Abstract
Cognitive abnormalities, including memory deficits, are common in heart failure (HF). Brain structures, including the hippocampus, fornix, and thalamus participate in memory processing, and most show structural injury and functional deficits in HF. The mammillary bodies and fornix play essential roles in spatial and working memory processing, interact with other structures, and may also be injured in HF. We assessed mammillary body volumes and cross-sectional fornix areas in 17 HF and 50 control subjects using high-resolution T1-weighted magnetic resonance images. Mammillary body volumes and fornix cross-sectional areas were significantly reduced bilaterally in HF, and these differences remained after controlling age, gender, and intracranial volume. Mammillary body and fornix injury may contribute to the compromised spatial and working memory deficits in HF. Pathological processes eliciting the damage may include injury accompanying hypoxic/ischemic processes in pathologic HF perfusion and breathing, and thiamine deficiency accompanying diuretic use and nutritional malabsorption in the condition.
Keywords: Anterior thalamus, Magnetic resonance imaging, Hippocampus, Thiamine, Ischemia, Memory
INTRODUCTION
Heart failure (HF) patients show multiple neuropsychological deficits, including emotional and cognitive abnormalities, and especially spatial and short-term memory deficits (Almeida and Flicker, 2001; Callegari et al., 2002; Ferguson et al., 1992; Grubb et al., 2000; Jiang et al., 2007). The neuropsychological deficits in HF likely result from compromised integrity of brain structures mediating the neural functions. Brain regions with structural injury, including scattered infarcts, appear on routine magnetic resonance imaging (MRI) (Almeida et al., 2005; Schmidt et al., 1991), and sites of gray matter volume loss (Woo et al., 2003), together with metabolic alterations (Lee et al., 2001) occur in the syndrome and may contribute to neuropsychological deficiencies in the condition.
The extent of memory and other cognitive deficits in HF are so severe that self-care and other quality-of life aspects are compromised significantly (Vogels et al., 2007). The available evidence, derived from voxel-based morphometry or T2-relaxometry procedures (Woo et al., 2008; Woo et al., 2003), indicates that injury appears within hippocampal and thalamic brain sites in HF that may contribute to the emotional and cognitive dysfunction in the condition, including memory processing (Lavenex et al., 2006; Ridley et al., 2004). Other structures, such as the fornix and mammillary bodies serve significant roles in memory and affective circuitry (Buckley et al., 2004; Paredes et al., 2000; Santin et al., 1999), but both traditional volumetric procedures and T2-relaxometry are inherently so low resolution that adequate assessment of small structures, such as the mammillary bodies is precluded. The bodies receive fibers from the hippocampus via the fornix, send efferents to the anterior and dorsal thalamus (Aggleton et al., 2005; Shibata, 1992), and are essential to route signals between brain areas integrating memory information. The consequences of injury to the anterior and dorsomedial thalamus, hippocampus, and mammillary bodies, or to interconnecting fibers are severe; such damage accompanies other conditions characterized by major anterograde and other memory deficits. One such condition, Wernicke-Korsakoff’s syndrome, results from injury induced by toxicity from chronic alcohol consumption accompanied by loss of neuroprotection from thiamine deficiency (van Asselen et al., 2005). Alzheimer’s disease also shows mammillary body and fornix injury and severe memory deficits (Copenhaver et al., 2006), and conditions associated with diuresis, anorexia, or intestinal malabsorption all present signs of anterograde memory loss (Harper, 2006). Mammillary body volumes are reduced in obstructive sleep apnea patients (Kumar et al., 2008), and these patients also exhibit compromised memory and cognitive functions (Bedard et al., 1991; Naegele et al., 1995).
The cognitive and memory deficits found in HF suggest the possibility that the mammillary bodies or afferent fibers have been injured in a similar fashion as found in other conditions with significant memory-deficiencies. The aim was to evaluate mammillary body volumes and fornix cross-sectional areas in HF and control subjects using high-resolution structural MRI and manual volume tracing procedures. We hypothesized that the mammillary bodies and fornix fibers in HF patients would show damage compared to control subjects.
MATERIALS AND METHODS
Subjects
Seventeen HF (mean age ± SD, 54.4 ± 8.1 years; range, 40–65 years; male, 12; LVEF, 0.28 ± 0.07, NYHA Class II, 16, III, 1) and 50 control subjects (age, 50.6 ± 7.0 years; range, 40–66 years; male, 29) were studied. Heart failure subjects were diagnosed based on national diagnostic criteria (Radford et al., 2005), and recruited from the Ahmanson-University of California at Los Angeles (UCLA) Cardiomyopathy Center and Los Angeles community. Four HF subjects had type II diabetes. The underlying HF etiology was ischemic in 5 subjects and idiopathic in 12 HF patients. None had evidence or history of alcohol-induced cardiomyopathy. All HF patients were treated with angiotensin receptor blockers or angiotension-converting enzyme inhibitors, beta blockers, and diuretics; body weight and medication doses were stabilized for at least six months before MRI studies. Control subjects were healthy, without clinical history of cardiovascular, stroke, respiratory, or neurological disorder, and recruited through greater Los Angeles area. Exclusion criteria for HF and control subjects were claustrophobia, non-removable metal such as braces, embolic coils, pacemakers/implantable cardioverter-defibrillators, and stents, and inability to lay supine, and body weight more than 125 kg (scanner limitation). Control and HF subjects provided written and informed consent prior to the study, and the study protocol was approved by the Institutional Review Board at UCLA.
Depressive and anxiety symptoms
Depressive symptoms were evaluated using the Beck Depression Inventory (BDI)-II (Beck et al., 1996), and anxious symptoms were evaluated by the Beck Anxiety Inventory (BAI) (Beck et al., 1988) in both HF and control subjects. Both instruments are self-report questionnaires, commonly used in medical condition, and were administered immediately before or after the MRI studies.
Magnetic resonance imaging
All brain studies were performed using a 3.0 Tesla MRI scanner (Magnetom Tim-Trio, Siemens, Erlangen, Germany), while subjects lay supine. Motion was minimized by using foam pads on both sides of the head. Two high-resolution T1-weighted image volumes, covering the entire brain, were collected using a magnetization prepared rapid acquisition gradient-echo sequence [repetition time (TR) = 2200 ms; echo-time (TE) = 2.2 ms; inversion time = 900 ms; flip angle (FA) = 9°; matrix size = 256 × 256; field of view (FOV) = 230 × 230 mm; slice thickness = 1.0 mm]. Whole brain proton-density (PD) and T2-weighted images (TR = 10,000 ms; TE1, 2 = 17, 134 ms; FA = 130°; matrix size = 256 × 256; FOV = 230 × 230 mm; slice thickness = 4.0 mm) were also collected simultaneously, using a dual-echo turbo spin-echo pulse sequence in the axial plane for visual assessment of any structural abnormalities.
