Abstract
Background
Access to specialty alcoholism treatment in rural environments is limited and new treatment approaches are needed. The objective was to evaluate the efficacy of naltrexone alone and in combination with sertraline among Alaska Natives and other Alaskans living in rural settings. An exploratory aim examined whether the Asn40Asp polymorphism of the μ-opioid receptor gene (OPRMI) predicted response to naltrexone, as had been reported in Caucasians.
Methods
Randomized, controlled trial enrolling 101 Alaskans with alcohol dependence, including 68 American Indians/Alaska Natives. Participants received 16 weeks of either (1) placebo (placebo naltrexone + placebo sertraline), (2) naltrexone monotherapy (50 mg naltrexone + sertraline placebo) and (3) naltrexone + sertraline (100 mg) plus nine sessions of medical management and supportive advice. Primary outcomes included Time to First Heavy Drinking Day and Total Abstinence.
Results
Naltrexone monotherapy demonstrated significantly higher total abstinence (35%) compared with placebo (12%, p = 0027) and longer, but not statistically different, Time to First Heavy Drinking Day (p = 0.093). On secondary measures, naltrexone compared with placebo demonstrated significant improvements in percent days abstinent (p = 0.024) and drinking-related consequences (p = 0.02). Combined sertraline and naltrexone did not differ from naltrexone alone. The pattern of findings was generally similar for the American Indian/Alaska Native sub-sample. Naltrexone treatment response was significant within the group of 75 individuals who were homozygous for OPRM1 Asn40 allele. There was a small number of Asp40 carriers, precluding statistical testing of the effect of this allele on response.
Conclusions
Naltrexone can be used effectively to treat alcoholism in remote and rural communities, with evidence of benefit for American Indians and Alaska Natives. New models of care incorporating pharmacotherapy could reduce important health disparities related to alcoholism.
Keywords: Alcohol Dependence, Naltrexone, Alaska Natives, Minorities, Rural
Alcoholism presents A major public health problem in Alaska and other rural and remote areas, and for American Indians and Alaska Natives (AI/AN) in particular. Forty-two percent of AI/ANs reside in rural settings, as compared with 23% of Caucasians (Manson, 2004), and they experience significant health disparities related to alcoholism. This study addresses the National Institutes of Health mandate that clinical research include women and minorities as participants (National Institutes of Health, 1994). Despite this mandate, a recent review concluded that minorities continue to be under-represented in clinical trials reported in influential journals (Geller et al., 2006).
We conducted this study largely in response to concerns by Alaska Native tribal organizations about the impact alcoholism has upon their communities (Department of Health and Human Services, 2002), their interest in improving alcohol treatment effectiveness, and the under-representation of AI/ANs in alcoholism treatment and related research studies (Beals et al., 2005; Shore et al., 2002). A survey of Alaska Native people in remote Alaskan villages identified alcohol and other drug abuse as their greatest concern (Department of Health and Human Services, 2002; Patch Opinion Survey, 1997). Additionally, the primary collaborator on this study—a large Native health organization—designated alcoholism as one of its top health-related priorities by resolution and in its planning documents.
Both Native and non-Native Alaskans have higher prevalence rates of alcohol dependence and associated consequences such as suicide, homicide, sexual assault, fetal alcohol spectrum disorder, injuries and accidents (Centers for Disease Control and Prevention, 2002; Day and Lanier, 2003; Hill et al., 2004), as compared with the general U.S. population. Fourteen percent of the adult population of Alaska suffers from Abuse or Dependence on alcohol, a rate more than twice the national average for one year point prevalence (Department of Health and Human Services, 2002). The State of Alaska also has above average incidence rates for alcohol-related injury and death (Alaska Behavioral Health Survey, 2002; Alaska Department of Vital Statistics, 2002; Department of Health and Human Services, 2002). Unfortunately, the environment of Alaska makes access to specialty treatment difficult due to cost, transportation and scarcity of providers. Consequently, a pharmacotherapy for alcoholism, such as naltrexone, could greatly aid treatment in rural settings if found effective when used with a low-intensity outpatient approach by general medical providers and para-professionals.
Naltrexone, an opiate antagonist, was approved in 1994 by the Food and Drug Administration for use in the treatment of alcohol dependence based in part on evidence of efficacy from two clinical trials (Volpicelli et al., 1992; O’Malley et al., 1992). Subsequently, naltrexone has been tested in numerous studies (for reviews see Kranzler and Van Kirk, 2001; O’Malley and Froehlich, 2003a; Pettinati et al., 2006), with most studies finding naltrexone to be efficacious, including the largest study conducted to date (Anton et al., 2006). However, these investigations were conducted primarily at academic institutions in urban environments using specialized counseling, and few if any AI/AN participants were enrolled. In addition, the effects of naltrexone have been modest, raising the question of whether combined treatments may enhance effectiveness (Anton et al., 2006; Farren et al., 1997, 2000; Johnson et al., 2000; Kiefer et al., 2003; Mason, 2005; Petrakis et al., 2005).
Therapy with a selective serotonin reuptake inhibitor (SSRI) and naltrexone represents one possible combination approach based on animal and preliminary human studies. Using a limited access preclinical model, Zink and colleagues found greater suppression of drinking by the combination of fluoxetine and naltrexone compared to either drug alone (Zink et al., 1997). Moreover, Le demonstrated in animal models that fluoxetine reduced the ability of stress to reinstate drinking, whereas naltrexone reduced the ability of alcohol to reinstate drinking (Le et al., 1999). These data suggest that adding an SSRI to naltrexone could support abstinence by preventing stress-induced drinking. Consistent with this hypothesis, a small open label study found higher abstinence rates over 10 weeks among patients treated with sertraline and naltrexone compared with a matched sample of patients who received open label naltrexone alone (Farren et al., 1997, 2000). However, a recent study of depressed alcohol-dependent patients did not find an advantage to augmenting sertraline with naltrexone compared with sertraline and naltrexone placebo (Oslin, 2005).
Thus, the primary aims of this study were to: (1) replicate previous findings of reduced relapse to heavy drinking with naltrexone by comparing the efficacy of naltrexone to placebo in a sample living in rural Alaska, many of whom were AI/AN, using a model of care that could be applied in rural health care settings, and (2) determine whether combined therapy with sertraline and naltrexone results in better outcomes than naltrexone alone. We hypothesized that naltrexone monotherapy would result in lower rates of relapse to heavy drinking compared with placebo and that combined therapy would yield higher abstinence rates compared with naltrexone monotherapy. We did not test the effects of sertraline alone because SSRIs have shown limited effect in non-depressed alcohol dependent subjects (Pettinati et al., 2003) and resulted in poorer outcomes in patients with Type B alcoholism (Kranzler et al., 1996; Pettinati et al., 2006).
An exploratory aim examined whether genetic variation in the μ-opioid receptor gene (OPRM1) influenced naltrexone response. Naltrexone, an opioid receptor antagonist, targets μ-opioid receptors, although it also blocks other endogenous opioid receptors. The medication is thought to be efficacious, in part, by blocking the effects of endogeneous opioids that are released in response to alcohol (Gianoulakis et al., 1996; Marinelli et al., 2005; Olive et al., 2001) or alcohol-related cues. Based on studies (Beyer et al., 2004; Bond et al., 1998; Zhang et al., 2005) describing functional significance of a polymorphism of OPRM1, Asn40Asp (Bergen et al., 1997), several groups have investigated whether this polymorphism predicts differential response to naltrexone for the treatment of alcohol dependence (Anton et al., 2008; Gelernter et al., 2007; Oslin et al., 2003).
Two of the studies (Anton et al., 2008; Oslin et al., 2003) found that the alcohol-dependent individuals with at least one copy of the Asp40 allele respond positively to naltrexone compared with individuals homozygous for the Asn40 allele. In the COMBINE study by Anton and colleagues, a gene by treatment interaction was found among participants treated with a less intensive behavioral therapy (Medical Management) but not among those who in addition received Combined Behavioral Intervention. A third study, the Veterans Administration Cooperative Study, showed a significant effect of naltrexone treatment in those who provided DNA, but failed to replicate the findings of a significant interaction between the OPRM1 Asp40 polymorphism and naltrexone response in this sample which included 42 carriers of the Asp40 allele (Gelernter et al., 2007). Given the inconsistent findings in the literature, we sought to explore whether the Asn40Asp polymorphism would alter response to naltrexone in our sample, and to rule out a confounding effect of this polymorphism.
METHODS AND MATERIALS
Design Overview
Using two population samples, AI/ANs (n = 68) and non-AI/AN Alaskans (n = 33), 101 alcohol-dependent individuals from geographically isolated Alaskan communities were randomized within AI/AN status to three parallel treatment groups for 16 weeks (Fig. 1): placebo (placebo naltrexone + placebo sertraline); naltrexone monotherapy (50 mg naltrexone + placebo sertraline), and naltrexone + sertraline (naltrexone 50 mg + sertraline 100 mg). In addition, participants received up to nine (9) concurrent medical management and support sessions. Research assessments were obtained at each of these treatment sessions and at follow-up interviews held 26, 52, and 68 weeks following randomization. This report focuses on the 16-week treatment period, a time point where naltrexone treatment response has been manifested in other studies.
