by LDCV release. In addition to direct stimulation, DOP may be translocated to the plasma membrane in response to DOP agonists, chronic morphine exposure, peripheral inflammation, inflammatory mediators and chronic nociceptive stimuli (Cahill et al. 2001b; Bao et al. 2003; Morinville et al. 2003; Morinville et al. 2004a; Morinville et al. 2004b; Guan et al. 2005; Hack et al. 2005; Lucido et al. 2005; Patwardhan et al. 2005; Gendron et al. 2006; Gendron et al. 2007). As a consequence, sensitivity to DOP agonists is increased. For example, Cahill and colleagues demonstrated an increase in both intrathecal DOP agonist-induced analgesia and the number of plasma membrane-associated DOP-ir particles following chronic morphine treatment (Cahill et al. 2001b). This stimulus-triggered exocytosis and consequent surface insertion of DOP is reported to be dependent on the SP domain of preprotachykinin A present in DOP-containing LDCVs (Guan et al. 2005).
We report here that both DOP-ir and α2AAR-ir are highly co-localized with SP-ir at the light microscope level in primary afferent fiber terminals in both skin and spinal cord. This three-way co-localization raises two possibilities: a) the α2AAR is trafficked and stored within the same vesicles that contain SP and DOP (Zhang et al., 1998), and b) that the availability of α2AAR on the plasma membrane is controlled by the same regulatory factors that control DOP availability. Evaluation of these possibilities will require further anatomical studies by electron microscopy. We hypothesize that DOP agonist-mediated translocation of DOP will also result in translocation and subsequent enhanced availability of α2AAR. The presence of a small amount of the DOP agonist deltorphin-II would potentially result in a dramatic increase in α2AAR agonist efficacy as a result of vesicle fusion and receptor translocation. This scenario would similarly apply if α2AAR agonists cause vesicle fusion and receptor translocation in a manner similar to that already demonstrated for DOP agonists, resulting in mutual potentiation between DOP and α2AAR. This proposed model for DOP-α2AAR synergy is based entirely on co-operative receptor trafficking and would predict a greater-than-additive interaction between DOP-α2AAR in isolated synaptosomes as we currently demonstrate and represents an alternative hypothesis regarding the mechanism(s) underlying synergy that is supported by the current findings.
Conclusions
This study provides definitive evidence of extensive co-localization between α2AAR and DOP in SP-containing peripheral and central nerve terminals. α2AAR/DOP co-localization can be observed in axonal terminals and isolated nerve terminals. Agonists acting at α2AR and DOP inhibited K+-induced neuropeptide release from synaptosomes and interacted in a greater-than-additive manner, indicating a synergistic interaction. This supra-additive interaction in isolated nerve terminals provides a functional implication to the immunohistochemically demonstrated receptor co-localization. Taken together, these data raise the possibility that DOP and α2AAR hetero-oligomeric receptor complexes could exist in vivo and may represent a molecular substrate for the analgesic synergy commonly observed between agonists acting at these receptors.
OTHER ACKNOWLEDGEMENTS
We thank Jeromy Dooyema, Cory Goracke-Postle and H. Oanh Nguyen for technical support, Drs. Martin W. Wessendorf, Carolyn A. Fairbanks and Lucy Vulchanova for valuable discussions and MWW for use of the rat-derived anti-DOR antiserum.
Supported by NIH R01-DA-01933 and NIH R01-DA-15438 to GLW; NIH R01-DA-06299 to RE; NIH R21-DA-017075 and CIHR MOP-86691 to LSS.