Data analysis
High-resolution T1-weighted images were evaluated to ensure the absence of movement artifacts. T1-weighted images, and T2- and PD-weighted images of HF and control subjects were also visually evaluated for any gross pathology, such as infarcts, and for the presence of any mass lesions near the hypothalamus that might alter mammillary body or fornix morphology.
We used the statistical parametric mapping package SPM5 (Wellcome Department of Cognitive Neurology, UK; http://www.fil.ion.ucl.ac.uk/spm/), MRIcron (http://www.sph.sc.edu/comd/rorden/mricron/install.html), and Matlab-based (The MathWorks Inc, Natick, MA) custom software to process brain images.
Total intracranial volume calculation
High-resolution T1-weighted image volumes were averaged to increase signal-to-noise ratio, and images were reoriented (without warping) into a common space. The averaged and reoriented T1-weighted images were partitioned into gray matter, white matter, and CSF probability maps, using the unified segmentation (Ashburner and Friston, 2005) approach implemented in SPM5 [Gaussian per class ≥ (3, 2, 2, 5); bias full-width-at-half-maximum ≥ 70 mm cutoff; sampling distance ≥ 2]. Voxels with a probability value = 0.5 in gray, white, and CSF probability maps were calculated, and whole brain gray matter, white matter, and CSF volumes were derived by multiplying the counted voxels by the voxel volume. The total intracranial volume (TIV) was calculated by adding gray matter, white matter, and CSF volumes.
Mammillary body volume quantification
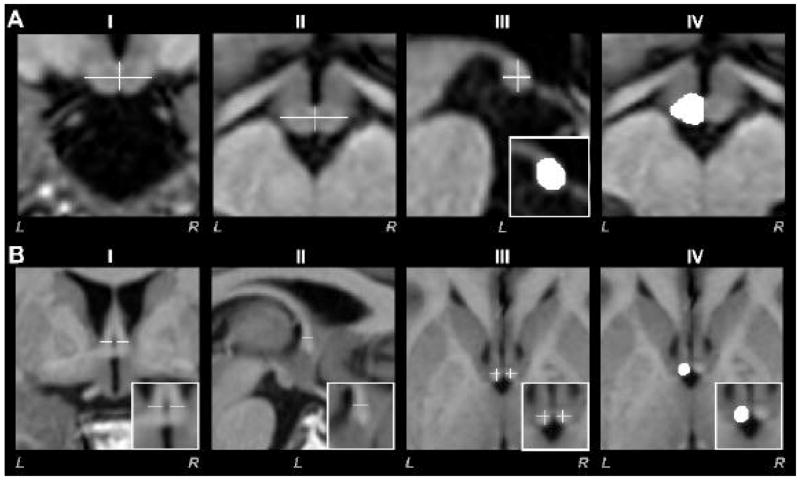
Brain areas containing the mammillary bodies were oversampled to a resolution of 0.2 × 0.2 × 0.2 mm, using averaged and reoriented T1-weighted images. Using MRIcron, a single investigator, unaware of subject group category, manually outlined body structures. Both bodies were differentiated from surrounding tissue parenchyma by their lighter gray color, and outlined after partitioning the midline from coronal and axial planes (Fig. 1A-I, II, crosshairs). Medial left or right mammillary body borders were outlined by tracing tissue in the sagittal plane of the midline (Fig. 1A-III). We determined the superior and inferior “mammillary notches” that indicated the superior and lateral boundaries of the body (Callen et al., 2001), using a central slice in the coronal view. Using the central coronal plane as a guideline, body outlines were traced; tracing continued through consecutive sagittal slices, moving medially-laterally from the medial section. Disappearance of the bodies in the central coronal section defined the lateral boundaries. Mammillary body boundaries were smoothed, moving from anterior to posterior regions in the coronal plane. Axial views were also checked for boundary smoothness (Fig. 1A-IV). The same tracing procedure was used for the remaining body. We counted outlined voxels in both mammillary bodies, and body volumes for each side were derived by multiplying the number of voxels by the voxel volume.
Fig. 1.
Brain images of different views show anatomical features used to calculate mammillary body volumes (A) and fornix cross-sectional areas (B). Midline borders for the left and right bodies were determined from coronal (A-I) and axial (A-II) views; vertical lines of the crosshairs used here indicate midline and medial borders, while extent to lateral borders are shown by horizontal lines in crosshairs. Caudal-rostral and dorsal-ventral extents were delineated using sagittal views (A-III; white crosshairs indicate mammillary body and corresponding outlined left body in white rectangle) after midline establishment, and coronal and axial views (A-IV) were used to assist determination of boundary edges. To assess fornix cross-sectional areas, coronal (B-I, white horizontal lines in magnified area within the white rectangle) and sagittal (B-II, white horizontal line in magnified area within the white rectangle) views were used to indicate fornix boundaries, providing crosshairs in axial views (B-III, white crosshairs within magnified area). The fornix cross-sectional area is outlined in axial view (B-IV, white structure within white rectangle). All images are in neurological convention (L = Left, R = Right).
Intra and inter subject reliabilities
Intra-subject reliability for mammillary body volume tracing was established by repeating the manual tracings and comparing mammillary body volumes in 8 randomly (5 controls, 3 HF) selected subjects by the original investigator. Another investigator also performed the manual tracing in the same subjects to evaluate inter-subject reliability. Intra-subject (r = 0.96, p < 0.001) and inter-subject (r = 0.98, p < 0.001) reliabilities were high for volume tracing.
Fornix cross-sectional area calculation
High-resolution averaged and reoriented T1-weighted images were oversampled (0.2 × 0.2 × 0.2 mm) in brain areas immediately dorsal to the septum containing the columns of the fornix. Using a coronal view, both left and right fornix fibers were marked before entering the septum parallel to the anterior commissure (Fig. 1B-I). The tags provided boundaries for the fibers in the medial-lateral direction. In a sagittal view, caudal-rostral borders were marked for both fornix columns (Fig. 1B-II). The coronal and sagittal boundaries provided borders for both columns (crosshairs in Fig.1B-III) in the axial view. Using axial views with the crosshairs, both left and right fornix cross-sectional areas were outlined separately (Fig. 1B-IV). Voxels from fornix cross-sectional views on each side were counted, and areas were calculated by multiplying the voxel count by the voxel area.
Statistical analysis
The Statistical Package for the Social Sciences (SPSS, V 15.0, Chicago, IL) was used for data analyses. Demographic and psychological variables of HF and control subjects were evaluated with Chi-square and independent t-tests. Between HF and control subjects, the mammillary body volume and cross-sectional fornix area differences were assessed with independent t-tests. We also performed a multivariate analysis of covariance (MANCOVA) to determine the mammillary body volume and fornix cross-sectional area differences between HF and control groups, with age, gender, and TIV included as covariates. All tests were two-tailed, and significance levels were established at p < 0.05.
