Abstract
Background
Whether HIV seroconverters are presenting with lower CD4 counts over the HIV epidemic is controversial. Additional data on whether HIV may have become more virulent on a population level, as measured by post-seroconversion CD4 counts, may provide important insights regarding HIV pathogenesis.
Methods
To determine if the post-seroconversion CD4 counts have changed over calendar time, we evaluated 2,174 HIV seroconverters as part of a large cohort study (1985-2007). Participants were documented HIV seroconverters who were antiretroviral naïve and had a CD4 count within six months of HIV diagnosis. Multiple linear regression models were used to assess trends in initial CD4 counts.
Results
The mean initial CD4 count decreased during the study period: 632 cells/mm3 in 1985-1990, 553 cells/mm3 in 1991-1995, 493 cells/mm3 in 1996-2001, and 514 cells/mm3 in 2002-2007. During the time periods, the percentage of seroconverters with an initial CD4 count of <350 cells/mm3 was 12%, 21%, 26%, and 25%, respectively. In the multiple linear model, the mean CD4 count in 1991-1995 was -65 cells/mm3 (p<0.0001), 1996-2001 was -107 cells/mm3 (p<0.0001), and 2002-2007 was -102 cells/mm3 (p<0.0001) compared to 1985-1990. Similar trends occurred in the CD4 percentage and total lymphocyte count. African-Americans and Whites had similar decreases in initial CD4 counts during the epidemic.
Discussion
A significant decline in initial CD4 counts among U.S. HIV seroconverters occurred during the epidemic. These data provide an important clinical correlate to studies suggesting that HIV may have adapted to the host resulting in a more virulent infection.
Keywords: HIV, CD4 cell counts, seroconverters, virulence, military
Background
CD4 cell counts are the most important clinical marker for immune competence and disease progression among HIV-infected persons [1], and are key determinants of the initiation of highly active antiretroviral therapy (HAART) [2]. Most clinicians expect a several-year window between HIV seroconversion and the need for antiretroviral therapy. However, some reports have suggested that HIV-infected persons are presenting with lower CD4 cell counts in recent years and are experiencing rapid HIV progression requiring the introduction of HAART shortly after diagnosis [3-5]. Some of these data may be confounded, as patients may have been infected with HIV for several years before their actual diagnosis.
Studies examining trends in post-seroconversion CD4 cell counts among documented HIV seroconverters over the HIV epidemic are conflicting. The most recent study examining a cohort of HIV seroconverters from Europe, Australia, and Canada suggested that initial CD4 cell counts have declined from 1985 to 2002 [4, 6]. However, several previous studies found no significant trends in post-seroconversion CD4 cell counts over various times during the HIV epidemic [7-12] or a decreasing trend only among certain HIV-transmission groups [13]. Some studies have had methodological difficulties or short time periods of evaluation [11, 14-17].
Whether HIV patients are presenting with lower CD4 cell counts over the course of the HIV epidemic remains unclear and no recent study has been conducted in a U.S.-based population. If CD4 cell counts are declining, this could suggest that HIV has become more virulent, providing valuable information to clinicians as well as important insights regarding HIV pathogenesis on a population level. We evaluated documented HIV seroconverters who were racially diverse and from various geographic areas within the U.S. to evaluate if the initial CD4 cell counts at diagnosis has changed during the HIV epidemic.
Methods
Study cohort
We evaluated documented HIV-1 seroconverters from 1985-2007 as part of a large cohort study to determine if post-seroconversion CD4 cell counts have changed over calendar time. The Tri-Service AIDS Clinical Consortium (TACC) HIV Natural History Study (NHS) is an ongoing, prospective observational cohort of HIV-infected Department of Defense (DoD) beneficiaries (active duty members, retirees, and dependents) at seven U.S. military medical centers. All active duty U.S. military personnel are confirmed HIV-negative prior to enlistment and undergo routine HIV screening every one to five years. Since the time of study initiation in 1985, the NHS has enrolled over 4,900 participants. HIV participants in the study mainly acquired HIV from sexual transmission; an estimated <1% of our cohort utilized illicit drugs related to military drug policies and testing procedures [18].
Participants in this study were documented HIV seroconverters with a window of four years or less (mean 1.5 years, standard deviation (SD) 0.9 years) between the last HIV negative and first HIV positive dates. HIV positive tests were assessed by ELISA and confirmed by Western Blot. All included participants were antiretroviral naïve and had a CD4 cell count within six months after HIV diagnosis. This sub-study involving 2,174 participants was approved by the governing central institutional review board.
Data collection
Following enrollment, data collected among participants included demographic information (age, gender, and self-reported race/ethnicity); body mass index (BMI); medical histories including medication use; and clinical laboratory studies including white blood cell (WBC) counts, total lymphocyte counts, CD8 cell counts, CD4 cell counts and CD4 cell percentages (flow cytometry); and HIV viral load (Roche, Amplicor). Viral load testing became available for our study cohort in approximately 1996; viral loads measured within 6 months after HIV diagnosis were used when available. All laboratory tests were performed at the participating military medical centers which are accredited by Clinical Laboratory Improvement Amendments (CLIA).