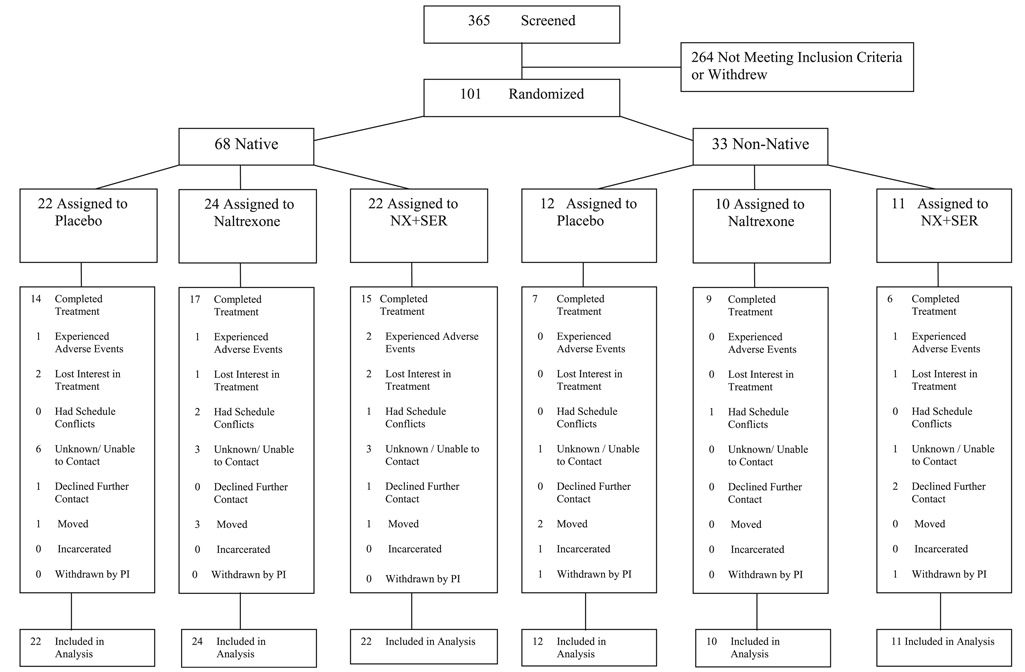
Fig. 1.
Study profile. Note: Multiple reasons for study discontinuation could be given.
Setting and Participants
One hundred and one individuals aged 18 to 65 diagnosed with alcohol dependence were recruited from January 2002 through April 2005 for participation. Five communities took part in the study. Tribal names and locations are not provided in keeping with a convention adopted by several investigators who have studied small, discrete populations including AI/AN communities (Robin et al., 1997; Shore et al., 1987). There are 569 federally recognized Native American tribal entities in the United States distinguished by their heterogeneity in culture, beliefs, practices, and language. Over 200 of these tribal entities are geographically located in Alaska.
Initially, documentation of AI/AN ancestry was required for participation. In October 2003, eligibility was extended to non-Native Alaskans with the result that 68 AI/AN, and 33 non-Native Alaskans were studied in this trial. Race/ethnicity was initially identified by the participant and then further defined by the investigator via questions about the participant having tribal enrollment or a Certificate of Indian Blood.
Individuals were eligible for inclusion if they met criteria for current alcohol dependence according to the Diagnostic and Statistical Manual of Mental Disorders – Fourth Edition (American Psychiatric Association, 2000) and reported drinking more than 14 drinks (women) or 21 drinks (men) per week, with at least two heavy drinking days during a 30 day period within the 90 days prior to baseline. At least four days and no more than 30 days of abstinence were required prior to the first dose of medication. Individuals were offered the option of inpatient or outpatient detoxification if needed but were required to be abstinent from detoxification medications for at least four days prior to randomization. Reasons for exclusion included no reliable contact by telephone, plans to be away for >21 consecutive days (e.g., fishing or travel), legal involvement that might interfere with participation, a current DSM-IV diagnosis for cocaine, opioid, or amphetamine abuse or dependence, current opiate use, psychiatric conditions that required use of psychotropic medications, a condition jeopardizing safety (e.g., suicidality, psychosis), and medical conditions that would contraindícate the use of sertraline or naltrexone or interfere with study participation (e.g., history of unstable or severe cardiovascular disease, serum liver enzymes > 3 times the upper limit of normal, pregnancy, nursing or lack of reliable contraception for women, or use of monoamine oxidase inhibitors or Type 1C anti-arrhythmics). Individuals were excluded who had a Clinical Institute Withdrawal Assessment - Alcohol (CIWA-Ar) (Sullivan et al., 1989) score of >8 prior to randomization, were receiving other substance abuse treatment, or required more intensive treatment.
In preparation for the study, focus groups composed of tribal, community, and human service personnel were held at potential research sites to create and promote culturally sensitive procedures and assessments, to lay groundwork for recruitment and to enlist the support of the community, a practice consistent with other studies among AI/AN communities (Beals et al., 2003; Manson et al., 2004; Mohatt et al., 2004; Roubideaux et al., 2000; Rubin et al., 2002). Participants for the clinical trial were recruited from the community at-large, by referral from health clinics and treatment centers, and through advertisements. Oral consent was obtained prior to screening by phone or in-person, and written informed consent was obtained by a research assistant at the beginning of the first intake appointment. Consent was obtained again by the on-site principal investigator or research nurse prior to randomization. The protocol was approved by the Yale Human Investigation Committee, the Alaska Area and National Indian Health Service Institutional Review Boards, and the NIAAA Institutional Review Board. A Certificate of Confidentiality was obtained from the National Institute on Alcohol Abuse and Alcoholism (NIAAA). A Data and Safety Monitoring Board reviewed safety measures every six months independent of group assignment. Participants were paid up to $160 for completing research assessments over 68 weeks.
Entrance into the study took place following physical examination, laboratory tests including liver function tests, urine drug screen, and administration of the CIWA to evaluate alcohol withdrawal. A research or clinical psychologist with extensive experience with AI/AN people administered the Structured Clinical Interview for DSM-IV (First et al., 1997) and the Family History Assessment Module (FHAM; Rice et al., 1995). Both the SCID and the Schedule for Affective Disorders and Schizophrenia, Lifetime (Endicott and Spitzer, 1978; Spitzer et al., 1989), another semi-structured clinical interview, have been found to be reliable in studies of addictions and other psychiatric diseases among Native Americans (Beals et al., 2002; Boehnlein et al., 1993; Shore et al., 1987). Final interviews were conducted by board certified psychiatrists, experienced working with Native people. A breath alcohol level (BAL) reading of 0.00 was required prior to randomization and BAL was measured before each patient visit. A blood sample for DNA analysis was taken on the day of randomization once final eligibility was determined. All participants were advised about alternative treatment options, including naltrexone, and active referrals were made for those who were ineligible or declined to participate.
Randomization and Interventions
Pharmacotherapy
Naltrexone or matching placebo was titrated over the first week beginning with 12.5 mg for one day, 25 mg for two days, and 50 mg thereafter for 16 weeks, which is the approved dose of naltrexone. Naltrexone supplies were purchased from Mallinckrodt Pharmaceuticals (St. Louis, MI). Participants also took 50 mg of sertraline or matching placebo for two weeks at which time the dose was increased to 100 mg daily (two 50 mg or placebo tablets). Dose reductions were permitted to address issues of tolerability. This dose was selected based on the doses typically used in the treatment of depression (Donohue, 1995; Gregor et al., 1994), the preclinical literature on the effects of sertraline on alcohol consumption (Gill et al., 1988; Higgins et al., 1992) and a pilot study (Farren et al., 2000). Sertraline supplies were donated by Pfizer Pharmaceuticals. At the end of the 16-week treatment period, sertraline was reduced to 50 mg and naltrexone reduced to 25 mg daily with both medications discontinued after four days.
Naltrexone and sertraline study medications were dispensed in separate bottles, which had caps with an electronic microchip that monitored the time and day that the pill bottle was opened as a measure of presumed dosing. These electronic records were used to compute a measure of medication adherence (number of bottle openings/total number of possible days in the study).
Randomization
Study participants were randomized to conditions in blocks of 12 within Native and non-Native groups and within study site (study headquarters, all other sites). All study staff and participants were kept blind to treatment condition other than the pharmacists, who did not interact directly with participants.
Treatment Procedures
Initial screening occurred within the participant’s home community. The first 82 participants were evaluated further and initiated into treatment at the main study site, traveling primarily by plane if they resided within outlying communities. Following a counseling session in which the study nurse administered the initial medication doses, participants were given a two-week supply of study medication and returned to their home communities for all other care. Ultimately, the capacity for completing final eligibility determination and initiating treatment was developed at an additional site in order to reduce the need for client travel and accommodate the larger population base of that town. Following the initial counseling session, participants were seen weekly for four weeks, bi-weekly for one month, and once a month in the final two months.
To enhance generalizability to rural and remote health care systems, pharmacotherapy was provided within a model of counseling that could be delivered by paraprofessionals collaborating with medical providers or by medical staff alone. Native health care organizations employ personnel with similar qualifications in remote clinics. The approach of advice and medical management (MM) was adapted from the Medication Management manual of the COMBINE study (Pettinati et al., 2004b). While a single medical professional provided all aspects of the MM counseling in the COMBINE study (Anton et al., 2006), we divided these functions between two providers: (a) 9 medical providers (e.g., nurses, physician assistants) performed medical monitoring functions (e.g., side effects, dose adjustments); and (b) 13 paraprofessionals or master’s-level clinicians who provided advice and support for medication compliance and abstinence from alcohol. Training of providers included review of manuals, role plays, supervision, and feedback based on review of audiotapes of sessions. Audiotapes of the first two sessions for the first two clients of each therapist and at least 1 tape per client thereafter were rated by supervising clinicians using checklists adapted from the COMBINE study (Anton et al., 2006).
Outcomes and Follow-up
The assessment battery generally paralleled that of the COMBINE study (COMBINE Study Research Group, 2003) with modifications made to accommodate the population and setting. The focus of this report is on measures of drinking behavior and consequences during treatment. The Alcohol Dependence Scale (Skinner and Allen, 1982) was administered at baseline. The Timeline Follow Back (TLFB) method (Sobell and Sobell, 1992) was used to determine frequency and quantity of alcohol consumption at intake and at each appointment and the Drinker’s Inventory of Consequences (DrInC) (Miller et al., 1995) was administered at intake, week 8 and week 16 to measure drinking-related consequences. Craving for alcohol was measured with the Alcohol Urge Questionnaire (Bohn et al., 1995), and depressive symptoms were monitored with the Center for Epidemiologic Studies Depression Scale (CES-D) (Somervell et al., 1993).