Correlations between age, body mass index (BMI), BDI-II, BAI, and cross-sectional fornix areas with mammillary body volumes in HF and control subjects combined, and in HF subjects alone were assessed with Pearson’s correlation procedures. Intra- and inter-subject reliabilities were also established by comparing traced mammillary body volumes with Pearson’s correlation.
RESULTS
Demographic data and psychological measures from HF and control subjects are summarized in Table 1. Significant differences in BMI, BDI-II, and BAI appeared between control and HF subjects. However, no significant differences in age or gender emerged between groups.
Table 1.
Demographics and characteristics of HF and control subjects
| Demographics/Characteristics | HF (n = 17) | Controls (n = 50) | p value |
|---|---|---|---|
| Age (Mean ± SD, years) | 54.4 ± 8.1 | 50.6 ± 7.0 | 0.07 |
| Gender (Male: Female) | 12: 5 | 29: 21 | 0.36 |
| BMI (Mean ± SD, kg/m2) | 29.4 ± 5.7 | 24.9 ± 4.0 | 0.001 |
| LVEF (Mean ± SD) | 0.28 ± 0.07 | – | – |
| BDI-II (Mean ± SD) | 10.4 ± 7.6 | 4.3 ± 4.3 | 0.005a |
| BAI (Mean ± SD) | 9.1 ± 7.1 | 4.4 ± 5.3 | 0.005 |
SD = Standard deviation; BMI = Body mass index; LVEF = Left ventricular ejection fraction; BDI-II = Beck Depression Inventory-II; BAI = Beck Anxiety Inventory;
= Equal variance not assumed.
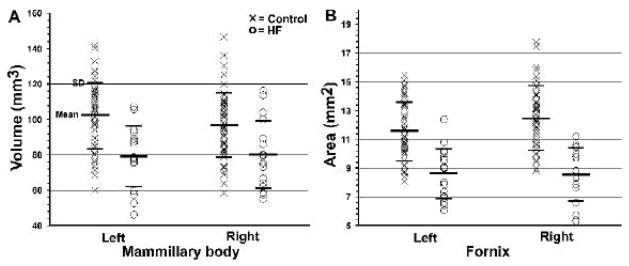
Reduced mammillary body volumes and fornix cross-sectional areas in HF were visually apparent on high-resolution T1-weighted images from most individual HF subjects (Fig. 2, Fig. 3), and this impression was confirmed with statistical assessment. In control subjects, the average left mammillary body volume was 102.19 ± 18.45 mm3, and the right mammillary body volume was 96.71 ± 18.19 mm3 (Fig. 4A). The average left side body volume for HF subjects was 79.09 ± 17.28 mm3, and the right side volume was 79.91 ±19.30 mm3 (Fig. 4A). Both left and right mammillary body volumes differed significantly between the HF and control groups (independent t-test; left, p < 0.001; right, p < 0.003). Significant volume differences remained between control and HF groups when evaluated with MANCOVA, with age, gender, and TIV included as covariates (left, p < 0.001; right, p < 0.01).
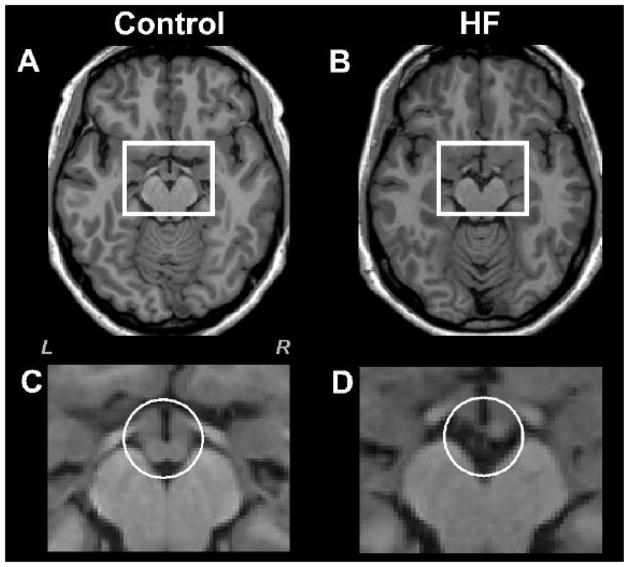
Fig. 2.
High-resolution T1-weighted images show mammillary bodies in a control (A) and HF (B) subject (white rectangles). All brain images are in neurological convention (L = Left, R = Right). Brain images (C) and (D) show magnified areas within the rectangles of the control (A) and HF subject (B). The right mammillary body in HF subject is smaller, and the left body is almost missing, compared to the control subject (C vs D, white circles).
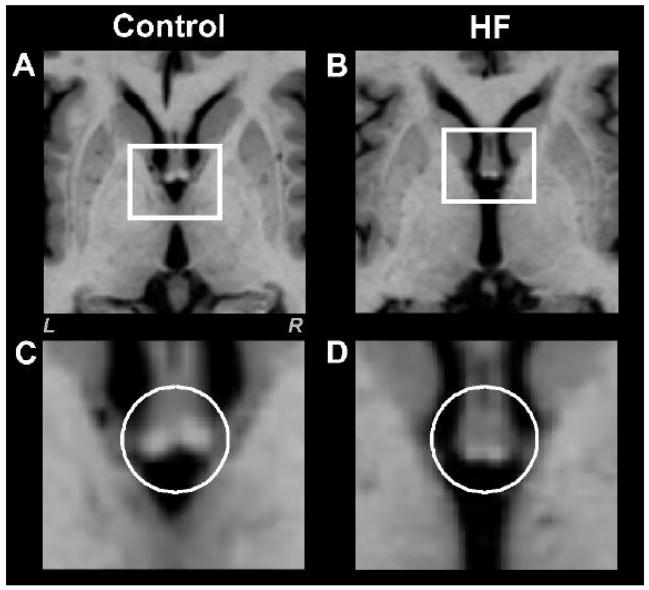
Fig. 3.
High-resolution T1-weighted oversampled images show fornix cross-sectional areas in a control (A) and HF (B) subject (white rectangles). Brain images (C) and (D) show magnified areas within the rectangles of the control (A) and HF subject (B). Both fornix areas are smaller in the HF than the control subject (C vs D, white circles). Figure conventions are the same as in Fig. 2.
Fig. 4.
Scatter plot (A) shows individual mammillary body volumes and plot (B) shows fornix cross-sectional areas in control (×) and HF (O) subjects. Both left and right mammillary body volumes and fornix cross-sectional areas differed significantly between the groups.
The average left side fornix cross-sectional area in control subjects was 11.55 ± 2.02 mm2, and the right cross-sectional area was 12.48 ± 2.25 mm2 (Fig. 4B). In HF subjects, the average left side fornix cross-sectional area was 8.62 ± 1.76 mm2, and for the right side was 8.55 ± 1.90 mm2 (Fig. 4B). Both left and right fornix cross-sectional areas were significantly reduced in HF, compared to control subjects (independent t-test; left, p < 0.001; right, p < 0.001), and these differences remained after controlling for age, gender, and TIV (MANCOVA; left, p < 0.001; right, p < 0.001).
