Abstract
Tissue inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors of matrix metalloproteinases which are involved in normal cellular processes and also in cancer development and progression. The purpose of this study was to evaluate polymorphisms in the TIMP-2 and TIMP-3 genes for their associations with breast cancer susceptibility and survival. Using data from the Shanghai Breast Cancer Study, 19 SNPs for each gene were evaluated for associations with breast cancer risk among 1,062 cases and 1,069 controls; associations with disease-free and overall survival were evaluated among the cases. For TIMP-2, women with the rs7501477 TT genotype were 3 times more likely to be breast cancer cases than women with the CC genotype (OR: 2.9, 95% CI: 1.2-7.0). For TIMP-3, women with the rs9609643 AA genotype were 60% less likely to be breast cancer cases than women with the GG genotype (OR: 0.4, 95% CI: 0.2-1.0), whereas women with the rs8136803 TT genotype were 5 times more likely to be cases than women with the GG genotype (OR: 5.1, 95% CI: 1.1-24.3). Further, breast cancer cases with rs8136803 TT were almost four times more likely to have decreased disease-free survival (HR: 3.9, 95% CI: 1.4-10.6) and had a trend towards decreased overall survival (HR: 1.9, 95% CI: 0.6-6.1). An important study limitation was that these 3 SNPs (rs7501477, rs9609643, rs8136803) had low minor allele frequencies which resulted in small numbers of homozygote individuals. Genetic variation in the TIMP-2 and TIMP-3 genes may contribute to individual differences in breast cancer susceptibility and survival.
Keywords: breast cancer, epidemiology, genetic susceptibility, TIMP-2, TIMP-3, polymorphisms, SNPs, survival
Introduction
Known germline mutations in high-penetrance breast cancer susceptibility genes, such as BRCA-1 and BRCA-2, account for only 5-10% of all breast cancers because of their low mutation frequency in the general population. In contrast, common low-penetrant genetic factors likely contribute to the susceptibility of developing breast cancer for most sporadic cases.1 Polymorphisms in these low-penetrance but high-prevalence genes may, in combination, have a greater influence on cancer risk and prognosis, and their identification is an important goal. Relevant biological pathways include cell-cycle control and extracellular matrix (ECM) remodeling, among others.
The human matrix metalloproteinase (MMP) family consists of over 20 proteolytic enzymes that can degrade all components of the ECM, and are known contributors to tumor cell invasion and metastasis.2-8 MMPs also have non-matrix substrates, and can therefore influence not only ECM remodeling, but also cell growth, apoptosis, cell migration, and cell-cell communication, further supporting their involvement in multiple stages of carcinogenesis and tumor control processes.3;6;9 The expression and activity of the MMPs are tightly regulated at 3 major levels: gene transcription, pro-enzyme activation, and enzymatic activity inhibition.10;11
Tissue inhibitors of metalloproteinases (TIMPs) are endogenous inhibitors of activated MMPs that contribute to normal functions such as tissue repair after injury and development, as well as to pathologic states such as cardiovascular disease and cancer. Traditionally thought to suppress cancer growth and metastasis, the TIMP genes have recently been found to have additional paradoxical effects on tumorigenesis.11;12 For example, TIMPs have been shown to be involved in cell growth stimulation and inhibition of apoptosis, thereby favoring early tumor initiation and growth.13;14 In addition, TIMPs may promote tumor angiogenesis either indirectly by inhibiting MMPs that help generate angiogenic inhibitors such as angiostatin and endostatin, or directly through an inhibitory effect on endothelial cell proliferation.15;16
There are four known TIMP genes (TIMP-1-4) which have both MMP-dependent and MMP-independent effects in many cell types including the breast.11;17 TIMP-2 is normally expressed in breast stromal tissue; however, increased expression has been found in ductal carcinoma in situ and in invasive breast carcinomas.18;19TIMP-2 has been found to stimulate cell growth and inhibit apoptosis in breast cancer cells, as well as to inhibit endothelial cell growth and abrogate angiogenesis.11;17;20 Increased expression of TIMP-2 in breast cancer tissue has also been associated with tumor recurrence and development of metastasis.21-23
TIMP-3 is a cell-cycle-regulated gene that is normally found in the breast epithelium; reduced TIMP-3 expression in breast tumor and peri-tumoral tissues has been linked to cell cycle deregulation and tumor cell proliferation.24TIMP-3 has been found to induce apoptosis in both normal and malignant cells and also to inhibit endothelial cell motility and proliferation.11;20 In addition to inhibiting tumor growth, TIMP-3 has also been found to be a potent inhibitor of angiogenesis.25 Reduced expression of TIMP-3 in breast cancer tissue has been associated with poor disease-free survival.24;26
Functional polymorphisms in the TIMP genes could lead to either increased or decreased activities, which in turn could cause an imbalance in the TIMP/MMP ratio, and thus impact cancer development and progression. Studies have begun to evaluate the association between TIMP polymorphisms and cancer risk and survival,27-29 however, only one TIMP-2 SNP and one TIMP-3 SNP have been considered, and studies in breast cancer are sparse.30;31 The purpose of the present study was to systematically evaluate polymorphisms in TIMP-2 and TIMP-3, and to characterize their association with breast cancer susceptibility and survival.
Methods
Study population
The Shanghai Breast Cancer Study is a population-based case-control study of women in urban Shanghai, China, which has previously been described in detail.32 Briefly, cases were women diagnosed with breast cancer between August 1996 and March 1998, 25-64 years of age, without a previous cancer diagnosis, and alive at the time of interview. Cases were identified via a rapid case-reporting system supplemented by the population-based Shanghai Cancer Registry; diagnoses were confirmed by two senior pathologists. Controls were women without a previous cancer diagnosis randomly selected from the general population using the Shanghai Resident Registry, a population registry of adult residents in urban Shanghai. Structured questionnaires were used to obtain detailed information on demographic, reproductive, and behavioral factors. Of eligible participants, 1,459 (91.1%) cases and 1,556 (90.3%) controls completed in-person interviews and 1,193 cases (81.8%) and 1,310 controls (84.2%) donated blood samples. Information about clinicopathological characteristics, including cancer stage, treatment, and estrogen receptor (ER) and progesterone receptor (PR) status was obtained by medical record review using a standard protocol.
Patients were followed through July 2005 by active follow-up and death certificate linkage with the Shanghai Center for Disease Control and Prevention. Of the 1,459 breast cancer cases, 1,378 (94.4%) patients were either contacted directly, or if deceased, contact was made with the next of kin (N=266). Status of the remaining 77 patients was determined by death registry linkage; 47 were found be deceased. The 30 remaining patients were assumed to be alive six months prior to the date of death certificate linkage to allow for any possible delay of record entry. Four subjects had insufficient information for record linkage and were considered to be lost to follow-up.
