Abstract
Background
A role for vitamin D deficiency in Parkinson disease (PD) has recently been proposed.
Objective
To compare the prevalence of vitamin D deficiency in a research database cohort of patients with PD with the prevalence in age-matched healthy controls and patients with Alzheimer disease (AD).
Design
Survey study and blinded comparison of plasma 25-hydroxyvitamin D (25[OH]D) concentrations of stored samples in a clinical research database at Emory University School of Medicine.
Setting
Referral center (PD and AD patients), primary care clinics, and community setting (controls).
Participants
Participants were recruited into the study between May 1992 and March 2007. Every fifth consecutively enrolled PD patient was selected from the clinical research database. Unrelated AD (n=97) and control (n=99) participants were randomly selected from the database after matching for age, sex, race, APOE genotype, and geographic location.
Main Outcome Measures
Prevalence of suboptimal vitamin D and mean 25(OH)D concentrations.
Results
Significantly more patients with PD (55%) had insufficient vitamin D than did controls (36%) or patients with AD (41%; P=.02, χ2 test). The mean (SD) 25(OH)D concentration in the PD cohort was significantly lower than in the AD and control cohorts (31.9 [13.6] ng/mL vs 34.8 [15.4] ng/mL and 37.0 [14.5] ng/mL, respectively; P=.03).
Conclusions
This report of 25(OH)D concentrations in a predominantly white PD cohort demonstrates a significantly higher prevalence of hypovitaminosis in PD vs both healthy controls and patients with AD. These data support a possible role of vitamin D insufficiency in PD. Further studies are needed to determine the factors contributing to these differences and elucidate the potential role of vitamin D in pathogenesis and clinical course of PD.
Vitamin D is important for maintaining many physiologic functions, and vitamin D deficiency is associated with increased risk of disease. Optimal balance, muscle strength, and innate immunity1–3 require adequate vitamin D levels; vitamin D deficiency is associated with increased risk for several types of cancer, as well as autoimmune and cardiovascular disorders. 1,3–7 Vitamin D also regulates processes known to go awry in multiple sclerosis, Parkinson disease (PD), and other neurodegenerative disorders, including neurotrophin, inducible nitric oxide synthase, glutathione and monoamine synthesis, and apoptosis.8,9 The enzyme 25-hydroxyvitamin D-1α-hydroxylase (1α-OHase) converts the storage 25-hydroxyvitaminD(25[OH]D) form to the biologically active vitamin D form, 1,25-dihydroxyvitamin D. Both 1α-OHase and vitamin D receptors (VDRs) are expressed in many extrarenal tissues, including muscle and brain. Given the high prevalence rates of vitamin D deficiency in such varied populations as elderly patients, chronically ill patients, and healthy young adults1 and the widespread distribution of the VDRs and 1α-OHase in brain and muscle,10,11 optimal vitamin D status may be important for preventing or treating neurodegenerative disorders.
Active 1,25-dihydroxyvitamin D binds to VDRs and regulates approximately 200 genes involved in cell differentiation, proliferation, and apoptosis.1 In the brain, VDRs localize in the nucleus, whereas 1α-OHase is distributed throughout the cytosol. In particular, hippocampal and substantia nigra cells demonstrate high concentrations of VDRs and 1α-OHase.10 Additional epidemiologic, animal, and human data support the concept that vitamin D deficiency may be involved in the pathogenesis, progression, and clinical manifestations of PD.12 Limited previous reports13–16 of vitamin D deficiency in PD focus on skeletal health associations in elderly (aged ≥65 years) Asian populations, but these reports have not been confirmed in white populations. Furthermore, if vitamin D insufficiency is acquired as a result of chronic neurodegeneration, one would expect the prevalence of vitamin D to be similar in 2 populations with different neurodegenerative diseases. This report summarizes the prevalence of vitamin D deficiency in a research database cohort of patients with PD and 2 comparison cohorts—one of age-matched healthy controls and another of patients with Alzheimer disease (AD), also a slowly progressive neurodegenerative disease.
