Abstract
Processing task-irrelevant emotional information may compromise attention performance, particularly among those showing elevated threat sensitivity. If threat-sensitive individuals are able to recruit attentional control to inhibit emotional processing, however, they may show few decrements in attention performance. To examine this hypothesis, attention performance was measured in three domains—alerting, orienting, and executive attention. Task-irrelevant fearful, sad, and happy faces were presented for 50 ms before each trial of the attention task to create a mildly competitive emotional context. Electroencephalographic recordings were made from 64 scalp electrodes to generate event-related potentials (ERPs) to the faces. Participants reporting high threat sensitivity showed enhanced ERPs thought to reflect emotional processing (P200) and attentional control (P100 and N200). Enhanced N200 following fearful faces was linked to sustained and even slightly improved executive attention performance (reduced conflict interference) among high threat-sensitive individuals, but with decrements in executive attention among low threat-sensitive individuals. Results are discussed in terms of cognitive processing efficiency and the balance between threat sensitivity and attentional control in relation to executive attention performance. Results may have implications for understanding automatic and voluntary attentional biases related to anxiety.
Keywords: Cognitive control, Event-related brain potentials, Behavioral inhibition system, Emotion-attention interactions
Preferential processing of negative emotional information may compromise attention performance (Cacioppo and Berntson, 1994; Hare et al., 2005; Simpson et al., 2000). This relatively automatic ‘negativity bias’ is adaptive because it facilitates rapid processing of threat, but may also deplete the resources available for more voluntary control of attention performance (Bishop et al., 2004; Desimone and Duncan, 1995; Kieras et al., 2000; Miller and Cohen, 2001).
Individuals who show behavioral inhibition system (BIS) sensitivity and anxious mood are thought to show enhanced negativity biases, particularly towards threat and fear-related stimuli (Bishop et al., 2004; Carver and Scheier, 1998; Gray and McNaughton, 2000; Higgins et al., 1997; Leen-Feldner et al., 2004; Mathews and Mackintosh, 1998). Threat-sensitivity is more likely to interfere with attention when it exceeds an optimal level: for example, elevated anxiety has been shown to increase the negative impact of threat-related emotional stimuli on executive attention (Jazbec et al., 2005; Mathews and Mackintosh, 1998; Wood et al., 2001) such as conflict interference tasks (Fenske and Eastwood, 2003; Williams et al., 1996).
BIS-sensitive individuals, however, vary in the degree to which they recruit cognitive control resources to inhibit attention towards emotional information (Gray and Burgess, 2004) and in the degree to which this attentional control supports attention performance (Derryberry and Reed, 2002). For example, in a study with anxious adults, those showing high versus low dispositional attentional control showed reduced threat biases (orienting towards threatening cues; Derryberry and Reed, 2002). In other research, high BIS sensitivity has been linked to improved spatial working memory (Gray, 2001), but this likely depends on increased activation in the medial frontal cortex (Gray et al., 2005). Indeed, negative emotional states and information may actually enhance the responsiveness of prefrontal systems related to cognitive control (Gray et al., 2002; Potts et al., 2006). Such findings suggest that, although increased neural activation may reflect reduced neural efficiency (Gray et al., 2002), those showing high BIS sensitivity may require greater cognitive control resources to modulate emotional reactivity and facilitate cognitive performance (Gray and Braver, 2002). Another implication of a neural efficiency framework is that low BIS-sensitive individuals, who presumably show less reactivity, should not require enhanced recruitment of cognitive control in emotional contexts. Those who do show more neural activation related to cognitive control might be cognitively “inefficient” and thus be vulnerable to emotion interference effects that impair performance (Compton, 2003; Eysenck and Calvo, 1992; Hanoch and Vitouch, 2004). Scalp-recorded event-related potentials (ERPs) provide a powerful measure of cognitive processing efficiency because they capture very early and rapid stages of emotional and attentional processing that may be particularly difficult to measure via behavioral performance (De Pascalis et al., 2005). ERP responses to human faces have received considerable empirical attention due to their social significance and affective salience; in particular, very early ERP responses reflecting relatively automatic emotional and attentional processing have been targeted (Eimer and Holmes, 2002; Pizzagalli et al., 1999; Sato et al., 2001). As early as 80–100 ms, negative emotional faces compared to neutral faces elicit enhanced ERPs in posterior regions reflecting enhanced visual attention (Hillyard et al., 1995; Smith et al., 2003). For example, a negative posterior negative deflection around 170 ms, enhanced in the right hemisphere, may be specifically sensitive to negative emotional faces (Batty and Taylor, 2003; Bentin et al., 1996; Eger et al., 2003; Pizzagalli et al., 1999; Righart and de Gelder, 2006). Some studies, however, fail to document emotional sensitivity of the N170 (Eimer and Holmes, 2002) and have found both earlier and later latencies of the component (Pizzagalli et al., 1999; Sato et al., 2001). The posterior P200 (180–250 ms) may also reflect emotional significance processing and is enhanced for negative emotional stimuli (Carretié et al., 2001; Correll et al., 2006; Schutter et al., 2004).
When emotional stimuli are irrelevant to performing a target task, ERP responses related to the cognitive control of attention may also emerge. Positive deflections in posterior cortical areas around 100 ms, or P100, are thought to reflect automatic suppression of unattended stimuli and the recruitment of cognitive and attentional control over conflicting or emotional information (Hillyard et al., 1995; Mangun and Hillyard, 1995; Näätänen and Picton, 1987; Tendolkar et al., 2005). Later in the processing stream (200–350 ms), a range of ERP responses generated from areas of the medial frontal cortex, such as the anterior cingulate cortex, have been linked to cognitive and attentional control processes (Gehring and Willoughby, 2002; Luu et al., 2000; Parasuraman, 1998; Potts et al., 2006; Yeung et al., 2005). For example, the N200 is enhanced during tasks requiring monitoring of “crosstalk”, or conflicting information and response options, and is thought to signal the extent to which attentional control is required (Nieuwenhuis et al., 2003; van Veen and Carter, 2002). N200 and other early frontally generated negative ERP responses may reflect a “gating” mechanism in the medial frontal cortex through which motivationally significant information gains access to cognitive control systems.
In summary, the combination of enhanced threat sensitivity and high cognitive control may represent a “balance” that facilitates attention performance when negative emotional information competes for attention (Derryberry and Reed, 2002; Gray, 2004; Matthews and Mackintosh, 1998); for those showing low threat sensitivity, however, greater recruitment of cognitive control might represent an “imbalance” marking inefficient cognitive processing. ERPs provide a highly appropriate measurement approach because they capture very early interactions between affect and cognitive control, in particular in relation to salient emotional stimuli like human emotional faces.
To examine these questions, electroencephalographic (EEG) recordings were made while participants completed the Attention Network Test (ANT; Fan et al., 2002). This task was modified to include briefly presented (50 ms) task-irrelevant emotional faces before each trial (Dennis and Chen, 2007; Dennis et al., in press), thus providing a mildly competitive emotional context, which may facilitate detection of individual differences in attentional biases (Matthews and Mackintosh, 1998). There were three emotional face types varying in threat-relevance and valence: fearful (most threat-related), sad (negative but less threat related), and happy (positive and not threat related). One face type was presented per block of trials in order to create three distinct emotional contexts. Emotional faces were chosen as distracter stimuli because they are salient and motivationally significant. Faces are processed extremely rapidly while competing for attentional resources; thus, early-occurring ERPs (0–300 ms) related to emotional processing of faces and cognitive control were targeted.
