Summary
Universal nevirapine (NVP) therapy (provision of the drug without HIV testing) has been suggested as potentially superior to targeted NVP therapy (provision of the drug to seropositive patients identified through voluntary HIV counseling and testing [VCT]) for perinatal HIV prevention in low-resource, high-prevalence settings. The authors postulated that uptake (the proportion of women who accept the strategy when offered) may be higher for universal therapy, since it does not require a woman to learn her serostatus; they further postulated that adherence (the proportion of women who actually ingest the NVP tablet at labor onset) may be higher for targeted therapy, since knowledge of serostatus could motivate better adherence. Two clinics in Lusaka, Zambia were assigned to provide either the targeted or universal strategy. Halfway through the study period, the approach offered at each clinic was crossed over. Adherence was assessed by liquid chromatographic assay for NVP of cord blood. Regarding uptake, 1524 pregnant women were offered participation, and 1025 (67%) accepted. Of 694 women offered enrollment in the universal strategy, 496 (71%) accepted; of 830 women offered enrollment in the targeted strategy, 529 (64%) accepted (p < .01). Uptake was similar at both clinics for the universal strategy: 250 of 339 (74%) at clinic A and 246 of 355 (69%) at clinic B (p [H11505] .2), but differed significantly between clinics for the targeted strategy: 229 of 316 (72%) at clinic A and 300 of 514 (58%) at clinic B (RR, 1.51; 95% CI, 1.23, 1.86). Increased uptake correlated with having been offered the universal rather than the targeted strategy (AOR, 1.5; 95% CI, 1.1, 2.1), attendance at clinic A (AOR, 1.4; 95% CI, 1.01, 2.0), and maternal report of a prior fetal or infant death (AOR, 1.6; 95% CI, 1.1, 2.5). Regarding adherence, in the universal strategy, 40 of 103 women (39%) were nonadherent compared with 25 of 98 women (26%) in the targeted strategy (RR, 1.5; 95% CI, 1.004, 2.3). Failure to adhere correlated with participation in the universal strategy (AOR, 2.0; 95% CI, 1.04, 4.2) and illiteracy (AOR, 2.6; 95% CI, 1.2, 5.3). In high-prevalence settings with adequate VCT services, uptake of NVP using the universal or targeted approach appears comparable. However, the universal strategy may result in better uptake in clinics with less well-functioning VCT services (as with clinic B). Adherence to the single-dose NVP intervention was lower among women who did not learn their HIV status. Programs that seek to save the greatest possible number of infants from perinatal HIV acquisition should consider a combination approach, in which women who desire HIV testing can access NVP through a targeted strategy, and women who do not desire testing can access NVP through a universal strategy.
Keywords: Perinatal, Nevirapine, Uptake, Adherence, Presumptive treatment
Ninety percent of global mother-to-child HIV transmission occurs in sub-Saharan Africa, with an estimated 800,000 new perinatal infections in 2001 (1). Since essentially all infected infants will die prematurely, the impact of pediatric AIDS has already reversed hard-won gains in childhood mortality in much of the developing world (2). Nevirapine (NVP) provided as a single dose to the mother at the onset of labor and to the infant within 72 hours of birth reduces HIV transmission by 47% at 12 to 14 weeks of life compared with a very short course of zidovudine (3). Its benefit, although mitigated to some degree, persists even with breast-feeding (4).
Economic analyses have confirmed that intrapartum NVP would be a cost-effective use of scarce health care resources even in the poorest of countries. Marseille et al. (5) proposed that a strategy of universal NVP administration (offering the drug to all pregnant women in a population, without testing them for HIV) would be economically favorable to a strategy of targeted NVP administration (offering NVP to only those women identified as seropositive) in certain high-prevalence, low-resource settings. These cost-effectiveness results are driven primarily by the low cost of NVP (<$4 per patient regimen when purchased over the counter or free if obtained from the manufacturer's donation program) (6) and the comparatively high cost of voluntary HIV counseling and testing (VCT), estimated at $7 to $8 per person in high-prevalence settings where more posttest counseling of seropositive patients is done (7). Despite its hypothesized ease of administration and superior cost-effectiveness, the idea of offering NVP to pregnant women of unknown HIV serostatus is controversial (8,9).
