Introduction
Agonist-induced internalization of G-protein coupled receptors (GPCRs) is an important function to maintain homeostatic control in the cell and to regulate cell surface expression of receptors (Ferguson, 2001; Gainetdinov et al., 2004). GPCR internalization primarily occurs through an agonist-driven association with an arrestin protein which functions as a scaffold to target the receptors to clathrin-coated pits and subsequent endosomes (Ferguson, 2001; Gainetdinov et al., 2004). There are four known arrestins, although only two are expressed outside of the visual system (Lefkowitz and Shenoy, 2005). These non-visual arrestins are referred to as arrestin2 and arrestin3, also known as β-arrestin1 and β-arrestin2, respectively. A major area of inquiry has been directed at establishing receptor specificity of the non-visual arrestins and whether or not there is redundancy of function in these molecules. Initial investigations by Oakley et al. (Oakley et al., 2000 and 2001) suggested that there are two classes of GPCRs: class A receptors, which bind arrestin3 with greater affinity than arrestin2, and class B receptors which bind both arrestin2 and arrestin3 with high affinity. Lefkowitz and colleagues have also shown using cells derived from arrestin2 or arrestin3 knockout mice, that internalization of different GPCRs can indeed be selectively mediated by either arrestin2 or arrestin3 (Kohout et al., 2001).
The D2 dopamine receptor (DAR) is an important drug target in neuropsychiatry and understanding its regulation is critical to the development of improved therapies involving the modulation of D2-mediated signaling. While it is known that agonist-induced internalization of the D2 receptor is mediated by arrestins (Ito et al., 1999; Kim et al., 2001), the arrestin specificity of this process is not known with certainty. In heterologous expression systems, the D2 receptor appears to interact equally well with either arresin2 or arrestin3 (Macey et al., 2004, Namkung and Sibley, unpublished observations) suggesting that it might be a class B receptor. In contrast, Macey et al. (2004) have suggested that arrestin2 selectively mediates D2 DAR internalization in neostriatal neurons in culture. Conversely, Caron and colleagues have suggested that the D2 receptor selectively associates with arrestin3 in the brain (Beaulieu et al., 2005). In the current study, we have further investigated the arrestin selectivity of agonist-induced D2 DAR internalization using arrestin3 knockout mice and conclusively find that arrestin3 is required for D2 receptor internalization.
Materials and Methods
The D2-like agonist 2-methoxy-N-propylnorapomorphine (MNPA) was synthesized by Christer Halldin at the Karolinska Institute. MNPA exhibits high affinity (∼ 1 nM) for both the D2 and D3 receptors (Skinbjerg et al., in press), whereas its affinity for D4 receptors is undetermined. The arrestin3-deficient mice (Bohn et al., 1999) were a kind gift from Dr. Robert J. Lefkowitz and were generated and characterized as described (Bjork et al., 2008). Homozygous wild-type and arrestin3-deficient mice were obtained by breeding heterozygote mice. Male mice from 3-6 months of age were used for experimentation. The brains were removed rapidly and frozen in powdered dry ice, then cut in 10 μm coronal sections (1.34-0.02 mm from bregma) on a cryostat. Sections were thaw-mounted onto glass slides and allowed to air dry at room temperature before processing. Sections were hydrated for 5 min in phosphate buffered saline (PBS, pH 7.2) followed by a 30 min incubation in PBS containing 2% ascorbic acid and either 100 μM dopamine (Sigma-Aldrich), 100 nM MNPA, 50 nM SKF-81297 (Sigma-Aldrich), or PBS-ascorbate buffer for controls. Blocking experiments were performed by pre-treating the sections with the D2-like antagonist eticlopride (100 nM, Sigma-Aldrich) for 10 min followed by incubation in the internalization cocktail containing both agonist and antagonist. All incubations were carried out at room temperature in a foil-covered moisture box to minimize evaporation and exposure to light. Sections were then fixed for 5 min in freshly made 4% paraformaldehyde in PBS, rinsed for 5 min in PBS and incubated at 4°C overnight in primary anti-D2 dopamine receptor antisera (1:200), raised against a synthetic peptide representing the amino terminal 18 residues of the receptor (McVittie et al., 1991). The characterization and specificity of this antiserum, which recognizes both D2S and D2L isoforms, has been previously described (McVittie et al., 1991; Ariano et al., 1993; Cepeda et al., 2001). The next day, the sections were rinsed twice for 15 min in PBS and incubated for 1.5 h at 4°C in CY3 fluorescently labeled anti-rabbit secondary antisera (Jackson ImmunoResearch, Inc.). The incubation was terminated by two rinses (15 min each) in PBS, followed by a brief dip in ddH2O and left to air dry in a light-protected container.
