Abstract
Although deficiencies in emotional responding have been linked to externalizing behaviors in children, little is known about how discrete response systems (e.g., expressive, physiological) are coordinated during emotional challenge among these youth. We examined time-linked correspondence of sad facial expressions and autonomic reactivity during an empathy-eliciting task among boys with disruptive behavior disorders (n = 31) and controls (n = 23). For controls, sad facial expressions were associated with reduced sympathetic (lower skin conductance level, lengthened cardiac preejection period [PEP]) and increased parasympathetic (higher respiratory sinus arrhythmia [RSA]) activity. In contrast, no correspondence between facial expressions and autonomic reactivity was observed among boys with conduct problems. Furthermore, low correspondence between facial expressions and PEP predicted externalizing symptom severity, whereas low correspondence between facial expressions and RSA predicted internalizing symptom severity.
Keywords: Conduct problems, Disruptive behavior, Facial expression, Sadness, Autonomic reactivity
Conduct disorder (CD) and oppositional defiant disorder (ODD), jointly labeled as disruptive behavior disorders (DBDs) in the DSM-IV-TR (American Psychiatric Association, 2000), are among the most common mental health problems facing youth (Achenbach & Howell, 1993; Knitzer, Steinberg, & Fleisch, 1991). There is now a growing body of literature suggesting that children and adolescents with DBDs have difficulties with several domains of empathic responding, including emotional processing and self-appraisals of emotional experiences (de Wied, Goudena, & Matthys, 2005; Douglas & Strayer, 1996; Herpertz et al., 2005; Strand & Nowicki, 1999). However, other components of empathic responding such as displays of sad facial expressions and autonomic reactivity have only begun to receive empirical attention (Blair, 1999; de Wied et al., 2005; Douglas & Strayer, 1996; Herpertz et al., 2005).
Functionalist theories of emotion suggests that the synchronization of emotional response systems plays a critical role in emotional health and that desynchronization contributes to the emergence and maintenance of psychopathology (see Ekman, 1992; Kring, Kerr, Smith, & Neale, 1993; Levenson, 1994; Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005; Sloan, Strauss, Quirk, & Sajatovic, 1997). According to this perspective, as emotions unfold over time, the coordination of emotional appraisal, expressive behavior, and physiological responses should improve the reliability and precision of emotion cues and promote efficient behavioral and social responses (Levenson, 1994). To date, empirical support for this hypothesis has been mixed (Feldman-Barrett, 2006), with some authors finding low to moderate correlations between experiential, behavioral, and physiological response domains (Buck, 1977; Gross & Levenson, 1993,1997; Kettunen, Ravaja, Näätänen, Keltikangas-Jarvinen, 2000; Lanzetta, Cartwright-Smith, & Kleck, 1976; Mauss et al., 2005), others reporting discrepancies in the direction of effects (Notarius & Levenson, 1979), and still others reporting no relationship at all (Mauss, Wilhelm, & Gross, 2004).
Recently, several researchers have suggested that inconsistent findings may be the result of methodological limitations in prior research (for a review, see Mauss et al., 2005). For example, within-participant designs assessing correlations between facial sadness and physiology for an individual over time may be more sensitive and more relevant to questions of response coherence than more commonly used between-participants designs that assess whether people who tend to show more facial sadness also have stronger physiological responses.
In addition, few of the studies conducted to date in which response coherence has been examined have included differentiated measures of autonomic nervous system (ANS) responding. Yet empirical work has demonstrated that parasympathetic (PNS) and sympathetic (SNS) nervous system influences on heart rate often operate with considerable independence in affecting cardiac chronotropy (Berntson, Cacioppo, Quigley, & Fabro, 1994; Cacioppo, Uchino, & Berntson, 1994). This suggests that studies of response coherence that include measures of physiological responding may benefit from using independent indicators of PNS (respiratory sinus arrhythmia) and SNS (electrodermal responding, cardiac preejection period) activation rather than using measures that are influenced concurrently by both autonomic branches (heart rate). For example, response coherence between electrodermal responding (EDR) and facial affect assessed using a within-participant design during episodes of sadness is moderate to high in samples of healthy adults (Mauss et al., 2005).
Preliminary research suggests that there are marked individual differences in the degree of response coherence and that deficits in emotion regulation capabilities and/or attempts to control emotional expression may cause uncoupling of response domains for some individuals (Mauss et al., 2005). Along these lines, dissociation of emotion response systems has been noted in those with depression and schizophrenia, two disorders that are characterized by dysregulated emotion, and among individuals low in dispositional emotion expressivity (Gross, John, & Richards, 2000; Kring et al., 1993; Sloan et al., 1997). Yet surprisingly, no research has explored the extent to which youth with DBDs may exhibit less correspondence across emotional response systems (e.g., facial expression, autonomic reactivity) than their typically developing peers. In this article, time-linked correspondence between facial expressions of sadness and autonomic responding during a sad emotion induction are compared between boys with DBDs and typically developing controls.
Until recently, evidence for deficits in empathic responding among youth with DBDs came primarily from studies of nonverbal processing abilities and self-reported emotional empathy. Nonverbal processing of affect, or the ability to read and decode emotions in faces and vocal tones, is critical for social functioning and is a principal component of empathy (Elfenbein, Marsh, & Ambady, 2002). Children with DBDs are less accurate at decoding emotion in both faces and voices. For example, children with psychopathic tendencies show selective impairments in the recognition of both sad and fearful vocal tones and facial expressions (Stevens, Charman, & Blair, 2001). Furthermore, clinically identified children diagnosed with CD do not decode emotion in faces as well as age-matched controls (Strand & Nowicki, 1999). Youth with DBDs also self-report lower emotional arousal in response to affective pictures and describe fewer concordant emotional responses to other’s expressions of affect, particularly sadness and anger (de Wied et al. 2005; Douglas & Strayer, 1996; Herpertz et al., 2005). These findings are not surprising given the extent to which emotional sensitivity conflicts with aggressive or otherwise delinquent acts that are characteristic of these youth (Beauchaine, Gartner, & Hagen, 2000; Blair, 1995).
Among the various components of emotional responding, facial expressions have emerged as a promising means of evaluating state reactivity to emotionally evocative stimuli and appear to be particularly relevant for studies of empathy (Ekman, 1992). Humans tend to unconsciously mimic facial expressions that are congruent with the emotions they perceive, and this mimicry appears to be important for efficiently and accurately detecting emotional states in others (Niedenthal, Brauer, Halberstadt, & Innes-Ker, 2001). Compared with simply observing emotional subject matter, generating facial muscle movements that imitate another’s emotional state evokes heightened activation in neural circuits that are implicated in the experience of emotion, such as the inferior frontal cortex, superior temporal cortex, insula, and amygdala (Carr, Iacoboni, Dubeau, Mazziotta, & Luigi Lenzi, 2003; Iacoboni, 2005). Thus, mimicry may play an important role in the ability to empathize with the emotions of others. Facial mimicry of sadness is of particular interest, as it is important in emotional contagion, or vicariously sharing the emotions of others, which is thought to precede empathic responding (Hess & Blairy, 2001).
Although few studies have investigated facial expressions in youth with DBDs, preliminary findings have begun to emerge. While taking an IQ test, externalizing boys exaggerated facial expressions of anger, but not of fear or sadness (Keltner, Moffitt, & Stouthamer-Loeber, 1995). In contrast, facial mimicry of angry facial expressions is limited among boys with DBDs, suggesting that they may be deficient in their ability to identify with angry emotional states in others (de Wied, van Boxtel, Zaalberg, Goudena, & Matthys, 2006). Thus, although preliminary research suggests that facial expressions of sadness may not be impaired in youth with DBDs, no research has investigated facial sadness in these youth using tasks designed specifically to elicit sad emotional states (Keltner et al., 1995). Thus, further research is needed.
There is also evidence that viewing another person in distress elicits corresponding autonomic nervous system (ANS) responses that indicate emotional states (Eisenberg et al., 1988; Levenson & Ruef, 1992; Tsai, Levenson, & Cartenson, 2000; Walbott, 1991). These autonomic response patterns appear to be consistent across cultures and serve to recruit the physiological resources needed to regulate and respond to emotional cues (Levenson, 1992; Levenson & Ruef, 1992). The ANS also mediates expressive changes in the eyes and facial muscles that are important for emotional communication (Levenson, 2003). Thus, different patterns of ANS responding appear to be important for empathy reactions.