In a prior publication, we used decision-analysis modeling to understand the clinical effectiveness and cost-effectiveness of strategies for NVP implementation. That investigation found the optimal choice between the universal and targeted approach hinged critically on two important areas of data uncertainty and could not be confidently made without additional information (10). First, it is possible that women who are offered access to NVP under a universal strategy may be more likely to uptake (i.e., participate in) the intervention, since it does not require them to learn their HIV status, than if a targeted approach were used. Thus, a universal strategy theoretically could reach a larger segment of the at-risk population. Second, it is possible that HIV-infected women participating in a universal strategy may be less likely to adhere to therapy (i.e., actually ingest the NVP tablet) than would women participating in a targeted strategy, since the latter know their status and may be more likely to perceive tangible infant benefit from taking the NVP. Our analysis found that a relatively small differential between strategies in uptake or adherence could cause the clinical effectiveness and cost-effectiveness balance to be tipped in favor of one or the other strategy. In response to these uncertainties, we launched a clinical trial to assess the uptake of the two strategies in a field setting and to measure the effect of HIV serostatus knowledge on likelihood of adherence to single-dose NVP.
METHODS
We conducted a prospective, clinic-level randomized trial of two strategies for perinatal NVP administration. The study was conducted in Lusaka, the capital city of the Republic of Zambia, at two district health facilities that provide comprehensive obstetric services (antenatal, delivery, and postnatal care) to “low-risk” women. From nine eligible clinics, we selected these two based on comparable obstetric volume, their service of demographically similar but geographically discreet populations, their similar staff-to-patient ratios, and their similar proportions of (more highly trained) registered midwives to enrolled midwives. In antenatal care, women who had completed 34 weeks gestation by the midwife's best estimate were offered enrollment into the study; thereafter, they were seen weekly until delivery. In an effort to enhance the generalizability of the study's results, we used liberal inclusion criteria but did exclude women who were aged <16 years (the age of legal majority in Zambia) or who indicated that they were unwilling to return for delivery or infant follow-up evaluation. We also would have excluded women with known or suspected allergy to NVP or related agents had such a candidate presented to us. Eligible women in each treatment arm received an educational message in a group session regarding the high prevalence of HIV among pregnant women in Lusaka, the risk of perinatal transmission from infected mothers, the ability of NVP to reduce transmission risk, and infant feeding options. A script was provided to the midwife to ensure consistency of this message from day to day.
In the targeted strategy, women were counseled and offered voluntary HIV testing. Those who accepted testing and were found to be HIV infected were then offered enrollment into the clinical trial. In the universal strategy, women were counseled and offered enrollment into the study without HIV testing. To identify correlates of strategy uptake, we collected limited anonymous demographic data on all women offered participation, including those who declined. After informed consent was obtained, participants were issued a single 200-mg NVP tablet to take home, with instructions to ingest it at the onset of labor. When participants presented in labor, they were asked whether they had taken their NVP tablet and, if so, at what time. Those who reported not having taken it were issued another one immediately. Women were also asked whether they had disclosed their possession of the NVP to another person. At delivery, a specimen of umbilical cord blood was obtained for NVP assay. For ethical reasons, women enrolled in the universal strategy were offered HIV testing postpartum after the cord blood had been obtained.
A coin toss determined that during the first half of the study period, the universal strategy would be offered at clinic A and the targeted strategy at clinic B. Once half the intended number of HIV-infected participants was enrolled, the strategy offered at each facility was crossed over to the complementary one. While the targeted strategy enrolled HIV-infected women only, this was not possible in the universal strategy since women did not learn their status. We compensated for this by enrolling more women in the universal strategy to have comparable numbers of infected women in each study arm.
