Abstract
Objective
We evaluated the utility of weight-for-length (defined as gm/cm3, “ponderal index”) as a complementary measure of growth in infants in neonatal intensive care units (NICUs).
Study design
Secondary analysis of infants (n=1214) 26-29 weeks at birth, included in a registry database (1991-2003), who had growth data at birth and discharge. Weight-for-age and weight-for-length were categorized as small (<10th percentile), appropriate or large (>90th percentile).
Results
Statistical agreement between the weight-for-age and weight-for-length measures was poor (kappa=0.02 at birth, 0.10 at discharge, Bowker test for symmetry p<0.0001). From birth to discharge, the percent of small-for-age infants increased from 12% to 21%, and the percent of small-for-length infants decreased from 10% to 4%; the percent of large-for-age infants remained similar (<1%), and the percent of large-for-length infants increased from 5% to 17%. At discharge, 92% of small-for-age infants were appropriate or large-for-length, and 19% of appropriate-for-age infants were large-for-length.
Conclusions
Weight-for-age and weight-for-length are complementary measures. Weight-for-length or other measure of body proportionality should be considered for inclusion in routine growth monitoring of infants in the NICU.
Keywords: Growth status, growth, weight-for-age, weight/length3, ponderal index, weight/length ratio, obesity, overweight, underweight, small-for-gestational age, nutrition
A body proportionality index provides an assessment of body mass relative to length or height. Body mass index (BMI = weight/height2) in children and adults is known to be highly correlated with body fatness and risk of related diseases (1). Thus, BMI has become an important part of health assessment.
Prematurity is the only period during the lifecycle for which a body proportionality index, such as BMI, is not routinely used to assess body size. Even though comparing an infant's weight to fetuses of the same gestational age, or weight-for-age (2-7) is an excellent measure of overall size, it cannot detect situations where weight growth exceeds or fails to keep up with growth in the infant's length. This is potentially a problem for infants whose weight is appropriate for their age but low or high for their length (i.e. have a body disproportionality) as they may have growth and developmental issues that are overlooked in the usual clinical setting. Alternatively, small-for-age infants could be long and thin or short and fat, implying different mechanisms of altered growth.
Based on current methods of growth assessment, many infants are discharged from the neonatal intensive care unit (NICU) small-for-age (8, 9, 10), which is defined < 10th percentile weight-for-age (2, 11, 12). This affects the majority of infants who are small-for-age at birth, but also includes a sizeable proportion of infants born appropriate-for-age at birth especially at the younger gestational ages (9). Yet clinical observations suggest a contrast; many infants classified as small or appropriate-for-age upon visual examination seem to have appropriate or even abundant fat stores.
The purpose of this study was to examine the categorization of weight growth status in preterm infants using the weight/length3 ratio-for-age or “ponderal index” (weight-for-length) method compared with the current standard of weight-for-age, to determine whether routine measurement of weight-for-length or other measure of body proportionality in addition to weight-for-age may be justified. We hypothesized that the categorization of weight growth status as small, appropriate or large by these two methods would have modest to poor agreement, and that at discharge a significant proportion of infants classified as small-for-age would be considered appropriate or large weight-for-length.
Methods
This study was a secondary analysis of infants cared for in Cincinnati's three NICUs using an existing database: the National Institute of Child Health and Human Development (NICHD) Neonatal Research Network registry. This database (“Cincinnati very low birth weight cohort”) includes all infants admitted to the NICUs within 14 days of birth who weighed 401 to 1500 grams at birth between 1991 and 2003 (n=3975). Gestational age (GA) was based on obstetrical best estimate (using last menstrual period dates, prenatal ultrasound); obstetrical GA was missing for five infants for whom Ballard score (from neonatologist exam) was used. GA was presented in completed weeks. This study was approved by the Cincinnati Children's Hospital Medical Center and Drexel University Institutional Review Boards.
