Summary
The basic helix-loop-helix (bHLH) transcription factor Hand2 has been implicated in the development of multiple organs, including craniofacial organs. Mice carrying Hand2 hypomorphic alleles (Hand2LoxP/−) display a cleft palate phenotype. A specific deletion of the Hand2 branchial arch-specific enhancer also leads to a hypoplastic mandible and cleft palate formation in mice. However, the underlying mechanism of Hand2 regulation of palate development remains unknown. Here we show that Hand2 is expressed in both the epithelium and mesenchyme of the developing palate. While mesenchymal specific inactivation of Hand2 has no impact on palate development, epithelial specific deletion of Hand2 creates a cleft palate phenotype. Hand2 appears to exert distinct roles in the anterior and posterior palate. In the anterior palate of Hand2LoxP/− mice, premature death of periderm cells and a down-regulation of Shh are observed in the medial edge epithelium (MEE), accompanied by a decreased level of cell proliferation in the palatal mesenchyme. In the posterior palate, a lower dose of Hand2 causes aberrant periderm cell death on the surface of the epithelium, triggering abnormal fusion between the palatal shelf and mandible and preventing palatal shelf elevation. We further demonstrate that BMP activities are essential for the expression of Hand2 in the palate. We conclude that Hand2 is an intrinsic regulator in the epithelium and is required for palate development.
Keywords: Hand2, cleft palate, hypomorphic, craniofacial development
Introduction
Among the most frequent congenital disorders is a cleft palate. Palate development is a multistep morphogenetic process and is controlled by a highly coordinated genetic network. It forms by union of the primary palate of the frontonasal process and a pair of the secondary palatal shelves. In mammals, the secondary palatal shelves first arise from the paired maxillary process and grow vertically along the developing tongue. A rapid elevation positions the palatal shelves horizontally above the tongue. Following fusion of the palatal shelves and disappearance of the midline seam, an intact palatal shelf finally forms separating the nasal cavity from the oral cavity (Ferguson, 1988). A cleft palate can result from intrinsic or extrinsic disruptions at any of these steps. Intrinsic defects of palate development include palatal shelves outgrowth deficiency, failure in elevation, and persistence of a midline seam. For example, in Shox2−/− or Msx1−/− embryos, outgrowth defects result in shortened palatal shelves that fail to make contact at the midline (Zhang et al., 2002; Yu et al., 2005; Gu et al., 2008). However, in mice lacking Fgf10, Jagged2, or Pax9, the palatal shelves fail to elevate due to abnormal palatal shelf-mandible/tongue fusion or internal force deficiency (Jiang et al., 1998; Peters et al., 1998; Rice et al., 2004; Alappat et al., 2005; Casey et al., 2006). The persistence of the midline seam also represents a cause of cleft palate formation, such as in Tgfβ3 mutants (Kaartinen et al., 1995; Proetzel et al., 1995; Taya et al., 1999). Malformation of other craniofacial structures could also cause cleft palate formation. In mice lacking Ryk, the deformed tongue physically obstructs the palatal shelves elevation leading to cleft palate formation (Halford et al., 2000).
Hand genes encode proteins belonging to the basic helix-loop-helix (bHLH) family of transcription factors (Massari and Murre, 2000). Hand2 is expressed in a variety of cell types during embryogenesis and has been implicated in development of a number of tissues and organs including the peripheral nervous system, limb bud, heart and pharyngeal arches (Srivastava et al., 1995; 1997; Charite et al., 2000; Morikawa et al. 2005; 2007; Barbosa et al., 2007; D’Autreaux et al., 2007). For example, in the developing heart, Hand2 is expressed in the right ventricle, and right ventricle apoptosis is seen in mice lacking Hand2 (Srivastava et al., 1997). During limb development, Hand2 is expressed in the posterior limb mesenchyme, overlapping with the Shh expression domain. Altered Hand2 activity leads to limb bud defects accompanied by changes in Shh expression, indicating that Hand2 acts as an upstream regulator of Shh (Charite et al., 2000; Fernandez-Teran et al., 2000; McFadden et al., 2002). During craniofacial development, Hand2 is expressed in the cranial neural crest (CNC) derived mesenchyme of the first and second branchial arches (Clouthier et al., 2000). Mice carrying a specific deletion of the Hand2 branchial arch-specific enhancer exhibit severe mandibular hypoplasia, which is thought to be causative of a cleft palate defect (Yanagisawa et al., 2003). We have recently reported that mice carrying a Hand2-null allele and a floxed Hand2 allele (Hand2LoxP/−) exhibit complete cleft of the secondary palate with a 100% penetrance, despite normal mandible formation, suggesting a direct involvement of Hand2 in the regulation of palate development (Morikawa et al., 2007).
In the present work, we analyzed the cellular and molecular etiology of the cleft palate in Hand2LoxP/− mice, and investigated its tissue specific requirement during palatogenesis. We demonstrate that Hand2 activity is required in the palatal epithelium where it controls cell proliferation via Shh and Bmp2 in the anterior palate. Intriguingly, in the posterior palate, the Hand2 hypomorphic condition leads to abnormal fusion of the palatal shelf and mandible. Hand2 therefore regulates palate development through two distinct mechanisms.
Materials and Methods
Animals
The generation and genotyping of Hand2 floxed, Hand2+/−, and Osr2-Cre animals have been described previously (Srivastava et al., 1997; Lan et al., 2007; Morikawa et al., 2007). Wnt1-Cre transgenic mice (Danielian et al., 1998) and the R26R conditional reporter line (Soriano, 1999) were obtained from Jackson Laboratories (Bar Harbor, ME). To create Pitx2-Cre transgenic animals, the 1.3-kb mouse Pitx2 P1 promoter and the 17-kb first branchial arch enhancer fragments (17-P1-ΔASE) (kindly provided by Dr. Hiroshi Hamada of Osaka University, Japan; Shiratori et al., 2001) were linked with the Cre gene. The transgenic construct (Pitx2-Cre) was released from the vector, gel-purified, and suspended in injection buffer, as described previously (Zhang et al., 2000). To generate pMes-Nog transgenic mice, the coding sequence of the mouse Noggin gene was cloned into pMES-IRES-Egfp vector in front of the IRES-Egfp sequence under the control of the chick β-actin promoter. A STOP cassette flanked by LoxP sequences was inserted between the β-actin promoter and the Noggin sequence. The transgenic construct is named pMes-Nog. Pronuclear injection and embryo transfer were performed according to a published protocol (Hogan et al., 1994). The integration of Pitx2-Cre or pMes-Nog transgene was determined by polymerase chain reaction (PCR) using genomic DNA extracted from the tail tip of founders. The specificity of the Pitx2 promoter/enhancer was determined by LacZ activity in mice carrying the Pitx2-Cre transgene and the R26R conditional reporter allele (Chai et al., 2000). Embryos were collected from timed pregnant mice and fixed in 4% paraformaldehyde (PFA) in PBS at 4°C for overnight. All the genetically engineered mice used in this study were maintained in B6/C57 background. All animal studies were approved by the Tulane University Institutional Animal Care and Use Committee.
Cell proliferation and TUNEL assays
Cell proliferation rate was monitored by BrdU incorpotation. BrdU was injected into the peritoneal of timed pregnant mice for one hour at a dose of 1.5 ml of labeling reagent/100 g body weight using the BrdU labeling and Detection Kit II from Roche. Samples were fixed in Carnoy’s fixative, ethanol-dehydrated, paraffin-embedded, and sectioned at 5-μm. The sections were subjected to immunodetection according to the manufacturer’s protocol. Three individual embryos of both wild type and mutant were subjected for cell proliferation assay, and three adjacent sections from each sample were counted for BrdU labeling. BrdU-labeled cells were counted and presented as percentage of total nuclei within arbitrarily defined areas, and Student’s t-test was used to determine if significance of differences and P values were present in the mutant samples and wild type controls. The TUNEL assay was performed to detect cell apoptosis in the developing palate in paraffin sections as described previously (Alappat et al., 2005).
