Abstract
Breast cancer is one of the most clear-cut examples of a solid tumor in which systemic cues play a decisive part in its development. The breast tissue is constantly subjected to changes in hormone levels and modifications in the microenvironment. This scenario is even more striking during tumor development because of the dramatic loss or aberration of basement membrane (BM) and myoepithelial cells and the gain of peritumoral myofibroblasts. We suggest that the microenvironment, defined here as all components of the mammary gland other than luminal and/or tumor epithelial cells, might be instrumental in maintaining organ integrity and in promoting, and at times even initiating, breast cancer development. As such, the tumor microenvironment and its constituents, alone or in combination, might serve as promising targets for therapy.
Breast cancer and stroma: for better or worse
Tumor development is a prolonged and circuitous process and, similar to what has been postulated for normal organ homeostasis [1], should be regarded as a continuous reciprocal interaction between tumor cells and their surrounding microenvironment [2], in which stromal cells and the extracellular matrix (ECM) have decisive roles. The other cell types and the ECM constituents that surround the tumors are different from those that are contained in normal tissue (reviewed in [3]). In the normal breast, the luminal epithelial microenvironment includes myoepithelial cells, basement membrane (BM) and the collective complex referred to as ‘stroma’ (fibroblasts, vasculature, immune cells and interstitial ECM). In invasive breast cancer, myoepithelial cells and BM are essentially lost, and tumor cells are in direct contact with a remodeled interstitial stroma comprising fibroblasts and myofibroblasts, tumor vasculature and a considerable number of infiltrating immune cells, such as lymphocytes, macrophages and mast cells. Although several of the molecular mechanisms underlying the emergence of the individual cellular phenotypes have now been elucidated, the full potential of the tumor microenvironment as a possible target for diagnosis, prognosis and therapy has yet to be appreciated, understood and applied.
Until recently, the acceptance of a microenvironmental contribution to tumor development was limited mostly to that of a role for angiogenesis in solid tumors beyond a certain size [4]. The more recent, albeit broad, focus on the importance of the microenvironment, in particular inflammation, in essence represents a renaissance of the historic view of cancer as a never-healing wound [5]; in chickens, wounding itself can be a co-carcinogen that creates a conducive tissue environment for Rous sarcoma virus (RSV) tumorigenesis (reviewed in [6]), and bacterial or viral infections in humans induce chronic inflammation, leading to a significantly increased risk of cancer (reviewed in [7]). Thus, although sporadic or inherited mutations in crucial genes might represent initiating events in tumorigenesis, chronic inflammation favors the selection of additional features in initiated cells that might promote their full malignant transition (reviewed in [8]).
Our understanding of the importance of the microenvironment has now shifted: whereas previously it was regarded as merely providing passive support for epithelial cells during carcinoma, the microenvironment is now considered an active contributor to tumor progression. The recent discoveries that the capability of the microenvironment, in this case the bone marrow, is the sole cause of hematopoietic disorders [9,10] bear witness to the possibility that the tumor microenvironment essentially could control the course of breast tumor progression.
Here, we discuss experimental evidence for the role of the activated stroma in the tumor microenvironment, and we discuss evidence of a tumor-promoting effect of the microenvironment in physiological settings. Finally, we focus on tissue integrity, ECM organization and tissue architecture as determinants of epithelial cell fate. The process of invasive breast tumor development is illustrated schematically in Figure 1.
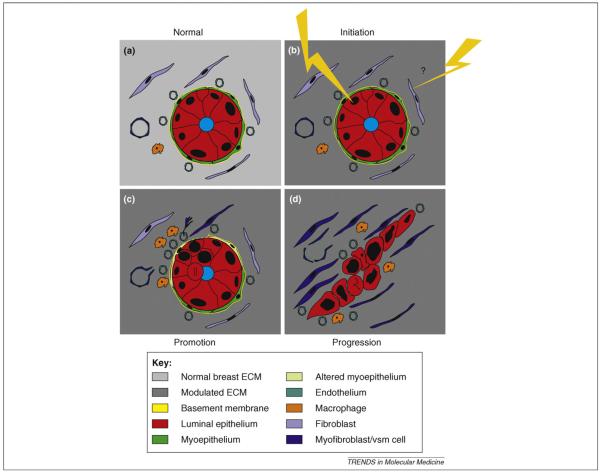
Figure 1.
Breast cancer development is an intricate and involved process implicating reciprocal interactions between epithelial cells, the stromal cells and the ECM. (a) Normal breast is characterized by a high level of architectural integrity. Polarized, luminal epithelium is surrounded by myoepithelium, and the entire epithelium is separated from the surrounding interstitial stroma by an intact BM. The stroma comprises vasculature, fibroblasts and macrophages embedded in BM and ECM characteristic of normal breast. (b) The classical perception of initiation of tumor development is that cancer originates from mutagenic or epigenetic insults to a single cell in the epithelium (lightning). Recent studies suggest that LOH or epigenetic events in stromal cells might promote or even initiate genetic instability and tumor development. Likewise, changes in ECM composition, genetic defects in specific ECM molecules or global changes, such as those occurring due to aging, might be implicated. (c) Promotion of tumor development relies on the proliferation of mutated epithelial cells (double bar depicts mitosis) and the alteration and/or loss of myoepithelial cells and BM. Resident fibroblasts are converted into myofibroblasts, and other stromal cells, such as resident vascular smooth muscle (vsm) cells, blood-borne fibrocytes and/or bone-marrow-derived mesenchymal cells, might be recruited from the vasculature to participate in direct epithelial—stromal interactions. Hypoxia leads to macrophage infiltration and angiogenesis. From this point on, the ECM is subjected to major structural and functional changes. (d) Progression to invasive breast carcinoma is characterized by a complete loss or alteration of myoepithelial cells and BM, and the highly aberrant tumor cells are now surrounded by a fully activated stroma, often characterized by desmoplasia that allows and indeed encourages invasion. Tumor cells also might invade vessels to establish metastases in other organs (not depicted).
The activated stroma revisited
Myofibroblasts represent the most abundant cell type in the tumor stroma of invasive breast carcinomas (for a review, see [3]). Myofibroblasts were shown originally to derive mainly from interstitial and perivascular fibroblasts, except for a subpopulation of highly smooth-muscle-differentiated myofibroblasts from venous smooth muscle cells [11] (Figure 2). Bone-marrow-derived mesenchymal stem cells or fibrocytes might also contribute to reactive stroma. Experimental models have demonstrated that mesenchymal stem cells enhance invasion, motility and metastasis of human breast carcinoma cell lines [12]; however, the relevance of this interaction in breast carcinomas in humans still awaits confirmation. Likewise, although recruitment of fibrocytes is a pronounced phenomenon in wound healing and fibroses [13,14], their contribution to human breast cancer has not been documented convincingly.