Mammillary body volumes showed no significant correlations with BMI, age, or BAI in combined HF and control subjects, or in HF subjects alone. However, a significant negative correlation appeared between mammillary body volumes and BDI-II scores in combined HF and control subjects (left, r = − 0.28, p < 0.025; right, r = − 0.27, p < 0.031). Mammillary body volumes also positively correlated with the corresponding fornix cross-sectional area (left, r = 0.42, p < 0.001; right, r = 0.32, p < 0.009) when HF and controls are combined.
DISCUSSION
Heart failure subjects showed reduced mammillary body volumes and cross-sectional areas of the fornix, compared to similar age- and gender- distributed control subjects. Mammillary body volumes correlated positively with fornix cross-sectional area for the corresponding side in combined HF and control groups. A substantial body of both animal and human evidence links both mammillary body and fornix damage to severe anterograde and spatial memory deficits (Buckley et al., 2004; Vann and Aggleton, 2004), suggesting that the memory issues in HF have a basis in structural injury.
The mammillary bodies with their fiber projections to other significant structures serve essential roles in learning and memory, including spatial and anterograde memory. The bodies are composed of medial and lateral nuclei, which receive fibers via the fornix from the hippocampus (Aggleton et al., 2005). Mammillary body or fornix damage results in navigation and spatial delayed-alternation task deficits in multiple species (Holmes et al., 1983; Irle and Markowitsch, 1982; Rosenstock et al., 1977; Vann and Aggleton, 2003; Whishaw and Maaswinkel, 1998). Spatial navigation is encoded with information from head-direction and angular velocity cells distributed in several brain sites, including the hippocampus (Leutgeb et al., 2000), anterior and dorsolateral thalamus (Blair and Sharp, 1995; Mizumori and Williams, 1993), postsubicular cortex (Taube, 1998), lateral mammillary body nuclei (Blair et al., 1998), and the dorsal tegmental nucleus of Gudden (Sharp et al., 2001). Medial mammillary body cells, which activate with the hippocampal theta rhythm, are associated with spatial memory performance (Bland et al., 1995; Kirk et al., 1996). Lateral mammillary body nuclei also participate in spatial working memory tasks, based on animal oxidative metabolic studies (Conejo et al., 2004); however, spatial navigation and learning appear to be principal functions. The imaging procedures used here were insufficient to partition lateral and medial portions of the mammillary bodies. Nevertheless, the substantial loss overall in the bodies suggests that both spatial learning and navigation would be impaired in HF patients.
Mammillary body and thalamic lesions also affect anterograde memory (Kapur et al., 1996; Langlais et al., 1992; Tanaka et al., 1997). Fornix injury elicits long term memory deficits, while mammillary body damage impairs episodic memory recall (Tsivilis et al., 2008). The hippocampus, its projecting fornix fibers to the mammillary bodies, and the mammillary projections to the anterior thalamus are especially important for affective and cognitive regulation, including memory processing (Aggleton and Brown, 1999; Aggleton et al., 2005; Shibata, 1992). We earlier described functional impairment (Woo et al., 2005; Woo et al., 2007) and structural injury in HF (Woo et al., 2008; Woo et al., 2003) in most of these regions, including hippocampal, fornix, and thalamic areas; the limited resolution of the measurement techniques precluded adequate evaluation of the mammillary bodies. The collective findings suggest a system which regulates cognitive and affective behaviors, and processes memory, is damaged in HF, and may underlie several of the deficits found in the condition.
Significant negative correlations emerged between mammillary body volume and BDI-II scores. Classical brain structures that mediate mood regulation include the hippocampus, anterior cingulate and frontal cortices, and amygdala (Blumberg et al., 2003; Brooks et al., 2008; MacQueen et al., 2003; Rajkowska et al., 1999), and several of these structures show injury in HF. The mammillary bodies and fornix fibers are uniquely sited between hippocampal and thalamic areas to modify transmission for mood regulation. The mammillary bodies project to the anterior and dorsal thalamus, and the ventral anterior and dorsomedial thalamic nuclei interact with anterior cingulate and frontal cortices (Kaitz and Robertson, 1981; Vogt et al., 1987; Yeterian and Pandya, 1988); the latter structures have significant roles in depression (Brooks et al., 2008; Rajkowska et al., 1999). However, since the mammillary bodies are so closely integrated with hippocampal and other structures classically associated with depressive signs upon injury, it is unclear whether mammillary body damage or it’s associated structures underlie the correlations with BDI scores.
The mechanisms underlying the fornix fiber injury and mammillary body volume loss here are unknown. The fornix fiber loss may stem from the hippocampal damage, found earlier in HF, with the fornix fiber loss resulting in mammillary cell injury (Loftus et al., 2000). Initial hippocampal damage may stem from significant alterations in cerebral perfusion found in HF, secondary to low cardiac output, with localized alterations in flow (Alves et al., 2005). In addition, HF subjects show a very high incidence of sleep-disordered breathing, with both obstructive sleep apnea and Cheyne-Stokes breathing appearing (Spaak et al., 2005). Impaired breathing leads to significant cerebral blood flow and blood pressure changes during apneic events, leading to potential ischemic or hypoxic exposure in brain sites. Central alterations in perfusion accompanying HF or apnea in the condition are more likely than cerebro-arteriosclerotic disease to cause the injuries, since we found no major tissue infarcts in the HF patients.
The hippocampus and thalamus both show significant injury in obstructive sleep apnea patients (Macey et al., 2002; Macey et al., 2008). Animal studies modeling sleep disordered breathing by intermittent hypoxia exposure during sleep show tissue injury in multiple areas, including limbic and cerebellar sites (Gozal et al., 2001; Pae et al., 2005; Veasey et al., 2004). Successive intermittent hypoxic periods trigger a variety of injurious processes, including oxidative and inflammatory sequences which can elicit neural injury (Ohga et al., 2003; Veasey et al., 2004; Zhan et al., 2005).
An additional possibility is that the mammillary bodies, being the recipient of long fibers of the fornix from the hippocampus, may be subjected to excitotoxic injury triggered by ischemic or hypoxic processes in a comparable fashion as cerebellar Purkinje cells are damaged by ischemia-induced excitotoxic injury with excessive activation of climbing fibers from the inferior olive (Pae et al., 2005; Welsh et al., 2002). Hyper-excited fornix fibers may injure mammillary body neurons following ischemia/hypoxia; excitotoxic injury has been suggested as a mode of injury in hippocampal neurons following excitation of projecting Schaeffer’s collaterals during seizure discharge (Ang et al., 2006).