Maternal HIV status was assessed using a dual rapid test algorithm that has been previously validated in our setting (Determine HIV-1/2, Abbott Laboratories, Abbott Park, IL, U.S.A., and Capillus HIV-1/HIV-2, Trinity Biotech, Wicklow Co, Ireland) (11). Women with discordant rapid test results (n = 3) were offered perinatal NVP prophylaxis but were excluded from the analysis. Among the infected women, a CD4 lymphocyte count was estimated using an enzyme-linked immunoassay (TRAx CD4, Innogenetics, Atlanta, GA, U.S.A.) performed on lysed whole blood. This assay has excellent correlation with results from flow cytometry and hematology when the CD4 lymphocyte count is >200 cells/cm3 (12). We screened for syphilis with a single, nontreponemal test (RPR Immunotrep, Omega Diagnostics, Clacks, U.K.) and treated all patients with positive results with intramuscular penicillin, consistent with the current standard of care in the Lusaka Urban District Clinics.
We performed quantitative assay for NVP using a method developed at the University of Alabama at Birmingham Clinical Pharmacology Infectious Diseases Laboratory. NVP was isolated from 0.1 mL of plasma using a simple liquid extraction process. NVP and plasma interferences in the extract were then separated via isocratic reversed-phase HPLC assay (Microsorb MV C8, 4.6 × 250 mm, 5 μm; Varian Inc.) with UV detection at 284 nm. Detector responses of unknown patient samples were compared with known concentrations of an internal standard (5,11-dihydro-6H-11-ethyl-4-methyl-dipyrido[3,2-b:2′,3′-e](1,4)diazepin-one, C14H14N4O; supplied by Boehringer Ingelheim Pharmaceuticals, Inc.). This assay is capable of measuring concentrations in the range of 25 to 10,000 ng/mL (ng NVP per mL plasma) (13). NVP crosses the placenta within minutes of ingestion. We have previously reported that of 183 women in whom NVP ingestion was directly observed by study personnel, 182 (99.4%) had drug detectable in the cord blood, making cord blood HPLC an excellent objective measure of adherence (14).
The study was designed with 95% power to detect an absolute difference of 20% in adherence between the universal and targeted groups (two-tailed α < .05), an effect that would be considered policy relevant based on our decision-analysis work (10). Adherence rates were compared between study arms using the χ2 test and by generating relative risks with Taylor Series 95% confidence limits. To assess the comparability of various demographic factors between the two arms, continuous variables were analyzed by unpaired, two-tailed Student t-tests and dichotomous variables by χ2 or Fisher exact tests. Multivariable logistic regression analyses were performed using variables that were significant at the p < .1 level in univariate analysis using the LOGISTIC procedure, SAS System release 8.01 for Windows (SAS Institute, Cary, NC). This study was approved by the University of Alabama at Birmingham Institutional Review Board and by the University of Zambia Research Ethics Committee. Prior to indicating willingness to participate in the protocol by signature or thumbprint, each participant underwent a detailed informed consent process where the consent document was read aloud and questions answered.
RESULTS
Uptake
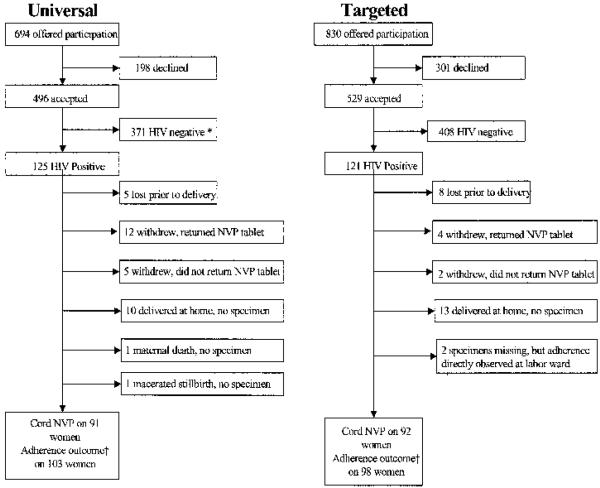
Between September 2000 and May 2001, we approached 1524 women for enrollment in the clinical trial, and 1025 (67%) accepted (Fig. 1). Of the 694 women offered enrollment in the universal strategy, 496 (71%) elected to participate. Of the 830 women offered enrollment in the targeted strategy, 529 (64%) elected to participate (p < .01). The HIV seroprevalence was similar in the universal (124 of 496; 25%) and targeted (122 of 529; 23%) groups (RR, 1.06; 95% CI, 0.91–1.22). Up-take was similar at both clinics for the universal strategy, 250 of 339 (74%) at clinic A and 246 of 355 (69%) at clinic B (RR, 1.06; 95% CI, 0.97, 1.2), but differed significantly between clinics for the targeted strategy, 229 of 316 (72%) at clinic A and 300 of 514 (58%) at clinic B (RR, 1.51; 95% CI, 1.23, 1.86).