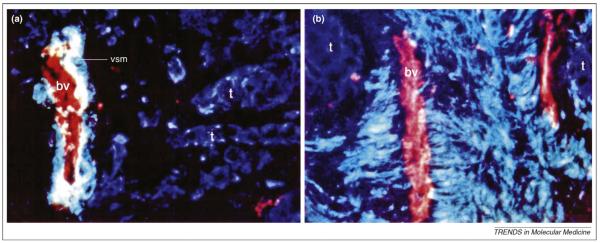
Figure 2.
Vascular smooth muscle cells recruited from venous blood vessels might contribute to reactive stroma. Using triple-staining of cryosections of human breast tumors, we demonstrated that (a) in tumors with no or few smooth-muscle-differentiated myofibroblasts, blood vessels (bv) are intact, whereas (b) in tumors with foci of distinct smooth-muscle-differentiated myofibroblasts, blood vessels within the same area are dramatically stripped of vascular smooth muscle cells. Tumor cells (t) were recognized by cytokeratin 18 (blue), and endothelial cells and vascular smooth muscle cells (vsm) of blood vessels were labeled by endothelial surface antigen (red) and smooth-muscle-myosin heavy chain (green), respectively. We suggest that such differences in myofibroblast differentation could be used to classify patients in terms of the kind of treatment needed. Reproduced from Ref. [11] with permission from the American Society for Clinical Investigation. Magnification: (a) x400; (b) x640.
Upon transplantation of a breast cancer cell line to mice transplanted with bone marrow from double-mutant recombinase-activating gene-1 (RAG-1)-/- β-gal transgenic and green fluorescent protein (GFP) transgenic mice, 20% of the myofibroblasts in the resultant tumors were bone-marrow-derived [15]. In another study, SV40 large T antigen-, human telomerase reverse transcriptase (hTERT)- and RasV12-transfected human breast cells recruited Rag-1null and GFP transgenic bone-marrow-derived cells to comprise ∼90% of the tumor-associated cells, of which the majority expressed α-smooth muscle (α-sm) actin [16]. Given that cancer is a tissue-specific disease and that human breast stroma differs appreciably from the stroma of mouse mammary gland [3], we believe that additional markers of human fibrocytes are needed. This is particularly pertinent to breast tissue. Currently, the most widely used marker of fibrocytes, CD34, is expressed by all fibroblasts in normal breast but is lost in α-sm-actin-positive myofibroblasts [17].
We have shown previously that resident, normal fibroblasts readily undergo conversion to myofibroblasts in response to tumor cells in culture (reviewed in [3,18]). Multiple studies have documented that the converted stroma in turn supports cancer cell growth and metastasis [3,18–22]. That the reactive stroma itself, once converted by any means, might also signal to epithelial cells and eventually convert them to cancer cells is beginning to be appreciated.
A comparison of stromal cells isolated from tumors and normal tissues, respectively, suggests that stromal cells might indeed provide cues for malignancy. In recombination studies of human prostate-derived cells, normal fibroblasts support growth arrest and normal histology of the epithelium, whereas so-called cancer-associated fibroblasts (CAFs) direct immortalized prostatic epithelial cells towards adenocarcinomas [23]. The myoepithelial cells isolated from ductal carcinoma in situ (DCIS) were shown to be grossly abnormal and to secrete many cytokines and matrix metalloproteinases (MMPs) that normal myoepithelial cells did not [24]. Thus, stromal as well as myoepithelial cells might be a switch for overt malignancy in luminal epithelial cells. Conversely, an experimental model of DCIS has demonstrated that only myoepithelial cells isolated from normal glands can inhibit progression of DCIS to invasive carcinoma [24].
Evidence that not only certain cell populations but also changes in the microenvironment as a whole can promote tumorigenesis also comes from studies of irradiated mouse mammary gland stroma. Ionizing radiation is a known albeit weak carcinogen in the mammary epithelium. In addition to its widely accepted DNA-damaging (i.e. mutagenic) effect, irradiation elicits persistent microenvironmental alterations in ECM composition and cytokine activities. Transplantation of a p53-null mouse mammary epithelial cell line to cleared mammary fat pads showed that tumor incidence was 81% in irradiated animals but only 19% in sham-irradiated animals. Experiments in animals with one-sided irradiation confirmed that tumors formed only on the irradiated side [25].
It is intriguing to note the dramatic epigenetic changes that were clearly transmitted to progenies after combined irradiation and transforming growth factor-β (TGF-β) treatment of single human breast epithelial cells [26]. There was a heritable disruption of epithelial cell polarity and multicellular organization in three-dimensional (3D) assays in the entire surviving population, indicating non-mutational but heritable mechanisms [26]. Of note, the stroma itself also has been suggested as a target of the chemical carcinogen N-nitrosomethylurea, which was previously thought to exert its effect only through direct mutation induced in the epithelium [27]. However, confirmation of these latter findings via the use of marked stromal cells would be essential for interpretation of the data.
The stromal compartment also includes mast cells, macrophages and T-cell subtypes, all of which are emerging as essential modulators of tumor growth, and more research clearly needs to be done in this area. The influence of immune cell infiltrates on tumor development might be favorable or adverse, depending on the cell types involved. Mast cells are attracted to tumors by tumor-derived chemoattractants and affect tumor development in accordance with local tumor conditions. In a mouse tumor model, recruitment of mast cells was required for angiogenesis and expansion of Myc-induced pancreatic islet tumors [28], whereas in human breast cancer, the presence of mast cells might be an independent, favorable prognostic factor [29]. Macrophages, as a major population of infiltrating cells in the tumor stroma, are considered to have an overall tumor-supporting role. The attraction of tumor-associated macrophages is thought to be caused by hypoxia, which stimulates macrophages to perform pro-angiogenic functions [30]. Pollard and coworkers, who were the first to describe the importance of macrophages in breast tumors, developed a transgenic mouse model that displays a progression series from benign tumor to malignancy. The results show a marked increase in macrophage infiltration prior to the transition to malignancy, which is associated with angiogenesis, thus showing that the microenvironment is directly involved in the development of the high-density tumor vasculature that is required for tumor progression (reviewed in [31]). These findings highlight the crucial role that the host stromal reaction, including the inflammatory cell infiltrate, has in modulating cancer progression. Immune cells, which seem to be very widely involved in tumor development, are easily accessible, making them a potential and attractive tool for tumor therapy, as discussed below.