In vitro palatal shelf culture
Palatal shelf culture was carried out in Trowell type organ cultures with chemically defined medium, as described previously (Taya et al., 1999; Zhang et al., 2002). Briefly, paired secondary palatal shelves were isolated from individual E13.5 embryos obtained from the mating between Hand2LoxP/LoxP and Hand2+/− mice, and placed in contact with the MEE of each palatal shelf facing the other in each organ culture. Body tissues from each embryo were subsequently subjected to genotyping. Samples were cultured for 3 days prior to being harvested.
Histology, in situ hybridization, and immunohistochemical staining
For histological analysis and in situ hybridization assays, samples fixed in 4% paraformaldehyde (PFA) at 4°C for overnight, dehydrated through a graded ethanol series, and then processed for paraffin sectioning. Serial sections were made at 10-μm and subjected to standard Hematoxylin/Eosin staining or to section in situ hybridization using non-radioactive riboprobes, as described previously (St. Amand et al., 2000). For immunohistochemical staining, samples were fixed in 4% PFA, washed with 30% sucrose/PBS and embedded in O.C.T. compound (Tissue-Tek), and cryo-sectioned. Sections were washed in PBS (containing 0.1% Triton-X-100) and blocked with 10% horse serum/0.1% BSA/0.1% Triton-X-100 in PBS for 1 hour prior to being incubated with Anti-Hand2 antibody (R&D systems) at 4°C overnight. For negative controls, the primary antibody was omitted. Biotinylated Anti-Goat IgG (H+L) (Vector Laboratories) was used as secondary antibody. After extensive washing in PBS, samples were stained with DAPI (Invitrogen), mounted with VECTASHIELD Fluorescent Mounting Media (Vector Laboratories), and the results were examined under a fluorescent microscope and digitally recorded.
Real-time PCR
Paired palatal shelves isolated from individual E13.5 wild type, Hand2LoxP/+, and Hand2LoxP/− embryos were subjected to RNA extraction with RNase Mini Kit (Qiagene) and reversely transcribed using SuperScript III First Strand Synthesis System (Invitrogen). Hand2 transcripts were quantified by real-time PCR using the primers (upper: 5′-TACCAGCTACATCGCCTACCT-3′; and lower: 5′-CAGCTTCACTCCAGGCCACT-3′). Gapdh was used as a reference control. Real-time PCR was performed using the iCycleriQ™ Real-time Detection System (Bio-Rad).
Results
Hypomorphic Hand2 mice exhibit complete cleft of secondary palate
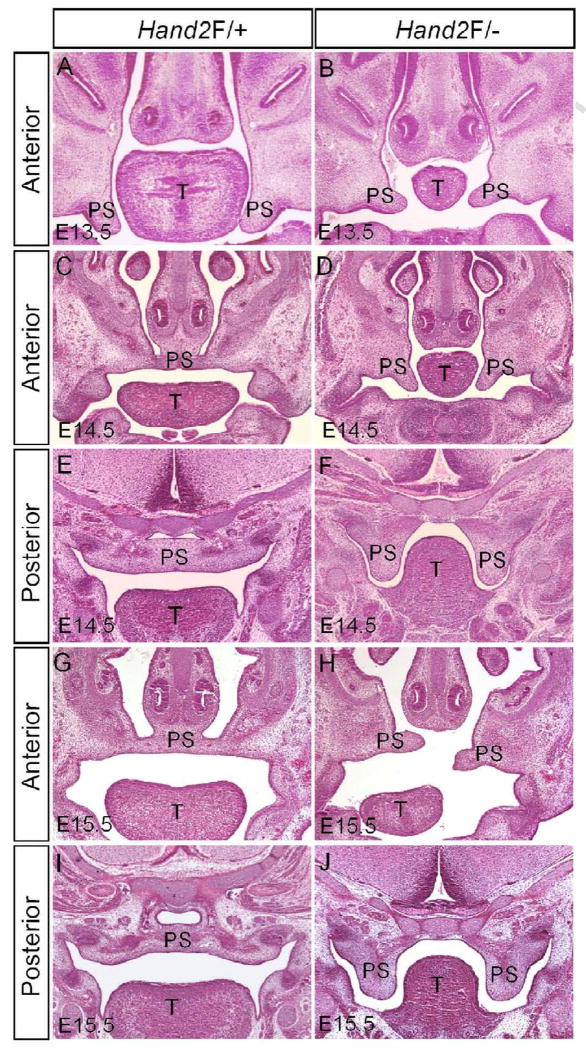
Previous studies have shown that Hand2 is involved in craniofacial development including palatogenesis, intrinsically or extrinsically (Ruest et al., 2003; Yanagisawa et al., 2003; Barbosa et al., 2007). We previously reported that mice bearing one Hand2 null and one hypomorphic (Hand2LoxP/−) alleles display a complete cleft secondary palate with 100% penetrance (Morikawa et al., 2007). To establish a role for Hand2 in palate development, we began with a histological analysis of palatal defects in Hand2LoxP/− mice. Morphologically, the developing palatal shelves from E11.5 and E12.5 and2LoxP/− embryos were indiscernible from the wild type controls (data not shown). At E13.5 when the normal palatal shelves take a vertical position on both sides of the tongue, the mutant palatal shelves assumed the similar position but appeared slightly smaller as compared to the littermate controls (Fig. 1A, 1B). A pronounced aberration in the mutant palate was observed at E14.5 when the wild type palatal shelves have elevated to above the tongue and begun to fuse at the midline (Fig. 1C–F). In Hand2LoxP/− embryos, the palatal shelves failed to elevate and remained in a vertical position on the sides of the hypoplastic tongue (Fig. 1D, 1F). At E15.5, the mutant palate displayed distinct developmental defects along the anterior-posterior (A–P) axis. Though delayed, the anterior palatal shelves elevated to the horizontal level, but appeared short and misaligned (Fig. 1H). However, the posterior portion of the palate did not elevate (Fig. 1J). These observations suggest that Hand2 may exert distinct roles along the A–P axis of the developing palate during palatogenesis.
Figure 1.
Hand2LoxP/− mice exhibit hindrance of palate shelf elevation. (A) A section of an E13.5 wild type embryo shows normal developing palatal shelves. (B) The palatal shelves of an E13.5 Hand2LoxP/− embryo appear relatively small. (C–F) At E14.5, palatal shelves in the wild type control have already elevated to the position above the tongue and fused at the midline (C, E). However, in Hand2LoxP/− mice, the palatal shelves remain in a vertical position (D, F). (G–J) Wild type controls at E15.5 are shown in (G) and (I). In the mutant, the anterior portion of the palatal shelves elevates to the horizontal position above the tongue, but exhibits a misalignment (H), while the posterior portion remains in the vertical position (J). T, tongue; PS, palatal shelf.
Hand2 is expressed in the developing palate
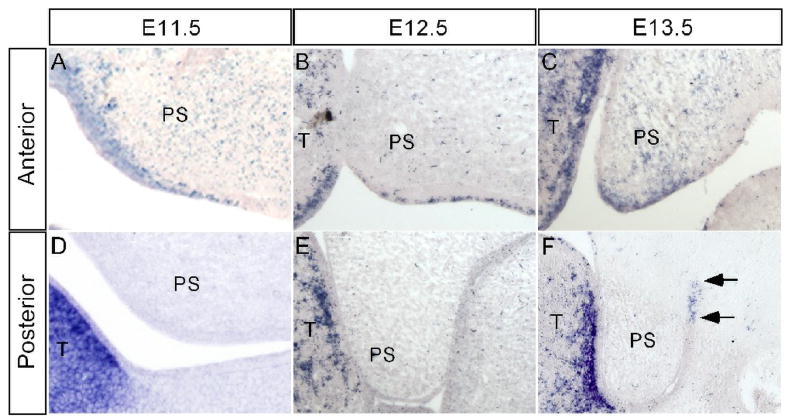
Hand2 is expressed in the neural crest derived mesenchyme of first and second pharyngeal arches (Yanagisawa et al., 2003), however, its expression in the developing palatal shelf has not been examined. The cleft palate defect seen in mice lacking Hand2 expression in branchial arches was attributed to the formation of a severely hypoplastic mandible (Yanagisawa et al., 2003). To differentiate if Hand2 acts intrinsically or extrinsically to regulate palate development, we examined Hand2 expression in the developing palatal shelf from E11.5 to E13.5 by in situ hybridization. Our results showed that Hand2 is expressed in the developing palatal shelves from E11.5 through E13.5, exhibiting differential expression in the anterior and posterior palate. In the anterior palate, Hand2 expression was detected in the epithelium as well as in the mesenchyme in a punctuate pattern (Fig. 2A–C). At E13.5, the expression in the palatal epithelium is mainly restricted in the medial edge epithelium (MEE) (Fig. 2C). The expression in the MEE was not detectable at E14.0 when the palatal shelves elevate to the dorsum of the tongue (data not shown). In contrast, in the posterior palate, we did not detect Hand2 expression at E11.5 and E12.5 (Fig. 2D, 2E). However, at E13.5 a restricted expression domain was observed in the epithelial cells of the lateral junction of the palatal shelf and mandible (Fig. 2F). These observations strongly suggest that Hand2 plays a direct role in regulating palate development.