REFERENCES
- Aantaa R, Marjamaki A, Scheinin M. Molecular pharmacology of alpha 2-adrenoceptor subtypes. Ann Med. 1995;27(4):439–449. doi: 10.3109/07853899709002452. [DOI] [PubMed] [Google Scholar]
- Abbadie C, Pan YX, Pasternak GW. Differential distribution in rat brain of mu opioid receptor carboxy terminal splice variants MOR-1C-like and MOR-1-like immunoreactivity: evidence for region-specific processing. J Comp Neurol. 2000;419(2):244–256. doi: 10.1002/(sici)1096-9861(20000403)419:2<244::aid-cne8>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- Aicher SA, Sharma S, Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural localization of mu-opiate receptors and substance p in the dorsal horn. Synapse. 2000;36(1):12–20. doi: 10.1002/(SICI)1098-2396(200004)36:1<12::AID-SYN2>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Alguacil LF, Morales L. Alpha-2 Adrenoceptor Ligands and Opioid Drugs: Pharmacological Interactions of Therapeutic Interest. Current Neuropharmacology. 2004;2(4):343–352. [Google Scholar]
- Arvidsson U, Dado RJ, Riedl M, Lee JH, Law PY, Loh HH, Elde R, Wessendorf MW. Delta-opioid receptor immunoreactivity: distribution in brain stem and spinal cord and relationship to biogenic amines and enkephalin. J Neurosci. 1995a;15:1215–1235. doi: 10.1523/JNEUROSCI.15-02-01215.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arvidsson U, Riedl M, Chakrabarti S, Lee JH, Nakano AH, Dado RJ, Loh HH, Law PY, Wessendorf MW, Elde R. Distribution and targeting of a mu-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995b;15(5 Pt 1):3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao L, Jin SX, Zhang C, Wang LH, Xu ZZ, Zhang FX, Wang LC, Ning FS, Cai HJ, Guan JS, Xiao HS, Xu ZQ, He C, Hokfelt T, Zhou Z, Zhang X. Activation of delta opioid receptors induces receptor insertion and neuropeptide secretion. Neuron. 2003;37(1):121–133. doi: 10.1016/s0896-6273(02)01103-0. [DOI] [PubMed] [Google Scholar]
- Besse D, Lombard MC, Zajac JM, Roques BP, Besson JM. Pre- and postsynaptic distribution of mu, delta and kappa opioid receptors in the superficial layers of the cervical dorsal horn of the rat spinal cord. Brain Research. 1990;521(1-2):15–22. doi: 10.1016/0006-8993(90)91519-m. [DOI] [PubMed] [Google Scholar]
- Bulenger S, Marullo S, Bouvier M. Emerging role of homo- and heterodimerization in G-protein-coupled receptor biosynthesis and maturation. Trends Pharmacol Sci. 2005;26(3):131–137. doi: 10.1016/j.tips.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Cahill CM, McClellan KA, Morinville A, Hoffert C, Hubatsch D, O’Donnell D, Beaudet A. Immunohistochemical distribution of delta opioid receptors in the rat central nervous system: evidence for somatodendritic labeling and antigen-specific cellular compartmentalization. J Comp Neurol. 2001a;440(1):65–84. doi: 10.1002/cne.1370. [DOI] [PubMed] [Google Scholar]
- Cahill CM, Morinville A, Lee MC, Vincent JP, Collier B, Beaudet A. Prolonged morphine treatment targets delta opioid receptors to neuronal plasma membranes and enhances delta-mediated antinociception. J Neurosci. 2001b;21(19):7598–7607. doi: 10.1523/JNEUROSCI.21-19-07598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Svingos AL, Wang H, Clarke CL, Jenab S, Beczkowska IW, Inturrisi CE, Pickel VM. Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of delta-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci. 1995;15(9):5976–5988. doi: 10.1523/JNEUROSCI.15-09-05976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung K, Lee WT, Carlton SM. The effects of dorsal rhizotomy and spinal cord isolation on calcitonin gene-related peptide-labeled terminals in the rat lumbar dorsal horn. Neurosci Lett. 1988;90(1-2):27–32. doi: 10.1016/0304-3940(88)90781-1. [DOI] [PubMed] [Google Scholar]