SNP selection
Polymorphisms were selected by searching Han Chinese data from the HapMap Project33 using the Tagger program.34 Haplotype tagging SNPs (htSNPs) were selected to cover SNPs with a linkage disequilibrium (LD) r2 of 0.90 or greater in the TIMP-2 and TIMP-3 genes ± 5 kb with a minor allele frequency (MAF) of at least 0.05. Known or potentially functional SNPs were forced into the htSNP selection process. During assay design, two TIMP-2 htSNPs failed (rs11547635 and rs130300) and were replaced by rs5754312 and rs135029. Nineteen SNPs for each gene were selected and genotyped (Table 2).
Table 2. Association of TIMP-2 and TIMP-3 SNPs with Breast Cancer, the Shanghai Breast Cancer Study.
| Gene | SNP | region | Major/Minor Allele * | Minor Allele Frequency * | OR (95% CI) 1 | |
|---|---|---|---|---|---|---|
| AB 2 | BB 2 | |||||
| TIMP-2 | rs7501477 | promoter | G/T | 9.8% | 1.0 (0.8-1.3) | 2.9 (1.2-7.1) |
| TIMP-2 | rs4789932 | promoter | T/C | 33.2% | 1.2 (1.0-1.4) | 1.2 (0.9-1.6) |
| TIMP-2 | rs7212662 | intron 1 | T/G | 26.1% | 1.0 (0.9-1.2) | 1.0 (0.7-1.3) |
| TIMP-2 | rs6416835 | intron 1 | A/G | 40.4% | 1.1 (0.9-1.4) | 1.2 (0.9-1.6) |
| TIMP-2 | rs6501266 | intron 1 | T/C | 29.6% | 1.0 (0.8-1.2) | 1.2 (0.9-1.6) |
| TIMP-2 | rs7211674 | intron 1 | A/C | 28.9% | 1.1 (0.9-1.4) | 1.1 (0.8-1.6) |
| TIMP-2 | rs4789860 | intron 1 | A/G | 23.7% | 1.0 (0.9-1.2) | 1.3 (0.9-1.8) |
| TIMP-2 | rs4789936 | intron 1 | G/A | 27.7% | 1.0 (0.9-1.2) | 1.1 (0.8-1.6) |
| TIMP-2 | rs2376999 | intron 1 | T/C | 22.9% | 1.1 (0.9-1.3) | 1.1 (0.7-1.6) |
| TIMP-2 | rs2003241 | intron 1 | A/G | 18.0% | 1.0 (0.8-1.2) | 1.0 (0.6-1.7) |
| TIMP-2 | rs7502935 | intron 1 | G/A | 36.2% | 0.9 (0.8-1.1) | 0.8 (0.6-1.0) |
| TIMP-2 | rs11654470 | intron 1 | T/C | 24.5% | 1.3 (1.1-1.5) | 1.3 (0.9-1.9) |
| TIMP-2 | rs8064344 | intron 1 | T/C | 41.5% | 1.2 (1.0-1.5) | 1.3 (1.0-1.7) |
| TIMP-2 | rs4796813 | intron 1 | G/A | 17.1% | 1.0 (0.8-1.2) | 0.8 (0.5-1.4) |
| TIMP-2 | rs7218237 | intron 1 | G/T | 8.8% | 1.2 (1.0-1.5) | 1.0 (0.4-2.6) |
| TIMP-2 | rs2277698 | exon 3 | G/A | 20.6% | 1.4 (1.2-1.7) | 1.2 (0.8-1.8) |
| TIMP-2 | rs9905930 | intron 3 | C/A | 17.2% | 1.2 (1.0-1.5) | 1.1 (0.7-1.8) |
| TIMP-2 | rs16971783 | intron 3 | T/A | 7.3% | 1.1 (0.8-1.4) | 0.9 (0.3-2.3) |
| TIMP-2 | rs9916809 | intron 3 | C/A | 7.2% | 1.3 (1.1-1.7) | 1.2 (0.3-4.2) |
| TIMP-3 | rs5754289 | promoter | C/T | 5.1% | 1.1 (0.8-1.5) | 2.0 (0.6-6.9) |
| TIMP-3 | rs5754290 | promoter | G/A | 7.7% | 1.2 (0.9-1.5) | 1.5 (0.6-3.6) |
| TIMP-3 | rs9606994 | promoter | A/G | 46.2% | 1.1 (0.9-1.3) | 1.2 (0.9-1.5) |
| TIMP-3 | rs1962223 | promoter | G/C | 37.4% | 1.1 (0.9-1.3) | 1.0 (0.8-1.3) |
| TIMP-3 | rs9619311 | promoter | T/C | 7.8% | 1.2 (0.9-1.5) | 1.4 (0.6-3.2) |
| TIMP-3 | rs738992 | intron 1 | T/C | 48.4% | 1.0 (0.8-1.2) | 1.1 (0.9-1.4) |
| TIMP-3 | rs80272 | intron 1 | T/C | 12.3% | 1.1 (0.9-1.4) | 1.4 (0.7-3.0) |
| TIMP-3 | rs8140818 | intron 1 | T/C | 5.4% | 1.1 (0.8-1.4) | 4.7 (1.0-22.5) |
| TIMP-3 | rs242077 | intron 1 | G/A | 43.7% | 1.0 (0.8-1.2) | 1.0 (0.7-1.2) |
| TIMP-3 | rs715572 | intron 1 | G/A | 34.2% | 1.0 (0.8-1.2) | 0.9 (0.7-1.2) |
| TIMP-3 | rs242072 | intron 1 | T/C | 49.1% | 1.0 (0.8-1.2) | 1.0 (0.8-1.3) |
| TIMP-3 | rs8136803 | intron 1 | G/T | 5.4% | 1.1 (0.8-1.4) | 5.1 (1.1-24.3) |
| TIMP-3 | rs135029 | intron 1 | G/A | 14.6% | 1.1 (0.9-1.4) | 1.0 (0.6-1.8) |
| TIMP-3 | rs5754312 | intron 1 | A/T | 44.5% | 1.0 (0.8-1.2) | 1.0 (0.8-1.3) |
| TIMP-3 | rs2283884 | intron 2 | A/T | 38.0% | 1.0 (0.8-1.2) | 1.2 (0.9-1.5) |
| TIMP-3 | rs9609643 | intron 2 | G/A | 14.2% | 0.9 (0.7-1.1) | 0.4 (0.2-1.0) |
| TIMP-3 | rs137485 | intron 4 | T/A | 14.1% | 1.1 (0.9-1.3) | 1.5 (0.7-3.1) |
| TIMP-3 | rs137487 | 3′ FR | G/A | 43.0% | 1.0 (0.8-1.2) | 1.0 (0.8-1.3) |
| TIMP-3 | rs137489 | 3′ FR | T/C | 47.8% | 1.0 (0.8-1.2) | 0.9 (0.7-1.2) |
Major/minor alleles and frequencies, as determined by the distribution among the controls
Odds Ratio, 95% Confidence Interval from 1,062 cases and 1,069 controls; bold values represent p<0.05
AA common homozygotes (reference group), AB heterozygotes, BB rare allele homozygotes
DNA extraction and SNP genotyping
Genomic DNA was extracted from buffy coats using Puregene's DNA Purification kits (Gentra Systems, Minneapolis, MN) or Qiagen's DNA Purification kits (Qiagen, Valencia, CA). Of the 2,503 participants who donated blood samples, 2,219 (88.7%) were genotyped for the 19 TIMP-2 SNPs and 19 TIMP-3 SNPS with the Targeted Genotyping System (Affymetrix, Santa Clara, CA) using an advanced Molecular Inversion Probe (MIP) method.35 Successful genotyping data were obtained from 2,131 (96.0%) of the samples. Consistency rates for blinded duplicates (N=39) and HapMap samples (N=12) averaged 99.6%. Laboratory personnel were blinded to the case-control status of all samples.