The ANT provides reliable estimates of three distinct attention functions: alerting, orienting, and executive attention (Fan et al., 2002; Fossella et al., 2002). These three systems vary in the degree to which they are driven by relatively automatic or voluntary attentional mechanisms (Derryberry and Reed, 2002; Posner and Petersen, 1990): alerting and orienting are supported by the more automatic posterior attention system and executive attention by the more voluntary anterior attention system, including the anterior cingulate cortex. Therefore, ERP responses to task-irrelevant emotional stimuli linked to the anterior attention system (such as the frontally generated N200) may have more of an impact on executive attention performance than ERPs linked to more automatic emotional and attentional processing operations.
There were two hypotheses: (1) after viewing task-irrelevant emotional faces, threat-sensitive individuals will show enhanced ERPs related to emotional processing (N170 and P200) and recruitment of attentional control (P100 and N200), particularly following fearful faces; (2) enhanced ERP responses related to more deliberate attentional control (N200) will reduce the negative impact of threat-related fearful faces on executive attention performance in the high threat-sensitive group, but in the low threat-sensitive group may reflect inefficient cognitive processing and predict reduced attention performance.
1. Methods
1.1. Participants
Participants were 36 adults, aged 18–40 (M = 21.42, S.D. = 5.25; 26 females) recruited through the psychology participant research pool at a university in New York City, and screened for identified psychological or neurological impairments. Self-reported race and ethnicity was as follows: 16 Caucasian, 2 African American, 10 Hispanic, 7 Asian, and 1 “Other”.
1.2. Procedures and measures
Participants spent approximately 2 h in the laboratory. They completed a series of questionnaires immediately after consent procedures and before electrophysiological recording. This report includes the 24-item BIS/BAS Questionnaire, which has demonstrated test–retest reliability and validity (Carver and White, 1994). This measure yields a behavioral inhibition system sensitivity score, as well as a behavioral activation system sensitivity score. Higher scores indicate higher sensitivity. Only the BIS score was used in the present analyses. Questions included “Criticism or scolding hurts me quite a bit” and “I feel pretty worried or upset when I think or know somebody is angry at me.” Questions are rated on a 1–4 scale, with 1 being very true for me and 4 being very false for me. Low and high BIS groups were generated based on a median split (50th percentile = 3.14; N = 20 in the low BIS group and N = 16 in the high BIS group) and did not differ on demographic variables, number of EEG artifacts, or number of response errors. The average BIS sensitivity score was M = 3.19, S.D. = 0.47, range = 2.29–4.00. Its correlation with self-report of trait anxiety (Spielberger, 1983) was r = .68, p < .001, suggesting that this measure of BIS sensitivity captures aspects of mood and threat sensitivity that are relevant to dispositional anxiety.
After the questionnaire period, EEG was recorded while subjects were administered the ANT (Fan et al., 2002), which was modified to present emotional faces before each task trial. The ANT was presented via E-Prime software (Psychological Software Tools, Pittsburgh, PA) on an IBM personal computer running Window XP, presenting to a 14-in. IBM monitor. Participants viewed the screen from a distance of 65 cm, and responses were collected via two buttons on the mouse. The ANT, illustrated in Fig. 1, combines a cued reaction time and flanker task (Eriksen and Eriksen, 1974). It quantifies the efficiency of three attention systems by measuring how response times to the target flanker task are influenced by alerting and spatial cues and flankers. Following presentation of the inter-trial faces over a fixation cross, a cue is presented, followed by the target arrow, which randomly appears either above or below the fixation cross and is surrounded on the left and right by four “flanker” stimuli. Participants indicate with one of two alternative button presses whether the central target arrow point left or right.
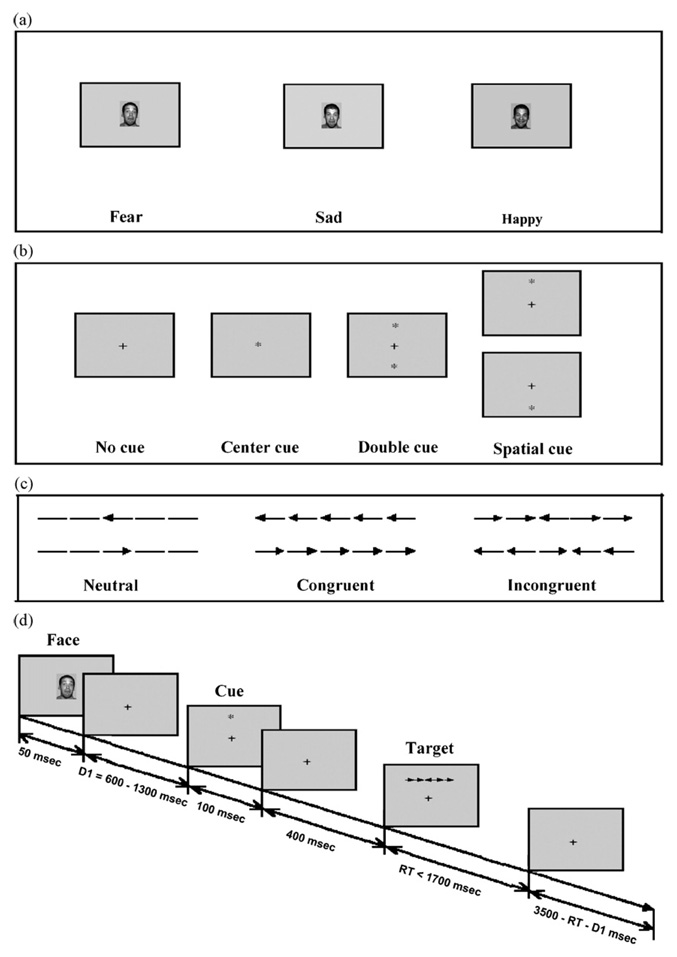
Fig. 1.
Diagram of experimental design (based on Fan et al., 2002).
Fig. 1a shows the inter-trial stimuli and Fig. 1b shows the cue conditions. Cues modulate whether subjects are alerted to the impending stimulus, and whether subjects are oriented ahead of time to the location of the target. Cues are no cues, double cues (asterisk appears above and below the fixation), center cues (asterisk appears superimposed over the fixation), and spatial cues (asterisk appears above or below the fixation to indicate the location of the subsequent target). Fig. 1c shows the flanker stimuli: congruent flankers point in the same direction as the central target arrow, incongruent flankers point in the opposite direction, and neutral flankers have no directional information.
The experiment consisted of a 24-trial full-feedback practice block (reaction time, whether answer was correct, and cumulative success rate) followed by three blocks of feedback-free trials (96 trials per block). Block order was counterbalanced across subjects. The ANT was modified in this study by presenting fearful, sad, or happy faces over the central fixation cross at the beginning of each trial, one face type per block. Fearful, sad, and happy faces were taken from a battery developed by the Research Network on Early Experience and Brain Development (Tottenham et al., 2002, April). The faces used in this study were selected based on normative ratings of the faces for emotional facial expressions, and obtaining equal representation of gender and ethnicity. Participants were informed that there would be faces presented at the beginning of each trial of the task, but were not instructed to attend or not attend. Because faces were completely unrelated and uninformative for performance of the primary ANT task, this design provides a simple but effective way to examine processing of task-irrelevant emotional stimuli and its effects on attention performance.
Efficiency of the three attentional networks, alerting, orienting, and executive attention, is determined by measuring how response times to the flanker displays are influenced by alerting cues, spatial cues, and flanker type (see Fan et al., 2002 for additional details). These efficiency scores are used rather than individual reaction time scores. Using correct trials only, the efficiency of alerting is calculated as RT following no cue—RT double cue. The double cue was used because it diffuses attention between the two potential target locations while alerting the participant to the arrival of the target. Higher scores indicate greater alerting efficiency due to presence of cues. The efficiency of orienting is calculated as RT following center cue—RT spatial cue. Higher scores indicate greater orienting efficiency due to presence of spatially predictive information of one cue, while controlling for alerting effects in the other. The efficiency of executive attention is calculated in terms of conflict interference – RT to incongruent flankers – RT to congruent flankers. Higher scores indicate greater conflict interference or less efficient executive attention.