Infants were excluded for factors expected to negatively affect growth status (total n=380), including mortality within first 12 hours (n=249), one or more major congenital anomalies (including central nervous system, congenital heart, gastrointestinal, or genitourinary defects; chromosomal abnormalities; n=97), and intrauterine infection exposure (any TORCH infections, untreated maternal syphilis or HIV; n=37). Healthy multiples were not excluded as these infants represent normal variation in size of infants in the NICU. Infants were not excluded for postnatal factors as our goal was to capture the postnatal change in size of infants in the NICU in our sample. After the above exclusions, 3595 infants were eligible for the analysis.
The sample was further restricted to 26 to 29 weeks gestational age at birth (n=1750). This was because very low birth weight cohorts like this over-represent small-for-age infants over 29 weeks GA at birth, as previously reported (13, 14). Also the sample was restricted to infants ≥26 weeks gestational age at birth because the Lubchenco growth curves start at 26 weeks (2, 12)
Extreme outliers for weight and length measurements were excluded to avoid distortion of our growth outcome as done in other growth studies (6, 7). Extreme outliers were defined as values more than two times the interquartile range (25th to 75th percentiles around the median) for each gestational age (15). Twenty-seven extreme values were excluded (11 weight or length at birth, 16 at discharge) however three infants had two outliers each and nine also had missing data (eliminated in the next step). Outliers represented 1% or less of the data available for analyses at each time point, and appeared to be due to measurement or recording errors as either weight or length was affected for all but two cases (where both weight and length were outliers based on GA).
The final sample included 1214 infants who had weight and length measurements at birth and discharge from NICU to home (n=1075), another hospital (n=80) or died (n=59). This end timepoint will be referred to as “discharge” based on the majority of infants.
Assessment of growth status
The only available growth curves for the assessment of weight-for-age and weight-for-length accommodating infants between 26 to 42 weeks gestational age, based on intrauterine growth data (versus postnatal growth data) from the same dataset and presented in percentiles were the Lubchenco growth curves (2, 11, 12). These curves were used to categorize weight growth status at birth and discharge.
Weight-for-gestational age (weight-for-age)
Using the Lubchenco weight-for-age growth curve, each infant's weight at birth and discharge was plotted against gestational age and then categorized as “small-for-age” if <10th percentile for weight-for-gestational age, “appropriate-for-age” if 10-90th percentiles for weight-for-gestational age, or “large-for-age” if >90th percentile for weight-for-gestational age.
Weight/length3 ratio-for-age (weight-for-length)
The Lubchenco growth curves use weight/length3 ratio-for-age as the measure of body proportionality. The weight/length3 ratio was calculated as (weight divided by length cubed)*100 (in gm/cm3) and plotted against gestational age (12). At birth and discharge, each infant's weight-for-length was categorized as “small-for-length”, “appropriate-for-length”, or “large-for-length”, if <10th, 10-90th or >90th percentile for weight/length3 ratio-for-age, respectively.
Data Analysis
Data analyses were conducted using SAS 9.1 (SAS Institute Inc.; Cary, NC; 2002-2003). Concordance coefficients (i.e., kappa statistics) and Bowker's test for symmetry were used to evaluate agreement and discordance (or lack of agreement) respectively between the weight-for-age and weight-for-length methods. High agreement was defined as a kappa of at least 0.8 (16). High agreement between the weight-for-age and weight-for-length methods would mean that these categorize infant weight growth status similarly (e.g., small infants are typically categorized as small by both methods). In turn, lack of agreement would mean that the two methods categorize infant weight growth status differently, and thus each provides different information about weight growth status. In Table I, the center diagonal line made up of the small/small, appropriate/appropriate, and large/large cells shows the infants for which there was agreement between methods.
