SUMMARY
Cyclin A, the first cyclin ever cloned, is thought to be an essential component of the cell cycle engine. Mammalian cells encode two A-type cyclins, testis-specific cyclin A1 and ubiquitously expressed cyclin A2. Here we tested the requirement for cyclin A function using conditional knockout mice lacking both A-type cyclins. We found that acute ablation of cyclin A in fibroblasts did not affect cell proliferation, but led to prolonged expression of cyclin E across the cell cycle. However, combined ablation of all A- and E-type cyclins extinguished cell division. Hence, in fibroblasts cyclins A and E play redundant roles in cell proliferation. In contrast, ablation of cyclin A in bone marrow obliterated hematopoiesis. We found that cyclin A function was essential for proliferation of hematopoietic and embryonal stem cells. In these compartments cyclin A-Cdk complexes are expressed at particularly high levels, which may render stem cells dependent on cyclin A.
INTRODUCTION
Replication of genetic material during cell division in Metazoan organisms is thought to be driven by cyclin A. Cyclin A was the first cyclin cloned in any organism (Swenson et al., 1986). It was originally described as a protein with periodic expression pattern in clam embryos (Evans et al., 1983). Subsequently, cyclin A genes have been found in all multicellular organisms, including humans (Pines and Hunter, 1990). While only a single cyclin A gene is present in the genomes of C. elegans and Drosophila, mammalian cells express two A-type cyclins, A1 and A2 (Nieduszynski et al., 2002). Cyclin A1 is expressed almost exclusively in the testes, during meiosis in the male germline (Sweeney et al., 1996; Yang et al., 1997), and male knockout mice lacking cyclin A1 are sterile due to an arrest in meiotic prophase at the diplotene stage, just before the first meiotic division (Liu et al., 1998; van der Meer et al., 2004). The second mammalian A-type cyclin, cyclin A2 is ubiquitously expressed in all proliferating cells, and is generally considered to be the mammalian S phase cyclin (Hochegger et al., 2008; Pines and Hunter, 1990; Yam et al., 2002). During cell cycle progression cyclin A2 is induced at the beginning of the S phase (Erlandsson et al., 2000; Girard et al., 1991). Once induced, cyclin A2 binds and activates its catalytic partners, cyclin-dependent kinases Cdk2 and Cdk1. These cyclin A2-Cdk complexes phosphorylate critical proteins that play role in DNA synthesis, thereby driving S phase progression. Cyclin A2 is expressed throughout the S and G2 phases, and is rapidly degraded upon entry of cells into mitosis (Geley et al., 2001; den Elzen and Pines, 2001). Injection of anti-cyclin A2 antibodies or antisense constructs into in vitro cultured fibroblasts or other cell types blocked DNA synthesis, consistent with the essential function for cyclin A in DNA replication (Girard et al., 1991; Pagano et al., 1992; Zindy et al., 1992). In addition, cyclin A2 was postulated to play a role in entry of cells into mitosis (Swenson et al., 1986), and injection of anti-cyclin A2 antibodies into in vitro cultured fibroblasts, or inhibiting cyclin A2 function by p21Cip1 during the G2 phase blocked G2→M phase progression (Furuno et al., 1999; Pagano et al., 1992). An essential function for cyclin A in cell proliferation is supported by the observations that cyclin A2 knockout mouse embryos died shortly after implantation (Murphy et al., 1997). These studies have led to the current model that the “core” components of the cell cycle machinery (cyclins A and B) represent absolutely essential elements of the cell cycle engine (Hochegger et al., 2008; Murphy et al., 1997).
In the work described below, we decided to revisit the requirement for cyclin A function in cell proliferation using conditional cyclin A knockout mice.
RESULTS
Generation and Characterization of Conditional Cyclin A2 Knockout Mice
To obtain a conditional cyclin A2 allele, we inserted loxP sites into the first and seventh intron of the murine cyclin A2 gene (Figure 1A). The gene-targeting construct was introduced into embryonal stem (ES) cells, and heterozygous cyclin A2f/+ (Af denotes the “floxed” allele) ES were obtained through homologous recombination (Figures 1A and 1B). Cyclin A2f/+ ES cells were then injected into mouse blastocysts, and homozygous cyclin A2f/f animals were generated using standard procedures (Geng et al., 2003). Cyclin A2f/f mice were viable and phenotypically normal (data not shown), consistent with our expectation that the “floxed” cyclin A2 allele is functionally wild-type.
Figure 1. Generation of Cyclin A2f/f Mice.
(A) Cyclin A2 gene targeting strategy. Coding exons are shown as filled boxes and are numbered. Neo, neomycin phosphotransferase; loxP and FRT sequences are indicated, as light blue triangles and dark blue rectangles, respectively. Restriction enzymes recognition sites: K, KpnI; Sb, SnaBI; X, XbaI; N, NdeI; Sp, SphI; Sl, SalI; S, SmaI. Solid black lines represent Southern blotting probes A and probe B used to screen for homologous recombination. Arrows denote PCR primers used for genotyping animals (p1, p2, p4,) or for nested PCR (1M, 2M, 2L, 4L). Also shown is the conditional cyclin A2flox locus, and the deleted cyclin A2Δ allele after Cre- mediated recombination. (B) Southern blot nalysis of genomic DNA extracted from wild-type (WT) and A2f/+ ES cell clones. DNA was digested with KpnI and SphI and hybridized with probe A (5' end screening) or digested with NdeI and hybridized with probe B (3' end screening). The sizes of WT and “floxed” alleles are shown. (C) Expected and observed frequency of E7.5 embryos obtained in cyclin A2Δ+ x A2Δ+ cross. (D) Western blot analysis of cyclin A2 in wild-type mouse embryo fibroblasts (WT), A2f/f cells, A2f/f cells transduced with a retrovirus expressing an inactive, point-mutant version of Cre (A2f/f +control), Af/f cells transduced with Cre-expressing virus (A2Δ/Δ) or A1-/-A2f/f cells transduced with Cre (A1-/-A2Δ/Δ).
In order to verify that deletion of the “floxed” cyclin A2 sequences resulted in a functionally null allele, we crossed cyclin A2f/f mice with a “deleter” Meox2-Cre strain (Tallquist and Soriano, 2000), and generated cyclin A2Δ/+ mice (A2Δ denotes the deleted cyclin A2 allele). We then intercrossed cyclin A2Δ/+heterozygotes, sacrificed pregnant females 7 days post coitum and genotyped the embryos. No cyclin A2Δ/Δ embryos were observed (Figure 1C),consisted with the early embryonic lethality associated with a cyclin A-null phenotype. Hence, deletion of the “floxed” cyclin A2 sequences converts the conditional cyclin A2 allele into a functionally null allele.
Analyses of Cyclin A-null Fibroblasts
We next derived fibroblasts from conditional cyclin A2 knockout embryos and cultured them in vitro. Transduction of cyclin A2f/f cells with Cre-expressing retroviruses led to essentially complete loss of cyclin A2 protein (Figure 1D). Very unexpectedly, we found that an acute ablation of cyclin A2 had no major impact on cell proliferation. Thus, cyclin A2Δ/Δ cells normally increased cell number during in vitro culture (Figure 2A), and normally re-entered the cell cycle from quiescence (Figure 2B). However, analyses of cell cycle progression using propidium iodide and anti-BrdU staining revealed that ablation of cyclin A2 increased the fraction of cells in S and G2/M phases, with concomitant decrease in the G1 population (Figures 2C and 2D).
Figure 2. Analyses of Cyclin A2Δ/Δ and A1-/-A2Δ/Δ Fibroblasts.