Mammillary body volume loss classically develops in conditions associated with thiamine deficiency, including Wernicke-Korsakoff’s syndrome accompanying chronic alcoholism (van Asselen et al., 2005), cerebral beriberi (Kornreich et al., 2005), dialysis, and anorexia (Harper, 2006). Wernicke-Korsakoff’s syndrome shows anterograde memory deficits, together with other neuropsychological inadequacies; the syndrome typically develops from alcohol toxicity, assisted by insufficient intake or absorption of thiamine. Thiamine plays significant roles in functioning of cells and neurons, and deficiency can introduce brain tissue changes through altered carbohydrate metabolism, which can lead to mitochondrial injury resulting in tissue necrosis, apoptosis (Singleton and Martin, 2001), and oxidative stress (Calingasan et al., 1999). In animal models, reduced thiamine levels elicit severe cognitive and spatial memory deficits which correlate with injury to multiple brain sites, including the mammillary bodies (Langlais and Savage, 1995). Early intervention with thiamine in Wernicke-Korsakoff’s syndrome improves memory performance, but delayed treatment fails to reverse memory loss (Harding et al., 2000). The appearance of Korsakoff’s-like symptoms in other circumstances in which thiamine deficiency appears, such as dialysis, anorexia, intestinal bypass or other malabsorption conditions provides clues to potential mechanisms operating in HF. Use of diuretics and malabsorption are common features of HF, and likely contribute to the markedly-reduced levels of thiamine found in this condition (da Cunha et al., 2002; Hanninen et al., 2006).
Thiamine deficiency also is prevalent in both Type I and Type II diabetes, with patients showing 76% reduced thiamine levels (Thornalley et al., 2007), likely resulting from frequent urination with loss of essential nutrition (Drivsholm et al., 2005). HF patients have a high incidence of diabetes, up to 48% in hospitalized HF patients (Deswal et al., 2004; Rathore et al., 2003); up to 22% of diabetic patients develop HF in older ages (Bertoni et al., 2004; Thrainsdottir et al., 2005). High glucose levels induce tissue injury (Vincent et al., 2002), and thiamine protects cells, axons, and the vasculature system against increased levels of glucose (Thornalley, 2005).
We speculate that processes accompanying alterations in cerebral perfusion and sleep-disordered breathing, operating without the neuroprotective benefit of adequate thiamine, damage hippocampal, thalamic, fornix and mammillary body structures and their projections in HF, and underlie a portion of the severe neurocognitive disturbances accompanying the condition. Interventions that target these etiologies for neurological damage (treatment of sleep-disordered breathing, stabilization/maintenance of cerebral perfusion, thiamine supplementation) may provide neuroprotection and/or alleviate neurocognitive abnormalities in HF.
Conclusions
Significantly reduced mammillary body volumes and fornix cross-sectional areas occur in HF patients over control subjects; both fornix fiber loss and diminished mammillary body volume likely contribute to anterograde and spatial memory deficits found in the syndrome. The mechanisms underlying the neuropathology are unknown, but may develop from reduced perfusion in the condition, assisted by compromised blood flow and hypoxia/ischemia accompanying sleep-disordered breathing that is common in HF. The frequent use of diuretics and the commonly-encountered nutritional malabsorption in HF may lead to nutritional deficiencies; thiamine, in particular, plays an essential neuroprotective role from excitotoxic and other processes injurious to neurons in other conditions associated with mammillary body damage.
Acknowledgments
The authors thank Ms. Rebecca Harper, Mr. Edwin Valladares, Dr. Rebecca Cross, and Dr. Stacy Serber for assistance with data collection. This research was supported by NR-009116 and HL-60296.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aggleton JP, Brown MW. Episodic memory, amnesia, and the hippocampal-anterior thalamic axis. Behav Brain Sci. 1999;22:425–444. [PubMed] [Google Scholar]
- Aggleton JP, Vann SD, Saunders RC. Projections from the hippocampal region to the mammillary bodies in macaque monkeys. Eur J Neurosci. 2005;22:2519–2530. doi: 10.1111/j.1460-9568.2005.04450.x. [DOI] [PubMed] [Google Scholar]
- Almeida JR, Alves TC, Wajngarten M, Rays J, Castro CC, Cordeiro Q, Telles RM, Fraguas RJ, Busatto GF. Late-life depression, heart failure and frontal white matter hyperintensity: a structural magnetic resonance imaging study. Braz J Med Biol Res. 2005;38:431–436. doi: 10.1590/s0100-879x2005000300014. [DOI] [PubMed] [Google Scholar]
- Almeida OP, Flicker L. The mind of a failing heart: a systematic review of the association between congestive heart failure and cognitive functioning. Intern Med J. 2001;31:290–295. doi: 10.1046/j.1445-5994.2001.00067.x. [DOI] [PubMed] [Google Scholar]
- Alves TC, Rays J, Fraguas R, Jr, Wajngarten M, Meneghetti JC, Prando S, Busatto GF. Localized cerebral blood flow reductions in patients with heart failure: a study using 99mTc-HMPAO SPECT. J Neuroimaging. 2005;15:150–156. doi: 10.1177/1051228404272880. [DOI] [PubMed] [Google Scholar]
- Ang CW, Carlson GC, Coulter DA. Massive and specific dysregulation of direct cortical input to the hippocampus in temporal lobe epilepsy. J Neurosci. 2006;26:11850–11856. doi: 10.1523/JNEUROSCI.2354-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26:839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory-II. San Antonio, Texas: The Psychological Corporation; 1996. [Google Scholar]
- Bedard MA, Montplaisir J, Richer F, Rouleau I, Malo J. Obstructive sleep apnea syndrome: pathogenesis of neuropsychological deficits. J Clin Exp Neuropsychol. 1991;13:950–964. doi: 10.1080/01688639108405110. [DOI] [PubMed] [Google Scholar]
- Bertoni AG, Hundley WG, Massing MW, Bonds DE, Burke GL, Goff DC., Jr Heart failure prevalence, incidence, and mortality in the elderly with diabetes. Diabetes Care. 2004;27:699–703. doi: 10.2337/diacare.27.3.699. [DOI] [PubMed] [Google Scholar]
