Abstract
SQSTM1/p62 is a multidomain/scaffold for the atypical protein kinase Cs (aPKC). Phosphorylation of AMPA receptors by PKC has been shown to regulate their insertion in the postsynaptic membrane. Here, we directly tested whether p62 could interact with AMPA receptor subunits and influence their trafficking and phosphorylation. GluR1 receptor intracellular loop L2–3 and the ZZ-type zinc finger domain of p62 are essential for the interaction between these two proteins. In this context, both p62 and aPKC-mediated phosphorylation were necessary for surface delivery of the receptor. Our findings reveal that p62 is the first protein identified that interacts with a region of the GluR receptor other than the C-terminal tail. Furthermore, mice deficient in p62 displayed impaired hippocampal CA1 long-term potentiation (LTP), along with diminished surface expression of GluR1 and phos-phorylation of S818. Lastly, we identify a conserved sequence (ISExSL) shared by all p62 interacting-aPKC substrates. These findings support a model where p62 interaction and aPKC phosphorylation act together to mediate AMPA receptor trafficking and long-term synaptic plasticity in the hippocampus.© 2008 Wiley-Liss, Inc.
Keywords: GluR1, aPKC, LTP, phosphorylation, surface delivery
INTRODUCTION
α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)-type glutamate receptors mediate the fastest excitatory synaptic transmission in the mammalian brain. Structurally, AMPA receptors are heterotetrameric cation channels composed of four subunits: GluR1–4, each of which comprises about 900 amino acids with a molecular weight of about 105 kDa, and shares 68–74% amino acid sequence identity. Each AMPA receptor subunit consists of an extracellular N-terminus, three transmembrane spanning domains (TM1, TM3 and TM4), a re-entrant transmembrane domain (TM2), and an intracellular C-terminus. Therefore, the transmembrane domains form two intracellular loops (L1–2 and L2–3) and one extracellular loop (L3–4). The re-entrant TM2 domain contributes to the cation pore channel. The C-terminal cytoplasmic tail of each AMPA receptor subunit is unique and could be associated with specific regulatory proteins, which are related to receptor modification, trafficking, and signaling (Hollmann and Heinemann, 1994; Dingledine et al., 1999).
It is widely believed that long-term potentiation (LTP) and long-term depression (LTD), form the cellular and molecular basis for neuronal plasticity, including learning and memory (Bliss and Collingridge, 1993; Malenka and Nicoll, 1999; Malinow and Malenka, 2002). Trafficking of AMPA receptors to and away from synapses is a mechanism to modulate synaptic strength (Lynch and Baudry, 1984; Malenka and Nicoll, 1999). During LTP expression, additional AMPA receptors are delivered to the postsynaptic membrane (Shi et al., 1999). In contrast, LTD induces receptor internalization (Sheng and Lee, 2001). Therefore, alterations in synaptic strength are directly related to the receptor exocytosis and endocytosis (Malinow and Malenka, 2002). AMPA receptor trafficking to the surface is primarily regulated by two mechanisms: (1) receptor associated proteins that aid in delivery of the receptor to the surface membrane (Hayashi et al., 2000; Fukata, 2005; Palmer et al., 2005), and (2) phosphorylation (Roche et al., 1996; Song and Huganir, 2002; Lee et al., 2000, 2003; Oh et al., 2006; Shepherd and Huganir, 2007). For example, two phosphorylation sites in the C-terminus of GluR1 subunit exist, S845 is a protein kinase A (PKA) site, whereas S831 is a site phosphorylated by calcium/calmodulin-dependent protein kinase II (CaMKII) and protein kinase C (PKC). Recently, an additional phosphorylation site at S818 has been identified for PKC (Boehm et al., 2006). However, the detailed cellular and molecular mechanism for AMPA receptor trafficking is not yet fully understood.
Several members of the PKC family directly phosphorylate AMPA receptor subunits to mediate plasticity (Carvalho et al., 1999; Chung et al., 2000; McDonald et al., 2001; Correia et al., 2003; Boehm et al., 2006; Lee et al., 2007). The hippocampus is the primary brain region responsible for learning and memory. The atypical PKC (aPKC) PKCi/λ and PKMζ are two abundant atypical PKC isoforms expressed in hippocampus (Hernandez et al., 2003; Oster et al., 2004). Likewise, the aPKC scaffold, p62/Sequestosome 1 (SQSTM1), is also primarily expressed in hippocampus (Gong et al., 1999). However, p62 binds to the regulatory region of the aPKCs (Puls et al., 1997; Sanchez et al., 1998), whereas PKMζ lacks this region and therefore would not be expected to interact with p62. Furthermore, mice deficient in p62 exhibit loss of numerous hippocampus-dependent behaviors (Babu et al., 2008). The possibility that the aPKC adaptor functions as a scaffold for aPKC-mediated phosphorylation of the AMPA receptor and possible regulation of trafficking has not been investigated. p62 is a cytoplasmic and membrane-associated protein that possesses six domains: a PB1 domain consisting of SH2 binding motif and aPKC interacting domain (AID), a ZZ-type Zinc finger domain for interaction with aPKC substrates, a tumor necrosis factor receptor-associated factor 6 binding domain, two PEST domains for proteolytic recognition, and a ubiquitin-associated (UBA) domain (Moscat et al., 2007). These domains endow p62 with the ability to associate with many other proteins and enables p62 to serve as a scaffold to recruit substrates of aPKC through its PB1 domain and ZZ-type finger domain (Puls et al., 1997; Gong et al., 1999; Sanz et al., 1999; Cariou et al., 2002; Croci et al., 2003; Kim, 2006). In addition, p62 has also been shown to function as a shuttling protein for endocytosis of polyubiquitinated proteins through interaction with its UBA domain (Geetha et al., 2005).
Here we demonstrate that p62 is an AMPA receptor interacting protein (RIP). The interaction between p62 and AMPA receptor is mediated through the AMPA receptor subunit intracellular loop L2–3 and the ZZ-type Zinc finger domain of p62. Furthermore, LTP was significantly reduced in mice lacking p62 along with a parallel decrease in surface GluR1 and GluR1-pS818 phosphorylation. Altogether, our results reveal that p62 and aPKC play a critical role in synaptic plasticity via regulating AMPA receptor trafficking and phosphorylation.
MATERIALS AND METHODS
Generation of p62 Knock-Out Mice
Knock out (KO) mice (p62−/−) were generated as described previously (Duran et al., 2004). For the duration of the study, all mice were housed in a pathogen free barrier environment. The animals were handled according to the NIH and Auburn University IACUC guidelines.
Antibodies
The mouse GluR1 and GFP monoclonal, rabbit HA, and Myc polyclonal antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA). GluR1 polyclonal, GluR2 and GluR3 monoclonal antibodies, GluR1 phosphorylation antibody pS818 were a kind gift from R.L. Huganir from Johns Hopkins University (Baltimore, MD). P62 monoclonal antibody and PKCi/λ monoclonal antibody were from BD Biosciences (San Jose, CA). Rabbit p62 polyclonal antibody was raised against the full-length p62 (Geetha et al., 2005).
Generation of GluR1 Deletion Constructs
The GluR1 cDNA deletions were performed with a Quick-Change® II XL Site-Directed Mutagenesis Kit according to the manufacturer’s standard protocol (Stratagene, LA Jolla, CA). The primers used for loop L1–2 deletion: Forward 5′-GGAGT GAGCGTCGTCCTCTTCCTGGT CAGCTTTGGCATATT CAACAGCCTGTGGTTCTCC-3′; Backward 5′-GGAGAAC CACAGG CTGTTGAATATGCCAAAGCTGACCAGGAA GAGGACGACGCTCACTCC-3′. The primers used for loop L2–3 deletion: Forward 5′ -CCCTGGGGGCCTTCATGCAG CAAGGATGTATCG TCGGCGGCGTCTGGTGGTTCTTC AC-3′; Backward 5′-GTGAAGAACCACCAGACGCCGC CG ACGATACATCCTTGCTGCATGAAGGCCCCCAGGG-3′. The deletions were confirmed by DNA sequencing and the absence of any nonspecific mutation was confirmed for the GluR1 cDNA.
Preparation of Mouse Brain Lysate
Adult mouse brains were homogenized in ice-cold Triton lysis buffer [50 mM Tris-HCl pH 7.5, 150 mM NaCl, 10 mM NaF, 0.5% Triton X-100, 1 mM Na3VO4, 1 mM phenylmeth-ylsulfonyl fluoride (PMSF), 2 µg/ml leupeptin, and 2 µg/ml aprotinin] plus SDS at different concentration range. The homogenate was sonicated on ice for 10 s three times, followed by rotating the lysates for 30 min, and centrifuged for 10 min at 48°C. The protein concentration was estimated by Bio-Rad DC assay (Hercules, CA), with 1 mg/ml bovine serum albumin (Sigma-Aldrich, St. Louis, MO) as a standard.
HEK Cell Culture and Transfection
Human embryonic kidney (HEK) 293 cells were maintained as previously described (Geetha et al., 2005). The HEK cells were transfected with the calcium phosphate method by using a Mammalian Cell Transfection Kit (Millipore, Billerica, MA) or with cationic lipid method by using Lipofectamine™ 2000 Transfection Reagent (Invitrogen, Carlsbad, CA). The cells were harvested and lysed with Triton lysis buffer (Geetha et al., 2005). Protein concentration was estimated by Bradford assay.
Coimmunoprecipitation and Western Blotting
HEK Cell lysates (750 µg) or brain lysates (1 mg) were diluted in l ml lysis buffer and incubated with 4 µg of primary antibodies at 4°C for 3 h. The immunoprecipitates were collected with agarose-coupled secondary antibodies (Sigma) for at least 2 h and then were washed three times with lysis buffer. Proteins bound to the antibodies were released with protein sodium dodecyl-sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer (2% SDS, 1% β-mercaptoethanol, 0.005% Bromophenol blue, 2% glycerol, and 0.05 M Tris-HCl, pH 6.8).The brain lysates, cell lysates, brain-slice lysate, or immunoprecipitated proteins were resolved by 10% SDS-PAGE under reducing conditions (1% β-mercaptoethanol) and transferred onto nitrocellulose membranes (Amersham Biosciences). The blots were incubated with appropriate antibodies at 4°C overnight, followed by incubation with horseradish peroxidase-conjugated secondary antibodies (Amersham Biosciences) the blots were developed by enhanced chemiluminescence and Hyperfilm (Amersham Biosciences). The captured images were scanned and quantified using the UN-SCAN-IT gel software (Silk Scientific, Orem, UT).