Statistical methods
Statistical analyses included summaries of baseline characteristics of the study population and the immune cell counts (means and standard deviations for continuous variables and percentages for categorical variables). The study period of 1985-2007 was a priori divided into four calendar periods by time of HIV diagnosis: two time periods in the pre-HAART era (1985-1990 and 1991-1995) and two periods in the HAART era (1996-2001 and 2002-2007). Chi-square testing was utilized to compare characteristics between the four calendar periods.
Linear regression models were used to assess trends in initial CD4 cell counts across calendar periods. Models were adjusted for length of seroconverting window, time from positive HIV test to initial CD4 cell count, age, gender, race, BMI, enrollment site, and HIV viral load categorized into standard groupings when available (n=1,267) and categorized as ‘missing’ when not available (n=907); this will be referred to as the fully adjusted model. These same fully adjusted regression models were repeated for each of CD4 percentage, CD8 cell, total lymphocyte, and WBC counts. Predicted means of CD4 cell counts were computed by period using marginal weighting to reflect the actual distribution of adjusting characteristics in this cohort; tests of pairwise differences used the Tukey-Kramer adjustment for multiple comparisons. In addition to adjusting for race in our fully adjusted models, a period by race interaction was added to the fully adjusted model and tested, as well as separate models by race performed. Similarly, we considered a period by enrollment site interaction. All analyses were conducted using SAS (version 9, Cary, NC).
Results
Baseline Characteristics
The study population included 2,174 HIV seroconverters with a mean age of 29 years (SD 7), 96% were male, and race was White/non-Hispanic 44%, African American 45%, and other 11% (Table 1). Thirty-five percent of participants had a seroconverting window of <1 year, 41% had a window of 1-2 years duration, 17% between 2-3 years, and 7% between 3-4 years. Ninety-three percent of the study cohort had a CD4 cell count within three months of HIV diagnosis.
Table 1.
Baseline Clinical and Laboratory Characteristics of HIV-Seroconverters by Calendar Periods, 1985-2007
| Characteristic | All Periods N=2174 |
1985-1990 N=562 |
1991-1995 N=630 |
1996-2001 N=553 |
2002-2007 N=429 |
p-value |
|---|---|---|---|---|---|---|
| Age at HIV diagnosis, years, mean, SD | 28.7 (6.7) | 27.3 (5.9) | 28.6 (6.4) | 29.4 (6.9) | 30.0(7.6) | <0.0001 |
| Sex, female | 92 (4.2%) | 23 (4.1%) | 38 (6.0%) | 20 (3.6%) | 11 (2.6%) | 0.04 |
| Race | <0.0001 | |||||
| White/non-Hispanic | 948 (43.6%) | 286 (50.9%) | 264 (41.9%) | 203 (36.7%) | 195 (45.5%) | |
| African American | 971 (44.7%) | 229 (40.8%) | 298 (47.3%) | 274 (49.6%) | 170 (39.6%) | |
| Other | 255 (11.7%) | 47 (8.4%) | 68 (10.8%) | 76 (13.7%) | 64 (14.9%) | |
| BMIa, kg/m2 | <0.0001 | |||||
| Mean, SD | 25.0 (3.5) | 23.5 (2.6) | 24.7 (3.3) | 25.3 (3.5) | 25.9 (3.9) | |
| Missing | 1048 (48.2%) | 414 (73.7%) | 290 (46.0%) | 177 (32.0%) | 167 (38.9%) | |
| <24.9 | 594 (27.3%) | 111 (19.8%) | 185 (29.4%) | 180 (32.6%) | 118 (27.5%) | |
| 25-30 | 436 (20.1%) | 35 (6.2%) | 137 (21.8%) | 158 (28.6%) | 106 (24.7%) | |
| >30 | 96 (4.4%) | 2 (0.4%) | 18 (2.9%) | 38 (6.9%) | 38 (8.9%) | |
| Seroconverting Window | <0.0001 | |||||
| Mean, SD | 1.45(0.88) | 1.42 (0.77) | 1.62 (0.95) | 1.39 (0.89) | 1.35 (0.84) | |
| <1 year | 769 (35.4%) | 176 (31.3%) | 194 (30.8%) | 225 (40.7%) | 174 (40.6%) | |
| 1 - <2 years | 880 (40.5%) | 264 (47.0%) | 237 (37.6%) | 210 (38.0%) | 169 (39.4%) | |
| 2 - <3 years | 374 (17.2%) | 101 (18.0%) | 131 (20.8%) | 79 (14.3%) | 63 (14.7%) | |
| 3 -4 years | 151 (7.0%) | 21 (3.7%) | 68 (10.8%) | 39 (7.1%) | 23 (5.4%) | |
