Abstract
Purpose
This study tested the hypothesis that the type of dose-fractionation regimen determines the ability of radiotherapy to synergize with anti-CTLA-4 antibody.
Experimental design
TSA mouse breast carcinoma cells were injected s.c. into syngeneic mice at two separate sites, defined as a “primary” site that was irradiated, and a “secondary” site outside the radiotherapy field. When both tumors were palpable mice were randomly assigned to 8 groups receiving no radiotherapy or 3 distinct regimens of radiotherapy (20 Gy × 1, 8 Gy × 3 or 6 Gy × 5 fractions in consecutive days) in combination or not with 9H10 mAb against CTLA-4. Mice were followed for tumors growth/regression. Similar experiments were conducted in the MCA38 mouse colon carcinoma model.
Results
In either of the 2 models tested treatment with 9H10 alone had no detectable effect. Each of the radiotherapy regimens caused comparable growth delay of the primary tumors, but had no effect on the secondary tumors, outside the radiation field. Conversely, the combination of 9H10 and either fractionated radiotherapy regimens achieved enhanced tumor response at the primary site (p<0.0001). Moreover, an abscopal effect, defined as a significant growth inhibition of the tumor outside the field occurred only in mice treated with the combination of 9H10 and fractionated radiotherapy (p<0.01). Frequency of CD8+ T cells showing tumor-specific IFNγ production was proportional to the inhibition of the secondary tumor.
Conclusions
Fractionated, but not single dose radiotherapy, induces an abscopal effect when in combination with anti-CTLA-4 antibody, in two preclinical carcinoma models.
Keywords: Metastatic cancer, radiotherapy, CTLA-4, abscopal effect, tumor immunity
INTRODUCTION
Ionizing radiation therapy is an effective tool for local tumor control, and plays an important role in the treatment of breast and other cancers. In the setting of metastatic disease, however, the role of radiotherapy is generally limited to symptoms’ palliation. We have previously proposed a partnership between local radiation and immunotherapy in the treatment of cancer (1). Recent evidence that radiation induces an immunogenic tumor cell death and alters the tumor microenvironment to enhance recruitment of anti-tumor T cells supports the hypothesis that radiation can enhance both the priming and effector phase of the anti-tumor immune response (2-5). Clinical observations consistent with this hypothesis, however, are very rare. One such observation is known as the “abscopal effect” and refers to tumor regression seen outside of the field of radiation, implying an indirect anti-tumor effect induced by local radiotherapy (6-9). The paucity of evidence that radiotherapy can promote therapeutically effective anti-tumor immunity is not surprising, considering that successful vaccination often does not translate into clinical tumor responses (10). Development of tolerance and immunosuppression in tumor-bearing hosts have been identified as major obstacles to the success of immunotherapy in general (11), and may also impair the immune-mediated abscopal effect induced by radiation (12).
Employing 4T1, a syngeneic mouse breast cancer model that spontaneously metastasizes systemically soon after implantation, we have previously shown that local radiotherapy to the primary subcutaneous tumor induces a CD8 T cell-mediated immune response inhibiting lung micrometastases when combined with a strategy of blocking the CTLA-4 receptor to overcome T cell tolerance (13). In this model, CTLA-4 blockade as single modality did not significantly inhibit lung metastases or extend mice survival. The strength of the CD8 anti-tumor T cell response triggered by radiotherapy and CTLA-4 blockade was regulated by invariant natural killer T cells, and in a fraction of the mice was sufficient to cause the complete regression of the well-established primary irradiated tumor (14). The latter was facilitated by radiation-induced release of CXCL16, a chemokine enhancing the recruitment of activated CD8 cells to the irradiated tumor site (5).
Before translating these findings to the clinic we elected to explore whether different dose-fractionation regimens have an influence on the abscopal effect observed. We chose the TSA breast cancer and the MCA38 colon cancer mouse models to test whether radiotherapy to one tumor nodule in combination with CTLA-4 blockade can induce an immune-mediated abscopal effect in a second, palpable, tumor nodule outside the radiation field. A single large dose and two fractionated radiotherapy regimens had similar ability to control tumor growth at the irradiated primary site, but no effect on the secondary tumor outside of the treatment field. CTLA-4 blockade as single modality did not have any effect on either tumor. However, when CTLA-4 blockade was combined with radiotherapy there was enhanced inhibition of the primary, as well as the secondary (abscopal effect) tumors. Regression of the secondary tumor was proportional to the frequency of tumor-specific T cells, consistent with an immune-mediated effect. Surprisingly, in either model, the abscopal effect was seen with either fractionated but not with the single dose radiotherapy regimen. Overall, these data support testing the combination of radiotherapy with immunomodulatory antibodies in patients with metastatic disease, and suggest that the schedule and dose per fraction of radiotherapy may be critical determinants of its ability to synergize with immunotherapy,
MATERIALS AND METHODS
Mice
Six to eight week old BALB/c and C57BL/6 mice were obtained from Taconic Animal Laboratory (Germantown, NY), and maintained under pathogen-free conditions in the animal facility at New York University Langone Medical Center. All animal experiments were performed according to protocols approved by the Institutional Animal Care and Use Committee of New York University.