As depicted in Fig. 1d, each trial consisted of six events: (1) face (fearful, sad, or happy; 50 ms); (2) fixation period (variable 600–1300 ms); (3) cue condition (no cue, center cue, double cue, spatial cue; 100 ms); (4) fixation period (400 ms); (5) simultaneously presented target and flanker stimuli (terminated at response up to 1700 ms); (6) post-target fixation period (varied, based on the first fixation and reaction time for that trial). Each trial lasted for 4050 ms.
1.3. Psychophysiological recording and data analysis
EEG activity was recorded continuously via 64Ag/AgCl scalp electrodes embedded in an elasticized nylon cap. Recordings were re-referenced off-line to an average reference. Eye movements were monitored by electro-oculogram (EOG) signals from electrodes placed parallel and below the left eye and from electrodes lateral to each eye. EEG and EOG signals were amplified with a band pass of .16–100 Hz by BioSemi ActiveTwo amplifier and sampled at 512 Hz (BioSemi, Amsterdam, NL). The continuous EEG recordings were then filtered with a low-cutoff frequency of 1 Hz and a high-cutoff frequency of 30 Hz. Stimulus-locked data were segmented into epochs from 200 ms before to 800 ms after stimulus onset. The raw EEG epochs were passed through a computerized artifact scan batch. Trials with EEG or EOG activity remaining above ±100 µV were excluded from further analysis. Trial acceptance rates were M = 82.21, S.D. = 20.89, range 57.29–98.96.
1.4. Data reduction and ERP components
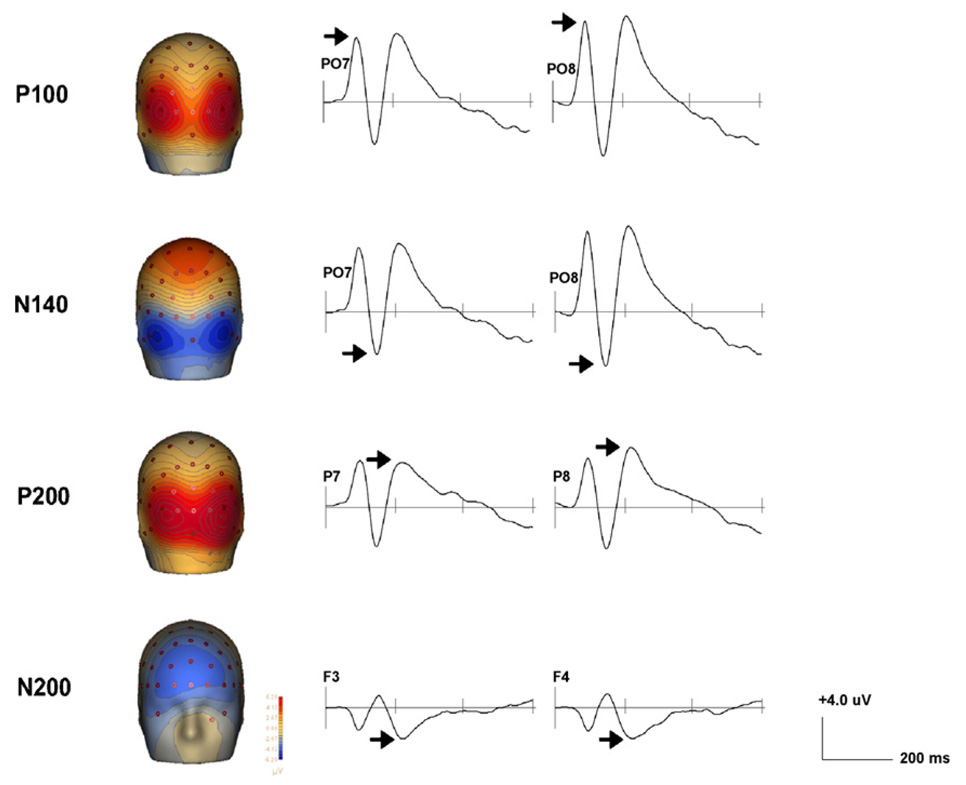
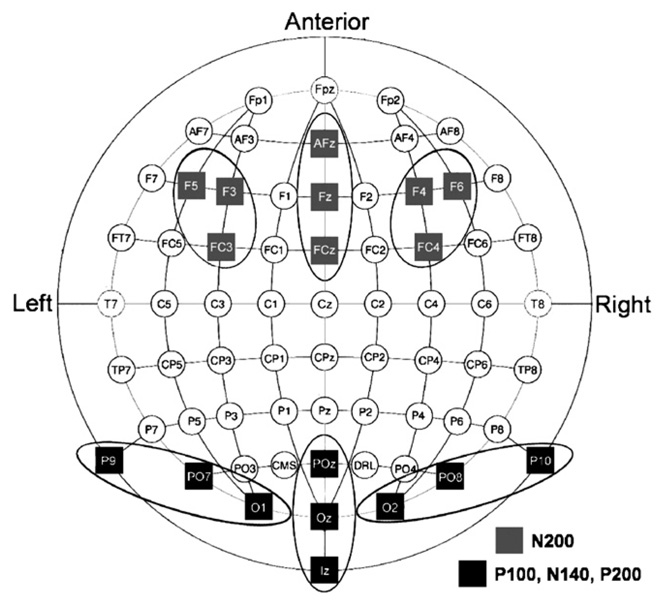
ERPs emerging during the first 300 ms following presentation of faces were targeted. Using Brain Electrical Source Analysis version 5.1 (BESA; MEGIS Software GmbH, Munich, Germany) average waveforms across all face conditions revealed four deflections: P100 (maximal at 90 ms), N140 (maximal at 140 ms, perhaps reflecting a reduced-latency N170), and the posterior P200 and anterior N200 (maximal at 220 ms). Fig. 2 shows scalp topography and representative waveforms for each. Peak amplitudes during the period 20 ms before and after the average maximum peak were generated for each component per emotion: (1) P100 was defined as the maximum positive peak between 70 and 110 ms at posterior leads; (2) the N140 was defined as the maximum negative peak between 120 and 160 ms at posterior leads; (3) the P200 was defined as the maximum positive peak between 200 and 240 ms at posterior leads; (4) the N200 was defined as the maximum negative peak between 200 and 240 ms at anterior leads. The placement of electrodes in the ActiveTwo 64-channel electrode array and the electrode regions over which data were averaged for each component are shown in Fig. 3. Electrode regions were chosen by identifying electrodes showing maximal amplitudes along with two flanking electrodes.
Fig. 2.
Scalp topography and representative waveforms for each ERP component. Contour lines are spaced every 0.50 µV.
Fig. 3.
Topographical diagram of electrodes included in analyses.
2. Results
2.1. Descriptive statistics
Table 1 presents ERP amplitudes and attention scores for correct trials only. Within each domain of attention, efficiency scores following each face type were highly inter-correlated (r = .69–.71). Because there were no gender-related hypotheses and no gender differences in study variables emerged, gender was not included in analyses reported below.
Table 1.
