Abstract
Objective
We demonstrate the feasibility of implementing a referral and management system for cryotherapy-ineligible women in a “screen-and-treat” cervical cancer prevention program targeting HIV-infected women in Zambia.
Methods
We established criteria for patient referral, developed a training program for loop electrosurgical excision procedure (LEEP) providers, and adapted LEEP to a resource-constrained setting.
Results
We successfully trained 15 nurses to perform visual inspection with acetic acid (VIA) followed by immediate cryotherapy. Women with positive tests but ineligible for cryotherapy were referred for further evaluation. We trained four Zambian physicians to evaluate referrals, perform punch biopsy, LEEP, and manage intra-operative and post-operative complications. From January 2006 through October 2007, a total of 8823 women (41.5% HIV seropositive) were evaluated by nurses in outlying prevention clinics; of these, 1477 (16.7%) were referred for physician evaluation based on established criteria. Of the 875 (59.2% of 1147 referred) that presented for evaluation, 748 (8.4% of total screened) underwent histologic evaluation in the form of punch biopsy or LEEP. Complications associated with LEEP included anesthesia reaction (n=2) which spontaneously resolved, intra-operative (n=12) and post-operative (n=2) bleeding managed by local measures, and post-operative infection (n=12) managed with antibiotics.
Conclusion
With adaptations for a resource-constrained environment, we have demonstrated that performing LEEP is feasible and safe, with low rates of complications that can be managed locally. It is important to establish referral and management systems using LEEP-based excisional evaluation for women with cryotherapy-ineligible lesions in VIA-based “screen-and-treat” protocols nested within HIV-care programs in resource-constrained settings.
Keywords: Cervix, Cancer, Loop electrosurgical excision procedure (LEEP), HIV, Zambia
Introduction
Invasive cervical cancer is a preventable disease diagnosed in 493,000 women and resulting in 274,000 deaths worldwide each year [1]. Greater than 20% of the world's annual deaths from cervical cancer occur in sub-Saharan African countries, like Zambia, where cervical cancer is the most common malignancy and the leading cause of cancer-related deaths among women [1,2]. Limited or non-existent resources for screening and treatment of precancerous lesions result in the vast majority of women presenting with incurable disease at the time of diagnosis [2].
Human papillomavirus (HPV) is more prevalent among HIV-infected women and co-infection with HPV and HIV leads to higher and more rapid progression rates of cervical intraepithelial neoplasia (CIN), the precursor to cervical cancer [3]. Increasing numbers of HIV-infected women in resource-constrained nations are gaining access to antiretroviral therapy (ART) [4,5]. This may lead to increasing life expectancy, higher cumulative risk for persistent oncogenic HPV infections and subsequent development of CIN [6,7]. A significant proportion of HIV-infected women seeking antiretroviral therapy (ART) in Zambia harbor high-risk HPV-infection and CIN at baseline; thus it is critical to provide frequent screening and appropriate treatment services to these women to lower their risk of developing invasive cervical cancer [8,9].
Single-visit “screen-and-treat” programs using visual inspection with acetic acid (VIA) linked to cryotherapy have been shown to be effective methods of cervical cancer prevention in resource-constrained settings [10–12]. We recently established Zambia's first VIA-based cervical cancer prevention program, implemented as a routine healthcare service for women attending government-operated health clinics [13,14]. Building upon an existing HIV-care and treatment infrastructure, we targeted service provision to HIV-infected women who were accessing antiretroviral therapy and HIV care through a President's Emergency Plan for AIDS Relief (PEPFAR)-funded program [15]. Trained nurses served as primary health care providers and performed VIA and cryotherapy, while referring women with cryotherapy-ineligible lesions to a central, university hospital-based clinic. At the referral clinic, trained physicians performed punch biopsy and/or loop electrosurgical excision procedure (LEEP), an office-based surgical technique shown to be safe and effective for the management of precancerous cervical lesions (cervical intraepithelial neoplasia—CIN) [16]. This paper describes the feasibility and operationalization of providing referral and management services, inclusive of LEEP-based excisional evaluation, within a cervical cancer prevention program targeting HIV seropositive women in a resource-constrained sub-Saharan African nation.