Cell line and reagents
TSA is a BALB/c mouse-derived poorly immunogenic mammary carcinoma cell line (15) and MCA38 is a C57BL/6 mouse-derived poorly immunogenic colon carcinoma (16). TSA and MCA38 cells were cultured in DMEM medium (Invitrogen Corporation, Carlsbad, CA) supplemented with 2mM L-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin, 2.5 × 10-5 M 2-mercapthoethanol, and 10% FBS (Gemini Bio-Products Woodland, CA) (complete medium). These cells were found to be free of contamination by Mycoplasma by the Mycoplasma detection kit (Roche Diagnostics, Chicago, IL). Anti-CTLA-4 hamster mAb 9H10 was purified as previously described (13).
Tumor challenge and treatment
BALB/c and C57BL/6 mice were injected s.c. with 1×105 TSA and 5×105 MCA38 cells, respectively, in the right flank on Day 0 (primary tumor) and in the left flank on Day 2 (secondary tumor). Perpendicular tumor diameters were measured with a Vernier caliper, and tumor volumes were calculated as length × width2 × 0.52. On Day 12, when both tumors were palpable (average volume for TSA: 32 mm3 and 21 mm3 for primary ands secondary tumors, respectively; average volume for MCA38: 50 mm3 and 25 mm3 for primary and secondary tumors, respectively) animals were randomly assigned to various treatment groups, as indicated. Radiotherapy was administered as previously described (13), with some modifications. Briefly, all mice (including mice receiving mock radiation) were lightly anesthetized by i.p. injection of Avertin (240mg/kg), positioned on a dedicated plexiglass tray and the whole body was protected by lead shielding, except for the area of the tumor to be irradiated. Radiotherapy was delivered to a field including the tumor with 5 mm margins using a Clinac 2300 C/D Linear Accelerator (Varian Medical Systems, Palo Alto, Ca.) fitted with a 25 mm RadioSurgery conical collimator (BrainLAB AG, Feldkirchen, Germany), which is designed to deliver very sharp and limited radiation dose fields. Superflab bolus (1.5cm tissue equivalent material) was placed over the tumor, and a source to skin distance (SSD) of 100 cm was set. Radiation was delivered at 600 cGy/min with 6 MV x-rays. Mice received a single dose of 20 Gy, 3 fractions of 8 Gy, or 5 fractions of 6 Gy in consecutive days (Figure 1). CTLA-4 blocking mAb 9H10 or vehicle (phosphate-buffered saline, PBS) was administered i.p. at the dose of 200 μg/mouse (10 mg/kg) on days 14, 17 and 20. In some experiments, 9H10 was administered on days 12, 15, 18, or 14, 17, and 20, as indicated in figure legends. Tumor growth was evaluated every 2 to 3 days until day 35. All mice were sacrificed on day 35, and tumors harvested and weighed.
Figure 1. Tumor model and treatment schedule.
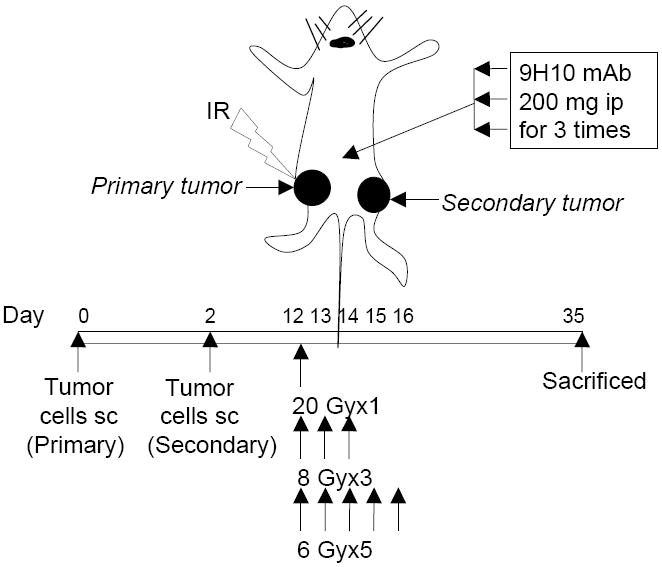
Immunocompetent mice were injected s.c. with syngeneic TSA cells (1 × 105) into the right (defined as “primary” tumor) and left (defined as “secondary” tumor) flank on day 0 and 2, respectively. Ionizing radiation (IR) was administered locally exclusively to the primary tumor with the rest of the body shielded, in a single dose or multiple fractions given in consecutive days starting on day 12. CTLA-4 blocking mAb 9H10 was given i.p every three days for three times starting on day 12, 14 or 16, as indicated. Primary and secondary tumor volumes were measured until day 35, at which time mice were sacrificed and tumors weighed.
Immunostaining of tumor sections
Tumors from treated and untreated mice were harvested at Day 35 post tumor inoculation, fixed for 1 h at 4°C in 4% paraformaldehyde followed by overnight incubation in 30% sucrose, and frozen in optimum cutting temperature (OCT) medium. Sections (8 μm) were incubated with 0.1% Tween-20 and 0.01% Triton-X100 for 20 minutes, followed by 4% rat serum in 4% BSA/PBS for an additional 30 minutes. Sections were stained with PE-Texas-Red-conjugated rat anti-mouse CD4 or PE-conjugated rat anti-mouse CD8α (Caltag, Carlsbad, CA), and counterstained with 5 μg/ml DAPI (Sigma). Images were obtained using a Nikon Eclipse 800 deconvolution microscope. Number of CD4 and CD8 T cells were counted in three randomly selected (20X) fields in each tumor.