FIG. 1.
Trial profile: *371 HIV-uninfected women in the universal arm were followed in the study, but their cord blood was not assayed for NVP, and they are not reported on here. †Composite adherence outcome includes those from whom cord blood specimens were available plus those who returned their NVP tablet plus (in the targeted group only) and two patients from whom cord blood specimens were missing but in whom NVP ingestion was directly observed by study staff.
Factors associated in univariate analysis with uptake of either strategy included antenatal attendance at clinic A (71% vs. 59%; p < .001), having been offered the universal rather than the targeted strategy (70% vs. 59%; p < .001), maternal report of a prior fetal or infant death (69% vs. 53%; p = .002), and multiparity (68% vs. 44%; p = .001). In a logistic regression model, having been offered the universal rather than the targeted strategy (AOR, 1.5; 95% CI, 1.1, 2.1), attendance at clinic A (AOR, 1.4; 95% CI, 1.01, 2.0), and report of prior fetal or infant death (AOR, 1.6; 95% CI, 1.1, 2.5) remained significant.
Nonadherence to Therapy
The 372 uninfected women (75%) in the universal arm were followed in the study, but their cord blood was not analyzed for NVP. The 407 uninfected women (77%) in the targeted arm were not followed. Baseline characteristics of the 246 HIV-infected women in the study are shown in Table 1, stratified by study arm. Participants in the universal strategy did not differ significantly from those in the targeted strategy with respect to age, income, obstetric history, CD4 lymphocyte count estimate at entry, number of lifetime sexual partners, body mass index, or general knowledge of HIV as assessed by a 10-point questionnaire. Participants in the universal strategy had an average of 1 additional year of education compared with participants in the targeted strategy.
TABLE 1.
Characteristics of women at enrollment
| Characteristic | Universal (n = 125) |
Targeted (n = 121) |
p |
|---|---|---|---|
| Mean (SD) age, years | 25.6 (5.1) | 25.6 (4.7) | .96 |
| Mean (SD) education, years | 7.2 (2.5) | 6.3 (2.8) | .01 |
| Mean (SD) weekly income, USD | 16.4 (32.6) | 13.4 (23.1) | .45 |
| Median parity (range) | 3 (1–7) | 3 (1–8) | .39 |
| Median number of children who have died (range)a | 0 (0–4) | 0 (0–2) | .59 |
| Median number of years cohabitating with current partner (range) | 4 (0–16) | 4 (0–22) | .95 |
| Mean Body Mass Index at 36 weeks' gestation (range) | 25.5 (3.4) | 25.0 (3.3) | .26 |
| Mean number of lifetime sexual partners (SD) | 2.6 (1.5) | 2.3 (1.6) | .14 |
| Median HIV knowledge composite score (range) | 9 (5–10) | 9 (5–10) | .79 |
| Median CD4 lymphocyte estimate (cells/μL) at entry (IQR)b | 342 (216–509) | 329 (210–467) | .38 |
| Number with positive syphilis screening test at enrollment (proportion) | 24 (0.19) | 19 (0.15) | .54 |
Prior to the index pregnancy, not including stillbirths.
Calculated from the enzyme-linked immune assay (TRAx CD4).