Materials and methods
From January 2006 to October 2007, we implemented VIA-based “screen-and-treat” cervical cancer prevention services in 13 outlying primary care government-operated clinics and a tertiary care hospital in Lusaka, Zambia. We describe the steps taken to effectively implement the referral and management system for cryotherapy-ineligible women needing further evaluation.
Targeting HIV-infected women
Zambian Cervical Cancer Prevention Program clinics have been developed within the context of an HIV-care and treatment program supported through PEPFAR. Given the high risk of cervical precancer and cancer in HIV-infected women in Zambia[8], we undertake the following focused activities for enrolling HIV-infected women: first, we train HIV peer educators (lay health workers) to deliver messages in the community about the importance of cervical cancer screening in HIV-infected women as they sensitize women to be tested for HIV. Second, cervical cancer peer educators (lay health workers) give daily cervical cancer prevention talks in the HIV-care and treatment clinics in each of the facilities where our cervical cancer prevention services are offered. Third, cervical cancer peer educators navigate HIV-infected women who are interested in being screened into cervical cancer prevention clinics. Fourth, staff members in the HIV-care and treatment clinics are strongly encouraged to refer every HIV-infected woman to the clinics to be screened for cervical cancer. And fifth our nurses educate patients about the high risk of cervical cancer in HIV-infected women and are trained DCT (diagnostic counseling and testing for HIV) counselors.
Establishing a referral system
Following VIA-based “screen-and-treat” protocol, nurses performed immediate cryotherapy and effectively referred patients requiring further evaluation [17]. Referral reasons included visual characteristics that are predictive of extensive high-grade disease or invasion or abnormalities that precluded successful cryotherapy [18]. See Table 1. Women requiring referral were given an appointment at the tertiary care hospital, University Teaching Hospital (UTH), within 1–2 weeks. In evaluating referred patients, physicians repeated VIA and reviewed nurse referral reasons. Physicians performed punch biopsy or LEEP when indicated. Women with clinically invasive cancer assessed to be at risk for hemorrhage following punch biopsy were referred to another setting within UTH better prepared to manage such events. Patients with other complaints and gynecologic disorders unrelated to a positive VIA test were referred to the appropriate gynecologic services at the University Teaching Hospital.
Table 1.
Ineligibility criteria for cryotherapy
| Incomplete visualization of acetowhite lesion |
| Acetowhite lesion occupying ≥3 quadrants or ≥75% of the transformation zone |
| Acetowhite lesion unable to be entirely covered by the tip of the cryoprobe |
| Presence of a cervical lesion (e.g. polyp) or anatomic defect (e.g. scarring or fibrosis) that prevents flush contact between acetowhite lesion/transformation zone and the cryoprobe |
| Suspicious of cancer |
Training of physicians to perform LEEP
Because LEEP was not previously available in Zambia, we instituted a comprehensive training curriculum for physicians at UTH (Fig. 1). We developed a four-step plan that included didactic sessions and practical training. Physician-trainees first familiarized themselves with the equipment using simulated models [19]. They observed 10 LEEP procedures performed by experienced gynecologists (GP and MM), performed 25 procedures under close supervision of experienced gynecologists, then performed 25 additional procedures with the experienced gynecologist in close proximity. Once the trainee appropriately performed a total of 50 LEEPs, he/she was certified to have completed training and eligible to practice independently. In our program the process was usually completed within 4 weeks, after which time the trainee received a certificate of competency in LEEP provision and management of complications.
Fig. 1.