Ex vivo production of IFN-γ by spleen cells
Spleen cells (1×106) from TSA tumor-bearing mice were cultured in 24-well tissue culture plates with 2.5×105 irradiated (20 Gy) TSA cells for 24 hrs in 1-ml fresh RPMI 1640 medium supplemented with 2 mM L-glutamine, 100 U/ml penicillin,100 μg/ml streptomycin, 50 μM 2-mercapthoethanol, 10% FBS (T cell medium). The supernatants were collected and stored at −80°C. IFNγ was measured in cell-free supernatants of duplicate wells by ELISA (Diaclone Tepnel, Lifecodes Corp. Stamford, CT). Tumor-specific IFNγ production was calculated by subtracting the background values measured in supernatants of spleen cells cultured with medium alone.
Flow cytometry analysis of IFN-γ producing CD8 T cells
For in vitro re-stimulation, 3.5×106 spleen cells from TSA tumor-bearing mice were cultured with the TSA-derived immunodominant CD8 epitope AH1 peptide [SPSYVYHQF] (1 μM) (15), whereas spleen cells from MCA38 tumor-bearing mice were cultured with 1×106 irradiated (50 Gy) MCA38 cells. After 5 days culture in 24-well tissue culture plates in 2-ml T cell medium supplemented with 10 U/ml human rIL-2 (provided by the National Cancer Institute BRB Preclinical Repository) the percentage of CD8 T cells producing IFN-γ was determined. Briefly, T cells were cultured for 16 hrs with irradiated TSA or MCA38 target cell or with irrelevant target RMA-S Ld cells preloaded with MCMV peptide (17) at 1:1 ratio in the presence of 1 μl/ml of Brefaldin A, washed and incubated with rat anti-mouse CD16/CD32 mAb (2.4G2) to block nonspecific binding and then stained with CD8α-PE-Cy5 and IFN-γ-FITC or control antibodies according to the manufacture’s instructions (BD PharMingen). Cells were analyzed using a FACScan flow cytometer and FlowJo version 8.7.1 (Tree Star, Ashland, OR).
Statistical analysis
Random coefficients regression was used to model log tumor volume and log tumor weight as functions of elapsed time from treatment onset and to compare treatment regimens with respect to tumor growth rate. Separate analyses were conducted to assess the effect of treatment on the growth of primary and secondary tumors. The logs of tumor weight and of tumor volume were used in place of the observed data to better satisfy underlying distributional assumptions and since changes over time in tumor volume and weight were well approximated as log-linear. The use of random coefficients regression permits a separate tumor growth curve to be fit to the data from each animal. The treatments are then compared on the basis of aggregate tumor growth models; for a given treatment the aggregate growth model is a single curve describing the average change in tumor volume among animals receiving the treatment. The model to predict log tumor weight or volume each included level of RT exposure and the variable identifying whether the animal received PBS or 9H10 as fixed classification factors and terms representing the interaction of these factors. The models also included time from treatment onset as a numeric factor and terms representing the interaction of time with treatment. To account for statistical dependencies among data derived for a single animal, the covariance structure for was modeled by assuming observations to be correlated only when acquired from the same animal. All reported p values are two-sided and were declared statistically significant at the 5% level. The statistical computations were carried out using SAS for Windows, version 9.0 (SAS Institute, Cary, NC).
RESULTS
Fractionated but not single dose radiotherapy synergizes with anti-CTLA-4 antibody in the TSA breast cancer model
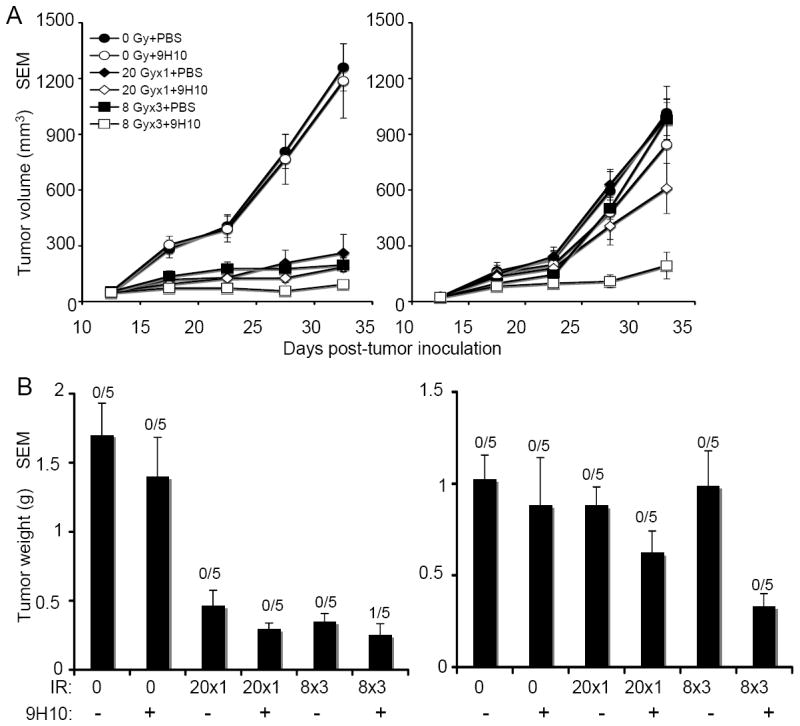
We have previously shown in the 4T1 mouse model of metastatic breast cancer that local radiotherapy in combination with CTLA-4 blockade induces an anti-tumor immune response inhibiting systemic growth of micrometastases (13). To determine whether the induced anti-tumor immune response could be effective against larger “metastatic” tumor nodules, we employed the TSA mouse mammary carcinoma cells injected at two separate sites, as illustrated in Figure 1. Similarly to 4T1, TSA is a poorly immunogenic carcinoma with ability to shed spontaneous metastases. In contrast to 4T1, however, TSA cells metastasize with a delay of few weeks from initial implantation (18), providing a window where the potential effects of the spontaneoulsy shed tumor cells on the growth of the two subcutaneously implanted tumors is negligible. To mimic the clinical setting in which radiotherapy is applied to the largest (symptomatic) nodule, the site designated as “primary” and receiving local radiation was injected two days earlier than the “secondary” site outside the field of radiation. On day 12, when both tumors were palpable, mice were randomly assigned to eight treatment groups receiving mock radiation, one dose of 20 Gy, three fractions of 8 Gy, or 5 fractions of 6 Gy to the primary tumor (Figure 1). CTLA-4 blocking mAb 9H10 was administered to half of the mice in each radiation group three times, on days 14, 17, and 20.