METHODS
STUDY POPULATION
Three hundred individuals were selected for 3 cohorts (100 patients with PD, 100 patients with AD, and 100 healthy controls) from 2019 participants in an existing research registry database, the Clinical Research in Neurology (CRIN) database, maintained by the Department of Neurology, Emory University School of Medicine. The umbrella structure, enrollment, and sample handling protocols for the CRIN database have been approved by the Emory University institutional review board. All participants provided informed consent and were recruited between May 1992 and March 2007. The CRIN database participants with AD (n=844) and PD (n=699) were recruited sequentially from the memory and movement disorder clinics. The CRIN database control participants (n=476) without known neurologic disease were recruited mostly from general medical clinics and community educational events.
Participants were assigned a research diagnosis of AD, PD, or control based on comprehensive evaluation by experienced subspecialty cognitive or movement disorder neurologists (M.L.E. and M.R.D.) or by 2 or more subspecialty neurologists after review of available medical information. The most recent clinical impressions were used for participants with longitudinal clinical assessments. Patients with a history of stroke, transient ischemic attack, restless legs syndrome, or essential tremor were excluded from all 3 groups. The PD participants had their conditions diagnosed based on the presence of 2 or more cardinal motor features of PD,17 definite response to levodopa, and the absence of features suggestive of other parkinsonian syndromes. The AD participants were diagnosed as having AD using widely accepted criteria, including supportive neuropsychological testing,18 and were free of other neurologic diagnoses. The control participants had no history of neurologic disease (by examination where available and by self-report for all others) and had Mini-Mental State Examination scores of 25 or higher. Participants related to any previous participant in the 3 CRIN database groups were excluded to minimize recruitment bias.
PARTICIPANT SELECTION
Every fifth participant with PD from the CRIN database was selected (in chronologic order of enrollment) until the PD cohort sample size reached 100. To control for known confounding variables (age, geographic latitude, and race), as well as potential confounding predictor variables (sex and APOE genotype), AD (n=100) and control (n=100) cohorts were chosen by random selection after matching for age (±5 years), except for the youngest AD patient, who was matched within 10 years (Table), race, APOE genotype, sex, and residence in the southeastern United States.
Table.
Demographic Data and Vitamin D Status in PD Patients, AD Patients, and Matched Healthy Controls
| P Valuea | ||||||
|---|---|---|---|---|---|---|
| PD Patients (n=100) |
AD Patients (n=97) |
Healthy Controls (n=99) |
PD Patients vs Controls |
AD Patients vs Controls |
PD Patients vs AD Patients |
|
| Demographic | ||||||
| Age, mean (range), y | 65.4 (37–88) | 66.4 (47–88) | 65.7 (39–89) | .84 | .59 | .47 |
| Sex, % | ||||||
| Male | 57 | 57 | 57 | … | … | … |
| Female | 43 | 43 | 43 | … | … | … |
| Race, No. | ||||||
| White | 96 | 94 | 96 | … | … | … |
| African American | 1 | 1 | 1 | … | … | … |
| Asian | 1 | 1 | 1 | … | … | … |
| Hispanic | 2 | 1 | 1 | … | … | … |
| Symptom duration, mean (SD) [range], y | 7.6 (5.4) [0–28] | 3.6 (2.6) [0–16] | … | |||
| Season sample drawn, % | .01 | .29 | .26 | |||
| Summer to fall ( July–December) | 57 | 47 | 39 | |||
| Winter to spring ( January–June) | 43 | 52 | 61 | |||
| Vitamin D statusb | ||||||
| 25(OH)D level, mean (SD) [range], ng/mL | 31.9 (13.6) [12.1–68.9] | 34.8 (15.4) [7.5–81.2] | 37.0 (14.5) [11.1–81.9] | .01 | .30 | .12 |
| Prevalence of vitamin D insufficiency (25[OH]D, ≤30 ng/mL), % | 55.0 | 41.2 | 36.4 | .008 | .48 | .05 |
| Vitamin D status by season | … | … | … | … | … | … |
| Summer to fall ( July–December)c | ||||||
| 25(OH)D, mean (SD), ng/mL | 32.3 (13.8) (n=57) | 35.1 (16.6) (n=46) | 39.85 (13.8) (n=39) | .13 | .86 | .20 |
| Prevalence of vitamin D insufficiency, % | 54 | 44 | 31 | .02 | .16 | .27 |
| Winter to spring ( January–June)d | … | … | … | … | … | … |
| 25(OH)D, mean (SD), ng/mL | 31.4 (13.5) (n=43) | 34.7 (14.6) (n=50) | 35.1 (14.8) (n=60) | .02 | .16 | .36 |
| Prevalence of vitamin D insufficiency, % | 56 | 38 | 40 | .11 | .83 | .09 |
Abbreviations: AD, Alzheimer disease; 25(OH)D, 25-hydroxyvitamin D; PD, Parkinson disease.