- Blair HT, Cho J, Sharp PE. Role of the lateral mammillary nucleus in the rat head direction circuit: a combined single unit recording and lesion study. Neuron. 1998;21:1387–1397. doi: 10.1016/s0896-6273(00)80657-1. [DOI] [PubMed] [Google Scholar]
- Blair HT, Sharp PE. Anticipatory head direction signals in anterior thalamus: evidence for a thalamocortical circuit that integrates angular head motion to compute head direction. J Neurosci. 1995;15:6260–6270. doi: 10.1523/JNEUROSCI.15-09-06260.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bland BH, Konopacki J, Kirk IJ, Oddie SD, Dickson CT. Discharge patterns of hippocampal theta-related cells in the caudal diencephalon of the urethan-anesthetized rat. J Neurophysiol. 1995;74:322–333. doi: 10.1152/jn.1995.74.1.322. [DOI] [PubMed] [Google Scholar]
- Blumberg HP, Kaufman J, Martin A, Whiteman R, Zhang JH, Gore JC, Charney DS, Krystal JH, Peterson BS. Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry. 2003;60:1201–1208. doi: 10.1001/archpsyc.60.12.1201. [DOI] [PubMed] [Google Scholar]
- Brooks JO, 3rd, Wang PW, Bonner JC, Rosen AC, Hoblyn JC, Hill SJ, Ketter TA. Decreased prefrontal, anterior cingulate, insula, and ventral striatal metabolism in medication-free depressed outpatients with bipolar disorder. J Psychiatr Res. 2008 doi: 10.1016/j.jpsychires.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley MJ, Charles DP, Browning PG, Gaffan D. Learning and retrieval of concurrently presented spatial discrimination tasks: role of the fornix. Behav Neurosci. 2004;118:138–149. doi: 10.1037/0735-7044.118.1.138. [DOI] [PubMed] [Google Scholar]
- Calingasan NY, Chun WJ, Park LC, Uchida K, Gibson GE. Oxidative stress is associated with region-specific neuronal death during thiamine deficiency. J Neuropathol Exp Neurol. 1999;58:946–958. doi: 10.1097/00005072-199909000-00005. [DOI] [PubMed] [Google Scholar]
- Callegari S, Majani G, Giardini A, Pierobon A, Opasich C, Cobelli F, Tavazzi L. Relationship between cognitive impairment and clinical status in chronic heart failure patients. Monaldi Arch Chest Dis. 2002;58:19–25. [PubMed] [Google Scholar]
- Callen DJ, Black SE, Gao F, Caldwell CB, Szalai JP. Beyond the hippocampus: MRI volumetry confirms widespread limbic atrophy in AD. Neurology. 2001;57:1669–1674. doi: 10.1212/wnl.57.9.1669. [DOI] [PubMed] [Google Scholar]
- Conejo NM, Gonzalez-Pardo H, Vallejo G, Arias JL. Involvement of the mammillary bodies in spatial working memory revealed by cytochrome oxidase activity. Brain Res. 2004;1011:107–114. doi: 10.1016/j.brainres.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Copenhaver BR, Rabin LA, Saykin AJ, Roth RM, Wishart HA, Flashman LA, Santulli RB, McHugh TL, Mamourian AC. The fornix and mammillary bodies in older adults with Alzheimer’s disease, mild cognitive impairment, and cognitive complaints: a volumetric MRI study. Psychiatry Res. 2006;147:93–103. doi: 10.1016/j.pscychresns.2006.01.015. [DOI] [PubMed] [Google Scholar]
- da Cunha S, Albanesi Filho FM, da Cunha Bastos VL, Antelo DS, Souza MM. Thiamin, selenium, and copper levels in patients with idiopathic dilated cardiomyopathy taking diuretics. Arq Bras Cardiol. 2002;79:460–465. doi: 10.1590/s0066-782x2002001400003. [DOI] [PubMed] [Google Scholar]
- Deswal A, Petersen NJ, Souchek J, Ashton CM, Wray NP. Impact of race on health care utilization and outcomes in veterans with congestive heart failure. J Am Coll Cardiol. 2004;43:778–784. doi: 10.1016/j.jacc.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Drivsholm T, de Fine Olivarius N, Nielsen AB, Siersma V. Symptoms, signs and complications in newly diagnosed type 2 diabetic patients, and their relationship to glycaemia, blood pressure and weight. Diabetologia. 2005;48:210–214. doi: 10.1007/s00125-004-1625-y. [DOI] [PubMed] [Google Scholar]
- Ferguson DW, Berg WJ, Roach PJ, Oren RM, Mark AL. Effects of heart failure on baroreflex control of sympathetic neural activity. Am J Cardiol. 1992;69:523–531. doi: 10.1016/0002-9149(92)90998-e. [DOI] [PubMed] [Google Scholar]
- Gozal D, Daniel JM, Dohanich GP. Behavioral and anatomical correlates of chronic episodic hypoxia during sleep in the rat. J Neurosci. 2001;21:2442–2450. doi: 10.1523/JNEUROSCI.21-07-02442.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grubb NR, Simpson C, Fox KA. Memory function in patients with stable, moderate to severe cardiac failure. Am Heart J. 2000;140:E1–E5. doi: 10.1067/mhj.2000.106647. [DOI] [PubMed] [Google Scholar]
- Hanninen SA, Darling PB, Sole MJ, Barr A, Keith ME. The prevalence of thiamin deficiency in hospitalized patients with congestive heart failure. J Am Coll Cardiol. 2006;47:354–361. doi: 10.1016/j.jacc.2005.08.060. [DOI] [PubMed] [Google Scholar]
- Harding A, Halliday G, Caine D, Kril J. Degeneration of anterior thalamic nuclei differentiates alcoholics with amnesia. Brain. 2000;123 (Pt 1):141–154. doi: 10.1093/brain/123.1.141. [DOI] [PubMed] [Google Scholar]
- Harper C. Thiamine (vitamin B1) deficiency and associated brain damage is still common throughout the world and prevention is simple and safe! Eur. J Neurol. 2006;13:1078–1082. doi: 10.1111/j.1468-1331.2006.01530.x. [DOI] [PubMed] [Google Scholar]
- Holmes EJ, Jacobson S, Stein BM, Butters N. Ablations of the mammillary nuclei in monkeys: effects on postoperative memory. Exp Neurol. 1983;81:97–113. doi: 10.1016/0014-4886(83)90160-7. [DOI] [PubMed] [Google Scholar]
- Irle E, Markowitsch HJ. Single and combined lesions of the cats thalamic mediodorsal nucleus and the mamillary bodies lead to severe deficits in the acquisition of an alternation task. Behav Brain Res. 1982;6:147–165. doi: 10.1016/0166-4328(82)90011-0. [DOI] [PubMed] [Google Scholar]
- Jiang W, Kuchibhatla M, Clary GL, Cuffe MS, Christopher EJ, Alexander JD, Califf RM, Krishnan RR, O’Connor CM. Relationship between depressive symptoms and long-term mortality in patients with heart failure. Am Heart J. 2007;154:102–108. doi: 10.1016/j.ahj.2007.03.043. [DOI] [PubMed] [Google Scholar]
- Kaitz SS, Robertson RT. Thalamic connections with limbic cortex. II Corticothalamic projections. J Comp Neurol. 1981;195:527–545. doi: 10.1002/cne.901950309. [DOI] [PubMed] [Google Scholar]