| Time from HIV diagnosis to first CD4 cell count | <0.0001 | |||||
| Mean, SD | 42.8 (30.2) | 47.9 (32.7) | 44.8 (31.7) | 39.7 (27.9) | 37.5 (25.8) | |
| -30 to 29 days | 799 (36.8%) | 170 (30.3%) | 212 (33.7%) | 226 (40.9%) | 191 (44.5%) | |
| 30-89 days | 1212 (55.8%) | 336 (59.8%) | 358 (56.8%) | 297 (53.7%) | 221 (51.5%) | |
| 90-149 days | 135 (6.2%) | 47 (8.4%) | 50 (7.9%) | 24 (4.3%) | 14 (3.3%) | |
| 150-182 days | 28 (1.3%) | 9 (1.6%) | 10 (1.6%) | 6 (1.1%) | 3 (0.7%) | |
| Baseline Viral Loadb, copies/ml | <0.0001 | |||||
| Mean, SD | 4.3 (0.9) | 4.2 (0.8) | 4.2 (0.8) | 4.3 (0.9) | 4.2 (1.0) | |
| Missing | 907 (41.7%) | 468(83.3%) | 427 (67.8%) | 12 (2.2%) | 0(0%) | |
| ≤1000 | 126 (5.8%) | 10 (1.8%) | 19 (3.0%) | 48 (8.7%) | 49 (11.4%) | |
| 1001-4000 | 128 (5.9%) | 9 (1.6%) | 23 (3.7%) | 57 (10.3%) | 39 (9.1%) | |
| 4001-10000 | 150 (6.9%) | 12 (2.1%) | 27 (4.3%) | 66 (11.9%) | 45 (10.5%) | |
| 10001-50000 | 434 (20.0%) | 37 (6.6%) | 85 (13.5%) | 170 (30.7%) | 142 (33.1%) | |
| 50001-100000 | 207 (9.5%) | 11 (2.0%) | 31 (4.9%) | 99 (17.9%) | 66 (15.4%) | |
| >100000 | 222 (10.2%) | 15 (2.7%) | 18 (2.9%) | 101 (18.3%) | 88 (20.5%) | |
BMI categorized as ≤24.9 kg/m2 as underweight or normal weight at HIV diagnosis, 25-29.9 kg/m2 as overweight, and ≥30 kg/m2 as obese.
HIV viral load became widely available in our study cohort in 1996. Mean viral load is computed among those with value
The baseline characteristics of HIV seroconverters during the four calendar periods are shown in Table 1. Comparisons between baseline characteristics showed a lower percentage of White/non-Hispanics and higher percentage other races in more recent years (p<0.0001) as well as an increasing age at HIV diagnosis (p<0.0001) and BMI (p<0.0001) over time. HIV viral loads were different between the time periods when missing values were included; this was due to missing values for 77% of participants in 1985-1995 compared to 1% of participants from 1996-2007. Excluding missing values, there were no significant differences in the baseline values of HIV viral loads over the study period (X2=13, p=0.60). Finally, there were differences over the four time periods in the length of the seroconverting window (p<0.0001) and the time to initial CD4 cell count (p=0.001); both appeared to be shorter during recent years.
Descriptive Trends and Unadjusted Regression Analyses of CD4 Cell Counts during the HIV Epidemic
Mean initial CD4 cell counts were: 632 cells/mm3 for 1985-1990, 553 cells/mm3 for 1991-1995, 493 cells/mm3 for 1996-2001, and 514 cells/mm3 for 2002-2007 (Table 2). In the unadjusted regression models, compared to HIV seroconverters in 1985-1990, the mean initial CD4 cell count in 1991-1995 was 79 cells/mm3 less (p<0.0001), in 1996-2001 was 140 cells/mm3 less (p<0.0001), and in 2002-2007 was 118 cells/mm3 less (p<0.0001) (Table 3). There were no significant differences in the initial CD4 cell count between the time periods of 1996-2001 and 2002-2007 in the unadjusted model (p=0.52). Similar trends were noted when examining individual years over the 23-year period (results not shown).
Table 2.
Mean Values (Standard Deviations) for Immune-Related Cell Counts per Calendar Period, 1985-2007
| Laboratory Value | All Periods | 1985-1990 | 1991-1995 | 1996-2001 | 2002-2007 |
|---|---|---|---|---|---|
| CD4 Cell Count, cells/mm3 | 551 (250) | 632 (276) | 553 (249) | 493 (214) | 514 (229) |
| CD4 Cell Percentage, % | 28 (9) | 30 (9) | 28 (9) | 27 (9) | 27 (9) |
| CD8 Cell Count, cells/mm3 | 963 (564) | 997 (442) | 1010 (810) | 897 (429) | 935 (399) |
| Total Lymphocyte Count, cells/mm3 |
2013 (792) | 2143 (675) | 2039 (1005) | 1868 (648) | 1926 (567) |
| White Blood Count, cells/ml |
5513 (2027) | 5870 (2059) | 5461 (2344) | 5257 (1815) | 5449 (1560) |
Table 3.