It is now well known that children and adolescents with DBDs exhibit patterns of underresponsiveness in both the sympathetic and parasympathetic branches of the ANS (e.g., Beauchaine, Gatzke-Kopp, & Mead, 2007; Beauchaine, Katkin, Strassberg, & Snarr, 2001; Crowell et al., 2006; Ortiz & Raine, 2004). These findings extend to a number of stimulus conditions, including (a) responding for monetary incentives, (b) viewing escalating conflict scenes among child actors, and (c) viewing both pleasant (pictures of pets or family scenes) and unpleasant (pictures of wounded children and violent scenes) images (e.g., Beauchaine et al., 2001; Herpertz et al., 2005). In the only study in which autonomic reactivity was assessed specifically during distress cues in such a population, Blair (1999) reported lower skin conductance responses to expressive emotional slides (e.g., a person crying) among clinic-referred children with psychopathic tendencies compared with controls. Although these same deficits were not observed in boys with DBDs who did not exhibit psychopathic traits, the sample size was limited, and only one measure of autonomic activity (electrodermal responding) was recorded. Thus, it remains unclear whether youth with DBDs show broader patterns of deficient autonomic responding when viewing others in distress. However, these findings suggest that boys with DBDs may not show a more global pattern of abnormal autonomic responding during sad emotional states.
In addition, research conducted with DBD samples to date has focused almost exclusively on reactivity within single emotional response systems. The few studies that have sampled multiple domains of emotional responding among youth with DBDs have indicated functional compromises across systems, underscoring the importance of measuring multiple domains of emotional reactivity. Children with CD, for example, self-report low levels of arousal to unpleasant pictures and show autonomic hyporesponsiveness to positive, negative, and neutral emotional stimuli (Herpertz et al., 2005). Thus, although much progress has been made in delineating the univariate emotional deficits exhibited by youth with DBDs, it remains unclear how their emotion response systems are coordinated. One possibility is that certain deficits (such as responses to sadness) may not be observable unless multiple response systems are considered.
In this study, we used multilevel modeling techniques to evaluate time-linked correspondence of facial expressions of sadness and ANS reactivity in youth with DBDs and their typically developing peers. We use the term correspondence somewhat broadly to refer to time-linked correlations between any two emotional response systems. This is the first study to apply a multilevel modeling approach to questions of response coherence. This procedure should be more sensitive to detecting effects than more traditional approaches given increased power associated with direct modeling of hierarchically structured data at all levels of nesting (i.e., repeated measures nested within individuals nested within groups).
In addition, although many theories of response coherence are predicated on the fact that incoherent emotional responding across systems marks ill health, to our knowledge this is the first study to use such methodologically rigorous procedures to test whether the level of coherence differs between individuals with and without psychopathology. This study addresses a critical hypothesis—that boys with DBDs show less correspondence across emotional response systems than control boys—regardless of their overall levels of emotional responding. We examined how emotional response systems are coordinated in boys with DBDs and their age-matched peers during a sad emotion induction. We hypothesized that boys with DBDs would exhibit (a) fewer expressions of sadness, (b) less physiological reactivity, and (c) less correspondence between facial expressions of sadness and autonomic reactivity during an empathy-eliciting film than controls.
Method
Participants
Data for this study were drawn from a larger investigation of the autonomic substrates of childhood emotional and behavioral problems. Parents of children were recruited through fliers, newspaper ads, and community outpatient clinics serving suburban and urban communities in and around Seattle, Washington. One version of these ads described the behavioral characteristics of ODD and CD and informed parents that they could earn $75 for participating with their child. A second version recruited well-adjusted children. Parents of potential child participants were interviewed by telephone using a computerized diagnostic interview that included portions of the Child Symptom Inventory (CSI; Gadow & Sprafkin, 1997). The parent version of the CSI is a dimensionalized DSM-IV checklist with established test-retest reliability and adequate predictive validity compared with psychiatric diagnoses of ODD and CD (Gadow & Sprafkin, 2002). Parent responses on the CSI also demonstrate concurrent validity with the aggressive behavior and delinquency subscales on the Child Behavior Checklist (CBCL; Achenbach, 1991). Ratings are rendered across four anchors (0 = never, 1 = sometimes, 2 = often, 3 = very often), with scores of 2 or higher considered positive for each diagnostic criterion. Children were considered positive for a given disorder if enough symptoms were endorsed at 2 or higher to meet DSM-IV criteria.
Families were invited to participate if they met inclusion criteria but failed to meet exclusion criteria for one of the study groups. Boys were placed in the DBD group if they met diagnostic criteria for ODD and/or CD based on the CSI. Controls were required to be diagnosis free on all CSI-assessed disorders. Scores on all psychopathology scales are reported by group in Table 1. Convergent evidence of psychopathology was obtained from the CBCL, parent report (Achenbach, 1991). Scores obtained on the CBCL aggression, attention, and anxious/depressed scales are also reported by group in Table 1.
Table 1.
Descriptive Statistics for Boys in the Disruptive Behavior Disorder (DBD) and Control Groups
| Variable | DBD (n = 31) mean (SD) | Control (n = 23) mean (SD) | Effect size (d) | F(l,52) | P |
|---|---|---|---|---|---|
| Age | 9.8 (1.4) | 10.5(1.5) | 0.5 | 1.39 | .24 |
| Family income (thousands) | 52.3 (43.8) | 66.4 (22.2) | 0.4 | 2.77 | .10 |
| Single parent homes | 42% | 19% | 0.6 | 3.42 | .07 |
| Minority status | 23% | 9% | 0.4 | 1.83 | .18 |
| CBCL aggression T score | 79.2 (7.5) | 50.8 (2.0) | 5.2 | 310.00 | <.01 |
| CBCL attention problems T score | 74.2 (11.0) | 52.9 (6.5) | 2.4 | 68.46 | <.01 |
| CBCL anxious/depressed T score | 73.5 (10.6) | 52.9 (3.8) | 2.6 | 78.90 | <.01 |
| CSI CD symptom count | 2.3 (2.0) | 0.0 (0.0) | 1.6 | 28.71 | <.01 |
| Diagnostic criteria met | 51% | 0% | |||
| CSI ODD symptom count | 6.4 (1.6) | 0.3 (0.7) | 8.9 | 291.30 | <.01 |
| Diagnostic criteria met | 94% | 0% | |||
| CSI ADHD symptom count | 11.8 (4.4) | 2.3 (3.4) | 2.4 | 75.65 | <.01 |
| Diagnostic criteria met | 41% | 0% | |||
| CSI MDD symptom count | 1.3 (2.3) | 0.0 (0.0) | 0.8 | 8.23 | <.01 |
| Diagnostic criteria met | 10% | 0% | |||
| CSI dysthymia symptom count | 1.0 (1.8) | 0.0 (0.0) | 0.8 | 6.87 | .01 |
| Diagnostic criteria met | 23% | 0% | |||
| Facial sadnessa | |||||
| Baseline | 0.4 (0.6) | 0.2 (0.4) | 0.4 | 2.23 | .14 |
| Second-by-second scoring | 0.3 (0.5) | 0.4 (0.5) | 0.2 | 1.20 | .64 |
| Epoch scoring (30 s) | 2.0 (1.4) | 2.4 (1.8) | 0.3 | 0.95 | .34 |
| Baseline physiological data | |||||
| Skin conductance (µS) | 0.03 (0.1) | 0.05 (0.1) | 0.2 | 1.21 | .28 |
| Preejection period (ms) | 102.3 (20.1) | 99.3 (18.1) | 0.2 | 0.32 | .57 |
| Respiratory sinus arrhythmia (ms2) | 9.1 (1.4) | 9.1 (1.4) | 0.0 | 0.00 | .99 |
| Task physiological datab | |||||
| Skin conductance (µS) | 0.3 (0.3) | 0.6 (0.8) | 0.3 | — | — |
| Preejection period (ms) | 105.5 (18.2) | 100.1 (18.6) | 0.3 | — | — |
| Respiratory sinus arrhythmia (ms2) | 9.1 (1.3) | 9.3 (1.4) | 0.2 | — | — |
Notes. DBD: disruptive behavior disorder; CSI: Child Symptom Inventory; CD: conduct disorder; ODD: oppositional defiant disorder; ADHD: attention deficit hyperactive disorder; MDD: major depressive disorder.
Facial sadness values are not comparable across conditions given differences in scoring protocols.
Group differences in physiological responding during the task are not presented here because they were evaluated in the multilevel models and are reported in text.
The sample included 31 boys with DBDs and 23 controls, ranging in age from 9 to 13. The racial/ethnic composition included 16.7% minority participants (7.4% African American, 3.7% American Indian, 1.9% Asian American, 3.8% other). Parents reported a median family income of $50,000. Demographic information is reported in Table 1.