Immunocytochemistry
For localization of AMPA receptor subunits and p62, HEK cells were cotransfected with Myc- or HA-tagged p62 and GluR1, GluR2, or GluR3 with or without GFP tag as indicated in the figures. After 48 h, the cells were fixed, permeabilized, and incubated with rabbit anti-Myc or HA IgG and mouse anti-GluR1, GluR2, or GluR3 IgG for the AMPA receptor subunit construct without GFP tag. The cells were labeled with Texas Red-conjugated antirabbit antibodies (red) and Oregon Green-conjugated antimouse antibodies (Green). Localization was determined by confocal immunofluorescence microscopy and analyzed on a Bio-Rad MRC 1024 Laser Scanning Confocal Microscope.
Hippocampal Slice Preparation
Adult mice were anesthetized, decapitated, and brains were rapidly isolated in ice cold dissection buffer (250 mM sucrose, 25 mM NaHCO3, 25 mM glucose, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, and 1.5 mM MgCl2, pH 7.3). Hippocampal slices (transverse, 300 µm thickness) were cut in ice cold dissection buffer continuously bubbled with a mix of 95% O2 and 5% CO2 using a Tissue Sectioning System (Vibratome, St. Louis, MO). The slices were then transferred to an incubating chamber filled with artificial cerebrospinal fluid (ACSF) (124 mM NaCl, 25 mM NaHCO3, 25 mM glucose, 2.5 mM KCl, 1.25 mM NaH2PO4, 2 mM CaCl2, and 1.5 mM MgCl2, pH 7.3), equilibrated with a mix of 95% O2 and 5% CO2, and incubated at 22–24°C for 1.5 h before use.
Electrophysiology
Field excitatory postsynaptic potentials (fEPSPs) from the Shaffer collateral-CA1 synapses in hippocampal slices from adult mice were recorded at 22–24°C (Bukalo and Dityatev, 2006). Current intensity of test stimuli was set to produce 50% of subthreshold maximum and the test stimuli was applied every 15 s. Stimulus response and paired pulse ratio experiments were performed by varying the stimulus intensity and interpulse interval. LTP was induced by five trains of theta burst stimulation (TBS) (Each train consisted of 10 bursts at 5 Hz; each burst consisted of four pulses delivered at 100 Hz with 15 s interval). Values of LTP were calculated as the increase in the mean slopes of fEPSPs measured 50–60 min after TBS. NMDA receptor mediated fEPSPs were evoked in low Mg2+ (0.1 mM) ACSF supplemented with 10 mM 2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline (NBQX) purchased from Tocris, Ellisville, MO.
Surface Biotinylation
To measure surface level of GluR1 biotinylation was conducted essentially as described (Chan et al., 2006). In brief, acute hippocampal slices from adult mice were transferred to ice-cold ACSF for 2 min, followed by biotinylation in 1 mg/ ml of EZ-Link Sulfo-NHS-SS-Biotin (Pierce) for 45 min with slow agitation at room temperature. The slices were rinsed in cold Tris-based ACSF (HEPES replaced by Tris) for three times to quench free biotin. The slices then lysed in cold homogenized buffer (50 mM NaCl, 10 mM EDTA, 10 mM EGTA, 1 mM Na3VO4, 50 mM NaF, 25 mM NaPPi, 1 mM β-glycerophosphate, 1 mM PMSF, 1 µM microcystine, 2 µg/ml aproptinin, 2 µg/ml leupeptin, 1% Triton X-100, and 50 mM HEPES, pH 7.5). The homogenates were sonicated the on ice for 5 s three times and rotated at 4°C for 1 h. The lysates were centrifuged for 10 min to pellet the insoluble fraction. The protein concentration was examined with the Bradford dye (Bio-Rad). Samples (40 µg) were subjected to SDS-PAGE and Western blot to examine the total GluR1 in the whole lysates (both surface and intracellular fractions). For surface GluR1 analysis, 400 µg protein of each sample in 500 µl homogenization buffer was precipitated with 50 µl of 50% avidin-agarose beads (Pierce, Rockford, IL) for 3 h at 4°C. The beads were pelleted and rinsed three times with homogenization buffer, followed by boiling in 60 µl 1X SDS sample buffer. The samples (surface fraction and total lysates) were subjected to SDS-PAGE and Western blotting with appropriate antibodies.
Statistics
Data were expressed as the mean ± standard error of mean (SEM) Statistical analyses were performed using Excel (Microsoft, Redmond, WA). P-values <0.05 were judged statistically significant.
RESULTS
p62 Interacts with AMPA Receptor Subunits
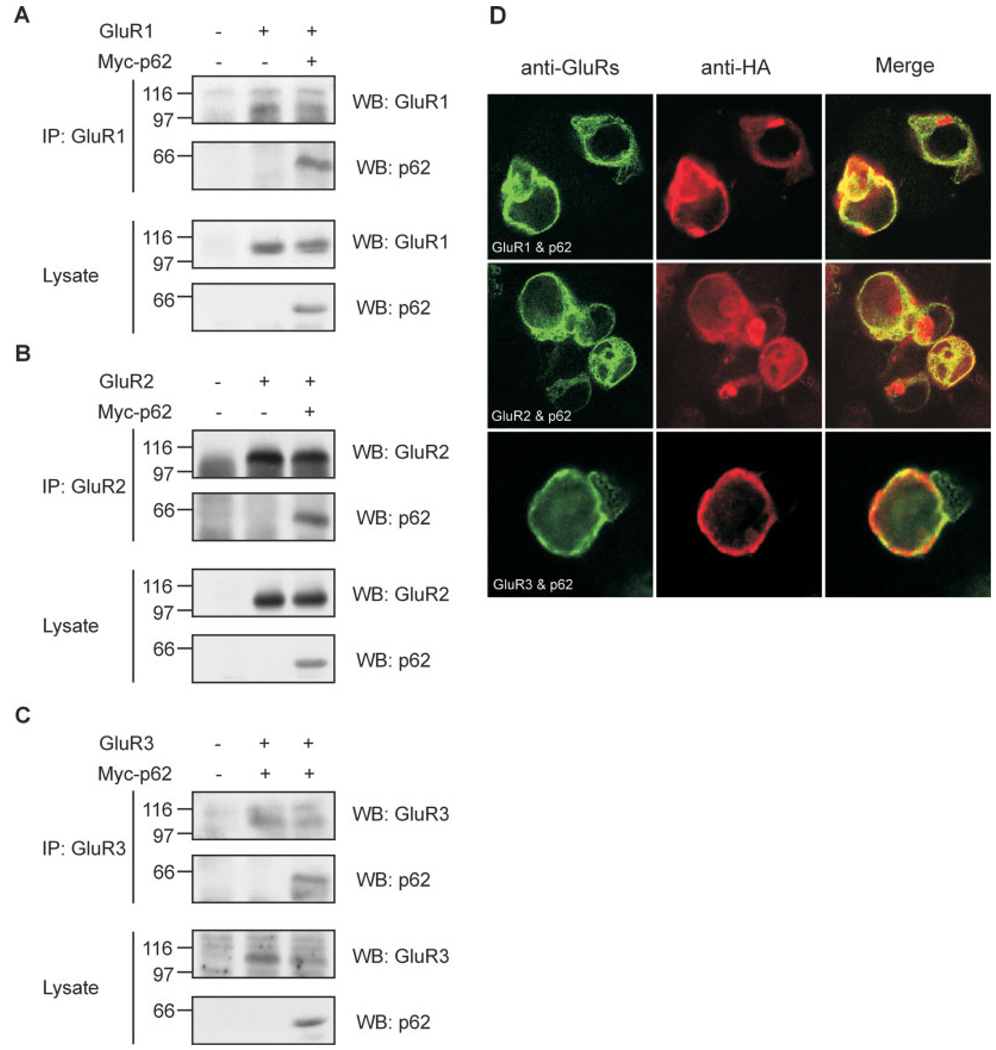
All PKC isoforms, including aPKCs, phosphorylate the AMPA receptor subunit GluR1 both in vitro and in vivo (Boehm et al., 2006; Lee et al., 2007). Therefore, we hypothesized an interaction between the AMPA receptor and the aPKC adaptor, p62, might occur. To examine this possibility, HEK cells were cotransfected with AMPA receptor subunit GluR1, GluR2, or GluR3 and Myc-tagged p62 cDNA constructs followed by immunoprecipitation with appropriate antibodies (Fig. 1A–C). Western blot revealed that the AMPA receptor subunits GluR1–3, which are expressed in the adult mammalian hippocampus, interact with p62 in vitro (Fig. 1–C). As an alternative means to examine interaction, colocalization of GluR1–3 and p62 were also tested by cotransfection of HEK cells, followed by immunofluorescent microscopy. GluR1, GluR2, and GluR3 colocalized with p62 at the cell surface (Fig. 1D).