5′-Nuclease Genotyping
Genomic DNA was extracted from peripheral blood mononuclear cells. Genotyping for the OPRM1 A118G polymorphism was performed using the TaqMan® 5′ nuclease genotyping assay. The assay probes (Assay ID C890074, rs1799971) were obtained as an Assay-On-Demand from Applied Biosystems (ABI; Foster City, CA). Ten nanograms of genomic DNA was used in each assay and the reaction run according to the manufacturer’s protocol. Genotype was determined by end-point analysis using an ABI 7900 Sequence Detector. The genotyping error rate was 0%. Genotyping completion rate was 95/96. No significant deviation from Hardy-Weinberg equilibrium was found. All genotyping was done at the Laboratory of Neurogenetics, NIAAA and performed blind to treatment assignment and outcome data, on an anonymized dataset.
Statistical Analysis
The primary outcome measures were total abstinence (TA) during the 16-week treatment period and Time to First Heavy Drinking Day (TFHD). Heavy drinking was defined as consuming five or more drinks on a single occasion for men or four or more drinks for women (Anton et al., 2006). Consistent with the approach used in the COMBINE study (Anton et al., 2006), individuals lost to follow-up were assumed to have failed on the day following their last recorded data point. Total abstinence was defined by alcohol abstinence on all days for the 16 week treatment period with those lost to follow-up coded as non-abstinent. Covariates included site, Native classification (Native, non-Native) and percent days abstinent in the 120 days prior to treatment. Categorical data were analyzed using standard chi-square tests, and time-to-event analyses were performed using the Kaplan-Meier method for data display and the Maximum Likelihood method for comparison of the groups with covariates.
Secondary outcomes were compared using mixed general linear models with groups as fixed effects and week as a repeated measure for continuous measures. Logistic regression analyses were used to compare groups on categorical outcomes. Effect size estimates were computed using odds ratios for categorical outcomes, hazard ratios for time-to-event analyses, and Cohen’s d (Cohen, 1988) for continuous measures.
The placebo condition and the combination condition were compared with the naltrexone-only group using planned contrasts, with data from all three groups being used to estimate error variance. The contrast between the placebo condition and the naltrexone group was included to meet the aim of replicating prior naltrexone findings, and the contrast between the combination group and the naltrexone-only group was included to meet the aim of testing whether there is an advantage of combined treatment over naltrexone alone. Covariates included site, Native classification, and the baseline value of the dependent measure.
The intention-to-treat (ITT) analyses are based on the entire sample of Native and non-Native participants. Results are also presented separately for the AI/AN subsample as pre-specified by the protocol. Analyses of treatment efficacy were repeated using the models specified above within a subset of individuals who were homozygous for the Asn40 allele. The small number of Asp40 carriers precluded statistical evaluation of an effect of this allele on treatment response.
Based on a review of the literature, a moderate effect size was identified (h = 0.45) for the comparison of naltrexone with placebo on relapse to heavy drinking. A moderate effect size of the same magnitude also was specified for the combination of naltrexone and sertraline to naltrexone only on abstinence. With this estimated effect size, a two-sided alpha of 0.05 was selected together with power of 0.80 lead to an initially targeted total sample size of 198.
The statistical packages SAS Version 9.1 for Windows (SAS Institute Incorporated, Cary, NC) was used to perform all data analyses.
RESULTS
Participant Characteristics
Figure 1 shows the flow of participants throughout the study. Three hundred sixty-five were initially screened and 101 were assigned to treatment conditions. Twenty-six percent of the sample was recruited from a small town that was the site of the study’s headquarters, 68% from a larger town, and 6% from villages or very small town settings. As shown in Table 1, the three treatment groups were comparable for all baseline demographic and clinical characteristics. Sixty-seven percent (n = 68) of the sample was of AI/AN descent consisting of 12 tribal groups; 65 of 68 were of Alaska Native ancestry. The non-Native participants (n = 33) were primarily White (n = 30, 10 per treatment condition), two were African American (1 placebo, 1 combination group), and 1 was Hispanic (placebo group).
Table 1.
Baseline Characteristics
| Placebo (n = 34) | NX (n = 34) | NX + SER (n = 33) | Overall | p-values | |
|---|---|---|---|---|---|
| ITT sample | |||||
| Demographics | |||||
| Age (yr, mean, SD) | 38.8 (10.41) | 42.0(10.67) | 39.1 (8.03) | 40.0 (9.80) | 0.34 |
| Sex (% male) | 62 | 65 | 73 | 66 | 0.62 |
| Native (%) | 65 | 71 | 67 | 67 | 0.87 |
| Education (% ≤high school) | 68 | 56 | 58 | 60 | 0.56 |
| Married or common law (%) | 47 | 35 | 27 | 37 | 0.24 |
| Full or part time employment (%) | 62 | 59 | 58 | 59 | 0.94 |
| Family history of alcoholism (%) | 70 | 88 | 87 | 81 | 0.10 |
| Smoker (%) | 59 | 62 | 58 | 59 | 0.94 |
| Clinical characteristics | |||||
| Percentage of days abstinent (mean, SD)a | 43.6 (25.50) | 40.6 (26.86) | 43.2 (25.29) | 42.5 (25.67) | 0.88 |
| Percentage of days heavy drinking (mean, SD)a | 54.6 (25.01) | 53.5 (26.61) | 55.5 (25.62) | 54.5 (25.51) | 0.95 |
| Drinks per drinking day (mean, SD)a | 17.6 (12.70) | 16.5 (8.44) | 19.6(13.10) | 17.9(11.55) | 0.54 |
| Early onset of alcohol dependence (%) | 50 | 47 | 45 | 48 | 0.93 |
| Alcohol dependence scale (mean, SD) | 18.0 (6.93) | 21.7(8.62) | 18.8 (8.27) | 19.5(8.04) | 0.14 |
| DrInC total score (mean, SD) | 51.0 (22.46) | 53.0(19.90) | 54.4 (22.04) | 52.8(21.32) | 0.81 |
| Alcohol urge questionnaire (mean, SD) | 11.9(7.77) | 10.2 (5.95) | 9.2 (4.72) | 10.5(6.33) | 0.23 |
| CES depression score (mean, SD) | 16.4 (10.67) | 18.2(10.17) | 16.7(11.05) | 17.1 (10.55) | 0.76 |
| Gamma-glutamyltransferase (mean, SD) | 59.1 (64.78) | 66.6(74.17) | 75.8 (88.64) | 67.1 (75.86) | 0.68 |
| History of inpatient treatment (%) | 15 | 24 | 24 | 21 | 0.56 |
| History of emergency treatment (%) | 26 | 26 | 27 | 27 | 1.00 |
| History of outpatient treatment (%) | 38 | 47 | 36 | 41 | 0.63 |
| Past AA participation (%) | 24 | 32 | 21 | 26 | 0.54 |
| (n = 22) | (n = 24) | (n = 22) | |||
| Native subsample | |||||
| Demographics | |||||
| Age (yr, mean, SD) | 35.6 (8.86) | 39.4 (9.92) | 40.1 (7.39) | 38.4 (8.90) | 0.20 |
| Sex (% male) | 55 | 58 | 68 | 60 | 0.63 |
| Education (% ≤high school) | 77 | 67 | 64 | 69 | 0.59 |
| Married or common-law marriage (%) | 50 | 38 | 27 | 38 | 0.30 |
| Full or part-time employment (%) | 55 | 50 | 50 | 51 | 0.94 |
| Family history of alcoholism (%) | 77 | 96 | 95 | 89 | 0.08 |
| Smoker (%) | 55 | 71 | 50 | 59 | 0.32 |
| Clinical Characteristics | |||||
| Percentage of days abstinence (mean, SD)a | 52.5 (24.45) | 43.0(23.15) | 50.9 (22.30) | 48.7 (23.34) | 0.34 |
| Percentage of days heavy drinking (mean, SD)a | 46.4 (24.64) | 50.2 (22.49) | 48.0 (22.72) | 48.2 (22.98) | 0.85 |
| Drinks per Drinking Day (Mean, SD)a | 18.0 (14.90) | 16.5 (8.65) | 22.0 (15.25) | 18.8(13.17) | 0.36 |
| Alcohol dependence scale (mean, SD) | 18.3 (6.17) | 22.7 (8.23) | 21.2 (7.92) | 20.8 (7.62) | 0.15 |
| Early onset of alcohol dependence (%) | 55 | 50 | 50 | 48 | 0.94 |
| DrInC total score (mean, SD) | 50.5 (24.70) | 55.2(19.24) | 54.7 (25.44) | 53.5 (22.93) | 0.76 |
| Alcohol urge questionnaire (mean, SD) | 11.9 (8.89) | 10.9 (6.54) | 9.3 (4.89) | 10.7(6.96) | 0.46 |
| CES depression score (mean, SD) | 15.9 (9.94) | 18.5(10.37) | 18.0(11.94) | 17.5(10.67) | 0.70 |
| Gamma-glutamyltransferase (mean, SD) | 48.5 (42.21) | 60.8 (69.84) | 93.5 (104.65) | 67.3 (77.43) | 0.15 |
| History of inpatient treatment (%) | 14 | 25 | 32 | 24 | 0.36 |
| History of emergency treatment (%) | 23 | 21 | 27 | 24 | 0.87 |
| History of outpatient treatment (%) | 32 | 46 | 41 | 40 | 0.62 |
| Past AA participation (%) | 32 | 33 | 32 | 32 | 0.99 |
During 120 days of baseline.