The 246 HIV-infected study participants delivered 246 live born infants (5 sets of twins) and 5 stillbirths. One mother died with severe pneumonia at term. Her infant was live born, but no NVP adherence data were available since cord blood was not obtained. Cord blood was obtainable from the four fresh stillbirths but not from one that was macerated. Thirteen women (5.3%) were lost to follow-up, 23 (9.3%) delivered at home or a nonstudy facility, and 23 (9.3%) withdrew. Examination of the study pharmacy records revealed that 16 of 23 women who withdrew from the study returned their NVP tablet. In addition, two women (both in the targeted arm) were directly observed taking their NVP tablet, but their cord blood specimen is missing. Thus, we measured nonadherence in two ways: 1) by only considering those women from whom a cord blood specimen was available, and 2) by creating a composite adherence measure that considered not only those women in whom cord blood was available but also those who returned the tablet to the pharmacy and those directly observed ingesting the tablet. When the data were limited to only those women in whom cord blood NVP was available, we found that 32 of 102 women (31%) enrolled in the universal strategy were nonadherent compared with 23 of 110 women (21%) nonadherent in the targeted strategy (RR, 1.4; 95% CI, 0.84, 2.3). When the above-described composite measure of adherence was considered, we found that 40 of 103 women (39%) enrolled in the universal strategy were nonadherent compared with 25 of 98 women (26%) nonadherent in the targeted strategy (RR, 1.5; 95% CI, 1.004, 2.3; Table 2).
TABLE 2.
Nonadherencea to therapy under the two study strategies
| Study arm |
Number without NVP detected in cord blood |
Total known not to adhere |
|---|---|---|
| Universal | 28/91 (31%) | 40/103 (39%) |
| Targeted | 21/92 (23%) | 25/98 (26%) |
| p = 0.23 | p = 0.044 | |
| RR = 1.4; 95% CI: 0.83, 2.2 | RR = 1.5; 95% CI: 1.004, 2.3 |
Nonadherence is defined as the proportion of women who did not ingest their nevirapine (NVP) tablet, and can be measured by restricting the dataset to only those women from whom cord blood specimens are available or by generating a composite adherence outcome that also includes those women who returned their NVP tablet to the pharmacy (n = 16) and those women from whom cord blood specimens are missing but who took their NVP tablet in the presence of study staff (n = 2).
Other predictors of failure to ingest the NVP tablet in univariable analysis included having been pregnant at least once before (RR, 2.0; 95% CI, 1.0, 3.8) and illiteracy in one's primary language (RR, 1.3; 95% CI, 1.1, 1.7). The following characteristics did not predict failure to adhere: clinic of participation, maternal age, marital status, prior stillbirth, prior infant death, positive syphilis test in index pregnancy, CD4 count, reading or understanding of English, family income, number of lifetime sexual partners, reported use of traditional medicines during the index pregnancy, reporting that the index pregnancy was planned, and disclosure of their possession of the NVP tablet to their partner (Table 3). In multivariable analysis, using the composite nonadherence measure as the response variable, participation in the universal strategy (AOR, 2.0; 95% CI, 1.04, 4.2) and illiteracy in primary language (AOR, 1.8; 95% CI, 1.1, 3.0) remained independently associated with nonadherence.
TABLE 3.
Univariate predictors of nonadherence
| Characteristic | Number (%) not adheringa |
RR (95% CI) | |
|---|---|---|---|
| Study arm | Universal | 40/103 (0.39) | |
| target | 25/98 (0.26) | 1.5 (1.0, 2.3) | |
| Parity (including index pregnancy) | 1 | 8/44 (0.18) | |
| 2+ | 42/118 (0.36) | 2.0 (1.0, 3.8) | |
| Reading of primary language | Some | 24/121 (0.20) | |
| None | 22/60 (0.37) | 1.8 (1.1, 3.0) |
Nonadherence in this table is defined as absent nevirapine (NVP) in umbilical cord blood or withdrawal from the study with documented return of the NVP tablet to the pharmacy. Some of the data in this table were obtained via questionnaire at discharge from the delivery unit. Therefore, data on women who withdrew from the study prior to the delivery visit are missing.
Other Study Outcomes
One hundred twenty-four women (66%) told at least one person that they had the NVP tablet; 114 women (61%) told their partner, and 49 women (26%) told a person other than their partner (categories not mutually exclusive). Women enrolled in the targeted strategy were less likely to have disclosed their possession of the tablet to anyone than were women in the universal arm (56% vs. 75%; RR, 0.56; 95% CI, 0.38–0.87). In univariate analysis, clinic of care, age, parity, marital status, history of neonatal or fetal demise, education, CD4 count, positive syphilis serology, literacy, and reported use of traditional medicines in the index pregnancy were not associated with disclosure of possession of the NVP tablet (data not shown).