- Kapur N, Thompson S, Cook P, Lang D, Brice J. Anterograde but not retrograde memory loss following combined mammillary body and medial thalamic lesions. Neuropsychologia. 1996;34:1–8. doi: 10.1016/0028-3932(95)00058-5. [DOI] [PubMed] [Google Scholar]
- Kirk IJ, Oddie SD, Konopacki J, Bland BH. Evidence for differential control of posterior hypothalamic, supramammillary, and medial mammillary theta-related cellular discharge by ascending and descending pathways. J Neurosci. 1996;16:5547–5554. doi: 10.1523/JNEUROSCI.16-17-05547.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornreich L, Bron-Harlev E, Hoffmann C, Schwarz M, Konen O, Schoenfeld T, Straussberg R, Nahum E, Ibrahim AK, Eshel G, Horev G. Thiamine deficiency in infants: MR findings in the brain. Am J Neuroradiol. 2005;26:1668–1674. [PMC free article] [PubMed] [Google Scholar]
- Kumar R, Birrer BV, Macey PM, Woo MA, Gupta RK, Yan-Go FL, Harper RM. Reduced mammillary body volume in patients with obstructive sleep apnea. Neurosci Lett. 2008;438:330–334. doi: 10.1016/j.neulet.2008.04.071. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Mandel RJ, Mair RG. Diencephalic lesions, learning impairments, and intact retrograde memory following acute thiamine deficiency in the rat. Behav Brain Res. 1992;48:177–185. doi: 10.1016/s0166-4328(05)80155-x. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Savage LM. Thiamine deficiency in rats produces cognitive and memory deficits on spatial tasks that correlate with tissue loss in diencephalon, cortex and white matter. Behav Brain Res. 1995;68:75–89. doi: 10.1016/0166-4328(94)00162-9. [DOI] [PubMed] [Google Scholar]
- Lavenex PB, Amaral DG, Lavenex P. Hippocampal lesion prevents spatial relational learning in adult macaque monkeys. J Neurosci. 2006;26:4546–4558. doi: 10.1523/JNEUROSCI.5412-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CW, Lee JH, Lim TH, Yang HS, Hong MK, Song JK, Park SW, Park SJ, Kim JJ. Prognostic significance of cerebral metabolic abnormalities in patients with congestive heart failure. Circulation. 2001;103:2784–2787. doi: 10.1161/01.cir.103.23.2784. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Ragozzino KE, Mizumori SJ. Convergence of head direction and place information in the CA1 region of hippocampus. Neuroscience. 2000;100:11–19. doi: 10.1016/s0306-4522(00)00258-x. [DOI] [PubMed] [Google Scholar]
- Loftus M, Knight RT, Amaral DG. An analysis of atrophy in the medial mammillary nucleus following hippocampal and fornix lesions in humans and nonhuman primates. Exp Neurol. 2000;163:180–190. doi: 10.1006/exnr.2000.7361. [DOI] [PubMed] [Google Scholar]
- Macey PM, Henderson LA, Macey KE, Alger JR, Frysinger RC, Woo MA, Harper RK, Yan-Go FL, Harper RM. Brain morphology associated with obstructive sleep apnea. Am J Respir Crit Care Med. 2002;166:1382–1387. doi: 10.1164/rccm.200201-050OC. [DOI] [PubMed] [Google Scholar]
- Macey PM, Kumar R, Woo MA, Valladares EM, Yan-Go FL, Harper RM. Brain structural changes in obstructive sleep apnea. Sleep. 2008;31:967–977. [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, Young LT. Course of illness, hippocampal function, and hippocampal volume in major depression. Proc Natl Acad Sci U S A. 2003;100:1387–1392. doi: 10.1073/pnas.0337481100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizumori SJ, Williams JD. Directionally selective mnemonic properties of neurons in the lateral dorsal nucleus of the thalamus of rats. J Neurosci. 1993;13:4015–4028. doi: 10.1523/JNEUROSCI.13-09-04015.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naegele B, Thouvard V, Pepin JL, Levy P, Bonnet C, Perret JE, Pellat J, Feuerstein C. Deficits of cognitive executive functions in patients with sleep apnea syndrome. Sleep. 1995;18:43–52. [PubMed] [Google Scholar]
- Ohga E, Tomita T, Wada H, Yamamoto H, Nagase T, Ouchi Y. Effects of obstructive sleep apnea on circulating ICAM-1, IL-8, and MCP-1. J Appl Physiol. 2003;94:179–184. doi: 10.1152/japplphysiol.00177.2002. [DOI] [PubMed] [Google Scholar]
- Pae EK, Chien P, Harper RM. Intermittent hypoxia damages cerebellar cortex and deep nuclei. Neurosci Lett. 2005;375:123–128. doi: 10.1016/j.neulet.2004.10.091. [DOI] [PubMed] [Google Scholar]
- Paredes J, Winters RW, Schneiderman N, McCabe PM. Afferents to the central nucleus of the amygdala and functional subdivisions of the periaqueductal gray: neuroanatomical substrates for affective behavior. Brain Res. 2000;887:157–173. doi: 10.1016/s0006-8993(00)02972-3. [DOI] [PubMed] [Google Scholar]
- Radford MJ, Arnold JM, Bennett SJ, Cinquegrani MP, Cleland JG, Havranek EP, Heidenreich PA, Rutherford JD, Spertus JA, Stevenson LW, Goff DC, Grover FL, Malenka DJ, Peterson ED, Redberg RF. ACC/AHA key data elements and definitions for measuring the clinical management and outcomes of patients with chronic heart failure. Circulation. 2005;112:1888–1916. doi: 10.1161/CIRCULATIONAHA.105.170073. [DOI] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, Overholser JC, Roth BL, Stockmeier CA. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rathore SS, Foody JM, Wang Y, Smith GL, Herrin J, Masoudi FA, Wolfe P, Havranek EP, Ordin DL, Krumholz HM. Race, quality of care, and outcomes of elderly patients hospitalized with heart failure. JAMA. 2003;289:2517–2524. doi: 10.1001/jama.289.19.2517. [DOI] [PubMed] [Google Scholar]
- Ridley RM, Baker HF, Mills DA, Green ME, Cummings RM. Topographical memory impairments after unilateral lesions of the anterior thalamus and contralateral inferotemporal cortex. Neuropsychologia. 2004;42:1178–1191. doi: 10.1016/j.neuropsychologia.2004.02.005. [DOI] [PubMed] [Google Scholar]
- Rosenstock J, Field TD, Greene E. The role of mammillary bodies in spatial memory. Exp Neurol. 1977;55:340–352. doi: 10.1016/0014-4886(77)90005-x. [DOI] [PubMed] [Google Scholar]
- Santin LJ, Rubio S, Begega A, Arias JL. Effects of mammillary body lesions on spatial reference and working memory tasks. Behav Brain Res. 1999;102:137–150. doi: 10.1016/s0166-4328(99)00011-x. [DOI] [PubMed] [Google Scholar]