Descriptive statistics for ERP amplitudes and attention performance scores
| Region | Emotion | |||
|---|---|---|---|---|
| Fear M (S.D.) | Sad M (S.D.) | Happy M (S.D.) | Total M (S.D.) | |
| P100 | ||||
| Right | 5.15 (2.84) | 5.10 (3.11) | 5.14 (3.21) | 5.13 (2.94) |
| Left | 4.44 (2.74) | 4.89 (2.78) | 4.56 (2.62) | 4.63 (2.62) |
| Midline | 2.39 (2.20) | 2.41 (2.57) | 2.27 (2.37) | 2.36 (2.26) |
| N140 | ||||
| Right | −5.89 (4.30) | −5.44 (4.04) | −5.60 (4.24) | −5.64 (4.10) |
| Left | −4.89 (3.57) | −4.67 (3.50) | −4.64 (3.53) | −4.74 (3.42) |
| Midline | −3.66 (2.95) | −3.39 (3.13) | −3.56 (2.86) | −3.54 (2.86) |
| P200 | ||||
| Right | 6.90 (4.16) | 6.97 (3.89) | 6.69 (3.82) | 6.85 (3.85) |
| Left | 5.94 (3.53) | 6.25 (3.35) | 6.09 (2.95) | 6.10 (3.18) |
| Midline | 5.25 (2.73) | 5.24 (2.48) | 5.14 (2.34) | 5.21 (2.34) |
| N200 | ||||
| Right | −3.37 (1.80) | −3.39 (1.78) | −3.24 (1.61) | −3.33 (1.64) |
| Left | −2.89 (1.77) | −2.64 (1.64) | −2.86 (1.59) | −2.78 (1.54) |
| Midline | −3.06 (2.00) | −3.05 (2.02) | −2.84 (1.64) | −2.98 (1.78) |
| Alerting | 25.06 (28.98) | 33.72 (35.41) | 26.67 (34.87) | 28.48 (21.53) |
| Orienting | 42.07 (33.84) | 43.50 (31.00) | 43.28 (35.96) | 42.95 (25.00) |
| Executive attention | 101.00 (46.81) | 88.49 (47.63) | 102.01 (47.84) | 97.16 (42.37) |
| Error rates (%) | 3 (4) | 4 (4) | 3 (3) | 3 (4) |
2.2. Emotional faces and arousal
At the completion of the attention task, participants rated each face using the Self-Assessment Mannequin technique (Lang et al., 1998). Faces were rated on a 1–5 scale for arousal, with 5 indicating highly arousing, and a 1–5 scale for valence, with 1 being very positive and 5 being very negative. Although there were high levels of inter-correlation among emotions for arousal and valence ratings (r = .54–.88), sad faces were rated as less arousing than fearful, t(35) = −4.64, p < .001, and happy faces, t(35) = −3.08, p < .01 (sad M = 1.32, S.D. = 0.81; fearful M = 1.63, S.D. = 0.89; happy M = 1.78, S.D. = 0.99). Happy faces were rated as more positive than fearful, t(35) = −7.82, p < .001, and sad faces, t(35) = −9.10, p < .001, and fearful faces more positive than sad faces, t(35) = −6.04, p < .001 (sad M = 3.56, S.D. = 0.59; fearful M = 3.19, S.D. = 0.51; happy M = 2.04, S.D. = 0.56). Thus, overall, sad faces were perceived as less arousing but more negative than fearful and happy faces.
To confirm the characterization of ERP components related to emotional processing, it was examined whether links between BIS sensitivity and arousal ratings for faces depended on ERP responses, particularly N140 and P200. That is, it was predicted that faces would be perceived as arousing mainly when greater emotional processing resources were recruited after viewing them. To test this, a series of hierarchical regressions were conducted in which the dependent variable was subjective ratings of arousal when viewing fearful, sad, and happy faces. P100, N140, P200, and N200 amplitudes at maximal electrode regions were entered in the first step, BIS was entered in the second step, and the interactions between BIS and ERP amplitudes were entered in the third step. If the interaction term’s contribution to r2 was significant (p< .05), moderation effects were plotted using simple regression equations (Lang et al., 1998). These recast the significant interactions as the regression of one criterion on one predictor (Aiken and West, 1991; Finney et al., 1984). The criterion on the y-axis was plotted against two levels of the predictor (BIS), 1 S.D. below the mean (low) and 1 S.D. above the mean (high). Plotted regression lines represent two levels of the moderator (high and low ERP amplitudes also one standard deviation below and above the mean). Predictor variables were centered to reduce problems of lack of invariance of regression coefficients and multicollinearity (Aiken and West, 1991). Table 2 lists regression coefficients with all variables entered. As BIS sensitivity increased, arousal increased but only when P200 amplitudes were relatively low. The low BIS group reported predicted increases in arousal to faces when P200 amplitudes to faces were relatively high (Fig. 4). Regressions with other ERPs did not reach significance.
Table 2.
Regressions: effects of BIS and ERP amplitudes on arousal and executive attention
| Steps and predictors | F | B | B | R | ΔF |
|---|---|---|---|---|---|
| Arousal | |||||
| Step 1. P200—fear faces | <1 | .03 | .13 | .00 | <1 |
| Step 2. BIS | <1 | −.07 | −.04 | .01 | <1 |
| Step 3. P200 × BIS | −2.17* | −.18 | −.41 | .14 | 4.72* |
| Step 1. P200—sad faces | <1 | −.004 | −.02 | .04 | 1.22 |
| Step 2. BIS | <1 | −.12 | −.07 | .04 | <1 |
| Step 3. P200 × BIS | −2.00* | −.16 | −.37 | .14 | 4.01* |
| Executive attention | |||||
| Step 1. N200—fear faces | <1 | −.01 | −.02 | .01 | <1 |
| Step 2. BIS | <1 | .07 | .05 | .01 | <1 |
| Step 3. N200 × BIS | −2.12* | −.24 | −.37 | .13 | 4.49* |
p < .05. Coefficients are those generated when all steps are entered.
Fig. 4.
The association between BIS and arousal varied depending on the amplitude of P200 following fearful and sad faces.
2.3. Links between BIS and ERP measures of emotional and attentional processing
Next, it was tested whether increased BIS sensitivity was related to enhanced ERP responses related to emotional processing and the recruitment of cognitive control. Consistent with this, correlational analyses (see Table 3) showed that BIS sensitivity was positively correlated with P100, P200, and N200 amplitudes, indicating that as BIS sensitivity increased, ERP responses increased. This question was further examined by conducting four 2 (BIS: low versus high) × 3 (Emotion: fear, sad, happy) × 3 (Electrode Region: right, midline, left) repeated-measure ANOVAs, one for each component. Dependent variables were peak amplitudes at right, left, and midline (a) posterior electrode regions for components P100, N140, and P200; and (b) anterior electrode regions for N200. Greenhouse–Geisser corrections were inspected but did not differ from uncorrected values. Significant effects were followed with LSD tests or paired t-test.
Table 3.
