Abstract
Background
Early serous carcinomas (SCAs) predominate in the fimbria of BRCA+ women. An entity in non-neoplastic mucosa sharing several properties of SCA - the “p53 signature” - has been described in the distal fallopian tube and proposed as a precursor to SCA. This study compared the prevalence of p53 signatures in both ovarian cortical inclusion cysts (CICs) and fallopian tubes from BRCA+ women and explored their relationship.
Design
All tissues from 75 completely excised ovaries and tubes obtained during prophylactic surgery were studied by conventional microscopy, immunostaining for p53, and in selected cases, ã-H2AX (DNA damage). P53 signatures were defined as 12 or more consecutive p53-positive secretory cell nuclei. Their prevalence in fallopian tubes and CICs was recorded, compared to an existing database of consecutive women without a suspicion of BRCA+ or ovarian cancer, and correlated with the presence of CICs.
Results
Tubal p53 signatures were detected in 29 of 75 cases (38%); 20 of 30 (66%) signatures examined were ã-H2AX-positive. One ovary contained a small γgative p53 signature on the ovarian surface; no p53-positive CICs were identified. Prevalence of BRCA+ p53 tubal signatures was similar in women with unknown BRCA status (38 v 33%). Presence of p53 signatures did not correlate with number of CICs.
Conclusions
p53 signatures were common in the fallopian tubes of BRCA+ women, were not identified in CICs, and did not correlate with the latter. The tubal p53 signature merits serious consideration as an important early event in serous carcinogenesis in BRCA+ women.
Introduction
Approximately 25,000 women in the United States develop ovarian cancer each year, and over one half die of their disease.1 The majority of these tumors are epithelial and have been traditionally presumed to arise from the ovarian surface epithelium (OSE). OSE has been defined as a modified mesothelium with Müllerian characteristics. One theory has held that a mesothelial to Müllerian transformation or “metaplasia” occurs in these cells, leading to Müllerian inclusions in the ovarian cortex. Another proposes that salpingeal epithelial cells are transported to the ovarian surface and give rise to inclusion cysts. In either model, inclusions resulting from these origins occasionally undergo neoplastic transformation, leading to a wide range of epithelial neoplasms in the ovary.2, 3 This model is most strongly supported by tumors that are indisputably ovarian in origin, including mucinous, endometrioid, and low-grade serous neoplasms. Many of these tumors are confined to the ovary when discovered and do not involve the ovarian surface. In contrast to the above tumors, serous carcinomas have a more widespread distribution when detected, including the involvement of peritoneal surfaces. 4 Although some are associated with endometriotic cysts, the usual presentation for these tumors is bilateral ovarian involvement, including the ovarian surface, hence their designation as ovarian surface epithelial tumors.4
Although an origin in the OSE has been proposed for these tumors, little direct evidence has emerged to link high-grade serous carcinomas to this epithelium. This has been due in part to the extensive nature of most of these tumors, precluding assignment of a site of origin. Studies searching for early neoplasia in the ovarian surface epithelium have yielded conflicting results. Earlier studies reported focal p53 immuno-positive inclusion cysts in the ovaries of women with serous carcinomas, postulating an origin from these sites.5 Similar changes were not found in the OSE of women with BRCA mutations.6 Others have described dysplastic changes in the ovarian surface epithelium.2, 7 Other candidate sites of origin, including the peritoneum, have been proposed for cases with minimal ovarian involvement. The fallopian tube has been proposed as a primary site for a minority of serous carcinomas, with an estimated ovarian to fallopian tube serous cancer prevalence of 50:1.