- Cuello AC, Galfre G, Milstein C. Detection of substance P in the central nervous system by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979;76(7):3532–3536. doi: 10.1073/pnas.76.7.3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dado RJ, Law PY, Loh HH, Elde R. Immunofluorescent identification of a delta (delta)-opioid receptor on primary afferent nerve terminals. Neuroreport. 1993;5(3):341–344. doi: 10.1097/00001756-199312000-00041. [DOI] [PubMed] [Google Scholar]
- Ding YQ, Nomura S, Kaneko T, Mizuno N. Co-localization of mu-opioid receptor-like and substance P-like immunoreactivities in axon terminals within the superficial layers of the medullary and spinal dorsal horns of the rat. Neurosci Lett. 1995;198(1):45–48. doi: 10.1016/0304-3940(95)11960-5. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Nguyen HO, Grocholski BM, Wilcox GL. Moxonidine, a selective imidazoline-alpha2 -adrenergic receptor agonist, produces spinal synergistic antihyperalgesia with morphine in nerve-injured mice. Anesthesiology. 2000a;93(3):765–773. doi: 10.1097/00000542-200009000-00026. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Posthumus IJ, Kitto KF, Stone LS, Wilcox GL. Moxonidine, a selective imidazoline/alpha(2) adrenergic receptor agonist, synergizes with morphine and deltorphin II to inhibit substance P-induced behavior in mice. Pain. 2000b;84(1):13–20. doi: 10.1016/S0304-3959(99)00171-2. [DOI] [PubMed] [Google Scholar]
- Fairbanks CA, Stone LS, Kitto KF, Nguyen HO, Posthumus IJ, Wilcox GL. alpha(2C)-Adrenergic receptors mediate spinal analgesia and adrenergic-opioid synergy. J Pharmacol Exp Ther. 2002;300(1):282–290. doi: 10.1124/jpet.300.1.282. [DOI] [PubMed] [Google Scholar]
- Fried G, Franck J, Brodin E, Born W, Fischer JA, Hiort W, Hokfelt T. Evidence for differential storage of calcitonin gene-related peptide, substance P and serotonin in synaptosomal vesicles of rat spinal cord. Brain Res. 1989;499(2):315–324. doi: 10.1016/0006-8993(89)90780-4. [DOI] [PubMed] [Google Scholar]
- Gendron L, Esdaile MJ, Mennicken F, Pan H, O’Donnell D, Vincent JP, Devi LA, Cahill CM, Stroh T, Beaudet A. Morphine priming in rats with chronic inflammation reveals a dichotomy between antihyperalgesic and antinociceptive properties of deltorphin. Neuroscience. 2007;144(1):263–274. doi: 10.1016/j.neuroscience.2006.08.077. [DOI] [PubMed] [Google Scholar]
- Gendron L, Lucido AL, Mennicken F, O’Donnell D, Vincent JP, Stroh T, Beaudet A. Morphine and pain-related stimuli enhance cell surface availability of somatic delta-opioid receptors in rat dorsal root ganglia. J Neurosci. 2006;26(3):953–962. doi: 10.1523/JNEUROSCI.3598-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, O’Dowd BF, Lee SP. G-protein-coupled receptor oligomerization and its potential for drug discovery. Nat Rev Drug Discov. 2002;1(10):808–820. doi: 10.1038/nrd913. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Miller RJ, Hammond DL. Inhibitory actions of delta 1-, delta 2-, and mu-opioid receptor agonists on excitatory transmission in lamina II neurons of adult rat spinal cord. Journal Of Neuroscience. 1994;14(8):4965–4971. doi: 10.1523/JNEUROSCI.14-08-04965.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goracke-Postle CJ, Nguyen HO, Stone LS, Fairbanks CA. Release of tritiated agmatine from spinal synaptosomes. Neuroreport. 2006;17(1):13–17. doi: 10.1097/01.wnr.0000192739.38653.aa. [DOI] [PubMed] [Google Scholar]