Post-seroconversion CD4 Cell Counts by Calendar Period during the Course of the HIV Epidemic. Unadjusted and Adjusted Linear Regression Models (n=2174)
| Unadjusted Model | Adjusted Modela | |||||
|---|---|---|---|---|---|---|
| Factor | Estimate | Standard Error |
P-value | Estimate | Standard Error |
P-value |
| Seroconverting Period | ||||||
| 1985-1990 | Referent | Referent | ||||
| 1991-1995 | -79.0 | 14.2 | <0.0001 | -65.0 | 14.2 | <0.0001 |
| 1996-2001 | -139.5 | 14.6 | <0.0001 | -106.9 | 20.1 | <0.0001 |
| 2002-2007 | -118.0 | 15.6 | <0.0001 | -101.5 | 20.7 | <0.0001 |
| Seroconverting Window | ||||||
| <1 year | 56.3 | 21.0 | 0.007 | |||
| 1 - <2 years | 35.7 | 20.7 | 0.08 | |||
| 2- <3 years | 8.8 | 22.3 | 0.69 | |||
| 3-4 years | Referent | |||||
| Time from HIV diagnosis to CD4 count |
||||||
| -30 to 29 days | 63.5 | 44.5 | 0.15 | |||
| 30-89 days | 49.3 | 44.1 | 0.26 | |||
| 90-149 days | 12.4 | 47.8 | 0.80 | |||
| 150-182 days | Referent | |||||
| Baseline Viral Load, copies/ml |
||||||
| Missing | 133.0 | 22.6 | <0.0001 | |||
| ≤1000 | 289.9 | 26.0 | <0.0001 | |||
| 1001-4000 | 228.3 | 25.8 | <0.0001 | |||
| 4001-10000 | 142.1 | 24.5 | <0.0001 | |||
| 10001-50000 | 94.7 | 19.2 | <0.0001 | |||
| 50001-100000 | 56.9 | 22.3 | 0.01 | |||
| >100000 | Referent | |||||
| Age per 10 years | -20.5 | 7.8 | 0.008 | |||
| Race | ||||||
| White/non-Hispanic | 46.5 | 16.4 | 0.005 | |||
| African American | 6.8 | 16.4 | 0.68 | |||
| Other | Referent | |||||
| Gender, female | 32.4 | 24.9 | 0.19 | |||
| BMI Categoryb, kg/m2 | ||||||
| Missing | -7.2 | 14.4 | 0.62 | |||
| ≤24.9 | -19.5 | 14.7 | 0.18 | |||
| 25-29.9 | Referent | |||||
| ≥30 | -13.7 | 26.1 | 0.60 | |||
BMI, body mass index;
Adjusted for all variables in the table as well as site of enrollment
BMI categorized as <24.9 kg/m2 as underweight or normal weight at HIV diagnosis, >30 kg/m2 as obese, and 25-30 kg/m2 as overweight
Trends in CD4 cell counts categorized as <200, 200-349, 350-499, and >500 cells/mm3 were also significantly different over the four time periods (X2=65.4, p<0.0001). The percentage of HIV seroconverters with an initial CD4 cell count of <200 cells/mm3 at HIV diagnosis was 2%, 4%, 5%, and 5% from earliest to most recent time periods, respectively. Similarly, a CD4 cell count <350 cells/mm3 was noted among 12%, 21%, 26%, and 25% over the four time periods. CD4 cell counts of >500 cells/mm3 occurred among 65%, 54%, 45%, and 46%, respectively.
Multiple Regression Modeling of CD4 Cell Trends during the HIV Epidemic
Multiple linear regression models were created adjusting for all covariates to examine potential changes in the initial CD4 cell counts during the HIV epidemic (Table 3). In the fully adjusted multiple regression model, the mean initial CD4 cell count of seroconverters in 1991-1995 was 65 cells/mm3 lower (p<0.0001), in 1996-2001 was 107 cells/mm3 lower (p<0.0001), and in 2002-2007 was 102 cells/mm3 lower (p<0.0001) compared to 1985-1990. We also compared the mean CD4 cell counts among seroconverters during 2002-2007 and 1996-2001 and found no difference (p=0.98). Significant predictors of a higher initial CD4 cell count in the adjusted model also included a shorter seroconverting window, lower initial HIV viral load, younger age, and White/non-Hispanic race (Table 3). There were no significant relationships between the initial CD4 cell count and time to first CD4 cell count, gender, or BMI category. In addition, we examined enrollment site specific trends, which were similar for all sites to the overall cohort (results not shown). Fully adjusted multiple regression models were repeated limiting the analysis to only participants with HIV seroconverting windows of ≤2 years with similar results. In addition, we re-analyzed our data excluding participants with CD4 counts measured potentially during their acute seroconverting illness (time from estimated seroconversion to first CD4 count of ≤90 days) and found similar results (not shown).