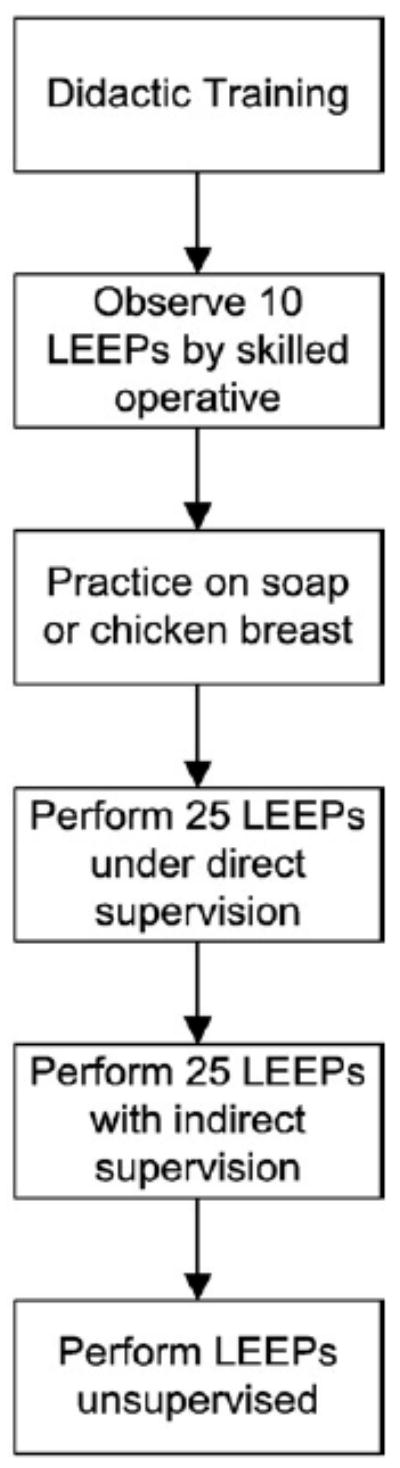
LEEP training.
Adapting LEEP to a resource-constrained environment
We encountered numerous challenges to implementing routine and reliable LEEP services in this resource-constrained setting. In Table 2, we list several of these barriers as well as the local solutions devised to address them.
Table 2.
Common challenges and simple solutions for managing a loop electrosurgical excision procedure clinic in a resource-constrained setting
| Challenge | Solution |
|---|---|
| Cervicitis/vaginitis at time of evaluation | Low grade infection: concomitant treatment of infection a and cervical lesion. |
| High-grade infection (copious yellow discharge or upper abdominal symptoms/signs): treat infection a first and give appointment date for LEEP in 2 weeks. | |
| Voltage mismatch | Step-down unit to adjust voltage to in-country requirements. |
| Voltage irregularities | Voltage regulator to filter incoming current to stable outgoing current. |
| Inadequate hemostatic equipment | Use ferric subsulfate solution and manual pressure when interruption of electrical current results in inability to cauterize. Keep suture kit on hand in case of emergency. In the absence of a proper needle driver use sponge forceps or any type of available clamp as needle holder. |
| Water shortage | Keep 10–20 l of clean water in the clinic as a reserve for when water supply is interrupted. |
| Waste disposal | Coordinate with hospital biohazardous waste disposal. If that fails, find a non-government organization (NGO) partner to create regular collection times and consider building an incinerator. |
| Trained assistants | Train a layperson to sterilize equipment, autoclaves, prepare exam room, give health talks, and assist LEEPs. |
| Take care to explain special instructions for insulated speculums. If same sterilization techniques for non-insulated speculums are used for insulated speculums, they will be destroyed after one or two uses. | |
| Non-availability of cervical needles, syringes, and anesthetic | Consult local dentists as dental needles, syringes and anesthetic can be used for cervical anesthesia. |
| Inadequate pathology services | Investigate local laboratory capacity, as it may not meet increased demands created by the LEEP program. |
| |
| Explain expected time delay for histopathology results to clients. | |
| Timely delivery of supplies | Develop a forecasting, delivery and storage system. Take into account supply shortages, driver/vehicle requirements for transport, and time lag between ordering and receiving supplies. |
| Out of country supply orders | For equipment purchased outside the country, shipment should include a very specific, itemized listing of package contents (order number, quantity, shipping codes and any other information) to identify and track equipment. |
| Equipment cost | Sterilize and reuse electrodes by cleaning in soapy water followed by 10 min of decontamination in 0.5% chlorine solution, water rinse, and 30 min autoclaving. Use regular needles rather than specialty (Potocky™) needles. |
Cervicitis and vaginitis treated in accordance with WHO syndromic treatment guidelines.