Statistical analysis
Hardy-Weinberg equilibrium (HWE) was tested by comparing differences in the observed and expected genotype distributions for the cases and controls separately. Case and control characteristics were compared with the χ2 test or t-test when appropriate. The associations between TIMP-2 and TIMP-3 polymorphisms and risk of breast cancer were estimated by odds ratios (ORs) and 95% confidence intervals (95% CIs) using logistic regression analyses: additive, dominant, and recessive models were applied. Covariates considered included age, education, age at menarche, age at first live birth among parous women, age at menopause among postmenopausal women, use of oral contraceptives, use of estrogen replacement therapy, family history of breast cancer, history of breast fibroadenomas, body-mass index (BMI, kg/m2), waist-to-hip ratio (WHR) and regular physical activity in the decade preceding diagnosis.
Disease-free survival (DF) was calculated for cases using the time from cancer diagnosis to disease relapse or death; overall survival (OS) was calculated using the time from cancer diagnosis to death. Censoring occurred at the date of last contact or 6 months prior to the date of record linkage. Differences in Kaplan-Meier survival functions were evaluated with the log-rank test. Hazard ratios (HRs) and corresponding 95% CIs were determined by proportional hazards regression. Covariates included age at diagnosis, menopausal status, disease stage, estrogen (ER) and progesterone receptor (PR) status, and cancer treatment, including chemotherapy, radiotherapy, and tamoxifen drug therapy.
Linkage disequilibrium between polymorphisms within each gene was assessed by Haploview.36 Associations between haplotypes37 and breast cancer risk and survival were analyzed with HAPSTAT software.38 Haplotype analysis covariates included age and education for risk models, and age and disease stage for survival models. All other analyses were performed using Statistical Analysis System software (Version 9.1; SAS Institute, Cary, NC). All statistical tests were two-tailed and p-values of ≤ 0.05 were interpreted as statistically significant.
Results
Table 1 presents demographic, reproductive, and behavioral risk factors by case-control status in participants of the Shanghai Breast Cancer Study for whom genotyping information was available. Consistent with the findings from the parent study and prior epidemiologic studies, early age at menarche, late age at menopause, late age at first live birth, prior history of fibroadenomas, high BMI or WHR, and low physical activity were found to be associated with the risk of breast cancer.
Table 1. Characteristics of Study Participants, The Shanghai Breast Cancer Study.
| Population Characteristics | Controls (N=1,069) | Cases (N=1,062) | p-value * |
|---|---|---|---|
| Demographic Factors | |||
| Age (years) | 47.0 ± 8.7 | 47.5 ± 7.9 | 0.203 |
| Education | |||
| Elementary school or less | 158 (14.8%) | 130 (12.2%) | |
| Middle school | 454 (42.5%) | 473 (44.5%) | 0.213 |
| High school or more | 457 (42.8%) | 459 (43.2%) | |
| Reproductive Risk Factors | |||
| Age at menarche (years) | 14.7 ± 1.7 | 14.5 ± 1.6 | <0.001 |
| Age at menopause (years)1 | 47.2 ± 5.0 | 48.1 ± 4.8 | 0.019 |
| Age at first live birth (years)2 | 26.2 ± 3.8 | 26.8 ± 4.1 | <0.001 |
| Used oral contraceptives | 226 (21.1%) | 228 (21.5%) | 0.854 |
| Used estrogen replacement therapy | 28 (2.6%) | 28 (2.6%) | 0.974 |
| Additional Risk Factors | |||
| First degree relative with breast cancer | 30 (2.8%) | 36 (3.4%) | 0.437 |
| Ever had breast fibroadenomas | 50 (4.7%) | 104 (9.8%) | <0.001 |
| Body mass index (kg/m2) | 23.3 ± 3.4 | 23.6 ± 3.4 | 0.020 |
| Waist-to-hip ratio | 0.80 ± 0.06 | 0.81 ± 0.06 | <0.001 |
| Regular physical activity | 272 (25.5%) | 202 (19.0%) | <0.001 |
Bold values considered to be significant p<0.05
Of the 38 SNPs evaluated for TIMP-2 and TIMP-3, only two were found to deviate from HWE after adjusting for multiple testing. rs7212662 deviated from HWE among both cases and controls and was not included in further analyses; rs9609643 deviated from HWE among breast cancer patients, but was in accordance with HWE among controls.