Procedure
Active informed consent was obtained from parents and youth according to procedures approved by the University of Washington Human Subjects Review Committee. Participants were instructed to avoid caffeine and over-the-counter medications 24 h prior to their visit. Participants were also asked to discontinue stimulants (if applicable)—most of which have very short half lives—36 h prior to the laboratory visit to minimize effects on psychophysiological recordings. Use of other medications was fairly low in this sample. Nine participants (16.7%) reported stimulant use, 2 participants (3.7%) reported antidepressant use, and 1 participant (1.9%) reported atomoxetine use. All analyses were initially run with these boys dropped from the models and then refit with them included. No significant differences were observed between models, so only the models with all participants included are presented. All information obtained from participant families was protected by a Certificate of Confidentiality from the National Institute of Mental Health.
During the visit, boys were seated in a sound-attenuated room and filmed by a concealed camera. Boys first participated in a monetary incentive and extinction task that lasted approximately 30 min and has been described in detail elsewhere (e.g., Beauchaine et al., 2001). Then, following a 5-min baseline, participants underwent the sad emotion induction procedure.1 This involved watching a 3-min scene from The Champ, a film that has been used extensively in prior research to elicit emotional sadness, as indicated by subjective, behavioral, physiological, and neural responses among both adolescents and adults (e.g., Crowell et al., 2005; Goldin et al., 2005; Gross & Levenson, 1995; Hewig etal., 2005; Hutcherson, Goldin, Ochsner, Gabrieli, & Gross, 2005). The film shows a young boy responding to the death of his father. He reacts with disbelief and then becomes increasingly inconsolable. For purposes of the present study, an emotional film clip offered several advantages over other procedures commonly used to elicit emotions, such as still picture paradigms. Most importantly, we were interested in assessing time-linked synchronization of emotional reactivity across autonomic and facial channels, a goal that could not be accomplished efficiently using discrete stimulus presentations. Sadness was targeted because the degree of sadness induced by viewing another person in distress quantifies empathic responding, the development of which is compromised among boys with behavior problems (Duan, 2000; Eisenberg et al., 1988; Hess & Blairy, 2001). Boys’ psychophysiological responding across a number of channels (see below) was continuously recorded during the video. Families were recompensed $75 for participation.
Psychophysiological Measures
Skin conductance level (SCL)
To assess electrodermal responding during the task, SCL was acquired continuously using a Grass Model 15LT Physiodata Amplifier System with a 15A12 DC amplifier (West Warwick, RI). Two 0.8-cm2 Ag-AgCl electrodes were applied to the thenar eminence of the participant’s nondominant hand using adhesive masking collars and Parker Laboratories Signa Gel (Fairfield, NJ). The SCL signal was recorded in microSiemens and digitized at 1 kHz using the Grass PolyVIEW software system. Electrodermal responding reflects cholinergically mediated influences of the sympathetic nervous system (SNS). Increased SCL indicates SNS activation. All SCL data were within-person centered for use in the multilevel models, described below, to minimize hydration artifacts (Ben-Shakhar, 1985).
Cardiac preejection period (PEP)
Cardiac activity was monitored continuously during the task via electrocardiographic (ECG) and impedance cardiographic (ICG) signals acquired by a HIC 2000 Impedance Cardiograph (Chapel Hill, NC) using the spot electrode configuration described by Qu, Zhang, Webster, and Tompkins (1986). Both the ECG and ICG waveforms were digitized at 1 kHz. Digitized ECG and ICG signals were ensemble-averaged in 30-s epochs using COP-WIN 5.06. PEP was indexed as the time between left-ventricular depolarization, indicated by onset of the ECG Q-wave, and the initiation of ejection of blood into the aorta, indicated by the ICG B-wave. PEP is a validated measure of β-adrenergically mediated SNS-linked cardiac activity (see Sherwood, Allen, Obrist, & Langer, 1986; Sherwood et al., 1990). Shorter PEPs indicate increased SNS activation.
Respiratory sinus arrhythmia (RSA)
RSA was indexed by extracting the high frequency component (>0.15 Hz) of the R-R time series using spectral analysis. High-frequency spectral densities were calculated in 30-s epochs using the Medistar Nevrokard software system and normalized through natural log transformations, as is common practice. The ECG signal was acquired through the ICG electrodes, which were arranged in a modified Lead II configuration. RSA is a well-validated measure of parasympathetic nervous system (PNS) activity (see Berntson et al., 1997). Increases in RSA reflect greater PNS activation.
Facial Sadness
Emotion elicitation condition
Videotapes of participants observing the film clip were coded by two trained research assistants using the Emotional Behavior Coding System (EBCS; Gross, 1996). Coders were blind to participant diagnostic status and were required to attend an 8-week training course prior to beginning coding. During the training, coders were required to learn the individual muscular actions and the prototype expression for sadness described by Ekman and Friesen (1975), which serve as the basis for the EBCS codes. The sadness code of the EBCS categorizes the degree of facial sadness expressed each second based on the presence and valence (intensity) of emotion expression. In accord with EBCS guidelines, coders first observed the participant for 2 min at baseline to familiarize themselves with the participants’ natural resting facial expressions. While coding, scores were assigned during the sad emotion induction based upon movement away from this baseline. Sadness was coded on a 4-point scale, including 0 (no sadness), 1 (slight down turning of the mouth or a slight upturning of the inner eye-brow), 2 (any moderate sign of sadness, including clear upturning of the inner eyebrow or mouth, slight quivering of the chin or mouth, widening of the nostrils), and 3 (multiple moderate signs of sadness). Scores were assigned each second to match the temporal sequencing of the SCL data.
For PEP and RSA, which cannot be scored reliably on a second-to-second basis, a single sadness value was assigned to each 30-s epoch. This enabled us to match the timing of the cardiac measures. To capture both the duration (number of seconds spent appearing sad in the 30-s period) and the intensity of sadness, codes were assigned on a scale of 0 (0 s spent showing any signs of sadness) to 6 (at least 3 s showing multiple moderate signs of sadness). For example, to obtain a 2 across the 30-s epoch, participants needed to show at least 3 s of 1 level sadness within that epoch. To obtain a 4 across the 30-s epoch, participants needed to show at least 2 s of 2 level sadness within that epoch. This coding scheme carries more information than a simple average because it weights higher valenced (less subtle) facial expressions of sadness more heavily.
Baseline condition
Because group differences in facial sadness at baseline could affect outcomes observed during the emotion elicitation, we also assessed facial expressions during the 5-min rest period preceding the task. This was accomplished using a modified version of the ECBS procedure described above. Global scores of facial sadness (rather than changes from baseline) were assigned to each participant across the entire 5-min rest period on a scale of 0 (no facial sadness) to 3 (multiple signs of sadness).
To assess reliability, 15% of the data were assigned randomly to both coders, who were unaware of which data were used for assessing interrater agreement. The average between-observer agreement, using a ± 1-s window, was 89% for the second-by-second data. The intraclass correlation coefficient was adequate for both the second-by-second data (using a ± 1-s window), ρ = .74, and for the 30-s windowed data, ρ = .73. These reliability statistics are consistent with prior research using the EBCS to code facial displays of sadness (e.g., Rottenberg, Kasch, Gross, & Gotlib, 2002).
Results
Preliminary Analyses
Descriptive statistics
Descriptive statistics for each measure are reported by group in Table 1. Prior to analyses, distributions of all variables were examined for adherence to distributional assumptions of the inferential statistics used. Preliminary analyses evaluated the significance of group differences in each of the psychopathology scales using analyses of variance (ANOVAs). Effect sizes (Cohen’s d) are also reported. As expected, given our recruitment strategy, group differences in CSI symptoms of CD, ODD, hyperactivity, inattention, MDD, and dysthymia were significant, all Fs > 6.87, ps ≤ .01, with large effect sizes (d = 0.8–8.9).
Baseline effects
During the 5-min baseline, there were no significant differences in autonomic activity for any of the measures, all Fs < 3.03, all ps > .09 (see Table 1). In addition, few expressions of facial sadness were observed at baseline for boys with DBDs or for controls (see Table 1), with no group difference found, F = 2.23, p = .14.2
Multilevel Modeling Analyses
During the video clip, boys showed facial expressions of sadness that were above baseline levels 22% of the time, which is consistent with figures reported in prior research. This suggests that the clip was effective in eliciting state sadness (see Rottenberg et al., 2002). Next, analyses exploring group differences in levels of each of the assessed measures were conducted using HLM 6.0 (Raudenbush, Bryk, Cheong, & Congdon, 2004). One of the advantages of the multilevel modeling approach is that it provides simultaneous estimates of both within-participant effects (e.g., the 180 repeated observations of SCL and facial sadness for each person) and between-participants effects (e.g., individual differences in the relationship between SCL and facial sadness between people or between groups). Full maximum likelihood models followed the general form presented below for facial sadness:
At Level 1 the repeated observations for each participant were modeled. For example, in the equation above, sadnessti represents the 180 repeated observations of sadness (one observation per second for 3 min) for each person. At Level 2, individual variation among participants was assessed, and group comparisons were conducted using a dummy coded time invariant variable (1 = DBD, 0 = control). Thus, in the Level 2 equation above, the β01 term captures whether there are differences between groups in average levels of sadness. This set of analyses tested the hypotheses that boys with DBDs would exhibit (a) fewer expressions of sadness and (b) less physiological reactivity than controls. No significant group differences were found on any of the assessed measures: sadness, β01 = −.34, p = .34; SCL, β01 = .07, p =.54; PEP, β01 = −5.37, p = .30; RSA, β01 = −.12, p = .75. Thus, contrary to the first two study hypotheses, boys with DBDs and controls showed similar levels of facial sadness, SCL, PEP, and RSA during the emotion induction.