Pathology
Tissue specimens were reviewed in Zambia by a United Kingdom-trained, board-certified Zambian senior pathologist (VM) in the Pathology department of UTH.
Post-surgical follow-up
Women were asked to return to the Gynecologic Cancer Prevention Unit for pathology results and follow-up 6 weeks after punch biopsy or LEEP. Gynecologic complaints prompted pelvic examination. Women with preinvasive cancer received appointments for six- and 12-month post-LEEP repeat VIA examination in the clinics from which they were referred. Those with stages IA1 to IB2 were referred to the Gynecology department at UTH for appropriate surgery and those with >stage IIA were referred to the newly established Cancer Disease Hospital within UTH for radiation therapy or palliative care.
Results
Integration in pre-existing infrastructures
One of the keys to sustainability of a new program is integration into pre-existing infrastructures. Towards this end we placed our screening and LEEP program at government-operated health facilities. One of the program co-directors (MM) is a full-time university-based Zambian gynecologist; the other program co-director (GP) is a U.S. trained and board-certified gynecologic oncologist and citizen but has received a full-time appointment as Honorary Professor in the Department of Obstetrics and Gynecology at the same university. The University Senate recently approved an application for the Cervical Cancer Prevention Program to become an official rotation for Zambian medical students and Obstetrics and Gynecology residents/registrars as part of their formal medical training. The Ministry of Health has signed off on the development of a curriculum that would lead to a Cervical Cancer Prevention Nurse Certificate, on par with Maternal Child Health, Nurse Midwifery, etc.
Referral for LEEP
Since the initial roll-out of our “screen-and-treat” program in January 2006 until October 2007, we screened a total of 8823 women. These include 3661 (41.5%) who were HIV-infected (HIV seropositive), 2534 (28.7%) HIV seronegative, and 2628 (29.8%) of unknown HIV serostatus. HIV-infected women have access to ART through the PEPFAR-supported HIV-care and treatment program in the Lusaka District Health Management clinics if they meet World Health Organization laboratory and clinical criteria. Among our HIV-infected patients, 69.3% had documented use of ART. A total of 2378 (26.9%) women were treated with cryotherapy for positive VIA tests while 1477 (16.7%) were referred for physician evaluation. Despite a lack of any formal patient tracking system, 875 women (59.2% of those referred) kept their appointments for a physician evaluation at the Gynecologic Cancer Prevention Unit at the University Teaching Hospital. Physician evaluation revealed 106 women with gynecologic conditions that did not require histological evaluation of the cervix and 21 women with clinically evident invasive cervical cancer who were assessed to be at risk for hemorrhage following punch biopsy and thus referred to another setting within UTH better prepared to manage such events. 748 women (8.4% of total women screened) underwent LEEP. These included 465 (62.2%) who were HIV-infected, 116 (15.5%) who were HIV seronegative and 167 (22.3%) whose HIV serostatus was unknown at the time of screening.