Associations between TIMP-2 and TIMP-3 polymorphisms and breast cancer risk are shown in Table 2. Estimates are adjusted for age, education, age at menarche, age at menopause, age at first live birth, BMI, and WHR. For all SNPs, major allele homozygotes serve as the reference group, and heterozygotes and minor allele homozygotes are separately compared. Dominant and recessive models were also considered when appropriate. Homozygotes for the TIMP-2 rs7501477 minor allele (T) had an increased risk of breast cancer (OR: 2.9, 95% CI: 1.2-7.0), this was significant in a recessive fashion (p=0.020). Carrying a minor allele in five other TIMP-2 SNPs (rs4789932, rs11654470, rs8064344, rs2277698, and rs9916809) was associated with modest increases in breast cancer risk with ORs ranging from 1.2 to 1.4, which were statistically significant in dominant models. For TIMP-3, homozygotes for the rare T allele for rs8136803 had a significantly increased risk of breast cancer (OR 5.1, 95% CI 1.1-24.3), although the small number of homozygotes (N=8) affected the stability of the point estimate. Homozygote women for the TIMP-3 rs9609643 minor A allele had a marginally significant decreased risk of breast cancer (OR 0.4, 95% CI: 0.2-1.0) when compared to women homozygous for the major G allele (p=0.06). When associations between SNPs and breast cancer risk were estimated in models that included adjustment for only age and education, results were materially unaltered (data not shown). Stratification by menopausal status did not appreciably alter the effect estimates and there were no significant interactions found (data not shown).
The LD structures of the TIMP-2 and TIMP-3 genes among the controls were used to determine haplotype blocks (Supplemental Figure 1). Four haplotype blocks were identified for TIMP-2 and five for TIMP-3. Haplotype analysis results were generally consistent with single SNP analysis, and did not reveal any additional SNPs to be associated with breast cancer risk (Table 3).
Table 3. Haplotype Analysis of TIMP-2 and TIMP-3 and Breast Cancer Risk, The Shanghai Breast Cancer Study.
| Additive Models | Dominant Models | Recessive Models | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Haplotype1 | Frequency 2 | OR 3 | 95% CI 3 | p-value | OR 3 | 95% CI 3 | p-value | OR 3 | 95% CI 3 | p-value |
| TIMP-2 | Block 1: rs6416835 and rs6501266 | ||||||||||
| H1: AT | 61.1 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||
| H2: GC | 29.5 | 1.1 | 0.9-1.2 | 0.333 | 1.0 | 0.9-1.2 | 0.890 | 1.2 | 0.9-1.5 | 0.139 | |
| H3: GT | 9.3 | 1.1 | 0.9-1.4 | 0.230 | 1.2 | 0.9-1.5 | 0.139 | 0.6 | 0.2-1.4 | 0.196 | |
| TIMP-2 | Block 2: rs4789860 and rs4789936 | ||||||||||
| H1: AG | 72.1 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||
| H2: GA | 23.6 | 1.1 | 0.9-1.2 | 0.446 | 1.0 | 0.9-1.2 | 0.656 | 1.1 | 0.9-1.5 | 0.419 | |
| TIMP-2 | Block 3: rs2376999, rs2003241, rs7502935 | ||||||||||
| H1: TAG | 40.8 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||
| H2: TAA | 35.8 | 0.9 | 0.8-1.0 | 0.164 | 0.9 | 0.8-1.1 | 0.313 | 0.9 | 0.7-1.1 | 0.310 | |
| H3: CGG | 17.4 | 1.0 | 0.8-1.1 | 0.705 | 1.0 | 0.8-1.2 | 0.941 | 1.0 | 0.7-1.4 | 0.907 | |
| H4: CAG | 5.2 | 1.1 | 0.9-1.5 | 0.384 | 1.2 | 0.9-1.5 | 0.313 | 1.2 | 0.4-3.3 | 0.751 | |
| TIMP-2 | Block 4: rs11654470, rs8064344, rs4796813, rs7218237, rs2277698 | ||||||||||
| H1: TTGGG | 58.2 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||
| H2: TCAGG | 16.9 | 1.0 | 0.9-1.2 | 0.651 | 1.1 | 0.9-1.3 | 0.424 | 0.8 | 0.5-1.2 | 0.278 | |
| H3: CCGGA | 11.8 | 1.2 | 1.0-1.5 | 0.021 | 1.2 | 1.0-1.5 | 0.035 | 1.3 | 0.8-2.0 | 0.271 | |
| H4: CCGTA | 8.5 | 1.2 | 1.0-1.5 | 0.041 | 1.3 | 1.0-1.6 | 0.031 | 1.0 | 0.5-1.9 | 0.917 | |
| TIMP-3 | Block 1: rs5754289, rs5754290, rs9606994, rs1962223, and rs9619311 | ||||||||||
| H1: CGAGT | 53.1 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||
| H2: CGGCT | 36.8 | 1.1 | 0.9-1.2 | 0.441 | 1.1 | 0.9-1.3 | 0.424 | 1.0 | 0.8-1.2 | 0.938 | |
| TIMP-3 | Block 2: rs738992 and rs80272 | ||||||||||
| H1: TT | 51.3 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||
| H2: CT | 36.6 | 1.0 | 0.9-1.2 | 0.682 | 1.0 | 0.9-1.3 | 0.550 | 1.0 | 0.8-1.2 | 0.708 | |
| H3: CC | 11.7 | 1.1 | 0.9-1.4 | 0.212 | 1.1 | 0.9-1.4 | 0.196 | 1.0 | 0.6-1.7 | 0.872 | |
| TIMP-3 | Block 3: rs8140818, rs242077, rs715572, rs242072, and rs8136803 | ||||||||||
| H1: TGGTG | 50.9 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||
| H2: TAACG | 33.8 | 1.0 | 0.9-1.1 | 0.969 | 1.0 | 0.9-1.2 | 0.993 | 1.0 | 0.8-1.2 | 0.806 | |
| H3: TAGCG | 9.4 | 1.0 | 0.8-1.3 | 0.737 | 1.0 | 0.8-1.3 | 0.803 | 1.1 | 0.6-2.0 | 0.859 | |
| H4: CGGCT | 5.3 | 1.2 | 0.9-1.5 | 0.229 | 1.1 | 0.8-1.5 | 0.442 | 2.1 | 1.0-4.7 | 0.061 | |
| TIMP-3 | Block 4: rs5754312, rs2283884, and rs9609643 | ||||||||||
| H1: AAG | 41.0 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||
| H2: TTG | 37.8 | 1.0 | 0.9-1.1 | 0.956 | 0.9 | 0.8-1.1 | 0.506 | 1.2 | 1.0-1.5 | 0.062 | |
| H3: AAA | 14.1 | 0.8 | 0.7-1.0 | 0.055 | 0.9 | 0.7-1.1 | 0.244 | 0.3 | 0.1-0.7 | 0.005 | |
| H4: TAG | 6.9 | 0.8 | 0.6-1.1 | 0.154 | 0.8 | 0.6-1.1 | 0.112 | 1.2 | 0.5-2.9 | 0.753 | |
| TIMP-3 | Block 5: rs137485, rs137487, and rs137489 | ||||||||||
| H1: TGC | 47.4 | 1.0 | reference | 1.0 | reference | 1.0 | reference | ||||
| H2: TAT | 28.8 | 1.0 | 0.9-1.1 | 0.908 | 1.1 | 0.9-1.3 | 0.376 | 0.8 | 0.6-1.1 | 0.125 | |
| H3: AAT | 13.7 | 1.1 | 0.9-1.3 | 0.437 | 1.1 | 0.9-1.4 | 0.153 | 0.8 | 0.5-1.3 | 0.412 | |
| H4: TGT | 9.6 | 1.0 | 0.8-1.2 | 0.983 | 1.1 | 0.8-1.3 | 0.654 | 0.8 | 0.4-1.6 | 0.522 | |
Bold letters indicate less common alleles
Frequency of haplotype among controls
Age and education adjusted estimates of effect
Bold values considered to be significant p<0.05
Table 4 shows the associations between breast cancer clinicopathological factors and disease-free (DF) and overall survival (OS) among the 1,062 cases. Proportional hazards regression models adjusted for age at diagnosis and stage of disease only, as well as models adjusted for age, disease stage, menopausal status, ER/PR status, and treatment (chemotherapy, radiotherapy, and tamoxifen drug therapy) are shown. As expected, advanced disease stage was associated with decreased disease-free and overall survival in all models. However, post-menopausal status was associated with worse disease-free and overall survival, which may reflect that routine breast cancer screening was not performed in the study population when the study began. In addition, ER/PR status was not associated with survival in this patient population, possibly due to the high number of patients with missing hormone receptor status information.