Table 1.
| Weight-for-Age c | |||||
|---|---|---|---|---|---|
| Small | Appropriate | Large | |||
| AT BIRTH | n(%) | 149 (12.3) | 1062 (87.5) | 3 (0.2) | |
| Weight-for-Length c | Small | 117 (9.6) | 22.1% | 7.9% | 0.0% |
| Appropriate | 1034 (85.2) | 77.2% | 86.3% | 100.0% | |
| Large | 63 (5.2) | 0.7% | 5.8% | 0.0% | |
| AT DISCHARGE | n(%) | 259 (21.3) | 944 (77.8) | 11 (0.9) | |
| Weight-for-Length c | Small | 55 (4.5) | 8.5% | 3.5% | 0.0% |
| Appropriate | 949 (78.2) | 80.7% | 77.9% | 45.5% | |
| Large | 210 (17.3) | 10.8% | 18.6% | 54.5% | |
Weight-for-gestational age (weight-for-age) and Weight/Length3 ratio-for-age (weight-for-length) based on Lubchenco fetal growth charts (Lubchenco Pediatrics 1966)
Unshaded cells present column percentages
Small, appropriate and large defined as <10th, 10-90th, and >90th percentile weight-for-age or length, as appropriate
Results
Infant characteristics were presented as mean±SD as variables were approximately normal except for GA at birth and the means and medians were equivalent. At birth, infants were 27.7±1.1 weeks gestation, 1054±218gm weight and 36.3±2.7cm length. At discharge, infants were 35.5±3.1 weeks gestation, 2213±589gm weight and 43.3±3.5cm length. The sample was comprised of 49.3% females and was 68.3% Caucasian. Based on the Lubchenco weight cutoffs for the 90th percentile weight-for-age, our sample was made up of less than the expected 10% of large weight-for-age infants at birth (Table I), however the Lubchenco weight cutoffs are higher than those of the newer Riddle growth curve based on a more racially diverse sample of infants (5). Using the newer Riddle curve, our sample had 11.8% LGA infants. (The Riddle curves could not be used for this study because these do not include a weight-for-length curve.)
Comparison between weight growth status assessment methods
At birth
We found poor agreement at birth between the weight-for-age and weight-for-length methods of weight growth status assessment in assigning small, appropriate and large categories to the infants in our sample (kappa=0.1; Bowker test p<0.0001; Table I). Among the infants who were small-for-age at birth, some were small, most were appropriate and a few were large according to weight-for-length. Of the infants who were appropriate-for-age at birth, most were also appropriate-for-length but some were small or large. Results for the infants who were large-for-age at birth are limited by small sample size.
At discharge
We found significant discordance at discharge between the weight-for-age and weight-for-length methods of weight growth status assessment (kappa 0.02; Bowker's test p<0.0001; Table I) which was primarily due to small-for-age infants being mostly appropriate-for-length. Of the 944 infants considered appropriate-for-age, 22.1% were found to be inappropriate-for-length (Table I) and of the 949 considered appropriate-for-length, 22.5% were found to be inappropriate-for-age (data not shown); again illustrating that the two measures categorize infants differently.
Change in weight growth status over time
Table II shows the distribution by weight growth status category (small, appropriate, large) of the overall rise in large-for-length infants and decline in small-for-length infants from birth to discharge. Of the 210 large-for-length infants by discharge, only 11 were large-for-age, and most started as appropriate-for-age (86.7%) and appropriate-for-length (88.1%) at birth (data not shown).
Table 2.
| Birth Weight-for-Length c | |||||
|---|---|---|---|---|---|
| Small | Appropriate | Large | |||
| n(%) | 117 (9.6) | 1034 (85.2) | 63 (5.2) | ||
| Discharge Weight-for-Length c | Small | 55 (4.5) | 7.7% | 4.2% | 4.8% |
| Appropriate | 949 (78.2) | 81.2% | 77.9% | 76.2% | |
| Large | 210 (17.3) | 11.1% | 17.9% | 19.0% | |
Weight/Length3 ratio-for-age (weight-for-length) based on Lubchenco fetal growth charts (Lubchenco Pediatrics 1966)
Unshaded cells present column percentages
Small, appropriate and large defined as <10th, 10-90th, and >90th percentile weight-for-age or length, as appropriate
The overall percent of small-for-age infants almost doubled from birth to discharge (Table I). Although weight-for-age categorization indicates that the majority of small-for-age infants at birth remained small-for-age at discharge, including length in the categorization shows that most of the infants were appropriate weight-for-length (Figure 1). Of the infants who were born appropriate-for-age, most remained appropriately-sized based on weight-for-age and weight-for-length at discharge (Figure 2). Figures 1 and 2 also illustrate that a substantial proportion of small-for-age and appropriate-for-age infants at birth became large-for-length by discharge.