Intra-operative and post-operative complications
Complications commonly cited for LEEP are peri-operative/intra-operative bleeding, post-operative bleeding, cervical infection and cervical stenosis [18]. Intra-operative bleeding is described as bleeding that requires vaginal packing or suturing for hemostasis. Mild to moderate post-operative bleeding is defined as bleeding that prompts a return visit to the clinic requiring re-cauterization or application of Monsel's solution, while severe post-operative bleeding is defined as bleeding that requires hospital admission, blood transfusion or suturing. Two women experienced uncontrollable trembling of the lower extremities after the cervical injection of 2% lidocaine with 1:80,000 epinephrine which spontaneously resolved in 5 min. Twelve women who underwent LEEP experienced intra-operative bleeding and 2 experienced post-operative bleeding that prompted a return visit to the clinic. None required blood transfusions or intravenous fluids and all were managed successfully with local measures in the clinic, on an outpatient basis. Of the 12 women who experienced intra-operative bleeding uncontrolled by cautery, 10 had arterial bleeders at the surgical site and 2 had incidental vaginal sidewall lacerations. Cervical arterial bleeding was controlled by the local application of a hemostatic agent (Monsel's solution) coupled with vaginal packing in 3 patients and suturing in the remaining 7 patients. Patients treated with vaginal packing were asked to lie still on a clinic bench for 2 h after which time the pack was removed and they were discharged home. The 2 cases of bleeding secondary to vaginal lacerations were controlled by suturing. No anesthesia was required in any of the cases requiring suturing. Two women had excessive bleeding from the surgical site 24 h post-excision, requiring an application of Monsel's solution to the cervix and immediate discharge from the clinic. None of our patients experienced severe post-operative bleeding. Twelve women returned with vaginal discharge consistent with a post-operative surgical site infection, for which antibiotics were prescribed and the condition resolved. None of our patients reported complaints consistent with cervical stenosis. Complications by HIV serostatus are listed in Table 3.
Table 3.
Intra- and post-operative complications
| LEEP complications | HIV seropositive (n=465) |
HIV seronegative (n=116) |
Unknown HIV serostatus (n=116) |
|---|---|---|---|
| Intra-operative | |||
| Anesthetic reaction | 1 (0.002%) | 0 (0%) | 1 (0.006%) |
| Intra-operative bleeding a | 6 (0.012%) | 3 (0.025%) | 3 (0.018%) |
| Post-operative | |||
| Mild to moderate post-operative bleeding b | 1 (0.002%) | 0 (0%) | 1 (0.006%) |
| Cervical infection | 4 (0.008%) | 6 (0.05%) | 2 (0.012%) |
Note: percentages denote cell proportions within total of each column, i.e. HIV serostatus.
Defined as bleeding that requires suturing or vaginal packing for hemostasis.
Defined as bleeding that prompts a return visit to clinic and requires re-cauterization or application of Monsel's solution.
Pathology
Among women who underwent histologic evaluation, 155 had CIN I, 144 had CIN II/III, and 149 had invasive cancer (58% microinvasive). Disease prevalence according to HIV serostatus is presented in Table 4.
Table 4.
Disease prevalence according to HIV serostatus
| Pathologic diagnosis | Overall | Proportion of pathologic diagnoses by HIV serostatus | ||
|---|---|---|---|---|
| HIV seropositive | HIV seronegative | Unknown HIV serostatus | ||
| CIN I | 155 | 63% | 21% | 16% |
| CIN II/III | 144 | 74% | 15% | 11% |
| Invasive cancer | 149 | 60% | 27% | 13% |
Discussion
We established a referral and management system for women screened and treated by nurses in a single-visit “screen-and-treat” cervical cancer prevention program targeting HIV-infected women in a resource-constrained setting in Zambia. By establishing criteria for referral, training physicians to perform LEEP, and adapting LEEP to the environmental context, we successfully operationalized the program. Referral criteria enabled nurses to triage patients for immediate cryotherapy or referral for physician evaluation. Our LEEP training program produced four physicians with a high degree of skill in both performing the procedure and managing complications. Based on our case volume the training was completed within 4 weeks.