Treatment Adherence
The groups did not differ significantly on measures of medication compliance or the number of treatment sessions attended (all p-values >0.20). Mean adherence to naltrexone was 60%, 67% and 59% for the placebo, naltrexone monotherapy group and the combination group, respectively. Mean adherence to sertraline in the three groups was similar to mean adherence to naltrexone: 61% in the placebo group, 66% in the naltrexone monotherapy group, and 58% in the combination group. Overall adherence was highly correlated for the two medications (r = 0.90, p < 0.0001) as was the time to medication discontinuation for those who did not complete treatment (r = 0.87, p < 0.0001). The mean number of treatment sessions attended was 5 (placebo), 6 (naltrexone), and 5 (naltrexone + sertraline). The percentage of subjects who provided complete drinking timeline data was comparable across groups (91%, 91%, and 88% for the placebo, naltrexone monotherapy, and combination groups respectively).
Biological Verification of Alcohol Drinking
Percent change in gamma-glutamyltransferase (GGT) from baseline to the last data point was analyzed as a verification of self-report abstinence for those with repeat GGT levels. Participants (n = 25) who reported total abstinence for 16 weeks had a 30% decrease in GGT, while those who reported any drinking (n = 68) had a reduction of only 3% (p = 0.054, effect size = 0.50), providing validation of self-reported abstinence.
Adverse Events
Significant differences were found for nausea (p = 0.03), sleepiness (p = 0.001), and dizziness (p = 0.04), with the highest rates observed in the naltrexone + sertraline group (Table 2). The rate of serious adverse events during treatment was comparable among groups, with overnight hospitalization for alcohol treatment the most common event.
Table 2.
Any Adverse Events by Treatment Group
| Placebo |
Naltrexone |
NX + SER |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Percent | n | Percent | n | Percent | DF | Chi-square | p-value | |
| Adverse events | |||||||||
| Nausea | 16 | 47 | 20 | 59 | 25 | 78 | 2 | 6.790 | 0.034 |
| Dry mouth | 16 | 47 | 16 | 47 | 23 | 72 | 2 | 5.414 | 0.067 |
| Sleepiness | 9 | 26 | 12 | 35 | 22 | 69 | 2 | 13.271 | 0.001 |
| Dizziness | 7 | 21 | 8 | 24 | 15 | 47 | 2 | 6.451 | 0.040 |
| Serious adverse eventsa | |||||||||
| Alcohol related | 3 | 9 | 2 | 6 | 0 | 0 | 2 | 2.865 | 0.239 |
| Other | 1 | 3 | 2 | 6 | 4 | 12 | 2 | 2.275 | 0.321 |
| Any | 4 | 12 | 4 | 12 | 4 | 12 | 2 | 0.003 | 0.999 |
For individual adverse events that were not serious, these numbers and percent refer to individuals reporting a particular event at any level of severity at any point during treatment.
Number (%) of individuals who reported one or more serious adverse events.
Primary Efficacy Outcomes
Primary Outcome: ITT Sample
Table 3 presents the estimated effects, statistics, and p-values for the comparisons between placebo and naltrexone only, and between the combination condition and naltrexone only for the two primary outcomes, TFHD and TA, and for secondary outcomes. These data are presented first for the ITT sample (n = 101) and then for the AI/AN subsample (n = 67).
Table 3.
Treatment Outcomes
| NX versus PL |
NX versus NX + Ser |
||||||
|---|---|---|---|---|---|---|---|
| Placebo (n = 34) |
NX (n = 34) |
NX + SER (n = 33) |
p-value | OR/HR/ES (LL/UL) | p-value | OR/HR/ES (LL/UL) | |
| ITT sample | |||||||
| Primary outcomes | |||||||
| Total abstinence (n, %) | 4(12) | 12(35) | 10 (30) | 0.027 | 4.37 (1.19/16.11)a | 0.634 | 1.30 (0.45/3.77)a |
| Relapse to a heavy | 28 (82) | 22 (65) | 22 (67) | 0.093 | 1.64 (0.92/2.90)b | 0.874 | 0.95 (0.53/1.73)b |
| drinking day (n, %) | |||||||
| Secondary outcomes | |||||||
| Percent days abstinent (Mean, SD) |
85.7 (2.96) | 94.8 (3.09) | 96.3 (3.08) | 0.024 | 0.53(0.03/1.02)c | 0.710 | 0.09 (−0.40/0.58)c |
| Percent days heavy drinking (Mean, SD) |
11.2 (2.92) | 3.7 (3.02) | 3.0 (3.04) | 0.059 | 0.44 (−0.05/0.93)c | 0.859 | 0.04 (−0.45/0.53)c |
| Drinks per drinking day (Mean, SD) |
3.9 (0.82) | 3.6 (0.85) | 1.9 (0.85) | 0.728 | 0.08 (−0.40/0.56)c | 0.131 | 0.35 (−0.14/0.85)c |
| GGT change from baseline (Mean, SD) |
19.2 (13.46) | 23.2 (13.39) | 43.2(13.61) | 0.964 | 0.05 (−0.44/0.55)c | 0.427 | 0.26 (−0.23/0.76)c |
| DrInC (n, % reporting any problems) |
22 (76) | 14(45) | 14 (47) | 0.020 | 0.264 (0.09/0.81)a | 0.948 | 0.97 (0.35/2.68)a |
| Alcohol Urge Questionnaire (Mean, SD) |
12.8 (0.96) | 12.3 (0.97) | 13.5(1.03) | 0.671 | 0.11 (−0.42/0.64)c | 0.353 | 0.23 (−0.30/0.77)c |
| CES-Depression (Mean, SD) | 10.1 (0.93) | 10.9 (0.94) | 9.8 (0.97) | 0.520 | 0.16 (−0.37/0.69)c | 0.383 | 0.22 (−0.32/0.76)c |
| (n = 22) | (n = 24) | (n = 22) | |||||
| Native subsample | |||||||
| Primary outcomes | |||||||
| Total abstinence (n, %) | 4(18) | 8(33) | 8(36) | 0.224 | 2.42 (0.58/10.11)a | 0.880 | 0.91 (0.26/3.20)a |
| Relapse to a heavy drinking day (n, %) |
17(77) | 16(67) | 14 (64) | 0.224 | 1.56 (0.76/3.20)b | 0.972 | 0.99 (0.48/2.04)b |
| Secondary outcomes | |||||||
| Percent days abstinent | 88.8 (3.03) | 96.6 (2.90) | 96.9 (3.02) | 0.060 | 0.56 (−0.05/1.16)c | 0.930 | 0.03 (−0.57/0.62)c |
| Percent days heavy drinking | 8.5 (2.91) | 2.8 (2.76) | 0.6 (2.90) | 0.142 | 0.43 (−0.17/1.03)c | 0.578 | 0.16 (−0.43/0.75)c |
| Drinks per drinking day | 4.2 (0.93) | 4.0 (0.89) | 1.9 (0.92) | 0.830 | 0.06 (−0.53/0.65)c | 0.095 | 0.50 (−0.10/1.10)c |
| GGT change from baseline | 21.8(18.81) | 17.2 (16.98) | 61.0(18.52) | 0.975 | 0.06 (−0.55/0.66)c | 0.141 | 0.54 (−0.08/1.16)c |
| DrInC (n, % reporting any problems) | 13(72) | 8(38) | 8(42) | 0.026 | 0.20 (0.05/0.83)a | 0.715 | 0.785 (0.21/2.88)a |
| Alcohol Urge Questionnaire | 11.2 (1.25) | 12.6(1.16) | 13.4(1.33) | 0.405 | 0.27 (−0.39/0.93)c | 0.637 | 0.15 (−0.50/0.81)c |
| CES-Depression | 10.2 (1.21) | 11.8 (1.12) | 10.2 (1.21) | 0.295 | 0.34 (−0.32/0.99)c | 0.310 | 0.33 (−0.32/0.98)c |
OR, odds ratio; HR, hazard ratio; ES, effect size estimates are reported as Cohen’s d-values; LL/UL, lower limit/upper limit.
OR.
HR.
ES.
On TFHD, the placebo group showed a more rapid return to heavy drinking compared with the naltrexone-only group, although this difference did not reach statistical significance (p = 0.09; Fig. 2A). Naltrexone + sertraline was similar to naltrexone only (p = 0.87).
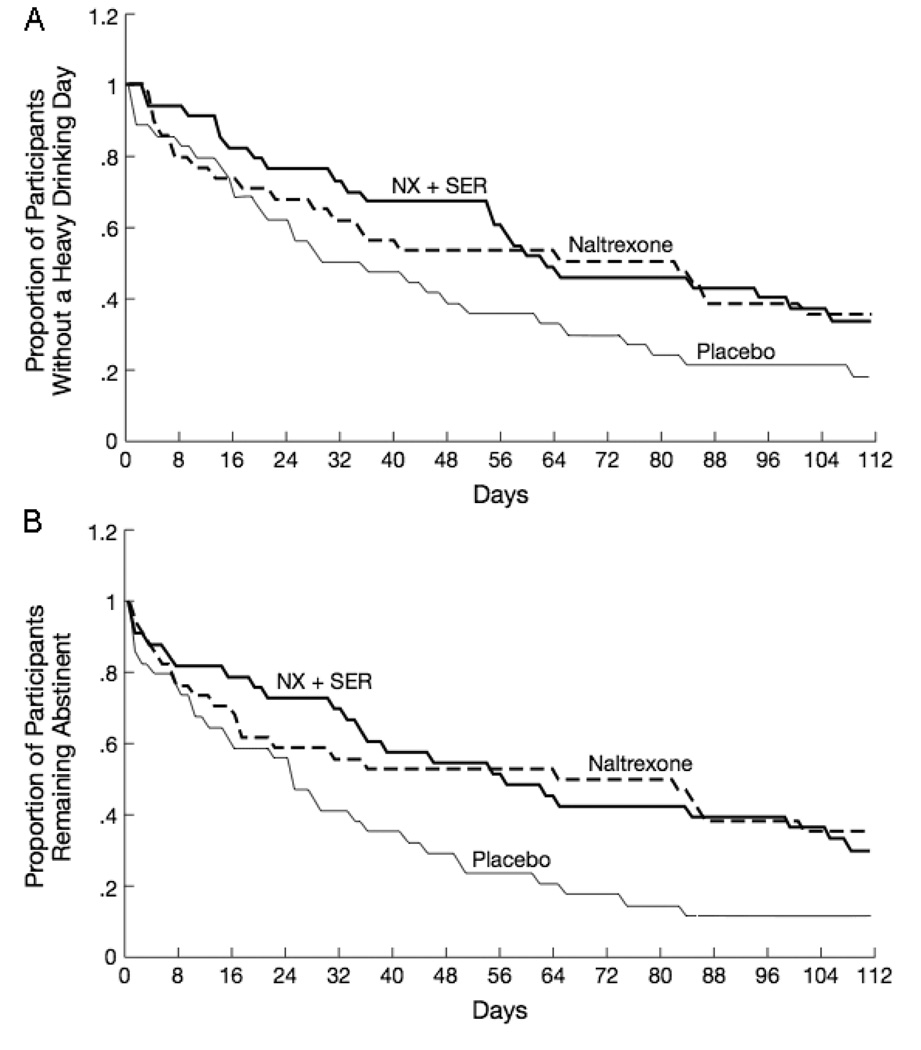
Fig. 2.