Transgenic mouse models have also pointed to the importance of ECM and TGF-β signaling in tumor development. Disruption of the ECM in the mammary gland of normal mice ([32], discussed in more detail below) led to the formation of reactive stroma [33] long before tumors were formed [34]. Importantly, knockout of TGF-β receptor II revealed prostatic hyperplasia in the stroma, as well as in adjacent epithelium, leading to prostatic intraepithelial neoplasia, a presumed forerunner of carcinoma [35]. In spite of the fact that TGF-β signaling has so far not been found to be inactivated during the normal course of tumor pathogenesis, these latter studies provide a small but growing number of counter-examples to the assumption that mutations in epithelial cells are a required initiating event for neoplastic change.
Collectively, these studies in our view lend credence to the fact that a physiologically abnormal microenvironment could promote, as well as possibly initiate, mammary or other types of epithelial tumors.
Pregnancy, parity and aging as risk factors in breast cancer
The breast undergoes profound tissue remodeling depending on hormonal status, which varies due to monthly fluctuations in the levels of sex hormones and changes dramatically during pregnancy, lactation and involution. It has long been recognized that early pregnancy is associated with a reduction in a woman’s lifetime risk of developing breast cancer. A recent experimental study in rats implicated microenvironmental factors as possible causative agents. Injection of chemically induced cancer cells into cleared fat pads of virgin rats and twice-parous rats, respectively, demonstrated that the stroma of parous rats restricted tumor development and, in addition, provided cues to support normal ductal outgrowths from cancer epithelial cells [36].
It has long been postulated that the observed lifetime protective effect of an early full-term pregnancy against estrogen-receptor-positive breast cancers among postmenopausal women is due to the complete cellular differentiation that the gland undergoes during the process of pregnancy ([37] and references therein). This belief has recently been questioned experimentally: it seems that it is not differentiation per se but exposure to estrogen at a critical stage of the gland’s development that causes the protective effect [38].
However, the long-term protective effect of pregnancy is not constant and varies with age at the time of the first pregnancy; moreover, pregnancy is associated with its own paradoxical but relatively transient increase in risk of breast cancer, which often leads to metastasis with poor prognosis. The increase of risk at first pregnancy peaks 6.5 years post-partum and persists for up to 15 years after parturition [39]. That such a window of time can be defined suggests that an event subsequent to pregnancy is involved in breast cancer progression. One obvious post-partum change in mammary gland physiology after pregnancy is gland involution, during which a tissue-remodeling program similar to those of inflammation and wound healing is activated [40]. Mammary gland involution has been investigated widely in rodent models. Thus, the stroma from mammary glands undergoing weaning-induced involution contains elevated levels of MMPs (including MMP-3), urokinase plasminogen activator, fibrillar collagen and proteolytic fragments of laminin and fibronectin [41–43]. The possible effect of ECM components has been tested directly: MDA-MB-231 cells were xenografted to mammary fat pads with extracts of endogenous mammary ECM from quiescent, nulliparous and involuting rat glands, respectively. Although the volumes of the resultant tumors were the same, the ability to metastasize was highly dependent on the source of the matrix: ECM from involuting glands significantly promoted tumor cell metastasis [43]. This suggests that the physiological status of the microenvironment might determine epithelial cell potential and fate, even in the form of tissue extract. Although the underlying molecular mechanisms still await elucidation, the prominent proteolytic modulators of the breast tissue microenvironment, MMPs, undoubtedly have an important role in the process. In the experiment above, the stromal ECM of the mammary glands from involuting rats was collected between days four and six after weaning, coinciding with the MMP-3-dependent phase of involution [44].
We have shown a role for MMP-3 in tumor development in physiological settings by creating MMP-3 transgenic mice in which the BM was disrupted in the middle of pregnancy and mammary glands underwent ‘involution’ in mid-pregnancy instead of during weaning [32]. As these animals aged, they developed mammary tumors [34]. Although MMP-3 does not seem to influence breast cancer susceptibility in humans, the MMP-3 5A/6A promoter polymorphism seems to be linked to a higher risk for metastasis among breast cancer patients [45]. The molecular pathways by which MMP-3 might induce epithelial—mesenchymal transition (EMT) as well as genomic instability, two crucial events in the process of malignant progression, are now being dissected [46]. Exposure of mouse mammary epithelial cells to MMP-3 cleaves E-cadherin, alters the cytoskeleton and cell shape and provokes the expression of Rac1b. This in turn causes a rise in cellular reactive oxygen species (ROS) and an increase in the transcription factor Snail, leading to EMT. The above scenario can be achieved by elevating ROS, thus bypassing MMP-3, or by expression of Rac1b [46].
MMP-3 has also been implicated in overall age-associated risk of cancer development. Although most age-related cancers arise from epithelial cells, accumulation of senescent stromal cells can favor tumor development by both compromising tissue renewal capacity and secreting cytokines and proteolytic enzymes, which alter tissue homeostasis. Thus, senescent fibroblasts have been shown to support epithelial proliferation and to alter epithelial morphology and functional differentiation in part through high-level secretion of MMP-3 [47].
Collectively, these data indicate that changes in the microenvironment, pathological as well as physiological, influence cell behavior and gene expression profiles to promote tumor development and progression. At least some of these effects can be mimicked by disruption of BM organization and 3D tissue structure.
Stromal ECM and risk of breast cancer
Mammographic breast density, a poorly understood parameter, is positively associated with increase in breast cancer risk, that is, increased susceptibility to breast cancer and decreased detection of cancer by mammography [48,49]. Breast density is positively associated with tumor size, lymph node status and lymphatic or vascular invasion [48]. For women younger than 56 years, 26% of all breast cancers and 50% of cancers detected less than 12 months after a negative screening were attributable to density in 50% or more of the mammogram [49]. In women with dense breast tissue, detection of tumors might be prevented by density or tumors might grow quickly between examinations, or both [48,49]. The relative contribution from different ECM components to breast density has yet to be fully analyzed and reported, but these findings in our view support the importance of context, that is, the epithelial microenvironment prior to tumor development. It is important now to investigate not only how the microenvironment could promote or even initiate tumors but also whether the type of tumor developed could be different in different microenvironments.