Figure 1. Discharge weight growth status of infants who were small weight-for-age at birth (n=149).
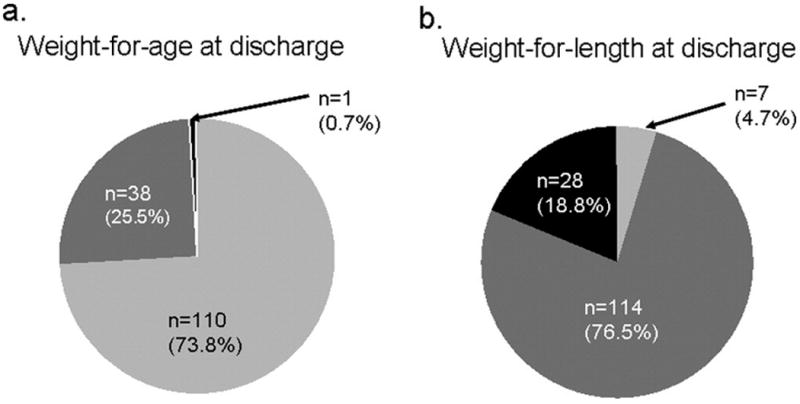
 Small at Discharge (<10th percentile weight or length-for-age)
Small at Discharge (<10th percentile weight or length-for-age)
 Appropriate at Discharge (10-90th percentile or length-for-age)
Appropriate at Discharge (10-90th percentile or length-for-age)
 Large at Discharge (<10th percentile weight or length-for-age)
Large at Discharge (<10th percentile weight or length-for-age)
Weight-for-gestational age (weight-for-age) and weight/length3 ratio-for-age (weight-for-length) based on Lubchenco fetal growth charts (Lubchenco Pediatrics 1966)
Figure 2. Discharge weight growth status of infants who were appropriate weight-for-age at birth (n=1062).
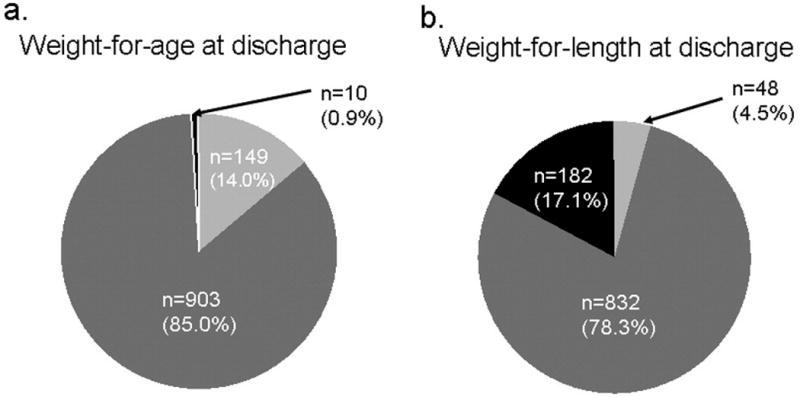
 Small at Discharge (<10th percentile weight or length-for-age)
Small at Discharge (<10th percentile weight or length-for-age)
 Appropriate at Discharge (10-90th percentile or length-for-age)
Appropriate at Discharge (10-90th percentile or length-for-age)
 Large at Discharge (<10th percentile weight or length-for-age)
Large at Discharge (<10th percentile weight or length-for-age)
Weight-for-gestational age (weight-for-age) and weight/length3 ratio-for-age (weight-for-length) based on Lubchenco fetal growth charts (Lubchenco Pediatrics 1966)
Discussion
Using weight-for-age to assess weight growth status indicates that preterm infants fall behind in their growth between birth and discharge, as percent small-for-age increases from 12% to 21%. Weight-for-length gives a very different impression: percent large-for-length increases from 5% to 17%, indicating possible disproportionate growth and early adiposity. Using the combination of the two, the vast majority of small-for-age infants are appropriate weight-for-length at discharge, and most of the large-for-length infants are appropriate weight-for-age. This discordance of classification suggests the need for greater understanding of the functional consequences of these different growth patterns, while suggesting the need to monitor both weight-for-age and weight-for-length, or other measure of proportionality.