- Goracke-Postle CJ, Overland AC, Riedl MS, Stone LS, Fairbanks CA. Potassium- and capsaicin-induced release of agmatine from spinal nerve terminals. J Neurochem. 2007;102(6):1738–1748. doi: 10.1111/j.1471-4159.2007.04647.x. [DOI] [PubMed] [Google Scholar]
- Gray EG, Whittaker VP. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- Guan JS, Xu ZZ, Gao H, He SQ, Ma GQ, Sun T, Wang LH, Zhang ZN, Lena I, Kitchen I, Elde R, Zimmer A, He C, Pei G, Bao L, Zhang X. Interaction with vesicle luminal protachykinin regulates surface expression of delta-opioid receptors and opioid analgesia. Cell. 2005;122(4):619–631. doi: 10.1016/j.cell.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Guo XH, Fairbanks CA, Stone LS, Loh HH. DPDPE-UK14,304 synergy is retained in mu opioid receptor knockout mice. Pain. 2003;104(1-2):209–217. doi: 10.1016/s0304-3959(03)00007-1. [DOI] [PubMed] [Google Scholar]
- Hack SP, Bagley EE, Chieng BC, Christie MJ. Induction of delta-opioid receptor function in the midbrain after chronic morphine treatment. J Neurosci. 2005;25(12):3192–3198. doi: 10.1523/JNEUROSCI.4585-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho RH, DePalatis LR. Substance P immunoreactivity in the median eminence of the North American opossum and domestic fowl. Brain Res. 1980;189(2):565–569. doi: 10.1016/0006-8993(80)90370-4. [DOI] [PubMed] [Google Scholar]
- Hylden JL, Wilcox GL. Pharmacological characterization of substance P-induced nociception in mice: modulation by opioid and noradrenergic agonists at the spinal level. J Pharmacol Exp Ther. 1983;226(2):398–404. [PubMed] [Google Scholar]
- Jessell TM, Iversen LL. Opiate analgesics inhibit substance P release from rat trigeminal nucleus. Nature. 1977;286:549–551. doi: 10.1038/268549a0. [DOI] [PubMed] [Google Scholar]
- Jordan BA, Gomes I, Rios C, Filipovska J, Devi LA. Functional interactions between mu opioid and alpha 2A-adrenergic receptors. Mol Pharmacol. 2003;64(6):1317–1324. doi: 10.1124/mol.64.6.1317. [DOI] [PubMed] [Google Scholar]
- Kamisaki Y, Hamada T, Maeda K, Ishimura M, Itoh T. Presynaptic alpha 2 adrenoceptors inhibit glutamate release from rat spinal cord synaptosomes. J Neurochem. 1993;60(2):522–526. doi: 10.1111/j.1471-4159.1993.tb03180.x. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Kumamoto E, Furue H, Yoshimura M. Alpha 2 adrenoceptor-mediated presynaptic inhibition of primary afferent glutamatergic transmission in rat substantia gelatinosa neurons. Anesthesiology. 2003;98(3):682–689. doi: 10.1097/00000542-200303000-00016. [DOI] [PubMed] [Google Scholar]
- Kieffer BL. Opioids: first lessons from knockout mice [Review] Trends in Pharmacological Sciences. 1999;20(1):19–26. doi: 10.1016/s0165-6147(98)01279-6. [DOI] [PubMed] [Google Scholar]
- Kohno T, Kumamoto E, Higashi H, Shimoji K, Yoshimura M. Actions of opioids on excitatory and inhibitory transmission in substantia gelatinosa of adult rat spinal cord. J Physiol. 1999;518(Pt 3):803–813. doi: 10.1111/j.1469-7793.1999.0803p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo I, Marvizon JC, Song B, Salgado F, Codeluppi S, Hua XY, Yaksh TL. Inhibition by spinal mu- and delta-opioid agonists of afferent-evoked substance P release. J Neurosci. 2005;25(14):3651–3660. doi: 10.1523/JNEUROSCI.0252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuraishi Y, Hirota N, Sato Y, Kaneko S, Satoh M, Takagi H. Noradrenergic inhibition of the release of substance P from the primary afferents in the rabbit spinal dorsal horn. Brain Res. 1985;359:177–182. doi: 10.1016/0006-8993(85)91426-x. [DOI] [PubMed] [Google Scholar]