Once breast tumors are formed, they are often characterized by the presence of an extremely dense collagenous stroma, the so-called desmoplastic response, which is produced by myofibroblasts in the interstitium. Desmoplasia is a prominent feature of the most frequent breast carcinoma lesion, ‘infiltrating ductal carcinoma not otherwise specified’, which constitutes 75–80% of all breast tumors [3]. The desmoplastic response forms stromal tissue that can vary from being predominantly cellular, with accumulated fibroblasts and myofibroblasts and little collagenous tissue, to dense and collagenous, with few stromal cells present [50]. How the nature of the desmoplastic reaction relates to the composition of the ECM and breast density before tumor development is not known. Nevertheless, direct histopathological analyses have shown increased expression of molecular markers of desmoplasia, such as collagen, lumican, dEcoRIn and syndecan-1, in the stroma of women with high density breasts [51,52].
The relevance of the varieties of desmoplasia for tumor progression might be difficult to discern, but the presence of fibrotic foci, defined as radiating fibrosclerotic scars with varying cellularity, in the desmoplastic stroma has been shown to be a significant histopathological parameter of worse prognosis [53]. Recent discoveries further imply that the physical properties of a tissue might determine specification, morphology, differentiation and function. It has been shown recently that matrix elasticity per se directs lineage differentiation of naïve mesenchymal stem cells, thus implicating physical properties of the microenvironment in fate determination [54], and it is likely that similar mechanisms might apply to tumor development. Another elegant study addressed whether desmoplasia and tissue stiffening might actively promote malignant behavior [55]. Compression analyses of normal and malignant mammary tissues from transgenic mice showed that normal tissue was very soft, whereas tumors and adjacent stroma were relatively stiff. Human breast epithelial cell lines were then cultured in matrices with stiffness ranging between that of normal tissue to that of tumor tissue. Increased collagen content of the substrate compromised tissue organization, inhibited lumen formation and destabilized cell—cell junctions in non-malignant structures. In addition, increase in substrata rigidity enhanced growth by inducing Rho-generated cytoskeletal tension to promote focal adhesion assembly and increased extracellular-signal-regulated kinase activation [55]. Thus, normal tissue function (i.e. homeostasis) is favored by a ‘soft’ microenvironment and low tension, whereas tumorigenic behavior is initiated when the ECM becomes chronically stiffer and/or when mechanotransduced tension rises [55].
These studies point to the possibility that breast density and rigidity might govern the tensional homeostasis needed to maintain normal cell differentiation, morphology and function and, as a consequence, any imbalance in the dynamic reciprocity between the epithelium and its microenvironment could be an incentive for the tissue to undergo malignant transformation. Irrespective of whether changes in the microenvironment, including modification of stromal characteristics, might precede epithelial cell changes, future research could be helped by the development of additional spontaneous progression series [56] and an analysis of how the progression can be altered in vivo and in 3D cultures [57].
Genetic evidence for direct involvement of stroma in carcinogenesis
In addition to molecular portraits of entire human breast tumors and breast cancer cell lines [57], specific stromal cell profiles have now started to appear, and more needs to be done. Originally, a so-called ‘wound response signature’, defined as a general transcriptional response of cultured fibroblasts to serum, provided a possible link between cancer progression and wound healing [58], and sub-sequently more specific profiles have been described. Based on the hypothesis that different types of fibroblastic tumors represent expansion of different subpopulations of fibroblasts, the expression profiles of two fibroblastic tumors, solitary fibrous tumor (SFT) and desmoid-type fibromatosis (DTF), were determined [59]. A set of genes that distinguishes the two profiles was defined and, upon application to breast carcinomas, two groups of breast carcinomas with significant differences in overall survival were revealed, with the DTF profile having a more favorable outcome [59]. Although these are interesting findings, we believe that the use of only two stromal profiles for drawing survival conclusions might be too simple an approach and, in light of current knowledge on the importance of other luminal microenvironment cell types, such as myoepithelial cells and macrophages, the need for multiple stromal profiles is even more essential [24,31,60].
The stromal profiles are also being explored at the genome level. In germ-line BRCA1/2 (see Glossary)-related breast cancers, the average frequencies of loss of heterozygosity or allelic imbalance (LOH/AI) in stroma and epithelium were reported to be similar, whereas in sporadic breast cancer, the average epithelial LOH/AI frequency far exceeds the average stromal LOH/AI frequency [61]. Further scrutiny has revealed that stroma-specific LOH/AI is associated with mutations in the somatic tumor suppressor gene TP53 and regional lymph-node metastases in sporadic but not hereditary breast cancers, suggesting that genomic alterations in stromal cells contribute to the clinical outcome [62]. However, because genetic alterations in the stroma of sporadic breast cancer can be distinct from, and hence independent of, those of adjacent malignant epithelium, the epithelial and stromal mutations are not derived from a common progenitor but rather seem to both undergo similar selection pressures in the tumor microenvironment [63]. In some instances, stromal changes might not even be restricted to the microenvironment: recently, frequent genetic alterations have been revealed in normal skin in breast cancer patients [64]. In spite of the limited sample number (twelve), these findings provide evidence of a genetic basis for the aberrant behavior of explanted normal skin fibroblasts from patients with breast cancer, a phenomenon that was originally described more than two decades ago [65]. It is tempting to speculate that a broader spectrum of LOH/AI affecting multiple cell populations in the organ might ultimately be manifested in a more ‘localized’ carcinoma [64].
Nevertheless, translation of stromal cell signatures into clinical relevance is still far from accepted. Comparison of the expression profiles of morphologically normal stromal tissue from patients with breast cancer or undergoing breast reduction mammoplasty did not show molecular signatures that distinguished breast reduction tissue from normal stroma adjacent to the tumor — apparently contradicting the hypothesis that normal stromal gene sets are predictive of clinical characteristics [66]. However, normal and tumor-adjacent stroma samples were taken exclusively from the (extra-)interlobular stromal compartment, and it is possible that further scrutiny of intralobular stroma might reveal additional differences. More correlations between clinicopathological features and LOH/AI exist in the intralobular stroma than in the tumor epithelium, suggesting first that the intralobular stroma is indeed the relevant source of information and second that stromal genomic alterations might in fact account for clinical diversity [67]. However, there are some caveats in using intralobular stroma, including the risk of sampling stray cells from the epithelial compartment as well as ‘converted’ tumor cells [19].