Descriptive Trends and Regression Analyses of Other Immune Cells
The mean initial CD4 cell percentage declined over the four time periods: 30%, 28%, 27%, and 27% from earliest to most recent period (Table 2). In the unadjusted regression models, compared to 1985-1990, the mean initial CD4 cell percentage was 2% lower in 1991-1995 (p=0.0007), 3% lower in 1996-2001 (p<0.0001), and 3% lower in 2002-2007 (p<0.0001) (Table 4). In the fully adjusted multiple regression model, compared to 1985-1990, the mean initial CD4 percentage had significantly declined in 1991-1995 (-1.5%, p=0.006), in 1996-2001 (-1.7%, p=0.03), and in 2002-2007 (-2.3%, p=0.003). The mean initial CD8 cell count varied over time (Table 2); however, there were no statistically significant differences in the adjusted model comparing values among seroconverters in 1985-1990 with the other three time periods (Table 4).
Table 4.
Unadjusted and Adjusted Linear Regression Models for Post-Seroconversion CD4 Percentage, CD8 Cell Count, Total Lymphocyte Count, and White Blood Cell Count by Calendar Period, 1985-2007.
| Unadjusted Model | Adjusted Modela | |||||
|---|---|---|---|---|---|---|
| Factor (Number Evaluable) | Estimate | Standard Error |
P-value | Estimate | Standard Error |
P- value |
| CD4 Cell Percentage, % | ||||||
| N=1981 | ||||||
| Seroconverting Period | ||||||
| 1985-1990 (428) | Referent | Referent | ||||
| 1991-1995 (574) | -1.9 | 0.6 | 0.0007 | -1.5 | 0.6 | 0.006 |
| 1996-2001 (545) | -2.8 | 0.6 | <0.0001 | -1.7 | 0.7 | 0.03 |
| 2002-2007 (229) | -3.1 | 0.6 | <0.0 001 | -2.3 | 0.8 | 0.003 |
| CD8 Cell Count, cells/mm3 | ||||||
| N=2134 | ||||||
| Seroconverting Period | ||||||
| 1985-1990 (544) | Referent | Referent | ||||
| 1991-1995 (595) | 12.9 | 33.0 | 0.70 | 30.1 | 34.9 | 0.39 |
| 1996-2001 (538) | -99.5 | 33.9 | 0.003 | -70.2 | 49.0 | 0.15 |
| 2002-2007 (229) | -62.1 | 36.2 | 0.09 | -52.8 | 50.3 | 0.29 |
|
Total Lymphocyte Count, cells/mm3 |
||||||
| N=1826 | ||||||
| Seroconverting Period | ||||||
| 1985-1990 (535) | Referent | Referent | ||||
| 1991-1995 (613) | -104.3 | 46.4 | 0.02 | -45.7 | 48.9 | 0.35 |
| 1996-2001 (449) | -274.9 | 50.0 | <0.0001 | -172.2 | 71.2 | 0.02 |
| 2002-2007 (124) | -217.0 | 62.8 | 0.0006 | -239.6 | 78.3 | 0.002 |
|
White Blood Cell Count, cells/ml |
||||||
| N=2058 | ||||||
| Seroconverting Period | ||||||
| 1985-1990 (452) | Referent | Referent | ||||
| 1991-1995 (572) | -409.6 | 118.4 | 0.0006 | -257.9 | 124.1 | 0.04 |
| 1996-2001 (537) | -613.5 | 121.9 | <.0001 | -235.8 | 175.8 | 0.18 |
| 2002-2007 (224) | -421.1 | 138.5 | 0.002 | -114.7 | 186.1 | 0.54 |
Adjusted for length of seroconverting window, time from positive HIV test to CD4 cell count, age, gender, race, body mass index, enrollment site, and HIV viral load. Some data from covariates were missing, as detailed in Table
The initial total lymphocyte count mean value showed an overall decline over the study period (Table 2). In the final adjusted regression model, the mean total lymphocyte count was 46 cells/mm3 less in 1991-1995 (p=0.35), 172 cells/mm3 less in 1996-2001 (p=0.02), and 240 cells/mm3 less in 2002-2007 (p=0.002) compared to 1985-1990 (Table 4). Finally, the mean initial WBC count fluctuated over the study period (Table 2). In the final adjusted model, the WBC count was lower in 1991-1995 compared to 1985-1990 (-258 cells, p=0.04); there were no significant differences in the WBC counts between 1985-1990 and the two most recent time periods.