(A) In vitro proliferation of fibroblasts transduced with control virus (+control) or with Cre-expressing retrovirus (A2Δ/Δ and A1-/-A2Δ/Δ). Equal numbers of cells were plated at the beginning of the experiment. Cells were counted every day for 7 days. (B) Cell cycle reentry analysis. Cells transduced as above were rendered quiescent by serum deprivation and then stimulated to re-enter the cell cycle by addition of serum. Entry into S phase was gauged by measuring [3H]-thymidine uptake. (C) Cell cycle distribution of asynchronously growing mouse embryonal fibroblasts cultured in vitro. Cells were transduced as above, pulsed with bromodeoxyuridine (BrdU) for 1 hr, then stained with anti-BrdU antibodies and with propidium iodide followed by FACS analysis. The percentages of cells in particular phases of cell cycle are shown. (D) Histogram representation of the data shown in (C). For each genotype, we analyzed 4 independent fibroblast cultures, prepared from 4 different embryos. Shown are mean values ±SD. The significance of differences was analyzed by the t-test.
To rule out the possible contribution from cyclin A1, we crossed cyclin A2f/f mice with cyclin A1-null animals (Liu et al., 1998, Ji et al., 2005), and generated cyclin A1-/-A2f/f mice. Fibroblasts were isolated from cyclin A1-/-A2f/f embryos, cultured in vitro and transduced with Cre-expressing viruses, thereby ablating all cyclin A expression (Figures 1D and S1A). We found that cells lacking all A-type cyclins proliferated normally in culture (Figure 2A) and entered the S phase from G0 without any delay (Figure 2B). Again, analyses of cell cycle profile revealed increased fraction of cells in S and G2/M phases with concomitant decrease in the G1 population (Figures 2C and 2D), similar to that seen in cyclin A2Δ/Δ cells.
To further investigate these cell cycle alterations, we compared the length of G1, S and G2/M phases in control versus in cyclin A1-/-A2Δ/Δ MEFs using pulse-chase experiments. Specifically, we pulsed in vitro cultured MEFs with BrdU, and followed the progression of BrdU-labeled cells through S, G2/M and G1 phases (see Supplemental Experimental Procedures). We found that ablation of cyclin A resulted in increased length of the S phase (from approx. 7 hrs in control to approx. 8 hrs in cyclin A1-/-A2Δ/Δ) and also prolonged the G2/M phases (from 5 to 6 hrs). In contrast, the length of G1 phase was approx. 1.5 hrs shorter upon cyclin A2 ablation. These analyses are consistent with the increased fraction of cyclin A1-/-A2Δ/Δ cells in S and G2/M phases, and decreased percentage in G1 (Figure 2C and 2D), and with overall normal cell division time in cyclin A-null cells (Figure 2A).
To elucidate the molecular basis of cell division in the absence of A-cyclins, we first compared the expression of various components of the cell cycle machinery between control versus cyclin A-null (A1-/-A2Δ/Δ) cells. We found that the levels of cyclins D1, D2, B, Cdk1, Cdk2, Cdk4, Cdk6 and p27Kip1, as well as Cdk2-associated kinase activity were essentially unchanged in cyclin A-null fibroblasts, while the activity of Cdk1-kinase was modestly decreased (Figures 3A and 3C). In contrast, we found that cyclin E was markedly upregulated following acute cyclin A shutdown (Figures 3A and 3B).
Figure 3. Molecular Analyses of A1-/-A2Δ/ΔFibroblasts.
(A) The levels of cell cycle regulators in cyclin A-deficient fibroblasts. Lysates were prepared from cyclin A1-/-A2f/f fibroblasts transduced with control (Control) or Cre-encoding viruses (A1-/-A2Δ/Δ), immunoblotted and probed with the indicated antibodies. (B) Similar analysis as in (A) using A2Δ/Δ and A1-/-A2Δ/Δ cells. (C) Cdk1 and Cdk2 were immunoprecipitated from lysates prepared as above, and subjected to in vitro kinase reactions with histone H1 as a substrate. (D) Levels of cell cycle regulators during cell cycle progression. Cells were arrested in G0 by serum-deprivation, and stimulated to re-enter the cell cycle by addition of serum. Cells were collected at the indicated time-points following serum stimulation and analyzed by Western blotting. Arrow indicates cyclin D2. (E) Quantification of cyclin E1 and A2 levels at different time-points during reentry into the cell cycle.
We also analyzed the expression levels of cyclins and Cdk across cell cycle progression. In control cells, cyclin E levels peaked in early S phase, and declined thereafter when most cells were traversing the S phase (Figures 3D and 3E). In cyclin A-null cells, however, cyclin E assumed a broad cell cycle expression pattern resembling the combined expression of cyclins E plus A in wild-type cells (Figure 3E). On the other hand, the pattern of cyclin D1, D2 and D3 expression was comparable between control and mutant cells (Figure 3D and data not shown). We therefore hypothesized that cyclin E was responsible for the relatively normal proliferation of cyclin A-null cells.
To extend these findings, we analyzed phosphorylation status of a panel of cyclin A-Cdk1/2 and E-Cdk1/2-substrates in cyclin A-deficient cells, using phospho-specific antibodies. We observed essentially unperturbed phosphorylation of these proteins in cyclin A1-/-A2Δ/Δ MEFs (Figure S2A). We also verified normal levels of several E2F target genes in cyclin A-null cells (Figure S2B), indicating that phosphorylation and concommittant functional inactivation of the retinoblastoma protein and other “pocket” proteins by Cdk1/2-containing complexes proceeded normally in the absence of cyclin A. These findings were consistent with the possibility that cyclin E-Cdk1/2 complexes might perform cyclin A-Cdk1/2 functions in cyclin A-null MEFs.
Generation and Analyses of Cells Lacking All A-type and E-type Cyclins
To rigorously test this possibility we decided to combine ablation of A-type and E-type cyclins, and to generate cells lacking all these proteins. Of note, mammalian cells express two E-type cyclins, E1 and E2 (Sherr and Roberts, 2004). Ablation of both E-cyclins in mice led to embryonal lethality at day 10.75 of gestation due to placental abnormalities (Geng et al., 2003; Parisi et al., 2003). In order to obtain cyclin E-null fibroblasts, we previously turned to the tetraploid blastocyst complementation rescue method, which essentially provided cyclin E-null embryos with wild-type (tetraploid) placentas (Geng et al., 2003). Using this method, we by-passed the placental failure, and obtained day 13.5 cyclin E1-/-E2-/- embryos, from which we derived in vitro cultures of embryonal fibroblasts. Cyclin E1-/-E2-/- fibroblasts proliferated relatively normally during conditions of continuous cell growth, but they were unable to re-enter the cell cycle from quiescence. These cyclin E-null cells expressed normal levels of cyclin A2, and had normal cyclin A2-associated kinase activity (Geng et al., 2003).