In the absence of radiotherapy, 9H10 administration did not have any effect on either primary or secondary tumors (Figure 2). Radiotherapy as single modality caused significant growth delay of the primary tumor that was comparable for all regimens used but had no effect on secondary tumors (Figure 2 A). Radiotherapy and 9H10 showed a significant interaction (p<0.001) on the primary tumor growth only when given in three fractions of 8Gy and 5 fractions of 6 Gy, causing enhanced tumor inhibition in comparison to radiation alone and complete regression in the majority of mice (Figure 2 B, left panel). Importantly, growth of the secondary tumors was significantly inhibited (p<0.01) only in mice treated with fractionated but not single dose radiotherapy in combination with 9H10, and in two mice treated with three fractions of 8 Gy the secondary tumor completely regressed (Figure 2 B, right panel).
Figure 2. The abscopal effect is induced in TSA tumor-bearing mice by fractionated radiation in combination with anti-CTL-4 antibody.
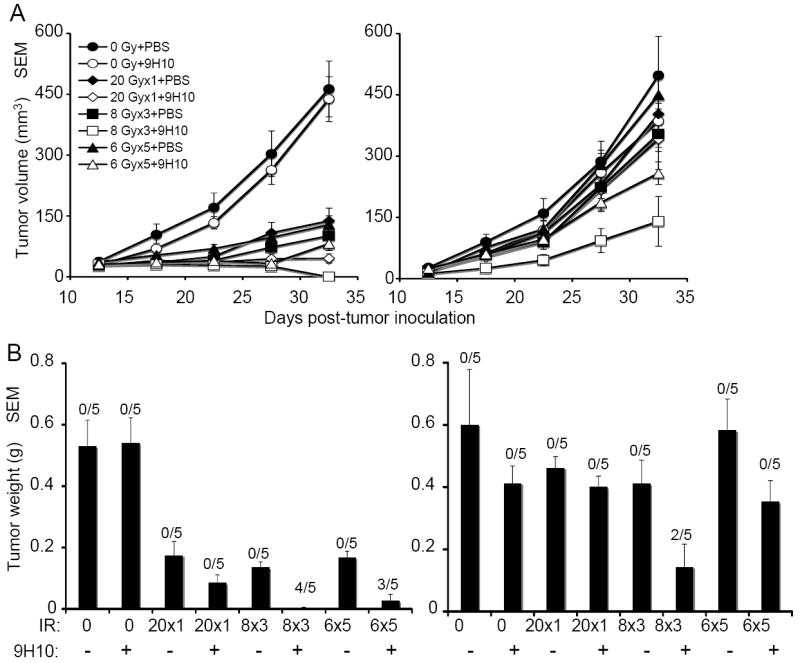
(A) Tumor growth delay of primary irradiated tumors (left panel) and secondary non-irradiated tumor (right panel) in mice treated with PBS (closed circles), 9H10 (open circles), 20 Gy × 1 + PBS (closed diamonds), 20 Gy × 1 + 9H10 (open diamonds), 8 Gy × 3 + PBS (closed squares), 8 Gy × 3 + 9H10 (open squares), 6 Gy × 5 + PBS (closed triangles), or 6 Gy × 5 + 9H10 (open triangles). 9H10 was given on days 14, 17, and 20. Data are the mean ± SE of 5 mice/group. (B) Tumor weight of primary (left panel) and secondary (right panel) tumors at day 35. Data are the mean ± SE. The number of mice with complete tumor regression over the total number of mice per group is indicated. Data shown are from one of two independent experiments with similar results.
These data indicate that different radiation regimens causing similar direct effects in terms of growth inhibition of the irradiated tumor convey a different propensity to induce an abscopal effect in combination with CTLA-4 blockade.