Figure 2.
Expression of Hand2 in the early developing palatal shelf. (A–C) In the anterior palatal shelf, Hand2 is expressed in the epithelium including MEE and mesenchyme at E11.5 (A), E12.5 (B) and E13.5 (C). (D–F) In the posterior palatal shelf, Hand2 expression is not detectable at E11.5 (D) and E12.5 (E). However, at E13.5, Hand2 transcripts (arrows) are specifically detected in the epithelium of the lateral junction of the palatal shelf and mandible (F). T, tongue; PS, palatal shelf.
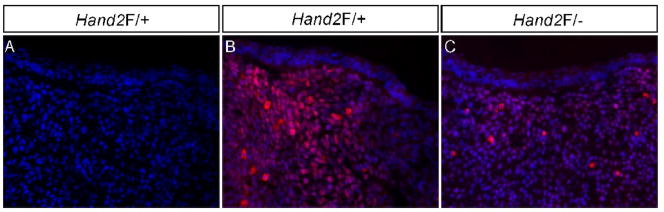
We previously demonstrated that the LoxP site insertion does not alter Hand2 expression pattern in developing embryos including the craniofacial region but creates a hypomorphic allele (Morikawa et al., 2007). To confirm that the insertion of LoxP sites reduces Hand2 expression in the developing palate, we performed quantitative RT-PCR to determine Hand2 expression levels in the palatal shelves of E13.5 wild type, Hand2LoxP/+, and Hand2LoxP/− embryos. Hand2 expression levels were reduced by 16% (±6%) in the Hand2LoxP/+ palatal shelves, and 66% (±6%) in the Hand2LoxP/− palates, as compared to wild type controls (data not shown). To further confirm a reduced Hand2 protein level in Hand2LoxP/− embryo, we performed immunohistochemical studies. We examined E13.5 mandible due to the known high expression of Hand2 (Yanagisawa et al., 2003). The immunostaining results demonstrate a significant reduction of Hand2 protein in Hand2LoxP/− mandible as compared to that in Hand2Loxp/+ sample (Fig. 3).
Figure 3.
Reduction of Hand2 protein in Hand2LoxP/− mandible. (A) A negative control for immunohistochemical staining. (B) Immunohistochemical staining shows abundant Hand2-positve cells in an E13.5 Hand2F/+ mandible. (C) An E13.5 Hand2F/− mandible exhibits significantly reduced immunostaining signals for Hand2 protein in the mesenchymal cells.
Hand2 function is required in the palatal epithelium
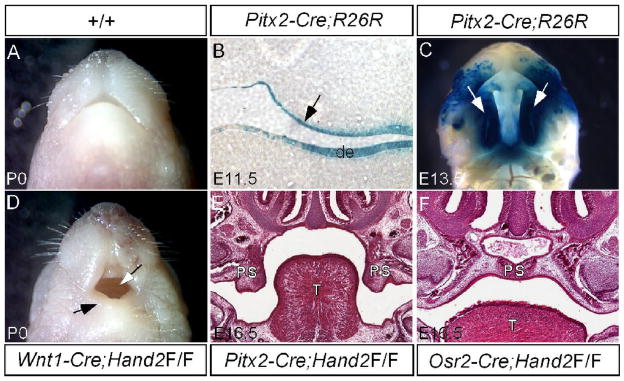
Since Hand2 is expressed in both the epithelial and mesenchymal components of the developing palate, we determined the tissue requirement for Hand2 during palate development. We took a tissue-specific gene deletion approach to inactivate Hand2. The palatal mesenchyme derives from CNC cells (Ito et al., 2003). We inactivated Hand2 in the CNC cell-derived palatal mesenchyme using the Wnt1-Cre transgenic line. Although a hypoplastic mandible was observed in Wnt1-Cre;Hand2LoxP/LoxP mice, a cleft palate defect was not found (Fig. 4D). To confirm this observation, we used another Cre deleter mouse line, Osr2-Cre, which expresses Cre in the palatal mesenchyme from the beginning of palatogenesis (Lan et al., 2007). Mice carrying Hand2LoxP/LoxP and Osr2-Cre transgenic allele had a normal palate (Fig. 4F). We therefore conclude that Hand2 expression in the palatal mesenchyme is dispensable for palate development. To determine the requirement of Hand2 in the palatal epithelium, we generated a Pitx2-Cre transgenic line using the 1.3-kb mouse Pitx2 P1 promoter and the 17-kb first branchial arch enhancer fragments (Shiratori et al., 2001) to drive Cre expression. Pitx2-Cre mice exhibit Cre activities in the palatal epithelium and dental epithelium at E11.5 and entire palatal epithelium at E13.5 (Fig. 4B, 4C). Upon inactivation of Hand2 in the palatal epithelium, a wide open cleft palate defect was observed in Pitx2-Cre;Hand2LoxP/LoxP mice (Fig. 4E). It has been previously reported that Cre activity alone could cause dramatic developmental defects in certain transgenic lines, including up-regulated apoptosis in many embryonic tissues (Naiche and Papaioannou, 2007). None of the Cre expressing lines used in this study showed a cleft palate defect. We examined if Cre activity alone would cause any alternations in cell proliferation, apoptosis, and gene expression in the developing palate. In the mesenchyme of E13.5 Wnt1-Cre palatal shelves, we did not observe changes in cell proliferation or the gene expression changes including Bmp2, Fgf10, and Sox9 (data not shown). We also did not observe aberrant apoptosis in the epithelium of E13.5 Pitx2-Cre palate (data not shown). We thus conclude that Cre activity does not exert detrimental effects to the developing palate and Hand2 is an essential factor required in the epithelial component to regulate palate development.
Figure 4.
Epithelial specific inactivation of Hand2 leads to cleft palate formation. (A) A newborn wild type mouse shows a normal mandible. (B) A section through oral cavity of an E11.5 Pitx2-Cre;R26R embryo shows specific LacZ staining in the palatal epithelium (arrow) and dental epithelium (de). (C) Whole mount LacZ staining of an E13.5 Pitx2-Cre;R26R embryo demonstrates Cre activity in the entire palatal shelf (arrows). (D) A newborn Wnt1-Cre;Hand2LoxP/LoxP mouse (labeled as Wnt1-Cre;Hand2F/F) exhibits a hypoplastic mandible (black arrow) and an intact palate (white arrow). (E) A section through oral cavity of an E16.5 Pitx2-Cre;Hand2LoxP/LoxP embryo (labeled as Pitx2-Cre;Hand2F/F) shows cleft palate defect. Note the palatal shelves remain in a vertical position. (F) A section through oral cavity of an E16.5 Osr2-Cre;Hand2LoxP/LoxP embryo (labeled as Osr2-Cre;Hand2F/F) shows a normal formed palate. T, tongue; de, dental epithelium; PS, palatal shelf.
Hand2 functions through Shh signaling to regulate palate development
The fact that Hand2 is expressed and required in the palatal epithelium prompted us to test if the reduced Hand2 expression in the Hand2LoxP/− palatal shelves could cause a failure in palate fusion. We placed paired palatal shelves isolated from E13.5 Hand2LoxP/+ and Hand2LoxP/− embryos in an in vitro organ culture with the MEE of the shelves in contact. Histological analyses of cultured samples demonstrated successful fusion of palatal shelves from either Hand2LoxP/+ (10/12) or Hand2LoxP/− (10/13) mice, as determined by the disappearance of the midline seam and the establishment of the mesenchymal continuity (data not shown). We therefore rule out the possibility that failure of fusion contributes to cleft palate defect in Hand2LoxP/− mice.