Images of striatal tissue sections were captured using epifluorescence microscopy (Olympus BX41 at 20× magnifications). The exposure time for images was established using control sections, and the duration of time was kept constant for all treatment groups. Fluorescence intensity was quantified by measuring the luminosity via the Adobe Photoshop CS2 histogram function as previously described (Ariano et al., 2005). Briefly, D2 DAR positive cells in the striatum were visually located and measured for luminosity with a minimum value of 20% above background. Background was defined as the luminosity of the fiber bundles perforating the striatum. A total of 18-21 images from each group were acquired from the agonist treatment experiments, and 7 images from each group from the blocking experiments were measured. Luminosity values from treatment groups were compared to controls and analyzed using Student's two-tailed t-test.
Results and Discussion
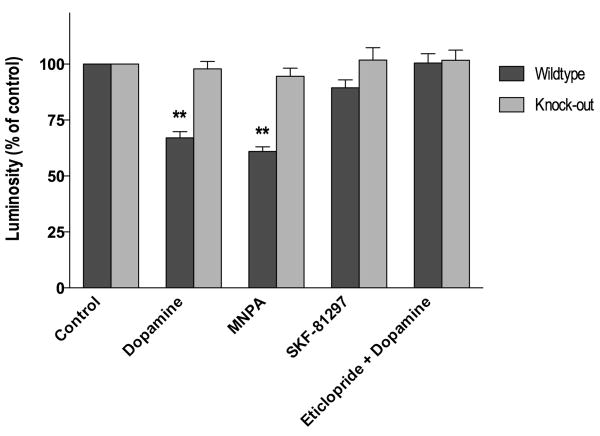
We have previously used striatal tissue slices and immunohistochemistry to study agonist-induced internalization of the D1 dopamine receptor (Ariano et al., 1997). One advantage of using the tissue slice system is that cellular architecture is maintained which is not the case when the tissue is disrupted in order to prepare primary neurons. Visualization of the D2 DARs within striatal tissue slices revealed that receptor immunofluorence was primarily observed within the somata of medium-diameter striatal neurons and secondarily within the neuropil (Fig. 1). There did not appear to be any qualitative or quantitative differences of D2 DAR staining between wild-type and arrestin3-deficient mouse tissue (cf. Fig. 1 control panels). Pretreatment of the tissue with either dopamine or the D2-like agonist, MNPA, prior to receptor staining, resulted in a ∼35% reduction in the receptor immunofluorescence throughout the slices (Figs. 1 and 2). As the tissue slices were not permeabilized, and the D2 DAR antiserum employed is directed to an extracellular epitope on the receptor (McVittie et al., 1991), the loss of receptor staining is interpreted to be due to receptor internalization, as we have previously shown for the D1 DAR in similar experiments (Ariano et al., 1997). Strikingly, neither dopamine nor MNPA pretreatment had any effect on D2 DAR staining in striatal tissue from the arrestin3-deficient mice (Figs. 1 and 2). This is especially notable given that the expression of arrestin2 is normal in these mice (Bohn et al., 1999).
Fig. 1.