Effect of anti-CTLA-4 antibody administration schedule on TSA tumor inhibition in mice treated with radiotherapy
To determine whether the time of administration of 9H10 mAb relative to radiotherapy could play a role in its ability to induce an abscopal effect, 9H10 treatment was started on different days. As single modality, administration of 9H10 starting on day 12 did not show any effect on TSA tumor growth, similarly to what was observed when 9H10 was started on day 14 (Figure 2 and 3 A). Importantly, administration of 9H10 on day 12, 15 and 18 did not enhance the inhibition of either the primary or secondary tumors by a single 20 Gy dose yielding results similar to the delayed administration on day 14, 17 and 20 (Figure 3 A). Consistent with these results, no significant interaction between 20 Gy and 9H10 started on day 12 was observed (p=0.21 and 0.42 for primary and secondary tumors, respectively). Therefore, the observed differential ability of single dose and fractionated radiotherapy to induce an abscopal effect (Figure 2) can not be explained by the two days interval between the 20 Gy irradiation and the beginning of immunotherapy.
Figure 3. Effect of time of administration of anti-CTLA-4 antibody on the abscopal effect induced by radiotherapy in TSA tumor-bearing mice.
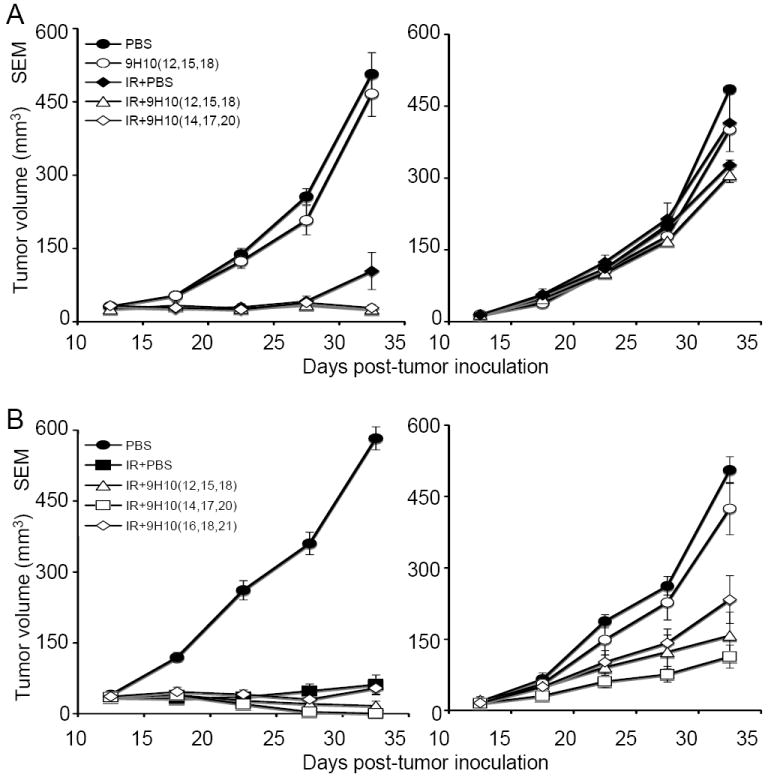
(A) Tumor growth delay of primary irradiated tumors (left panel) and secondary non-irradiated tumor (right panel) in mice treated with PBS (closed circles), 9H10 givenatday 12, 15, 18 (open circles), 20 Gy × 1 + PBS (closed diamonds), 20 Gy × 1 + 9H10 givenatday 12, 15, 18 (open triangles), or 20 Gy × 1 + 9H10 given at day 14, 17, 20 (open diamonds). Data are the mean ± SE of 5 mice/group. No complete regression of either primary or secondary tumors was observed in any of the treatment arms. (B) Tumor growth delay of primary irradiated tumors (left panel) and secondary non-irradiated tumor (right panel) in mice treated with PBS (closed circles), 8 Gy × 3 + PBS (closed squares), 8 Gy × 3 + 9H10 given at day 12, 15, 18 (open triangles), 8Gy × 3 + 9H10 given at day 14, 17, 20 (open squares), and 8 Gy × 3 + 9H10 given at day 16, 18, 21 (open diamonds). Data are the mean ± SE of 6 mice/group. Complete regression was seen in 3 of 6 primary and 1 of 6 secondary tumors in mice treated with 8 Gy × 3 + 9H10 given at day 12, 15, 18; in 5 of 6 primary and 1 of 6 secondary tumors in mice treated with 8 Gy × 3 + 9H10 given at day 14, 17, 20; and in 1 of 6 primary and 1 of 6 secondary tumors in mice treated with 8 Gy × 3 + 9H10 given at day 16, 18, 21.
Next, we tested whether starting 9H10 mAb treatment two days before the conclusion of fractionated radiotherapy (day 12), at conclusion (day 14) or two days later (day 16) affected the inhibition of tumor growth observed in mice treated with three fractions of 8 Gy. Primary tumor inhibition was significantly enhanced by administration of 9H10 on days 12, 15 and 18 or 14, 17 and 20 as compared to radiotherapy alone (p<0.001 for both schedules) (Figure 3 B). Although 5 out of 6 primary tumors completely regressed with 3 fractions of 8 Gy plus 9H10 started at day 14, and only 3 out of 6 completely regressed when 9H10 was started at day 12, this difference was not statistically significant (p=0.08). Likewise, the growth of secondary tumors was significantly inhibited in both groups of mice (p<0.05 compared to control mice) and there was no significant difference when 9H10 mAb administration was started on day 12 or 14 (p=0.9) (Figure 3 B). Delaying administration of 9H10 mAb until day 16 reduced the therapeutic effect, with only 1 out of 6 primary tumors showing complete regression, and a reduced growth inhibition of the secondary tumors (Figure 3 B). This suggests that delaying immunotherapy may reduce its potential benefit. Of note, however, is the fact that complete regression of one secondary tumor was obtained in mice receiving fractionated radiotherapy to the primary tumor even when CTLA-4 blockade was started on day 16, whereas in mice receiving single dose radiotherapy to the primary tumor early administration of 9H10 on day 12 did not induce a significant abscopal effect.