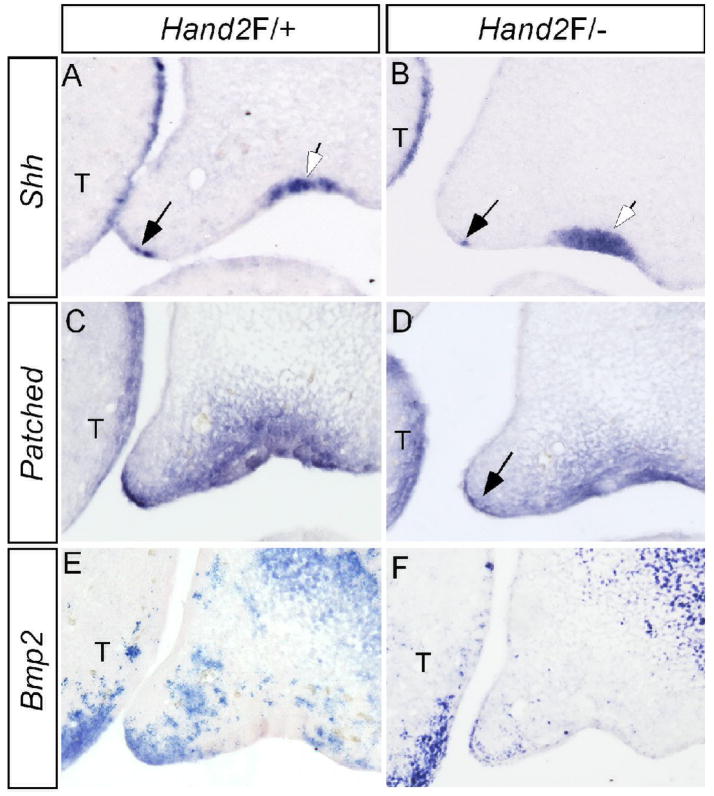
Shh signaling has been implicated in palatogenesis (Zhang et al., 2002; Rice et al., 2004; Gritli-Linde, 2007). In the anterior portion of developing palate, Shh is expressed in the MEE, overlapping with Hand2 and acts to regulate palatal growth via Bmp2 (Zhang et al., 2002). Since Hand2 is both sufficient and necessary for Shh expression in the developing limb (Charite et al., 2000; Fernandez-Teran et al., 2000; McFadden et al., 2002), we determined if Hand2 also functions through the Shh signaling pathway to regulate palate development. Our in situ hybridization studies revealed a down-regulation, but not complete elimination, of Shh expression in the MEE of the anterior palate in Hand2LoxP/− mice (Fig. 5A, 5B). Interestingly, Shh expression in the future regal epithelium was not altered in the mutant embryos, indicating a specific regulation of Shh by Hand2 in the MEE. Consistent with the down-regulation of Shh in the MEE, the Shh downstream targets Ptc and Bmp2 also exhibited attenuated expression in the palatal mesenchyme adjacent to the MEE (Fig. 5C–F).
Figure 5.
Down-regulation of Shh and its downstream genes in the palatal shelf of Hand2 hypomorphic mice. (A) Expression of Shh is detected in the MEE (black arrow) and the rugal epithelium (white arrow) of an E13.5 control palate. (B) Shh expression is significantly down-regulated in the MEE (black arrow), but remains unaffected in the rugal epithelium (white arrow) of an E13.5 Hand2LoxP/− embryo. (C, E) Expression of Ptc (C) and Bmp2 (E) in E13.5 control palatal shelves. (D) Ptc expression is down-regulated in the palatal shelf of an E13.5 Hand2LoxP/− embryo. Note specific gene down-regulation in the palatal mesenchyme adjacent to the MEE (arrow). (F) Bmp2 expression is reduced in the palatal shelf of an E13.5 Hand2LoxP/− embryo. Note the expression in the maxillary mesenchyme and the tongue is not affected. T, tongue
The expression of Shh in the MEE of developing palatal shelves is known to be controlled by palatal mesenchyme expressed Bmp4 and Fgf10 (Zhang et al., 2002; Rice et al., 2004). To ensure that the down-regulation of Shh in the MEE of Hand2Loxp/− palate is a direct consequence of reduced Hand2 activity in the MEE, we examined the expression of Bmp4 and Fgf10 in E13.5 Hand2Loxp/− palatal shelves. The expression of these two genes is unaltered in the Hand2LoxP/− palate (data not shown), indicating that Hand2 functions as an upstream regulator of Shh in the MEE of developing palatal shelves.
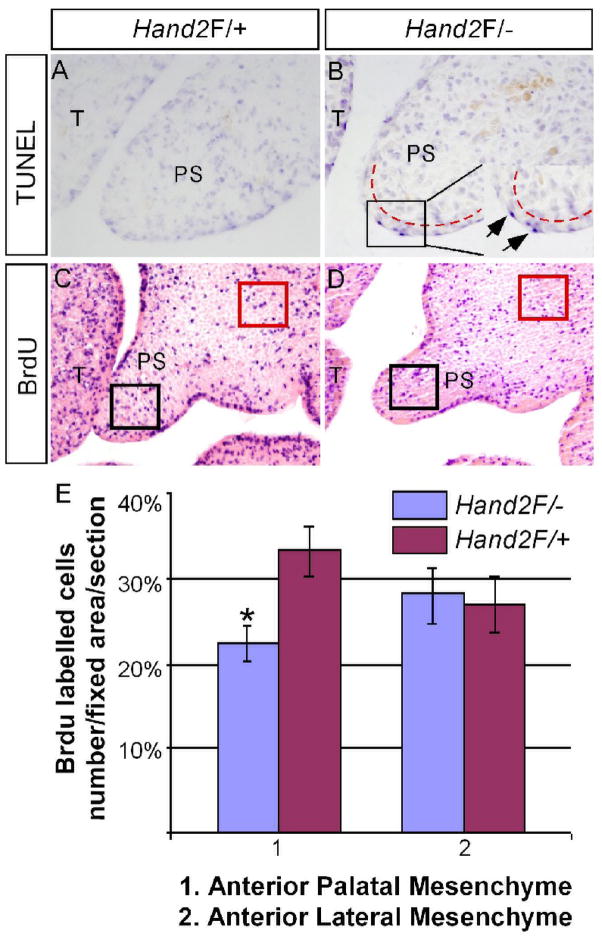
Shh signaling has been demonstrated to regulate cell proliferation in the palatal mesenchyme via Bmp2 (Zhang et al., 2002). Consistent with a down-regulation of Shh and Bmp2, cell proliferation level in the palatal mesenchyme of E13.5 Hand2LoxP/− mice was significantly reduced as compared to the wild type controls (Fig. 6C–E, P<0.01). However, cell proliferation rate was not changed in the palate of E12.5 Hand2LoxP/− embryo, consistent with an unaltered morphology at this developmental stage (data not shown). Since programmed cell death is thought to attribute to the cellular defects in the developing heart and branchial arch of Hand2 null embryos, we examined the levels of apoptosis using TUNEL assays. Excess apoptotic cells in the MEE were found in the Hand2LoxP/− palate (Fig. 6A and B). A close examination revealed that apoptosis was restricted to the periderm cells, the surface layer of the epithelium, but not in the basal layer epithelial cells (Fig. 6B). Thus in the anterior palate, altered cell proliferation and apoptosis represent major cellular defects contributing to cleft palate formation in Hand2LoxP/− mice.
Figure 6.
Aberrant cell proliferation and apoptosis in the palatal shelf of Hand2 hypomorphic mice. (A, B) TUNEL assay on the anterior portion of palate from E13.5 control (A) and Hand2LoxP/− (B) embryos shows abnormal apoptotic cells (arrows in the insert of panel B) in the MEE of the mutant. Note the apoptotic cells are periderm cells. The red dash lines demarcate the boundary of epithelium and mesenchyme. (C, D) BrdU labeling assays show a reduced level of cell proliferation in the anterior palate of E13.5 Hand2LoxP/− embryo (D), as compared to the control (C). (E) Comparison of BrdU-labeled cells in fixed areas of palate and lateral maxillary mesenchyme in controls and Hand2LoxP/− embryo. Standard deviation values were indicated as the error bars. *: P < 0.01. T, tongue; PS, palatal shelf.