Demographic effects
Correlational analyses were used to examine the relations among (a) mean levels of facial sadness, (b) mean levels of each of the psychophysiological measures during the emotion induction, (c) adolescents’ racial/ethnic group membership, and (d) family income. This ensured that any observed effects were not artifacts of demographic differences within the sample. Results indicated no significant effects for race/ethnicity or parental income on the assessed variables (all rs < .20, all ps > .14). Demographic variables were therefore omitted from further analyses in order to maximize power.
Response coherence analyses
HLM with full maximum likelihood estimation was used to compare the response coherence of groups.3 For each psychophysiological measure, a two-level model was constructed (Bryk & Raudenbush, 1992). The SCL model was as follows:
At Level 1 the relationship between facial sadness and SCL was assessed by modeling the 180 second-by-second repeated observations of SCL and facial expression data for each participant, with facial sadness included as a time-varying covariate. At Level 2, variation in coherence among individuals was assessed, and group comparisons were conducted using a dummy coded time invariant variable (1 = DBD, 0 = control).
Identical procedures were followed for PEP and RSA, with two exceptions. First, because PEP and RSA were scored in 30-s epochs, data were modeled across the six 30-s epochs rather than across seconds. Second, because the time effect was not significant when added to the Level 1 equation in either model (PEP: β10 = .22, p = .23; RSA: β10 = .00, p = .76), the term was dropped in order to correctly identify the Level 1 model while maximizing degrees of freedom (Bryk & Raudenbush, 1992). The final RSA model is shown below:
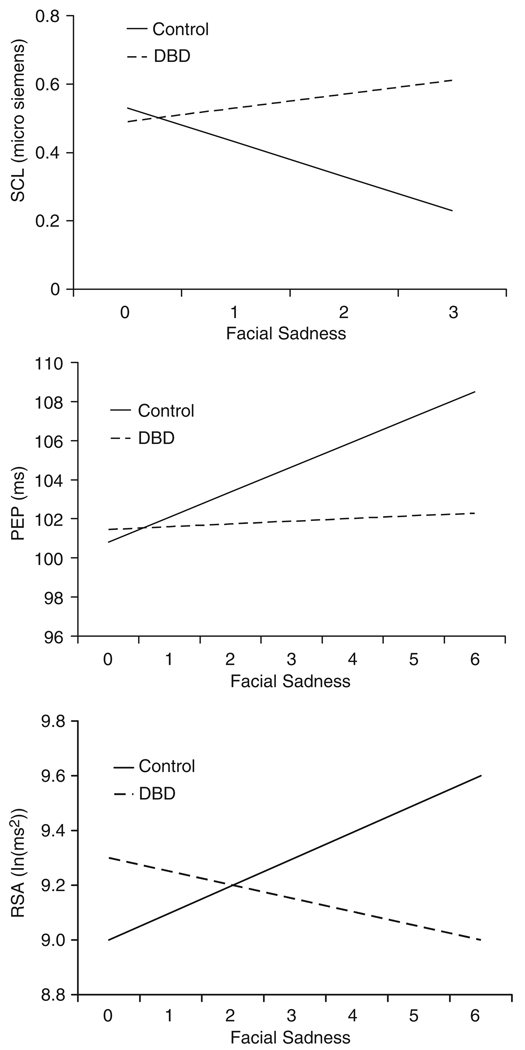
Analyses revealed significant associations between facial sadness and (a) decreased SCL, β20 = −.09, p = .03; (b) lengthened PEP, β10 = 1.19, p <.001; and (c) increased RSA, β10 = .09, p = .02. Thus, expressions of sadness were associated with reduced SNS and increased PNS activity. However, each of these findings was moderated by a significant group effect: SCL, β21 = .15, p = .01; PEP, β11 = −1.04, p < .0l; RSA, β11 = −.13, p = .03. Associations between facial sadness and autonomic responding are depicted by group in Figure 1. These findings are consistent with our third hypothesis, that boys with DBDs would exhibit less correspondence between facial expressions of sadness and autonomic reactivity to the emotion induction.
Figure 1.
Associations between facial expressions of sadness and skin conductance level (SCL; top panel), preejection period (PEP; middle panel), and respiratory sinus arrhythmia (RSA; bottom panel) for boys with DBDs (dashed lines) and controls (solid lines). Note that facial sadness ranged from 0 (no sadness) to 3 (highest level of sadness) for SCL, and from 0 (no sadness) to 6 (highest level of sadness) for PEP and RSA because of the different temporal resolutions of measures.
Follow-up group contrasts indicated that for each variable, significant associations between facial expression and autonomic responding were observed among controls, all ps < .05, but not among boys with DBDs, all ps ≥ .49. Without these contrasts, we could not rule out the possibility that boys with DBDs exhibited significant, albeit diminished, coherence relative to controls. Thus, controls evidenced a pattern of response coherence in which the PNS was activated and the SNS was deactivated during expressions of sadness. In contrast, facial expressions and autonomic responses were not significantly related for boys with DBDs. Effect sizes (Cohen’s d) separating boys with DBDs from controls on HLM slopes, which represent the correspondence between facial expressions and autonomic responding, were .70 for SCL, .87 for PEP, and .40 for RSA.
As is often the case, a greater number of boys with DBDs were symptomatic for ADHD (42%) and depression (7%) than controls (0% and 0%, respectively). Given this, we also explored whether the effects reported above were independently attributable to DBDs, above any effects of MDD, dysthymia, and ADHD. To accomplish this, all models were rerun with scores for (1) MDD, then (2) dysthymia, and finally (3) hyperactivity added as Level 2 time-invariant covariates for each of the psycho-physiological measures. MDD, dysthymia, and hyperactivity did not moderate the relationships between facial sadness and any of the assessed autonomic indices. Moreover, all but one group difference in the relationship between facial sadness and the physiological measures remained significant after controlling for MDD (SCL, β21 = .14, p = .03; PEP, β11 = −1.09, p < .01; RSA, β11 = .14, p = .03), dysthymia (SCL, β21 = .14, p = .03; PEP, β11= −1.06, p < .01; RSA, β11 = −.13, p = .04), and ADHD (SCL, β21 = .15, p = .03; PEP, β11 = −1.04, p < 0.1; RSA, β11 = −.10,.p = .12).4
Relation between Response Coherence and Problem Behaviors
In our final set of analyses, we evaluated associations between response coherence and measures of both internalizing and externalizing psychopathology. These analyses are summarized in Table 2, where we present correlations between individual slopes obtained from the HLM analyses, which represent response coherence, and each of the problem behavior scales from the CSI. These analyses yielded an interesting pattern of results. First, PEP slopes were negatively correlated with all externalizing behavior scores, including CD, ODD, hyperactivity, and inattention. Because response coherence for PEP was signified by positive slopes (see Figure 1), this indicates that low correspondence between PEP reactivity and facial expressions of sadness were associated with externalizing behaviors. No such pattern was observed linking PEP to internalizing behaviors.
Table 2.
Bivariate (and Partial) Correlations between Response Coherence Slopes and Problem Behavior Scores
| Variable | PEP slope | SCL slope | RSA slope |
|---|---|---|---|
| Physiological measures | |||
| PEP slope | — | ||
| SCL slope | −.23 | — | |
| RSA slope | −.25 | −.15 | — |
| Externalizing scales | |||
| ASI conduct | −.31* (−.09) | .32* (−.02) | −.21 (−.03) |
| disorder | |||
| ASI oppositional | −.33* (−.02) | .34* (−.12) | −.17 (.20) |
| defiant disorder | |||
| ASI hyperactivity | −.35* (−.09) | .32* (−.19) | −.06 (−.06) |
| ASI inattention | −.37* (.21) | .31* (.10) | −.11 (−.12) |
| Internalizing measures | |||
| ASI major | −.16 (.04) | .31* (.01) | −.32* (−.20) |
| depressive disorder | |||
| ASI dysthymia | −.13 (−.11) | .35* (−.05) | −.37* (.25) |
Notes. PEP: preejection period; SCL: skin conductance level; RSA: respiratory sinus arrhythmia; ASI: Adolescent Symptom Inventory (scale scores). Slopes represent the correspondence between each autonomic measure (PEP, SCL, RSA) and facial expressions of sadness during the film. These slopes were extracted from the HLM analyses for each participant. Partial correlations reflect the correspondence between response coherence slopes and each externalizing measure, controlling for all others, and each internalizing measure, controlling for the other.
p ≤ .05, two-tailed.