Models Including Race-Specific Period Effects
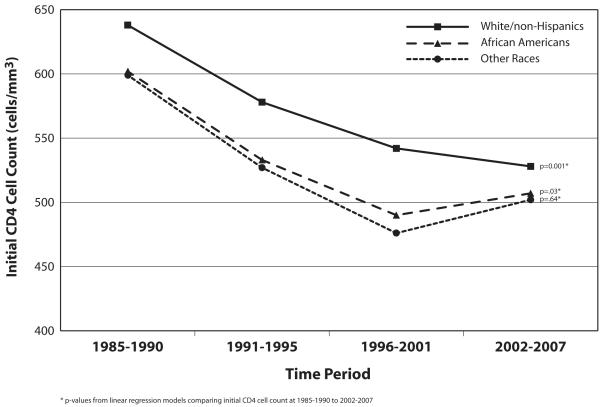
Adjusted linear regression models were repeated including a period by race interaction for CD4 counts. Overall interactions between race and period were not significant (p=0.96). We also created separate models to examine changes in the initial CD4 cell counts by race since immune cell numbers are different among the races (typically lower in African Americans) and since trends in CD4 counts over time may vary by race. The adjusted mean initial CD4 cell counts by race are shown in the Figure. Mean CD4 cell counts among Whites were significantly different across the periods, with the largest difference between 1985-1990 and 2002-2007 (-111 cells, p=0.001). The mean initial CD4 cell counts per calendar period for African Americans were also examined (Figure) and were significantly different between 1985-1990 and 1996-2001 (-111 cells, p=0.001), similar to the decline seen from 1985-1990 to 2002-2007 (-94 cells, p=0.03). There were no significant differences in the initial CD4 counts between 1996-2001 and 2002-2007 among either Whites or African Americans. Among other races (non-White/non-Hispanic or African American), a decrease in initial CD4 cell counts from 1985-1990 to 2002-2007 of 97 cells was noted, but this comparison (p=0.64) nor any of the other period comparisons were statistically significant, likely due to the small sample size of this group.
Fig. 1.
Discussion
Our study was conducted to determine if HIV-infected persons are presenting with lower CD4 cell counts during the HIV epidemic. We and other investigators have observed that patients entering HIV care more recently may be presenting with lower initial CD4 cell counts and requiring antiretroviral therapy initiation earlier in their disease course. Several reports in the literature have highlighted such occurrences [5], however were confounded by the lack of known HIV seroconversion dates. In order to avoid lead time bias due to uncertain dates of HIV infection, we evaluated HIV seroconverters who had documented seroconversion windows.
We found that the initial CD4 cell count among documented U.S. HIV seroconverters significantly declined during the HIV epidemic. Our findings confirm results from the CASCADE collaboration conducted among HIV-infected persons in Europe, Australia, and Canada [4]. Although some prior studies did not find decreases in initial CD4 counts over time, they often suffered from methodological difficulties or short study intervals [7, 8, 11, 14-17]. Several studies examining the rate of HIV progression after diagnosis also have found that more recent seroconverters have a faster progression to low CD4 counts [4, 13].
Possible explanations for the decline in the CD4 count include changes in the host, virus, or environment over time. Given the faster replication cycle of the virus compared to the host and the lack of known significant environmental changes, the most plausible explanation may be that the virus evolved. Since early HIV-specific cytotoxic T lymphocyte (CTL) responses mediated by human leukocyte antigen (HLA) recognition are associated with initial CD4 cell counts [19], one hypothesis is the virus evolved with adaption to the host resulting in poorer CTL responses and early CD4 cell depletion [20, 21]. Other potential changes may include alterations in viral subtype, syncytium-inducing or the CXCR4 phenotype [12, 22, 23]; however, these viral characteristics are not known to have significantly changed among seroconverters during the U.S. epidemic. Studies examining viral isolates for adaption correlating with the decline in the CD4 counts are needed; prior studies have been limited by small sample size and narrow study periods [24].
The initial CD4 cell counts appeared to decline early in the HIV epidemic, with more recent periods showing stabilization. Our findings suggest that if viral evolution was the cause of the CD4 decline early in the epidemic, it may have been inhibited by the introduction of HAART perhaps by increasing transmission of primary resistant HIV strains with impaired virulence or by loss of viral fitness and diversity known to occur with antiretroviral use [25, 26].
Our study noted that the initial CD4 count decreased by 102 cells/mm3 during the HIV epidemic; prior studies have shown that early CD4 counts after seroconversion are important predictors of HIV disease progression, suggesting that our findings may be important to HIV-infected persons and their physicians. In our study, 25% of HIV patients are currently presenting with CD4 cell counts of <350 cells/mm3, the threshold for HAART initiation [2], and 5% with a CD4 count <200 cells/mm3 meeting the definition of AIDS. These data highlight the clinical importance of early HIV diagnosis and reconfirms the need for routine HIV testing.
Our study is the first to examine whether declines in the initial CD4 cell count over time varies by race. Given the racial diversity of our study population, we repeated our analyses stratified by race and noted that African Americans and White/non-Hispanics had similar decreases in initial CD4 cell counts. If the decline in CD4 cells over time is a result of viral evolution, this suggests that the virus adapted to a common immune determinant among both racial groups.
CD4 cell percentages also declined during the epidemic confirming that HIV seroconverters are truly presenting with lower immune counts since this measure is less dependent on the WBC count or the flow cytometry platform utilized. We also evaluated WBC counts over time to assure that alterations in these counts were not the reason for CD4 cell declines in our study; we found that their patterns of change were different from that of the CD4 counts. Lastly, we investigated the flow cytometry platforms at our clinical sites, and found no relationship between timing of changes in methodology and the observed CD4 trends.