Abnormal fusion between lateral palatal shelf and mandible leads to failed elevation of the posterior palate inHand2 LoxP/− mice
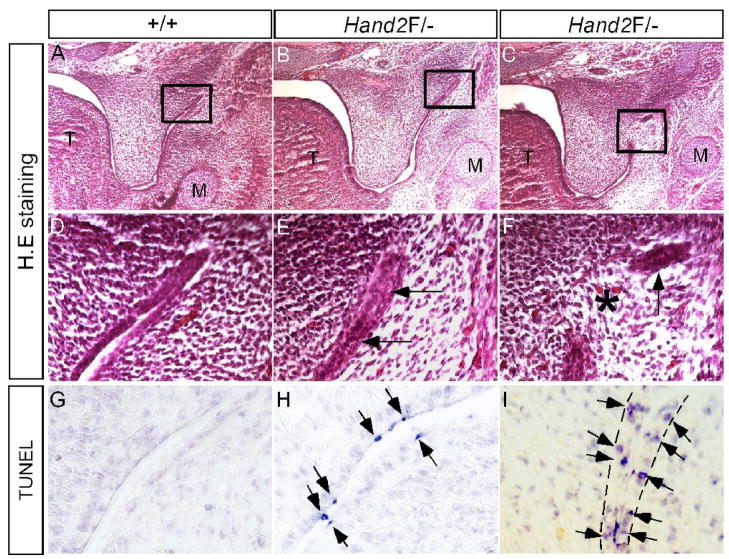
In Hand2LoxP/− mice, the anterior palate was delayed in elevation, but the posterior palate never elevated (Fig. 1). These observations indicate distinct roles for Hand2 in regulating palatogenesis along the A–P axis. Since Hand2 expression is restricted in the epithelial cells of the lateral junction of the palatal shelf and mandible at E13.5, a defect could occur in this specific region. An examination of serial histological sections revealed an abnormal adhesion/fusion of the posterior palate and mandible (Fig. 7A–F). The epithelia of the lateral junction of palatal shelf and mandible became adherent initially, followed by the disappearance of the adhesive epithelia, causing pathological fusion of the palatal shelf and mandible. We examined cell apoptosis in this specific fusion region in E13.5 embryo by TUNEL assay. As expected, at the lateral junction of the posterior palatal shelf and mandible of E13.5 Hand2LoxP/− embryos, apoptotic cells were found in the epithelia of the fusion region (Fig. 7G–I). Surprisingly, we identified a unique cell apoptotic pattern. Before the epithelial contact occurs in the lateral region, apoptosis was restricted to the periderm cells, similar to what was observed in the MEE of the anterior palate (Fig. 7H). However, when the epithelia made contact, apoptosis was observed in the basal layer epithelial cells (Fig. 7I), leading to a fusion between the palatal shelf and mandible. This aberrant fusion apparently results in hindrance of the palatal shelf elevation and thus contributes to the cleft palate formation in Hand2LoxP/− mice. Abnormal palate-mandible fusion has been reported in Jagged2−/− and Fgf10−/− mice (Jiang et al., 1998; Alappat et al., 2005; Casey et al., 2006). In Fgf10−/− palate, altered expression of Tgfβ3 and Jagged2 was thought to contribute to the aberrant fusion phenotype (Alappat et al., 2005). We thus asked if similar molecular defects could exist in Hand2LoxP/− palate. In situ hybridization studies revealed an unaltered expression of Tgfβ3 and Jagged2 in the epithelia of the lateral palate shelf and mandible junction in Hand2LoxP/− embryos (data not shown), suggesting involvement of a different genetic pathway.
Figure 7.
Pathological adhesion/fusion of the palatal shelf and mandible in Hand2 hypomorphic mice. (A, D, G) E13.5 wild type controls show normal histological structure (A, D) and absence of apoptotic cells in the lateral junction of the posterior palate and mandible. (B, E) An E13.5 Hand2LoxP/− embryo shows adherence of epithelia (arrows in E) of the lateral junction of the posterior palate and mandible. (C, F) An E13.5 Hand2LoxP/− embryo shows fusion of the palatal shelf and mandible at the lateral junction. Star denotes a confluence of mesenchyme, and arrow points to a remanent epithelium. (H) An E13.5 Hand2LoxP/− embryo shows apoptotic periderm cells (arrows) in the lateral junction right before epithelial contact/adherence. (I) An E13.5 Hand2LoxP/− embryo shows apoptotic basal layer epithelial cells (arrows) in the lateral junction fusion of the palatal shelf and mandible. The dash lines demarcate the epithelial boundary.
BMP activity is required for Hand2 expression in the developing palate
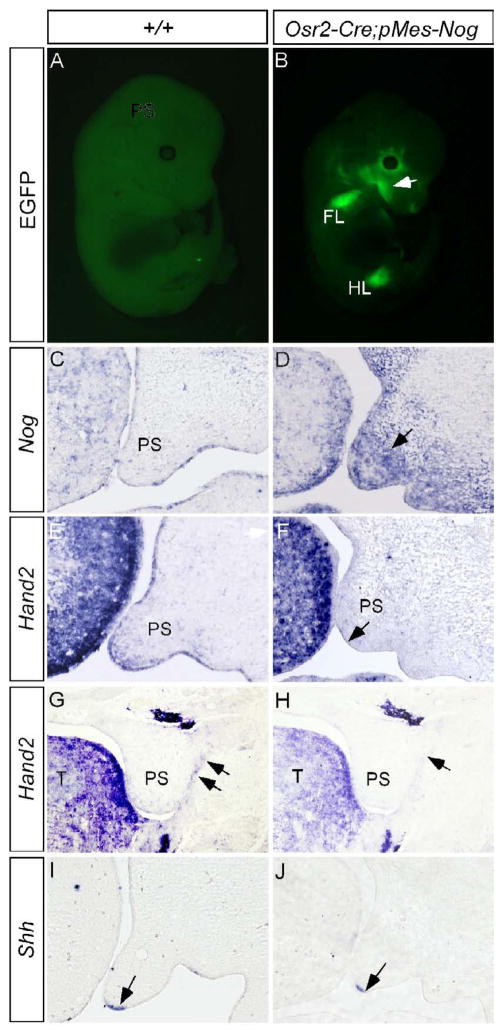
Our previous studies demonstrated a regulation of Shh in the MEE by Bmp4 in the anterior palate (Zhang et al., 2002). Since Hand2 regulates Shh in the MEE, we examined if Hand2 acts as a component in the Bmp4-Shh genetic pathway. To test this hypothesis, we took a loss-of-function approach by creating a conditional transgenic model which, upon crossing to a Cre line, expresses Noggin under the control of the chick β-actin promoter. The transgenic construct, named pMes-Nog, also contains an IRES-Egfp cassette following the Noggin transgene, to allow simultaneous expression of Egfp as a marker for the transgene expression. To ectopically express Noggin in the palatal mesenchyme, we generated binary transgenic embryos by compounding the pMes-Nog transgenic allele with Osr2-Cre allele. Egfp expression in the developing limbs and craniofacial region indicated successful activation and correct expression pattern of the transgene (Fig. 8A, 8B; Lan et al., 2007). In the developing palatal shelf, Noggin is not expressed in most regions of the palate with the exception of a small region in the anterior extremity (Fig. 8C, and unpublished data). In the palatal shelves of Osr2-Cre;pMes-Nog mice, abundant Noggin transcripts were detected (Fig. 8D). Overexpression of Noggin to the palatal mesenchyme caused retarded palatal growth (Fig. 8F) and eventually led to a complete clefting of the secondary palate (Xiong, W. and Chen, Y.P., unpublished observations). In Osr2-Cre;pMes-Nog mice, we observed a greatly reduced expression of Hand2 not only in the MEE but also in the mesenchyme of the anterior palate (Fig. 8E, 8F). Associated with this change in Hand2 expression is a down-regulation of Shh in the MEE (Fig. 8I, 8J). However, a down-regulation of Fgf10 expression in the Osr2-Cre;pMes-Nog palate may also contribute, at least partially, to this down-regulation of Shh in the MEE (data not shown). In addition, we also observed a repression of Hand2 expression in the epithelial cells of the lateral junction of the posterior palatal shelf and mandible (Fig. 8G, 8H). Consistent with this repressed Hand2 expression is an aberrant fusion of posterior palate and mandible, identical to that observed in Hand2LoxP/− embryo (Xiong, W. and Chen, Y.P., unpublished results). BMP activities appear necessary for Hand2 expression in the developing palate.