Table 4. Breast Cancer Clinicopathological Factors, The Shanghai Breast Cancer Study.
| Variable | N (%) 1 | Disease-Free Survival | Overall Survival | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Events 2 | 5 yr % 3 | HR (95% CI) 4 | HR (95% CI) 5 | Deaths | 5 yr % 3 | HR (95% CI) 4 | HR (95% CI) 5 | ||
| Age at diagnosis | |||||||||
| 45 or younger | 479 (45.1) | 130 | 76.3 | 1.0 (reference) | 1.0 (reference) | 96 | 85.0 | 1.0 (reference) | 1.0 (reference) |
| Older than 45 | 583 (54.9) | 156 | 76.0 | 0.9 (0.7-1.1) | 0.7 (0.5-0.9) | 131 | 82.2 | 1.0 (0.8-1.3) | 0.8 (0.6-1.1) |
| Menopausal status | |||||||||
| Premenopausal | 717 (67.5) | 180 | 78.0 | 1.0 (reference) | 1.0 (reference) | 138 | 85.4 | 1.0 (reference) | 1.0 (reference) |
| Post-menopausal | 345 (32.5) | 106 | 72.5 | 1.9 (1.3-2.7) | 2.0 (1.4-2.9) | 89 | 79.6 | 1.8 (1.2-2.8) | 1.9 (1.2-2.9) |
| TNM Stage of Disease | |||||||||
| 0-I | 265 (26.7) | 38 | 88.1 | 1.0 (reference) | 1.0 (reference) | 23 | 92.6 | 1.0 (reference) | 1.0 (reference) |
| II | 617 (62.2) | 162 | 77.1 | 2.0 (1.4-2.9) | 1.7 (1.2-2.5) | 124 | 84.9 | 2.5 (1.6-3.8) | 2.1 (1.4-3.3) |
| III-IV | 110 (11.1) | 59 | 48.6 | 5.6 (3.7-8.4) | 3.8 (2.5-5.8) | 53 | 60.6 | 7.2 (4.4-11.8) | 4.5 (2.7-7.5) |
| ER/PR Status | |||||||||
| ER+ and PR+ | 389 (53.4) | 101 | 75.9 | 1.0 (reference) | 1.0 (reference) | 82 | 83.3 | 1.0 (reference) | 1.0 (reference) |
| ER+/PR- and ER-/PR+ | 147 (20.2) | 43 | 74.5 | 1.2 (0.8-1.7) | 1.2 (0.8-1.7) | 30 | 86.1 | 1.0 (0.7-1.6) | 1.0 (0.7-1.6) |
| ER- and PR- | 192 (26.4) | 47 | 79.8 | 1.0 (0.7-1.4) | 1.0 (0.7-1.4) | 32 | 86.2 | 0.8 (0.5-1.2) | 0.8 (0.5-1.2) |
| TIMP-3 rs8136803 | |||||||||
| GG | 937 (88.3) | 249 | 76.4 | 1.0 (reference) | 1.0 (reference) | 201 | 83.8 | 1.0 (reference) | 1.0 (reference) |
| GT | 116 (10.9) | 33 | 76.1 | 1.1 (0.8-1.6) | 1.1 (0.8-1.6) | 23 | 82.2 | 1.0 (0.6-1.5) | 1.0 (0.7-1.6) |
| TT | 8 (0.8) | 4 | ≤42.8 * | 4.8 (1.8-13.0) | 3.9 (1.4-10.6) | 3 | ≤62.5 * | 3.7 (1.2-11.5) | 1.9 (0.6-6.1) |
Of patients with variable information available; percentages may not sum to 100 due to rounding error. Stage data is missing for 70 cases and ER/PR status for 334 cases.
Disease progression, relapse, or death
Percent of patients alive 5 years after breast cancer diagnosis
Hazard Ratio and 95% Confidence Interval; estimates adjusted for age and stage of disease
HR estimates adjusted for age, stage of disease, menopausal status, ER/PR, and treatment (chemotherapy, radiotherapy, and tamoxifen)
Five year survival not estimatable due to censoring of all events after 5 years
Of the 38 TIMP-2 and -3 SNPs evaluated, only one was found to be associated with survival. Cases with the TIMP-3 rs8136803 TT genotype were almost 4 times more likely to have worse disease-free survival when compared to GG homozygotes (HR: 3.9, 95% CI: 1.4-10.6), even after adjusting for known prognostic factors including age at diagnosis, menopausal status, disease stage, ER and PR status, and cancer treatment. These women also had decreased overall survival (HR: 3.7, 95% CI: 1.2-11.5), although adjustment for additional clinicopathological factors attenuated this effect (HR: 1.9, 95% CI: 0.6-6.1). The small number of rare allele homozygote cases (N=8) resulted in imprecise estimates. The Kaplan-Meier survival functions and log rank p-values for all SNPs were consistent with regression analyses (data not shown); results for TIMP-3 rs8136803 are shown in Figure 1. Cases with the TT genotype had decreased disease-free survival (p=0.007), whereas overall survival time was not significantly affected (p=0.136). Five-year survival estimates for TT patients could only be approximated, because all outcomes after five years were censored. Haplotype survival analysis was in agreement with single SNP analysis (data not shown); only haplotypes with the rare allele of TIMP-3 rs8136803 (T) were associated with significantly reduced disease-free and marginally reduced overall survival in recessive models.