Limitations in our study include the male predominant population evaluated. Although HIV-positive persons in the U.S. are predominantly male suggesting that our findings may be generalizable, we could not evaluate changes in the initial CD4 count by gender. Secondly, viral load measures were unavailable early in the epidemic; hence, we were unable to determine if viral loads increased during the same periods as the CD4 declined. Thirdly, though our findings may be attributed to a change in the characteristics of the study population over time, we carefully adjusted for all known factors which may have influenced CD4 counts. Regarding potential biases regarding exclusion for ART initiation before the initial CD4 counts, <1% of participants were excluded due to this criteria. Fourthly, while other studies calculated the rate of CD4 cell decline by year [4, 6], our data showed that the pattern of CD4 cell decline was not linear; hence, data were presented by calendar periods. Finally, our cohort does not have data on transmission risk factors or viral characteristics such as baseline genotypes, replicative capacity, or co-receptor phenotype; of note, for those with subtype data in our network, >90% have remained subtype B suggesting that clade type does not account for the observed CD4 changes.
In summary, the initial CD4 cell counts among U.S. HIV seroconverters have significantly declined during the epidemic. The decrease in the post-seroconversion CD4 cell counts occurred early in the epidemic, with stabilization since the advent of HAART. These data may provide an important clinical correlate to studies suggesting that HIV may have adapted to the host resulting in a more virulent infection.
Acknowledgements
The authors have no commercial or other association that might pose a conflict of interest in this work.
Support for this work was provided by the Infectious Disease Clinical Research Program (IDCRP), Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD, of which the TriService AIDS Clinical Consortium (TACC) is a component. The IDCRP is a Department of Defense tri-service program executed through USUHS and the Henry M. Jackson Foundation for the Advancement of Military Medicine in collaboration with HHS/NIH/NIAID/DCR through Interagency Agreement HU0001-05-2-0011.
References
- 1.Mellors JW, Muñoz A, Giorgi JV, et al. Plasma viral load and CD4+ lymphocytes as prognostic markers of HIV-1 infection. Ann Intern Med. 1997;126:946–54. doi: 10.7326/0003-4819-126-12-199706150-00003. [DOI] [PubMed] [Google Scholar]
- 2.Hammer SM, Eron JJ, Jr, Reiss P, et al. Antiretroviral Treatment of Adult HIV Infection. 2008 Recommendations of the International AIDS Society—USA Panel. JAMA. 2008;300:555–70. doi: 10.1001/jama.300.5.555. [DOI] [PubMed] [Google Scholar]
- 3.Giorgi JV, Detels R. T-cell subset alterations in HIV-infected homosexual men: NIAID Multicenter AIDS Cohort Sutdy. Clin Immunol Immunopathol. 1989;52:10–8. doi: 10.1016/0090-1229(89)90188-8. [DOI] [PubMed] [Google Scholar]
- 4.Dorrucci M, Rezza G, Porter K, Phillips A. Concerted Action on Seroconversion to AIDS and Death in Europe Collaboration. Temporal trends in postseroconversion CD4 cell count and HIV load: the Concerted Action on Seroconversion to AIDS and Death in Europe Collaboration, 1985-2002. J Infect Dis. 2007;195:525–34. doi: 10.1086/510911. [DOI] [PubMed] [Google Scholar]
- 5.Markowitz M, Hohri H, Mehandru S, et al. Infection with multidrug resistant, dual-tropic HIV-1 and rapid progression to AIDS: a case report. Lancet. 2005;365:1031–8. doi: 10.1016/S0140-6736(05)71139-9. [DOI] [PubMed] [Google Scholar]
- 6.Dorrucci M, Phillips AN, Longo B, Rezza G. Italian Seroconversion Study. Changes over time in post-seroconversion CD4 cell counts in the Italian HIV-Seroconversion Study: 1985-2002. AIDS. 2005;19:331–5. [PubMed] [Google Scholar]
- 7.Holmberg SD, Conley LJ, Luby SP, Cohn S, Wong LC, Vlahov D. Recent infection with human immunodeficiency virus and possible rapid loss of CD4 T lymphocytes. J Acquir Immune Defic Syndr Hum Retrovirol. 1995;9:291–6. [PubMed] [Google Scholar]
- 8.Galai N, Lepri AC, Vlahov D, Pezzotti P, Sinicco A, Rezza G. Temporal trends of initial CD4 cell counts following human immunodeficiency virus seroconversion in Italy, 1985-1992. The Human Immunodeficiency Virus Italian Seroconversion Study. Am J Epidemiol. 1996;143:278–82. doi: 10.1093/oxfordjournals.aje.a008739. [DOI] [PubMed] [Google Scholar]
- 9.Keet IP, Veugelers PJ, Koot M, et al. Temporal trends of the natural history of HIV-1 infection following seroconversion between 1984 and 1993. AIDS. 1996;10:1601–2. doi: 10.1097/00002030-199611000-00025. [DOI] [PubMed] [Google Scholar]