Figure 8.
Overexpression of Noggin to the palatal mesenchyme down-regulates the expression of Hand2 and Shh. (A) An E13.5 wild type control embryo shows undetectable fluorescent signals. (B) An E13.5 Osr2-Cre;pMes-Nog embryo shows EGFP signal in the craniofacial region (arrow) and forelimb FL) and hindlimb (HL). (C) An E13.5 wild type control shows barely detectable Noggin transcripts in the developing palatal shelf. Note Noggin expression in the tongue. (D) Strong Noggin expression (arrow) is detected in the palatal mesenchyme of an E13.5 Osr2-Cre;pMes-Nog embryo. (E, G, I) The expression of Hand2 in the anterior palate (E) and the epithelial junction of posterior palate (G) and Shh expression (I) in the MEE are detected in E13.5 controlled palatal shelves. (F, H, J) The expression of Hand2 is down-regulated in the anterior palate (F) and the epithelial junction of posterior palate (H) of E13.5 Osr2-Cre;pMes-Nog embryo. Shh expression is also down-regulated in the MEE of E13.5 Osr2-Cre;pMes-Nog palate (J). Note unaltered strong Hand2 expression in the tongue in (F). Arrows in (F, G, H) point to the MEE, and in (G, H) point to the epithelial junction of posterior palate and mandible.
Discussion
In this study we investigated the cellular and molecular etiology of cleft palate in Hand2 hypomorphic mice. Our results reveal an indispensable role for Hand2 during palate development. Down-regulation of this transcription factor results in a number of cellular and molecular defects, including reduced cell proliferation and an aberrant fusion of the palatal shelf with mandible, which contribute to the formation of a cleft palate.
Epithelial expressed Hand2 is essential component regulating palatogenesis
During embryonic development, Hand2 is expressed in the developing pharyngeal arch and other organs (Srivastava et al., 1995; 1997; Charite et al., 2000; Clouthier, 2000; Fernandez-Teran et al., 2000; Yelon et al., 2000; McFadden et al., 2002; Miller et al., 2003; Barbosa et al., 2007). While Hand2 has been shown to play a critical role primarily in the mesenchymal component of a number of organs during the development, our work now demonstrates a role for Hand2 in the epithelium component during organogenesis. A role for Hand2 in palatogenesis was suggested from the observations that Hand2 is strongly expressed in the mandibular mesenchyme and a specific deletion of the Hand2 branchial arch-specific enhancer leads to mandibular hypoplasia and a cleft palate defect (Yanagisawa et al., 2003). However, the cleft palate phenotype in the mutant mice was considered as a secondary defect to the malformed mandible. In addition, the relatively weak, restricted and dynamic expression of Hand2 in the developing palate may have been overlooked due to its strong expression in the tongue and mandible. In this report, we show unambiguous Hand2 expression in both epithelial and mesenchymal components of the developing palate. In addition, our real-time PCR results further confirm the expression of Hand2 the developing palatal shelves, and a reduction in Hand2 expression in the palatal shelves of Hand2 hipomorphic mice. By using a conditional inactivation approach, it is evident that mesenchymally expressed Hand2 is dispensable during palate development. One possibility is that Hand1, another bHLH family member related to Hand2, is also expressed in the neural crest-derived mesenchyme and may functions redundantly with Hand2 to regulate palate development. In contrast, specific deletion of Hand2 in the palatal epithelium phenocopies the cleft palate defect seen in Hand2 hypomorphic mice. Although we cannot exclude the possibility that mandibular defects may contribute to a cleft palate formation in certain circumstance, the fact that a cleft palate defect is not associated with mandibular hypoplasia in Wnt1-Cre;Hand2LoxP/LoxP mice further support a direct role for Hand2 in palate development.
Hand2 acts downstream of BMP signaling to regulate Shh expression in the MEE
In developing limbs, Hand2 has been shown to regulate Shh expression in the ZPA (Charite et al., 2000; Fernandez-Teran et al., 2000). Although the underlying regulatory mechanisms remain to be determined, our results demonstrate the existence of similar genetic regulatory hierarchy in the developing palate. In Hand2 hypomorphic mice, the level of Shh expression in the MEE is significantly reduced but not completely abolished, as are the Shh downstream targets Ptc and Bmp2. The unaltered expression of Bmp4 and Fgf10 in Hand2LoxP/− palate further supports a role for Hand2 in the regulation of Shh expression. It is possible that Hand2 does not regulate Shh expression directly in the MEE. The excess periderm cell death in the MEE of Hand2LoxP/− palate may impair the MEE leading to a down-regulation of Shh in the MEE. Arguing against this, expression of Jagged2 in the Hand2LoxP/− palatal epithelium including the MEE was not affected, suggesting a normal epithelial structure. Although Shh has been shown to stimulate proliferation in many vertebrate organs, its mitotic effect is mediated by Bmp2 in the developing palate (Zhang et al., 2002). Therefore, the down-regulation of Bmp2 expression accounts for the reduced cell proliferation rate in the palatal mesenchyme of Hand2 hypomorphic mice. This reduced cell proliferation rate causes retarded growth of the palate, and eventually contributes to, at least partially, the formation of cleft palate in Hand2 hypomorphic mice.
Shh expression in the MEE is controlled by multiple signaling pathways, including BMP and FGF signaling (Zhang et al., 2002; Rice et al., 2004; reviewed in Grtili-Linde, 2007). As a transcriptional factor, Hand2 acts upstream of Shh, possibly directly regulating its expression. As demonstrated in our transgenic model in which Noggin, an antagonist of BMPs, is ectopically expressed in the palatal mesenchyme, Hand2 expression is inhibited, as is Shh, in the anterior palatal shelf. Interestingly, Hand2 expression is repressed in both the palatal epithelium and mesenhcyme, suggesting a similar regulatory mechanism in both tissue components. In addition, overexpression of Noggin also inhibits Hand2 expression in the epithelial junction of the posterior palate and mandible, leading to an abnormal fusion of palate and mandible identical to that seen in Hand2 hypomorphic embryos (unpublished data). The down-regulation of Fgf10 in the Osr2-Cre;pMes-Nog palate also suggests that Fgf10 may act as an intermediate between BMP action and Hand2 expression. Nevertheless, our results demonstrate a requirement of BMP activities for Hand2 expression in the developing palate. This regulatory pathway appears to be common during organogenesis, operating in several other developing systems, such as the heart and sympathetic neurons (Howard et al., 2000; Schlange et al., 2000; Liu et al., 2004).
Hand2 regulates epithelial adhesion/fusion by controlling cell apoptosis
It is noteworthy that ectopic cell death is always associated with Hand2 deficiency (Srivastava et al., 1997; Thomas et al., 1998; Yamagishi et al., 2001). This is also true in the developing palate when Hand2 levels fall below a threshold. We observed aberrant cell apoptosis in the MEE of the anterior palate and the epithelia of the lateral junction of the palatal shelf and mandible of Hand2 hypomorphic mice. Most interestingly, we observed two distinct steps of cell death in the epithelial junction of the palatal shelf and mandible: apoptosis of periderm cells before epithelial contact, and death of basal layer epithelial cells after epithelial contact/adhesion to create mesenchyme confluence, leading to fusion of the palatal shelf with the mandible. During normal palate fusion process, the periderm cell are shed before contact of the palatal shelves (Fitchett and Hay, 1989). This removal of periderm cells is thought to be essential for normal palate fusion, as artificial removal of periderm cells leads to degradation of underlying basal layer cells in MEE, a key step of palate fusion, and inhibition of cell death results in persistence of the medial edge seam (Cuervo and Covarrubias, 2004). It appears that similar to palate fusion, removal of periderm cells from surface of the palatal epithelium and oral epithelium allows abnormal adherence of the basal layer epithelial cells, and subsequently fusion of the palatal shelf and mandible.