Correlations between ERPs and study variables
| BIS | Arousal | Valence | Alerting | Orienting | Conflict | |
|---|---|---|---|---|---|---|
| Fear | ||||||
| P100 | .38* | −.09 | .10 | .09 | −.12 | .20 |
| N140 | −.02 | −.12 | −.17 | .01 | −.03 | −.16 |
| P200 | .42* | −.05 | −.26 | .19 | −.10 | −.01 |
| N200 | .46** | −.20 | −.19 | .17 | −.20 | .13 |
| Sad | ||||||
| P100 | .38* | −.10 | .30 | −.01 | .18 | .28 |
| N140 | .02 | −.15 | −.13 | −.21 | .23 | −.21 |
| P200 | .35* | −.19 | −.02 | −.01 | .17 | .07 |
| N200 | .33* | −.25 | .04 | −.11 | .09 | .15 |
| Happy | ||||||
| P100 | .39* | −.13 | .01 | −.10 | .13 | .08 |
| N140 | .05 | −.41* | −.02 | −.11 | −.02 | −.11 |
| P200 | .38* | −.09 | .22 | .06 | .19 | .06 |
| N200 | .29 | −.24 | .28 | −.03 | .17 | −.01 |
Note. ERPs were absolute values to aid interpretation of findings. To reduce the number of correlations, right hemisphere electrode values were used. Ampli-
P100 and P200 amplitudes were maximal at right and left lateralized compared to central sites, and N140 and N200 amplitudes were maximal at right compared to left and central sites, all t-test p < .001 for P100 and N140, p < .01 for P200, and p < .05 for N200. The effect of electrode region was not moderated by face type or BIS. As seen in Fig. 5, main effects of BIS were consistent with correlational analyses, showing that ERPs reflecting emotional processing (P200) and attentional inhibition (P100 and N200) were enhanced in the high versus low BIS group for all face types. For P100, (F(1,34) = 12.53, p < .001, partial η2 = .27; M = 5.33, S.D. = 2.37 versus M = 3.01, S.D. = 1.54, t(34) = −3.54, p < .001); for P200, (F(1,34) = 7.77, p < .01, partial η2 = .19; M = 7.39, S.D. = 3.10 versus M = 4.98, S.D. = 2.08, t(34) = −2.79, p < .01); and for N200, (F(1,34) = 6.70, p < .01, partial η2 = .17; M = −3.74, S.D. = 1.67 versus M = −2.47, S.D. = 1.28, t(34) = −2.59, p < .05). Effects did not reach significance for N140 amplitudes, and counter to prediction effects were not greater for fearful versus sad or happy faces.
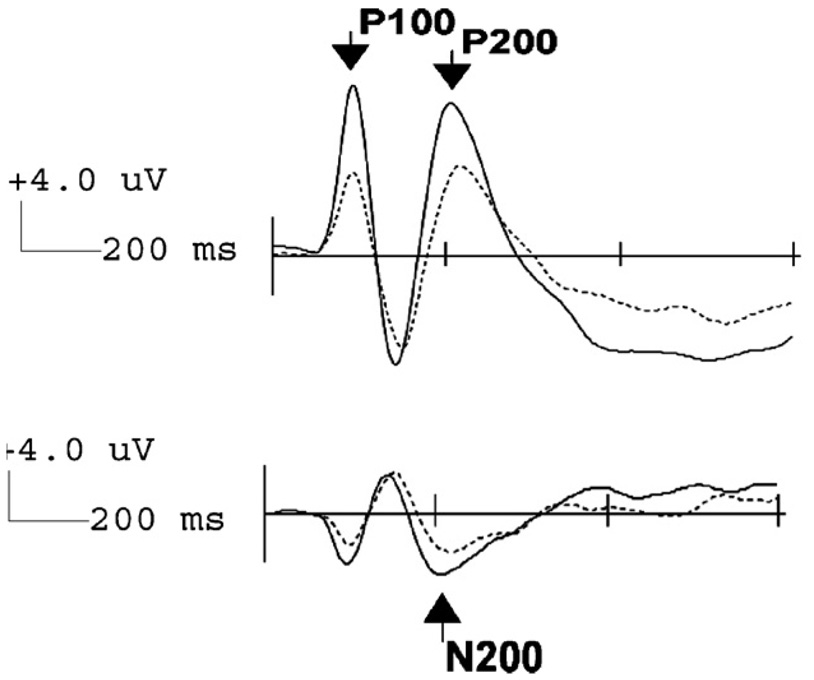
Fig. 5.
Grand-averaged ERP responses for high (solid line) vs. low (dashed line) BIS groups across all emotions for the P100 and P200 components (right posterior electrode PO8; top panel) and for N200 (right anterior electrode FC4; bottom panel). The amplitudes of these components were most pronounced in the high vs. low BIS group.
2.4. Attention performance: behavioral data
Before examining links between ERP responses and attention performance, it was of interest to test whether emotional factors alone influenced attention performance. Three 2 (low versus high BIS) × 3 (Emotion: fear, sad, happy) repeated measure ANOVAs were conducted to examine the effects of BIS and face type on attention performance (alerting, orienting, and executive attention separately). Multivariate effects did not reach significance. Within-subjects contrast for executive attention, however, showed a main effect of Emotion, F(1, 34) = 5, 23, p < .05: executive attention was improved (conflict interference reduced) following sad versus fearful, t(35) = 2.02, p < .05, and sad versus happy faces, t(35) = 2.02, p < .05.
2.5. Attention performance: effects of BIS and ERPs
Results thus far suggest that increased BIS sensitivity was linked to enhanced emotional processing and recruitment of cognitive control after viewing task-irrelevant emotional faces. The goal of the next set of analyses was to examine whether enhanced N200 will reduce the negative impact of threat-related fearful faces on executive attention among those showing high threat sensitivity, but in low threat-sensitive individuals interfere with attention performance. As seen in Table 3, ERPs alone were not correlated with attention performance.
In a series of hierarchical multiple regressions, the dependent variables were alerting, orienting, and executive attention (conflict) scores following presentation of fearful, sad, and happy faces. P100, N140, P200, and N200 amplitudes at maximal electrode regions were entered in the first step, BIS was entered in the second step, and the interactions between BIS and ERP amplitudes were entered in the third step. If the interaction term’s contribution to R2 was significant (p < .05), moderation effects were plotted using simple regression equations (Lang et al., 1998). The criterion on the y-axis was plotted against two levels of the predictor (BIS), 1 S.D. below the mean (low) and 1 S.D. above the mean (high). Plotted regression lines represent two levels of the moderator (high and low ERP amplitudes 1 S.D. below and above the mean). Table 2 lists regression coefficients with all variables entered.
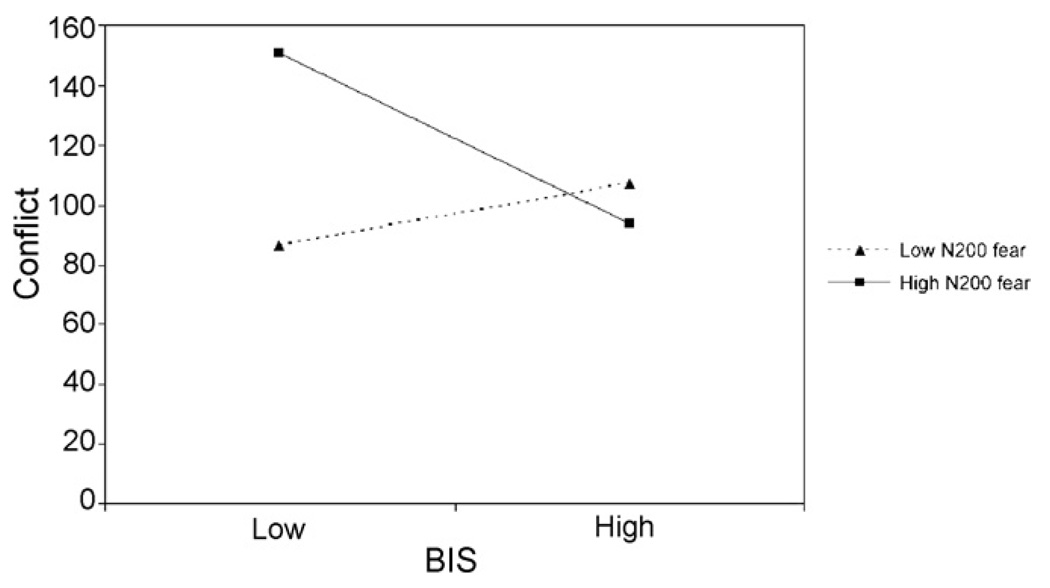
Analyses for alerting and orienting did not reach significance. Although behavioral effects suggested that executive attention was improved following sad faces, analyses with ERPs did not. Instead, as predicted, enhanced N200 reduced the negative impact of fearful faces on executive attention performance, but this depended on level of threat sensitivity: as BIS sensitivity increased, executive attention improved (conflict decreased) but only for those showing relatively high N200 to fearful faces (see Fig. 6). Low BIS combined with enhanced N200 was linked to the lowest executive attention scores. No other significant effects emerged.