Complications included intra-operative and post-operative bleeding managed effectively by local measures (vaginal packing, application of a haemostatic agent or suturing), self-limited anesthesia reaction, and post-operative infections that resolved with antibiotic treatment. Since the overall rate of complications was very low, the sample size was not adequate to allow us to compare for any significant difference in complications between HIV seropositive and HIV seronegative patients. Although our intra-operative complication rate is minimal and substantially documented, it is possible that post-operative complication rates are under-reported due to low patient compliance, despite our efforts to emphasize to patients and accompanying family members the importance of adhering to the prescribed post-surgical follow-up regimen. During normal working hours, patients have access to the Gynecologic Cancer Prevention Unit as well as the clinics from which they were referred, where the nurses have been trained to recognize, treat, and document post-operative complications. UTH is open 24 h a day and is 2–3 miles away from the communities surrounding the referral clinics with bus routes to the hospital. Studies of LEEP in HIV-infected women in high resource settings report comparable safety profiles [20]. Little has been documented regarding complications following ablational and excisional therapies for CIN in low resource nations, particularly among HIV-infected women. In a small study of 72 women in Zimbabwe, Chirenje et al. reported a 12-month cure rate of 86% in HIV-infected women compared to 100% in HIV seronegative women but did not discuss complications [21]. In a study of 60 HIV-infected women receiving LEEP in Thailand, no significant difference in complications could be demonstrated between HIV-infected women and the control group nor between HIV-infected women receiving antiretroviral therapy and those not receiving antiretroviral therapy [22]. We confirm the safety profile of LEEP in HIV-infected women in a resource-constrained setting with the largest such cohort reported based on our review of the English language medical literature. Despite encountering barriers common to low resource nations (see Table 2) we established LEEP services to effectively fulfill second tier needs of a VIA-based “screen-and-treat” cervical cancer prevention program. The provision of these services fills the gap between cryotherapy treatment and inpatient diagnostic/therapeutic procedures (conization, hysterectomy).
Major limitations to the sustainability and effectiveness of a LEEP program in a resource-constrained nation include limited availability of certain medical supplies, low patient follow-up, the cost to participants, and demands on pathology services. LEEP maintenance and replacement parts are unavailable within Zambia, requiring constant outsourcing. We are working to develop a more effective patient follow-up system as only 59.2% of women referred actually presented for evaluation. Reasons most often given for not presenting for evaluation include (1) difficulty getting partner support for post-treatment sexual abstinence (6 weeks), (2) fear of the procedure itself, and (3) travel costs to reach the hospital from outlying areas. As a service delivery program, we cannot offer incentives to patients to present for physician evaluation or to comply with prescribed post-surgical follow-up. However, we do not charge any fees to perform biopsy or LEEP and the UTH Pathology department charges only 5000 Zambian Kwacha (about US $1.40) to process and analyze tissue specimens. The cost of public transportation to reach UTH and return home from even the furthest areas of referral in Lusaka is 10,000 Zambian Kwacha (about US $2.80) or less. A successful referral and management program requires adequate pathology personnel and supplies. Historically, health care staff with these specialized pathology skills have been difficult to retain in Zambia (Personal communication, Victor Mudenda, MD). The overwhelming case burden and severe shortage of trained staff in the Pathology department have made it impossible to comment on margin status. Given the long time period that it takes to train pathologists, and their persistent brain drain once trained, we have begun exploring the possibility of establishing a digital pathology center within the UTH Department of Pathology to allow for automated bulk processing, faster interpretation of the tissue specimens and ability to report margin status. This system will also allow for distance consultation (telepathology) and improve overall quality of cervical cancer pathology as well as other pathology services at UTH by immediately increasing pathology manpower without the necessity of on-site presence.
The outcomes of our program emphasize the need for operations and implementation research in the following areas: (1) digital pathology to improve accuracy and efficiency of histopathology services and (2) interventions to increase patient adherence to follow-up recommendations. We plan to implement an appointment reminder system where patients receive a text message reminding them of their appointment the day before it is scheduled. We will call patients who miss appointments in order to reschedule. If the text message and phone call fail or patients do not have access to a phone, lay health workers will follow-up in person at the patient's home. Programmatic implications include a need to expand LEEP training to include nurses and allied healthcare personnel (e.g., physicians' assistants), increase training and interest among medical students and residents and creation of a national cervical cancer prevention strategy.