Among various causes of cleft palate is pathological palate-mandible or palate-tongue fusion (Gritli-Linde, 2007). Such aberrant fusions have been reported in mice carrying mutation in either Jagged2, Fgf10, or Irf6, causing cleft palate defect (Jiang et al., 1998; Rice et al., 2004; Alappat et al., 2005; Ingraham et al., 2006; Casey et al., 2006; Richardson et al., 2006). The delayed palate elevation in the anterior palate and failed elevation in the posterior palate of Hand2 hypomorphic mice further supports the notion that the abnormal palate-mandible fusion represents a major cause of cleft palate formation. It was previously shown that Jagged2 expression is required in the epithelium of oral cavity to prevent abnormal epithelial adhesion, while Tgfβ3 is needed for cell apoptosis in the MEE for palate fusion (Taya et al., 1999; Casey et al., 2006). In Fgf10 mutant palate, altered expression of Jagged2 and Tgfβ3 is associated with the abnormal palate-mandible fusion (Alappat et al., 2005). However, we did not detect a change in the expression of either gene in the adhesion/fusion region of the Hand2 hypomorphic palate, suggesting that Hand2 could either regulate a distinct pathway or act downstream of these two genes in the regulation of epithelial adhesion/fusion during palate development.
Acknowledgments
We would like to thank Dr. Hiroshi Hamada of Osaka University, Japan, for the mouse Pitx2 promoter construct. This work was supported by NIH grants R01DE14044 and R01DE12329 (Y.P.C.), R01DE13681 (R.J.), and an American Heart Association grant and support from NIH grant R01NS15547 (P.C.). Y.M. was supported by an AHA Postdoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alappat SR, Zhang Z, Suzuki K, Zhang X, Liu H, Jiang R, Yamada G, Chen YP. The cellular and molecular etiology of the cleft secondary palate in Fgf10 mutant mice. Dev Biol. 2005;277:102–113. doi: 10.1016/j.ydbio.2004.09.010. [DOI] [PubMed] [Google Scholar]
- Barbosa AC, Funato N, Chapman S, McKee MD, Richardson JA, Olson EN, Yanagisawa H. Hand transcription factors cooperatively regulate development of the distal midline mesenchyme. Dev Biol. 2007;310:154–168. doi: 10.1016/j.ydbio.2007.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey LM, Yu L, Eui-Sic C, Maltby KM, Thomas G, Jiang R. Jag2-Notch1 signaling regulates oral epithelial differentiation and palate development. Dev Dyn. 2006;235:1830–1844. doi: 10.1002/dvdy.20821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, Bringas P, Han J, Rowitch DH, Soriano P, McMahon AP, Sucov HM. Fate of the mammalian cranial neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Charite J, McFadden DG, Olson EN. The bHLH transcription factor dHAND controls Sonic hedgehog expression and establishment of the zone of polarizing activity during limb development. Development. 2000;127:2461–2470. doi: 10.1242/dev.127.11.2461. [DOI] [PubMed] [Google Scholar]
- Clouthier DE. Signaling pathways crucial for craniofacial development revealed by endothelin-A receptor-deficient mice. Dev Biol. 2000;217:10–24. doi: 10.1006/dbio.1999.9527. [DOI] [PubMed] [Google Scholar]
- Clouthier DE, Williams SC, Yanagisawa H, Wieduwilt M, Richardson JA, Yanagisawa M. Signaling Pathways Crucial for Craniofacial Development Revealed by Endothelin-A Receptor-Deficient Mice. Dev Biol. 2000;217:10–24. doi: 10.1006/dbio.1999.9527. [DOI] [PubMed] [Google Scholar]
- Cuervo R, Covarrubias L. Death is the major fate of medial edge epithelial cells and the cause of basal lamina degradation during palatogenesis. Development. 2004;131:15–24. doi: 10.1242/dev.00907. [DOI] [PubMed] [Google Scholar]
- D’Autreaux F, Morikawa Y, Cserjesi P, Gershon MD. Hand2 is necessary for terminal differentiation of enteric neurons from crest-derived precursors but not for their migration into the gut or for formation of glia. Development. 2007;134:2237–2249. doi: 10.1242/dev.003814. [DOI] [PubMed] [Google Scholar]
- Danielian PS, Puccino D, Rowitch DH, Michael SK, McMahon AP. Modification of gene activity in mouse embryos in utero by a tamoxifen-inducible form of Cre recombinase. Curr Biol. 1998;8:1323–1326. doi: 10.1016/s0960-9822(07)00562-3. [DOI] [PubMed] [Google Scholar]
- Ferguson MWJ. Palate development. Development. 1988;103:41–60. doi: 10.1242/dev.103.Supplement.41. [DOI] [PubMed] [Google Scholar]
- Fernandez-Teran M, Piedra ME, Kathiriya IS, Srivastava D, Rodriguez-Rey JC, Ros MA. Role of dHAND in the anterior-posterior polarization of the limb bud: implications for the Sonic hedgehog pathway. Development. 2000;127:2133–2142. doi: 10.1242/dev.127.10.2133. [DOI] [PubMed] [Google Scholar]
- Fitchett JE, Hay ED. Medial edge epithelium transforms to mesenchyme after embryonic palatal shelves fuse. Dev Biol. 1989;131:455–474. doi: 10.1016/s0012-1606(89)80017-x. [DOI] [PubMed] [Google Scholar]
- Gritli-Linde A. Molecular control of secondary palate development. Dev Biol. 2007;301:309–326. doi: 10.1016/j.ydbio.2006.07.042. [DOI] [PubMed] [Google Scholar]
- Gu S, Wei N, Yu XY, Jiang Y, Fei J, Chen YP. Mice with an anterior cleft of the palate survive neonatal lethality. Dev Dyn. 2008;237:1509–1516. doi: 10.1002/dvdy.21534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halford MM, Armes J, Buchert M, Meskenaite V, Gail D, Hibbs ML, Wilks AF, Farlie PG, Newgreen DF, Hovens CM, Stacker SA. Ryk-deficient mice exhibit craniofacial defects associated with perturbed Eph receptor crosstalk. Nat Genet. 2000;25:414–418. doi: 10.1038/78099. [DOI] [PubMed] [Google Scholar]
- Hogan B, Beddington R, Costantini F, Lacy E. Manipulating the mouse embryo: A laboratory manual. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1994. [Google Scholar]
- Howard MJ, Stanke M, Schneider C, Wu X, Rohrer H. The transcription factor dHAND is a downstream effector of BMPs in sympathetic neuron specification. Development. 2000;127:4073–4081. doi: 10.1242/dev.127.18.4073. [DOI] [PubMed] [Google Scholar]
- Ingraham CR, Kinoshita A, Kondo S, Yang B, Sajan S, Trout KJ, Malik MI, Dunnwald M, Goudy SL, Lovett M, et al. Abnormal skin, limb and craniofacial morphogenesis in mice deficient for interferon regulatory factor 6 (Irf6) Nat Genet. 2006;38:1335–1340. doi: 10.1083/ng1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y, Yeo JY, Chytil A, Han J, Bringas P, Jr, Nakajima A, Shuler CF, Moses HL, Chai Y. Conditional inactivation of Tgfr2 in cranial neurla crest causes cleft palate and calvaria defects. Development. 2003;130:5269–5280. doi: 10.1242/dev.00708. [DOI] [PubMed] [Google Scholar]