Despite the low reported incidence of tubal serous carcinoma in the literature, the potential role of the fallopian tube in the pathogenesis of ovarian serous carcinomas has not gone unnoticed.8 Recent studies of women with hereditary mutations in the BRCA1 and BRCA2 genes have reported detecting early carcinomas in the fallopian tube in a significant percentage of cases. Recently, Finch et al described a plausible origin in the fallopian tube for five of their seven cases, in the form of a non-invasive (intraepithelial) carcinoma.9 Medeiros et al reported that of five consecutive early carcinomas detected in BRCA+ women, all were in the fallopian tube, and were either in the fimbria or ampullary region, similar to other reports.10, 11 A follow-up study by Kindelberger et al reported that approximately one-half of carcinomas classified as ovarian serous neoplasms were associated with intra-epithelial carcinomas of the fimbria.12
Because p53 mutations occur in over 80% of high-grade pelvic serous carcinomas and because they are associated with accumulation of p53 protein, efforts to identify the early steps in pelvic carcinogenesis have concentrated on identifying p53 immunopositive ovarian cortical inclusions. One report described p53 positive inclusions in a small number of normal-appearing ovaries adjacent to advanced-stage ovarian cancers.5 (Hutson) Another study reported a statistically significant increase in p53 staining of cortical inclusion cysts in prophylactically removed ovaries for increased carcinoma risk versus normal ovaries.13 In contrast, another study systematically examined the ovaries from BRCA1 positive women and failed to identify p53-positive inclusions. 6 (Barakat)
Recently, we described an entity in the distal fallopian tube that shares many attributes with serous carcinomas, including strong p53 positivity, fimbrial location, secretory cell phenotype, evidence of DNA damage by localization of ã-H2AX, p53 mutations, and occasionally, direct continuity with intraepithelial carcinoma (Table 1). This entity, termed the “p53 signature” has been proposed as an early (or latent) precursor to tubal and some pelvic serous cancers.14
Table I.
Evidence supporting the distal fallopian tube as a source of pelvic epithelial malignancy in women with heterozygous BRCA mutations.
|
This study was designed to complement the above studies by expanding the number of fallopian tubes analyzed for p53 signatures in BRCA+ women, determine if p53 signatures were present in the ovaries of the same population, and determine if a relationship existed between the presence of p53 signatures and another possible risk factor for pelvic serous cancer, the cortical (Mullerian) inclusion cyst of the ovary (CIC).
Materials and Methods
Case material
This study was approved by the institutional review board at Brigham and Women’s Hospital. Consecutive cases of prophylactic salpingo-oophorectomies for a hereditary mutation in the BRCA1 or BRCA2 gene accessioned between February 2005 and December 2006 were examined. Controls consisted of a consecutively analyzed series of women with unknown BRCA status who did not have ovarian cancer, as previously reported. 14
Tissue processing and pathologic review
All fallopian tubes and ovaries were entirely submitted for histologic exam. Fallopian tubes were analyzed using a procedure for sectioning and extensively examining the fimbriated end (SEE-FIM protocol), as previously described.10 The fimbria was amputated, sectioned lengthwise and submitted with cross sections of the proximal tube at 2–3 mm intervals. Ovaries were sectioned at 2–3 mm intervals. All ovarian sections were reviewed and scored for the presence of cortical (Mullerian) inclusion cysts (CICs). The number of CICs was totaled for each ovary and a mean number for the entire group was computed.
Immunohistochemistry for the p53 and ã-H2AX antigens
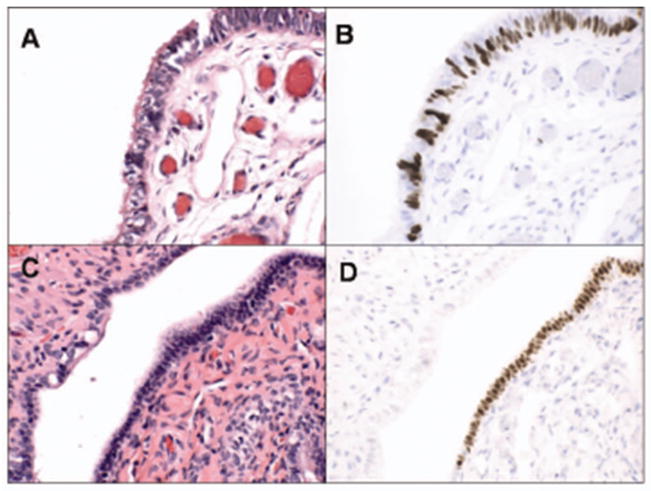
A monoclonal antibody to p53 was used to localize p53 protein (OP43, Oncogene Science, Cambridge, MA) in all sections from the fallopian tubes and ovaries. A positive score required strong immunostaining obscuring nuclear detail. A requirement of at least 12 consecutive p53-positive secretory cell nuclei was arbitrarily imposed to exclude sporadic staining, which is common, presumably physiologic, and usually limited to no more than 2–3 consecutive nuclei in the tubal epithelium. Although a threshold of 12 nuclei was established as a minimum, when detected, a minority of p53 signatures were at this threshold (Figure 1).