In order to obtain quadruple knockout cyclin E- and A-deficient embryos and fibroblasts, we again utilized tetraploid blastocyst complementation method. Specifically, we interbred cyclin A1-/-, A2f/f, E1-/- and E2-/- animals and generated compound heterozygotes (Figure 4A). We next intercrossed these animals, sacrificed females at day 3.5 post coitum, and derived ES cells from blastocyst-stage cyclin A1-/-A2f/fE1-/-E2-/- embryos. We next injected cyclin A1-/-A2f/fE1-/-E2-/- ES cells into tetraploid wild-type blastocysts, and implanted them into foster females for further development (Figure 4A). Since the tetraploid cells can contribute only to the extraembryonal tissues (Eggan et al., 2001), in the resulting chimeric embryos the embryo proper was derived entirely from the injected cyclin A1-/-A2f/fE1-/-E2-/- ES cells, while the placentas were “wild-type” (tetraploid). We sacrificed embryos at day 13.5 of gestation, derived in vitro cultures of cyclin A1-/-A2f/fE1-/-E2-/- fibroblasts and transduced these cells with viruses expressing Cre recombinase, thereby deleting cyclin A2 and rendering the cells null for all A- and E-type cyclins (Figures 4A, S1B and S3). Strikingly, combined ablation of cyclins A and E crippled cell proliferation (Figure 4E) and essentially extinguished incorporation of [3H]-thymidine and BrdU (Figures 4B, 4C, and S4), indicating an inability of cells to synthesize DNA. This, together with relatively normal S phase progression of cyclin E-null (E1-/-E2-/-) (Geng et al., 2003; Parisi et al., 2003) and cyclin A-null (A1-/-A2Δ/Δ) cells (Figure 2) reveals that either cyclin E or cyclin A must be present to allow DNA synthesis.
Figure 4. Analyses of Fibroblasts Lacking All A- and E-type Cyclins.
(A) Diagram illustrating generation of quadruple knockout fibroblasts via tetraploid complementation. Right-hand panel shows Western blot analysis of cyclin A2 levels in A1-/-A2f/fE1-/-E2-/- cells transduced with adenovirus encoding empty vector (Control) or Cre (A1-/-A2Δ/ΔE1-/-E2-/-). (B) Incorporation of [3H]-thymidine and (C) the percentage of BrdU-positive cells in A1-/-A2f/fE1-/-E2-/- fibroblasts transduced as above. Shown are mean values ± SD. (D) Upper panel: fibroblasts, cultured in medium containing 10% serum, were stained with propidium iodide and analyzed by FACS. Lower panel: cells were placed in serum-free medium for 3 days, stained with propidium iodide and analyzed by FACS. (E) In vitro proliferation of fibroblasts. Equal numbers of cells were plated at the beginning of the experiment. Cells were counted every day for 7 days. This experiment was performed using cells immortalized with dominant-negative p53, as cyclin E-deficient cells undergo premature senescence in culture (Geng et al., 2003). (F) The levels of the indicated proteins in cyclin A- and E-deficient fibroblasts, detected by Western blotting. (G) cyclin B1, Cdk1 or Cdk2 were immunoprecipitated from protein lysates, and used for in vitro kinase reactions with histone H1 as a substrate.
Surprisingly, staining of growth-arrested cyclin A1-/-A2Δ/Δ E1-/-E2-/- cells with propidium iodide revealed essentially unchanged percentage of cells with 2N DNA content (“G1”), cells containing between 2N and 4N DNA (“S”), and cells with 4N DNA content (“G2/M”), as compared to actively proliferating cyclin A1-/- A2f/f E1-/-E2-/- cells (see Figure 4D, upper panel). The unchanged fraction of cells containing between 2N and 4N DNA, together with extinguished thymidine and BrdU incorporation (Figures 4B and 4C) indicates intra-S phase arrest in cyclin A1-/-A2Δ/Δ E1-/-E2-/- cells. Moreover, ablation of cyclin A in quadruple knockout MEFs led to retention of G2/M cells with 4N DNA (in the absence of cell division). This indicates that in addition to the S phase block, cyclin A- and E-deficient cells arrest in G2/M, which is consistent with the proposed function of cyclin A not only in the S phase, but also during G2→M transition (Swenson et al., 1986; Furuno et al., 1999; Gong et al., 2007; Pagano et al., 1992).
We also performed additional experiments to confirm that cyclin A1-/-A2Δ/Δ E1-/-E2-/- MEFs are “locked” in S and G2/M phases. Thus, we placed proliferating cyclin A1-/-A2f/fE1-/-E2-/- and non-proliferating cyclin A1-/-A2Δ/Δ E1-/-E2-/-cells in serum-free medium, and we analyzed their cell cycle distribution by propidium iodide staining. As expected, upon serum starvation, proliferating cyclin A1-/-A2f/fE1-/-E2-/- cells exited the cell cycle, and arrested in G0/G1 (2N DNA), with concomitant decrease in S and G2/M fractions. In contrast, the DNA content of non-proliferating cyclin A1-/-A2Δ/Δ E1-/-E2-/- cells remained unchanged in serum-free medium (Figure 4D, lower panel). These observations further underscore that cyclin A1-/-A2Δ/Δ E1-/-E2-/- cells are “locked” in S and G2/M phases.
Analyses of cyclin A1-/-A2Δ/Δ E1-/-E2-/- cells revealed that the levels of cyclin B and Cdk1 were greatly decreased upon the loss of all A- and E-cyclins (Figure 4F). Also the levels of Cdk1-, Cdk2- and cyclin B-associated kinase were profoundly diminished upon cyclin A/E ablation (Figure 4G). These results are consistent the notion that cyclins A and E represent the redundant activators of Cdk1 and Cdk2. Whereas deletion of either A-type or E-type cyclins did not markedly affect Cdk1/2 catalytic activity (Figure 3C), the combined ablation of all A- and E-cyclins extinguished Cdk1 and Cdk2 kinase and arrested S- and G2/M phase progression.
Requirement for Cyclin A Function in Hematopoietic Stem Cells
Our observations that cyclin A function is dispensable for proliferation of fibroblasts prompted us to study the requirement for cyclin A in other cell types. We decided to focus on bone marrow cells, which contain several different hematopoietic lineages. To avoid ambiguity in interpreting the results, we chose to extinguish the expression of all A-type cyclins, by combining our conditional cyclin A2 knockout strain with cyclin A1-/- animals. Thus, we interbred cyclin A2f/f, A1-/- and MxCre mice, and generated cyclin A1-/-A2f/f MxCre animals. The MxCre strain expresses interferon-inducible Cre recombinase. Administration of double-stranded RNA polyI-polyC (pI-pC) to MxCre mice induces interferon leading to activation of Cre and results in a very efficient ablation of the “floxed” sequences in bone marrow cells, including in nearly 100% of hematopoietic stem cells (Kuhn et al., 1995).
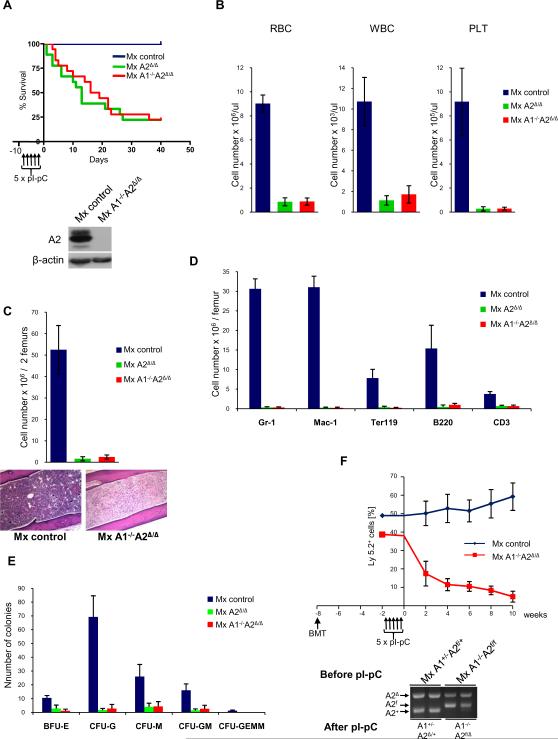
Administration of pI-pC to cyclin A1-/-A2f/f MxCre mice extinguished cyclin A expression in the bone marrow, and resulted in death of the majority of animals within 30 days (Figure 5A). In peripheral blood we observed substantially decreased numbers of red blood cells and white blood cells as well as platelets (Figure 5B), pointing to a severe anemia as a most likely cause of death. Analyses of bone marrow revealed greatly reduced numbers of bone marrow cells, with all lineages being equally affected (Figures 5C-E).