- Jiang R, Lan Y, Chapman HD, Shawber C, Norton CR, Serreze DV, Weinmaster G, Gridley T. Defects in limb, craniofacial, and thymic development in Jagged2 mutant mice. Genes Dev. 1998;12:1046–1057. doi: 10.1101/gad.12.7.1046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaartinen V, Volcken JK, Shuler C, Warburton D, Bu D, Heisterkamp N, Groffen J. Abnormal lung development and cleft palate in mice lacking TGF-β3 indicates defects of epithelial-mesenchymal interactions. Nat Genet. 1995;11:415–421. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- Lan Y, Wang Q, Ovitt CE, Jiang R. A unique mouse strain expressing Cre recombinase for tissue-specific analysis of gene function in palate and kidney development. Genesis. 2007;45:618–624. doi: 10.1002/dvg.20334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Selever J, Wang D, Lu MF, Moses KA, Schwartz RJ, Martin JF. Bmp4 signaling is required for outflow-tract septation and branchial-arch artery remodeling. Proc Natl Acad Sci USA. 2004;101:4489–4494. doi: 10.1073/pnas.0308466101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massari ME, Murre C. Helix-Loop-Helix Proteins: Regulators of Transcription in Eucaryotic Organisms. Mol Cell Biol. 2000;20:429–440. doi: 10.1128/mcb.20.2.429-440.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFadden DG, McAnally J, Richardson JA, Charite J, Olson EN. Misexpression of dHAND induces ectopic digits in the developing limb bud in the absence of direct DNA binding. Development. 2002;129:3077–3088. doi: 10.1242/dev.129.13.3077. [DOI] [PubMed] [Google Scholar]
- Miller CT, Yelon D, Stainier DYR, Kimmel CB. Two endothelin 1 effectors, hand2 and bapx1, pattern ventral pharyngeal cartilage and the jaw joint. Development. 2003;130:1353–1365. doi: 10.1242/dev.00339. [DOI] [PubMed] [Google Scholar]
- Morikawa Y, Dai YS, Hao J, Bonin C, Hwang S, Cserjesi P. The basic helix-loop-helix factor Hand2 regulates autonomic nervous system development. Dev Dyn. 2005;234:613–621. doi: 10.1002/dvdy.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morikawa Y, D’Autreaux F, Gershon MD, Cserjesi P. Hand2 determines the noradrenergic phenotype in the mouse sympathetic nervous system. Dev Biol. 2007;307:114–126. doi: 10.1016/j.ydbio.2007.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naiche LA, Papaioannou VE. Cre activity causes widespread apoptosis and lethal anemia during embryonic development. Genesis. 2007;45:768–775. doi: 10.1002/dvg.20353. [DOI] [PubMed] [Google Scholar]
- Peters H, Neubuser A, Kratochwil K, Balling R. Pax9-deficient mice lack pharyngeal pouch derivatives and teeth and exhibit craniofacial and limb abnormalities. Genes Dev. 1998;12:2735–2747. doi: 10.1101/gad.12.17.2735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proetzel G, Pawlowski SA, Wiles MV, Yin M, Boivin GP, Howles PN, Ding J, Ferguson MWJ, Doetschman T. Transforming growth factor-β3 is required for secondary palate fusion. Nat Genet. 1995;11:409–414. doi: 10.1038/ng1295-409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice R, Spencer-Dene B, Connor EC, Gritli-Linde A, McMahon AP, Dickson C, Thesleff I, Rice DPC. Disruption of Fgf10/Fgfr2b-coordinated epithelial-mesenchymal interactions cause cleft palate. J Clin Inves. 2004;113:1692–1770. doi: 10.1172/JCI20384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RJ, Dixon J, Malhotra S, Hardman MJ, Knowles L, Boot-Handford RP, Shore P, Whitmarsh A, Dixon MJ. Irf6 is a key determinant of the keratinocyte proliferation-differentiation switch. Nat Genet. 2006;38:1329–1334. doi: 10.1038/ng1894. [DOI] [PubMed] [Google Scholar]
- Ruest LB, Dager M, Yanagisawa H, Charite J, Hammer RE, Olson EN, Yanagisawa M, Clouthier DE. Dhand-cre transgenic mice reveal specific potential functions of dHAND during craniofacial development. Dev Biol. 2003;257:263–277. doi: 10.1016/s0012-1606(03)00068-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlange T, Andree B, Arnold HH, Brand T. BMP2 is required for early heart development during a distinct time period. Mech Dev. 2000;91:259–270. doi: 10.1016/s0925-4773(99)00311-1. [DOI] [PubMed] [Google Scholar]
- Shiratori H, Sakuma R, Watanabe M, Hashiguchi H, Mochida K, Sakai Y, Nishno J, Saijoh Y, Whitman M, Hamada H. Two-step regulation of left-right asymmetric expression of Pitx2: Initiation by Nodal signaling and maintenance by Nkx2. Mol Cell. 2001;7:137–149. doi: 10.1016/s1097-2765(01)00162-9. [DOI] [PubMed] [Google Scholar]
- Soriano P. Generalized LacZ expression with the ROSA26 Cre reporter strain. Nat Genet. 1999;21:70–71. doi: 10.1038/5007. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Cserjesi P, Olson EN. A subclass of bHLH proteins required for cardiac morphogenesis. Science. 1995;270:1995–1999. doi: 10.1126/science.270.5244.1995. [DOI] [PubMed] [Google Scholar]
- Srivastava D, Thomas T, Lin Q, Kirby ML, Brown D, Olson EN. Regulation of cardiac mesodermal and neural crest development by the bHLH transcription factor, dHAND. Nat Genet. 1997;16:154–160. doi: 10.1038/ng0697-154. [DOI] [PubMed] [Google Scholar]
- St Amand TR, Zhang Y, Semina EV, Zhang X, Hu Y, Nguyen L, Murray JC, Chen YP. Antagonistic signals between BMP4 and FGF8 define the expression of Pitx1 and Pitx2 in mouse tooth-forming anlage. Dev Biol. 2000;217:323–332. doi: 10.1006/dbio.1999.9547. [DOI] [PubMed] [Google Scholar]
- Taya Y, O’Kane S, Ferguson MWJ. Pathogenesis of cleft palate in TGF-β3 knockout mice. Development. 1999;126:3869–3870. doi: 10.1242/dev.126.17.3869. [DOI] [PubMed] [Google Scholar]
- Thomas T, Kurihara H, Yamagishi H, Kurihara Y, Yazaki Y, Olson EN, Srivastava D. A signaling cascade involving endothelin-1, dHAND and msx1 regulates development of neural-crest-derived branchial arch mesenchyme. Development. 1998;125:3005–3014. doi: 10.1242/dev.125.16.3005. [DOI] [PubMed] [Google Scholar]
- Yamagishi H, Yamagishi C, Nakagawa O, Harvey RP, Olson EN, Srivastava D. The Combinatorial Activities of Nkx2.5 and dHAND Are Essential for Cardiac Ventricle Formation. Dev Biol. 2001;239:190–203. doi: 10.1006/dbio.2001.0417. [DOI] [PubMed] [Google Scholar]
- Yanagisawa H, Clouthier DE, Richardson JA, Charite J, Olson EN. Targeted deletion of a branchial arch-specific enhancer reveals a role of dHAND in craniofacial development. Development. 2003;130:1069–1078. doi: 10.1242/dev.00337. [DOI] [PubMed] [Google Scholar]
- Yelon D, Ticho B, Halpern ME, Ruvinsky I, Ho RK, Silver LM, Stainier DY. The bHLH transcription factor hand2 plays parallel roles in zebrafish heart and pectoral fin development. Development. 2000;127:2573–2582. doi: 10.1242/dev.127.12.2573. [DOI] [PubMed] [Google Scholar]
- Yu L, Gu S, Alappat S, Song Y, Yan M, Zhang X, Zhang G, Jiang Y, Zhang Z, Zhang Y, Chen YP. Shox2-deficient mice exhibit a rare type of incomplete clefting of the secondary palate. Development. 2005;132:4397–4406. doi: 10.1242/dev.02013. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Song Y, Zhao X, Zhang X, Fermin C, Chen YP. Rescue of cleft palate in Msx1-deficient mice by transgenic Bmp4 reveals a network of BMP and Shh signaling in the regulation of mammalian palatogenesis. Development. 2002;129:4135–4146. doi: 10.1242/dev.129.17.4135. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Yu X, Zhang Y, Geronimo B, Lovlie A, Fromm SH, Chen YP. Targeted misexpression of constitutively active BMP receptor-IB causes bifurcation, duplication, and posterior transformation of digit in mouse limb. Dev Biol. 2000;220:154–167. doi: 10.1006/dbio.2000.9637. [DOI] [PubMed] [Google Scholar]