Fig. 6.
The association between BIS and executive attention performance varied depending on the amplitude of N200 following fearful faces.
3. Discussion
The present findings suggest that threat sensitivity in a typical range is not the “nemesis” of executive attention (Gray, 2004). Instead, it was the combination of relatively low BIS with enhanced N200 in response to fearful faces that was associated with clearly reduced executive attention performance. Findings are consistent with neural efficiency models and suggest that the balance between reactivity and control is an important basis for the emotional modulation of attention (Matthews and Mackintosh, 1998). These effects were notable because emotion stimuli were only mildly competitive: faces were extremely brief and presented well before the target task. Findings may have implications for normative and disrupted attentional processes related to anxiety (Compton, 2003; Gray, 2004; Gray and Burgess, 2004) and underscore the need to use measures that capture rapid stages of cognitive processing and that reflect neural efficiency.
There was some support for the hypothesis that enhanced N200 would reduce the negative impact of threat-related fearful faces on executive attention performance (Eysenck and Calvo, 1992; Gray and Braver, 2002). Findings, however, more strongly highlighted the impact of enhanced neural activation among those showing low BIS sensitivity. In this group, enhanced N200 to fearful faces was related to reduced executive attention performance. Since low threat-sensitive individuals tend to avoid threat stimuli when emotional intensity and attentional competition is low, as in the present study (Correll et al., 2006; Matthews and Mackintosh, 1998), enhanced N200 may specifically reflect increased efforts to inhibit attention towards fearful faces and reduced resources available for attention performance. This suggests that the ability to achieve an “optimal balance” between reactivity and control is closely associated with executive attention performance (Compton, 2003; Derryberry and Reed, 2002; Matthews and Mackintosh, 1998). Such short-term costs may be linked to more long-term risks. For example, one type of low reactivity, low positive emotionality, is associated with the development of mood problems (Derryberry and Rothbart, 1997; Shankman et al., 2005). Future research on low reactivity may reveal specific attentional and emotional processing biases and mental health consequences.
A significant portion of BIS-sensitive individuals may be at risk for reduced attentional efficiency, but are able to compensate by expending greater cognitive effort on the task. Consistent with this, relatively high BIS was associated with enhanced P100, P200, and N200 ERP responses. Some in the high BIS group, however, did not appear to show increased threat processing; this group would have recruited fewer attentional control resources (reduced N200 suggesting cognitive processing efficiency) and shown few decrements in attention. Indeed, it was the low BIS group only that reported greater subjective arousal when P200 amplitudes to faces were high. On average, the high BIS group might rapidly engage and then modulate their affective response, thus experiencing reduced arousal to these faces. Behavioral results of the present study hint that among threat-sensitive individuals negative emotional information might even “jump-start” attention performance, particularly when it does not greatly increase emotional arousal or cognitive load (Gray, 2004; Wood et al., 2001).
Counter to expectation, N140 amplitudes were not enhanced in the high BIS group, nor were they enhanced for negative versus positive emotional faces. The timing and distribution of the N140 is consistent with the N100, which reflects increased attention to attended stimuli; but is also consistent with the N170, which is enhanced for negative emotional faces (Righart and de Gelder, 2006; Batty and Taylor, 2003; Pizzagalli et al., 2002). Enhanced N140 might not have been detected in the present study because faces were extremely brief and were not explicit targets of attention. In addition, high BIS was not associated with enhanced ERPs to fearful faces, which would be expected given that fear is relatively threat-relevant (Compton, 2003; Gray, 2004; Gray and Burgess, 2004). In non-clinical groups, BIS sensitivity might bolster cross-emotion vigilance (Carver, 2004; Higgins and Kazdin, 2000). On the other hand, sad faces were rated as most negative, and thus might have been relatively salient. This could be due to the inclusion of closed mouth rather than opened mouth versions of each face. Valence ratings might have been attenuated for closed-mouth faces if an open mouth is more paradigmatic of threat and fear.
Given this, it was telling that the BIS-performance associations emerged selectively for fearful faces. The present study focused on relatively early and automatic processing stages, so it may be that emotional differentiation among types of negative emotions is minimized. However, at the stage that relatively voluntary attentional control (N200) is recruited to support attention performance, such differentiation may emerge (Compton, 2003). Because effects were significant only for executive attention, this supports the idea that N200 responses and executive attention performance share a common link to voluntary control processes of the anterior attention system (Fox et al., 2001; Wood et al., 2001).
Task characteristics are important to take into account when interpreting results. Participants were presented with extremely rapid irrelevant emotional information prior to each trial of the task. Thus, under conditions of relatively low competition and cognitive load, BIS sensitivity in a non-clinical range may enhance emotional processing and attentional control at extremely early cognitive stages and facilitate attention performance (Wood et al., 2001). Repeated presentation of faces in the present study might also have served as a mild mood induction, thus enhancing the emotional modulation of attention. Overall, this design complements previous research that focuses on interference effects emerging under conditions of direct competition, high emotional salience, or high threat sensitivity (Bishop et al., 2004; Mathews and Mackintosh, 1998; Mathews and MacLeod, 1985; Wood et al., 2001). Patterns of effects should be explored and replicated using additional tasks; for example, those directly manipulating mood might show greater affective enhancement and competition effects and visual oddball paradigms including attend and ignore conditions could provide a more precise manipulation of attentional control (Campanella et al., 2002).
In conclusion, this study was among the few using ERPs to document that the negative impact of threat-related stimuli is moderated by very early recruitment of attentional control combined with dispositional threat sensitivity. Facilitation of executive attention in threat-sensitive individuals was documented, as was, interference in those showing low threat-sensitivity. Evidence from this study add to the growing body of evidence that the emotional modulation of attention happens extremely rapidly with socially and emotionally salient stimuli such as faces (Derryberry and Reed, 2002; Eastwood et al., 2001). Future research should also target “later” neurophysiological responses such as the P300 (Nieuwenhuis et al., 2005) and late positive potential (Schupp et al., 2002), which may reflect more elaborated processes. Overall, findings suggest that an “optimal balance” between emotional reactivity and cognitive control may characterize adaptive functioning (Dennis and Chen, 2007). The present findings should be interpreted cautiously in relation to clinical disorders, but provide a basis for future research on emotion and attentional biases in anxiety disorders.
Acknowledgements
We would like to thank James Gordon for his invaluable aid during the initiation of this study and Gerard Bruder for his feedback on earlier versions of this manuscript. This research was supported by NIH Grants 5T34 GM007823, 5K01 MH075764-02, and 5S06GM060654-04, the latter two awarded to the first author.