- Lai J, Riedl M, Stone LS, Arvidsson U, Bilsky EJ, Wilcox GL, Elde R, Porreca F. Immunofluorescence analysis of antisense oligodeoxynucleotide-mediated ‘knock-down’ of the mouse delta opioid receptor in vitro and in vivo. Neurosci Lett. 1996;213(3):205–208. doi: 10.1016/0304-3940(96)12883-4. [DOI] [PubMed] [Google Scholar]
- Law PY, Loh HH. Regulation of opioid receptor activities [Review] Journal of Pharmacology & Experimental Therapeutics. 1999;289(2):607–624. [PubMed] [Google Scholar]
- Lawson SN, Perry MJ, Prabhakar E, McCarthy PW. Primary sensory neurones: Neurofilament, neuropeptides, and conduction velocity. Brain Res Bull. 1993;30:239–243. doi: 10.1016/0361-9230(93)90250-f. [DOI] [PubMed] [Google Scholar]
- Li X, Eisenach JC. alpha2A-adrenoceptor stimulation reduces capsaicin-induced glutamate release from spinal cord synaptosomes. J Pharmacol Exp Ther. 2001;299(3):939–944. [PubMed] [Google Scholar]
- Lucido AL, Morinville A, Gendron L, Stroh T, Beaudet A. Prolonged morphine treatment selectively increases membrane recruitment of delta-opioid receptors in mouse basal ganglia. J Mol Neurosci. 2005;25(3):207–214. doi: 10.1385/JMN:25:3:207. [DOI] [PubMed] [Google Scholar]
- MacDonald E, Kobilka BK, Scheinin M. Gene targeting--homing in on alpha 2-adrenoceptor-subtype function. Trends in Pharmacological Sciences. 1997;18(6):211–219. doi: 10.1016/s0165-6147(97)01063-8. [DOI] [PubMed] [Google Scholar]
- Mansour A, Fox CA, Akil H, Watson SJ. Opioid-receptor mRNA expression in the rat CNS: anatomical and functional implications. Trends Neurosci. 1995;18(1):22–29. doi: 10.1016/0166-2236(95)93946-u. [DOI] [PubMed] [Google Scholar]
- Monasky MS, Zinsmeister AR, Stevens CW, Yaksh TL. Interaction of intrathecal morphine and ST-91 on antinociception in the rat: dose-response analysis, antagonism and clearance. J Pharmacol Exp Ther. 1990;254(2):383–392. [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Aibak H, Rymar VV, Pradhan A, Hoffert C, Mennicken F, Stroh T, Sadikot AF, O’Donnell D, Clarke PB, Collier B, Henry JL, Vincent JP, Beaudet A. Morphine-induced changes in delta opioid receptor trafficking are linked to somatosensory processing in the rat spinal cord. J Neurosci. 2004a;24(24):5549–5559. doi: 10.1523/JNEUROSCI.2719-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Esdaile MJ, Aibak H, Collier B, Kieffer BL, Beaudet A. Regulation of delta-opioid receptor trafficking via mu-opioid receptor stimulation: evidence from mu-opioid receptor knock-out mice. J Neurosci. 2003;23(12):4888–4898. doi: 10.1523/JNEUROSCI.23-12-04888.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morinville A, Cahill CM, Kieffer B, Collier B, Beaudet A. Mu-opioid receptor knockout prevents changes in delta-opioid receptor trafficking induced by chronic inflammatory pain. Pain. 2004b;109(3):266–273. doi: 10.1016/j.pain.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Omote K, Kitahata L, Collins J, Nakatani K, Nakagawa I. The antinociceptive role ofμ and delta-opiate receptors and their interactions in the spinal dorsal horns of cats. Anesth Analg. 1990;71:23–28. doi: 10.1213/00000539-199007000-00004. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Harris S, Lloyd P, Messineo E. An isobolographic analysis of the antinociceptive effect of systemically and intrathecally administered combinations of clonidine and opiates. J Pharmacol Exp Ther. 1990a;255(3):1107–1116. [PubMed] [Google Scholar]