Figure 1.
Benign-appearing salpingeal epithelium (A&C) with strong nuclear staining for p53 (p53 signatures, B&D). Interrupted staining is due to the co-existing non-staining ciliated cells (B) in contrast to continuous staining (D) in a homogeneous population of secretory cells.
γ-H2AX, the phosphorylated form of the core histone H2AX, localizes to the vicinity of and is recognized as a marker for double-stranded DNA breakage (Upstate Cell Signaling Solution, Charlottesville, VA; monoclonal JBW301, 1:200). γ-H2AX is phosphorylated by the ATM/ATR kinases at a unique serine residue (S139) in its Cterminus at sites of DNA double strand breaks. 15, 16 The antibody staining was performed on cases with p53 signatures to determine the presence of co-localizing staining patterns. 17, 18 Punctate intranuclear staining was interpreted as a positive signal.
Statistical analysis
The following correlations were made: 1) frequency of p53 signatures in fallopian tubes and ovaries; 2) location in ovary versus tube, 3) location within ovary (CICs vs ovarian surface epithelium) or tube (fimbria versus tubal cross-sections); 4) comparison with women who did not have ovarian cancer or a history thereof; 5) evidence of DNA damage as determined by immunostaining for γ-H2AX; and 6) relationship to cortical inclusion cysts. We compared the mean number of CIC in women with versus without the p53 signature using the Student’s T-test.
Results
Material reviewed
Ovaries and fallopian tubes from 75 women were included in the study. The mean age of the population was 48 years with a range from 35 to 76. All ovaries and fallopian tubes were examined completely. Six cases harbored a malignancy, including two invasive and four intraepithelial carcinomas. A total of 486 and 605 tissue blocks from fallopian tubes and ovaries respectively from BRCA+ women were evaluated by immunohistochemical staining for p53. An average of 6.4 (range 2–10) and 8.2 (range 2–21) tubal and ovarian sections per case respectively were examined.
Frequency and location of p53 signatures in tubes and ovaries
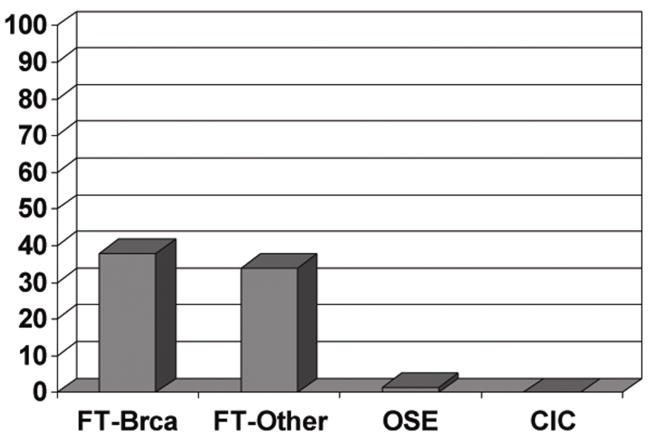
Twenty-nine of the 75 BRCA cases (38%) harbored at least one p53 signature in the fallopian tube (Figure 1). The prevalence of p53 signatures in the BRCA+ women (38%) is similar to that reported in women whose BRCA status is negative or unknown (33%) (Figure 2).14
Figure 2.
Graphic depiction (from left to right) of the distribution of p53 signatures in tubes from BRCA+ women, consecutive women untested for BRCA or negative for ovarian cancer from a prior study (right),14, BRCA+ OSE, and BRCA+ ovarian CICs.
A total of 39 signatures were identified in the fallopian tubes; eight cases contained more than one p53 signature. Examples of p53 signatures are shown in Figures 1 and 3. Of these 39 signatures, 29 occurred in the fimbriated end (74%) and 10 occurred in the proximal tube (26%). The average age of the women in the cases without p53 signatures (49 years) was similar to those with p53 signatures (48 years). Immunostaining of cortical inclusion cysts did not disclose any p53 signatures. One focus of moderate p53 positivity was identified on the ovarian surface in a cuboidal non-ciliated surface epithelium (Figure 2, Figure 3).