Figure 5. Impact of Cyclin A Ablation on Hematopoiesis.
(A) Survival of control A1+/-A2f/+MxCre (Mx Control), A2f/fMxCre (Mx A2Δ/Δ) and A1-/-A2f/fMxCre (Mx A1-/-A2Δ/Δ) mice following five doses of pI-pC. Each group consisted of 18 mice. Lower panel: deletion of cyclin A2 in bone marrow cells was verified by Western blotting 7 days after the last pI-pC injection. (B) Mean number of red blood cells (RBC), white blood cells (WBC) and platelets (PLT) in the peripheral blood of mice of the indicated genotypes (n=6 per group) after pI-pC administration. (C) Mean number of bone marrow cells per 2 femurs. Lower panel: histological sections of bones with bone marrow were stained with hematoxylin and eosin. Note acellular bone marrow in Mx A1-/-A2Δ/Δ animals. (D) Mean number of Gr-1+ (granulocyte), Mac-1+ (macrophage), Ter119+ (erythroid), B220+ (B cells) and CD3+ (T cells) in the bone marrow. Cells were stained with respective antibodies followed by FACS. (E) Mean number of myeloid colonies in methylcellulose cultures. 2 × 104 of bone marrow cells were isolated from mice (n=3 per group) 3 days after last administration of pl-pC (total 3 doses) and plated in duplicate. Colonies were counted after 12 to 14 days. (F) A total of 2.5 × 106 Ly5.2+ bone marrow cells from A1+/-A2f/+ MxCre (Mx control) or from A1-/-A2f/f MxCre (Mx A1-/-A2Δ/Δ) mice were adoptively transferred along with 2.5 × 106 wild-type (Ly5.1+) bone marrow cells into lethally irradiated (1000 Rad) Ly5.1+ congenic recipients (n=5 for each group) (BMT). After 6 weeks of engraftment, chimeric mice were treated with five doses of pl-pC, and the percentage of peripheral blood cells expressing Ly5.2 was determined biweekly by flow cytometry (shown are mean values for each time-point). Lower panel: at the end of experiment, genomic DNA was isolated from 5.2+ bone marrow cells and analyzed by PCR for the presence of A2+, A2f, and A2Δ alleles. In (A) - (F) Error bars denote SD. In (A) and (F) “time 0” corresponds to the last day of pl-pC injection.
Since in MxCre mice the deletion of the “floxed” sequences is not confined to bone marrow, we used bone marrow transplantation assays (Park et al., 2003) to ascertain that the observed phenotype was intrinsic to hematopoietic cells. To this end, we collected bone marrow cells from cyclin A1-/-A2f/f MxCre mice (never exposed to pI-pC), mixed them at 1:1 ratio with bone marrow cells derived from wild-type mice, and injected the cells into irradiated wild-type recipients. In this procedure, the injected hematopoietic stem cells reconstitute the bone marrow of recipient animals, which now represents a 1:1 mixture of cyclin A1-/-A2f/f MxCre and wild-type cells, while all other tissues of the recipient mouse are wild-type. Importantly, cyclin A1-/-A2f/f MxCre hematopoietic cells express a different haplotype of the Ly surface antigen (Ly5.2) than wild-type cells (Ly5.1), thereby allowing their unequivocal identification (Park et al., 2003). Following bone marrow reconstitution, we injected recipient mice with pI-pC, resulting in deletion of cyclin A selectively in Ly.5.2+ bone marrow hematopoietic cells. We found that deletion of cyclin A drastically reduced the numbers of Ly5.2+ blood cells (Figure 5F). At the end of observation period we sacrificed the mice, collected their bone marrow and determined that Ly5.2+ cells contributed to only ~5% of bone marrow cells. Flow-sorting of these residual Ly5.2+ bone marrow cells followed by PCR genotyping revealed that they arose from non-deleted cyclin A1-/-A2f/Δ hematopoietic stem cells (Figure 5F). Altogether, these analyses revealed that the requirement for cyclin A function is intrinsic to hematopoietic cells.
To determine at which stage of hematopoiesis cyclin A function becomes first rate-limiting, we collected bone marrow cells 7 days after cyclin A ablation, and flow-sorted hematopoietic stem cells (HSC), their immediate descendants (lineage-committed common myeloid progenitors, CMP, granulocyte-macrophage progenitors, GMP, and megakaryocyte-erythroid progenitors, MEP) as well as differentiated Mac-1+ (macrophage), Gr-1,+ (granulocyte) and Ter119+ (erythroid) cells (Akashi et al., 2000; Morrison et al., 1995). We found that ablation of cyclin A led to a rapid disappearance of hematopoietic stem cells as well as of lineage-committed HSC descendants, CMP, GMP and MEP (Figures 6A and B). In contrast, the proportions of the more differentiated Gr-1+, Mac-1+ and Ter119+ cells were unperturbed at this early time-point (Figure 6C and not shown). These analyses suggested that the primary defect following cyclin A ablation was at the HSC and early progenitor stage.
Figure 6. Analyses of Hematopoietic Stem Cells and Progenitors.
(A) Cells isolated from bone marrow of pl-pC treated A1+/-A2f/+MxCre (Mxcontrol), A2f/fMxCre (Mx A2Δ/Δ) and A1-/-A2f/fMxCre (Mx A1-/-A2Δ/Δ) mice were stained for the presence of hematopoietic stem cells (HSC), common myeloid progenitors (CMP), granulocyte-macrophage progenitors (GMP), and megakaryocyte-erythroid progenitors (MEP). The percentages of particular populations among all bone marrow cells are indicated. (B) Total numbers of HSC, CMP, GMP and MEP calculated per 2 femurs in animals treated as above. Error bars, SD. (C) bone marrow cells from the same animals as in (B) were stained for the presence of more differentiated granulocyte and monocyte precursors.
To test this possibility further, we flow-sorted hematopoietic stem cells from cyclin A1-/-A2f/f mice and transduced them with viruses encoding Cre and GFP. We again sorted GFP-positive HSC and cultured them - one cell per well - in a cocktail of cytokines that promotes HSC proliferation (Akashi et al., 2000). As control we used cyclin A1+/-A2f/+ HSC which were sorted and transduced in an identical fashion. As expected, Cre-transduced cyclin A1+/-A2f/+ HSC proliferated in vitro and formed colonies composed of up to over 1000 cells. PCR of individual colonies revealed that 29/31 of them arose from cyclin A1+/-A2Δ/+ HSC, confirming that Cre expression led to deletion of the “floxed” cyclin A2 alleles (Figures 7A and 7B). In striking contrast, Cre-transduced cyclin A1-/-A2f/f HSC failed to proliferate and never formed cyclin A1-/-A2Δ/Δ colonies. We did observe a few colonies in Cre-transduced cyclin A1-/-A2f/f HSC, however PCR analyses revealed they all arose from the partially recombined A1-/-A2f/Δ HSC (Figures 7A and 7B). These results indicate that cyclin A is critically required for proliferation of HSC.
Figure 7. Analyses of Hematopoietic Stem Cells and Embryonal Stem Cells.