SI conversion factor: To convert 25(OH)D to nanomoles per liter, multiply by 2.496.
Except where noted, pairwise P values via pooled t test and PROC GLM procedure. Ellipses indicate statistical test not relevant.
25(OH)D level (P=.03, analysis of variance using PROC GLM) and prevalence (P=.02, χ2 test) irrespective of season.
25(OH)D level (P=.28, analysis of variance) and prevalence (P=.17, χ2 test) for summer to fall months.
25(OH)D level (P=.07, analysis of variance) and prevalence (P=.07, χ2 test) for winter to spring months.
LABORATORY ANALYSIS OF 25-(OH)D CONCENTRATIONS
Stored plasma samples were analyzed with an enzyme-linked immunosorbent assay kit for 25(OH)D (Immunodiagnostic Systems Inc, Fountain Hills, Arizona) in 3 batches (with samples for each batch randomly selected from all 3 cohorts). The limit of detection is 2 ng/mL (to convert to nanomoles per liter, multiply by 2.496). Intra-assay and interassay coefficients of variation are less than 8% and less than 10%, respectively. On the basis of the 25(OH)D cut points used in our clinic practice and reviewed by Holick,1 we defined vitamin D insufficiency as a 25(OH)D concentration of 30 ng/mL or less and vitamin D deficiency as a 25(OH)D concentration of less than 20 ng/mL.
STATISTICAL ANALYSIS
In 4 samples (1 from the control cohort and 3 from the AD cohort), the 25(OH)D concentration was more than 3 SDs above the mean. These samples were excluded from analysis, leaving 100 individuals in the PD cohort, 97 in the AD cohort, and 99 in the control cohort. Microsoft Excel (Microsoft Inc, Seattle, Washington) and SAS statistical software, version 9.1 (SAS Institute Inc, Cary, North Carolina), were used for all statistical calculations, with a 2-sided significance level of .05 (α=.05).
The 25(OH)D concentrations were normally distributed; parametric tests were therefore used for analysis. Because 25 (OH)D concentrations are higher in the summer than in the winter, distribution for month and season of blood draw for participants in each cohort was examined. Finally, the AD and PD cohorts were evaluated to determine whether symptom duration correlated with the 25(OH)D concentration (8 of 97 participants in the AD cohort and 1 of 100 participants in the PD cohort had missing data).