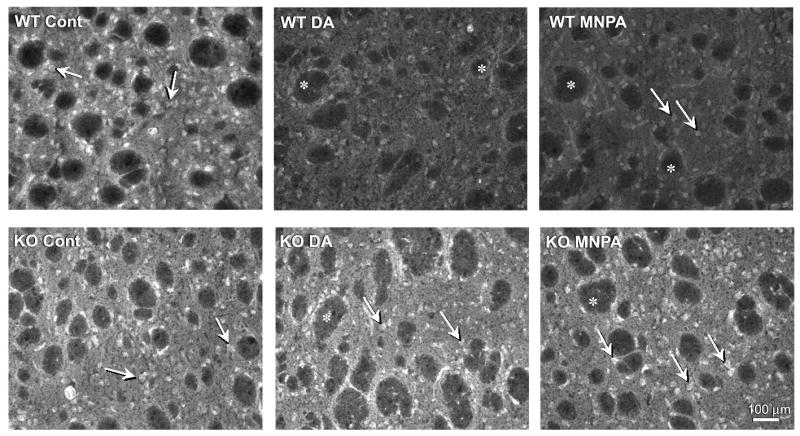
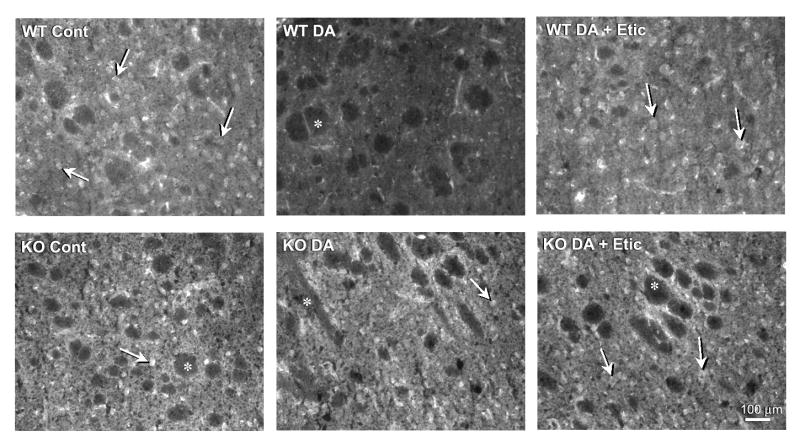
Agonist-induced internalization of D2 DARs in striatal slices from wild-type and arrestin3-deficient mouse brains. Fresh-frozen striatal slices were prepared and pre-incubated with either buffer (control), dopamine (100 μM), or MNPA (50 nM) for 30 min followed by fixation, immunostaining, and fluorescence microscopy as described in the Materials and Methods. Cell bodies of medium spiny neurons expressing the D2 DAR appear bright and clear and are uniformly distributed throughout the striatal tissue. Arrows denote representative staining in medium diameter neurons. Asterisks are placed in the unstained fiber bundles of the descending cortical fibers that penetrate the parenchyma of the rodent striatum. Upper Panels: tissue from wild-type mice show markedly decreased receptor staining after pretreatment with dopamine (WT DA) or MNPA (WT MNPA) compared to control (WT Cont). Lower Panels: D2 DAR immuno-reactivity in tissue from arrestin3-deficient mice (KO) is not affected by dopamine or MNPA pretreatment. This experiment was performed 2-5 times with the average results presented in Fig. 2.
Fig. 2.
Pharmacological characterization of agonist-induced D2 DAR internalization in striatal tissue slices. Fresh frozen striatal sections were pretreated with various agents for 30 min as described in Fig. 1 and the subsequently determined D2 DAR immunoreactivities were expressed as a percentage of the control groups. Both dopamine (100 μM) and MNPA (50 nM) caused a significant (33 ± 13 and 39 ± 9%, respectively, P <0.001) decrease of D2 DAR immunostaining in wild-type striatal slices, but had no effect in arrestin3 knockout tissue. Pretreatment with the D1 DAR agonist SKF-81297 (50 nM) did not affect D2 DAR immunostaining in either wild-type or arrestin3 knockout slices. The D2-like antagonist, eticlopride (100 nM), completely blocked the receptor internalization induced with dopamine (100 μM) treatment. All experiments were performed 2-5 times with three slices per slide.