Time to first heavy drinking day (A) and time to first day of any drinking (B) by medication condition.
On TA, the naltrexone-only group had a significantly higher rate of abstinence (35%) than the placebo group (12%) (p = 0.03). Total abstinence for the combination group (30%) was comparable to the naltrexone-only group. As follow-up, we conducted a secondary analysis of time to first day of drinking in the ITT sample (Fig. 2B). Naltrexone lengthened the time to the first day of drinking (p = 0.015). The onset of the first drinking day appeared to be delayed up through the first month of treatment in the combination group (see Fig. 2B), however, this apparent advantage disappeared and the combination group did not differ significantly from the naltrexone-only group in the survival analysis (p = 0.96). For TA, the number needed to treat is 4.25 for naltrexone compared to placebo and 20 for the combination compared with naltrexone only.
Secondary Outcomes: ITT Sample
Other measures of drinking behavior were consistent with the preceding analyses (see Table 3). The placebo group had significantly lower percent days abstinent (mean = 85.7, SD = 2.96) compared with the naltrexone-only group (mean = 94.8, SD = 3.09) which did not differ from the combination group (mean = 96.3, SD = 3.08). Although not statistically different, the percentage of heavy drinking days was lower for the naltrexone-only group (mean = 3.7 days, SD = 3.02) compared to the placebo group (mean = 11.2, SD = 2.92) and not different from the combination group (mean = 3.0, SD = 3.04). The number of drinks consumed per drinking day did not differ by treatment status.
A larger percentage of the placebo group reported experiencing one or more alcohol-related consequences (22 or 76%) on the DrInC compared with the naltrexone-only group (14 or 45%) (p = 0.020). The combination group was similar (14 or 47%) to the naltrexone group (p = 0.948). No statistically significant differences were observed on Alcohol Urge Questionnaire scores, CES-D scores or GGT.
Efficacy in Native Subsample
The general pattern of results for the Native subsample was consistent with the ITT results, although the strength of the findings was reduced in part due to the smaller sample size and the slightly better response to placebo among this subsample. Twice as many subjects in the naltrexone-only condition, compared with the placebo condition, were totally abstinent (8 vs. 4), but this was not statistically significant. During treatment, the percentage of days abstinent was 96.6% (SD = 2.90) for the naltrexone-only condition compared with 88.8% (SD = 3.03) for the placebo condition. The percentage of the sample reporting drinking-related consequences was significantly lower in the naltrexone group (38%) compared with the placebo group (72%; p = 0.026). Similar to the ITT analysis, the addition of sertraline did not result in additional improvements compared with naltrexone alone on any outcome measures.
OPRM1 Analyses
Usable DNA was obtained from 92 participants (59 of whom were AI/AN). The frequency of the Asp40 allele was 0.10 in the ITT sample and 0.11 in the AI/AN subsample. Because only 17 of the 92 genotyped participants had one or more copies of the Asp40 allele (placebo = 9, naltrexone only = 3; naltrexone + sertraline = 5), we restricted our statistical analyses of treatment response to participants who were homozygous for the Asn40 allele (placebo = 23; naltrexone only = 25; naltrexone + sertraline = 27).
The baseline characteristics of the 75 Asn40/Asn40 homozygotes did not differ between the three treatment groups (all p values >0.10). When the effect of treatments were compared within the Asn40 homozygous sub-sample, the pattern of results in this OPRM1 genotype-controlled subsample was similar to the results in the total sample, indicating that treatment efficacy was not dependent on presence of the Asp40 allele, which had been associated with enhanced naltrexone treatment response in some studies. Specifically there was a statistically significant advantage of naltrexone over placebo but no evidence of additional benefit from the addition of sertraline to naltrexone on TA (NX vs. PL p = 0.04, NX vs. NX-SER p = 0.56) and the percentage who reported a drinking-related problem during treatment (NX vs. PL p = 0.04, NX vs. NX + SER p = 0.85). Similar to the ITT analysis, TFHD was longer, although not significantly greater for the naltrexone-only group compared to placebo (NX vs. PL p = 0.14, NX vs. NX + SER p = 0.84). Figure 3 presents these outcome measures by treatment group within the Asn40/Asn40 homozygotes, and for the total group of individuals with DNA results.
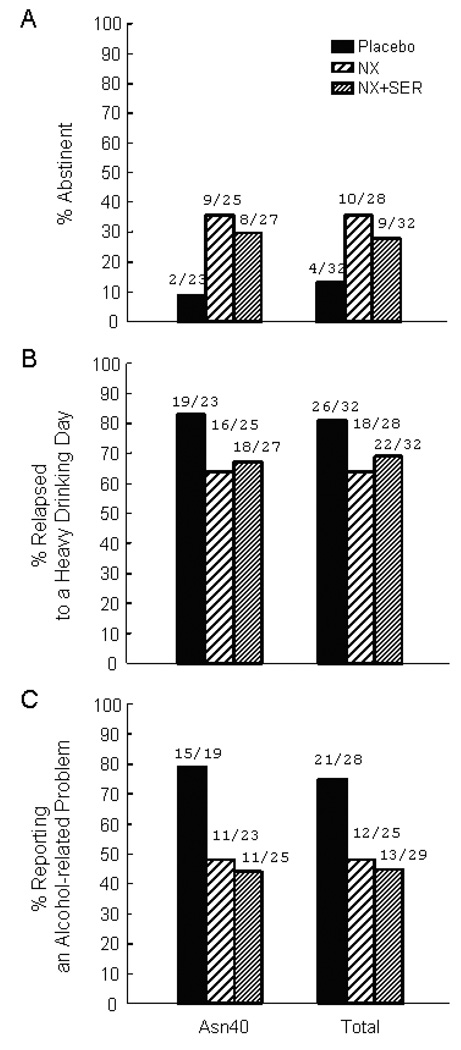
Fig. 3.
Percentage of individuals who were totally abstinent (A), relapsed to a heavy drinking day (B) or reported alcohol-related problems on the DriInC (C) during the 16-week treatment period by treatment condition within participants who were homozygous for the Asn40 allele (n = 75), carriers of the Asp40 allele (N = 17), and for the total sample of genotyped participants (n = 92).
When analyses were restricted to the subsample of Asn40/Asn40 homozygotes who were also AI/ANs, the pattern of findings was similar. However, in this smaller sample only the effect of naltrexone to reduce alcohol-related problems was significant.
DISCUSSION
Naltrexone was shown to be an efficacious treatment for alcohol dependence in geographically isolated and rural Alaskans, including those of AI/AN descent. This finding replicates and extends prior research in Caucasian, African American and Hispanic populations conducted in metropolitan academic settings, and is of relevance to health care practitioners whose patients have little access to specialty care for alcoholism. Approximately 20% of the United States population resides in rural areas; notably many of these areas have very high rates of alcohol abuse and dependence (Office of Applied Statistics, Substance Abuse and Mental Health Services, 2005; U.S. Census Bureau, 2000). The current study demonstrated a means to reach these underserved populations. Specifically, naltrexone provided in conjunction with counseling that can be used by non-specialists substantially improved abstinence rates and reduced drinking-related consequences with more modest effects on measures of heavy drinking. The addition of sertraline to naltrexone did not improve upon these outcomes.
Based on prior research, we predicted that naltrexone would reduce the risk of heavy drinking. Although this effect was not statistically significant (p = 0.09), the effect size (Cohen’s d = 0.44) was comparable to prior positive results in larger samples. In our study, however, this effect was not due to naltrexone reducing the risk of heavy drinkers following a lapse in abstinence given that all non-abstinent subjects who received naltrexone only met criteria for heavy drinking. Instead, naltrexone substantially improved total abstinence rates, nearly tripling the number of participants who were able to remain abstinent. Although a less common outcome of naltrexone, several studies found beneficial effects on total abstinence or time to first drink in patients who were medication compliant (Volpicelli et al., 1997), abstinent prior to randomization (O’Malley et al., 2007; Kranzler et al., 2004) or receiving an intervention supporting abstinence (O’Malley et al., 1992). The value of naltrexone may also be most apparent in studies that utilize less intensive behavioral therapies that can be used by non-specialists (Anton et al., 2006; Garbutt et al., 2005; Kranzler et al., 2004; O’Malley et al., 2003b).
The strength of the naltrexone effect on abstinence may arise from several factors. Compared with other trials, the sample had a higher rate of familial alcoholism and greater alcoholism severity. In previous research, family history of alcoholism has predicted benefit from naltrexone (King et al., 1997; Monterosso et al., 2001; O’Malley, 1999; Rubio et al., 2005), and greater alcoholism severity was associated with recoveries involving abstinence, rather than moderation (Dawson et al., 2005; Sobell and Sobell, 2006; Sobell et al., 1995, 1996). Finally, AI/AN social factors possibly played a role. AI/ANs may have a greater awareness of societal problems with alcohol. Consequently, Native communities may communicate a stronger andz more consistent sobriety message emphasizing total abstinence (Alaska Federation of Natives, 1999; Segal, 1998).