FIGURE 1.
p62 interacts with GluR1–3 subunits. A–C: HEK cells were cotransfected with GluR1, GluR2 or GluR3 and Myctagged p62. After 48 h, the cells were harvested and lysed with Triton lysis buffer. The cell lysates (750 µg) were immunoprecipitated with anti-GluR1, GluR2 or GluR3, and then Western blotted with anti-GluR1, GluR2 or GluR3, and antip62 (Upper panels). The lysates (50 µg) were also Western blotted with GluR1, GluR2 or GluR3, and p62 antibodies to verify expression of recombinant proteins (lower panels). D: HEK cells were cotransfected with GluR1 (upper panel), GluR2 (middle panel) or GluR3 (lowerpanel), and HA-tagged p62. After 48 h, the cells were fixed, and incubated with rabbit anti-HA IgG followed by labeling with Texas Red conjugated antirabbit antibodies (red) and mouse anti-GluR1, GluR2 or GluR3, and labeled with Oregon Green conjugated antimouse antibodies (Green). The colocalization of GluR2 or GluR3 and p62 was determined by confocal microscopy. GluR1, GluR2, and GluR3 colocalize with p62 in the cell membrane (yellow). All experiments were repeated three independent times with similar results. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
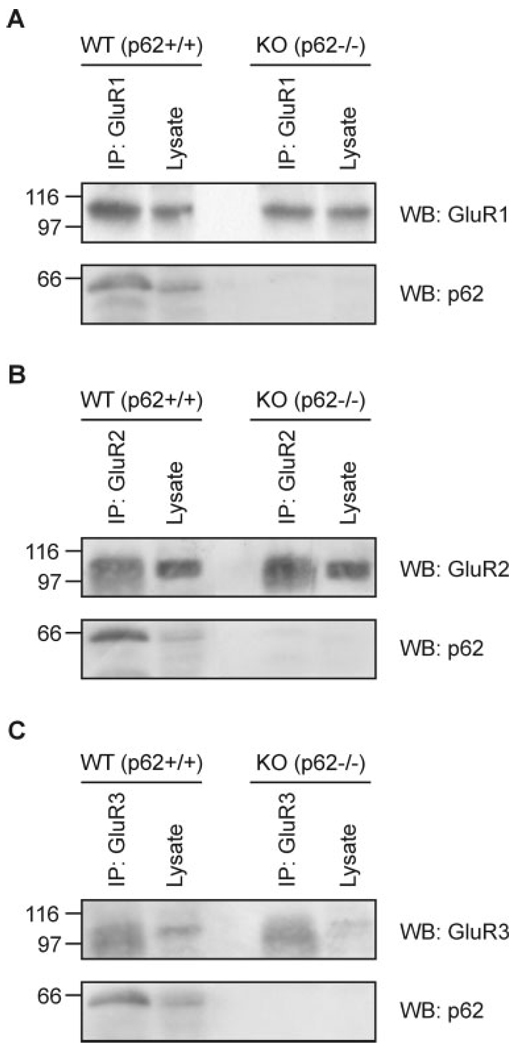
Next, the interaction between p62 and AMPA receptor subunits GluR1–3 in brain lysates prepared from adult wild-type (WT; +/+) and p62 knock-out (KO; −/−) mice was analyzed via coimmunoprecipitation. AMPA receptor subunits GluR1–3 interacted with p62 in the brain lysate from the WT mice; whereas the p62/AMPA receptor interaction was abolished in the p62 knock-out mice (KO) (Fig. 2A–C). As control, an absence of GluR antibody failed to pull-down p62 (not shown). These results validate that p62 interacts with all AMPA receptor subunits expressed in the adult mouse hippocampus both in vitro and in vivo and suggests a physiological role for this protein.
FIGURE 2.
AMPA receptor subunits GluR1, GluR2, and GluR3 interact with p62. A–C: Brain lysates (1 mg) of wild-type (WT) and p62 knock-out (KO) were immunoprecipitated with anti-GluR1, anti-GluR2, and anti-GluR3, and then Western blotted with relevant AMPA receptor subunit antibodies, and antip62. The lysates (40 µg) were also Western blotted with both AMPA receptor subunit and p62 antibodies. The findings are representative of three different experiments with similar results.
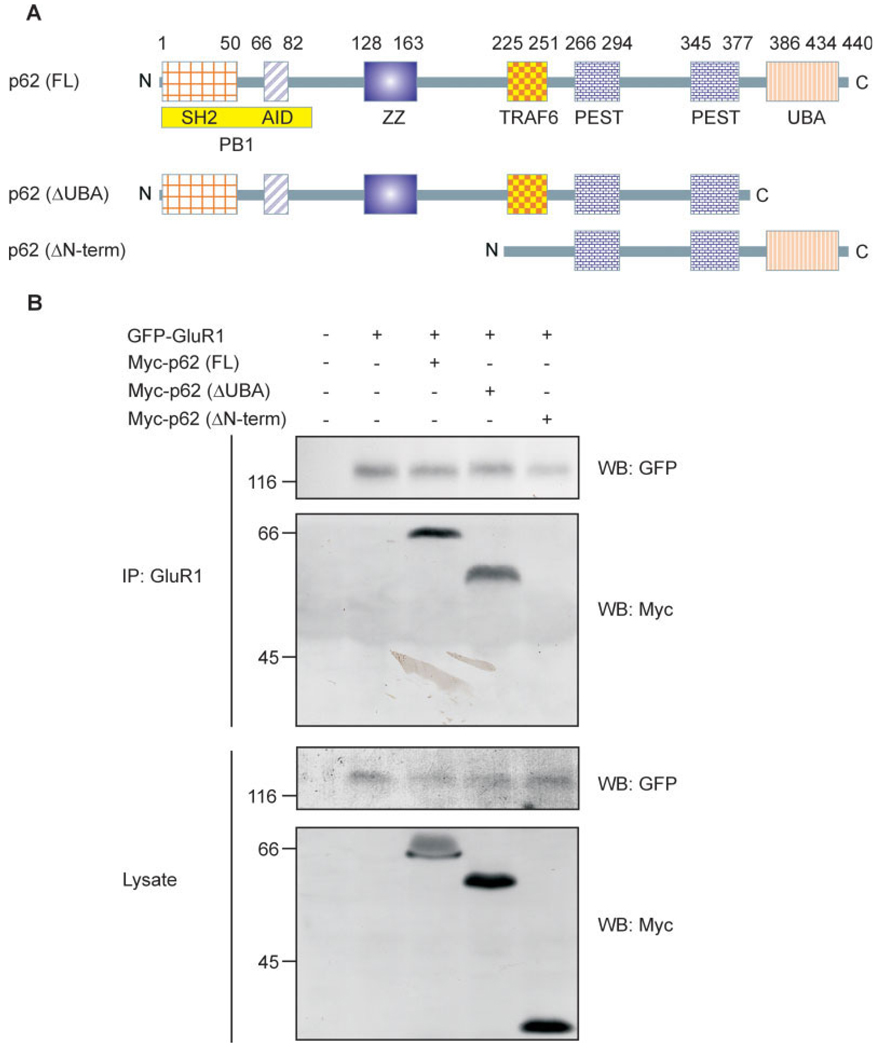
Mapping the Interaction of p62 with GluR1 Subunits
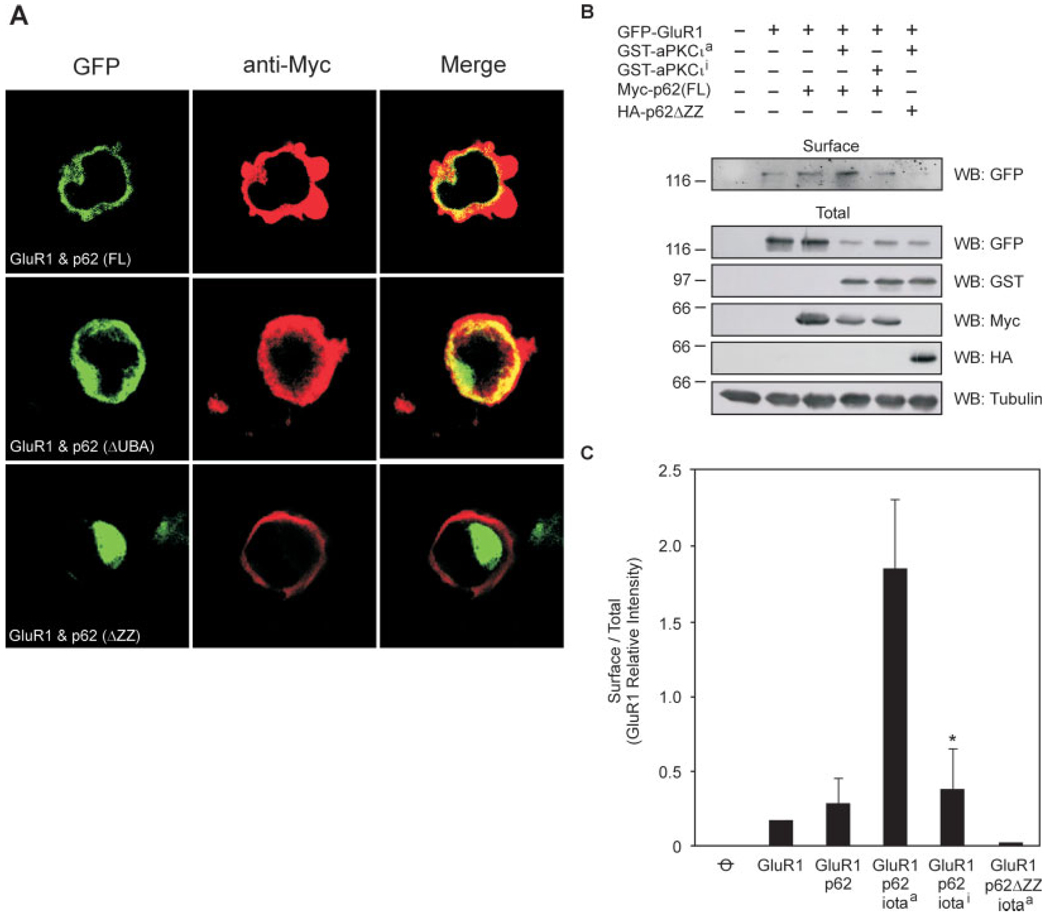
p62 possesses six domains, which enable the protein to recruit many different interactors (Moscat et al., 2007). To investigate which region of p62 is responsible for AMPA receptor interaction, C-terminal and N-terminal truncated p62 constructs were used to examine protein–protein interaction in transfected HEK cells by coimmunoprecipitation (Fig. 3A). Interestingly, the C-terminal truncated p62 (ΔUBA) still interacted with GluR1; whereas the N-terminal truncated p62 (ΔPB1, ΔZZ) lost the capacity to interact with GluR1 (Fig. 3B). These findings indicate that the N-terminus of p62 is essential for AMPA receptor interaction. There are three functional regions located on the p62 N-terminus: SH2 binding site, AID motif, and ZZ-type Zinc finger domain. To further investigate which domain in the N-terminus of p62 interacts with the AMPA receptor, a series of p62 deletion constructs were employed to test the capacity for GluR1 to interact with p62 by cotransfection of HEK cells and coimmunoprecipitation (Table 1). Among these p62 deletion constructs, the deletion of ZZ domain totally abolished the p62/GluR1 interaction. We conclude that the ZZ-type Zinc finger domain of p62 mediates interaction with the AMPA receptor. To examine if these interactions possessed a physiological consequence, we next examined whether p62 regulated GluR1 localization in transfected HEK cells by immunocytochemistry (Fig. 4A). Wild-type p62 and p62 (ΔUBA) colocalized with GluR1 in the cell membrane; whereas p62 (ΔZZ) failed to colocalize with GluR1. Interestingly, expression of GluR1 with the ΔZZ construct resulted in intracellular accumulation of GluR1 (Fig. 4A). These results indicate that interaction of GluR1 with the ZZ-type Zinc finger domain of p62 may be necessary for AMPA receptor surface expression. To further validate this observation, HEK cells were cotransfected with GluR1 along with p62 (WT or ΔZZ) in the presence or absence of active/inactivate GST-aPKC as shown (Fig. 4B). To measure the surface level of GluR1 biotinylation was conducted (Chan et al., 2006) followed by Western blot of the avidin-agarose beads with GFP to detect GluR1. Expression of the constructs was examined in the total cell lysates by blotting with antibodies to GFP, GST, Myc, HA, and Tubulin. The expression of GluR1 on the surface to total was normalized and graphed (Fig. 4C). Although p62 alone increased the expression of GluR1 at the cell surface, inclusion of active aPKC resulted in a significant increase in GluR1 at the cell surface. Expression of the catalytically inactivate aPKC construct, impaired this response. Likewise, absence of the GluR1 interaction site in p62 (ΔZZ) along with expression of active aPKC resulted in diminished surface expression of GluR1. Altogether, these findings reveal that p62 and aPKC play a coordinate role in regulating GluR1 surface expression.