Figure 1. TIMP-3 rs8136803 Kaplan-Meier Survival Functions.
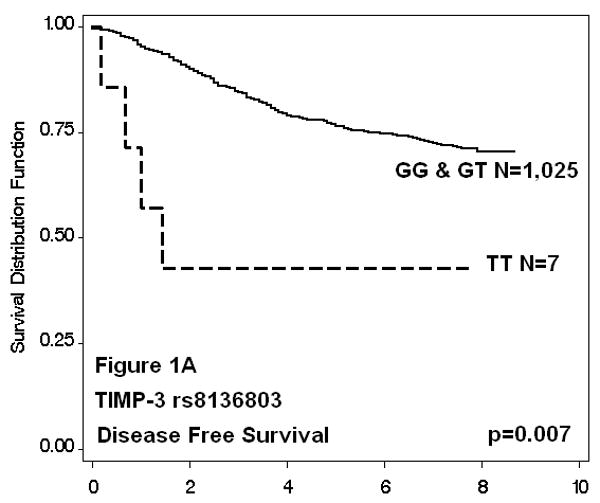
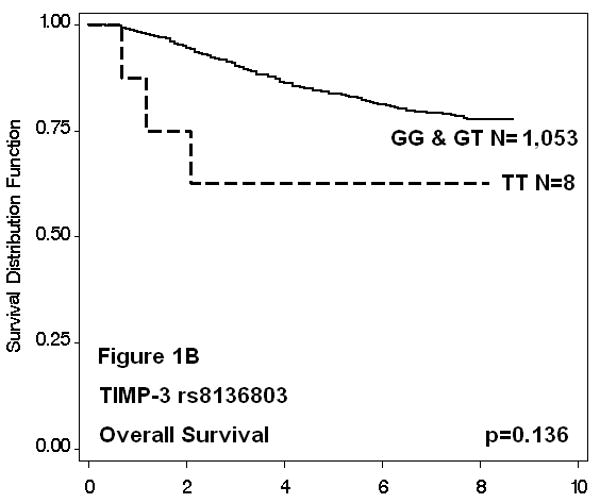
Discussion
Breast cancer is a leading cause of cancer deaths among women in Western countries and China. Because a relatively small percentage of breast cancers are attributed to highly penetrant germline mutations, we sought to examine other common, low-penetrant genetic variants that may affect breast cancer susceptibility and survival. MMPs and TIMPs are highly influential in cancer development and progression. In this study, we systematically evaluated polymorphisms in TIMP-2 and TIMP-3 for their association with breast cancer risk and survival in a large, population-based case-control study of Chinese women. For TIMP-2, we found that women with the promoter rs7501477 TT genotype were almost 3 times more likely to be breast cancer cases than women with the CC genotype. Women with either one or two copies of the minor alleles for five other TIMP-2 SNPs (rs4789932, rs11654470, rs8064344, rs2277698, and rs9916809) showed modest increases in breast cancer risk. We did not find any significant associations between TIMP-2 polymorphisms and breast cancer survival.
For TIMP-3, we found that women with the rs8136803 TT genotype were 5 times more likely to be breast cancer cases than women with the GG genotype, whereas women with the TIMP-3 rs9609643 AA genotype were 60% less likely to be breast cancer cases than women with the GG genotype. The effects of these two SNPs appeared to be independent, as a logistic regression model that included both polymorphisms yielded associations comparable to each SNP alone. However, their interaction could not be fully evaluated, as no individuals were found to have both rs8136803 TT and rs9609643 AA. In addition to being associated with breast cancer risk, rs8136803 was also found to be associated with decreased breast cancer survival in this population. Rare allele homozygotes (TT) were almost four times more likely to have decreased disease-free survival, and also tended to have shorter overall survival. The precision and significance of the estimates for this SNP were limited by its low minor allele frequency in our study population (5.4%) and its recessive effect.
TIMP-2 is an endogenous inhibitor of MMP-2; MMP-2 over-expression is generally thought to promote cancer invasion. We found that a genetic polymorphism in the promoter of TIMP-2 (rs7501477) was strongly associated with an increase in breast cancer risk. Although the functional significance of this polymorphism is not yet known, it is possible that it down-regulates the transcriptional activity of TIMP-2, or else tags a variation that does. To our knowledge, there has been only one other study that evaluated an association between a genetic polymorphism in TIMP-2 and breast cancer risk. Zhou and colleagues examined a promoter SNP (-418 G/C; rs8179090) in TIMP-2 whose functional significance is not known, but has been hypothesized to lead to transcriptional down-regulation because of its location within a consensus sequence for an Sp1-binding site.31 They found a reduced risk of breast cancer (OR 0.76; 95% CI: 0.58-0.99) for the variant allele compared to the common allele. In contrast, the variant allele has been associated with an increased risk of head and neck cancer,27 oral squamous cell cancer,28 and gastric cancer,29 in other studies. Unfortunately, we did not examine this particular SNP in our study.
TIMP-3 has been found to induce apoptosis in cancer cells39 and be a potent angiogenic inhibitor.25 We found that 2 intronic TIMP-3 polymorphisms (rs8136803 and rs9609643) were associated with increased and decreased breast cancer risk, respectively. The functional relevance of these polymorphisms is not known; they may directly impact TIMP-3 expression or activity, or they may be markers of other functionally relevant variations. As yet, there has been only one other study to examine a TIMP-3 SNP (-1296 T/C; rs9619311) for an association with breast cancer susceptibility. This functional significance of this promoter SNP is not known, but is thought to affect transcription factor binding sites. Lei and colleagues found a moderately increased risk of breast cancer among Swedish women who were carriers of the C allele (OR 1.25, 95% CI 1.05-1.50).30 We found a similar association, but our result did not reach statistical significance (OR 1.2, 95% CI 0.9-1.5), perhaps due to the lower MAF among Chinese. Also consistent with the findings of Lei et al.,30 we did not find a significant association between the TIMP-3 polymorphism rs9619311 and breast cancer survival.