Future directions
Aging constitutes the greatest risk for developing cancer. Since the risk increases exponentially with age, it is prudent to consider the possibility of preventing cancer from manifesting itself by protecting the stroma. Although this is not likely to happen soon, future advances in diagnosis, prognosis and therapy should focus on the contributions of individual effector elements of cancer, including key cytokines, ECM composition and stromal and immune cells, at the site of the tumor. Very recent studies have started to explore these new areas. Thus, DNA vaccination against tumor-associated macrophages [68], protection of stromal cells against tumor—stroma interactions by antioxidants [69], reversion of fibrotic-type stroma to active granulation tissue in skin tumors [70], tumor suppression in human embryonic stem cell microenvironment [71], and T-cell-directed destruction of tumor stroma [72] are all strategies that recognize and target the controlling function of stroma and focus on targets within it. Likewise, should it transpire that bone-marrow-derived mesenchymal stem cells and/or fibrocytes contribute significantly to human breast tumor stroma, as has been shown in other reactive fibroses [14], a potential new portal for the application of anticancer therapies could open.
Despite the dual role of TGF-β in normal function and cancer, breast tumor cells in which the TGF-β signaling pathway has gone awry have been shown to undergo apoptosis in response to rapamycin [73]. In the future, the simultaneous use of agents such as rapamycin incombination with TGF-β inhibitors might be even more effective in targeting tumor cells and at the same time might abrogate the cancer-promoting effect of the microenvironment.
Further resolution of the stromal component itself, in terms of cellular and ECM compositions and gene expression profiles combined with histological analysis, could improve risk assessment and add useful parameters of prognosis. One result of this approach is the recent recognition of the scar-like centre, the fibrotic focus, of invasive breast carcinomas as a surrogate marker for hypoxia and lymphangiogenesis, in addition to being a practical and reproducible histological prognostic parameter [74]. Another important finding is that ECM signatures identify breast cancer subgroups with different clinical outcome [75].
Cancer patients are treated still according to generalized guidelines. Ideally, the increasing knowledge about the stroma, once sufficiently verified by basic research, should rapidly translate into therapeutic strategies individually tailored to target the tumor cells as well as the tumor microenvironment. Irrespective of approach, however, a major hurdle to be resolved is the integration of novel information into current pathological and clinical procedures. For instance, although some techniques for revealing stromal characteristics, such as those applied for stroma-specific LOH/AI markers, are already established in the clinic, others, such as laser-capture microdissection, might be too advanced for routine clinical use [62], and more work is needed to see how they could be incorporated in the future. For a summary of outstanding questions, see Box 1.
Box 1. Outstanding questions.
More studies are required to characterize the stromal cell and ECM composition in normal breast tissue and how this changes when interacting with epithelial cells immediately before cancer initiation, as well as during cancer development and progression. The use of culture models of epithelial cell progression in 3D or in vivo models would help to resolve the following questions:
What is the contribution of subpopulations of stromal cells (bone marrow mesenchymal stem cells, fibrocytes or resident stromal cells) to breast tumor development?
Do changes in stromal cells and/or ECM, either inherited or sporadic, precede tumor initiation to any significant extent and, if so, how could the interactive process be prevented or aborted at an early time point?
Is prognosis related to the stromal cell make up of a tumor and, if so, can therapy be tailored to attack diverse stromal compartments in addition to conventional targeting of the tumor cells themselves?
Concluding remarks
It has been known for some time that microenvironments that can impose a normal tissue architecture can both suppress the malignant phenotype [76,77] and instruct otherwise malignant totipotent cells to give origin to differentiated cells and engage in normal organ development [78]. However, evidence is now accumulating that continuous input from the microenvironment might also determine the risk and course of tumor development in the adult mammary gland. To complement the advancing knowledge of stromal composition and characteristics before and after cancer development, the challenge now is to further develop experimental models to encompass the complexity of 3D tissue organization, multiple stromal cell populations and ECM composition while allowing for interpretation of the full significance of the microenvironment in the normal gland and in breast cancer [79,80]. These findings need to be translated into a double-barreled therapy that targets both the cancer cells and the particulate tumor stroma. Other approaches, such as the recruitment of useful bystanders (e.g. T lymphocytes), should also be considered for killing deserting tumor cells [81] or for destroying supporting stroma [72].
Undoubtedly, a more holistic and mechanistic view of tumor development will evolve into novel strategies of risk assessment, diagnosis, prognosis and therapy and, ideally, the attenuation and/or eradication of breast cancer.
Acknowledgements
Work from the authors’ laboratories is supported by grants from the Danish Research Council and the Danish Cancer Society (L.R-J.); a Distinguished Fellow Award from the Office of Science and the Office of Biological and Environmental Research of the US Department of Energy (M.J.B.); the Low Dose Radiation Program of the US Department of Energy (M.J.B.); National Cancer Institute awards R01CA064786 and U54CA126552 (M.J.B.); and Innovator Awards from the Department of Defence Breast Cancer Research Program (BC012005) (M.J.B.).
Glossary
- Adenocarcinoma
a malignant epithelial tumor derived from glandular epithelium.
- Angiogenesis
(angeion: vessel) sprouting and remodeling of existing blood vessels during development or induced in cancer.
- BRCA1/2
breast cancer susceptibility gene 1 or breast cancer susceptibility gene 2. BRCA is a tumor-supressing gene; if even one copy of it is mutated, cancer can develop.
- Desmoid
(desmos: band; eidos: form) very firm fibroma, often derived from fascia or tendon.
- Desmoplasia
(desmos: band; plasia: formation) desmoplasia is a host myofibroblast-mediated collagenous response that leads to a hard consistency of the majority of breast tumors.
- Epithelial—mesenchymal transition (EMT)
a fundamental process governing morphogenesis in multicellular organisms that might be reactivated during progression of carcinoma. The molecular mechanisms of EMT involve loss of epithelial cell polarity and the acquisition of a variety of mesenchymal phenotypic traits.
- Fibrocyte
blood-borne stellate shaped cell capable of forming collagen upon recruitment to connective tissue.
- Fibrosis
( fibra: fiber) induction of fibrous, often highly collagenous, tissue in an organ.
- Focal adhesion
focus of anchoring junctions that bind cells to ECM.