Overall, data indicate that the schedule of administration of 9H10 mAb relative to radiotherapy influences the therapeutic efficacy of this combination treatment. However, the radiotherapy regimen chosen is a fundamental determinant of the ability of the combination treatment to induce an abscopal effect.
Three fractions of 8 Gy are more effective than five fractions of 6 Gy in inducing anti-tumor immunity in combination with anti-CTLA-4 antibody
The data described above (Figure 2) suggested that among the two radiotherapy fractionation regimens the three fractions of 8 Gy protocol was the most effective for induction of the abscopal effect in combination with CTLA-4 blockade. To confirm this and examine the immunological mechanisms of the abscopal effect, mice bearing two separate TSA tumors were mock treated or given three fractions of 8 Gy, or 5 fractions of 6 Gy to the primary tumor in combination with 9H10 mAb given on days 14, 17 and 20. Radiotherapy plus 9H10 was very effective at inhibiting the growth of the irradiated (p<0.0001 compared to mock-treated mice for both regimens) as well as non-irradiated (p<0.0001 for 8 Gy × 3; p=0.015 for 6 Gy × 5 compared to mock-treated mice) tumor (Figure 4 A). However, 8 Gy × 3 was significantly more effective than 6 Gy × 5 at inhibiting the growth of both, the irradiated (p=0.038) and non-irradiated (p=0.014) tumors and complete regression of primary and secondary tumors was observed more frequently in mice receiving 8 Gy × 3 (Figure 4 B), supporting a superior therapeutic effect of this regimen when combined with CTLA-4 blockade.
Figure 4. Fractionated radiotherapy given to TSA tumor-bearing mice in 3 doses of 8 Gy is more effective than 5 doses of 6 Gy in synergizing with anti-CTLA-4 antibody.
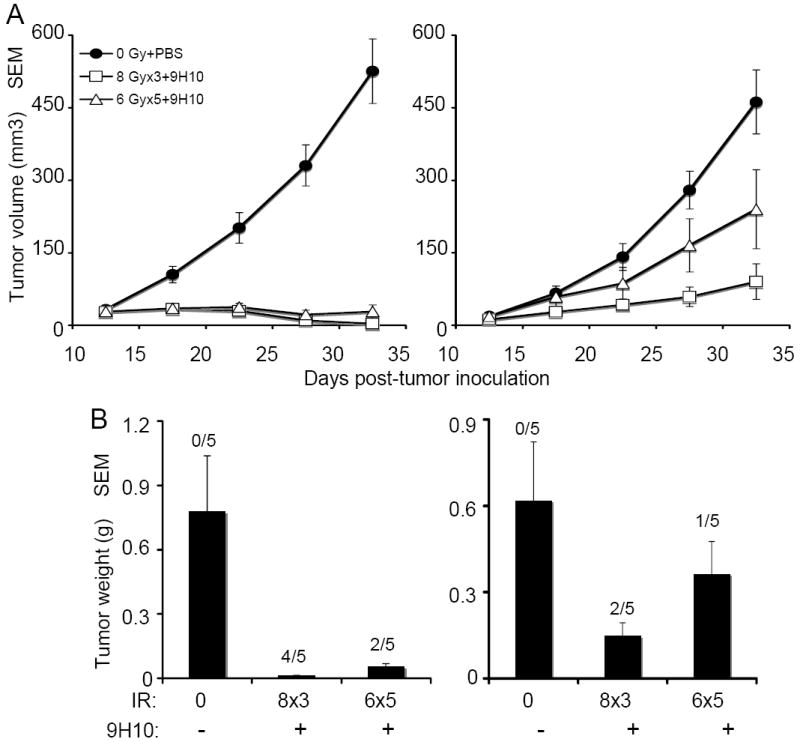
(A) Tumor growth delay of primary irradiated tumors (left panel) and secondary non-irradiated tumor (right panel) in mice treated with PBS (closed circles), 8 Gy × 3 + 9H10 (open squares), or 6 Gy × 5 + 9H10 (open triangles). 9H10 was given on days 14, 17, and 20. Data are the mean ± SE of 5 mice/group. (B) Tumor weight of primary (C) and secondary (D) tumors at day 35. Data are the mean ± SE. The number of mice with complete tumor regression over the total number of mice per group is indicated.
Analysis of secondary tumors for the presence of tumor-infiltrating lymphocytes (TIL) showed that, whereas in mice treated with radiotherapy and 9H10 as single modalities there was a minimal increase in the number of CD4+ and CD8+ TIL, treatment with 8 Gy × 3 and 9H10 caused a significant (p<0.05 compared to all other groups) increase in CD4+ and CD8+ TIL (Figure 5 A and B), suggesting that cell-mediated immunity was responsible for the abscopal effect. Consistent with this interpretation, ex vivo tumor-specific production of IFNγ by spleen cells was elevated only in mice that were effectively rejecting the secondary tumor (Figure 5 C). The frequency of CD8+ T cells showing tumor-specific IFNγ expression after in vitro restimulation with a CTL epitope known to be an immunodominant antigen in TSA cells (15) was also increased in treated mice that rejected secondary tumors but not in those that did not (Figure 5 C and D).