Exploratory analyses examining the influence of the OPRM1 gene on treatment response found that naltrexone was efficacious in individuals homozygous for the more common Asn40 allele. These findings in a group controlled for OPRM1 genotype are consistent with the main effect of naltrexone on reduced risk of relapse even when controlling for genotype in studies by Oslin et al., (2003) and Gelernter (Gelernter et al., 2007). However, the Oslin study found that carriers of the Asp40 allele were significantly less likely to relapse, and Anton et al. (2008) also found that the efficacy of naltrexone was most apparent in Asp40 carriers, with marginal efficacy in those who were homozygous for the Asn40 allele. The effect of the Asp40 allele is possibly population specific. In a small study (n = 29) blockade of the opioid system with naloxone was significantly increased in Asp40 carriers, but this effect was limited to individuals of European ancestry, and was not observed in those of Asian Ancestry (Hernandez-Avila et al., 2007). We were able to demonstrate that naltrexone treatment is effective in individuals homozygous for the Asn40 allele. The low frequency of the Asp40 allele in our study group prevented us from determining whether treatment response is improved in Asp40 carriers. To test this possibility we would need to recruit a larger cohort to obtain sufficient numbers of Asp40 carriers. The genotyping data does, however, allow us to rule out the possibility that the observed naltrexone treatment response is due to an unrecognized OPRM1 genotype stratification across treatment groups.
The study failed to confirm our hypothesis that the addition of sertraline to naltrexone would enhance abstinence rates. This stands in contrast to our preliminary study which was shorter in duration and involved comparisons between two matched groups of patients treated open label with either the combination or naltrexone monotherapy (Farren et al., 2000). In the current study, the onset of the first drinking day appeared to be delayed up through the first month of treatment for the combination group. This advantage dissipated rapidly, however, and the combination group and the naltrexone-only groups were comparable in the final analysis. From a heuristic perspective, one possible explanation for this pattern of response is that the tolerance developed to the salutary effects of sertraline. Consistent with this hypothesis, studies in mice have shown that tolerance develops rapidly to the early suppression of operant responding for alcohol by sertraline and that this effect was not overcome by higher doses (Gulley et al., 1995). Another possibility is that medication compliance suffers in response to adverse events with the combination leading to poorer compliance not only with sertraline but also with naltrexone given that medication compliance is highly correlated for the two drugs. Future analyses will explore these hypotheses. Because our study sample included many individuals with early onset of alcoholism, different results may have been obtained in patients with lower risk and severity, characteristics that have predicted positive response to monotherapy with sertraline (Brady et al., 2005; Pettinati et al., 2003, 2004a).
In our study, the outcomes associated with naltrexone treatment suggest that it is a viable approach to managing alcoholism in rural settings. However, there is room for improvement, including the potential for other forms of combination therapy. As discussed by Oslin (2005), future studies of combination therapies should consider alternative designs in which nonresponders to one medication are randomized to augmentation with the other medication or placebo. This would limit exposure to combination therapies that may be unnecessary and would more closely model clinical practice (Oslin, 2005). In pursuing research on combination therapies, it will be important to determine the optimal dose of each medication in order to minimize adverse events and maximize efficacy.
A number of cautions deserve mention. We originally planned to enroll 198 subjects of AI/AN ancestry, but unanticipated delays and slower recruitment than planned limited the final sample size. This reduced sample may have restricted our ability to find a significant difference between the combination group and naltrexone only. However, the magnitude of the observed differences was small; thus, a larger sample probably would not have yielded different findings. We also conducted secondary analyses in the Native subsample, thereby increasing the overall number of statistical comparisons. The heterogeneity of AI/AN populations suggests caution in generalizing from one community to another (Rodenhauser, 1994; Sack et al., 1994), however, we believe this information may aid clinicians, policy makers and researchers given the lack of data on naltrexone in this population. Because the study sample is clinically biased, it should not be considered representative of the general AI/AN population. Finally, actual clinical practice may yield lower success rates with more heterogeneous populations and less intensive evaluation and intervention than provided in our study.
In conclusion, accruing evidence suggests naltrexone offers an effective adjunct to the care of alcohol dependent patients by nonspecialists (Anton et al., 2006; Latt et al., 2002; O’Malley et al., 1995, 2003b). Our study extends this finding to individuals of AI/AN descent and to remote and rural environments, settings where more than 1/5 of the U.S. population receive their healthcare. If adopted, naltrexone in combination with medical management and treatment advice could increase access to care and reduce the consequences of alcoholism in these communities.
ACKNOWLEDGMENTS
This study was funded by the National Institute on Alcohol Abuse and Alcoholism and the National Center on Minority Health and Health Disparities: RO1AA12028 and KO5AA014715. Pfizer Pharmaceuticals donated study medications. These agencies had no role in the design, conduct or reporting of this study.
Dr. O’Malley, study Principal Investigator, had full access to all of the data in the study and takes responsibility for the integrity of the data and accuracy of the data analysis.
We wish to extend appreciation to project staff at the five study sites, study participants and participating communities, focus group and community members, project officers at NIAAA, members of the Alaska Area and National Indian Health Service, Yale and NIAAA Institutional Review Boards, members of the Data and Safety Monitoring Board, Gary Jenkins for assistance with genotyping, Conor Farren for early advice on the protocol, and James Hosking and Robert Makuch for statistical consultation.
Footnotes
CONFLICTS OF INTEREST
Dr. O’Malley has consulted to or received research supplies or funding from Alkermes, Bristol Myers Squibb, Eli Lilly, Forest Pharmaceuticals, Lipha Pharmaceuticals, Mallinckrodt, Ortho-McNeill/Johnson & Johnson, GlaxoSmithKline, Pfizer, and Sanofi-Aventis. Dr. Meandzija and Ms. Romano have participated in trials funded by Alkermes, Ortho/ McNeill, and Bristol Myers Squibb. Drs. O’Malley and Meandzija are inventors on unlicensed patents held by Yale University related to naltrexone and smoking cessation.
REFERENCES
- Alaska Behavioral Health Survey. Health Risks in Alaska Among Adults: 1999 Annual Report. Alaska Department of Health and Human Services; 2002. [Google Scholar]
- Alaska Department of Vital Statistics. Annual Report. Juneau, AK: Department of Health and Social Services, Divison of Public Health, Bureau of Vital Statistics; 2002. [Google Scholar]
- Alaska Federation of Natives. Alaska Natives Combating Substance Abuse and Related Violence through Self-Healing: A Report to the People. University of Alaska Anchorage: Center for Alcohol and Addition Studies, Institute for Circumpolar Health Studies; 1999. pp. 1–107. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders - Fourth Edition, Text Revision (DSM-IV-TR) Washington, DC: American. Psychiatric Press, Inc.; 2000. [Google Scholar]
- Anton RF, O’Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A. Combined pharmacotherapies and behavioral interventions for alcohol dependence - The COMBINE study: A randomized controlled trial. JAMA. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- Anton R, Oroszi G, O’Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beals J, Manson SM, Mitchell CM, Spicer P. Cultural specificity and comparison in psychiatric epidemiology: walking the tightrope in American Indian research. Cult Med Psychiatry. 2003;27:259–289. doi: 10.1023/a:1025347130953. [DOI] [PubMed] [Google Scholar]
- Beals J, Manson SM, Mitchell CM, Spicer P. Cultural specificity and comparison in psychiatric epidemiology: walking the tightrope in American Indian research. Cult Med Psychiatry. 2005;27:259–289. doi: 10.1023/a:1025347130953. [DOI] [PubMed] [Google Scholar]
- Beals J, Manson SM, Shore JH, Friedman M, Ashcraft M, Fairbank J, Schienger W. The prevalence of posttraumatic stress disorder among American Indian Vietnam veterans: disparities and context. J Trauma Stress. 2002;15:89–97. doi: 10.1023/A:1014894506325. [DOI] [PubMed] [Google Scholar]
- Bergen AW, Kokozka J, Peterson R, Long JC, Virkkunen M, Linnoila M, Goldman D. Mu opiod receptor gene variants; lack of association with alcohol dependence. Mol Psychiatry. 1997;2:490–494. doi: 10.1038/sj.mp.4000331. [DOI] [PubMed] [Google Scholar]
- Beyer A, Koch T, Schroder H, Schulz S, Hollt V. Effect of the Al18G polymorphism on binding affinity, potency, and agonist-medicated endocytosis desensitization, and resensitizaton of the human mu-opioid receptor. J Neurochem. 2004;89:553–560. doi: 10.1111/j.1471-4159.2004.02340.x. [DOI] [PubMed] [Google Scholar]