RESULTS
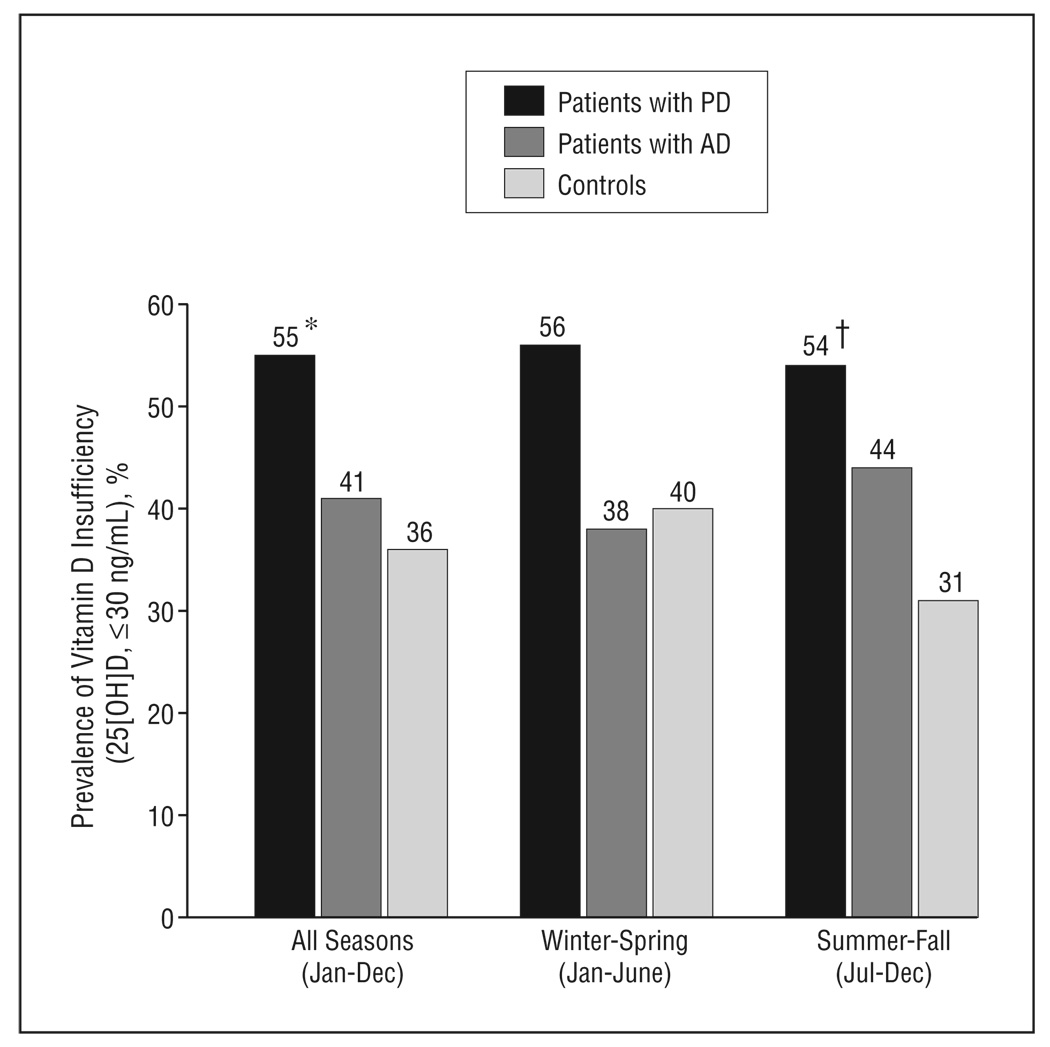
More than half of the PD participants (55%) had vitamin D insufficiency (25[OH]D,≤30 ng/mL) compared with approximately a third of the controls (Figure). Most participants in all 3 cohorts were white, reflecting the racial distribution of the PD population at our institution. All participants resided in southern latitudes (100% south of 39°N and 90% south of 37°N). The prevalence of vitamin D insufficiency was significantly higher in the PD cohort than in the AD and control cohorts (Table). Similarly, 23% of the PD cohort was vitamin D deficient (25[OH]D,<20 ng/mL) compared with 16% of the AD cohort and 10% of the control cohort (P=.01 for PD vs control participants and P=.18 for PD vs AD participants).
Figure.
Prevalence of vitamin D insufficiency in patients with Parkinson disease (PD), patients with Alzheimer disease (AD), and matched healthy controls. *P=.008 for PD patients vs controls and P=.05 for PD patients vs AD patients. †, P=.02 for patients with PD vs controls. 25[OH]D indicates 25-hydroxyvitamin D. (To convert 25(OH)D to nanomoles per liter, multiply by 2.496.)
A significantly higher portion of the samples from the PD cohort were drawn in the summer to fall (when vitamin D levels are higher) than in samples from the control group. Although not statistically significant, the portion of samples from the PD group drawn in the summer to fall was also higher than in the AD cohort. Evaluation of the symptom duration in the AD and PD cohorts revealed a longer duration in the PD cohort but no significant correlation between duration of disease symptoms and 25(OH)D concentration (P=.35 and .11, Pearson test, for the AD and PD patients, respectively) (Table).