Figure 5. The combination of fractionated radiotherapy with anti-CTLA-4 antibody enhances TIL in secondary TSA tumors and tumor-specific T cells producing IFNγ.
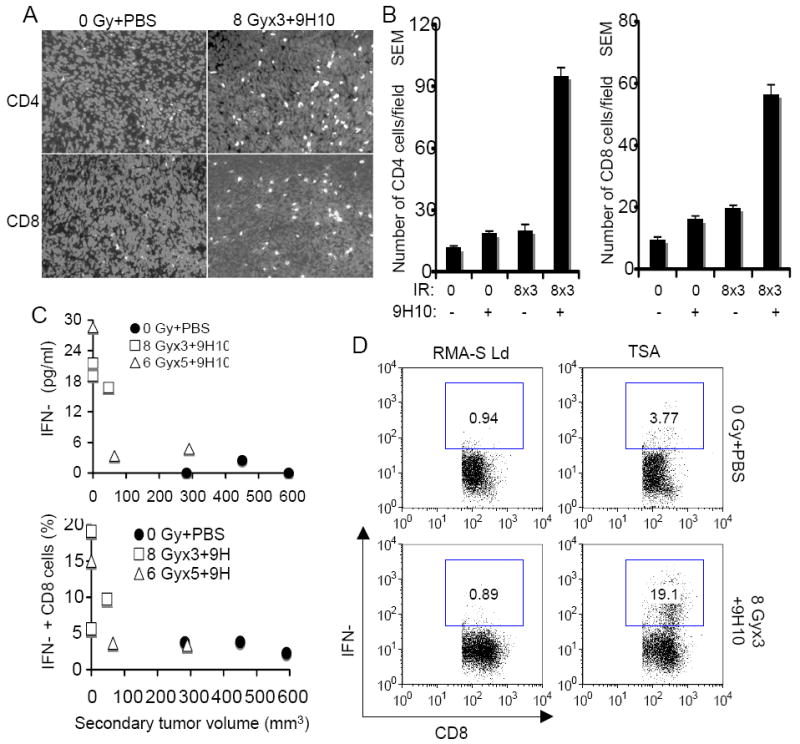
(A, B) Secondary tumors were excised at day 35 and analyzed by fluorescence microscopy for the presence of CD4+ and CD8+ T cells. (A) Representative fields showing CD4+ (top panels) and CD8+ (bottom panels) T cells (white) infiltrating secondary TSA tumors in mice treated as indicated. Nuclei were stained with DAPI (light gray). (B) Mean number ± SE of CD4+ and CD8+ TILs in three mice per group. Both CD4+ and CD8+ TIL were significantly increased in mice treated with the combination of 8 Gy × 3 + 9H10 (p<0.05 compared to all other groups), whereas radiation and 9H10 as single modalities did not have a significant effect. (C, D) Analysis of tumor-specific IFNγ production by spleen cells harvested at day 35 from mice in the various treatment groups. (C) IFNγ concentration in supernatants of total spleen cells isolated from mice treated with 0 Gy + PBS (closed circles), 8 Gy × 3 + 9H10 (open squares), or 6 Gy × 5 + 9H10 (open triangles) and cultured o.n. with irradiated TSA cells were plotted against the volume of the secondary tumor (Top panel). The percentage of CD8+ T cells expressing IFNγ when exposed to TSA cells as determined by intracellular staining (D) following in vitro restimulation with the TSA-derived immunodominant CD8 epitope AH1 was plotted against the volume of the secondary tumor (Bottom panel). Symbols are as above. Each symbol represents one animal. (D) Representative histograms showing the percentage of CD8+ T cells positive for IFNγ by intracellular staining and flow cytometry in response to TSA cells or the irrelevant target RMA-S-Ld. Samples were gated on CD8+ T cells.
Collectively, these results demonstrate that treatment with fractionated radiotherapy and CTLA-4 blockade induces tumor-specific T cell responses that, when sufficiently strong, are associated with complete rejection of tumors outside the radiation field.
Fractionated radiotherapy synergizes with anti-CTLA-4 antibody in the MCA38 colon cancer model
To determine whether the same effects of radiotherapy in combination with 9H10 would be seen in a different tumor type growing in mice of a different genetic background, we employed the MCA38 mouse colon carcinoma cells injected at two separate sites into C57BL/6 mice. On day 12, when both tumors were palpable, mice were randomly assigned to receive mock radiation, a single 20 Gy dose, or three fractions of 8 Gy to the primary tumor as described above (Figure 1), and 9H10 was administered to half of the mice in each treatment group on days 14, 17 and 20.