(A) The numbers of resulting colonies of the indicated genotypes following transduction of in vitro cultured control A1+/-A2f/+ and A1-/-A2f/f HSC with Cre. (B) Single-colony PCR of colonies derived from Cre-tranduced HSC. (C) The numbers of resulting colonies of the indicated genotypes following electroporation of A1+/-A2f/+, A1-/-A2f/f and A1-/-A2f/Δ embryonal stem cells with Cre. (D) Single-colony PCR of colonies derived from ES cells electroporated with Cre. (E) Upper panel: western blot analysis of in vitro cultured wild-type fibroblasts (MEF) and bone marrow cells (BM) probed with the indicated antibodies. Lower panel: Cdk2 was immunoprecipitated (IP) followed by immunoblotting with the indicated antibodies. (F) Cdk1 or Cdk2 were immunoprecipitated from wild-type MEFs or bone marrow cells (BM) and subjected to in vitro kinase reactions using histone H1 as a substrate. (G) Same analysis as in (E) using fibroblasts and ES cells.
Requirement for Cyclin A Function in Embryonal Stem Cells
These observations prompted us to analyze the requirement for cyclin A function in another stem cell type, namely embryonal stem cells. To ablate both A-cyclins, we interbred cyclin A1-/- and A2f/f animals, sacrificed pregnant females, collected blastocyst-stage embryos and derived A1-/-A2f/f, as well as control A1+/-A2f/+ ES cells. We then transduced ES cells with Cre-expressing retrovirus, plated out single cells and allowed them to form ES cell colonies. The genotype of individual colonies was determined by PCR. As expected, expression of Cre in control cyclin A1+/-A2f/+ ES cells converted them to A1+/-A2Δ/+ cells, which then proliferated and formed A1+/-A2Δ/+ ES cell colonies (78/92 analyzed, Figures 7C and 7D). In contrast, in case of Cre-transduced cyclin A1-/-A2f/f ES cells, we observed 106 ES cell colonies derived from cells with one deleted cyclin A2 allele (A1-/-A2Δ/f), but never detected any A1-/-A2Δ/Δ colonies (Figure 7C and 7D). Since it was formally possible that expression levels of Cre in ES cells were sufficient to delete only one, but not both “floxed” cyclin A2 alleles, we transduced A1-/-A2f/Δ ES cells with Cre-expressing retrovirus. Out of 135 ES cell colonies analyzed, none arose from cyclin A1-/-A2Δ/Δ cells (Figure 7C). These results indicate that deletion of both cyclin A2 alleles is incompatible with ES cell proliferation. Hence, as was the case in hematopoietic stem cells, cyclin A is also required for proliferation and colony formation of embryonal stem cells.
Molecular Analyses of Bone Marrow Cells and Embryonal Stem Cells
Our analyses described above revealed that while in fibroblasts cyclins E and A perform overlapping roles in cell cycle progression, the proliferation of HSC and ES cells critically depends on cyclin A. To begin to elucidate the molecular basis of this requirement, we compared the levels of cyclins E and A between hematopoietic bone marrow cells versus fibroblasts.
Our analyses revealed that the levels of cyclin E in bone marrow cells were dramatically lower than in fibroblasts. Even at long exposure times we could barely detect cyclin E signal in bone marrow cells (Figure 7E). A converse was true for cyclin A2 - the amount of this cyclin in bone marrow cells was substantially higher than that in fibroblasts (Figure 7E). Of note, in bone marrow samples cyclin A2 migrates at approx. 38kDa, unlike the 55kDa isoform seen in other compartments, as reported previously by Welm et al., (2002) (Figures 7E and S5A). This shorter cyclin A2 isoform was reported to lack N-terminal destruction box, but retained Cdk binding and activation, and hence possibly represents hyper-stable cyclin A species (Welm et al., 2002).
We also immunoprecipitated Cdk2 from bone marrow cells and from fibroblasts and probed immunoblots with anti-cyclin E and anti-cyclin A2 antibodies. Again, we found that cyclin E-Cdk2 complexes were essentially absent in bone marrow cells, while cyclin A-Cdk2 was more abundant in this compartment than in fibroblasts (Figure 7E). The overall levels of Cdk2-kinase activity were comparable between the two compartments, while Cdk1-associated kinase activity was markedly higher in bone marrow cells as compared to MEFs (Figure 7F). Collectively, these observations suggest that in hematopoietic cells, cyclin A represents the major S phase cyclin, while cyclin E is expressed at very low levels. Consequently, division of these cells critically depends on cyclin A. In contrast, fibroblasts express both E- and A-type cyclins, and each one of these cyclins is sufficient to drive normal cell cycle progression.
Our analyses of ES cells revealed that the levels of cyclin A2 in these cells were substantially higher than those observed in fibroblasts (Figure 7F). Immunoprecipitation of Cdk1 or Cdk2 followed by immunoblotting with anti-cyclin A2 antibodies indicated that cyclin A2-Cdk1 and A2-Cdk2 complexes are substantially more abundant in ES cells as compared to fibroblasts (Figures 7G and S5B). In contrast, the levels of cyclin E, and E-Cdk1 and E-Cdk2 complexes were comparable between ES cells and fibroblasts (Figure 7G). We propose that in hematopoietic cells and in ES cells, cyclin A-CDK complexes greatly predominate over cyclin E-CDK complexes, and this renders these cells dependent on cyclin A for cell cycle progression.
DISCUSSION
Cyclin A is considered an essential component of the cell cycle machinery. This notion is supported by the observations that Drosophila embryos arrested their proliferation as soon as maternal cyclin A stores became depleted (Lehner and O'Farrell, 1989; Lehner and O'Farrell, 1990), while cyclin A2 knockout mice died shortly after implantation (Murphy et al., 1997). In contrast, mice lacking G1 cyclins or Cdks displayed normal proliferation in the overwhelming majority of cell types, and died due to narrow, tissue-specific abnormalities (Sherr and Roberts, 2004). These observations led to the current model that there are essentially two types of cyclin proteins: those that are vital components of the cell cycle engine (cyclins A and B), and those involved in cell type-specific regulation of the cell cycle (G1 cyclins) (Hochegger et al., 2008; Murphy et al., 1997).
In this study we show that the early lethality of cyclin A-null embryos reflected the requirement for cyclin A function at this particular stage of embryo development, rather than an ubiquitous essential role for cyclin A in cell cycle machinery. We demonstrate that in fibroblasts - a cell type that has been most extensively studied in the cell cycle field - either cyclin A or cyclin E is sufficient to drive S phase entry and progression, as well as entry of cells into the M phase. Cyclin A-Cdk complexes are thought to phosphorylate distinct set of proteins than cyclin E-Cdk kinase, and cyclin A-Cdk is believed both to drive S phase progression as well as to prevent re-replication of genetic material (Yam et al., 2002). However, our analyses of the phosphorylation status of various Cdk1 and Cdk2 substrates in cyclin A-deficient (A1-/-A2Δ/Δ) and E-deficient (E1-/-E2-/-) MEFs revealed essentially unperturbed phosphorylation of these proteins (Figure S2A). Moreover, we found normal levels of E2F targets in cyclin A- and E-deficient cells, indicating normal functional inactivation of the pocket proteins (Figure S2B). Collectively, these analyses indicate that cyclin A- and E-associated kinases can phosphorylate the similar set of proteins in vivo, including proteins involved in centrosome duplication (nucleophosmin), DNA replication licensing (Cdc6), transcription (B-Myb) and cell cycle progression (pocket proteins).