The TIMP-3 intronic SNP, rs8136803, was associated not only with an increased risk of breast cancer, but also decreased survival in this study. To the best of our knowledge, no other study has examined this polymorphism in relation to cancer, nor is it known whether this SNP is functional. rs8136803 is reported to have a higher MAF among Africans than Asians or Caucasians,33 and validation of this association in an African population should be pursued. Of note, the entire TIMP-3 gene, which spans approximately 55 kb of DNA, is located within an exon of SYN-3, a neuronal-specific synapsin involved in vesicle neurotransmitter release. Therefore, although the role of SYN-3 in breast cancer development and progression is unknown, it is possible that the association with breast cancer observed for rs8136803 may be due to SYN-3 rather than TIMP-3.
An important limitation to our study was the small numbers of homozygote individuals with rare alleles which produced unstable odds and hazard ratio estimates. Studies with larger sample sizes are necessary to confirm our findings. In summary, we systematically investigated 38 polymorphisms across TIMP-2 and TIMP-3, and found several novel associations with breast cancer susceptibility and survival. Although the functional significance of these polymorphisms is not yet known, and some of the associations identified in our study could be the result of multiple comparisons, these findings do support a possible role for TIMPs in breast cancer development and progression. Future studies that include these polymorphisms may lead to a better understanding of genetic determinants of breast cancer risk and disease outcome.
Supplementary Material
Acknowledgments
The authors thank the participants and research staff of the Shanghai Breast Cancer Study. This research was supported by grants K07CA122827 (Peterson), R01CA64277 (Zheng), R01CA90899 (Zheng) and R01 CA118229 (Shu). Genotyping was conducted at the Vanderbilt Microarray Shared Resource; supported by the Vanderbilt Ingram Cancer Center (P30 CA68485), the Vanderbilt Diabetes Research and Training Center (P60 DK20593), the Vanderbilt Digestive Disease Center (P30 DK58404) and the Vanderbilt Vision Center (P30 EY08126).
Reference List
- 1.Dumitrescu RG, Cotarla I. Understanding breast cancer risk -- where do we stand in 2005? J Cell Mol Med. 2005;9:208–21. doi: 10.1111/j.1582-4934.2005.tb00350.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curran S, Murray GI. Matrix metalloproteinases: molecular aspects of their roles in tumour invasion and metastasis. Eur J Cancer. 2000;36:1621–30. doi: 10.1016/s0959-8049(00)00156-8. [DOI] [PubMed] [Google Scholar]
- 3.Egeblad M, Werb Z. New functions for the matrix metalloproteinases in cancer progression. Nat Rev Cancer. 2002;2:161–74. doi: 10.1038/nrc745. [DOI] [PubMed] [Google Scholar]
- 4.Freije JM, Balbin M, Pendas AM, Sanchez LM, Puente XS, Lopez-Otin C. Matrix metalloproteinases and tumor progression. Adv Exp Med Biol. 2003;532:91–107. doi: 10.1007/978-1-4615-0081-0_9. [DOI] [PubMed] [Google Scholar]
- 5.Hojilla CV, Mohammed FF, Khokha R. Matrix metalloproteinases and their tissue inhibitors direct cell fate during cancer development. Br J Cancer. 2003;89:1817–21. doi: 10.1038/sj.bjc.6601327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCawley LJ, Matrisian LM. Matrix metalloproteinases: they're not just for matrix anymore! Curr Opin Cell Biol. 2001;13:534–40. doi: 10.1016/s0955-0674(00)00248-9. [DOI] [PubMed] [Google Scholar]
- 7.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–33. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 8.Yoon SO, Park SJ, Yun CH, Chung AS. Roles of matrix metalloproteinases in tumor metastasis and angiogenesis. J Biochem Mol Biol. 2003;36:128–37. doi: 10.5483/bmbrep.2003.36.1.128. [DOI] [PubMed] [Google Scholar]
- 9.Spitz MR, Wu X, Mills G. Integrative epidemiology: from risk assessment to outcome prediction. J Clin Oncol. 2005;23:267–75. doi: 10.1200/JCO.2005.05.122. [DOI] [PubMed] [Google Scholar]
- 10.Folgueras AR, Pendas AM, Sanchez LM, Lopez-Otin C. Matrix metalloproteinases in cancer: from new functions to improved inhibition strategies. Int J Dev Biol. 2004;48:411–24. doi: 10.1387/ijdb.041811af. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y, Goldberg ID, Shi YE. Complex roles of tissue inhibitors of metalloproteinases in cancer. Oncogene. 2002;21:2245–52. doi: 10.1038/sj.onc.1205291. [DOI] [PubMed] [Google Scholar]
- 12.Woessner JF, Nagase H. Matrix Metalloproteinases and TIMPs. New York: Oxford University Press Inc.; 2000. [Google Scholar]
- 13.Jiang Y, Wang M, Celiker MY, Liu YE, Sang QX, Golberg ID, Shi YE. Stimulation of mammary tumorigenesis by systemic tissue inhibitor of matrix metalloproteinase 4 gene delivery. Cancer Res. 2001;61:2365–70. [PubMed] [Google Scholar]
- 14.Guedez L, McMarlin AJ, Kingma DW, Bennett TA, Stetler-Stevenson M, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinase-1 alters the tumorigenicity of Burkitt's lymphoma via divergent effects on tumor growth and angiogenesis. Am J Pathol. 2001;158:1207–15. doi: 10.1016/S0002-9440(10)64070-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy AN, Unsworth EJ, Stetler-Stevenson WG. Tissue inhibitor of metalloproteinases-2 inhibits bFGF-induced human microvascular endothelial cell proliferation. J Cell Physiol. 1993;157:351–8. doi: 10.1002/jcp.1041570219. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez HA, Kallenbach K, Seghezzi G, Grossi E, Colvin S, Schneider R, Mignatti P, Galloway A. Inhibition of endothelial cell migration by gene transfer of tissue inhibitor of metalloproteinases-1. J Surg Res. 1999;82:156–62. doi: 10.1006/jsre.1998.5534. [DOI] [PubMed] [Google Scholar]
- 17.Chirco R, Liu XW, Jung KK, Kim HR. Novel functions of TIMPs in cell signaling. Cancer Metastasis Rev. 2006;25:99–113. doi: 10.1007/s10555-006-7893-x. [DOI] [PubMed] [Google Scholar]
- 18.Brummer O, Athar S, Riethdorf L, Loning T, Herbst H. Matrix-metalloproteinases 1, 2, and 3 and their tissue inhibitors 1 and 2 in benign and malignant breast lesions: an in situ hybridization study. Virchows Arch. 1999;435:566–73. doi: 10.1007/s004280050442. [DOI] [PubMed] [Google Scholar]