- Ossipov MH, Harris S, Lloyd P, Messineo E, Lin BS, Bagley J. Antinociceptive interaction between opioids and medetomidine: systemic additivity and spinal synergy. Anesthesiology. 1990b;73(6):1227–1235. doi: 10.1097/00000542-199012000-00022. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lopez Y, Bian D, Nichols ML, Porreca F. Synergistic antinociceptive interactions of morphine and clonidine in rats with nerve-ligation injury. Anesthesiol. 1997;86(1):1–9. doi: 10.1097/00000542-199701000-00024. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Lozito R, Messineo E, Green J, Harris S, Lloyd P. Spinal antinociceptive synergy between clonidine and morphine, U69593, and DPDPE: isobolographic analysis. Life Sci. 1990c;47(16):PL71–76. doi: 10.1016/0024-3205(90)90530-5. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Suarez LJ, Spaulding TC. Antinociceptive interactions between alpha 2-adrenergic and opiate agonists at the spinal level in rodents. Anesth Analg. 1989;68(3):194–200. [PubMed] [Google Scholar]
- Patwardhan AM, Berg KA, Akopain AN, Jeske NA, Gamper N, Clarke WP, Hargreaves KM. Bradykinin-induced functional competence and trafficking of the delta-opioid receptor in trigeminal nociceptors. J Neurosci. 2005;25(39):8825–8832. doi: 10.1523/JNEUROSCI.0160-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipp M, Brede M, Hein L. Physiological significance of alpha(2)-adrenergic receptor subtype diversity: one receptor is not enough. Am J Physiol Regul Integr Comp Physiol. 2002;283(2):R287–295. doi: 10.1152/ajpregu.00123.2002. [DOI] [PubMed] [Google Scholar]
- Rios C, Gomes I, Devi LA. Interactions between delta opioid receptors and alpha-adrenoceptors. Clin Exp Pharmacol Physiol. 2004;31(11):833–836. doi: 10.1111/j.1440-1681.2004.04076.x. [DOI] [PubMed] [Google Scholar]
- Roerig SC, Lei S, Kitto K, Hylden JK, Wilcox GL. Spinal interactions between opioid and noradrenergic agonists in mice: multiplicativity involves delta and alpha-2 receptors. J Pharmacol Exp Ther. 1992;262(1):365–374. [PubMed] [Google Scholar]
- Shi TJS, Winzer-Serhan U, Leslie F, Hokfelt T. Distribution of alpha(2)-adrenoceptor mRNAs in the rat lumbar spinal cord in normal and axotomized rats. Neuroreport. 1999;10(13):2835–2839. doi: 10.1097/00001756-199909090-00025. [DOI] [PubMed] [Google Scholar]
- Sonohata M, Furue H, Katafuchi T, Yasaka T, Doi A, Kumamoto E, Yoshimura M. Actions of noradrenaline on substantia gelatinosa neurones in the rat spinal cord revealed by in vivo patch recording. J Physiol. 2004;555(Pt 2):515–526. doi: 10.1113/jphysiol.2003.054932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens CW, Monasky MS, Yaksh TL. Spinal infusion of opiate and alpha-2 agonists in rats: tolerance and cross-tolerance studies. J Pharmacol Exp Ther. 1988;244:63–70. [PubMed] [Google Scholar]
- Stone LS, Broberger C, Vulchanova L, Wilcox GL, Hokfelt T, Riedl MS, Elde R. Differential distribution of alpha2A and alpha2C adrenergic receptor immunoreactivity in the rat spinal cord. J Neurosci. 1998;18(15):5928–5937. doi: 10.1523/JNEUROSCI.18-15-05928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone LS, MacMillan LB, Kitto KF, Limbird LE, Wilcox GL. The alpha2a adrenergic receptor subtype mediates spinal analgesia evoked by alpha2 agonists and is necessary for spinal adrenergic-opioid synergy. J Neurosci. 1997;17(18):7157–7165. doi: 10.1523/JNEUROSCI.17-18-07157.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AF, Dashwood MR, Dickenson AH. Alpha 2-adrenoceptor modulation of nociception in rat spinal cord: location, effects and interactions with morphine. Eur J Pharmacol. 1987;138(2):169–177. doi: 10.1016/0014-2999(87)90430-4. [DOI] [PubMed] [Google Scholar]