Second, in direct contrast with the findings for PEP, RSA slopes were negatively correlated with all internalizing behavior scores, including major depression and dysthymia. Because response coherence for RSA was also signified by positive slopes (see Figure 1), this indicates that low correspondence between RSA reactivity and facial expressions of sadness were associated with internalizing behaviors. No such pattern was observed linking RSA reactivity to externalizing behaviors. Thus, response dissociation between PEP reactivity and facial expressions was specific to externalizing behaviors, whereas response dissociation between RSA reactivity and facial expressions was specific to internalizing behaviors.
Third, SCL slopes were positively correlated with all internalizing and externalizing behavior scores. Because response coherence for SCL was indexed by negative slopes (see Figure 1), this indicates that low correspondence between SCL reactivity and facial expressions of sadness were associated with both internalizing and externalizing outcomes.
Finally, we computed partial correlations between response coherence slopes and each externalizing measure (e.g., CD), controlling for all others (e.g., ODD, hyperactivity, inattention), and between response coherence slopes and each internalizing measure (e.g., MDD), controlling for the other (e.g., dysthymia). None of these partial correlations, which represent the independent effects of each psychopathology scale in predicting response coherence, were significant. Thus, the pattern of results summarized above applies broadly to externalizing and internalizing symptoms and not to any specific diagnostic class.
Discussion
In this study, we evaluated three hypotheses regarding facial expressions and autonomic responses of boys with DBDs during an empathy-eliciting film. First, we predicted fewer expressions of sadness to the scene of a boy with his dying father among boys with DBDs than among controls. This hypothesis was not supported. Boys with DBDs and controls demonstrated equivalent levels of facial sadness, both at baseline and during the film. Although the null finding at baseline is not surprising given the absence of eliciting stimuli, we expected a group difference during emotion induction, following from previous research demonstrating empathy deficits in boys with DBDs. Second, we predicted less physiological reactivity to the film among boys with DBDs than among controls, also marking expected deficits in empathy. This hypothesis was also unsupported.
One possible interpretation of these findings is that the film clip was ineffective in eliciting empathy among participants, regardless of their group status. However, several previous studies have demonstrated the effectiveness of the The Champ in evoking state sadness (Crowell et al., 2005; Goldin et al., 2005; Gross & Levenson, 1995; Hewig et al., 2005; Hutcherson et al., 2005). Furthermore, even though group differences in facial expressions and autonomic reactivity were not observed during the film, both groups demonstrated increased facial sadness and altered autonomic functioning compared with baseline. Thus, more likely explanations for the failure to find group effects are (a) differences in the sample recruited for this study compared with previous studies and (b) divergent methods used in this study compared with previous work.
Most data demonstrating empathy deficits in boys with DBDs have been either behavioral or self-report. In one of the few studies in which autonomic reactivity during a sad emotion induction was measured, no group differences between children with DBDs and controls in either RSA or heart rate emerged (Zahn-Waxler, Cole, Welsh, & Fox, 1995). In fact, we are aware of no findings linking deficient autonomic responding to deficits in empathy observed during emotion induction using films. In studies where empathy has been linked with autonomic responding among children, the relation has usually been correlational and indirect (e.g., boys with DBDs have generalized low autonomic arousal and exhibit limited empathy; Eisenberg et al., 1996). Moreover, although Liew et al. (2003) did measure skin conductance and heart rate, linking lower reactivity in each to less empathy, this study (a) was conducted with a normative sample and (b) linked ratings of empathy to autonomic reactivity to static facial expressions of emotion (slides). Finally, although Blair (1999) described a similar finding with skin conductance in youth scoring high on psychopathy, such individuals comprise a small subset of youth with DBDs who are especially recalcitrant (see, e.g., Fowles & Dindo, 2006). In fact, Blair found no significant differences in electrodermal responses to sad facial cues between controls and boys with DBDs who did not score high on psychopathy. Taken together, these findings suggest that boys with DBDs may not differ from controls in overall patterns of sympathetic or parasympathetic activity during the experience of sadness.
The finding of similar levels of facial sadness in boys with DBDs and controls during the emotion induction is also consistent with research showing comparable levels of facial sadness in externalizing and control boys during structured social interactions (Keltner et al., 1995). Although previous research has reported deficient facial reactivity to angry faces in youth with DBDs, this study is the first to explore facial reactions to a sad emotion induction, which may be more relevant to empathic responding (de Wied et al., 2006). There is now reasonable agreement that the vicarious experience of anger verses sadness evokes different physiological and neurological responses. For example, observing angry facial expressions is associated with activity in the right orbitofrontal and cortex, whereas viewing sad faces is associated with enhanced activity in the left amygdala (Blair, Morris, Frith, Perrett, & Dolan, 1999). Anger and sadness also evoke distinct autonomic reactions (Levenson, 1992) and have distinct social communicative functions. Anger conveys disapproval and signals others to withdraw, whereas sadness elicits comfort and support from others (Izard, 1991). Thus, the possibility that boys with DBDs are impaired in their facial reactions to angry but not sad emotional cues suggests that different biological and communicative mechanisms may be involved. Further research is needed to understand the mechanistic processes involved in these deficiencies.
Our third and final hypothesis was that boys with DBDs would exhibit less correspondence between facial expressions of sadness and autonomic reactivity during an empathy-eliciting film than controls. For all three autonomic measures, this hypothesis was supported. During the emotion induction, facial expressions of sadness were associated with reduced SNS activity (lower SCLs, lengthened PEPs) and increased PNS activity (higher RSA) among controls. In contrast, time-linked correspondence between facial expressions of sadness and autonomic reactivity was not observed among boys with DBDs. These findings cannot be attributed to a lack of emotional responding among boys with DBDs given that both groups exhibited similar levels of sad facial expressions and autonomic reactivity during the emotion induction. One way to interpret these findings is that when boys with DBDs communicate sadness facially, the autonomic nervous system is not responding adaptively to promote physiological homeostasis and facilitate social engagement behaviors (cf. Porges, 1995, 1997, 2001).
Taken together, these results replicate those observed among adults, suggesting that correspondence among emotional response systems reflects adaptive emotional processing, perhaps in part because emotional reactions are most efficient when discrete systems work collectively (Mauss et al., 2005). These findings provide some of the first empirical evidence that boys with DBDs exhibit deficiencies in the correspondence of affect-modulating systems that are necessary for effective emotion regulation. Had we examined only one system (e.g., facial sadness or the autonomic measures alone) these findings would not have been evident. This illustrates the importance of exploring multiple measures of emotional responding concurrently and demonstrates a potential role for emotional response coherence in elucidating emotional deficits among boys with DBDs.
Significant links between facial expressions of sadness and PNS-linked cardiac reactivity were not observed among boys with DBDs. Given evidence for RSA as a peripheral index of emotion regulation capabilities (e.g., Beauchaine, 2001; Beauchaine et al., 2007; Porges, 1995, 1997, 2001, 2007), this discordance may indicate that physiological dysregulation plays a central role in the inappropriate affect often displayed by youth with DBDs. In normal samples, expressions of emotion are accompanied by changes in RSA, with aberrant patterns of RSA and RSA reactivity observed among antisocial youth at baseline and during negative emotion induction (e.g., Beauchaine et al., 2001, 2007). The present study extends these findings and demonstrates that boys with DBDs fail to exhibit changes in RSA that are coordinated with their facial reactions to sad emotional stimuli. Vagal influences, as indexed by RSA, typically modulate sympathetic activity under conditions of emotional challenge (Porges, 1995, 1997, 2001, 2007). With neurobiological and facial reactions that are decoupled, boys with DBDs may lack the resources to effectively modulate their physiological arousal, rendering them less capable of responding appropriately to others’ distress (Fabes, Eisenberg, & Eisenbud, 1993).
Phylogenetic accounts of emotion regulation suggest that PNS-mediated strategies for modulating affect are preferred among mammals. When unsuccessful, emotional lability prevails, with the SNS driving behavior toward either fight or flight responding, an evolutionarily older strategy (see Beauchaine et al., 2007; Porges, 1995, 2007). In the present study, boys with DBDs exhibited deficits in the coordination of facial expressions of sadness and both PNS and SNS activity, which may mark abnormalities in emotion regulation capacities. Thus, combined PNS and SNS deficiencies during moments of emotional expression may have significant ramifications for emotional functioning.