Validity of Self-Report in Determining Adherence
Of the 171 women in whom self-report and cord NVP data were available, 21 (13 universal and 8 targeted) reported not having taken the medication. All but two of these (one universal, one targeted) were issued a second tablet, and all but these same two had NVP detected in the cord blood. By contrast, of the 150 women who reported having taken their tablet, 42 (28%) had no NVP detected. Thus, a reasonable estimate is that 75% of women (the 108 with NVP in cord blood plus the 21 women reporting not having taken it, or 129 of 171) correctly reported whether they took NVP.
DISCUSSION
Urban Zambian women were more likely to participate in the universal NVP strategy than in the targeted NVP strategy. Uptake of either strategy was more common among women who were multiparous, who reported a prior fetal or neonatal loss, and who attended clinic A. Despite reminders and the availability of replacement doses, 65 of 201 (32%) participants did not ingest the NVP tablet that was issued to them at antenatal care; nonadherence was more likely among those who did not learn their HIV status through voluntary HIV testing. Adherence as assessed by self-report correlated only 75% with the objective laboratory measure.
While the idea of administering NVP without HIV testing to pregnant women in settings such as ours has been examined in decision-analysis models (5,10,15) and by posing hypothetical questions to pregnant women (16), this is the first study to actually test the strategy in a field setting. Strengths of the study include its liberal inclusion criteria, its prospective design, and its use of a validated laboratory measurement to assess adherence to NVP. Its primary weakness is that patients were not randomized; thus, it is possible that unrecognized confounders are influencing our observations. We attempted to mitigate this effect by using a crossover design, which should balance clinic or neighborhood-attributable differences in the populations to some extent. Except for a small, but statistically significant, difference in education, women were comparable in the two treatment arms (Whether the difference between a sixth grade and a seventh grade education [Table 1] would result in any meaningful difference in adherence behavior seems doubtful, but since it was the less-adherent group that had more education, one would expect any bias introduced by this small disparity to actually be toward the null hypothesis.).
We elected to counsel women about the prevention strategy being offered, perform HIV testing (in the targeted arm), and enroll interested participants at the same antenatal visit. This approach differs from the standard model used in many clinical trials of therapeutic efficacy, where screening and enrollment are typically scheduled at different visits in an effort to maximize retention by allowing those likely to drop out to do so prior to enrollment. With this study, we sought to maximize the external validity of our results by mimicking as closely as possible the real circumstances under which women would gain access to NVP under the two studied strategies, were they to be implemented later as routine policy. It is worth noting, however, that counseling of all participants, irrespective of strategy, included the importance of ingesting the NVP tablet at labor onset and education in how to recognize the signs of labor onset.
We chose to use maternal drug adherence as a surrogate for the definitive outcome: the number of infected babies born to participants in each strategy. There were two reasons for this choice: first, use of perinatal infections as the primary study outcome would have increased the sample size requirement and the project budget by manyfold; second, we postulated that participation in the universal strategy would be higher than in the targeted strategy because many women did not wish to learn their HIV serostatus. Requiring each participant's infant to be tested for HIV infection might have acted as a disincentive for participation, thus biasing our results in favor of the targeted strategy. Given that the efficacy of NVP to prevent perinatal transmission has been proven in controlled clinical trials (3), we believe pharmacologically confirmed maternal drug dosing to be a valid surrogate for clinical effectiveness.
While we considered randomizing patients in this trial, the nature of the research question made such a design unattractive. For a woman to be enrolled into a randomized trial of universal versus targeted therapy, she would have to be willing to learn her HIV status since she would have a 50% chance of being assigned to targeted therapy. Since willingness to know one's HIV status may be linked to HIV risk perception, exclusion of these patients could give an inaccurate reflection of the true population-wide rates of adherence to universal therapy. Thus, we chose to offer the alternative strategies at matched clinics and to crossover the strategy offered at each clinic halfway through the study. The crossover was meant to minimize the effects of any possible differences between the two clinic populations, while the relatively short enrollment period served to minimize any potential time trend (e.g., due to increased awareness of HIV disease).