- Sullivan AF, Kalso EA, McQuay HJ, Dickenson AH. Evidence for the involvement of the mu but not delta opioid receptor subtype in the synergistic interaction between opioid and alpha 2 adrenergic antinociception in the rat spinal cord. Neurosci Lett. 1992;139(1):65–68. doi: 10.1016/0304-3940(92)90859-6. [DOI] [PubMed] [Google Scholar]
- Takano M, Takano Y, Yaksh TL. Release of calcitonin gene-related peptide (CGRP), substance P (SP), and vasoactive intestinal polypeptide (VIP) from rat spinal cord: modulation by alpha 2 agonists. Peptides. 1993;14(2):371–378. doi: 10.1016/0196-9781(93)90055-l. [DOI] [PubMed] [Google Scholar]
- Vilardaga JP, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ. Conformational cross-talk between alpha2A-adrenergic and mu-opioid receptors controls cell signaling. Nature chemical biology. 2008;4(2):126–131. doi: 10.1038/nchembio.64. [DOI] [PubMed] [Google Scholar]
- Walker SM, Goudas LC, Cousins MJ, Carr DB. Combination spinal analgesic chemotherapy: a systematic review. Anesth Analg. 2002;95(3):674–715. doi: 10.1097/00000539-200209000-00033. [DOI] [PubMed] [Google Scholar]
- Wessendorf MW, Elde RP. Characterization of an immunofluorescence technique for the demonstration of coexisting neurotransmitters within nerve fibers and terminals. J Histochem Cytochem. 1985;33(10):984–994. doi: 10.1177/33.10.2413102. [DOI] [PubMed] [Google Scholar]
- Wilcox GL, Carlsson KH, Jochim A, Jurna I. Mutual potentiation of antinociceptive effects of morphine and clonidine on motor and sensory responses in rat spinal cord. Brain Res. 1987;405(1):84–93. doi: 10.1016/0006-8993(87)90992-9. [DOI] [PubMed] [Google Scholar]
- Yaksh TL, Jessell TM, Gamse R, Mudge AW, Leeman SE. Intrathecal morphine inhibits substance P release from mammalian spinal cord in vivo. Nature. 1980;286:155–157. doi: 10.1038/286155a0. [DOI] [PubMed] [Google Scholar]
- Zachariou V, Goldstein BD. Delta-Opioid receptor modulation of the release of substance P-like immunoreactivity in the dorsal horn of the rat following mechanical or thermal noxious stimulation. Brain Research. 1996;736(1-2):305–314. doi: 10.1016/0006-8993(96)00718-4. [DOI] [PubMed] [Google Scholar]
- Zhang X, Bao L, Arvidsson U, Elde R, Hokfelt T. Localization and regulation of the delta-opioid receptor in dorsal root ganglia and spinal cord of the rat and monkey: evidence for association with the membrane of large dense-core vesicles. Neuroscience. 1998;82(4):1225–1242. doi: 10.1016/s0306-4522(97)00341-2. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Limbird LE. Hetero-oligomers of alpha2A-adrenergic and mu-opioid receptors do not lead to transactivation of G-proteins or altered endocytosis profiles. Biochem Soc Trans. 2004;32(Pt 5):856–860. doi: 10.1042/BST0320856. [DOI] [PubMed] [Google Scholar]
- Zhu Y, King MA, Schuller AG, Nitsche JF, Reidl M, Elde RP, Unterwald E, Pasternak GW, Pintar JE. Retention of supraspinal delta-like analgesia and loss of morphine tolerance in delta opioid receptor knockout mice. Neuron. 1999;24(1):243–252. doi: 10.1016/s0896-6273(00)80836-3. [DOI] [PubMed] [Google Scholar]