Analyses evaluating the strength of correspondence between emotional response coherence and individual psychopathology scales yielded an interesting pattern of results, which can be summarized as follows: (1) Deficits in response coherence between facial sadness and PEP were correlated exclusively with externalizing symptoms, including CD, ADHD, and ODD; (2) deficits in response coherence between facial sadness and RSA were correlated exclusively with internalizing symptoms, including major depression and dysthymia; and (3) deficits in response coherence between facial sadness and SCL were correlated with both externalizing and internalizing symptoms.
Because this pattern of results was not predicted and should therefore be replicated before being considered robust, we are reluctant to offer strong interpretations. Nevertheless, limited speculation is warranted. Prediction of externalizing symptoms by deficits in response coherence between facial sadness and PEP adds to a growing body of literature linking SNS deficiencies specifically to DBDs (e.g., Beauchaine et al., 2001, 2007). One possible yet tentative interpretation is that functional deficiencies within the dopaminergically mediated approach motivational system—which may be indicated by PEP changes to specific stimuli (Beauchaine et al., 2007; Brenner, Beauchaine, & Sylvers, 2005)—are reflected in response desynchrony. The approach motivational system mediates both appetitive and active avoidance behaviors, the latter of which facilitate escape from danger and threat (see Beauchaine, 2001; Beauchaine et al., 2001). In the present context, it is possible that sadness cues are not eliciting active avoidance motivations among children with DBDs. Again, this conjecture is speculative and requires further study.
Prediction of internalizing symptoms by deficits in response coherence between facial sadness and RSA is consistent with a growing literature linking vagal deficiencies to depressive, anxious, and self-injurious behaviors (e.g., Crowell et al. 2005; Rottenberg, Salomon, Gross, & Gotlib, 2005; Thayer, Friedman, & Borkovec, 1996). Yet reduced RSA is also observed consistently among externalizing children and adolescents (Beauchaine et al., 2001, 2007; Mezzacappa et al., 1997). Thus, replication of this finding is especially important before any claims of specificity to depressive symptoms are offered.
Finally, prediction of both externalizing and internalizing symptoms by deficits in response coherence between facial sadness and SCL is consistent with findings linking deficient electrodermal responding to both antisocial behavior and depression (e.g., Lorber, 2004; Sponheim, Allen, & Iacono, 1995). It is also well known that EDR is exquisitely sensitive to many types of stimuli, regardless of positive or negative valence (Dawson, Schell, & Filion, 2000). Again, we await future replications before offering a stronger interpretation.
Taken together, findings linking response dysynchrony to psychopathology must be juxtaposed with findings of no group differences in physiological reactivity to the emotion induction. Examination of descriptive statistics indicates the nonsignificant group differences in physiological measures were all in the expected direction—both at baseline and during the emotion induction—with the DBD group exhibiting lower skin conductance, longer preejection periods, and lower RSA than controls. However, effect sizes were too small to reach statistical significance. In contrast, effect sizes for differences in response coherence were all large. This discrepancy in effect sizes may reflect well-documented advantages of growth curve modeling, which HLM uses in the Level 1 models, over more traditional approaches to evaluating group differences in repeated measures over time (e.g., Rogosa, Brandt, & Zimowski, 1982; Speer & Greenbaum, 1995). If this is the case, evaluations of response coherence using multilevel modeling may offer an advantage over traditional methods in differentiating among clinical groups using physiological markers.
Limitations
Although this study advances our understanding of the relation between facial expressions of sadness and autonomic reactivity by assessing multiple response systems, there are nonetheless a number of limitations to consider. First, the sample size was relatively small, and the data were cross sectional. Consequently, we were unable to determine whether the pattern of results would have been different had we recruited boys with only ODD versus those with CD, the latter of whom tend to be older. Future longitudinal studies are needed to assess how patterns of response coherence change throughout childhood and adolescence for boys with DBDs. Second, the stimulus used elicited a particular type of emotion—sadness/empathy. Research suggests that different emotions generate specific ANS reactions (see Levenson, 1992). Using tasks designed specifically to elicit other emotions such as anger may elaborate patterns of response coherence and dysynchrony found in this study. Third, we focused on a particular form of psychopathology—DBDs. Future research exploring multiple and distinct psychopathological conditions (e.g., depression, anxiety, bipolar disorder) will help to uncover whether a lack of correspondence is a general marker of psychopathology or, alternatively, whether different patterns of incoherence are specific to different psychopathological conditions.
In addition, the lack of data from girls precluded exploration of potentially important sex differences in emotional response coherence. Moreover, although there is ample evidence for the reliability and validity of parental reports of behavior problems on the CSI, these data are were nonetheless limited by reliance on a single informant. Furthermore, we examined correspondence between two specific dimensions of emotional responding—ANS patterns and facial reactivity. Future research might address whether the patterns observed in this study are also found when other emotional response domains (e.g., self-report measures, EEG) are considered. Finally, we did not perform time-lagged analyses, which could uncover alternative patterns of response coherence across groups. For example, it is possible that boys with DBDs exhibit slower autonomic reactions to the experience of sadness, which might be detected by adding a time-lagged component to the HLM analyses. Although such a finding would be informative, it would not diminish findings presented here demonstrating clear differences in immediate response coherence among groups.
Conclusions
The results of this study indicate that although boys with DBDs show appropriate levels of facial and autonomic responding to elicitors of sadness, these systems are not as efficiently coupled as they are among controls. Such findings are consistent with functionalist theories of emotion, which argue that coordination across response systems should be associated with positive psychological adjustment and adaptive responding to social and environmental challenges. Although it has often been asserted that emotion response systems subserve adaptation, there has been little data on this question to date. In fact, our study may be the first to conduct this type of analysis using sophisticated multilevel modeling procedures to assess response coherence. Considered collectively, our results highlight the value of evaluating a broader array of emotional response systems than is typically examined in studies that focus on either biological or expressive components of emotional responding. Indeed, when multiple response systems are considered, it is possible to discriminate between boys with behavior problems and their age-matched, typically developing peers. Had we examined any particular emotional response system in isolation, these patterns would not have been evident.
Acknowledgments
This study was supported by grants from the National Science Foundation (GRFP DGE0203031) to Penny Marsh and the National Institute of Mental Health (R01 MH63699) to Theodore P. Beauchaine.
Footnotes
To determine whether there were carryover effects in the autonomic measures from the incentive task, we performed repeated measures analyses of variance in which (a) the last minute of responding during the 5-min baseline before the incentive task and (b) the last minute of responding during the 5-min baseline before the film were evaluated. No time effects, group effects, or Group × Time interactions were found for PEP, SCL, or RSA.
For investigative purposes, we also explored whether other forms of emotion (e.g., contemptuous affect) differed between groups. Fifteen percent of the boys showed transient expressions of mild contempt during the video (unilateral lip raise, eye roll), which typically lasted less than 5 s. However, there were no significant differences in contempt displayed between groups. Furthermore, we considered whether contemptuous affect interacted with diagnostic group status to predict patterns of autonomic responding in models paralleling those described for sadness above. No such effects were found.
For the HLM analyses, exact p values are reported for all probabilities at or above .001. Because HLM does not provide exact p values below this level, these effects are indicated by the inequality p < .001.
In a separate series of analyses, minority status and single parent status were entered as Level 2 time invariant covariates into all analyses to examine whether the reported effects were moderated by demographic factors. Neither demographic variable altered the relationships between facial sadness and any of the assessed autonomic indices. Moreover, group differences in the relationship between facial sadness and each of the physiological indicators remained after controlling for minority status (SCL, β21 = .14, p = .03; PEP, β11 = −1.02, p = .00; RSA, β11 = −.14, p = .03), and single parent status (SCL, β21 = .14, p = .03; PEP, β11 = −.99, p = .01; RSA, β11 = −.13, p = .06).