COMMENT
Vitamin D insufficiency is a common health problem in elderly individuals, who also have a high prevalence of neurodegenerative diseases. Vitamin D is primarily produced in the skin on exposure to UV-B radiation and is found in limited food sources1,19; advancing age, obesity, avoidance of sun exposure, residence in northerly latitudes, and darker skin pigmentation are associated with increased risk of vitamin D deficiency. Patients with chronic neurodegenerative diseases frequently have many risk factors for vitamin D insufficiency. Consistent with our a priori hypothesis, we found the prevalence of vitamin D insufficiency in the PD cohort to be significantly higher than in the control group. Surprisingly, the prevalence of vitamin D insufficiency in the PD cohort was higher than in the comparison cohort with AD.
The prevalence of vitamin D deficiency (25[OH]D, <20 ng/mL) in our PD cohort (23%) was lower than that previously observed in a Japanese PD cohort13 (50 of 71 patients with PD [85%]). Both insufficiency (36%) and deficiency (10%) in our control cohort were also less prevalent than reported in controls from a study of prostate cancer risk20 (58% insufficient [<32 mg/mL] and 16% deficient [<20 ng/mL]). Because all our study participants resided in southern latitudes and were predominantly white and a greater proportion of our samples were collected in the summer to fall, a lower prevalence of hypovitaminosis D in all our cohorts is not unexpected. Also, the Japanese participants were drawn from a hospital population, whereas participants in this study were recruited from an outpatient clinic. Previous studies14,21 indicate that hospitalized patients have lower mean vitamin D levels than age-matched participants recruited from the community. The higher prevalence of insufficiency in the PD cohorts compared with the control cohort is not unexpected given that PD may cause patients to have decreased activity levels and lower sunshine exposure.
However, the lower vitamin D levels in the PD vs AD cohort are intriguing. The typical course of AD is shorter than that of PD,22,23 and PD patients experience mobility problems more frequently than AD patients. Both factors could make a PD patient less likely to get sun exposure and account for the higher prevalence of vitamin D insufficiency. Supporting this notion, more severely affected PD patients with a longer mean (SD) disease duration (Hoehn and Yahr stages III–V, 7.1[3.8] years) reportedly had a higher prevalence of 25(OH)D deficiency than less severely affected patients with shorter disease duration (Hoehn and Yahr stages I–II, 4.1[2.3] years).13 In contrast, although the range and mean of symptom duration in our PD and AD cohorts were consistent with what one would expect to find in a neurology clinic, 25(OH)D insufficiency did not correlate with symptom duration in either cohort (AD or PD), suggesting that vitamin D insufficiency may be unique to PD. Alternatively, the possible correlation may be too weak to be detected with our current sample size.
Strengths of this analysis are that the sample size was relatively large (97–100 participants in each cohort) and that the cohorts were matched for sex (which, depending on cultural and social differences, could affect duration of sun exposure and degree of skin coverage or protection when in sunlight), age (known to affect susceptibility to vitamin D deficiency), and APOE genotyping (which has been postulated to be protective for vitamin D deficiency24). Age was not limited in our inclusion criteria, thus increasing the generalizability to PD and AD populations with younger and older patients. In addition, diagnoses were made by experienced subspecialty neurologists according to well-characterized criteria. Although this was a retrospective study, participants were identified and data were analyzed as if in a prospective cross-sectional study.
This analysis did not control for vitamin D intake, although in other populations vitamin D intake contributes little to 25(OH)D levels.25 In this cross-sectional study that lacks longitudinal data, we cannot address etiology for the clinical associations discussed. In addition, we do not have uniformly collected measures of PD severity (Hoehn and Yahr stage, Unified Parkinson Disease Rating Scale scores) or anthropomorphic and sun exposure data; therefore, we cannot assess what effect, if any, differences in body mass index and exposure to sunshine might have on these findings. Another weakness is that the portion of plasma samples drawn in the winter to spring and fall to summer were not matched across cohorts. Despite a significantly higher proportion of the samples from the PD cohort being drawn in the summer to fall, we still found higher rates of vitamin D insufficiency in the PD cohort compared with the AD and healthy control cohorts.