FIGURE 3.
N-terminus of p62 is responsible for AMPA receptor subunit interaction. A: Schematic diagram of Myc-tagged p62 constructs used for mapping of interaction between GluR1 and p62: p62 (WT), p62 (ΔUBA), and p62 (ΔN-term). B: HEK cells were cotransfected with GFP-GluR1 and Myc-tagged p62 (WT), p62 (ΔUBA), or p62 (ΔN-term). After 48 h, the cells were harvested and lysed with Triton lysis buffer. The cell lysates (750 µg) were immunoprecipitated with anti-GluR1, and then Western blotted with anti-GFP and anti-Myc (Upper 2 panels). The lysates (50 µg) were also Western blotted with anti-GFP and anti-Myc (Lower 2 panels) to verify expression of the constructs. These findings are representative of three similar experiments. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Table 1.
Mapping the Interaction of GluR1 with p62
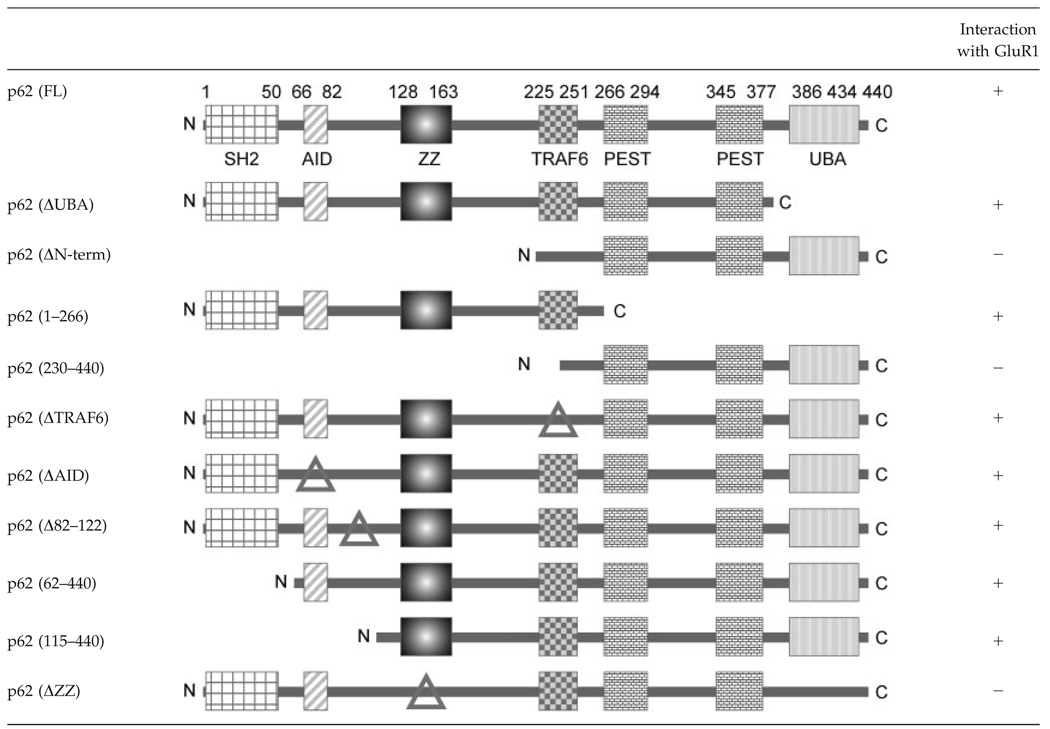 |
Δ: Indicate deletion.
FIGURE 4.
ZZ-type Zinc finger domain of p62 is necessary for GluR1 recruitment to the cell surface. A: HEK cells were cotransfected with GFP-tagged GluR1 and Myc-tagged p62 (WT), p62 (ΔUBA), or p62 (ΔZZ). After 48 h, the cells were fixed, followed by addition of rabbit anti-Myc IgG and labeled with Texas Red-conjugated antirabbit antibodies (red). The colocalization of GluR1 and p62 (yellow) was examined by confocal microscopy. B: HEK cells were cotransfected as shown with GFP-GluR1, GST aPKC active (a)/inactive (i), Myc-tagged full length (FL) p62 and HA-tagged DZZ p62. The surface expression of GluR1 was examined by biotinylation. A fraction (50 µg) of the total lysate was blotted with antibodies to GFP, GST, Myc, HA, or tubulin as shown. C: The blots were scanned and the relative expression of GluR1 on the surface was normalized to the total GluR1 expressed in each sample (X ± S.E.M., N = 3); GluR1/p62/iota active > GluR1/p62/iota inactive (*P < 0.05). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Intracellular Loop L2–3 of GluR1 is Critical for p62 Interaction
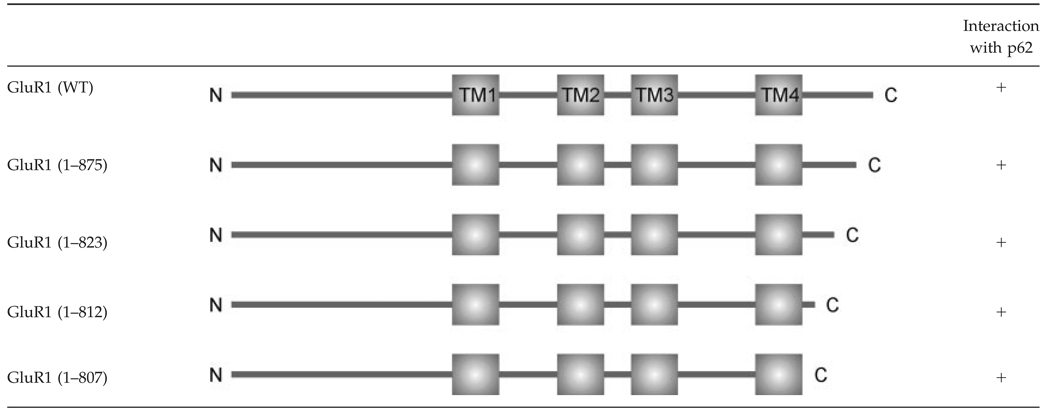
To date, most AMPA receptor associated proteins have been discovered to interact with the intracellular C-terminus of the receptor (Malinow and Malenka, 2002; Shepherd and Huganir, 2007). Therefore, we hypothesized that p62 might also interact with the AMPA receptor subunit C-terminus. To test this possibility, a series of C-terminal truncated GluR1 constructs were employed to map the interaction between the GluR1 and p62 in transfected HEK cells by coimmunoprecipitation (Shen et al., 2000). Surprisingly, all C-terminal truncated GluR1 constructs were observed to interact with p62. Total truncation of the GluR1 C-terminus did not impair interaction with p62 (Table 2).
Table 2.