- 10.CASCADE Collaboration Differences in CD4 cell counts at seroconversion and decline among 5739 HIV-1-infected individuals with well-estimated dates of seroconversion. JAIDS. 2003;34:76–83. doi: 10.1097/00126334-200309010-00012. [DOI] [PubMed] [Google Scholar]
- 11.Gorham ED, Garland FC, Mayers DL, et al. CD4 lymphocyte counts within 24 months of human immunodeficiency virus seroconversion. Findings in the U.S. Navy and Marine Corps. The Navy Retroviral Working Group. Arch Intern Med. 1993;153:869–76. [PubMed] [Google Scholar]
- 12.Müller V, Ledergerber B, Perrin L, et al. Swiss HIV Cohort Study. Stable virulence levels in the HIV epidemic of Switzerland over two decades. AIDS. 2006;20:889–94. doi: 10.1097/01.aids.0000218553.51908.6b. [DOI] [PubMed] [Google Scholar]
- 13.Vanhems P, Lambert J, Guerra M, Hirschel B, Allard R. Association between the rate of CD4+ T cell decrease and the year of human immuonodeficiency virus (HIV) type 1 seroconversion among persons enrolled in the Swiss HIV Cohort Sutdy. J Infect Dis. 1999;180:1803–8. doi: 10.1086/315110. [DOI] [PubMed] [Google Scholar]
- 14.Lepri A Cozzi, Phillips AN, Pezzotti P, Rezza G. Is the clinical course of HIV infection changing? Study’s censoring strategy may be source of bias. BMJ. 1997;315:1237. doi: 10.1136/bmj.315.7117.1237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinicco A, Fora R, Raiteri R, Sciandra M, Bechis G, Calvo MM, Gioannini P. Is the clinical course of HIV-1 changing? Cohort Study. BMJ. 1997;314:1232–7. doi: 10.1136/bmj.314.7089.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O’Brien TR, Hoover DR, Rosenberg PS, et al. Evaluation in secular trends in CD4+ lymphocyte loss among human immunodeficiency virus type 1 (HIV-1)-infected men with known dates of seroconversion. Am J Epidemiol. 1995;142:636–42. doi: 10.1093/oxfordjournals.aje.a117687. [DOI] [PubMed] [Google Scholar]
- 17.Weiss PJ, Brodine SK, Goforth RR, et al. Initial low CD4 lymphocyte counts in recent human immunodeficiency virus infection and lack of association with identified coinfections. J Infect Dis. 1992;166:1149–53. doi: 10.1093/infdis/166.5.1149. [DOI] [PubMed] [Google Scholar]
- 18.Brodine SK, Starkey MJ, Shaffer RA, et al. Diverse HIV-1 subtypes and clinical, laboratory and behavioral factors in a recently infected US military cohort. AIDS. 2003;17:2521–7. doi: 10.1097/00002030-200311210-00016. [DOI] [PubMed] [Google Scholar]
- 19.Koup RA, Safrit JT, Cao Y, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol. 1994;68:4650–5. doi: 10.1128/jvi.68.7.4650-4655.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vogel TU, Friedrich TC, O’Connor DH, et al. Escape in one of two cytotoxic T-lymphocyte epitopes bound by a high-frequency major histocompatibility complex class I molecule, Mamu-A*02: a paradigm for virus evolution and persistence? J Virol. 2002;76:11623–36. doi: 10.1128/JVI.76.22.11623-11636.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moore CB, John M, James IR, Christiansen FT, Witt CS, Mallal SA. Evidence of HIV-1 adaptation to HLA-restricted immune responses at a population level. Science. 2002;296:1439–43. doi: 10.1126/science.1069660. [DOI] [PubMed] [Google Scholar]
- 22.Kanki PJ, Hamel DJ, Sankalé JL, et al. Human immunodeficiency virus type 1 subtypes differ in disease progression. J Infect Dis. 1999;179:68–73. doi: 10.1086/314557. [DOI] [PubMed] [Google Scholar]
- 23.Kiwanuka N, Laeyendecker O, Robb M, et al. Effect of human immunodeficiency virus type 1 (HIV-1) subtype on disease progression in persons from Rakai, Uganda, with incident HIV-1 infection. J Infect Dis. 2008;197:707–13. doi: 10.1086/527416. [DOI] [PubMed] [Google Scholar]
- 24.Ariën KK, Troyer RM, Gali Y, Colebunders RL, Arts EJ, Vanham G. Replicative fitness of historical and recent HIV-1 isolates suggests HIV-1 attenuation over time. AIDS. 2005;19:1555–64. doi: 10.1097/01.aids.0000185989.16477.91. [DOI] [PubMed] [Google Scholar]
- 25.Troyer RM, Collins KR, Abraha A, et al. Changes in human immunodeficiency virus type 1 fitness and genetic diversity during disease progression. J Virol. 2005;79:9006–18. doi: 10.1128/JVI.79.14.9006-9018.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Karlsson AC, Deeks SG, Barbour JD, et al. Dual pressure from antiretroviral therapy and cell-mediated immune response on the human immunodeficiency virus type 1 protease gene. J Virol. 2003;77:6743–52. doi: 10.1128/JVI.77.12.6743-6752.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]