Increasing attention and resources are being targeted for implementing cervical cancer screening programs in the context of HIV-care programs in Africa and other resource-constrained settings. We are undertaking a cost effectiveness analysis of our program to provide additional information to others who plan to establish critically important referral centers for cryotherapy-ineligible women in resource-constrained environments like Zambia. Our experience, one of few in a real-world program setting, has highlighted the role of gynecologic evaluation and linked pathological diagnosis for lesions not treatable by cryotherapy and the need for inclusion of these components in a comprehensive cervical cancer prevention program for resource-constrained settings.
Acknowledgments
This work was supported by the President's Emergency Plan for AIDS Relief (PEPFAR) grant through the Elizabeth Glaser Pediatric AIDS Foundation from the Centers for Disease Control and Prevention Global AIDS Program, the National Institutes of Health through the Center for AIDS Research at UAB (NIH grant P30AI027767), the International Clinical Research Scholars/Vanderbilt-UAB AIDS International Training and Research Program (NIH grant D43TW001035 and R24 TW007988), Career Development award (NIH grant K23 AI01411), and an Elizabeth Glaser Pediatric AIDS Foundation Clinical Scholars Award.
Footnotes
Conflict of interest statement: The authors declare that they have no conflicts of interest to disclose.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. doi: 10.3322/canjclin.55.2.74. [DOI] [PubMed] [Google Scholar]
- 2.Kanavos P. The rising burden of cancer in the developing world. Ann Oncol. 2006;17(Suppl 8):viii15–23. doi: 10.1093/annonc/mdl983. [DOI] [PubMed] [Google Scholar]
- 3.Chirenje ZM. HIV and cancer of the cervix. Best Pract Res Clin Obstet Gynaecol. 2005;19:269–76. doi: 10.1016/j.bpobgyn.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 4.McCarthy M. The Global Fund: 5 years on. Lancet. 2007;370:307–8. doi: 10.1016/S0140-6736(07)61144-1. [DOI] [PubMed] [Google Scholar]
- 5.UNAIDS. 2007 AIDS epidemic update. Joint United Nations programme on HIV/AIDS. 2007 [Google Scholar]
- 6.Heard I, Palefsky JM, Kazatchkine MD. The impact of HIV antiviral therapy on human papillomavirus (HPV) infections and HPV-related diseases. Antivir Ther. 2004;9:13–22. [PubMed] [Google Scholar]
- 7.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55:244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Parham GP, Sahasrabuddhe VV, Mwanahamuntu MH, Shepherd BE, Hicks ML, Stringer EM, et al. Prevalence and predictors of squamous intraepithelial lesions of the cervix in HIV-infected women in Lusaka, Zambia. Gynecol Oncol. 2006;103:1017–22. doi: 10.1016/j.ygyno.2006.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sahasrabuddhe VV, Mwanahamuntu MH, Vermund SH, Huh WK, Lyon MD, Stringer JS, et al. Prevalence and distribution of HPV genotypes among HIV-infected women in Zambia. Br J Cancer. 2007;96:1480–3. doi: 10.1038/sj.bjc.6603737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Blumenthal PD, Lauterbach M, Sellors JW, Sankaranarayanan R. Training for cervical cancer prevention programs in low-resource settings: focus on visual inspection with acetic acid and cryotherapy. Int J Gynaecol Obstet. 2005;89(Suppl 2):S30–7. doi: 10.1016/j.ijgo.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 11.Denny L, Kuhn L, De Souza M, Pollack AE, Dupree W, Wright TC., Jr Screen-and-treat approaches for cervical cancer prevention in low-resource settings: a randomized controlled trial. Jama. 2005;294:2173–81. doi: 10.1001/jama.294.17.2173. [DOI] [PubMed] [Google Scholar]