- Hematopoietic disorder
hematopoiesis is the formation and development of blood cells from stem cells, which in adult mammals occurs in bone marrow. A hematopoietic disorder is a disruption of normal development.
- Hyperplasia
abnormal increase in the number of normal cells in normal arrangement in a tissue.
- Hypoxia
reduction of oxygen supply to tissue below physiological levels despite adequate perfusion of the tissue by blood.
- Immortalization
normal cells have a limited lifespan and are mortal, whereas cancer cells have undergone mutations to escape these restrictions and therefore as populations are “immortal”. Immortalization can be induced experimentally.
- Involution
degressive process, which all organs are submitted to in aging individuals. The breast in addition undergoes an involution process each time lactation is terminated.
- Luminal epithelial cells
cells that line the mammary ducts and alveoli.
- Lymph node status
a prognostic parameter based on assessment of the number of lymph nodes populated with metastasized cancer cells.
- Mammography
soft tissue X-ray of the breast gland without contrast injection.
- Rous sarcoma virus
the virus responsible for the classic first cell-free transmission of a solid tumor, the chicken sarcoma, first reported by Rous in 1911.
- Senescence
(senescentia: old age) a progressive process that cells undergo as they age, becoming incapable of further division unless immortalized.
- Totipotency
(totus: whole) characteristic of stem- or stem-like cells, which can generate all cell types in the organism.
References
- 1.Bissell MJ, et al. How does extracellular matrix direct gene expression? J. Theor. Biol. 1982;99:31–68. doi: 10.1016/0022-5193(82)90388-5. [DOI] [PubMed] [Google Scholar]
- 2.Bissell MJ, Radisky D. Putting tumors in context. Nat. Rev. Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rønnov-Jessen L, et al. Cellular changes involved in conversion of normal to malignant breast: the importance of the stromal reaction. Physiol. Rev. 1996;76:69–125. doi: 10.1152/physrev.1996.76.1.69. [DOI] [PubMed] [Google Scholar]
- 4.Folkman J. Angiogenesis and breast cancer. J. Clin. Oncol. 1994;12:441–443. doi: 10.1200/JCO.1994.12.3.441. [DOI] [PubMed] [Google Scholar]
- 5.Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N. Engl. J. Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- 6.Sieweke MH, Bissell MJ. The tumor promoting effect of wounding: a possible role for TGF-β-induced stromal alterations. Crit. Rev. Oncog. 1994;5:297–311. doi: 10.1615/critrevoncog.v5.i2-3.90. [DOI] [PubMed] [Google Scholar]
- 7.Lu H, et al. Inflammation, a key event in cancer development. Mol. Cancer Res. 2006;4:221–233. doi: 10.1158/1541-7786.MCR-05-0261. [DOI] [PubMed] [Google Scholar]
- 8.DeNardo DG, Coussens LM. Balancing immune response: crosstalk between adaptive and innate immune cells during breast cancer progression. Breast Cancer Res. 2007;9:212. doi: 10.1186/bcr1746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Walkley CR, et al. A microenvironment-induced myeloproliferative syndrome caused by retinoic acid receptor γ deficiency. Cell. 2007;129:1097–1110. doi: 10.1016/j.cell.2007.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Walkley CR, et al. Rb regulates interactions between hematopoietic stem cells and their bone marrow microenvironment. Cell. 2007;129:1081–1095. doi: 10.1016/j.cell.2007.03.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rønnov-Jessen L, et al. The origin of the myofibroblasts in breast cancer: recapitulation of tumor environment in culture unravels diversity and implicates converted fibroblasts and recruited smooth muscle cells. J. Clin. Invest. 1995;95:859–873. doi: 10.1172/JCI117736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449:557–565. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 13.Mori L, et al. Fibrocytes contribute to the myofibroblast population in wounded skin and originate from the bone marrow. Exp. Cell Res. 2005;304:81–90. doi: 10.1016/j.yexcr.2004.11.011. [DOI] [PubMed] [Google Scholar]
- 14.Bellini A, Mattoli S. The role of the fibrocyte, a bone marrow-derived mesenchymal progenitor, in reactive and reparative fibroses. Lab. Invest. 2007;87:858–870. doi: 10.1038/labinvest.3700654. [DOI] [PubMed] [Google Scholar]
- 15.Sangai T, et al. Effect of differences in cancer cells and tumor growth sites on recruiting bone marrow-derived endothelial cells and myofibroblasts in cancer-induced stroma. Int. J. Cancer. 2005;115:885–892. doi: 10.1002/ijc.20969. [DOI] [PubMed] [Google Scholar]
- 16.Gupta PB, et al. Systemic stromal effects of estrogen promote the growth of estrogen receptor-negative cancers. Cancer Res. 2007;67:2062–2071. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- 17.Barth PJ, et al. CD34+ fibrocytes in invasive ductal carcinoma, ductal carcinoma in situ, and benign breast lesions. Virchows Arch. 2002;440:298–303. doi: 10.1007/s004280100530. [DOI] [PubMed] [Google Scholar]
- 18.Rønnov-Jessen L. Stromal reaction to invasive cancer: the cellular origin of the myofibroblast and implications for tumor development. Breast J. 1996;2:320–339. [Google Scholar]
- 19.Petersen OW, et al. Epithelial to mesenchymal transition in human breast cancer can provide a non-malignant stroma. Am. J. Pathol. 2003;162:391–402. doi: 10.1016/S0002-9440(10)63834-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allinen M, et al. Molecular characterization of the tumor microenvironment in breast cancer. Cancer Cell. 2004;6:17–32. doi: 10.1016/j.ccr.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 21.Samoszuk M, et al. Clonogenic growth of human breast cancer cells co-cultured in direct contact with serum-activated fibroblasts. Breast Cancer Res. 2005;7:R274–R283. doi: 10.1186/bcr995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Orimo A, et al. Stromal fibroblasts present in invasive human breast carcinomas promote tumor growth and angiogenesis through elevated SDF-1/CXCL12 secretion. Cell. 2005;121:335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 23.Olumi AF, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59:5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu M, et al. Regulation of in situ to invasive breast carcinoma transition. Cancer Cell. 2008;13:394–406. doi: 10.1016/j.ccr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barcellos-Hoff MH, Ravani SA. Irradiated mammary gland stroma promotes the expression of tumorigenic potential by unirradiated epithelial cells. Cancer Res. 2000;60:1254–1260. [PubMed] [Google Scholar]