In contrast to the situation seen in fibroblasts, we found that cyclin A function was essential for proliferation of hematopoietic and embryonal stem cells. We found that in these two cell types cyclin A was expressed at particularly high levels, while cyclin E was essentially absent in the hematopoietic lineage. We propose that in the two stem cell types studied, cyclin A-Cdk complexes greatly predominate over cyclin E-Cdk complexes, and this renders these cells dependent on cyclin A for cell cycle progression. The proliferation of several stem cell types was shown to be driven by the Wnt → β catenin pathway (Nusse, 2008), and it is possible that this pathway feeds to the S phase machinery by inducing cyclin A. In contrast, mitogenic signals operating in fibroblasts may be capable of upregulating cyclins E and A equally.
An alternative possibility is that cyclin A-Cdk complexes play an essential, non-redundant function in stem cells by phosphorylating stem cell-specific cellular protein(s). Since the identity of these phosphorylation targets is currently unknown, one cannot test this hypothesis using biochemical means. However, this possibility can be verified genetically by creating a knock-in mouse strain in which cyclin A2 coding sequences would be replaced by those of cyclin E, and by assessing the ability of knock-in cyclin E to drive proliferation of cyclin A-dependent compartments (such as hematopoietic stem cells).
It also remains to be seen whether the function of cyclin A in stem cell compartments is kinase-dependent. Currently, the best-documented function of cyclin proteins is their ability to activate cyclin-dependent kinases (Sherr and Roberts, 2004). Hence, it seems likely that the function of cyclin A in vivo is mediated by cyclin A-Cdk1 and A-Cdk2 kinases. However, cyclin D-Cdk4 and D-Cdk6 complexes were shown to play kinase-independent functions during cell cycle progression (Sherr and Roberts, 2004). Moreover, cyclin E was recently found to play a kinase-independent function in cell cycle re-entry from quiescence (Geng et al., 2007). Creation of knock-in mice expressing kinase-inactive mutant versions of cyclin A would allow one to query the contribution of kinase-dependent and -independent functions of cyclin A to proliferation of cyclin A-dependent compartments.
It is surprising that altered levels of several cell cycle proteins selectively affected proliferation of stem cells. For example, deficiency in the polycomb family transcriptional repressor Bmi-1 caused increased levels of p16INK4a - an inhibitor of cyclin D-Cdk kinase - and led to greatly reduced self-renewal of neural stem cells and hematopoietic stem cells (Molofsky et al., 2003; Park et al., 2003). Consistent with these findings, it was shown that increased p16INK4a levels during aging contribute to age-dependent decline in regenerative capacity of pancreatic islet cells, neural stem cells and hematopoietic stem cells, while ablation of p16INK4a reversed these changes (Janzen et al., 2006; Krishnamurthy et al., 2006; Molofsky et al., 2006). Moreover, mice lacking all three D-type cyclins died due to an anemia caused by a proliferative failure of embryonal hematopoietic stem cells, while proliferation of the majority of other cell types proceeded unperturbed in the absence of D-cyclins (Kozar et al., 2004). Collectively, these observations suggest that wiring of cell cycle pathways in stem cells may operate in a more rigid and non-redundant fashion than in other cell types. This mechanism may allow stem cells to selectively respond to environmental cues by specifically upregulating a particular component of the cell cycle engine, and to undergo self-renewal, asymmetric division or cell differentiation.
It remains to be seen whether this strict requirement for particular cell cycle proteins also operates in cancer stem cells. If it does, a therapy targeting cyclin proteins might be highly selective in shutting off the proliferation of these cells within a developing tumor. Cyclin A2-overexpression was shown to endow cells with anchorage-independent growth (Barrett et al., 1995; Guadagno et al., 1993; Yang and Krauss, 1997), therefore blocking cyclin A2 function may revert an important step in oncogenic transformation. Also, the Myc oncogene has been postulated to signal by upregulating cyclin A (Barrett et al., 1995; Jansen-Durr et al., 1993; Qi et al., 2007), hence Myc-overexpressing human cancers might represent good candidates for anti-cyclin A therapy.
In an opposite setting, transiently stimulating the expression levels of particular cyclins, such as A- or D-cyclins might increase the proliferative potential of normal stem cells, thereby augmenting tissue regeneration after an injury, or relieving age-related functional changes in the stem cell compartment. Indeed, overexpression of cyclin A2 in transgenic mice was shown to improve cardiac regeneration after myocardial infarction by augmenting the proliferative capacity of cardiac progenitor cells (Cheng et al., 2007). Therefore, elucidation of the molecular functions of cell cycle regulators in different stem cell populations may have far reaching implications for treatments of various disease states.
EXPERIMENTAL PROCEDURES
Generation of Conditional Cyclin A2f/f Mice
Generation of cyclin A2 gene targeting construct, conditional cyclin A2 knockout ES cells and mice was described in Supplemental Experimental Procedures. Genotyping of cyclin A2 knockout mice was performed using primers p1 (5′- CGC AGC AGA AGC TCA AGA CTC GAC -3′), p2 (5′-TCT ACA TCC TAA TGC AAT GCC TGG-3′) and p4 (5′- CAC TCA CAC ACT TAG TGT CTC TGG -3′) by denaturing the DNA at 94°C for 3 min, followed by 3 0 cycles of amplification: 94°C for 1 min, 64°C for 1 min, 72°C for 1 min, and a final extension step at 72°C for 7 min. Wild-type cyclin A2 allele yields a 335bp product, cyclin A2f a 392bp product, and cyclin A2Δ a 580bp product.
Tetraploid Complementation, Derivation and Analyses of Fibroblasts
Cyclin E1-/-E2-/-A1-/-A2f/f ES cells were derived from blastocyst-stage embryos, cultured and injected into tetraploid blastocysts as described (Geng et al., 2003). Fibroblasts were derived from embryos at day 13.5 of gestation, cultured, pulsed with BrdU and [3H]-thymidine, and analyzed as described (Geng et al., 2003) (see Supplemental Experimental Procedures).
For transduction of fibroblasts with Cre, we used the hit-and-run system-based retroviruses expressing wild-type Cre, or, as a control, an enzymatically inactive Cre point-mutant (Silver and Livingston, 2001). Production of viral supernatants and infection of cells was performed as described (Silver and Livingston, 2001). Fibroblasts were infected twice for 4 hr, with a 24 hr interval between infections, using 10 MOI of virus. For analyses of A1-/-A2f/fE1-/-E2-/- fibroblasts, cells were infected twice, as above with 50 MOI of Adeno-Cre (from Vector Development Lab, Baylor College of Medicine) or the same amount of virus carrying an empty vector. Assays were performed after 48-72 hours.
Western Blotting
This was performed as described in Supplemental Experimental Procedures using antibodies against cyclin A2 (sc-596 from Santa Cruz, or c4710 from Sigma), D2, D3, Cdk2, Cdk6 (all from Santa Cruz), cyclin D1, Cdk4, Cdk1 (all from NeoMarkers), cyclins D1 and B1 (Sigma), p27 (BD Bioscience), actin (Chemicon) or cyclin E1 (kindly provided by Dr. B. Clurman). Densitometric analyses of band intensities were performed using ImageJ software http://rsb.info.nih.gov/ij/index.html.
Hematopoietic Analyses
8-10 week old cyclin A2f/fMxCre, A1-/-A2f/fMxCre or control A1+/-A2f/+MxCre mice were injected 5 times, every other day, with 400ug of polyinosinic-polycytidylic acid (pI-pC, Sigma). Peripheral blood analyses were performed using MASCOT Hemavet®850 counter (CDC Technologies). Staining and flow cytometry of bone marrow cells, as well as methylcellulose cultures were performed as described (Kozar et al., 2004).