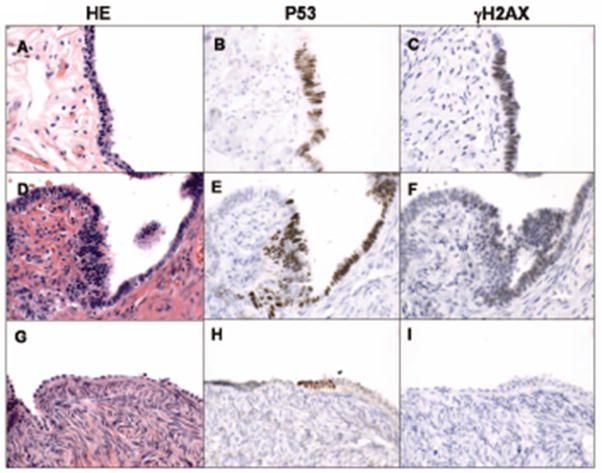
Figure 3.
Correlation of p53 signatures and ã-H2AX staining, a marker for DNA damage. Hematoxylin and eosin stained tube (A, D) and ovary (G), and respective p53 immunostaining (B, E, and H) and localization of ã-H2AX (C, F, and I). Note the lack of γ-H2AX localization in the ovary (I).
Correlation with evidence of DNA damage (γ-H2AX)
Of the 30 signatures from BRCA+ women stained with γ-H2AX, 20 were positive with variable punctate nuclear staining pattern (66%). Examples of positive immunohistochemical results for γ-H2AX localization with p53 are shown in Figure 3. The one p53-positive focus in the OSE was negative for γ-H2AX (Figure 3).
Correlation between p53 signatures and CICs
Overall, the average number of CICs identified per ovary was 6.6 (range 0–60). The differences in means between women with and without signatures was calculated. Among women with the p53 signature, the mean number of CICs was 6.38, in contrast to 6.86 for women without p53 signatures, which was not statistically significant (p=0.87).”
Discussion
There is an emerging shift in the relative frequencies of ovarian and fallopian tube cancer in BRCA+ women that has been generated by studies of women undergoing prophylactic salpingo-oophorectomy.9, 10, 11, 14, 19 In contrast to symptomatic BRCA+ women with ovarian cancer whose tumors have been classified as ovarian in over 90%, early cancers detected in the asymptomatic women have been attributed to the fallopian tubes in up to 100% of studies.19, 20 Supporting this origin has been a recently described entity in the benign tubal mucosa – the p53 signature – that shares several attributes with early serous carcinomas (tubal intraepithelial carcinomas), including strong staining for p53, predominately distal (fimbria) tubal location, involvement of secretory cells, and evidence of DNA damage with frequent p53 mutations.14 However, controversy exists over the magnitude of cases of serous carcinoma deserving assignment to the tube versus ovary. This is the first study to systematically compare the prevalence of this candidate precursor lesion in Müllerian epithelium of the fallopian tube and ovary from the same patient population and to correlate the presence of CICs with p53 signatures in either site.
The frequency of the p53 signature in this series of BRCA+ fallopian tubes was virtually identical to that of consecutively accessioned fallopian tubes from women with neither a strong family history of ovarian cancer nor concurrent ovarian cancer described in an earlier report (Figure 2). 14 The similarity in frequency of p53 signatures in the two groups suggests that the initiation of DNA damage and p53 accumulation is independent of BRCA mutation status and may serve as a generic early event in the pathogenesis of serous carcinomas arising in the distal fallopian tube.
The degree to which CICs play a role in serous carcinogenesis in BRCA+ women has been controversial. There is no question that the Mullerian epithelium in OSE, CICs or endometriosis is directly or indirectly responsible for a range of mucinous, endometrioid and low grade serous tumors. The degree to which BRCA status plays a role in the pathogenesis of these tumors is unclear, inasmuch as cancer risk in these patients is attributed primarily to the high grade serous carcinomas. 21 However, in a recent study, we found that 2 of 7 presumed primary tubal carcinomas in women with BRCA mutations were of the endometrioid type, suggesting that endometrioid carcinogenesis is enhanced, if to a lesser degree. 19 Investigators have focused principally on p53 mutations in CICs as an early event. A prior report has described p53 positive CICs adjacent to ovarian carcinomas. 5 A recent study has also implicated involvement of ovarian inclusions in this pathway by the discovery of p53 positive benign epithelia, often in concert with ovarian cancers.22 However, p53 positive CICs were not documented in another study of a similar population. 6 The findings in this study are consistent with the last study; only one p53 positive focus was detected on the ovarian surface and it was not associated with immunohistochemical evidence of DNA damage. No CICs contained positive staining.