In order to verify that the agonist-induced internalization is pharmacologically specific, we pretreated the tissue slices with a D1-selective agonist, SKF-81297. In contrast to dopamine or the D2-like agonist, MNPA, SKF-81297 treatment did not affect D2 DAR staining in the striatal slices from either the wild-type or arrestin3-deficient mice (Fig. 2). We next attempted to pharmacologically block the dopamine-induced receptor internalization using the D2-like antagonist, eticlopride. Figs. 2 and 3 show that this antagonist, indeed, completely blocks the dopamine-induced loss of D2 DAR staining in the wild-type tissue, whereas, again there is no effect of dopamine in the arrestin3-deficient tissue. These results support the notion that receptor activation is required for the loss of cell surface receptor staining in response to agonists. Taken together, these results further suggest that the expression of arrestin3 is required for D2 receptor internalization in vivo.
Fig. 3.
Pharmacological blockade of dopamine-induced receptor internalization. Fresh frozen striatal sections were treated with dopamine alone (100 μM) or dopamine plus eticlopride (100 nM) for 30 min as described in Fig. 1. Arrows denote representative staining in medium diameter neurons. Asterisks are placed in the unstained fiber bundles of the descending cortical fibers that penetrate the parenchyma of the rodent striatum. Upper Panels: dopamine pretreatment promotes a loss of receptor immunoreactivity in wild-type tissue and this is blocked by eticlopride. Lower Panels: there is no effect of dopamine on receptor internalization in arrestin3 knockout tissue in the presence of absence of eticlopride. This experiment was performed 2-5 times with the average results presented in Fig. 2.
Our current results agree with those of Caron and colleagues suggesting that the D2 DAR primarily interacts with arrestin3 in the brain (Beaulieu et al., 2005). However, they conflict with those of Macey et al. (2004), suggesting that arrestin2 mediates D2 DAR internalization in cultured neostriatal neurons. The reasons for this discrepancy are not clear, although one possible explanation is that the D2 DAR and arrestin3 are compartmentalized within neurons to facilitate their interactions. This compartmentalization may be lost during the tissue disruption and cellular dissociation that is required to prepare primary neuronal cell cultures. Additionally, the association of arrestin2 with D2 DAR may be a transient event associated with less mature neurons, as the primary cultures of Macey et al. (2004), used cells from E15-E18 striata. Our current reported studies used adult mice to examine the D2 DAR internalization. Further confirmation of the role of arrestin3 in D2 receptor signaling and regulation may come from PET imaging studies in live animals using radiolabeled agonist tracers (Seneca et al., 2008).
Acknowledgments
The authors are grateful to Dr. Robert J. Lefkowitz for providing the arrestin3-deficient mice. The authors would also like to thank David Cabrera for his technical assistance. This work was supported in part by the NINDS, NIMH, and NIAAA Intramural Research Programs of the National Institutes of Health.