- Boehnlein J, Kinzie J, Leung P, Matsunaga D, Johnson R, Shore J. The natural history of medical and psychiatric disorders in an American Indian community. Cult Med Psychiatry. 1993;16:543–554. doi: 10.1007/BF00053593. [DOI] [PubMed] [Google Scholar]
- Bohn MJ, Krahn D, Staehler BA. Development and initial validation of a measure of drinking urges in abstinent alcoholics. Alcohol Clin Exp Res. 1995;19:600–606. doi: 10.1111/j.1530-0277.1995.tb01554.x. [DOI] [PubMed] [Google Scholar]
- Bond C, LaForge KS, Tian M, Melia D, Zhang S, Borg L, Gong J, Schluger J, Strong J, Leal SM, Tischfield J, Kreek M, Yu L. Single-nucleotide polymorphism in the human mu opiod receptor gene alters â - endorphine binding and activity: possible implications for opiate addiction. Proc Natl Acad Sci U S A. 1998;95:9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady KT, Sonne S, Anton RF, Randall CL, Back SE, Simpson K. Sertraline in the treatment of co-occurring alcohol dependence and posttraumatic stress disorder. Alcohol Clin Exp Res. 2005;29:395–401. doi: 10.1097/01.alc.0000156129.98265.57. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. Fetal Alcohol Syndrome-Alaska, Arizona, Colorado, New York 1995–1997. MMWR. 2002;51:443–435. [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Lawrence Erlbaum Associates, Inc.; 1988. [Google Scholar]
- COMBINE Study Research Group. Testing combined pharmacotherapies and behavioral interventions in alcohol dependence: Rationale and methods. Alcohol Clin Exp Res. 2003;27:1107–1122. doi: 10.1097/00000374-200307000-00011. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, Ruan WJ. Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction. 2005;100:281–292. doi: 10.1111/j.1360-0443.2004.00964.x. [DOI] [PubMed] [Google Scholar]
- Day GE, Lanier AP. Alaska Native Mortality. Public Health Rep. 2003;118:518–530. doi: 10.1093/phr/118.6.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Department of Health and Human Services (Department of Public Health, Data and Evaluation Unit) Healthy Alaskans 2010 Volume 1: Targets for Improved Health Report. 2002. [Google Scholar]
- Donohue JM. A comparison of prescribing patterns of selective serotonin reuptake inhibitors in the treatment of depression in primary care in the United Kingdom. J Serotonin Res. 1995;1:47–51. [Google Scholar]
- Endicott J, Spitzer RL. A diagnostic interview: the schedule for affective disorders and schizophrenia. Arch Gen Psychiatry. 1978;35:837–844. doi: 10.1001/archpsyc.1978.01770310043002. [DOI] [PubMed] [Google Scholar]
- Farren CK, Catapano D, O’Malley S. Sertraline with naltrexone vs naltrexone alone in the treatment of alcohol dependence. Alcohol Clin Exp Res. 1997;21(suppl):64A. [Google Scholar]
- Farren CK, Rezvani AH, Overstreet D, O’Malley S. Combination pharmacotherapy in alcoholism: a novel treatment approach. CNS Spectrums. 2000;5:70–76. doi: 10.1017/s1092852900012839. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. SCID-I/P, Version 2.0 ed. New York, NY: New York State Psychiatric Institute; 1997. [Google Scholar]
- Garbutt JC, Kranzler HR, O’Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW, Vivitrex Study G. Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA. 2005;293:1617–1625. doi: 10.1001/jama.293.13.1617. [DOI] [PubMed] [Google Scholar]
- Gelernter J, Gueorgueva R, Kranzler H, Zhang H, Cramer J, Rosenheck R, Krystal JH. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA cooperative study. Alcohol Clin Exp Res. 2007;31:555–563. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- Geller S, Adams MG, Carnes M. Adherence to federal guidelines for reporting of sex and race/ethnicity in clinical trails. J Womens Health. 2006;15:1123–1131. doi: 10.1089/jwh.2006.15.1123. [DOI] [PubMed] [Google Scholar]
- Gianoulakis C, Krishnan B, Thavundayil J. Enhanced sensitivity of pituitary â-endorphin to ethanol in subjects at high risk of alcoholism. Arch Gen Psychiatry. 1996;53:250–257. doi: 10.1001/archpsyc.1996.01830030072011. [DOI] [PubMed] [Google Scholar]
- Gill K, Amit Z, Koe BK. Treatment with sertraline, a new serotonin reuptake inhibitor, reduces voluntary ethanol consumption in rats. Alcohol. 1988;5:349–354. doi: 10.1016/0741-8329(88)90019-5. [DOI] [PubMed] [Google Scholar]
- Gregor KJ, Overhage JM, Coons SJ, McDonald RC. Selective serotonin reuptake inhibitor dose titration in the naturalistic setting. Clin Ther. 1994;16:306–315. [PubMed] [Google Scholar]
- Gulley JM, McNamara C, Barbera TJ, Ritz MC, George FR. Selective serotonin reuptake inhibitors: effects of chronic treatment on ethanol-reinforced behavior in mice. Alcohol. 1995;12:177–181. doi: 10.1016/0741-8329(94)00079-s. [DOI] [PubMed] [Google Scholar]
- Hernandez-Avila CA, Covault J, Wand G, Zhang H, Gelernter J, Kranzler HR. Population-specific effects of the Asn40Asp polymorphism at the μ-opioid receptor gene (OPRM1) on EPA-axis activation. Pharmacogenetics and Genomics. 2007;17:1031–1038. doi: 10.1097/FPC.0b013e3282f0b99c. [DOI] [PubMed] [Google Scholar]
- Higgins GA, Tomkins DM, Fletcher PJ, Sellers EM. Effects of drugs influencing 5-HT function on ethanol drinking and feeding behavior in rats: studies using a drinkometer system. Neuroscience Biobehav Rev. 1992;16:535–552. doi: 10.1016/s0149-7634(05)80195-2. [DOI] [PubMed] [Google Scholar]
- Hill R, Wells RS, Andon H, Ballew C. Non-fatal injury hospitalizations among Alaska Natives, 1994–1999. Results from the Alaska Trauma Registry. 2004;46:37–48. [PubMed] [Google Scholar]
- Johnson BA, Ait-Daoud N, Prihoda TJ. Combining ondansetron and naltrexone effectively treats biologically predisposed alcoholics: from hypotheses to preliminary clinical evidence [In Process Citation] Alcohol Clin Exp Res. 2000;24:737–742. [PubMed] [Google Scholar]
- Kiefer F, Jahn H, Tarnaske T, Helwig E, Briken P, Holzbach R, Kampf P, Stracke R, Baehr M, Naber D, Wiedemann K. Comparing and combining naltrexone and acamprosate in relapse prevention of alcoholism: a double-blind, placebo-controlled study. Arch Gen Psychiatry. 2003;60:92–99. doi: 10.1001/archpsyc.60.1.92. [DOI] [PubMed] [Google Scholar]
- King AC, Volpicelli JR, Frazer A, O’Brien CP. Effect of naltrexone on subjective alcohol response in subjects at high and low risk for future alcohol dependence. Psychopharmacology. 1997;129:15–22. doi: 10.1007/s002130050156. [DOI] [PubMed] [Google Scholar]
- Kranzler HR, Burleson JA, Brown J, Babor TF. Fluoxetine treatment seems to reduce the beneficial effects of cognitive-behavioral therapy in type B alcoholics. Alcohol Clin Exp Res. 1996;20:1534–1541. doi: 10.1111/j.1530-0277.1996.tb01696.x. [DOI] [PubMed] [Google Scholar]
- Kranzler E, Van Kirk J. Efficacy of naltrexone and acamprosate for alcoholism treatment: a meta-analysis. Alcohol Clin Exp Res. 2001;25:1334–1341. [PubMed] [Google Scholar]
- Kranzler HR, Wesson DR, Billot L, DrugAbuse Sciences Naltrexone Depot Study G Naltrexone depot for treatment of alcohol dependence: a multicenter, randomized, placebo-controlled clinical trial. Alcohol Clin Exp Res. 2004;28:1051–1059. doi: 10.1097/01.alc.0000130804.08397.29. [DOI] [PubMed] [Google Scholar]
- Latt NC, Jurd S, Houseman J, Wutzke SE. Naltrexone in alcohol dependence: a randomized controlled trial of effectiveness in a standard clinical setting. Med J Aust. 2002;176:530–534. doi: 10.5694/j.1326-5377.2002.tb04550.x. [DOI] [PubMed] [Google Scholar]
- Le AD, Poulos CX, Harding S, Watchus J, Juzytsch W, Shaham Y. Effects of naltrexone and fluoxetine on alcohol self-administration and reinstatement of alcohol seeking induced by priming injections of alcohol and exposure to stress. Neuropsychopharmacology. 1999;21:435–444. doi: 10.1016/S0893-133X(99)00024-X. [DOI] [PubMed] [Google Scholar]
- Manson SM. Cultural Diversity Series: Meeting the Mental Health Needs of American Indians and Alaska Natives (2004) Prepared for the National Technical Assistance Center for State Mental Heath Planning, SAMHSA, DHHS; 2004. pp. 1–46. [Google Scholar]
- Manson SM, Garroutte E, Goins RT, Henderson PN. Access, relevance, and control in the research process: lessons from Indian country. J Aging Health. 2004;16:58S–77S. doi: 10.1177/0898264304268149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinelli P, Bai L, Quirion R, Gianoulakis C. A microdialysis profile of met-enkephalin release in the rat nucleus accumbens following alcohol administration. Alcohol Clin Exp Res. 2005;29:1821–1828. doi: 10.1097/01.alc.0000183008.62955.2e. [DOI] [PubMed] [Google Scholar]
- Mason BJ. Rationale for combining acamprosate and naltrexone for treating alcohol dependence. J Stud on Alcohol. 2005;66(SuppU5):148–156. doi: 10.15288/jsas.2005.s15.148. [DOI] [PubMed] [Google Scholar]
- Miller W, Tonigan S, Longabaugh R. The Drinker Inventory of Consequences (DrInC): An Instrument for Assessing Adverse Consequences of Alcohol Abuse, Vol. 4. Rockville, Maryland: U.U. Department of Health and Human Services; 1995. [Google Scholar]
- Mohatt GV, Hazel KL, Allen J, Stachelrodt M, Hensel C, Fath R. Unheard Alaska: culturally anchored participatory action research on sobriety with Alaska Natives. Amer J Comm. 2004;33:263–273. doi: 10.1023/b:ajcp.0000027011.12346.70. [DOI] [PubMed] [Google Scholar]
- Monterosso JR, Flannery BA, Pettinati HM, Oslin DW, Rukstalis M, O’Brien CP, Volpicelli JR. Predicting treatment response to naltrexone: the influence of craving and family history. Am J Addict. 2001;10:258–268. doi: 10.1080/105504901750532148. [DOI] [PubMed] [Google Scholar]
- National Institutes of Health. NIH guidelines on the inclusion of women and minorities as subjects in clinical research. Federal Register. 1994;59:14508. [Google Scholar]
- Office of Applied Statistics, Substance Abuse and Mental Health Services Administration. Alcohol dependence or abuse in substate areas. [Accessed April 11, 2008];The NSDUH Report. 2005 (Issue 25) http://www.oas.samhsa.gov/2k6/subStateAlc/subStateAlc.htm.