Although we and others have not demonstrated a compelling scenario of p53 mutations in CICs, the role of the OSE in serous carcinogenesis remains an important, if unproven, concept. Animal models of ovarian epithelial carcinogenesis have focused on genotoxic damage imposed by ovulation, in which OSE cells in the proximity of the ovulatory event undergo DNA damage.23 A similar scenario would explain the localization of p53 signatures and serous carcinomas to the distal fallopian tube, where the potential genotoxic impact of ovulation would be highest.14 In this study, we found only one focus of p53-positive epithelium in the ovarian surface, but the number could be underestimated if OSE were lost during tissue processing, as is often the case. Another report described focally intense p53 staining in the OSE of 14% of prophylactic specimens from BRCA+ women in contrast to none of controls, and linked it to expression of BTAK, which is over-expressed in immortalized human OSE cells and a high percentage of ovarian cancers.24, 25 Whether this staining pattern has biologic significance is unclear, inasmuch as we did not see evidence of DNA damage in the one p53-positive focus identified in the OSE in this study, and the p53 mutational status of these foci is unknown. Nevertheless, the experimental evidence supports a role of the OSE in the development of ovarian cancer. While a distal tubal origin can be implicated in nearly one-half of “ovarian” serous carcinomas, the origin of nearly one fourth of these tumors – surface carcinomas without either an obvious intra-parenchymal ovarian source or endosalpingeal involvement – remains unresolved and could be explained by an origin in the OSE.12
The recent studies implicating the fallopian tube in serous carcinogenesis and the presence of a candidate precursor in this site provide a compelling endorsement for further research to resolve the risk factors involved in this model of serous carcinogenesis. The high frequency of p53 signatures is in keeping with most precursor models, in whichp the early events are far more common than cancer outcomes.26 The overall risk of ovarian cancer has been reported as high as 60% in BRCA+ women, and the odds of detecting an early malignancy in prophylactic specimens approximately 6 percent.27 The high frequency of p53 signatures in fallopian tubes from all women, combined with the much higher risk of cancer in BRCA+ women, suggests that a germline BRCA mutation may serve as a promoter, enhancing the risk of transition from a p53 signature to malignancy. However, further studies are needed to determine if p53 signatures represent more than one biologic entity. In a prior study, we found that 8 of 14 p53 signatures contained p53 mutations, an approach that is complicated by the small amount of target tissue and low recovery of DNA. In this study, immunostaining for γ-H2AX was employed as a surrogate marker for DNA damage, a feature characteristic of serous carcinomas, with 66% of cases scoring positive. We are currently investigating the possible role of immunostaining with this marker to predict the presence of a p53 mutation.
The strength of association between the distal fallopian tube and serous carcinogenesis is a compelling endorsement for an epidemiologic analysis of the p53 signature, in women both with and without genetic risk factors for pelvic serous cancer (Table 1). Studies that systematically catalogue the frequency of p53 signatures and divine their epidemiologic correlates in these populations will clarify the role of this entity as a surrogate marker for ovarian cancer risk and shed light on its causation.