- Schmidt R, Fazekas F, Offenbacher H, Dusleag J, Lechner H. Brain magnetic resonance imaging and neuropsychologic evaluation of patients with idiopathic dilated cardiomyopathy. Stroke. 1991;22:195–199. doi: 10.1161/01.str.22.2.195. [DOI] [PubMed] [Google Scholar]
- Sharp PE, Tinkelman A, Cho J. Angular velocity and head direction signals recorded from the dorsal tegmental nucleus of gudden in the rat: implications for path integration in the head direction cell circuit. Behav Neurosci. 2001;115:571–588. [PubMed] [Google Scholar]
- Shibata H. Topographic organization of subcortical projections to the anterior thalamic nuclei in the rat. J Comp Neurol. 1992;323:117–127. doi: 10.1002/cne.903230110. [DOI] [PubMed] [Google Scholar]
- Singleton CK, Martin PR. Molecular mechanisms of thiamine utilization. Curr Mol Med. 2001;1:197–207. doi: 10.2174/1566524013363870. [DOI] [PubMed] [Google Scholar]
- Spaak J, Egri ZJ, Kubo T, Yu E, Ando S, Kaneko Y, Usui K, Bradley TD, Floras JS. Muscle sympathetic nerve activity during wakefulness in heart failure patients with and without sleep apnea. Hypertension. 2005;46:1327–1332. doi: 10.1161/01.HYP.0000193497.45200.66. [DOI] [PubMed] [Google Scholar]
- Tanaka Y, Miyazawa Y, Akaoka F, Yamada T. Amnesia following damage to the mammillary bodies. Neurology. 1997;48:160–165. doi: 10.1212/wnl.48.1.160. [DOI] [PubMed] [Google Scholar]
- Taube JS. Head direction cells and the neurophysiological basis for a sense of direction. Prog Neurobiol. 1998;55:225–256. doi: 10.1016/s0301-0082(98)00004-5. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ. The potential role of thiamine (vitamin B(1)) in diabetic complications. Curr Diabetes Rev. 2005;1:287–298. doi: 10.2174/157339905774574383. [DOI] [PubMed] [Google Scholar]
- Thornalley PJ, Babaei-Jadidi R, Al Ali H, Rabbani N, Antonysunil A, Larkin J, Ahmed A, Rayman G, Bodmer CW. High prevalence of low plasma thiamine concentration in diabetes linked to a marker of vascular disease. Diabetologia. 2007;50:2164–2170. doi: 10.1007/s00125-007-0771-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrainsdottir IS, Aspelund T, Thorgeirsson G, Gudnason V, Hardarson T, Malmberg K, Sigurdsson G, Ryden L. The association between glucose abnormalities and heart failure in the population-based Reykjavik study. Diabetes Care. 2005;28:612–616. doi: 10.2337/diacare.28.3.612. [DOI] [PubMed] [Google Scholar]
- Tsivilis D, Vann SD, Denby C, Roberts N, Mayes AR, Montaldi D, Aggleton JP. A disproportionate role for the fornix and mammillary bodies in recall versus recognition memory. Nat Neurosci. 2008;11:834–842. doi: 10.1038/nn.2149. [DOI] [PubMed] [Google Scholar]
- van Asselen M, Kessels RP, Wester AJ, Postma A. Spatial working memory and contextual cueing in patients with Korsakoff amnesia. J Clin Exp Neuropsychol. 2005;27:645–655. doi: 10.1081/13803390490919281. [DOI] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. Evidence of a spatial encoding deficit in rats with lesions of the mammillary bodies or mammillothalamic tract. J Neurosci. 2003;23:3506–3514. doi: 10.1523/JNEUROSCI.23-08-03506.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann SD, Aggleton JP. The mammillary bodies: two memory systems in one? Nat Rev Neurosci. 2004;5:35–44. doi: 10.1038/nrn1299. [DOI] [PubMed] [Google Scholar]
- Veasey SC, Davis CW, Fenik P, Zhan G, Hsu YJ, Pratico D, Gow A. Long-term intermittent hypoxia in mice: protracted hypersomnolence with oxidative injury to sleep-wake brain regions. Sleep. 2004;27:194–201. doi: 10.1093/sleep/27.2.194. [DOI] [PubMed] [Google Scholar]
- Vincent AM, Brownlee M, Russell JW. Oxidative stress and programmed cell death in diabetic neuropathy. Ann N Y Acad Sci. 2002;959:368–383. doi: 10.1111/j.1749-6632.2002.tb02108.x. [DOI] [PubMed] [Google Scholar]
- Vogels RL, Scheltens P, Schroeder-Tanka JM, Weinstein HC. Cognitive impairment in heart failure: a systematic review of the literature. Eur J Heart Fail. 2007;9:440–449. doi: 10.1016/j.ejheart.2006.11.001. [DOI] [PubMed] [Google Scholar]
- Vogt BA, Pandya DN, Rosene DL. Cingulate cortex of the rhesus monkey: I. Cytoarchitecture and thalamic afferents. J Comp Neurol. 1987;262:256–270. doi: 10.1002/cne.902620207. [DOI] [PubMed] [Google Scholar]
- Welsh JP, Yuen G, Placantonakis DG, Vu TQ, Haiss F, O’Hearn E, Molliver ME, Aicher SA. Why do Purkinje cells die so easily after global brain ischemia? Aldolase C, EAAT4, and the cerebellar contribution to posthypoxic myoclonus. Adv Neurol. 2002;89:331–359. [PubMed] [Google Scholar]
- Whishaw IQ, Maaswinkel H. Rats with fimbria-fornix lesions are impaired in path integration: a role for the hippocampus in “sense of direction”. J Neurosci. 1998;18:3050–3058. doi: 10.1523/JNEUROSCI.18-08-03050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MA, Kumar R, Macey PM, Fonarow GC, Harper RM. Brain injury in autonomic, emotional, and cognitive regulatory areas in patients with heart failure. J Card Fail. 2008 doi: 10.1016/j.cardfail.2008.10.020. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Fonarow GC, Hamilton MA, Harper RM. Regional brain gray matter loss in heart failure. J Appl Physiol. 2003;95:677–684. doi: 10.1152/japplphysiol.00101.2003. [DOI] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, Harper RM. Functional abnormalities in brain areas that mediate autonomic nervous system control in advanced heart failure. J Card Fail. 2005;11:437–446. doi: 10.1016/j.cardfail.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Woo MA, Macey PM, Keens PT, Kumar R, Fonarow GC, Hamilton MA, Harper RM. Aberrant central nervous system responses to the Valsalva maneuver in heart failure. Congest Heart Fail. 2007;13:29–35. doi: 10.1111/j.1527-5299.2007.05856.x. [DOI] [PubMed] [Google Scholar]
- Yeterian EH, Pandya DN. Corticothalamic connections of paralimbic regions in the rhesus monkey. J Comp Neurol. 1988;269:130–146. doi: 10.1002/cne.902690111. [DOI] [PubMed] [Google Scholar]
- Zhan G, Fenik P, Pratico D, Veasey SC. Inducible nitric oxide synthase in long-term intermittent hypoxia: hypersomnolence and brain injury. Am J Respir Crit Care Med. 2005;171:1414–1420. doi: 10.1164/rccm.200411-1564OC. [DOI] [PMC free article] [PubMed] [Google Scholar]