REFERENCES
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. Burlington, VT: University of Vermont Department of Psychiatry; 1991. [Google Scholar]
- Achenbach TM, Howell CT. Are American children’s problems getting worse? A 13-year comparison. Journal of the American Academy of Child & Adolescent Psychiatry. 1993;32:1145–1154. doi: 10.1097/00004583-199311000-00006. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed., text revision. Washington, DC: Author; 2000. [Google Scholar]
- Beauchaine TP. Vagal tone, development, and Gray’s motivational theory: Toward an integrated model of autonomic nervous system functioning in psychopathology. Development and Psychopathology. 2001;13:183–214. doi: 10.1017/s0954579401002012. [DOI] [PubMed] [Google Scholar]
- Beauchaine TP, Gartner JG, Hagen B. Comorbid depression and heart rate variability as predictors of aggressive and hyperactive symptom responsiveness during inpatient treatment of conduct-disordered, ADHD boys. Aggressive Behavior. 2000;26:425–441. [Google Scholar]
- Beauchaine TP, Gatzke-Kopp L, Mead HK. Polyvagal theory and developmental psychopathology: Emotion dysregulation and conduct problems from preschool to adolescence. Biological Psychology. 2007;74:174–184. doi: 10.1016/j.biopsycho.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchaine TP, Katkin ES, Strassberg Z, Snarr J. Disinhibitory psychopathology in male adolescents: Discriminating conduct disorder from attention-deficit/hyperactivity disorder through concurrent assessment of multiple autonomic states. Journal of Abnormal Psychology. 2001;110:610–624. doi: 10.1037//0021-843x.110.4.610. [DOI] [PubMed] [Google Scholar]
- Ben-Shakhar G. Standardization within individuals: A simple method to neutralize individual differences in psychophysiological responsivity. Psychophysiology. 1985;22:292–299. doi: 10.1111/j.1469-8986.1985.tb01603.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Bigger JT, Eckberg DL, Grossmann P, Kaufmann PG, Malik M, et al. Heart rate variability: Origins, methods, and interpretive caveats. Psychophysiology. 1997;34:623–648. doi: 10.1111/j.1469-8986.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT, Quigley KS, Fabro VT. Autonomic space and psychophysiological response. Psychophysiology. 1994;31:44–61. doi: 10.1111/j.1469-8986.1994.tb01024.x. [DOI] [PubMed] [Google Scholar]
- Blair RJR. A cognitive developmental approach to morality: Investigating the psychopath. Cognition. 1995;57:1–29. doi: 10.1016/0010-0277(95)00676-p. [DOI] [PubMed] [Google Scholar]
- Blair RJR. Responsiveness to distress cues in the child with psychopathic tendencies. Personality and Individual Differences. 1999;27:135–145. [Google Scholar]
- Blair RJR, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Brenner SL, Beauchaine TP, Sylvers PD. A comparison of psychophysiological and self-report measures of BAS and BIS activation. Psychophysiology. 2005;42:108–115. doi: 10.1111/j.1469-8986.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical linear models for social and behavioral research: Applications and data analysis methods. Newbury Park, CA: Sage; 1992. [Google Scholar]
- Buck R. Nonverbal communication of affect in preschool children: Relationships with personality and skin conductance. Journal of Personality and Social Psychology. 1977;35:225–236. doi: 10.1037//0022-3514.35.4.225. [DOI] [PubMed] [Google Scholar]
- Cacioppo JT, Uchino BN, Berntson GG. Individual differences in the autonomic origins of heart rate reactivity: The psychometrics of respiratory sinus arrhythmia and pre-ejection period. Psychophysiology. 1994;31:412–419. doi: 10.1111/j.1469-8986.1994.tb02449.x. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau M, Mazziotta JC, Luigi Lenzi G. Neural mechanisms of empathy in humans: A relay from neural systems for imitation to limbic areas. Proceedings of the National Academy of Sciences, USA. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowell S, Beauchaine TP, Gatzke-Kopp L, Sylvers P, Mead H, Chipman-Chacon J. Autonomic correlates of attention-deficit/hyperactivity disorder and oppositional defiant disorder in preschool children. Journal of Abnormal Psychology. 2006;115:174–178. doi: 10.1037/0021-843X.115.1.174. [DOI] [PubMed] [Google Scholar]
- Crowell S, Beauchaine TP, McCauley E, Smith C, Stevens AL, Sylvers P. Psychological, autonomic, and serotonergic correlates of parasuicidal behavior in adolescent girls. Development and Psychopathology. 2005;17:1105–1127. doi: 10.1017/s0954579405050522. [DOI] [PubMed] [Google Scholar]
- Dawson ME, Schell AM, Filion DL. The electrodermal system. In: Cacioppo IT, Tassinary LG, Berntson GG, editors. Handbook of psychophysiology. New York: Cambridge University Press; 2000. pp. 200–222. [Google Scholar]
- de Wied M, Goudena PP, Matthys W. Empathy in boys with disruptive behavior disorders. Journal of Child Psychology and Psychiatry. 2005;46:867–880. doi: 10.1111/j.1469-7610.2004.00389.x. [DOI] [PubMed] [Google Scholar]
- de Wied M, van Boxtel A, Zaalberg R, Goudena PP, Matthys W. Facial EMG responses to dynamic emotional facial expressions in boys with disruptive behavior disorders. Journal of Psychiatric Research. 2006;40:112–121. doi: 10.1016/j.jpsychires.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Douglas C, Strayer J. Empathy in conduct-disordered and comparison youth. Developmental Psychology. 1996;32:988–998. [Google Scholar]
- Duan C. Being empathic: The role of motivation to empathize and the nature of target emotions. Motivation and Emotion. 2000;24:29–50. [Google Scholar]
- Eisenberg N, Fabes RA, Murphy B, Karbon M, Smith M, Maszk P. The relations of children’s dispositional empathy-related responding to their emotionality, regulation, and social functioning. Developmental Psychology. 1996;32:195–209. [Google Scholar]
- Eisenberg N, Schaller M, Fabes R, Bustamante D, Mathy R, Shell R, et al. Differentiation of personal distress and sympathy in children and adults. Developmental Psychology. 1988;24:766–775. [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition and Emotion. 1992;6:169–200. [Google Scholar]
- Ekman P, Friesen WV. Unmasking the face. Engelwood Cliffs, NI: Prentice-Hall; 1975. [Google Scholar]
- Elfenbein HA, Marsh A, Ambady N. Emotional intelligence and the recognition of emotion from the face. In: Barrett LF, Salovey P, editors. The wisdom of feelings: Processes underlying emotional intelligence. New York: Guilford Press; 2002. pp. 37–59. [Google Scholar]
- Fabes RA, Eisenberg N, Eisenbud L. Behavioral and physiological correlates of children’s reactions to others in distress. Developmental Psychology. 1993;29:655–663. [Google Scholar]
- Feldman-Barrett L. Are emotions natural kinds? Perspectives on Psychological Science. 2006;1:28–58. doi: 10.1111/j.1745-6916.2006.00003.x. [DOI] [PubMed] [Google Scholar]
- Fowles DC, Dindo L. A dual-deficit model of psychopathy. In: Patrick CI, editor. Handbook of psychopathy. New York: Guilford Press; 2006. pp. 14–34. [Google Scholar]
- Gadow KD, Sprafkin J. Child Symptom Inventory 4 norms manual. Stony Brook, New York: Checkmate Plus; 1997. [Google Scholar]
- Gadow KD, Sprafkin I. Child Symptom Inventory 4: Screening and norms manual. Stony Brook, NY: Checkmate Plus; 2002. [Google Scholar]
- Goldin PR, Cendri AC, Hutcherson CA, Ochsner KN, Glover GH, Gabrieli JDE, et al. The neural bases of amusement and sadness: A comparison of block contrast and subject-specific emotion intensity regression approaches. NeuroImage. 2005;27:26–36. doi: 10.1016/j.neuroimage.2005.03.018. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion Expressive Behavior Coding System. Stanford, CA: Stanford University; 1996. [Google Scholar]
- Gross JJ, John O, Richards J. The dissociation of emotion expression from emotion experience: A personality perspective. Personality and Social Psychology Bulletin. 2000;26:712–726. [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64:970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotion elicitation using films. Cognition and Emotion. 1995;9:87–108. [Google Scholar]
- Gross JJ, Levenson RW. Hiding feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106:95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Mueller B, Qunaibi M, Lichterfeld C, Konrad K, Herpertz-Dahlmann B. Response to emotional stimuli in boys with conduct disorder. American Journal of Psychiatry. 2005;162:1100–1107. doi: 10.1176/appi.ajp.162.6.1100. [DOI] [PubMed] [Google Scholar]
- Hess U, Blairy S. Facial mimicry and emotional contagion to dynamic emotional facial expressions and their influence on decoding accuracy. International Journal of Psychophysiology. 2001;40:129–141. doi: 10.1016/s0167-8760(00)00161-6. [DOI] [PubMed] [Google Scholar]