References
- Ariano MA, Sortwell CE, Ray M, Altemus KL, Sibley DR, Levine MS. Agonist-induced morphologic decrease in cellular D1A dopamine receptor staining. Synapse. 1997;27(4):313–321. doi: 10.1002/(SICI)1098-2396(199712)27:4<313::AID-SYN5>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Fisher RS, Smyk-Randall E, Sibley DR, Levine MS. D2 dopamine receptor distribution in the rodent CNS using anti-peptide antisera. Brain Research. 1993;609:71–80. doi: 10.1016/0006-8993(93)90857-j. [DOI] [PubMed] [Google Scholar]
- Ariano MA, Wagle N, Grissell AE. Neuronal vulnerability in mouse models of Huntington's disease: membrane channel protein changes. J Neurosci Res. 2005;80(5):634–645. doi: 10.1002/jnr.20492. [DOI] [PubMed] [Google Scholar]
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122(2):261–273. doi: 10.1016/j.cell.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Bjork K, Rimondini R, Hansson AC, Terasmaa A, Hyytia P, Heilig M, Sommer WH. Modulation of voluntary ethanol consumption by beta-arrestin 2. Faseb J. 2008;22(7):2552–2560. doi: 10.1096/fj.07-102442. [DOI] [PubMed] [Google Scholar]
- Bohn LM, Lefkowitz RJ, Gainetdinov RR, Peppel K, Caron MG, Lin FT. Enhanced morphine analgesia in mice lacking beta-arrestin 2. Science. 1999;286(5449):2495–2498. doi: 10.1126/science.286.5449.2495. [DOI] [PubMed] [Google Scholar]
- Cepeda C, Hurst RS, Altemus KL, Flores-Hernandez J, Calvert CR, Jokel ES, Grandy DK, Low MJ, Rubinstein M, Ariano MA, Levine MS. Facilitated glutamatergic transmission in the striatum of D2 dopamine receptor mice. J Neurophysiol. 2001;85:659–670. doi: 10.1152/jn.2001.85.2.659. [DOI] [PubMed] [Google Scholar]
- Ferguson SS. Evolving concepts in G protein-coupled receptor endocytosis: the role in receptor desensitization and signaling. Pharmacol Rev. 2001;53(1):1–24. [PubMed] [Google Scholar]
- Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- Ito K, Haga T, Lameh J, Sadee W. Sequestration of dopamine D2 receptors depends on coexpression of G-protein-coupled receptor kinases 2 or 5. Eur J Biochem. 1999;260(1):112–119. doi: 10.1046/j.1432-1327.1999.00125.x. [DOI] [PubMed] [Google Scholar]
- Kim KM, Valenzano KJ, Robinson SR, Yao WD, Barak LS, Caron MG. Differential regulation of the dopamine D2 and D3 receptors by G protein-coupled receptor kinases and beta-arrestins. J Biol Chem. 2001;276(40):37409–37414. doi: 10.1074/jbc.M106728200. [DOI] [PubMed] [Google Scholar]
- Kohout TA, Lin FS, Perry SJ, Conner DA, Lefkowitz RJ. beta-Arrestin 1 and 2 differentially regulate heptahelical receptor signaling and trafficking. Proc Natl Acad Sci U S A. 2001;98(4):1601–1606. doi: 10.1073/pnas.041608198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitz RJ, Shenoy SK. Transduction of receptor signals by beta-arrestins. Science. 2005;308(5721):512–517. doi: 10.1126/science.1109237. [DOI] [PubMed] [Google Scholar]
- Macey TA, Gurevich VV, Neve KA. Preferential Interaction between the dopamine D2 receptor and Arrestin2 in neostriatal neurons. Mol Pharmacol. 2004;66(6):1635–1642. doi: 10.1124/mol.104.001495. [DOI] [PubMed] [Google Scholar]
- McVittie LD, Ariano MA, Sibley DR. Characterization of anti-peptide antibodies for the localization of D2 dopamine receptors in rat striatum. Proc Natl Acad Sci U S A. 1991;88(4):1441–1445. doi: 10.1073/pnas.88.4.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. Molecular determinants underlying the formation of stable intracellular G protein-coupled receptor-beta-arrestin complexes after receptor endocytosis*. J Biol Chem. 2001;276(22):19452–19460. doi: 10.1074/jbc.M101450200. [DOI] [PubMed] [Google Scholar]
- Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. Differential affinities of visual arrestin, beta arrestin1, and beta arrestin2 for G protein-coupled receptors delineate two major classes of receptors. J Biol Chem. 2000;275(22):17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- Seneca N, Zoghbi SS, Skinbjerg M, Liow JS, Hong J, Sibley DR, Pike VW, Halldin C, Innis RB. Occupancy of dopamine D(2/3) receptors in rat brain by endogenous dopamine measured with the agonist positron emission tomography radioligand [(11)C]MNPA. Synapse. 2008;62(10):756–763. doi: 10.1002/syn.20549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinbjerb M, Namkung Y, Halldin C, Innis RB, Sibley DR. Pharmacological characterization of 2-methoxy-N-propylnorapomorphine's (MNPA) interactions with D2 and D3 dopamine receptors. Synapse. 2009 doi: 10.1002/syn.20626. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]