The additional information provided by weight-for-length helps to illustrate a more encouraging picture at that time in NICUs than previous reports. The NICHD Neonatal Research Network (1995-1996) found 97% of very low birth weight infants were small-for-age by 36 weeks (8). The Pediatrix Medical Group (1997-2000) found 28% of preterm infants 23 to 34 weeks were small-for-age (9). Although comparisons between studies are difficult because different reference groups were used, these studies highlighted the need for extra attention to the postnatal growth and nutrition of preterm infants in the NICU.
Although calorie and protein recommendations of approximately 120kcal/kg/d and 3-4gm protein/kg/day for preterm infants were available (17-19) at the time that our study sample was in the NICU (1991-2003), it was common for these not to be met (20-22). Publication of the Network (8) and Pediatrix (9) study findings in the early 2000s, which was towards the end of our study period, helped to support the need for the “aggressive nutrition” practices (23) that are more common in NICUs today. Our results on preterm infants during the same time period as these two studies suggest NICU clinicians should seek more information (i.e., a body proportionality index) before providing extra nutrition to support rapid growth in all small-for-age infants. A higher calorie diet (assuming adequate protein intake) might be reserved for infants who are both small-for-age and small-for-length. Further study of this issue is warranted.
We showed the proportion of preterm infants considered large-for-length increased over time in the NICU (5 to 17% overall) and these infants were all either small-for-age or appropriate-for-age at birth. The changes in body composition that accompany this shift to large-for-length are unknown. Extrauterine growth rates that are greater than intrauterine rates (24, 25) may result in higher rates of body fat accretion. Some nutrition practices and environments that achieve rapid postnatal growth in weight have been shown to contribute to excess body fat. Formula-fed very low birth weight infants have higher accretion of body fat (26, 27) and less linear growth (27) postnatally compared with fetuses of the same gestational age. A high calorie diet has been shown to promote rapid postnatal fat accretion (28). Thus, the shift of small and appropriate-for-length infants to large-for-length may be a result of accelerated weight gain, poor linear growth or likely a combination both. Research on body composition in preterm infants, its long-term risks and impact of diet is needed. Detailed dietary data were not available for our analysis.
The relationship between small size and poor neurodevelopment has been explored for years. Even though poor neurodevelopment has been repeatedly associated with being born small in weight (29, 30) and in head size (31, 32), such an association with low weight-for-length has not been as well-documented. A report from the NICHD Neonatal Research Network's 18-month follow-up data included a personal communication indicating a positive association between low weight/length ratio and poor neurodevelopment (32). This relationship warrants a closer examination.
The American Academy of Pediatrics recommends that growth of preterm infants replicate that of fetuses of the same gestational age (17) and there are data available to describe in-utero changes in body composition (23). However, preterm infants in the ex-utero environment are known to grow (8, 9, 24, 25) and accrete nutrients (26-28) differently than fetuses in-utero. More research on postnatal growth, its composition and related outcomes is needed to better understand and define “ideal” for preterm infants.