In an effort to understand why participation rates in the targeted strategy were lower at clinic B than at clinic A, we commissioned an independent evaluation of the two study sites by an experienced team of social scientists and HIV counselors that was completed prior to informing clinic staff of the study results (17). While this team was told that recruitment was lower than expected in the study overall (motivating the independent assessment), they did not know that a differential existed between the two clinics. The evaluators reported generally lower morale among research and clinical staff at clinic B compared with clinic A. Although both facilities had similar obstetric volume and numbers of research and clinical staff, clinical space was somewhat more constrained at clinic B, a phenomenon that the team stated may have interfered with proper VCT. The evaluators also reported that clinic B staff had less well developed counseling skills than did the staff at the other site. They cited as an example that more than half of the staff at clinic B reported being “uncomfortable” or “very uncomfortable” conveying seropositive posttest results to their clients, while such a response was uncommon at clinic A. General understanding of the research protocol by implementing personnel was similar between sites.
Decision makers who are choosing among various strategies for NVP administration may consider a variety of criteria for what constitutes the “best” approach. For example, in settings of extreme resource constraints where there are insufficient resources to test and treat everyone, the universal strategy may be preferred if the primary concern is one of equity of drug access (16). Alternatively, if encouragement of HIV testing is paramount and it is feasible, then a targeted approach would be best. If one's primary aim is to achieve maximal NVP “coverage” of the at-risk population, a combination of the targeted and universal approaches should be considered (9). With a combined approach, all women would be counseled and offered HIV testing during antenatal care. NVP would then be offered to those identified as seropositive and to those who do not wish to be tested for HIV. The appeal of a combined approach is that it could potentially increase the population coverage of the drug, thus resulting in fewer perinatal infections than either approach alone. However, it also runs the theoretical risk of reducing the acceptance rate of VCT if women know they can access NVP without testing. Thus, when compared with a purely targeted strategy, a combined approach could result paradoxically in lower overall effectiveness if it were to discourage VCT and then if, as observed in the present trial, those who access NVP without testing do not actually take the medication. We are now initiating an evaluation of such a combined strategy in Zambia and Uganda. Because of the potential for more significant public health impact than a targeted approach alone, evaluations of combinations of approaches are a high priority for resource-limited settings with high HIV seroprevalence rates among women.
Acknowledgments
Jeff Stringer, Moses Sinkala, and Sten Vermund obtained funding for the study, designed it, oversaw its conduct and analysis, and co-wrote the manuscript. Julia Stout was responsible for day-to-day management of the study and data quality assurance in the field, and edited the manuscript. Robert Goldenberg and Rosemary Kumwenda participated in study design, general oversight, and analysis, and edited the manuscript. Ed Acosta participated in the study design, conducted the NVP assay, and edited the manuscript. Vicky Chapman managed the study database, participated in the analysis, and edited the manuscript.
The Elizabeth Glaser Pediatric AIDS Foundation funded this study as a basic research grant. Other than providing reviewer's comments on the study design, the Foundation had no role in the design, conduct, analysis, or presentation of the study. Investigator salary support was provided through various other US government and private foundation mechanisms, but none influenced the design, conduct, analysis, or presentation of the study.
This study was funded by the Elizabeth Glaser Pediatric AIDS Foundation (PG 51066), with complementary resources from the US National Institutes of Health (K23-HD01411, U01-AI47972, and D43-TW01035). Innogenetics Corporation provided TRAx CD4 assays free of charge.