- 26.Park CC, et al. Ionizing radiation induces heritable disruption of epithelial cell interactions. Proc. Natl. Acad. Sci. U. S. A. 2003;100:10728–10733. doi: 10.1073/pnas.1832185100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Maffini MV, et al. The stroma as a crucial target in rat mammary gland carcinogenesis. J. Cell Sci. 2004;117:1495–1502. doi: 10.1242/jcs.01000. [DOI] [PubMed] [Google Scholar]
- 28.Soucek L, et al. Mast cells are required for angiogenesis and macroscopic expansion of Myc-induced pancreatic islet tumors. Nat. Med. 2007;13:1211–1218. doi: 10.1038/nm1649. [DOI] [PubMed] [Google Scholar]
- 29.Rajput AB, et al. Stromal mast cells in invasive breast cancers are a marker of favourable prognosis: a study of 4444 cases. Breast Cancer Res. Treat. 2008;107:249–257. doi: 10.1007/s10549-007-9546-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Crowther M, et al. Microenvironmental influence on macrophage regulation of angiogenesis in wounds and malignant tumors. J. Leukoc. Biol. 2001;70:478–490. [PubMed] [Google Scholar]
- 31.Lin EY, Pollard JW. Tumor-associated macrophages press the angiogenic switch in breast cancer. Cancer Res. 2007;67:5064–5066. doi: 10.1158/0008-5472.CAN-07-0912. [DOI] [PubMed] [Google Scholar]
- 32.Sympson CJ, et al. Targeted expression of stromelysin-1 in mammary gland provides evidence for a role of proteinases in branching morphogenesis and the requirement for an intact basement membrane for tissue-specific gene expression. J. Cell Biol. 1994;125:681–693. doi: 10.1083/jcb.125.3.681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomasset N, et al. Expression of autoactivated stromelysin-1 in mammary glands of transgenic mice leads to a reactive stroma during early development. Am. J. Pathol. 1998;153:457–467. doi: 10.1016/S0002-9440(10)65589-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sternlicht MD, et al. The stromal proteinase MMP3/stromelysin-1 promotes mammary carcinogenesis. Cell. 1999;98:137–146. doi: 10.1016/s0092-8674(00)81009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bhowmick NA, et al. TGF-β signaling in fibroblasts modulates the oncogenic potential of adjacent epithelia. Science. 2004;303:848–851. doi: 10.1126/science.1090922. [DOI] [PubMed] [Google Scholar]
- 36.Maffini MV, et al. Stromal regulation of neoplastic development: age-dependent normalization of neoplastic mammary cells by mammary stroma. Am. J. Pathol. 2005;167:1405–1410. doi: 10.1016/S0002-9440(10)61227-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Balogh GA, et al. Genomic signature induced by pregnancy in the human breast. Int. J. Oncol. 2006;28:399–410. [PubMed] [Google Scholar]
- 38.Rajkumar L, et al. Short-term exposure to pregnancy levels of estrogen prevents mammary carcinogenesis. Proc. Natl. Acad. Sci. U. S. A. 2001;98:11755–11759. doi: 10.1073/pnas.201393798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Albrektsen G, et al. Breast cancer risk by age at birth, time since birth and time intervals between births: exploring interaction effects. Br. J. Cancer. 2005;92:167–175. doi: 10.1038/sj.bjc.6602302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schedin P. Pregnancy-associated breast cancer and metastasis. Nat. Rev. Cancer. 2006;6:281–291. doi: 10.1038/nrc1839. [DOI] [PubMed] [Google Scholar]
- 41.Lund LR, et al. Two distinct phases of apoptosis in mammary gland involution: proteinase-independent and -dependent pathways. Development. 1996;122:181–193. doi: 10.1242/dev.122.1.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schedin P, et al. Mammary ECM composition and function are altered by reproductive state. Mol. Carcinog. 2004;41:207–220. doi: 10.1002/mc.20058. [DOI] [PubMed] [Google Scholar]
- 43.McDaniel SM, et al. Remodeling of the mammary microenvironment after lactation promotes breast tumor cell metastasis. Am. J. Pathol. 2006;168:608–620. doi: 10.2353/ajpath.2006.050677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Talhouk RS, et al. Proteinases of the mammary gland: developmental regulation in vivo and vectorial secretion in culture. Development. 1991;112:439–449. doi: 10.1242/dev.112.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krippl P, et al. The 5A/6A polymorphism of the matrix metalloproteinase 3 gene promoter and breast cancer. Clin. Cancer Res. 2004;10:3518–3520. doi: 10.1158/1078-0432.CCR-04-0010. [DOI] [PubMed] [Google Scholar]
- 46.Radisky DC, et al. Rac1b and reactive oxygen species mediate MMP-3-induced EMT and genomic instability. Nature. 2005;436:123–127. doi: 10.1038/nature03688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Parrinello S, et al. Stromal—epithelial interactions in aging and cancer: senescent fibroblasts alter epithelial cell differentiation. J. Cell Sci. 2005;118:485–496. doi: 10.1242/jcs.01635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aiello EJ, et al. Association between mammographic breast density and breast cancer tumor characteristics. Cancer Epidemiol. Biomarkers Prev. 2005;14:662–668. doi: 10.1158/1055-9965.EPI-04-0327. [DOI] [PubMed] [Google Scholar]
- 49.Boyd NF, et al. Mammographic density and the risk and detection of breast cancer. N. Engl. J. Med. 2007;356:227–236. doi: 10.1056/NEJMoa062790. [DOI] [PubMed] [Google Scholar]
- 50.Walker RA. The complexities of breast cancer desmoplasia. Breast Cancer Res. 2001;3:143–145. doi: 10.1186/bcr287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alowami S, et al. Mammographic density is related to stroma and stromal proteoglycan expression. Breast Cancer Res. 2003;5:R129–R135. doi: 10.1186/bcr622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lundström E, et al. Expression of Syndecan-1 in histologically normal breast tissue from postmenopausal women with breast cancer according to mammographic density. Climacteric. 2006;9:277–282. doi: 10.1080/13697130600865741. [DOI] [PubMed] [Google Scholar]