Mapping the Interaction of p62 with GluR1
 |
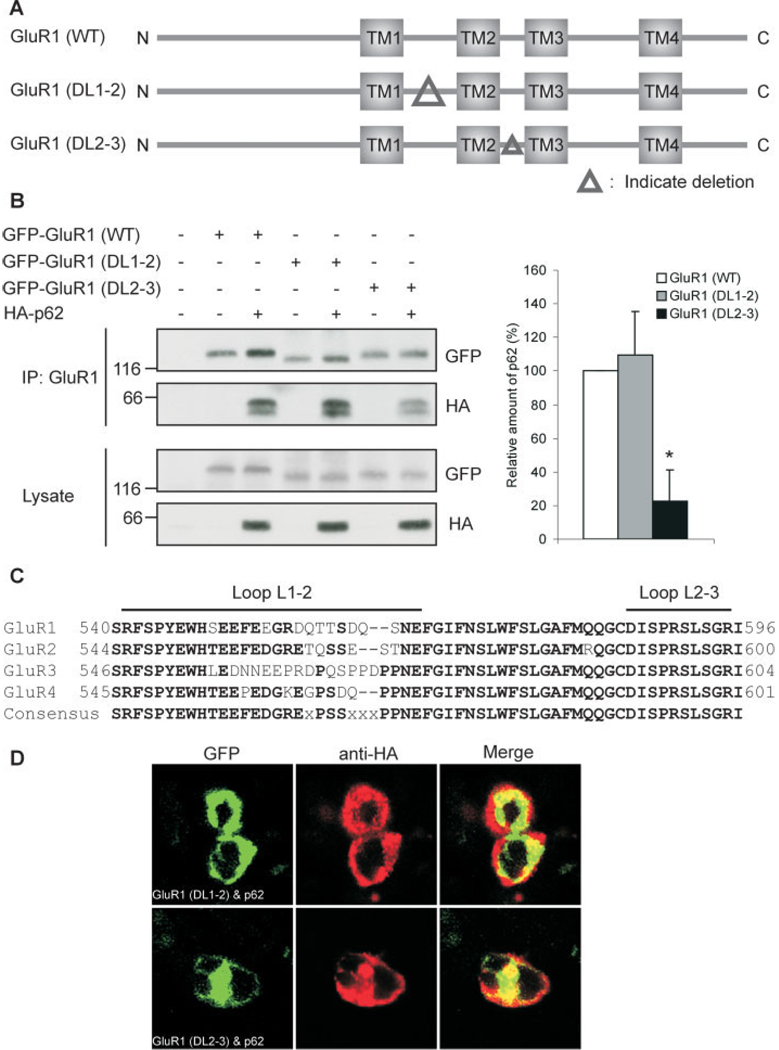
The three transmembrane domains of AMPA receptor subunit form three intracellular regions: C-terminus, loop TM1-TM2 (L1–2) and loop TM2-TM3 (L2–3) (Hollmann and Heinemann, 1994). As GluR1 C-terminus is not responsible for the interaction with p62, these two intracellular loops might be the p62 interaction site. To test this possibility, two GluR1 deletion constructs were generated: GluR1 (ΔL1–2) and GluR1 (ΔL2–3), which lacks the intracellular loop L1–2 and loop L2–3, respectively (Fig. 5A). Compared with the wild-type GluR1, deletion of L1–2 had no effect on GluR1/p62 interaction; whereas deletion of L2–3 significantly reduced the GluR1/p62 interaction to 78% of control (N 5 4, P < 0.005) (Fig. 5B). A residual degree of interaction was observed between the ΔL2–3 mutant and p62. As aPKC phosphorylates the C-term tail of GluR1 (Boehm et al., 2006), it is possible that endogenous aPKC may still recruit p62 to the GluR1 receptor or another C-term interacting protein may also interact with p62 as well. These results suggested that the intracellular loop L2–3 of GluR1 is critical for GluR1/p62 interaction. Alignment of GluR family members reveals that the intercellular loop L2–3 is completely conserved among all AMPA receptor subunits GluR1–4 (Fig. 5C). This finding is consistent with the observation that p62 interacts with all AMPA receptor subunits (Fig. 1A–D and Fig. 2A–C). The functional role for this interaction was tested in transfected HEK cells by immunocytochemistry. Interestingly, in the absence of the L1–2 region, GluR1 still colocalizes with p62 at the cell surface; whereas the deletion of L2–3, the p62 interaction site, impaired colocalization between GluR1/p62 at the cell membrane (Fig. 5D). Likewise, deletion of GluRΔL2–3 but not GluRΔL1–2 along with active aPKC and p62 resulted in diminished surface expression of biotinylated GluR1 (not shown). These results further suggest that interaction between p62 and the AMPA receptor may be necessary and functionally relevant.
FIGURE 5.
AMPA receptor subunits interact with p62 though the intracellular Loop L2–3. A: Schematic diagram of GFP-tagged GluR1 (WT), GluR1 (ΔL1–2), and GluR1 (ΔL2–3) mutant constructs. B: HEK cells were cotransfected with GFP-tagged GluR1 (WT), GluR1 (ΔL1–2), or GluR1 (ΔL2–3) and HA-tagged p62. After 48 h, the cells were harvested and lysed with Triton Lysis buffer. The cell lysates (750 µg) were immunoprecipitated with anti-GluR1, then Western blotted with anti-GluR1 and anti-HA (Left upper 2 panels). The lysates (50 µg) also were Western blotted with anti-GFP and anti-HA to verify expression of recombinant constructs. (Right) The relative interaction of p62 with GluR1 mutants was examined by coimmunoprecipitation and the resulting blots scanned. The experiment was repeated four independent times. The data represent the mean ± SEM (*N = 4, P < 0.005). C: Intracellular loop sequence alignment of AMPA receptor subunits GluR1–4. D: HEK cells were cotransfected with either GFP-tagged GluR1 (ΔL1–2) or GluR1 (ΔL2–3) and HA-tagged p62. After 48 h, the cells were fixed, followed by incubation with rabbit anti-HA IgG, and labeled with Texas Red conjugated antirabbit antibodies (red). The colocalization (yellow) was examined by confocal microscopy. GluR1 (ΔL1–2) colocalized with p62 in the cell membrane (yellow); whereas the deletion of intracellular loop L2– 3 significantly reduced the colocalization of GluR1 and p62 at the cell membrane. [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Absence of p62 Impairs LTP
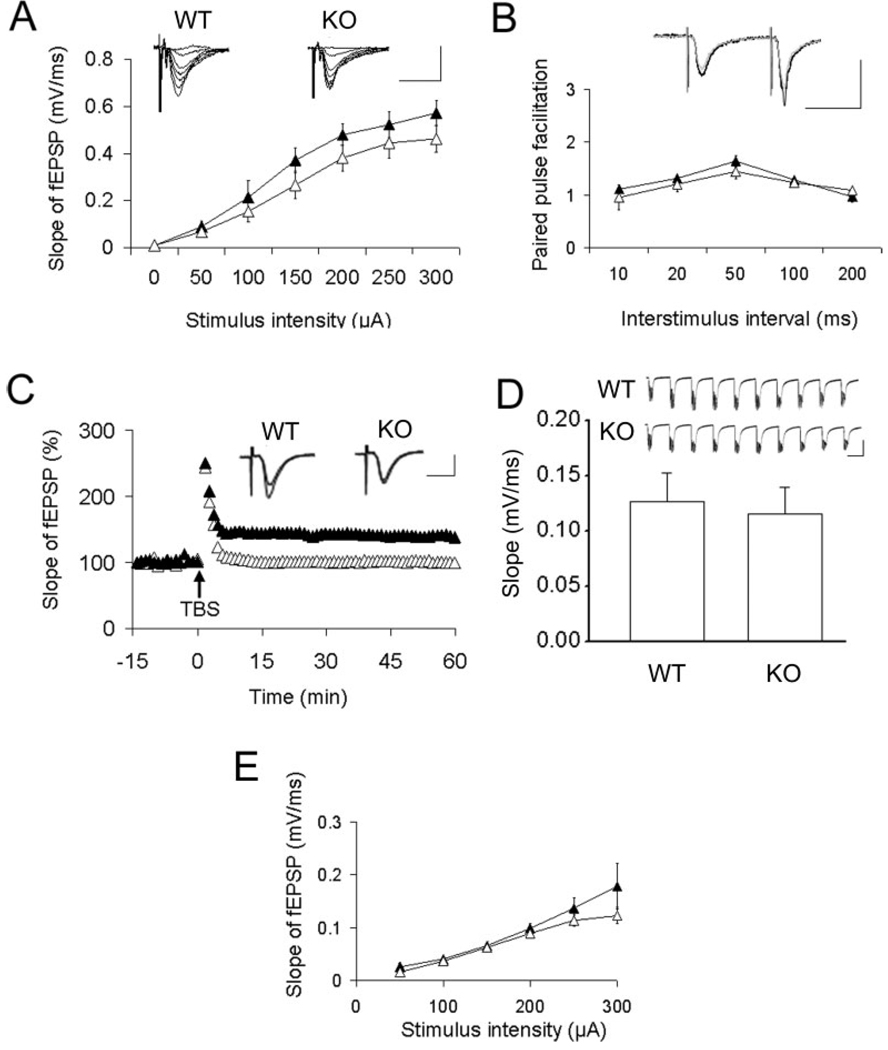
AMPA receptors mediate most excitatory neurotransmission in the central nervous system. We demonstrate that p62 interacts with AMPA receptors in vitro and in vivo and overexpression of p62 increased surface expression of the receptor. Therefore, it is possible that p62 might play an important role in synaptic plasticity. In addition, mice lacking p62 exhibit defective spatial learning, along with deficits in other hippocampus dependent tasks (Babu et al., 2008). The effect of p62 on basal synaptic transmission and synaptic plasticity in the Shaffer collateral-CA1 synapses of the hippocampus was examined. There were no significant differences in evoked fEPSPs across a range of stimulus intensities in slices obtained from mice lacking p62 compared with wild-type (WT) (Fig. 6A). Paired pulse facilitation (PPF) is a transient form of presynaptic-dependent short-term plasticity that is believed to be the result of enhanced probability of synaptic vesicle release (Zamanillo et al., 1999). PPF in the p62 knock-out mice was normal (Fig. 6B), consistent with the normal basal synaptic transmission. To further evaluate the properties of these synapses, hippocampal CA1 LTP induced by TBS was measured (Fig. 6C). Compared with WT mice, the LTP was abolished in the p62 KO mice; whereas post-tetanic potentiation in p62 KO mice remains normal. As there were no significant changes in stimulus-response curves or paired-pulse facilitation, the failure to induce LTP is not likely due to impaired basal synaptic transmission. As the form of LTP studied here is dependent on NMDAR-mediated induction (Huber et al., 1995; Morgan and Teyler, 2001), we studied the fEPSPs evoked first by TBS and found similar levels in both WT and KO (Fig. 6D), suggesting that there was no difference in NMDAR responses during the TBS priming. In addition, to test whether NMDAR component of fEPSP is altered or not, basal synaptic transmission mediated by NMDAR in the presence of low Mg2+ and AMPAR antagonist, NBQX, was examined. In this case, basal synaptic transmission in the KO mice was not significantly different from that of WT (Fig. 6E). Altogether, these findings indicate that p62 interaction with GluR1 has a physiological role.
FIGURE 6.