Analyses of Hematopoietic Stem Cells and Progenitors
Bone marrow cells were collected from cyclin A1-/-A2f/fMxCre, A2f/fMxCre and control A1+/-A2f/+MxCre mice 7 days after the last pI-pC dose. Hematopoietic stem cells (HSC) and lineage-committed progenitors were stained and analyzed essentially as described (Akashi et al., 2000) (see Supplemental Experimental Procedures). Cyclin A2 deletion in purified HSC was performed as described in Supplemental Experimental Procedures.
Analyses of ES Cells
Cyclin A1-/-A2f/f and control A1+/-A2f/+ ES cells were derived from blastocyst-stage embryos as described (Geng et al., 2003). 2 × 105 ES cells were transduced with hit-and-run retrovirus (Silver and Livingston, 2001), and plated at 5 × 103 cells per 10 cm plate with feeders. Colonies were evaluated after 8-12 days.
Supplementary Material
ACKNOWLEDGMENTS
We thank members of the Sicinski lab, Drs. R. Bronson, E. Sicinska, A. Minamishima, S. Kobayashi and B. Will for help and advice, B. Clurman for anti-cyclin E1 antibody and F. Liu for anti-phospho Smad3 antibody. This work was supported by R01CA132740 and R01CA108950 grants from the NIH (to P.S.) and R01 HD34915 (to D.J.W.). P.S. is a Scholar of The Leukemia and Lymphoma Society.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Akashi K, Traver D, Miyamoto T, Weissman IL. A clonogenic common myeloid progenitor that gives rise to all myeloid lineages. Nature. 2000;404:193–197. doi: 10.1038/35004599. [DOI] [PubMed] [Google Scholar]
- Barrett JF, Lewis BC, Hoang AT, Alvarez RJ, Jr., Dang CV. Cyclin A links c-Myc to adhesion-independent cell proliferation. J Biol Chem. 1995;270:15923–15925. doi: 10.1074/jbc.270.27.15923. [DOI] [PubMed] [Google Scholar]
- Cheng RK, Asai T, Tang H, Dashoush NH, Kara RJ, Costa KD, Naka Y, Wu EX, Wolgemuth DJ, Chaudhry HW. Cyclin A2 induces cardiac regeneration after myocardial infarction and prevents heart failure. Circ Res. 2007;100:1741–1748. doi: 10.1161/CIRCRESAHA.107.153544. [DOI] [PubMed] [Google Scholar]
- den Elzen N, Pines J. Cyclin A is destroyed in prometaphase and can delay chromosome alignment and anaphase. J Cell Biol. 2001;153:121–36. doi: 10.1083/jcb.153.1.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggan K, Akutsu H, Loring J, Jackson-Grusby L, Klemm M, Rideout WM, 3rd, Yanagimachi R, Jaenisch R. Hybrid vigor, fetal overgrowth, and viability of mice derived by nuclear cloning and tetraploid embryo complementation. Proc Natl Acad Sci U S A. 2001;98:6209–6214. doi: 10.1073/pnas.101118898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekholm SV, Zickert P, Reed SI, Zetterberg A. Accumulation of cyclin E is not a prerequisite for passage through the restriction point. Mol Cell Biol. 2001;21:3256–3265. doi: 10.1128/MCB.21.9.3256-3265.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlandsson F, Linnman C, Ekholm S, Bengtsson E, Zetterberg A. A detailed analysis of cyclin A accumulation at the G(1)/S border in normal and transformed cells. Exp Cell Res. 2000;259:86–95. doi: 10.1006/excr.2000.4889. [DOI] [PubMed] [Google Scholar]
- Evans T, Rosenthal ET, Youngblom J, Distel D, Hunt T. Cyclin: a protein specified by maternal mRNA in sea urchin eggs that is destroyed at each cleavage division. Cell. 1983;33:389–96. doi: 10.1016/0092-8674(83)90420-8. [DOI] [PubMed] [Google Scholar]
- Furuno N, den Elzen N, Pines J. Human cyclin A is required for mitosis until mid prophase. J Cell Biol. 1999;147:295–306. doi: 10.1083/jcb.147.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geley S, Kramer E, Gieffers C, Gannon J, Peters JM, Hunt T. Anaphase-promoting complex/cyclosome-dependent proteolysis of human cyclin A starts at the beginning of mitosis and is not subject to the spindle assembly checkpoint. J Cell Biol. 2001;153:137–48. doi: 10.1083/jcb.153.1.137. (2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng Y, Lee YM, Welcker M, Swanger J, Zagozdzon, et al. Kinase-independent function of cyclin E. Mol Cell. 2007;25:127–139. doi: 10.1016/j.molcel.2006.11.029. [DOI] [PubMed] [Google Scholar]
- Geng Y, Yu Q, Sicinska E, Das M, Schneider JE, Bhattacharya S, Rideout WM, Bronson RT, Gardner H, Sicinski P. Cyclin E ablation in the mouse. Cell. 2003;114:431–443. doi: 10.1016/s0092-8674(03)00645-7. [DOI] [PubMed] [Google Scholar]
- Girard F, Strausfeld U, Fernandez A, Lamb NJ. Cyclin A is required for the onset of DNA replication in mammalian fibroblasts. Cell. 1991;67:1169–1179. doi: 10.1016/0092-8674(91)90293-8. [DOI] [PubMed] [Google Scholar]
- Gong D, Pomerening JR, Myers JW, Gustavsson C, Jones JT, Hahn AT, Meyer T, Ferrell JE., Jr. Cyclin A2 regulates nuclear-envelope breakdown and the nuclear accumulation of cyclin B1. Curr Biol. 2007;17:85–91. doi: 10.1016/j.cub.2006.11.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guadagno TM, Ohtsubo M, Roberts JM, Assoian RK. A link between cyclin A expression and adhesion-dependent cell cycle progression [published erratum appears in Science 1994 Jan 28; 263(5146):455] Science. 1993;262:1572–1575. doi: 10.1126/science.8248807. [DOI] [PubMed] [Google Scholar]
- Hochegger H, Takeda S, Hunt T. Cyclin-dependent kinases and cell-cycle transitions: does one fit all? Nat Rev Mol Cell Biol. 2008;9:910–916. doi: 10.1038/nrm2510. [DOI] [PubMed] [Google Scholar]
- Jansen-Durr P, Meichle A, Steiner P, Pagano M, Finke K, Botz J, Wessbecher J, Draetta G, Eilers M. Differential modulation of cyclin gene expression by MYC. Proc Natl Acad Sci U S A. 1993;90:3685–3689. doi: 10.1073/pnas.90.8.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janzen V, Forkert R, Fleming HE, Saito Y, Waring MT, et al. Stem-cell ageing modified by the cyclin-dependent kinase inhibitor p16INK4a. Nature. 2006;443:421–426. doi: 10.1038/nature05159. [DOI] [PubMed] [Google Scholar]
- Ji P, Agrawal S, Diederichs S, Baumer N, Becker A, Cauvet T, et al. Cyclin A1, the alternative A-type cyclin, contributes to G1/S cell cycle progression in somatic cells. Oncogene. 2005;24:2739–2744. doi: 10.1038/sj.onc.1208356. [DOI] [PubMed] [Google Scholar]
- Kozar K, Ciemerych MA, Rebel VI, Shigematsu H, Zagozdzon A, Sicinska E, Geng Y, Yu Q, Bhattacharya S, Bronson RT, et al. Mouse development and cell proliferation in the absence of D-cyclins. Cell. 2004;118:477–491. doi: 10.1016/j.cell.2004.07.025. [DOI] [PubMed] [Google Scholar]