In summary, we found that PD patients have a higher prevalence of vitamin D insufficiency compared with patients with AD and healthy controls. These findings support the previously suggested need12 for further studies to assess what contribution a low 25(OH)D concentration adds to the risk of developing PD (vs other neurodegenerative disorders) and to determine whether correction of vitamin D insufficiency and deficiency will improve motor or nonmotor symptoms in PD. Finally, the finding of a high incidence of vitamin D deficiency in the PD and other cohorts highlights the importance of routinely checking the level of 25-(OH)D, particularly in elderly patients, since deficiency is strongly correlated with a higher incidence of osteoporosis, falls, and hip fractures and has been associated with a higher incidence of several forms of cancer and autoimmune disorders.
Acknowledgments
Funding/Support: This study was supported by National Institutes of Health (NIH)/National Center for Research Resources (NCRR) grant K12 RR017643 to the Emory Mentored Clinical Research Scholars Program (M.L.E.), NIH/NCRR grant M01 RR00039 to the Emory General Clinical Research Center (CRIN infrastructure support), NIH grant K23AR054334 (V.T.), NIH/National Institute of Aging grant P50AG025688 to the Emory Alzheimer’s Disease Research Center (A.R.), NIH/National Institute of Environmental Health Sciences grant ES012068 to the Emory University Collaborative Center for Parkinson’s Disease Environmental Research (A.R.), and funds from an anonymous donor.
Additional Contributions: We thank the CRIN database participants and the Alzheimer’s Disease Research Center research staff of the Movement Disorders Section, who support the CRIN database.
Footnotes
Financial Disclosure: None reported.
REFERENCES
- 1.Holick MF. Vitamin D deficiency. N Engl J Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 2.Nagpal S, Na S, Rathnachalam R. Noncalcemic actions of vitamin D receptor ligands. Endocr Rev. 2005;26(5):662–687. doi: 10.1210/er.2004-0002. [DOI] [PubMed] [Google Scholar]
- 3.Bischoff-Ferrari HA, Dietrich T, Orav EJ, et al. Higher 25-hydroxyvitamin D concentrations are associated with better lower-extremity function in both active and inactive persons aged > or =60 y. Am J Clin Nutr. 2004;80(3):752–758. doi: 10.1093/ajcn/80.3.752. [DOI] [PubMed] [Google Scholar]
- 4.Grant WB, Garland CF, Gorham ED. An estimate of cancer mortality rate reductions in Europe and the US with 1,000 IU of oral vitamin D per day. Recent Results Cancer Res. 2007;174:225–234. doi: 10.1007/978-3-540-37696-5_20. [DOI] [PubMed] [Google Scholar]
- 5.Gorham ED, Garland CF, Garland FC, et al. Optimal vitamin D status for colorectal cancer prevention: a quantitative meta analysis. Am J Prev Med. 2007;32(3):210–216. doi: 10.1016/j.amepre.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 6.Garland CF, Garland FC, Gorham ED, et al. The role of vitamin D in cancer prevention. Am J Public Health. 2006;96(2):252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zittermann A. Vitamin D and disease prevention with special reference to cardiovascular disease. Prog Biophys Mol Biol. 2006;92(1):39–48. doi: 10.1016/j.pbiomolbio.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Garcion E, Wion-Barbot N, Montero-Menei CN, Berger F, Wion D. New clues about vitamin D functions in the nervous system. Trends Endocrinol Metab. 2002;13(3):100–105. doi: 10.1016/s1043-2760(01)00547-1. [DOI] [PubMed] [Google Scholar]