Basal synaptic transmission and plasticity in hippocampal Shaffer collateral-CA1 synapses. A: Input/output curves and representative fEPSPs at increasing stimulus strength showed no differences between wild-type (WT) and p62 knock-out (KO) mice. Calibration: 1.5 mV, 20 ms. For each plot representative fEPSPs of p62 (−/−) animals are shown on right to that of WT. B: PPF showed no significant differences between the two groups of mice. Overlaid representative fEPSPs (black WT; gray KO) have 50 ms interstimulus interval. Calibration: 1.5 mV, 20 ms. C: Synaptic plasticity (long term potentiation, LTP) was induced by TBS and measured for 50–60 min after TBS. Compared with WT mice, the LTP was abolished in p62 KO mice; whereas post titanic potentiation (<2 min post TBS) in the KO remained normal. Traces show averages of 30 fEPSPs recorded before TBS overlaid with averages of 30 fEPSPs recorded 50 min after TBS. Calibration: 1 mV, 15 ms. D: Top panel: The fEPSPs evoked by the first TBS in WT and KO mice were similar. Calibration: 0.5 mV and 150 ms. The integral associated with the TBS wave form in KO vs. WT were calculated and group means were compared by analysis of variance (ANOVA). There was no significant difference between p62 WT and KO data (P < 0.05). E: The input output curves of NMDAR mediated fEPSPs did not show any significant difference between WT and KO. In all plots, WT data are represented by the filled triangles and KO data are represented by open triangles. The data are represented as X ± S.E.M (P < 0.05). Each experimental group contained recordings from 5 to 8 animals, 2 slices per animal.
p62 Regulates AMPA Receptor Phosphorylation
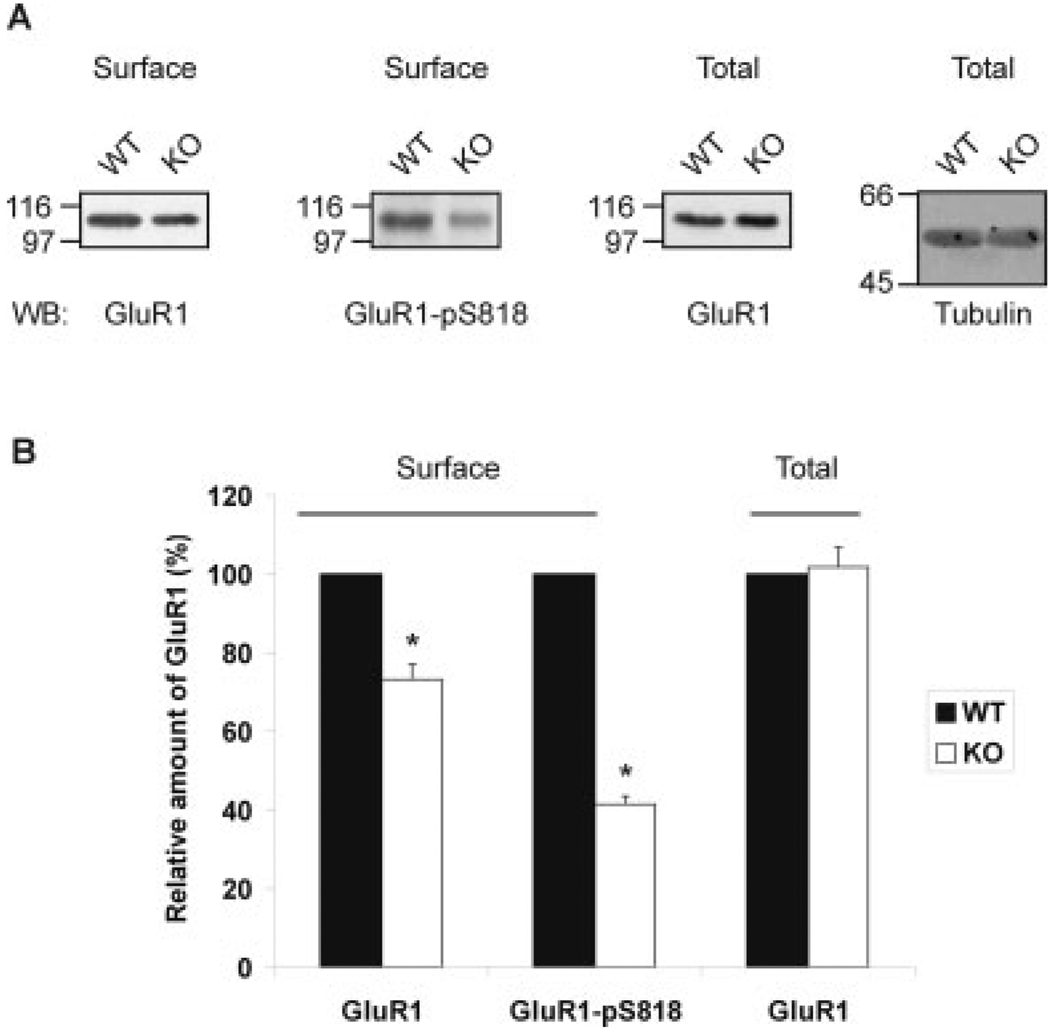
Phosphorylation of GluR1 regulates AMPA receptor trafficking to and from the postsynaptic membrane (Roche et al., 1996; Song and Huganir, 2002; Lee et al., 2000, 2003; Oh et al., 2006). A newly identified PKC phosphorylation site, S818, in GluR1 has been reported, which is necessary for AMPA receptor surface delivery and maintenance of GluR1 at the postsynaptic membrane (Boehm et al., 2006). Interestingly, GluR1 S818 is phosphorylated by all PKC isoforms, including the aPKCs. As hippocampal LTP in the p62 knock-out mice was impaired, and interaction with p62 was necessary for cell surface delivery of AMPA receptor in HEK cells, we hypothesized that an absence of p62 may also have an effect on both the amount of total GluR1 and phosphorylated GluR1 at the postsynaptic membrane. To investigate this possibility, we performed surface biotinylation and immunoblot analysis of acute hippocampal slices from 6-month-old wild-type (WT) and p62 knock-out (KO) mice. In the absence of p62, the surface level of GluR1 subunit as well as the degree of GluR1 S818 phosphorylation was significantly reduced; whereas the total amount of GluR1 subunit in the p62 knock-out mice was similar to that expressed in wild-type mice (Fig. 7A,B).
FIGURE 7.
p62 facilitates AMPA receptor surface delivery. A: Acute hippocampal slices from wild-type (WT) and p62 knock-out (KO) mice were biotinylated. The surface and total protein were separated by SDS-PAGE and Western blotted for GluR1, GluR1-pS818, and tubulin. B: The relative amount of GluR1 and GluR1-pS818 at the surface or total was determined by densitometric scan of the blots (n = 3). The relative differences X ± SEM were tested. *P < 0.05.
DISCUSSION
Several proteins, such as Stargazin, 4.1N, AP2, PI3-kinase, have been reported to regulate receptor trafficking and synaptic plasticity through interacting with AMPA receptors either directly or indirectly (Reviewed in Jiang et al., 2007; Shepherd and Huganir, 2007). In this study, we identified a novel AMPA RIP, p62/SQSTM1. The ZZ-type zinc finger domain of p62 and the intracellular loop L2–3 of AMPA receptor subunit are critical for these protein–protein interactions. Interestingly, p62 is the first protein that has been discovered to interact with the GluR receptor outside of its C-terminus with an impact on surface delivery and expression of LTP.
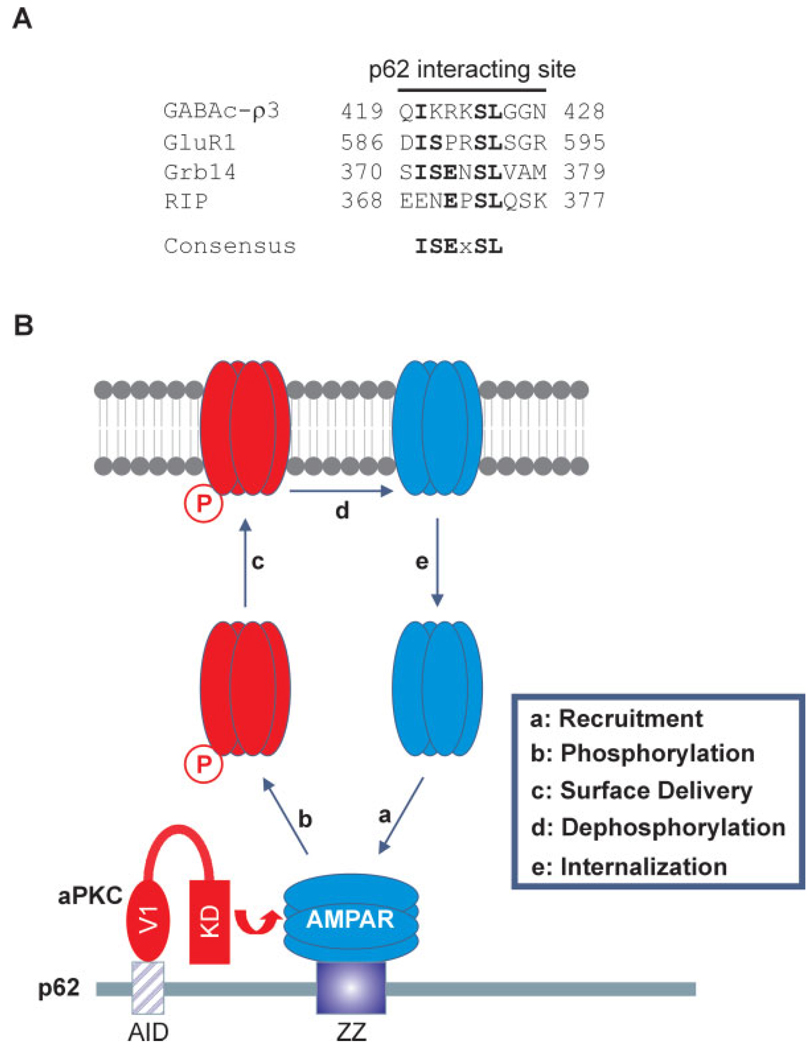
SQSTM1/p62 possesses six functional domains, which endow the protein with an ability to interact with many different molecules to exert multiple-functions. To date, most p62-interacting proteins have been observed to interact with its N-terminal ZZ-type zinc finger domain or the C-terminal UBA domain (Puls et al., 1997; Gong et al., 1999; Sanz et al., 1999; Cariou et al., 2002; Croci et al., 2003; Geetha et al., 2005; Kim, 2006). The UBA domain of p62 interacts with K63-polyubiquitinated membrane-bound proteins to initiate ubiquitin-dependent receptor endocytosis (Geetha et al., 2005), whereas the ZZ-type zinc finger domain interacts with substrates of aPKC (Puls et al., 1997; Gong et al., 1999; Sanz et al., 1999; Cariou et al., 2002; Croci et al., 2003; Kim, 2006). Therefore, p62 as a scaffold likely enables the kinase, aPKC, and the substrate, GluR1, to form a ternary complex. It is possible that the ZZ domain coordinates a correct folding of p62 to create an interaction surface. Thus far, several receptors and nonreceptor proteins have been discovered to interact with the ZZ-domain of p62. Those proteins include: dopamine D2 receptor (DAR), GABAC receptor subunit q1–3, growth factor receptor-bound protein 14 (Grb14), RIP, and potassium channel subunit Kvb2 (Table 3) (Puls et al., 1997; Gong et al., 1999; Sanz et al., 1999; Cariou et al., 2002; Croci et al., 2003; Kim, 2006). p62 interacts with the intracellular loop (TM3-TM4) of GABAC receptor, ID (intermediate domain) of RIP and PIR (phosphorylated insulin receptor) domain of Grb14; whereas in our study, the intracellular loop L2–3 of AMPA receptor subunits was revealed to be critical for p62 interaction. Alignment of all p62-interacting sites in each protein reveals a potential conserved consensus sequence, ISExSL (where x is any amino acid) (Fig. 8A). We hypothesize this site might serve as a putative protein interaction motif to recruit the substrate for phosphorylation by aPKCs. In fact, interactions of p62 with Kvβ2, GABAC receptor, RIP, and Grb14 are necessary for phosphorylation mediated by aPKC (Table 3).