- O’Malley SS. Naltrexone therapy: predictors of adverse events, medication compliance and treatment outcome. Symposium: naltrexone treatment of alcoholism: recent evidence from clinical research, in Paper Presented at the Research Society on Alcoholism Scientific Meeting; Santa Barbara, CA. 1999. [Google Scholar]
- O’Malley SS, Croop RS, Wroblewski JM, Labriola DF, Volpicelli JR. Naltrexone in the treatment of alcohol dependence: a combined analysis of two trials. Psychiatr Ann. 1995;25:681–688. [Google Scholar]
- O’Malley SS, Froehlich JC. Advances in the use of naltrexone: an integration of preclinical and clinical findings. Recent Dev in Alcohol. 2003a;16:217–245. [PubMed] [Google Scholar]
- O’Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Naltrexone and coping skills therapy for alcohol dependence. A controlled study. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Rounsaville BJ, Farren C, Namkoong K, Wu R, Robinson J, O’Connor PG. Initial and maintenance naltrexone treatment for alcohol dependence using primary care vs specialty care: a nested sequence of 3 randomized trials. Arch Intern Med. 2003b;163:1695–1704. doi: 10.1001/archinte.163.14.1695. [DOI] [PubMed] [Google Scholar]
- Olive M, Koenig H, Nannini M, Hodge C. Stimulation of endorphin neurotransmission in the nucleus accumbens by ethanol, cocaine, and amphetamine. J Neurosci. 2001;21:184. doi: 10.1523/JNEUROSCI.21-23-j0002.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW. Treatment of late-life depression complicated by alcohol dependence. Am J Geriatr Psychiatry. 2005;13:491–500. doi: 10.1176/appi.ajgp.13.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oslin DW, Berrettini W, Kranzler HR, Pettinati H, Gelernter J, Volpicelli JR, O’Brien CP. A functional polymorphism of the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28:1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- Patch Opinion Survey. Internal document. Alaska Health Consortium; 1997. Relevant Data on Health Status of Alaska; p. 13. [Google Scholar]
- Petrakis IL, Poling J, Levinson C, Nich C, Carroll K, Rounsaville B. Naltrexone and disulfiram in patients with alcohol dependence and comorbid psychiatric disorders. Biol Psychiatry. 2005;57:1128–1137. doi: 10.1016/j.biopsych.2005.02.016. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Dundon W, Lipkin C. Gender differences in response to sertraline pharmacotherapy in Type A alcohol dependence. Am J Addictions. 2004a;13:236–247. doi: 10.1080/10550490490459906. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Kranzler HR, Madaras J. In: The status of serotonin-selective pharmacotherapy in the treatment of alcohol dependence, in Recent Developments in Alcoholism. Galanter M, editor. Vol. 16. New York: New York; 2003. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, O’Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. The status of naltrexone in the treatment of alcohol dependence: Specific effects on heavy drinking. J Clin Psychiatry. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- Pettinati HM, Weiss RD, Miller WR, Donovan DM, Ernst DB, Rounsavile BJ. COMBINE monograph series, Volume 2. Medical Management Treatment Manual: A Clinical Research Guide for Medically Trained Clinicians Providing Pharmacotherapy as Part of the Treatment for Alcohol Dependence; Bethesda, MD: NIAAA, DHHS Publication No. (NIH) 04-5289; 2004b. [Google Scholar]
- Rice JP, Reich T, Bucolz AK, Neuman RJ, Fishman R, Rochberg N, Hesseibrock VM, Nurnberger JI, Scuckit MA, Begleiter E. Comparison of direct interview and family history diagnoses of alcohol dependence. Alcohol Clin Exp Res. 1995;19:1018–1023. doi: 10.1111/j.1530-0277.1995.tb00983.x. [DOI] [PubMed] [Google Scholar]
- Robin RW, Chester B, Rasmussen J, Jaranson J, Goldman D. Risk factors in the utilization of mental health and substance abuse services by American Indian men and women. Psychiatr Serv. 1997;48:826–832. doi: 10.1176/ps.48.6.826. [DOI] [PubMed] [Google Scholar]
- Rodenhauser P. Cultural barriers to mental health care delivery in Alaska. J Ment Health Admin. 1994;21:60–70. doi: 10.1007/BF02521346. [DOI] [PubMed] [Google Scholar]
- Roubideaux Y, Hodge FS, Weinmann S. Recruitment of American Indians and Alaska natives into clinical trials. Ann Epidemiol. 2000;10:S41–S48. doi: 10.1016/s1047-2797(00)00196-4. [DOI] [PubMed] [Google Scholar]
- Rubin RR, Fujimoto WY, Marrero DG, Brenneman T, Charleston JB, Edelstein SL, Fisher EB, Jordan R, Knowler WC, Lichterman LC, Prince M, Rowe PM, Group DR. The Diabetes Prevention Program: recruitment methods and results. Control Clin Trials. 2002;23:157–171. doi: 10.1016/s0197-2456(01)00184-2. [DOI] [PubMed] [Google Scholar]
- Rubio G, Ponce G, Rodriguez-Jimenez R, Jimenez-Arriero MA, Hoenicka J, Palomo T. Clinical predictors of response to naltrexone in alcoholic patients: who benefits most from treatment with naltrexone? Alcohol Alcohol. 2005;40:227–233. doi: 10.1093/alcalc/agh151. [DOI] [PubMed] [Google Scholar]
- Sack W, Beiser M, Baker-Brown G, Redshirt R. Depressive and suicidal symptoms in Indian school children: findings from the Flower of Two Soils. American Indian Alaska Native Mental Health Resource Monogram Series. 1994;4:81–94. doi: 10.5820/aian.mono04.1994.81. [DOI] [PubMed] [Google Scholar]
- Segal B. Drinking and drinking-related problems among Alaska Natives. Alcohol Health Res World. 1998;22:276–280. [PMC free article] [PubMed] [Google Scholar]
- Shore JH, Manson SM, Bloom J. A pilot study of depression among American Indian patients with research diagnostic criteria. Am Indian Alsk Native Ment Health Res. 1987;1:4–15. doi: 10.5820/aian.0102.1987.4. [DOI] [PubMed] [Google Scholar]
- Shore JH, Manson SM, Buchwald D. Screening for alcohol abuse among urban Native Americans in a primary care setting. Psychiatry Serv. 2002;53:757–760. doi: 10.1176/appi.ps.53.6.757. [DOI] [PubMed] [Google Scholar]
- Skinner HA, Allen BA. Alcohol dependence syndrome: measurement and validation. J Abnorm Psych. 1982;91:199–209. doi: 10.1037//0021-843x.91.3.199. [DOI] [PubMed] [Google Scholar]
- Sobell LC, Cunningham JA, Sobell MB. Recovery from alcohol problems with and without treatment: Prevalence in two population surveys. Am J Public Health. 1996;86:966–972. doi: 10.2105/ajph.86.7.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. In: Timeline follow-back: a technique for assessing self-reported ethanol consumption, in Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Allen J, Litten RZ, editors. Totowa, NJ: Humana Press, Inc.; 1992. pp. 4–72. [Google Scholar]
- Sobell MB, Sobell LC. Obstacles to the adoption of low risk drinking goals in the treatment of alcohol problems in the United States: A commentary. Add Res Theory. 2006;14:19–24. [Google Scholar]
- Sobell MB, Sobell LC, Glatt MM, Heather N, Anderson P, Weisner C, Duckert F, Kahler CW, Hore B, Buhringer G, Kufner H. Controlled drinking after 25 years: How important was the great debate? Addiction. 1995;90:1149–1153. [PubMed] [Google Scholar]
- Somervell P, Beals J, Kinzie J. Use of the CES-D in an American Indian village. Cult Med Psychiatry. 1993;16:503–517. doi: 10.1007/BF00053590. [DOI] [PubMed] [Google Scholar]
- Spitzer RL, Endicott J, Robins E. Research Diagnostic Criteria (RDC) for a Selected Group of Psychiatric Disorders. New York: Department of Research Assessment and Training, New York Psychiatric Institute; 1989. [Google Scholar]
- Sullivan JT, Sykora K, Schneiderman J, Naranjo CA, Sellers EM. Assessment of alcohol withdrawal: the evised Clinical Institute Withdrawal Assessment for Alcohol scale (CIWA-Ar) Br J Addict. 1989;84:1353–1357. doi: 10.1111/j.1360-0443.1989.tb00737.x. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau, Census 2000. Summary File 1, Matrix P1. [Accessed April 11, 2008];United States—Urban/Rural and Inside/Outside Metropolitan Area GCT-P1. Urban/Rural and Metropolitan/Nonmetropolitan Population: 2000 Data Set: Census 2000 Summary File 1 (SF 1) 100-Percent Data. 2000 http://factfinder.census.gov/servlet/GCTTable?_bm=y&geo_id=&ds_name=DEC_2000_SF1_U&_lang=en&redoLog=true&mt_name=DEC_2000_SF1_U_GCTP1_US1&format=US-1&CONTEXT=gct.
- Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP. Naltrexone in the treatment of alcohol dependence. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- Volpicelli JR, Rhines KC, Rhines JS, Volpicelli LA, et al. Naltrexone and alcohol dependence: role of subject compliance. Arch Gen Psychiatry. 1997;54:737–742. doi: 10.1001/archpsyc.1997.01830200071010. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang D, Johnson A, Papp A, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J Biol Chem. 2005;280:32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- Zink RW, Rohrbach K, Froehlich JC. Naltrexone and fluoxtine act synergistically to decrease alcohol intake. Alcohol Clin Exp Res. 1997;21(Suppl):104A. [Google Scholar]