Acknowledgments
Supported by the NCI (1 R21 CA124688; C. Crum, P.I. and P50 CA10500 (SPORE; D. Cramer, P.I.), Francis Ward Paine and TSA Pemberton Funds of the Division of Women’s and Perinatal Pathology, Department of Pathology, Brigham and Women’s Hospital, and grants from the Columbia Hospital for Women Research Foundation and the Charlotte Geyer Foundation (C. Crum).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Bibliography
- 1.Quirk JT, Natarajan N, Mettlin CJ. Age-specific ovarian cancer incidence rate patterns in the United States. Gynecol Oncol. 2005;99:248–50. doi: 10.1016/j.ygyno.2005.06.052. [DOI] [PubMed] [Google Scholar]
- 2.Deligdisch L, Gil J, Kerner H, Wu HS, Beck D, Gershoni-Baruch R. Ovarian dysplasia in prophylactic oophorectomy specimens: cytogenetic and morphometric correlations. Cancer. 1999;86:1544–50. [PubMed] [Google Scholar]
- 3.Bell DA, Scully RE. Early de novo ovarian carcinoma. A study of fourteen cases. Cancer. 1994;73:1859– 64. doi: 10.1002/1097-0142(19940401)73:7<1859::aid-cncr2820730714>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Scully RE, Young RH, Clement PB. Tumors of the ovary, maldeveloped gonads, fallopian tube and broad ligament. American Registry of Pathology, AFIP. 1998:51–79. [Google Scholar]
- 5.Hutson R, Ramsdale J, Wells M. p53 protein expression in putative precursor lesions of epithelial ovarian cancer. Histopathology. 1995;27:367–71. doi: 10.1111/j.1365-2559.1995.tb01528.x. [DOI] [PubMed] [Google Scholar]
- 6.Barakat RR, Federici MG, Saigo PE, Robson ME, Offit K, Boyd J. Absence of premalignant histologic, molecular, or cell biologic alterations in prophylactic oophorectomy specimens from BRCA1 heterozygotes. Cancer. 2000;89:383–90. doi: 10.1002/1097-0142(20000715)89:2<383::aid-cncr25>3.0.co;2-t. [DOI] [PubMed] [Google Scholar]
- 7.Piek JM, Van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. Dysplastic changes in prophylactically removed fallopian tubes of women predisposed to developing ovarian rcancer. J Pathol. 2001;195:451–456. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]
- 8.Piek JM, Kenemans P, Verheijen RH. Intraperitoneal serous adenocarcinoma: a critical appraisal of three hypotheses on its cause. Am J Obstet Gynecol. 2004;191:718–32. doi: 10.1016/j.ajog.2004.02.067. [DOI] [PubMed] [Google Scholar]
- 9.Finch A, Shaw P, Rosen B, Murphy J, Narod SA, Colgan RJ. Clinical and pathology findins of prophylactic salpingo-oophorectomies in 159 BRCA 1 and BRCA 2 carriers. Gynecologic Oncology. 2006;100:58–64. doi: 10.1016/j.ygyno.2005.06.065. [DOI] [PubMed] [Google Scholar]
- 10.Medeiros F, Muto MG, Lee Y, Elvin JA, Callahan MJ, Feltmate C, Garber JE, Cramer DW, Crum CP. The Tubal Fimbria is a Preferred Site for Early Adenocarcinoma in Women with Familial Ovarian Cancer Syndrome. Am J Surg Pathol. 2005 doi: 10.1097/01.pas.0000180854.28831.77. in press. [DOI] [PubMed] [Google Scholar]
- 11.Cass I, Holschneider C, Datta N, Barbuto D, Walts AE, Karlan BY. BRCA-Mutation-Associated Fallopian Tube Carcinoma: A Distinct Clinical Phenotype? Obstet Gynecol. 2005;106:1327–1334. doi: 10.1097/01.AOG.0000187892.78392.3f. [DOI] [PubMed] [Google Scholar]
- 12.Kindelberger D, Lee Y, Miron A, et al. Intraepithelial carcinoma of the fimbria and pelvic serous carcinoma: evidence for a causal relationship. Am J Surg Pathol. 2007;31:161–169. doi: 10.1097/01.pas.0000213335.40358.47. [DOI] [PubMed] [Google Scholar]
- 13.Schlosshauer PW, Cohen CJ, Penault-Llorca F, Miranda CR, Bignon YJ, Dauplat J, Deligdisch L. Prophylactic Oophorectomy. Cancer. 2003 Dec;98(12):2599–2606. doi: 10.1002/cncr.11848. [DOI] [PubMed] [Google Scholar]
- 14.Lee Y, Miron A, Drapkin R, Nucci MR, Medeiros F, Saleemuddin A, Garber J, Birch C, Mou H, Gordon RW, Cramer DW, McKeon FD, Crum CP. A candidate precursor to serous carcinoma that originates in the distal fallopian tube. J Pathol. 2007:21126–35. doi: 10.1002/path.2091. [DOI] [PubMed] [Google Scholar]