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Thousand Oaks, CA: Sage Publications Inc.; 1991. [Google Scholar]
- Batty M, Taylor MJ. Early processing of the six basic facial emotional expressions. Brain Research: Cognitive Brain Research. 2003;17:613–620. doi: 10.1016/s0926-6410(03)00174-5. [DOI] [PubMed] [Google Scholar]
- Bentin S, Allison T, Puce A, Perez E. Electrophysiological studies of face perception in humans. Journal of Cognitive Neuroscience. 1996;8:551–565. doi: 10.1162/jocn.1996.8.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop S, Duncan J, Brett M, Lawrence AD. Prefrontal cortical function and anxiety: controlling attention to threat-related stimuli. Nature Neuroscience. 2004;7:184–188. doi: 10.1038/nn1173. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Berntson GG. Relationship between attitudes and evaluative space: a critical review, with emphasis on the separability of positive and negative substrates. Psychological Bulletin. 1994;115:401–423. [Google Scholar]
- Campanella S, Gaspard C, Debatisse D, Bruyer R, Crommelinck M, Guerit JM. Discrimination of emotional facial expressions in a visual oddball task: an ERP study. Biological Psychology. 2002;59:171–186. doi: 10.1016/s0301-0511(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Carretié L, Mercado F, Tapia M, Hinojosa JA. Emotion, attention, and the ‘negativity bias’, studied through event-related potentials. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2001;41:75–85. doi: 10.1016/s0167-8760(00)00195-1. [DOI] [PubMed] [Google Scholar]
- Carver CS. Negative affects deriving from the behavioral approach system. Emotion. 2004;4:3–22. doi: 10.1037/1528-3542.4.1.3. [DOI] [PubMed] [Google Scholar]
- Carver CS, Scheier MF. On the Self-Regulation of Behavior. New York, NY: Cambridge University Press; 1998. [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: the BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. [Google Scholar]
- Compton RJ. The interface between emotion and attention: a review of evidence from psychology and neuroscience. Behavioral and Cognitive Neuroscience Reviews. 2003;2:115–129. doi: 10.1177/1534582303255278. [DOI] [PubMed] [Google Scholar]
- Correll J, Urland GR, Ito TA. Event-related potentials and the decision to shoot: the role of threat perception and cognitive control. Journal of Experimental Social Psychology. 2006;42:120–128. [Google Scholar]
- De Pascalis V, Arwari B, Matteucci M, Mazzocco A. Effects of emotional visual stimuli on auditory information processing: a test of J.A. Gray’s reinforcement sensitivity theory. Personality and Individual Differences. 2005;38:163–176. [Google Scholar]
- Dennis TA, Chen C. Emotional face processing and attention performance in three domains: Neurophysiological mechanisms and moderating effects of trait anxiety. International Journal of Psychophysiology. 2007;65:10–19. doi: 10.1016/j.ijpsycho.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis TA, Chen C, McCandliss BM. Threat-related attentional biases: an analysis of three attention systems. Depression and Anxiety. doi: 10.1002/da.20308. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derryberry D, Reed MA. Anxiety-related attentional biases and their regulation by attentional control. Journal of Abnormal Psychology. 2002;111:225–236. doi: 10.1037//0021-843x.111.2.225. [DOI] [PubMed] [Google Scholar]
- Derryberry D, Rothbart MK. Reactive and effortful processes in the organization of temperament. Development & Psychopathology. 1997;9:633–652. doi: 10.1017/s0954579497001375. [DOI] [PubMed] [Google Scholar]
- Desimone R, Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- Eastwood JD, Smilek D, Merikle PM. Differential attentional guidance by unattended faces expressing positive and negative emotion. Perception & Psychophysics. 2001;63:1004–1013. doi: 10.3758/bf03194519. [DOI] [PubMed] [Google Scholar]
- Eger E, Jedynak A, Iwaki T, Skrandies W. Rapid extraction of emotional expression: evidence from evoked potential fields during brief presentation of face stimuli. Neuropsychologia. 2003;41:808–817. doi: 10.1016/s0028-3932(02)00287-7. [DOI] [PubMed] [Google Scholar]
- Eimer M, Holmes A. An ERP study on the time course of emotional face processing. Neuroreport. 2002;13:427–431. doi: 10.1097/00001756-200203250-00013. [DOI] [PubMed] [Google Scholar]
- Eriksen BA, Eriksen CW. Effects of noise letters upon the identification of a target letter in a nonsearch task. Perception & Psychophysics. 1974;16:143–149. [Google Scholar]
- Eysenck MW, Calvo MG. Anxiety and performance: the processing efficiency theory. Cognition & Emotion. 1992;6:409–434. [Google Scholar]
- Fan J, McCandliss BD, Sommer T, Raz A, Posner MI. Testing the efficiency and independence of attentional networks. Journal of Cognitive Neuroscience. 2002;14:340–347. doi: 10.1162/089892902317361886. [DOI] [PubMed] [Google Scholar]
- Fenske MJ, Eastwood JD. Modulation of focused attention by faces expressing emotion: evidence from flanker tasks. Emotion. 2003;3:327–343. doi: 10.1037/1528-3542.3.4.327. [DOI] [PubMed] [Google Scholar]
- Finney JW, Mitchell RE, Cronkite RC, Moos RH. Methodological issues in estimating main and interactive effects: examples from coping/social support and stress field. Journal of Health and Social Behavior. 1984;25:85–98. [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, et al. Assessing the molecular genetics of attention networks [Electronic Version] BMC Neuroscience. 2002 doi: 10.1186/1471-2202-3-14. 3. Retrieved June 6, 2006, from http://www.biomedcentral.com/content/pdf/1471-2202-3-14.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox E, Russo R, Bowles R, Dutton K. Do threatening stimuli draw or hold visual attention in subclinical anxiety? Journal of Experimental Psychology: General. 2001;130:681–700. [PMC free article] [PubMed] [Google Scholar]
- Gehring WJ, Willoughby AR. The medial frontal cortex and the rapid processing of monetary gains and losses. Science. 2002;295:2279–2282. doi: 10.1126/science.1066893. [DOI] [PubMed] [Google Scholar]
- Gray JA, McNaughton N. The Neuropsychology of Anxiety. 2nd ed. New York, NY: Oxford; 2000. [Google Scholar]
- Gray JR. Emotional modulation of cognitive control: approach-with-drawal states double-dissociate spatial from verbal two-back task performance. Journal of Experimental Psychology: General. 2001;130:436–452. doi: 10.1037//0096-3445.130.3.436. [DOI] [PubMed] [Google Scholar]
- Gray JR. Integration of emotion and cognitive control. Current Directions in Psychological Science. 2004;13:46–48. [Google Scholar]
- Gray JR, Braver TS. Personality predicts working-memory-related activation in the caudal anterior cingulate cortex. Cognitive, Affective & Behavioral Neuroscience. 2002;2:64–75. doi: 10.3758/cabn.2.1.64. [DOI] [PubMed] [Google Scholar]
- Gray JR, Braver TS, Raichle ME. Integration of emotion and cognition in the lateral prefrontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2002;99:4115–4120. doi: 10.1073/pnas.062381899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray JR, Burgess GC. Personality differences in cognitive control? BAS, processing efficiency, and the prefrontal cortex. Journal of Research in Personality. 2004;38:35–36. [Google Scholar]
- Gray JR, Burgess GC, Schaefer A, Yarkoni T, Larsen RJ, Braver TS. Affective personality differences in neural processing efficiency confirmed using fMRI. Cognitive, Affective & Behavioral Neuroscience. 2005;5:182–190. doi: 10.3758/cabn.5.2.182. [DOI] [PubMed] [Google Scholar]
- Hanoch Y, Vitouch O. When less is more: Information, emotional arousal and the ecological reframing of the Yerkes-Dodson law. Theory & Psychology. 2004;14:427–452. [Google Scholar]