- 12.Sankaranarayanan R, Esmy PO, Rajkumar R, Muwonge R, Swaminathan R, Shanthakumari S, et al. Effect of visual screening on cervical cancer incidence and mortality in Tamil Nadu, India: a cluster-randomised trial. Lancet. 2007;370:398–406. doi: 10.1016/S0140-6736(07)61195-7. [DOI] [PubMed] [Google Scholar]
- 13.Mwanahamuntu M, Sahasrabuddhe V, Pfaendler K, Mudenda V, Hicks M, Vermund S, Stringer J, Parham G. Challenges and Successes in Linking Cervical Cancer Prevention and HIV Care in Zambia (under review) 2007 doi: 10.1097/QAD.0b013e3283236e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parham G, Mwanahamuntu M, Pfaendler K, Sahasrabuddhe V. Digital cervicography in a “screen and treat” program targeting HIV-infected women in Zambia: risk factors for positive results. 6th AORTIC conference on cancer in Africa; 2007; Cape Town, South Africa. [Google Scholar]
- 15.Stringer JS, Zulu I, Levy J, Stringer EM, Mwango A, Chi BH, et al. Rapid scale-up of antiretroviral therapy at primary care sites in Zambia: feasibility and early outcomes. Jama. 2006;296:782–93. doi: 10.1001/jama.296.7.782. [DOI] [PubMed] [Google Scholar]
- 16.Mitchell MF, Tortolero-Luna G, Cook E, Whittaker L, Rhodes-Morris H, Silva E. A randomized clinical trial of cryotherapy, laser vaporization, and loop electrosurgical excision for treatment of squamous intraepithelial lesions of the cervix. Obstet Gynecol. 1998;92:737–44. [PubMed] [Google Scholar]
- 17.Pfaendler K, Mwanahamuntu M, Sahasrabuddhe V, Stringer J, Vermund S, Parham G. Effective referral of women with histologic evaluation in a “see and treat” cervical cancer prevention program in Zambia. 6th AORTIC conference on cancer in Africa; 2007; Cape Town, South Africa. [Google Scholar]
- 18.Wright TC, Richart RM, Ferenczy A. Electrosurgery for HPV-related diseases of the lower genital tract: a practical handbook for diagnosis and treatment by loop electrosurgical excision and fulguration procedures. New City, New York, USA: Arthur Vision; Anjou, Quebec, Canada: BioVision; 1992. [Google Scholar]
- 19.Reeves KO, Young AE, Kaufman RH. A simple, inexpensive device for teaching the loop electrosurgical excision procedure. Obstet Gynecol. 1999;94:474–5. doi: 10.1016/s0029-7844(99)80050-7. [DOI] [PubMed] [Google Scholar]
- 20.Wright TC, Jr, Koulos J, Schnoll F, Swanbeck J, Ellerbrock TV, Chiasson MA, et al. Cervical intraepithelial neoplasia in women infected with the human immunodeficiency virus: outcome after loop electrosurgical excision. Gynecol Oncol. 1994;55:253–8. doi: 10.1006/gyno.1994.1286. [DOI] [PubMed] [Google Scholar]
- 21.Chirenje ZM, Rusakaniko S, Akino V, Munjoma M, Mlingo M. Effect of HIV disease in treatment outcome of cervical squamous intraepithelial lesions among zimbabwean women. J Low Genit Tract Dis. 2003;7:16–21. doi: 10.1097/00128360-200301000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Kietpeerakool C, Srisomboon J, Suprasert P, Phongnarisorn C, Charoenkwan K, Cheewakriangkrai C, et al. Outcomes of loop electrosurgical excision procedure for cervical neoplasia in human immunodeficiency virus-infected women. Int J Gynecol Cancer. 2006;16:1082–8. doi: 10.1111/j.1525-1438.2006.00518.x. [DOI] [PubMed] [Google Scholar]