- 53.Hasebe T, et al. Fibrotic focus in infiltrating ductal carcinoma of the breast: a significant histopathological prognostic parameter for predicting the long-term survival of the patients. Breast Cancer Res. Treat. 1998;49:195–208. doi: 10.1023/a:1006067513634. [DOI] [PubMed] [Google Scholar]
- 54.Engler AJ, et al. Matrix elasticity directs stem cell linage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 55.Paszek MJ, et al. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8:241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 56.Briand P, et al. Trisomy 7p and malignant transformation of human breast epithelial cells following epidermal growth factor withdrawel. Cancer Res. 1996;56:2039–2044. [PubMed] [Google Scholar]
- 57.Rizki A, et al. A human breast cell model of preinvasive transition. Cancer Res. 2008;68:1378–1387. doi: 10.1158/0008-5472.CAN-07-2225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chang HY, et al. Gene expression signature of fibroblast serum response predicts human cancer progression: similarities between tumors and wounds. PLoS Biol. 2004;2:e7. doi: 10.1371/journal.pbio.0020007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.West RB, et al. Determination of stromal signatures in breast carcinoma. PLoS Biol. 2005;3:e187. doi: 10.1371/journal.pbio.0030187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Condeelis J, Pollard JW. Macrophages: obligate partners for tumor cell migration, invasion, and metastasis. Cell. 2006;124:263–266. doi: 10.1016/j.cell.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 61.Weber F, et al. Total-genome analysis of BRCA1/2-related invasive carcinomas of the breast identifies tumor stroma as potential landscaper for neoplastic initiation. Am. J. Hum. Genet. 2006;78:961–972. doi: 10.1086/504090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Patocs A, et al. Breast-cancer stromal cells with TP53 mutations and nodal metastases. N. Engl. J. Med. 2007;357:2543–2551. doi: 10.1056/NEJMoa071825. [DOI] [PubMed] [Google Scholar]
- 63.Fukino K, et al. Combined total genome loss of heterozygosity scan of breast cancer stroma and epithelium reveals multiplicity of stromal targets. Cancer Res. 2004;64:7231–7236. doi: 10.1158/0008-5472.CAN-04-2866. [DOI] [PubMed] [Google Scholar]
- 64.Moinfar F, et al. Macro-environment of breast carcinoma: frequent genetic alterations in the normal appearing skins of patients with breast cancer. Mod. Pathol. 2008;21:639–646. doi: 10.1038/modpathol.2008.28. [DOI] [PubMed] [Google Scholar]
- 65.Schor SL, et al. Skin obtained from cancer patients display fetal-like behaviour in collagen gels. J. Cell Sci. 1985;73:235–244. doi: 10.1242/jcs.73.1.235. [DOI] [PubMed] [Google Scholar]
- 66.Finak G, et al. Gene expression signatures of morphologically normal breast tissue identify basal-like tumors. Breast Cancer Res. 2006;8:R58. doi: 10.1186/bcr1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fukino K, et al. Genomic instability within tumor stroma and clinicopathological characteristics of sporadic primary invasive breast cancer. J. Am. Med. Assoc. 2007;297:2103–2111. doi: 10.1001/jama.297.19.2103. [DOI] [PubMed] [Google Scholar]
- 68.Luo Y, et al. Targeting tumor-associated macrophages as a novel strategy against breast cancer. J. Clin. Invest. 2006;116:2132–2141. doi: 10.1172/JCI27648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cat B, et al. Enhancement of tumor invasion depends on trasdifferentiation of skin fibroblasts mediated by reactive oxygen species. J. Cell Sci. 2006;119:2727–2738. doi: 10.1242/jcs.03011. [DOI] [PubMed] [Google Scholar]
- 70.Willhauck MJ, et al. Reversion of tumor phenotype in surface transplants of skin SCC cells by scaffold-induced stroma modulation. Carcinogenesis. 2006;28:595–610. doi: 10.1093/carcin/bgl188. [DOI] [PubMed] [Google Scholar]
- 71.Postovit L-M, et al. Human embryonic stem cell microenvironment supresses the tumorigenic phenotype of aggressive cancer cells. Proc. Natl. Acad. Sci. U. S. A. 2008;105:4329–4334. doi: 10.1073/pnas.0800467105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang B, et al. Equilibrium between host and cancer caused by effector T cells killing tumor stroma. Cancer Res. 2008;68:1563–1571. doi: 10.1158/0008-5472.CAN-07-5324. [DOI] [PubMed] [Google Scholar]
- 73.Gadir N, et al. Defective TGF-β signaling sensitizes human cancer cells to rapamycin. Oncogene. 2007;27:1055–1062. doi: 10.1038/sj.onc.1210721. [DOI] [PubMed] [Google Scholar]
- 74.Van den Eynden GG, et al. A fibrotic focus is a prognostic factor and a surrogate marker for hypoxia and (lymph)angiogenesis breast cancer. Review of the literature and proposal on the criteria of evaluation. Histopathology. 2007;51:440–451. doi: 10.1111/j.1365-2559.2007.02761.x. [DOI] [PubMed] [Google Scholar]
- 75.Bergamaschi A, et al. Extracellular matrix signature identifies breast cancer subgroups with different clinical outcome. J. Pathol. 2008;214:357–367. doi: 10.1002/path.2278. [DOI] [PubMed] [Google Scholar]
- 76.Dolberg DS, Bissell MJ. Inability of Rous sarcoma virus to cause sarcomas in the avian embryo. Nature. 1984;309:552–556. doi: 10.1038/309552a0. [DOI] [PubMed] [Google Scholar]
- 77.Weaver VM, et al. Reversion of the malignant phenotype of human breast cells in 3-dimensional culture and in vivo using integrin blocking antibodies. J. Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Illmensee K, Mintz B. Totipotency and normal differentiation of single teratocarcinoma cells cloned by injection into blastocysts. Proc. Natl. Acad. Sci. U. S. A. 1976;73:549–553. doi: 10.1073/pnas.73.2.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Petersen OW, et al. The microenvironment of the breast: three-dimensional models to study the roles of the stroma and the extracellular matrix in function and dysfunction. Breast J. 1995;1:22–35. [Google Scholar]
- 80.Nelson CM, Bissell MJ. Modeling dynamic reciprocity: engineering three-dimensional culture models of breast architecture, function, and neoplastic transformation. Semin. Cancer Biol. 2005;15:342–352. doi: 10.1016/j.semcancer.2005.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bernhard H, et al. Adoptive transfer of autologous, HER2-specific, cytotoxic T lymphocytes for the treatment of HER2-overexpressing breast cancer. Cancer Immunol. Immunother. 2008;57:271–280. doi: 10.1007/s00262-007-0355-7. [DOI] [PMC free article] [PubMed] [Google Scholar]