- Hare TA, Tottenham N, Davidson MC, Glover GH, Casey BJ. Contributions of amygdala and striatal activity in emotion regulation. Biological Psychiatry. 2005;57:624–632. doi: 10.1016/j.biopsych.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Higgins ET, Kazdin AE. Encyclopedia of Psychology. vol. 7. Washington, DC: American Psychological Association; 2000. Self-regulation; pp. 218–220. [Google Scholar]
- Higgins ET, Shah J, Friedman R. Emotional responses to goal attainment: strength of regulatory focus as moderator. Journal of Personality and Social Psychology. 1997;72:515–525. doi: 10.1037//0022-3514.72.3.515. [DOI] [PubMed] [Google Scholar]
- Hillyard SA, Mangun GR, Woldorff MG, Luck SJ, Gazzaniga MS. The Cognitive Neurosciences. Cambridge, MA: The MIT Press; 1995. Neural systems mediating selective attention; pp. 665–681. [Google Scholar]
- Jazbec S, McClure E, Hardin M, Pine DS, Ernst M. Cognitive control under contingencies in anxious and depressed adolescents: an antisaccade task. Biological Psychiatry. 2005;58:632–639. doi: 10.1016/j.biopsych.2005.04.010. [DOI] [PubMed] [Google Scholar]
- Kieras DE, Meyer DE, Ballas JA, Lauber EJ, Braver TS, Cohen JD, et al. Control of Cognitive Processes: Attention and Performance XVIII. VII. Cambridge, MA: The MIT Press; 2000. Computational modeling of control; pp. 679–751. [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction manual and affective ratings (Tech. Rep. No. A-4) Gainsville, FL: University of Florida, The Center for Research in Psychophysiology; 1998. [Google Scholar]
- Leen-Feldner EW, Zvolensky MJ, Feldner MT, Lejuez CW. Behavioral inhibition: relation to negative emotion regulation and reactivity. Personality and Individual Differences. 2004;36:1235–1247. [Google Scholar]
- Luu P, Flaisch T, Tucker DM. Medial frontal cortex in action monitoring. Journal of Neuroscience. 2000;20:464–469. doi: 10.1523/JNEUROSCI.20-01-00464.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangun GR, Hillyard SA. Mechanisms and models of selective attention. In: Rugg MD, Coles MGH, editors. Electrophysiology of Mind: Event-Related Brain Potentials and Cognition. New York, NY: Oxford University Press; 1995. pp. 40–85. [Google Scholar]
- Mathews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy & Research. 1998;22:539–560. [Google Scholar]
- Mathews A, MacLeod C. Selective processing of threat cues in anxiety states. Behaviour Research & Therapy. 1985;23:563–569. doi: 10.1016/0005-7967(85)90104-4. [DOI] [PubMed] [Google Scholar]
- Matthews A, Mackintosh B. A cognitive model of selective processing in anxiety. Cognitive Therapy and Research. 1998;22:539–560. [Google Scholar]
- Miller EK, Cohen JD. An integrative theory of prefrontal cortex function. Annual Review of Neuroscience. 2001;24:167–202. doi: 10.1146/annurev.neuro.24.1.167. [DOI] [PubMed] [Google Scholar]
- Näätänen R, Picton TW. The N1 wave of the human electric and magnetic response to sound: a review and an analysis of the component structure. Psychophysiology. 1987;24:375–425. doi: 10.1111/j.1469-8986.1987.tb00311.x. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Aston-Jones G, Cohen JD. Decision making, the P3, and the locus coeruleus—norepinephrine system. Psychological Bulletin. 2005;131:510–532. doi: 10.1037/0033-2909.131.4.510. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Yeung N, Van Den Wildenberg W, Ridderinkhof KR. Electrophysiological correlates of anterior cingulate function in a go/no-go task: effects of response conflict and trial type frequency. Cognitive, Affective & Behavioral Neuroscience. 2003;3:17–26. doi: 10.3758/cabn.3.1.17. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. The Attentive Brain. Cambridge, MA: The MIT Press; 1998. [Google Scholar]
- Pizzagalli D, Regard M, Lehmann D. Rapid emotional face processing in the human right and left brain hemispheres: an ERP study. Neuroreport: For Rapid Communication of Neuroscience Research. 1999;10:2691–2698. doi: 10.1097/00001756-199909090-00001. [DOI] [PubMed] [Google Scholar]
- Pizzagalli DA, Lehmann D, Hendrick AM, Regard M, Pascual-Marqui RD, Davidson RJ. Affective judgments of faces modulate early activity (approximately 160 ms) within the fusiform gyri. Neuroimage. 2002;16:663–677. doi: 10.1006/nimg.2002.1126. [DOI] [PubMed] [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Potts GF, Martin LE, Burton P, Montague PR. When things are better or worse than expected: the medial frontal cortex and the allocation of processing resources. Journal of Cognitive Neuroscience. 2006;18:1112–1119. doi: 10.1162/jocn.2006.18.7.1112. [DOI] [PubMed] [Google Scholar]
- Righart R, de Gelder B. Context influences early perceptual analysis of faces—an electrophysiological study. Cerebral Cortex. 2006;16:1249–1257. doi: 10.1093/cercor/bhj066. (New York, NY, 1991) [DOI] [PubMed] [Google Scholar]
- Sato W, Kochiyama T, Yoshikawa S, Matsumura M. Emotional expression boosts early visual processing of the face: ERP recording and its decomposition by independent component analysis. Neuroreport. 2001;12:709–714. doi: 10.1097/00001756-200103260-00019. [DOI] [PubMed] [Google Scholar]
- Schupp HT, Cuthbert BN, Bradley M, Cacioppo JT, Ito T, Lang PJ. Affective picture processing: the late positive potential is modulated by motivational relevance. Psychophysiology. 2002;37:257–261. [PubMed] [Google Scholar]
- Schutter DJLG, de Haan EHF, van Honk J. Functionally dissociated aspects in anterior and posterior electrocortical processing of facial threat. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology. 2004;53:29–36. doi: 10.1016/j.ijpsycho.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Shankman SA, Tenke CE, Bruder GE, Durbin CE, Hayden EP, Klein DN. Low positive emotionality in young children: Association with EEG asymmetry. Development and Psychopathology. 2005;17:85–98. doi: 10.1017/s0954579405050054. [DOI] [PubMed] [Google Scholar]
- Simpson JR, Ongür D, Akbudak E, Conturo TE, Ollinger JM, Snyder AZ, et al. The emotional modulation of cognitive processing: an fMRI study. Journal of Cognitive Neuroscience. 2000;12:157–170. doi: 10.1162/089892900564019. [DOI] [PubMed] [Google Scholar]
- Smith NK, Cacioppo JT, Larsen JT, Chartrand TL. May I have your attention, please: electrocortical responses to positive and negative stimuli. Neuropsychologia. 2003;41:171–183. doi: 10.1016/s0028-3932(02)00147-1. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. State-Trait Anxiety Inventory Manual. Redwood City, CA: Mind Garden Inc.; 1983. [Google Scholar]
- Tendolkar I, Ruhrmann S, Brockhaus-Dumke A, Pauli M, Mueller R, Pukrop R, et al. Neural correlates of visuo-spatial attention during an antisaccade task in schizophrenia: an ERP study. The International Journal of Neuroscience. 2005;115:681–698. doi: 10.1080/00207450590887475. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Borscheid A, Ellertsen K, Marcus DJ, Nelson CA. Categorization of facial expressions in children and adults: establishing a larger stimulous set. Paper Presented at the Meeting of Cognitive Neuroscience Society; San Francisco. 2002. Apr, [Google Scholar]
- van Veen V, Carter CS. The anterior cingulate as a conflict monitor: fMRI and ERP studies. Physiology & Behavior. 2002;77:477–482. doi: 10.1016/s0031-9384(02)00930-7. [DOI] [PubMed] [Google Scholar]
- Williams JMG, Mathews A, MacLeod C. The emotional Stroop task and psychopathology. Psychological Bulletin. 1996;120:3–24. doi: 10.1037/0033-2909.120.1.3. [DOI] [PubMed] [Google Scholar]
- Wood J, Mathews A, Dalgleish T. Anxiety and cognitive inhibition. Emotion. 2001;1:166–181. doi: 10.1037/1528-3542.1.2.166. [DOI] [PubMed] [Google Scholar]
- Yeung N, Holroyd CB, Cohen JD. ERP correlates of feedback and reward processing in the presence and absence of response choice. Cerebral Cortex. 2005;15:535–544. doi: 10.1093/cercor/bhh153. [DOI] [PubMed] [Google Scholar]