In the meantime, the evaluation of preterm infant postnatal growth status continues to use available tools. Intrauterine growth curves illustrate “ideal” or fetal growth for preterm infants but not growth over time because there is a different group of newborn infants for each GA (2, 12). Postnatal growth curves in contrast illustrate actual growth of preterm infants over time but as a result do not evaluate if growth is ideal or not. Although intrauterine growth curves are intended for the evaluation of growth status at birth, they are used to assess the growth of preterm infants throughout their NICU stay due to the lack of a better assessment tool.
There are several limitations to our study. The “end” timepoint in our study included infants who were discharged home (the majority), transferred to another hospital or died. Although the variation included in this timepoint could have affected our results, the primary analyses (Table I) performed only on infants discharged home had the same results.
We used an early, historically accepted measure of body proportionality, Rohrer's ponderal index, which we defined as weight-for-length, because body proportionality in preterm infants has not been well characterized and no anthropometric measure has been fully or functionally validated. There is still much unknown about body proportionality indexes in preterm infants including their efficacy and utility in the care of preterm infants in the NICU.
Although the ideal body proportionality index for preterm infants remains unclear, the Lubchenco weight-for-length growth curve was used in this study because it met the needs of our study. Use of the Lubchenco curves has been criticized because these data are not generalizable to the current U.S. NICU population. However there were no reference curves for any weight-for-length ratio based on a large, contemporary U.S. dataset available at the time of our study. We found the Lubchenco weight cutoffs for 10th and 90th percentiles were higher than a more contemporary weight-for-age curve (5), which may have under- and over-represented the larger and smaller infants in our sample, respectively. Nonetheless, the lack of agreement between growth status assessment methods found in this study should be valid because the same reference data were used for both growth status assessment methods.
Lastly the accuracy of the clinically-measured growth measurements in our study may be a limitation. Although NICU weight measurements are considered to be reliable (33), length is more difficult to measure precisely in the clinical setting (33). However, length measurements can be highly reproducible in the research setting (34). Further, we demonstrated in a Cincinnati NICU that clinical length measurements (conducted by NICU nurses as usual practice) were not significantly different from repeated research measurements (using standardized technique and equipment) on average (Olsen, unpublished data). Thus, use of weight-for-length offers a potentially reliable assessment tool in the NICU setting.
The assessment of weight growth status of preterm infants should include both weight-for-age and a body proportionality index, such as weight-for-length, as they often provide different information. Combined these measures may provide clinicians with more information on which to make care decisions. Research to determine the “ideal” body proportionality index for preterm infants and understand its ability to predict later outcomes is needed.
Acknowledgments
Supported in part by a grant from the National Institute for Child Health and Human Development Cooperative Multicenter Neonatal Research Network, No. U10 HD27853. The authors declare no potential conflicts of interest.
Abbreviations
- NICU
neonatal intensive care unit
- NICHD