- Krishnamurthy J, Ramsey MR, Ligon KL, Torrice C, Koh A, Bonner-Weir S, Sharpless NE. p16INK4a induces an age-dependent decline in islet regenerative potential. Nature. 2006;443:453–457. doi: 10.1038/nature05092. [DOI] [PubMed] [Google Scholar]
- Kuhn R, Schwenk F, Aguet M, Rajewsky K. Inducible gene targeting in mice. Science. 1995;269:1427–1429. doi: 10.1126/science.7660125. [DOI] [PubMed] [Google Scholar]
- Lehner CF, O'Farrell PH. Expression and function of Drosophila cyclin A during embryonic cell cycle progression. Cell. 1989;56:957–968. doi: 10.1016/0092-8674(89)90629-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehner CF, O'Farrell PH. The roles of Drosophila cyclins A and B in mitotic control. Cell. 1990;61:535–547. doi: 10.1016/0092-8674(90)90535-m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu D, Matzuk MM, Sung WK, Guo Q, Wang P, Wolgemuth DJ. Cyclin A1 is required for meiosis in the male mouse. Nat Genet. 1998;20:377–380. doi: 10.1038/3855. [DOI] [PubMed] [Google Scholar]
- Molofsky AV, Pardal R, Iwashita T, Park IK, Clarke MF, Morrison SJ. Bmi-1 dependence distinguishes neural stem cell self-renewal from progenitor proliferation. Nature. 2003;425:962–967. doi: 10.1038/nature02060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molofsky AV, Slutsky SG, Joseph NM, He S, Pardal R, Krishnamurthy J, Sharpless NE, Morrison SJ. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 2006;443:448–452. doi: 10.1038/nature05091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SJ, Hemmati HD, Wandycz AM, Weissman IL. The purification and characterization of fetal liver hematopoietic stem cells. Proc Natl Acad Sci U S A. 1995;92:10302–10306. doi: 10.1073/pnas.92.22.10302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy M, Stinnakre MG, Senamaud-Beaufort C, Winston NJ, Sweeney C, Kubelka M, Carrington M, Brechot C, Sobczak-Thepot J. Delayed early embryonic lethality following disruption of the murine cyclin A2 gene. Nat Genet. 1997;15:83–86. doi: 10.1038/ng0197-83. [DOI] [PubMed] [Google Scholar]
- Nieduszynski CA, Murray J, Carrington M. Whole-genome analysis of animal A- and B-type cyclins. Genome Biol. 2002;3 doi: 10.1186/gb-2002-3-12-research0070. RESEARCH0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusse R. Wnt signaling and stem cell control. Cell Res. 2008;18:523–527. doi: 10.1038/cr.2008.47. [DOI] [PubMed] [Google Scholar]
- Pagano M, Pepperkok R, Verde F, Ansorge W, Draetta G. Cyclin A is required at two points in the human cell cycle. EMBO J. 1992;11:961–971. doi: 10.1002/j.1460-2075.1992.tb05135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi T, Beck AR, Rougier N, McNeil T, Lucian L, Werb Z, Amati B. Cyclins E1 and E2 are required for endoreplication in placental trophoblast giant cells. EMBO J. 2003;22:4794–4803. doi: 10.1093/emboj/cdg482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park IK, Qian D, Kiel M, Becker MW, Pihalja M, Weissman IL, Morrison SJ, Clarke MF. Bmi-1 is required for maintenance of adult self-renewing haematopoietic stem cells. Nature. 2003;423:302–305. doi: 10.1038/nature01587. [DOI] [PubMed] [Google Scholar]
- Pines J, Hunter T. Human cyclin A is adenovirus E1A-associated protein p60 and behaves differently from cyclin B. Nature. 1990;346:760–763. doi: 10.1038/346760a0. [DOI] [PubMed] [Google Scholar]
- Qi Y, Tu Y, Yang D, Chen Q, Xiao J, Chen Y, Fu J, Xiao X, Zhou Z. Cyclin A but not cyclin D1 is essential for c-myc-modulated cell-cycle progression. J Cell Physiol. 2007;210:63–71. doi: 10.1002/jcp.20816. [DOI] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM. Living with or without cyclins and cyclindependent kinases. Genes Dev. 2004;18:2699–2711. doi: 10.1101/gad.1256504. [DOI] [PubMed] [Google Scholar]
- Silver DP, Livingston DM. Self-excising retroviral vectors encoding the Cre recombinase overcome Cre-mediated cellular toxicity. Mol Cell. 2001;8:233–243. doi: 10.1016/s1097-2765(01)00295-7. [DOI] [PubMed] [Google Scholar]
- Sweeney C, Murphy M, Kubelka M, Ravnik SE, Hawkins CF, Wolgemuth DJ, Carrington M. A distinct cyclin A is expressed in germ cells in the mouse. Development. 1996;122:53–64. doi: 10.1242/dev.122.1.53. [DOI] [PubMed] [Google Scholar]
- Swenson KI, Farrell KM, Ruderman JV. The clam embryo protein cyclin A induces entry into M phase and the resumption of meiosis in Xenopus oocytes. Cell. 1986;47:861–870. doi: 10.1016/0092-8674(86)90801-9. [DOI] [PubMed] [Google Scholar]
- Tallquist MD, Soriano P. Epiblast-restricted Cre expression in MORE mice: a tool to distinguish embryonic vs. extra-embryonic gene function. Genesis. 2000;26:113–115. doi: 10.1002/(sici)1526-968x(200002)26:2<113::aid-gene3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- van der Meer T, Chan WY, Palazon LS, Nieduszynski C, Murphy M, Sobczak-Thepot J, Carrington M, Colledge WH. Cyclin A1 protein shows haplo-insufficiency for normal fertility in male mice. Reproduction. 2004;127:503–511. doi: 10.1530/rep.1.00131. [DOI] [PubMed] [Google Scholar]
- Welm AL, Timchenko NA, Ono Y, Sorimachi H, Radomska HS, Tenen DG, Lekstrom-Himes J, Darlington GJ. C/EBPalpha is required for proteolytic cleavage of cyclin A by calpain 3 in myeloid precursor cells. J Biol Chem. 2002;277:33848–33856. doi: 10.1074/jbc.M204096200. [DOI] [PubMed] [Google Scholar]
- Yam CH, Fung TK, Poon RY. Cyclin A in cell cycle control and cancer. Cell Mol Life Sci. 2002;59:1317–1326. doi: 10.1007/s00018-002-8510-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang JJ, Krauss RS. Extracellular ATP induces anchorage-independent expression of cyclin A and rescues the transformed phenotype of a ras-resistant mutant cell line. J Biol Chem. 1997;272:3103–3108. doi: 10.1074/jbc.272.5.3103. [DOI] [PubMed] [Google Scholar]
- Yang R, Morosetti R, Koeffler HP. Characterization of a second human cyclin A that is highly expressed in testis and in several leukemic cell lines. Cancer Res. 1997;57:913–920. [PubMed] [Google Scholar]
- Zindy F, Lamas E, Chenivesse X, Sobczak J, Wang J, Fesquet D, Henglein B, Brechot C. Cyclin A is required in S phase in normal epithelial cells. Biochem Biophys Res Commun. 1992;182:1144–1154. doi: 10.1016/0006-291x(92)91851-g. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.