- 9.Baksi SN, Hughes MJ. Chronic vitamin D deficiency in the weanling rat alters catecholamine metabolism in the cortex. Brain Res. 1982;242(2):387–390. doi: 10.1016/0006-8993(82)90331-6. [DOI] [PubMed] [Google Scholar]
- 10.Eyles DW, Smith S, Kinobe R, Hewison M, McGrath JJ. Distribution of the vitamin D receptor and 1α-hydroxylase in human brain. J Chem Neuroanat. 2005;29(1):21–30. doi: 10.1016/j.jchemneu.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Holick MF. Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr. 2004;79(3):362–371. doi: 10.1093/ajcn/79.3.362. [DOI] [PubMed] [Google Scholar]
- 12.Newmark HL, Newmark J. Vitamin D and Parkinson’s disease: a hypothesis. Mov Disord. 2007;22(4):461–468. doi: 10.1002/mds.21317. [DOI] [PubMed] [Google Scholar]
- 13.Sato Y, Kikuyama M, Oizumi K. High prevalence of vitamin D deficiency and reduced bone mass in Parkinson’s disease. Neurology. 1997;49(5):1273–1278. doi: 10.1212/wnl.49.5.1273. [DOI] [PubMed] [Google Scholar]
- 14.Sato Y, Honda Y, Iwamoto J, Kanoko T, Satoh K. Abnormal bone and calcium metabolism in immobilized Parkinson’s disease patients. Mov Disord. 2005;20(12):1598–1603. doi: 10.1002/mds.20658. [DOI] [PubMed] [Google Scholar]
- 15.Sato Y, Iwamoto J, Kanoko T, Satoh K. Alendronate and vitamin D2 for prevention of hip fracture in Parkinson’s disease: a randomized controlled trial. Mov Disord. 2006;21(7):924–929. doi: 10.1002/mds.20825. [DOI] [PubMed] [Google Scholar]
- 16.Vaserman N. Parkinson’s disease and osteoporosis. Joint Bone Spine. 2005;72(6):484–488. doi: 10.1016/j.jbspin.2004.04.012. [DOI] [PubMed] [Google Scholar]
- 17.Gelb DJ, Oliver E, Gilman S. Diagnostic criteria for Parkinson disease. Arch Neurol. 1999;56(1):33–39. doi: 10.1001/archneur.56.1.33. [DOI] [PubMed] [Google Scholar]
- 18.McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology. 1984;34(7):939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- 19.Lawson DE, Paul AA, Black AE, Cole TJ, Mandal AR, Davie M. Relative contributions of diet and sunlight to vitamin D state in the elderly. Br Med J. 1979;2(6185):303–305. doi: 10.1136/bmj.2.6185.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li H, Stampfer MJ, Hollis JBW, et al. A prospective study of plasma vitamin D metabolites, vitamin D receptor polymorphisms, and prostate cancer. PLoS Med. 2007;4(3):e103. doi: 10.1371/journal.pmed.0040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sato Y, Asoh T, Oizumi K. High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease. Bone. 1998;23(6):555–557. doi: 10.1016/s8756-3282(98)00134-3. [DOI] [PubMed] [Google Scholar]
- 22.Mölsä PK, Marttila RJ, Rinne UK. Survival and cause of death in Alzheimer’s disease and multi-infarct dementia. Acta Neurol Scand. 1986;74(2):103–107. doi: 10.1111/j.1600-0404.1986.tb04634.x. [DOI] [PubMed] [Google Scholar]
- 23.Ishihara LS, Cheesbrough A, Brayne C, Schrag A. Estimated life expectancy of Parkinson’s patients compared with the UK population. J Neurol Neurosurg Psychiatry. 2007;78(12):1304–1309. doi: 10.1136/jnnp.2006.100107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerdes LU. The common polymorphism of apolipoprotein E: geographical aspects and new pathophysiological relations. Clin Chem Lab Med. 2003;41(5):628–631. doi: 10.1515/CCLM.2003.094. [DOI] [PubMed] [Google Scholar]
- 25.Whiting SJ, Green TJ, Calvo MS. Vitamin D intakes in North America and Asia-Pacific countries are not sufficient to prevent vitamin D insufficiency. J Steroid Biochem Mol Biol. 2007;103(3–5):626–630. doi: 10.1016/j.jsbmb.2006.12.067. [DOI] [PubMed] [Google Scholar]