National Institute of Child Health and Human Development
- GA
Gestational age
- Small-for-age
<10th percentile weight-for-gestational age
- Appropriate-for-age
10-90th percentile weight-for-gestational age
- Large-for-age
>90th percentile weight-for-gestational age
- Small-for-length
<10th percentile weight/length3 ratio-for-age
- Appropriate-for-length
10-90th percentile weight/length3 ratio-for-age
- Large-for-length
>90th percentile weight/length3 ratio-for-age
- BMI
body mass index
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Dietz WH. Health consequences of obesity in youth: childhood predictors of adult disease. Pediatrics. 1998;101:518–25. [PubMed] [Google Scholar]
- 2.Lubchenco LO, Hansman C, Dressler M, Boyd E. Intrauterine growth as estimated from liveborn birth-weight data at 24 to 42 weeks of gestation. Pediatrics. 1963;32:793–800. [PubMed] [Google Scholar]
- 3.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–8. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 4.Fenton TR. A new growth chart for preterm babies: Babson and Benda's chart updated with recent data and a new format. BMC Pediatr. 2003;3:13. doi: 10.1186/1471-2431-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Riddle WR, Donlevy SC, Lafleur BJ, Rosenbloom ST, Shenai JP. Equations describing percentiles for birth weight, head circumference, and length of preterm infants. J Perinatol. 2006;26:556–61. doi: 10.1038/sj.jp.7211572. [DOI] [PubMed] [Google Scholar]
- 6.Arbuckle TE, Wilkins R, Sherman GJ. Birth weight percentiles by gestational age in Canada. Obstet Gynecol. 1993;81:39–48. [PubMed] [Google Scholar]
- 7.Beeby PJ, Bhutap T, Taylor LK. New South Wales population-based birthweight percentile charts. J Paediatr Child Health. 1996;32:512–8. doi: 10.1111/j.1440-1754.1996.tb00965.x. [DOI] [PubMed] [Google Scholar]
- 8.Lemons JA, Bauer CR, Oh W, Korones SB, Papile LA, Stoll BJ, et al. Very low birth weight outcomes of the National Institute of Child Health and Human Development neonatal research network, January 1995 through December 1996. NICHD Neonatal Research Network. Pediatrics. 2001;107:E1. doi: 10.1542/peds.107.1.e1. [DOI] [PubMed] [Google Scholar]
- 9.Clark RH, Thomas P, Peabody J. Extrauterine growth restriction remains a serious problem in prematurely born neonates. Pediatrics. 2003;111:986–90. doi: 10.1542/peds.111.5.986. [DOI] [PubMed] [Google Scholar]
- 10.Cooke RJ, Ainsworth SB, Fenton AC. Postnatal growth retardation: a universal problem in preterm infants. Arch Dis Child Fetal Neonatal Ed. 2004;89:F428–30. doi: 10.1136/adc.2001.004044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Battaglia FC, Lubchenco LO. A practical classification of newborn infants by weight and gestational age. J Pediatr. 1967;71:159–63. doi: 10.1016/s0022-3476(67)80066-0. [DOI] [PubMed] [Google Scholar]
- 12.Lubchenco LO, Hansman C, Boyd E. Intrauterine growth in length and head circumference as estimated from live births at gestational ages from 26 to 42 weeks. Pediatrics. 1966;37:403–8. [PubMed] [Google Scholar]
- 13.Bartels DB, Kreienbrock L, Dammann O, Wenzlaff P, Poets CF. Population based study on the outcome of small for gestational age newborns. Arch Dis Child Fetal Neonatal Ed. 2005;90:F53–9. doi: 10.1136/adc.2004.053892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Olsen I, Meinzen-Derr J, Lawson L, Morrow A. Unexpectedly high SGA rates by gestational age in VLBW cohort. PAS. 2005;57:2569. Abstract. [Google Scholar]
- 15.Tukey JW. Exploratory Data Analysis. Don Mills, Ontario: Addison-Wesley; 1977. [Google Scholar]
- 16.Altman DG. Practical statistics for medical research. London: Chapman and Hall; 1991. [Google Scholar]
- 17.American Academy of Pediatrics, Committee on Nutrition. Nutritional needs of low-birth-weight infants. Pediatrics. 1985;75:976–86. [PubMed] [Google Scholar]