Table 3.
Proteins Interacting with p62 Through ZZ-Type Zinc Finger Domain
| Binding site on protein | Role of p62 | Reference | |
|---|---|---|---|
| Receptor protein | |||
| Kvβ2 | Not mapped | Recruiting aPKC for phosphorylation | Gong et al., 1999 |
| D2 DAR | Not mapped | Ubiquitination related | Kim, 2006 |
| GABAC-ρ3 | Intracellular loop (TM3–TM4) | Recruiting aPKC for phosphorylation | Croci et al., 2003 |
| AMPA receptor | Intracellular loop (L2–3) | Trafficking | |
| Nonreceptor protein | |||
| RIP | ID (intermediate domain) | Linking aPKC to NF-ĸB activation | Sanz et al., 1999 |
| Grb14 | PIR (phosphorylated insulin receptor) domain | Recruiting aPKC for phosphorylation | Cariou et al., 2002 |
FIGURE 8.
p62 serves as a scaffold. A: Alignment of the p62 interaction sites in AMPA receptor subunits GluR1–4, GABAC receptor subunit ρ3, Grb14, and RIP indicates a conserved motif, ISExSL, shared by p62-interacting proteins. B: This model illustrates the role of p62 in synaptic targeting of AMPA receptor. p62 recruits AMPA receptor through its ZZ domain (a) for phosphorylation mediated by aPKC (b), then the phosphorylated AMPA receptor is delivered to the synaptic surface to mediate LTP (c). Once the receptor is dephosphorylated (d), the AMPA receptor is internalized (e), and can recycle back to the surface by phosphorylation (a). [Color figure can be viewed in the online issue, which is available at www.interscience.wiley.com.]
Receptor phosphorylation by CaMKII and PKC play critical roles in AMPA receptor trafficking. Four phosphorylation sites have been discovered in the GluR1 C-terminus: S818 and T840 are PKC sites, S831 is both a PKC and CaMII site, and S845 is phosphorylated by both PKA and cGKII (cGMP-dependent protein kinase II) (Roche et al., 1996; Esteban et al., 2003, Lee et al., 2003, 2007; Boehm et al., 2006; Serulle et al., 2007). In our study, surface delivery of GluR1 was not completely absent in hippocampus from mice deficient in p62. Therefore, other kinases/scaffold proteins may compensate for the deficit in p62. However, studies in HEK cells reveal that aPKC promotes surface expression of the receptor to a greater degree than GluR1 coexpressed with p62 alone. Altogether, these findings suggest that p62 and aPKC act together to mediate surface delivery of GluR1. These findings are similar to what has recently been reported for the PICK1 scaffold and phosphorylation by the classical PKCs in expression of mGluR7 surface expression (Suh et al., 2008). Our findings are in keeping with the reported role for phosphorylation in stabilizing the AMPA receptor in the synaptic membrane to mediate plasticity (Lee et al., 2000). The aPKC isoform, PKMζ, has a well-defined role in mediating late-phase LTP (Pastalkova et al., 2006), whereas, these findings reveal that PKCi/ζ which interact with p62, likely regulates the early phase of LTP. This idea would be consistent with the translocation of PKCi/ζ early after a tetanus that induces LTP (Sacktor et al., 1993). Therefore, as a scaffold p62 recruits GluR1 for phosphorylation by aPKC and also participates in trafficking to the membrane. In this context, p62 has been shown to interact with MAP1B a component of the cytoskeleton (Pankiv et al., 2007), and MAP1B knock-out mice are likewise deficient in LTP (Zervas et al., 2005). Thus, p62 may be directly involved in trafficking of the phosphorylated receptor to the cell surface by interaction with the cytoskeleton as well as in regulation of GluR1 phosphorylation by aPKC.
Collectively the electrophysiology and behavioral phenotype of p62 knock-out reveal a notable similarity with those reported for the GluR1 knock-out mice (Zamanillo et al., 1999; Reisel et al., 2002). Additionally, the p62 knock-out mice display mature-onset obesity (Rodriguez et al., 2006), which might amplify the deficits in AMPA receptor surface delivery and synaptic plasticity through oxidative stress. Altogether, our study reveals a novel and critical role for p62 along with the aPKC isoforms in AMPA receptor trafficking and synaptic plasticity.
Acknowledgments
We are grateful to Drs. A. Dityatev, Ramesh Babu, and Thangiah Geetha for advice and technical assistance, and Dr. G. Turrigiano for providing the GFP-GluR1 construct.
Grant sponsor: National Institutes of Health; Grant number: NS33661.
REFERENCES
- Babu JR, Seibenhener ML, Peng J, Strom AL, Kemppainen R, Cox N, Zhu H, Wooten MC, Diaz-Meco MT, Moscat J, Wooten MW. Genetic inactivation of p62 leads to accumulation of hyper-phosphorylated tau and neurodegeneration. J Neurochem. 2008;106:107–120. doi: 10.1111/j.1471-4159.2008.05340.x. [DOI] [PubMed] [Google Scholar]
- Bliss TVP, Collingridge GL. A synaptic model of memory: Long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Boehm J, Kang MG, Johnson RC, Esteban J, Huganir RL, Malinow R. Synaptic incorporation of AMPA receptors during LTP is controlled by a PKC phosphorylation site on GluR1. Neuron. 2006;51:213–225. doi: 10.1016/j.neuron.2006.06.013. [DOI] [PubMed] [Google Scholar]
- Bukalo O, Dityatev A. Analysis of neural cell functions in gene knockout mice: Electrophysiological investigation of synaptic plasticity in acute hippocampal slices. Methods Enzymol. 2006;417:52–66. doi: 10.1016/S0076-6879(06)17005-6. [DOI] [PubMed] [Google Scholar]
- Cariou B, Perdereau D, Cailliau K, Browaeys-Poly E, Bereziat V, Vasseur-Cognet M, Girard J, Burnol AF. The adapter protein ZIP binds Grb14 and regulates its inhibitory action on insulin signaling by recruiting protein kinase Cζ. Mol Cell Biol. 2002;22:6959–6970. doi: 10.1128/MCB.22.20.6959-6970.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho AL, Kameyama K, Huganir RL. Characterization of phosphorylation sites on the glutamate receptor 4 subunit of the AMPA receptors. J Neurosci. 1999;19:4748–4754. doi: 10.1523/JNEUROSCI.19-12-04748.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan CS, Weeber EJ, Zong L, Fuchs E, Sweatt JD, Davis RL. β1-intergrins are required for hippocampal AMPA receptor-dependent synaptic transmission, synaptic plasticity, and working memeory. J Neurosci. 2006;26:223–233. doi: 10.1523/JNEUROSCI.4110-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Xia J, Scannevin RH, Zhang X, Huganir RL. Phosphorylation of the AMPA receptor subunit GluR2 differentially regulates its interaction with PDZ domain-containing proteins. J Neurosci. 2000;20:7258–7267. doi: 10.1523/JNEUROSCI.20-19-07258.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Correia SS, Duarte CB, Faro CJ, Pires EV, Carvalho AL. Protein kinase C γ associates directly with the GluR4 α-amino-3-hydroxy-5-methyl-4-isoxazole propionate receptor subunit. J Biol Chem. 2003;278:6307–6313. doi: 10.1074/jbc.M205587200. [DOI] [PubMed] [Google Scholar]
- Croci C, Brandstatter JH, Enz R. ZIP3, a new splice variant of the PKC-ζ-interacting protein family, binds to GABAC receptors, PKC-ζ, and Kvβ2. J Biol Chem. 2003;278:6128–6135. doi: 10.1074/jbc.M205162200. [DOI] [PubMed] [Google Scholar]
- Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- Duran A, Serrano M, Leitgas M, Flores J, Picard S, Brown J, Moscat J, Diaz-Meco MT. The atypical PKC-interacting protein p62 is an important mediator of RANK-activated osteoclastogenesis. Dev Cell. 2004;6:303–309. doi: 10.1016/s1534-5807(03)00403-9. [DOI] [PubMed] [Google Scholar]
- Esteban JA, Shi SH, Wilson C, Nuriya M, Huganir RL, Malinow R. PKA phosphorylation of AMPA receptor subunits controls synaptic trafficking underlying plasticity. Nat Neurosci. 2003;6:136–143. doi: 10.1038/nn997. [DOI] [PubMed] [Google Scholar]
- Fukata Y, Tzingounis AV, Trinidad JC, Fukata M, Burlingame AL, Nicoll RA, Bredt DS. Molecular constituents of neuronal AMPA receptors. J Cell Biol. 2005;169:399–404. doi: 10.1083/jcb.200501121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha T, Jiang J, Wooten MW. The nerve growth factor receptor TrkA is K63 polyubiquitinated and degraded by the proteasome. Mol Cell. 2005;20:301–312. doi: 10.1016/j.molcel.2005.09.014. [DOI] [PubMed] [Google Scholar]
- Gong J, Bezanilla M, van Huizen R, Derin R, Li M. Differential stimulation of PKC phosphorylation of potassium channels by ZIP1 and ZIP2. Science. 1999;285:1565–1569. doi: 10.1126/science.285.5433.1565. [DOI] [PubMed] [Google Scholar]
- Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP, CaMKII: Requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- Hernandez AI, Blace N, Crary JF, Serrano PA, Meitges M, Libien J, Weinstein G, Tcherapanov A, Sacktor TC. Protein kinase Mζ synthesis from a brain mRNA encoding an independent protein kinase Cζ catalytic domain. J Biol Chem. 2003;278:40305–40316. doi: 10.1074/jbc.M307065200. [DOI] [PubMed] [Google Scholar]
- Hollmann M, Heinemann S. Cloned glutamate receptors. Annu Rev Neurosci. 1994;17:31–108. doi: 10.1146/annurev.ne.17.030194.000335. [DOI] [PubMed] [Google Scholar]
- Huber KM, Mauk MD, Kelly PT. Distinct LTP induction mechanisms: Contribution of NMDA receptors and voltage-dependent calcium channels. J Neurophysiol. 1995;73:270–279. doi: 10.1152/jn.1995.73.1.270. [DOI] [PubMed] [Google Scholar]
- Jiang J, Suppiramaniam V, Wooten MW. Posttranslational modifications and receptor-associated proteins in AMPA receptor trafficking and synaptic plasticity. Neurosignals. 2007;15:266–282. doi: 10.1159/000105517. [DOI] [PubMed] [Google Scholar]
- Kim O. The interaction of the D2 dopamine receptor with ZIP is regulated by ubiquitination. SFN Conference; Oct. 14–18, 2006; Atlanta, GA. 2006. [Google Scholar]
- Lee HK, Barbarosie M, Kameyama K, Bear MF, Huganir RL. Regulation of distinct AMPA receptor phosphorylation sites during bidirectional synaptic plasticity. Nature. 2000;405:955–959. doi: 10.1038/35016089. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Han JS, Man H, Kim CH, Rumbaugh G, Yu S, Ding L, He C, Petralia RS, Wenthold RJ, Gallagher M, Huganir RL. Phosphorylation of AMPA receptor GluR1 subunit is required for synaptic plasticity and retention of spatial memory. Cell. 2003;112:631–643. doi: 10.1016/s0092-8674(03)00122-3. [DOI] [PubMed] [Google Scholar]
- Lee HK, Takamiya K, Kameyama K, He K, Yu S, Rossetti L, Wilen D, Huganir RL. Identification and characterization of a novel phosphorylation site on the GluR1 subunit of AMPA receptors. Mol Cell Neurosci. 2007;36:86–94. doi: 10.1016/j.mcn.2007.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, Baudry M. The biochemistry of memory: A new and specific hypothesis. Science. 1984;224:1057–1063. doi: 10.1126/science.6144182. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Nicoll RA. Long-term potentiation-A decade of progress. Science. 1999;285:1870–1874. doi: 10.1126/science.285.5435.1870. [DOI] [PubMed] [Google Scholar]
- Malinow R, Malenka RC. AMPA receptors trafficking and synaptic plasticity. Annu Rev Neurosci. 2002;25:103–126. doi: 10.1146/annurev.neuro.25.112701.142758. [DOI] [PubMed] [Google Scholar]
- McDonald BJ, Chung HJ, Huganir RL. Identification of protein kinase C phosphorylation sites within the AMPA receptor GluR2 subunit. Neuropharmacology. 2001;41:672–679. doi: 10.1016/s0028-3908(01)00129-0. [DOI] [PubMed] [Google Scholar]
- Morgan SL, Teyler TJ. Electrical stimuli patterned after the theta-rhythm induce multiple forms of LTP. J Neurophysiol. 2001;86:1289–1296. doi: 10.1152/jn.2001.86.3.1289. [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Wooten MW. Signal integration and diversification through the p62 scaffold protein. Trends Biochem Sci. 2007;32:95–100. doi: 10.1016/j.tibs.2006.12.002. [DOI] [PubMed] [Google Scholar]
- Oh MC, Derkach VA, Guire ES, Soderling TR. Extrasynaptic membrane trafficking regulated by GluR1 Serine 845 phosphorylation primes AMPA receptors for long-term potentiation. J Biol Chem. 2006;281:752–758. doi: 10.1074/jbc.M509677200. [DOI] [PubMed] [Google Scholar]
- Oster H, Eichele G, Leitges M. Differential expression of atypical PKCs in the adult mouse brain. Brain Res Mol Brain Res. 2004;127:79–88. doi: 10.1016/j.molbrainres.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Palmer CL, Cotton L, Henley JM. The molecular pharmacology and cell biology of A-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid receptors. Pharmacol Rev. 2005;57:253–277. doi: 10.1124/pr.57.2.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankiv S, Clausen TH, Lamark T, Brech A, Bruun J, Outzen H, Overvatan A, Bjorkoy G, Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007;282:24131–24145. doi: 10.1074/jbc.M702824200. [DOI] [PubMed] [Google Scholar]
- Pastalkova E, Serrano P, Pinkhasova D, Wallace E, Fenton AA, Sacktor TC. Storage of spatial information by the maintenance mechanism of LTP. Science. 2006;313:1141–1144. doi: 10.1126/science.1128657. [DOI] [PubMed] [Google Scholar]
- Puls A, Schmidt S, Grawe F, Stabel S. Interaction of protein kinase C ζ with ZIP, a novel protein kinase C-binding protein. Proc Natl Acad Sci USA. 1997;94:6191–6196. doi: 10.1073/pnas.94.12.6191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisel D, Bannerman DM, Schmitt WB, Deason RM, Flint J, Borchardt T, Seeburg PH, Rawlins JNP. Spatial memory dissociations in mice lacking GluR1. Nat Neurosci. 2002;5:868–873. doi: 10.1038/nn910. [DOI] [PubMed] [Google Scholar]
- Roche KW, O’Brien RJ, Mammen AL, Bernhardt J, Huganir RL. Characterization of multiple phosphorylation sites on the AMPA receptor GluR1 subunit. Neuron. 1996;16:1179–1188. doi: 10.1016/s0896-6273(00)80144-0. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Duran A, Selloum M, Champy M, Diez-Guerra F, Flores J, Serrano M, Auwerx J, Diaz-Meco MT, Moscat J. Mature-onset obesity and insulin resistance in mice deficient in the signalling adapter p62. Cell Metabolism. 2006;3:211–222. doi: 10.1016/j.cmet.2006.01.011. [DOI] [PubMed] [Google Scholar]
- Sacktor TC, Osten P, Valsamis H, Jiang X, Naik MU, Sublette E. Persistent activation of the zeta isoform of protein kinase C in the maintenance of long term potentiation. Proc Natl Acad Sci USA. 1993;90:8342–8346. doi: 10.1073/pnas.90.18.8342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz L, Sanchez P, Lallena M-J, Diaz-Meco MT, Moscat J. The interaction of p62 with RIP links the atypical PKCs to NF-κB activation. EMBO J. 1999;18:3044–3053. doi: 10.1093/emboj/18.11.3044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez P, DeCarcer G, Sandovall IV, Moscat J, Diaz-Meco MT. Localization of atypical protein kinase C isoforms into lysosome-targeted endosomes through interaction with p62. Mol Cell Biol. 1998;18:3069–3080. doi: 10.1128/mcb.18.5.3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serulle Y, Zhang S, Ninan I, Puzzo D, McCarthy M, Khatri L, Arancio O, Ziff EB. A GluR1-cGKII interaction regulates AMPA receptor trafficking. Neuron. 2007;56:670–688. doi: 10.1016/j.neuron.2007.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen L, Liang F, Walensky LD, Huganir RL. Regulation of AMPA receptor GluR1 subunit surface expression by a 4.1N-linked actin cytoskeletal association. J Neurosci. 2000;20:7932–7940. doi: 10.1523/JNEUROSCI.20-21-07932.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng M, Lee SH. AMPA receptor trafficking and the control of synaptic transmission. Cell. 2001;105:825–828. doi: 10.1016/s0092-8674(01)00406-8. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Ann Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Shi S, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1815. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- Song I, Huganir RL. Regulation of AMPA receptors during synaptic plasticity. Trends Neurosci. 2002;25:578–588. doi: 10.1016/s0166-2236(02)02270-1. [DOI] [PubMed] [Google Scholar]
- Suh YH, Pelkey KA, Lavezzari G, Roche PA, Huganir RL, McBain DJ, Roche KW. Corequirement of PICK1 binding and PKC phosphorylation for stable surface expression of the metabotropic glutamate receptor mGluR7. Neuron. 2008;58:736–748. doi: 10.1016/j.neuron.2008.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanillo D, Sprengel R, Hvalby O, Jensen V, Burnashev N, Rozov A, Kaiser KM, Koster HJ, Borchardt T, Worley P, Lubke J, Frotscher M, Kelly PH, Sommer B, Andersen P, Seeburg PH, Sakmann B. Importance of AMPA receptors for hippocampal synaptic plasticity but not for spatial learning. Science. 1999;284:1805–1811. doi: 10.1126/science.284.5421.1805. [DOI] [PubMed] [Google Scholar]
- Zervas M, Opitz T, Edelmann W, Wainer B, Kucherlapati R, Stanton PK. Impaired hippocampal long-term potentiation in microtubule-associated protein 1B-deficient mice. J Neurosci Res. 2005;82:83–92. doi: 10.1002/jnr.20624. [DOI] [PubMed] [Google Scholar]