- 15.Thiriet C, Hayes JJ. Chromatin in need of a fix: phosphorylation of H2AX connects chromatin to DNA repair. Mol Cell. 2005;18:617–22. doi: 10.1016/j.molcel.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Capetillo O, Lee A, Nussenzweig M, Nussenzweig A. H2AX: the histone guardian of the genome. DNA Repair (Amst) 2004;3:959–67. doi: 10.1016/j.dnarep.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 17.Rios-Doria J, Fay A, Velkova A, Monteiro AN. DNA damage response: determining the fate of phosphorylated histone H2AX. Cancer Biol Ther. 2006;5:142–4. doi: 10.4161/cbt.5.2.2530. [DOI] [PubMed] [Google Scholar]
- 18.Motoyama N, Naka K. DNA damage tumor suppressor genes and genomic instability. Current Opinion in Genetics and Development. 2004;14:11–16. doi: 10.1016/j.gde.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 19.Callahan MJ, Crum CP, Medeiros F, Kindelberger DW, Elvin JA, Garber JE, Feltmate CM, Berkowitz RS, Muto MG. Primary fallopian tube malignancies in BRCA-positive women undergoing surgery for ovarian cancer risk reduction. J Clin Oncol. 2007;25:3985–90. doi: 10.1200/JCO.2007.12.2622. [DOI] [PubMed] [Google Scholar]
- 20.Piek JM, Torrenga B, Hermsen B, Verheijen RH, Zweemer RP, Gille JJ, Kenemans P, van Diest PJ, Menko FH. Histopathological characteristics of BRCA1- and BRCA2-associated intraperitoneal cancer: a clinic-based study. Fam Cancer. 2003;2:73–8. doi: 10.1023/a:1025700807451. [DOI] [PubMed] [Google Scholar]
- 21.Werness BA, Ramus SJ, DiCioccio RA, Whittemore AS, Garlinghouse-Jones K, Oakley-Girvan I, Tsukada Y, Harrington P, Gayther SA, Ponder BA, Piver MS. Histopathology, FIGO stage, and BRCA mutation status of ovarian cancers from the Gilda Radner Familial Ovarian Cancer Registry. Int J Gynecol Pathol. 2004 Jan;23(1):29–34. doi: 10.1097/01.pgp.0000101083.35393.cd. [DOI] [PubMed] [Google Scholar]
- 22.Kerner R, Sabo E, Gershoni-Baruch R, Beck D, Ben-Izhak O. Expression of cell cycle regulatory proteins in ovaries prophylactically removed from Jewish Ashkenazi BRCA1 and BRCA2 mutation carriers: correlation with histopathology. Gynecol Oncol. 2005 Nov;99(2):367–75. doi: 10.1016/j.ygyno.2005.06.041. [DOI] [PubMed] [Google Scholar]
- 23.Murdoch WJ, Martinchick JF. Oxidative damage to DNA of ovarian surface epithelial cells affected by ovulation: carcinogenic implication and chemoprevention. Exp Biol Med (Maywood) 2004;229:546–52. doi: 10.1177/153537020422900613. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Z, Singh M, Davidson S, Rosen DG, Yang G, Liu J. Activation of BTAK expression in primary orvarian surface epithelial cells of prophylactic ovaries. Mod Pathol. 2007;20:1078–84. doi: 10.1038/modpathol.3800945. [DOI] [PubMed] [Google Scholar]
- 25.Chung CM, Man C, Jin Y, Jin C, Guan XY, Wang Q, Wan TS, Cheung AL, Tsao SW. Amplification and overexpression of aurora kinase A (AURKA) in immortalized human ovarian epithelial (HOSE) cells. Mol Carcinog. 2005;43:165–74. doi: 10.1002/mc.20098. [DOI] [PubMed] [Google Scholar]
- 26.Mutter GL, Ince TA, Baak JP, Kust GA, Zhou XP, Eng C. Molecular identification of latent precancers in histologically normal endometrium. Cancer Res. 2001;61:4311–4. [PubMed] [Google Scholar]
- 27.Easton DF, Ford D, Bishop DT. Breast and ovarian cancer incidence in BRCA1-mutation carriers. Breast Cancer Linkage Consortium. Am J Hum Genet. 1995;56:265–71. [PMC free article] [PubMed] [Google Scholar]
- 28.Piek JM, van Diest PJ, Zweemer RP, Jansen JW, Poort-Keesom RJ, Menko FH, Gille JJ, Jongsma AP, Pals G, Kenemans P, Verheijen RH. Dysplastic changes in prophylactically removed Fallopian tubes of women predisposed to developing ovarian cancer. J Pathol. 2001;195:451–6. doi: 10.1002/path.1000. [DOI] [PubMed] [Google Scholar]