- 18.Ziegler EE. Protein in premature feeding. Nutrition. 1994;10:69–71. [PubMed] [Google Scholar]
- 19.Tsang RC, Lucas A, Uauy R, Zlotkin S, editors. Nutritional Needs of the Preterm Infant: Scientific Basis and Practical Guidelines. Baltimore: Williams & Wilkins; 1993. [Google Scholar]
- 20.Carlson SJ, Ziegler EE. Nutrient intakes and growth of very low birth weight infants. J Perinatol. 1998;18:252–8. [PubMed] [Google Scholar]
- 21.Embleton NE, Pang N, Cooke RJ. Postnatal malnutrition and growth retardation: an inevitable consequence of current recommendations in preterm infants? Pediatrics. 2001;107:270–3. doi: 10.1542/peds.107.2.270. [DOI] [PubMed] [Google Scholar]
- 22.Olsen IE, Richardson DK, Schmid CH, Ausman LM, Dwyer JT. Intersite differences in weight growth velocity of extremely premature infants. Pediatrics. 2002;110:1125–32. doi: 10.1542/peds.110.6.1125. [DOI] [PubMed] [Google Scholar]
- 23.Ziegler EE, Thureen PJ, Carlson SJ. Aggressive nutrition of the very low birthweight infant. Clin Perinatol. 2002;29:225–44. doi: 10.1016/s0095-5108(02)00007-6. [DOI] [PubMed] [Google Scholar]
- 24.Cooke R, Embleton N, Rigo J, Carrie A, Haschke F, Ziegler E. High protein pre-term infant formula: effect on nutrient balance, metabolic status and growth. Pediatr Res. 2006;59:265–70. doi: 10.1203/01.pdr.0000196376.99101.34. [DOI] [PubMed] [Google Scholar]
- 25.Ehrenkranz RA, Dusick AM, Vohr BR, Wright LL, Wrage LA, Poole WK. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics. 2006;117:1253–61. doi: 10.1542/peds.2005-1368. [DOI] [PubMed] [Google Scholar]
- 26.Reichman B, Chessex P, Putet G, Verellen G, Smith JM, Heim T, et al. Diet, fat accretion, and growth in premature infants. N Engl J Med. 1981;305:1495–500. doi: 10.1056/NEJM198112173052503. [DOI] [PubMed] [Google Scholar]
- 27.Bhatia J, Rassin DK. Growth and total body water in premature infants fed “in-utero” or “ex-utero”. Acta Paediatr Scand. 1988;77:326–31. doi: 10.1111/j.1651-2227.1988.tb10656.x. [DOI] [PubMed] [Google Scholar]
- 28.Kashyap S, Schulze KF, Forsyth M, Zucker C, Dell RB, Ramakrishnan R, et al. Growth, nutrient retention, and metabolic response in low birth weight infants fed varying intakes of protein and energy. J Pediatr. 1988;113:713–21. doi: 10.1016/s0022-3476(88)80388-3. [DOI] [PubMed] [Google Scholar]
- 29.Vohr BR, Wright LL, Dusick AM, Mele L, Verter J, Steichen JJ, et al. Neurodevelopmental and functional outcomes of extremely low birth weight infants in the National Institute of Child Health and Human Development Neonatal Research Network, 1993-1994. Pediatrics. 2000;105:1216–26. doi: 10.1542/peds.105.6.1216. [DOI] [PubMed] [Google Scholar]
- 30.Hack M, Flannery DJ, Schluchter M, Cartar L, Borawski E, Klein N. Outcomes in young adulthood for very-low-birth-weight infants. N Engl J Med. 2002;346:149–57. doi: 10.1056/NEJMoa010856. [DOI] [PubMed] [Google Scholar]
- 31.Hack M, Breslau N, Weissman B, Aram D, Klein N, Borawski E. Effect of very low birth weight and subnormal head size on cognitive abilities at school age. N Engl J Med. 1991;325:231–7. doi: 10.1056/NEJM199107253250403. [DOI] [PubMed] [Google Scholar]
- 32.Dusick AM, Poindexter BB, Ehrenkranz RA, Lemons JA. Growth failure in the preterm infant: can we catch up? Semin Perinatol. 2003;27:302–10. doi: 10.1016/s0146-0005(03)00044-2. [DOI] [PubMed] [Google Scholar]
- 33.Johnson TS, Engstrom JL, Gelhar DK. Intra- and interexaminer reliability of anthropometric measurements of term infants. J Pediatr Gastroenterol Nutr. 1997;24:497–505. doi: 10.1097/00005176-199705000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Griffin IJ, Pang NM, Perring J, Cooke RJ. Knee-heel length measurement in healthy preterm infants. Arch Dis Child Fetal Neonatal Ed. 1999;81:F50–5. doi: 10.1136/fn.81.1.f50. [DOI] [PMC free article] [PubMed] [Google Scholar]