- Hewig J, Hagemann D, Seifert J, Gollwitzer M, Naumann E, Bartussek D. A revised film set for the induction of basic emotions. Cognition and Emotion. 2005;19:1095–1109. [Google Scholar]
- Hutcherson CAC, Goldin PR, Ochsner KN, Gabrieli JDE, Gross JJ. Attention and emotion: Does rating emotion alter neural responses to amusing and sad films? NeuroImage. 2005;27:656–668. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Understanding others: Imitation, language, empathy. In: Hurley S, Chater N, editors. Perspectives on imitation: From cognitive neuroscience to social science: Vol. 1: Mechanisms of imitation and imitation in animals. Cambridge, MA: MIT Press; 2005. pp. 77–99. [Google Scholar]
- Izard CE. The psychology of emotions. New York: Plenum; 1991. [Google Scholar]
- Keltner D, Moffitt T, Stouthamer-Loeber M. Facial expressions of emotion and psycho pathology in adolescent boys. Journal of Abnormal Psychology. 1995;104:644–652. doi: 10.1037//0021-843x.104.4.644. [DOI] [PubMed] [Google Scholar]
- Kettunen J, Ravaja N, Näätänen P, Keltikangas-Jarvinen L. The relationship of respiratory sinus arrhythmia to the co-activation of autonomic and facial responses during the Rorschach test. Psychophysiology. 2000;37:242–250. [PubMed] [Google Scholar]
- Knitzer J, Steinberg Z, Fleisch B. Schools, children’s mental health, and the advocacy challenge. Journal of Clinical Child Psychology. 1991;20:102–111. [Google Scholar]
- Kring A, Kerr S, Smith D, Neale J. Flat affect in schizophrenia does not reflect diminished subjective experience of emotion. Journal of Abnormal Psychology. 1993;102:507–517. doi: 10.1037//0021-843x.102.4.507. [DOI] [PubMed] [Google Scholar]
- Lanzetta JT, Cartwright-Smith J, Kleck RE. Effects of nonverbal dissimulation on emotional experience and autonomic arousal. Journal of Personality and Social Psychology. 1976;33:354–370. doi: 10.1037//0022-3514.33.3.354. [DOI] [PubMed] [Google Scholar]
- Levenson RW. Autonomic nervous system differences among emotions. Psychological Science. 1992;3:23–27. [Google Scholar]
- Levenson RW. Human emotions: A functional view. In: Ekman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. New York: Oxford University Press; 1994. pp. 123–126. [Google Scholar]
- Levenson RW. Blood, sweat and fears: The autonomic architecture of emotion. Annals of the New York Academy of Sciences. 2003;1000:348–366. doi: 10.1196/annals.1280.016. [DOI] [PubMed] [Google Scholar]
- Levenson RW, Ruef AM. Empathy: A physiological substrate. Journal of Personality and Social Psychology. 1992;63:234–246. [PubMed] [Google Scholar]
- Liew J, Eisenberg N, Losoya SH, Fabes RA, Guthrie IK, Murphy BC. Children’s physiological indices of empathy and their socioemotional adjustment: Does caregivers’ expressivity matter? Journal of Family Psychology. 2003;17:584–597. doi: 10.1037/0893-3200.17.4.584. [DOI] [PubMed] [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: A meta-analysis. Psychological Bulletin. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Mauss I, Levenson R, McCarter L, Wilhelm F, Gross JJ. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5:175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mauss I, Wilhelm FH, Gross JJ. Is there less to social anxiety than meets the eye? Emotion experience, expression, and bodily responding. Cognition and Emotion. 2004;18:631–642. [Google Scholar]
- Mezzacappa E, Tremblay RE, Kindlon D, Saul JP, Arseneault L, Seguin J, et al. Anxiety, antisocial behavior, and heart rate regulation in adolescent males. Journal of Child Psychology and Psychiatry. 1997;38:457–469. doi: 10.1111/j.1469-7610.1997.tb01531.x. [DOI] [PubMed] [Google Scholar]
- Niedenthal P, Brauer M, Halberstadt J, Innes-Ker A. When did her smile drop? Facial mimicry and the influences of emotional state on the detection of change in emotional expression. Cognition and Emotion. 2001;15:853–864. [Google Scholar]
- Notarius CI, Levenson RW. Expressive tendencies and physiological response to stress. Journal of Personality and Social Psychology. 1979;37:1204–1210. doi: 10.1037//0022-3514.37.7.1204. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: A meta-analysis. Journal of the American Academy of Child & Adolescent Psychiatry. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Porges SW. Orienting in a defensive world: Mammalian modifications of our evolutionary heritage. A polyvagal theory. Psychophysiology. 1995;32:301–318. doi: 10.1111/j.1469-8986.1995.tb01213.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. Emotion: An evolutionary by-product of the neural regulation of the autonomic nervous system. Annals of the New York Academy of Sciences. 1997;807:62–77. doi: 10.1111/j.1749-6632.1997.tb51913.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: Phylogenetic substrates of a social nervous system. International Journal of Psychophysiology. 2001;42:123–146. doi: 10.1016/s0167-8760(01)00162-3. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biological Psychology. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qu M, Zhang Y, Webster JG, Tompkins WJ. Motion artifact from spot and band electrodes during impedance cardiography. Transactions on Biomedical Engineering. 1986;11:1029–1036. doi: 10.1109/TBME.1986.325869. [DOI] [PubMed] [Google Scholar]
- Raudenbush S, Bryk A, Cheong YF, Congdon R. HLM 6: Hierarchical linear and nonlinear modeling. Lincolnwood, IL: Scientific Software International; 2004. [Google Scholar]
- Rogosa D, Brandt D, Zimowski M. A growth curve approach to the measurement of change. Psychological Bulletin. 1982;92:726–748. [Google Scholar]
- Rottenberg J, Kasch KL, Gross JJ, Gotlib IH. Sadness and amusement reactivity differentially predict concurrent and prospective functioning in major depressive disorder. Emotion. 2002;2:135–146. doi: 10.1037/1528-3542.2.2.135. [DOI] [PubMed] [Google Scholar]
- Rottenberg J, Salomon K, Gross JJ, Gotlib IH. Vagal withdrawal to a sad film predicts recovery from depression. Psychophysiology. 2005;42:277–281. doi: 10.1111/j.1469-8986.2005.00289.x. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Fahrenberg J, Kelsey RM, Lovallo WR, van Doornen LJ. Methodological guidelines for impedance cardiography. Psychophysiology. 1990;27:1–23. doi: 10.1111/j.1469-8986.1990.tb02171.x. [DOI] [PubMed] [Google Scholar]
- Sherwood A, Allen MT, Obrist PA, Langer AW. Evaluation of beta-adrenergic influences on cardiovascular and metabolic adjustments to physical and psychological stress. Psychophysiology. 1986;23:89–104. doi: 10.1111/j.1469-8986.1986.tb00602.x. [DOI] [PubMed] [Google Scholar]
- Sloan DM, Strauss ME, Quirk SW, Sajatovic M. Subjective and expressive emotional response in depression. Journal of Affective Disorders. 1997;46:135–141. doi: 10.1016/s0165-0327(97)00097-9. [DOI] [PubMed] [Google Scholar]
- Speer DC, Greenbaum PE. Five methods for computing individual client change and improvement rates: Support for an individual growth curve approach. Journal of Consulting and Clinical Psychology. 1995;63:1044–1048. doi: 10.1037//0022-006x.63.6.1044. [DOI] [PubMed] [Google Scholar]
- Sponheim SR, Allen JJ, Iacono WG. Selected psycho-physoiological measures in depression: The significance of electrodermal activity, electroencephalographic asymmetries, and contingent negative variation to behavioral and neurobiological aspects of depression. In: Miller GA, editor. The behavioral high risk paradigm in psycho pathology. New York: Springer; 1995. pp. 222–249. [Google Scholar]
- Stevens D, Charman T, Blair RJR. Recognition of emotion in facial expressions and vocal tones in children with psychopathic tendencies. Journal of Genetic Psychology. 2001;162:201–211. doi: 10.1080/00221320109597961. [DOI] [PubMed] [Google Scholar]
- Strand K, Nowicki S. Receptive nonverbal processing ability and locus of control orientation in children and adolescents with conduct disorder. Behavioral Disorders. 1999;24:102–108. [Google Scholar]
- Thayer JF, Friedman BH, Borkovec TD. Autonomic characteristics of generalized anxiety and worry. Biological Psychiatry. 1996;39:255–266. doi: 10.1016/0006-3223(95)00136-0. [DOI] [PubMed] [Google Scholar]
- Tsai JL, Levenson RW, Carstensen L. Autonomic, subjective, and expressive responses to emotional films in older and younger Chinese Americans and European Americans. Psychology and Aging. 2000;15:684–693. doi: 10.1037//0882-7974.15.4.684. [DOI] [PubMed] [Google Scholar]
- Walbott HG. Recognition of emotion from facial expression via imitation? Some indirect evidence for an old theory. British Journal of Social Psychology. 1991;30:207–219. doi: 10.1111/j.2044-8309.1991.tb00939.x. [DOI] [PubMed] [Google Scholar]
- Zahn-Waxler C, Cole PM, Welsh JD, Fox NA. Psychophysiological correlates of empathy and prosocial behaviors in preschool children with behavior problems. Development and Psychopathology. 1995;7:27–48. [Google Scholar]