- 19.Kim HJ, Park CI, Park BW, Lee HD, Jung WH. Expression of MT-1 MMP, MMP2, MMP9 and TIMP2 mRNAs in ductal carcinoma in situ and invasive ductal carcinoma of the breast. Yonsei Med J. 2006;47:333–42. doi: 10.3349/ymj.2006.47.3.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mannello F, Luchetti F, Falcieri E, Papa S. Multiple roles of matrix metalloproteinases during apoptosis. Apoptosis. 2005;10:19–24. doi: 10.1007/s10495-005-6058-7. [DOI] [PubMed] [Google Scholar]
- 21.Zhang YG, DU J, Tian XX, Zhong YF, Fang WG. Expression of E-cadherin, beta-catenin, cathepsin D, gelatinases and their inhibitors in invasive ductal breast carcinomas. Chin Med J. 2007;120:1597–605. [PubMed] [Google Scholar]
- 22.Ree AH, Florenes VA, Berg JP, Maelandsmo GM, Nesland JM, Fodstad O. High levels of messenger RNAs for tissue inhibitors of metalloproteinases (TIMP-1 and TIMP-2) in primary breast carcinomas are associated with development of distant metastases. Clin Cancer Res. 1997;3:1623–8. [PubMed] [Google Scholar]
- 23.Remacle A, McCarthy K, Noel A, Maguire T, McDermott E, O'Higgins N, Foidart JM, Duffy MJ. High levels of TIMP-2 correlate with adverse prognosis in breast cancer. Int J Cancer. 2000;89:118–21. doi: 10.1002/(sici)1097-0215(20000320)89:2<118::aid-ijc3>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 24.Mylona E, Magkou C, Giannopoulou I, Agrogiannis G, Markaki S, Keramopoulos A, Nakopoulou L. Expression of tissue inhibitor of matrix metalloproteinases (TIMP)-3 protein in invasive breast carcinoma: relation to tumor phenotype and clinical outcome. Breast Cancer Res. 2006;8:57. doi: 10.1186/bcr1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi JH, Ebrahem Q, Moore N, Murphy G, Claesson-Welsh L, Bond M, Baker A, Anand-Apte B. A novel function for tissue inhibitor of metalloproteinases-3 (TIMP3): inhibition of angiogenesis by blockage of VEGF binding to VEGF receptor-2. Nat Med. 2003;9:407–15. doi: 10.1038/nm846. [DOI] [PubMed] [Google Scholar]
- 26.Kotzsch M, Farthmann J, Meye A, Fuessel S, Baretton G, Tjan-Heijnen VC, Schmitt M, Luther T, Sweep FC, Magdolen V, Span PN. Prognostic relevance of uPAR-del4/5 and TIMP-3 mRNA expression levels in breast cancer. Eur J Cancer. 2005;41:2760–8. doi: 10.1016/j.ejca.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 27.O'Charoenrat P, Khantapura P. The role of genetic polymorphisms in the promoters of the matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 genes in head and neck cancer. Oral Oncol. 2006;42:257–67. doi: 10.1016/j.oraloncology.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 28.Vairaktaris E, Yapijakis C, Yiannopoulos A, Vassiliou S, Serefoglou Z, Vylliotis A, Nkenke E, Derka S, Critselis E, Avgoustidis D, Neukam FW, Patsouris E. Strong association of the tissue inhibitor of metalloproteinase-2 polymorphism with an increased risk of oral squamous cell carcinoma in Europeans. Oncol Rep. 2007;17:963–8. [PubMed] [Google Scholar]
- 29.Yang L, Gu HJ, Zhu HJ, Sun QM, Cong RH, Zhou B, Tang NP, Wang B. Tissue inhibitor of metalloproteinase-2 G-418C polymorphism is associated with an increased risk of gastric cancer in a Chinese population. Eur J Surg Oncol. 2007;34:636–41. doi: 10.1016/j.ejso.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Lei H, Hemminki K, Altieri A, Johansson R, Enquist K, Hallmans G, Lenner P, Forsti A. Promoter polymorphisms in matrix metalloproteinases and their inhibitors: few associations with breast cancer susceptibility and progression. Breast Cancer Res Treat. 2007;103:61–9. doi: 10.1007/s10549-006-9345-2. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y, Yu C, Miao X, Tan W, Liang G, Xiong P, Sun T, Lin D. Substantial reduction in risk of breast cancer associated with genetic polymorphisms in the promoters of the matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 genes. Carcinogenesis. 2004;25:399–404. doi: 10.1093/carcin/bgh020. [DOI] [PubMed] [Google Scholar]
- 32.Gao YT, Shu XO, Dai Q, Potter JD, Brinton LA, Wen W, Sellers TA, Kushi LH, Ruan Z, Bostick RM, Jin F, Zheng W. Association of menstrual and reproductive factors with breast cancer risk: results from the Shanghai Breast Cancer Study. Int J Cancer. 2000;87:295–300. doi: 10.1002/1097-0215(20000715)87:2<295::aid-ijc23>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 33.The International HapMap Project. Nature. 2003;426:789–96. doi: 10.1038/nature02168. [DOI] [PubMed] [Google Scholar]
- 34.de Bakker PI, McVean G, Sabeti PC, Miretti MM, Green T, Marchini J, Ke X, Monsuur AJ, Whittaker P, Delgado M, Morrison J, Richardson A, et al. A high-resolution HLA and SNP haplotype map for disease association studies in the extended human MHC. Nat Genet. 2006;38:1166–72. doi: 10.1038/ng1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hardenbol P, Yu F, Belmont J, Mackenzie J, Bruckner C, Brundage T, Boudreau A, Chow S, Eberle J, Erbilgin A, Falkowski M, Fitzgerald R, et al. Highly multiplexed molecular inversion probe genotyping: over 10,000 targeted SNPs genotyped in a single tube assay. Genome Res. 2005;15:269–75. doi: 10.1101/gr.3185605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005;21:263–5. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 37.Gabriel SB, Schaffner SF, Nguyen H, Moore JM, Roy J, Blumenstiel B, Higgins J, DeFelice M, Lochner A, Faggart M, Liu-Cordero SN, Rotimi C, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–9. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 38.Lin DY, Zeng D, Millikan R. Maximum likelihood estimation of haplotype effects and haplotype-environment interactions in association studies. Genet Epidemiol. 2005;29:299–312. doi: 10.1002/gepi.20098. [DOI] [PubMed] [Google Scholar]
- 39.Baker AH, George SJ, Zaltsman AB, Murphy G, Newby AC. Inhibition of invasion and induction of apoptotic cell death of cancer cell lines by overexpression of TIMP-3. Br J Cancer. 1999;79:1347–55. doi: 10.1038/sj.bjc.6690217. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