REFERENCES
- 1.UNAIDS . Report on the global HIV/AIDS epidemic: July 2002. UNAIDS; Geneva: 2002. [Google Scholar]
- 2.Dabis F, Ekpini E. HIV-1/AIDS and maternal and child health in Africa. Lancet. 2002;359:2097–2104. doi: 10.1016/S0140-6736(02)08909-2. [DOI] [PubMed] [Google Scholar]
- 3.Guay LA, Musoke P, Fleming T, et al. Intrapartum and neonatal single-dose nevirapine compared with zidovudine for prevention of mother-to-child transmission of HIV-1 in Kampala, Uganda: HIVNET 012 randomised trial. Lancet. 1999;354:795–802. doi: 10.1016/S0140-6736(99)80008-7. [DOI] [PubMed] [Google Scholar]
- 4.Fowler MG, Mwatha A, Guay L, et al. Effect of nevirapine (NVP) for perinatal HIV prevention appears greatest among women with most advanced disease: subgroup analyses of HIVNET 012 [abstract 120]. Presented at the 9th Conference on Retroviruses and Opportunistic Infections; Seattle. February 24–28, 2002. [Google Scholar]
- 5.Marseille E, Kahn J, Mmiro F, et al. Cost effectiveness of single-dose nevirapine regimen for mothers and babies to decrease vertical HIV-1 transmission in sub-Saharan Africa. Lancet. 1999;354:803–809. doi: 10.1016/S0140-6736(99)80009-9. [DOI] [PubMed] [Google Scholar]
- 6.Boerhringer-Ingelheim VIRAMUNE® Donation Programme for the Prevention of Mother-to-Child Transmission of HIV-1. Available at: http://www.viramune-donation-program.org. Accessed February 13, 2003.
- 7.Marseille E. Countywide implementation of mother to child HIV prevention programs [abstract 098]. Presented at the 3rd Conference on Global Strategies for the Prevention of HIV Transmission from Mothers to Infants; Kampala, Uganda. September 9–13, 2001. [Google Scholar]
- 8.Campa A, Shor-Posner G, Baum M. HIVNET nevirapine trials [letter] Lancet. 1999;354:1816. doi: 10.1016/S0140-6736(05)70582-1. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization . Use of nevirapine for the prevention of mother-to-child transmission of HIV among women of unknown serostatus: report of a technical consultation. WHO Press; Geneva: 2002. [Google Scholar]
- 10.Stringer JSA, Rouse DJ, Vermund SH, et al. Cost-effective use of nevirapine to prevent vertical HIV transmission in sub-Saharan Africa. J Acquir Immune Defic Syndr. 2000;24:369–377. doi: 10.1097/00126334-200008010-00012. [DOI] [PubMed] [Google Scholar]
- 11.McKenna SL, Muyinda GK, Roth D, et al. Rapid HIV testing and counseling for voluntary testing centers in Africa. AIDS. 1997;11:S103–110. [PubMed] [Google Scholar]
- 12.Paxton H, Pins M, Denton G, et al. Comparison of CD4 cell count by a simple enzyme-linked immunosorbent assay using the TRAx CD4 test kit and by flow cytometry and hematology. Clin Diag Lab Immunol. 1995;2:104–114. doi: 10.1128/cdli.2.1.104-114.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jayaraj A, Alexander J, Price C, et al. A rapid and sensitive HPLC-UV method for the quantitation of an anti-HIV agent, nevirapine, and its solid phase extractable metabolites in biologic fluids. Pharm Res. 1992;9:S334. [Google Scholar]
- 14.Stringer JSA, Sinkala M, Goldenberg RL, et al. A pilot study of nevirapine administered upon presentation in labor without HIV testing (abstract 300). Presented at the 3rd Conference on Global Strategies for the Prevention of HIV Transmission from Mothers to Infants; Kampala, Uganda. September 9–13. [Google Scholar]
- 15.Stringer JSA, Sinkala M, Rouse DJ, et al. Effect of nevirapine toxicity on choice of perinatal HIV prevention strategies. Am J Public Health. 2002;92:365–366. doi: 10.2105/ajph.92.3.365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sinkala M, Stout JP, Goldenberg RL, et al. Zambian women's attitudes on mass nevirapine to prevent perinatal transmission of HIV. Lancet. 2001;358:1611–1612. doi: 10.1016/S0140-6736(01)06662-4. [DOI] [PubMed] [Google Scholar]
- 17.Sinkala M, Kwapa P, Chilufya M, et al. Knowledge, satisfaction, and informed consent in nevirapine administration among nurse-midwives and postpartum women in Lusaka, Zambia (abstract 404). Presented at the 3rd Conference on Global Strategies for the Prevention of HIV Transmission from Mothers to Infants; Kampala, Uganda. September 9–13. [Google Scholar]