Similarly to what observed in the TSA model, 9H10 administration as single modality did not have any effect on growth of primary or secondary MCA38 tumors (Figure 6 A). Radiation alone caused a significant (p<0.0001) growth delay of primary irradiated tumors that was similar at 20 Gy × 1 and 8 Gy × 3, but had no effect on secondary tumors. Addition of 9H10 treatment to mice treated with 8 Gy × 3 significantly improved growth inhibition of primary tumors compared to radiation alone (p=0.0049), and caused a marked inhibition of secondary tumors (p=0.0001) (Figure 6 A and B). The combination of 9H10 with radiation at 20 Gy × 1 failed to significantly enhance the inhibition of primary tumors (p=0.145) and, although it modestly reduced the growth of secondary tumors, the effect was significantly less than that observed with 8 Gy × 3 + 9H10 (p<0.0001) (Figure 6 A and B).
Figure 6. The abscopal effect is induced in MCA38 tumor-bearing mice by fractionated radiation in combination with anti-CTL-4 antibody.
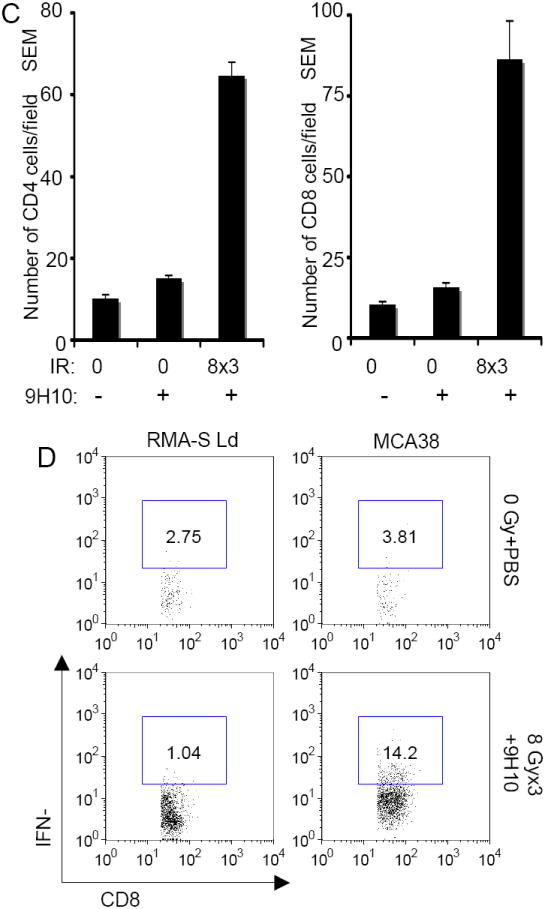
C57BL/6 mice were injected with syngeneic MCA38 colon carcinoma cells (5 × 105) s.c. into the right and left flank as outlined in Figure 1. (A) Tumor growth delay of primary irradiated tumors (left panel) and secondary non-irradiated tumor (right panel) in mice treated with PBS (closed circles), 9H10 (open circles), 20 Gy × 1 + PBS (closed diamonds), 20 Gy × 1 + 9H10 (open diamonds), 8 Gy × 3 + PBS (closed squares), 8 Gy × 3 + 9H10 (open squares). 9H10 was given on days 14, 17, and 20. Data are the mean ± SE of 5 mice/group. (B) Tumor weight of primary (left panel) and secondary (right panel) tumors at day 35. Data are the mean ± SE. The number of mice with complete tumor regression over the total number of mice per group is indicated. (C) Secondary tumors were excised at day 35 and analyzed by fluorescence microscopy for the presence of CD4+ and CD8+ T cells. Data are the mean number ± SE of CD4+ and CD8+ TILs in three mice per group. Both CD4+ and CD8+ TIL were significantly increased in mice treated with the combination of 8 Gy × 3 + 9H10 (p<0.05 compared to all other groups). (D) Analysis of tumor-specific IFNγ production by spleen cells harvested at day 35 from treated and untreated mice and re-stimulated in vitro with irradiated MCA38 cells. Histograms show the percentage of CD8+ T cells positive for IFNγ by intracellular staining and flow cytometry in response to MCA38 cells or the irrelevant target RMA-S-Ld. Samples were gated on CD8+ T cells. Spleen cells from 3 mice in each treatment group were pooled.
Therefore, in the MCA38 model fractionated more than single dose radiation triggered an abscopal effect when combined with CTLA-4 blockade.
Administration of 9H10 as single modality did not enhance significantly CD4+ and CD8+ TIL in secondary MCA38 tumors, whereas TIL were markedly increased in mice treated with 8 Gy × 3 + 9H10 (p<0.05 for both CD4+ and CD8+ T cells, compared to control and 9H10 alone) (Figure 6 C). The frequency of CD8+ T cells showing tumor-specific IFNγ expression after in vitro re-stimulation with MCA38 cells was also increased in mice treated with 8 Gy × 3 and 9H10 confirming the development of tumor-specific immunity following treatment (Figure 6 D).
DISCUSSION
In this study we show in a breast and colon carcinoma models that fractionated local radiotherapy to one palpable tumor can synergize with CTLA-4 blockade to induce anti-tumor T cells immunity and inhibit a second palpable tumor outside of the radiation field. This abscopal effect was not seen with radiotherapy alone. Although localized tumor irradiation by itself has been shown to enhance the generation of tumor-specific T cells in both pre-clinical models as well as in patients, the therapeutic effects of this response remains undetermined (3, 19). Clearly, irradiation to the primary tumor was required in our models to induce growth inhibition of the secondary tumors outside the field, since CTLA-4 blockade by itself was ineffective (Figures 2 and 6). This is consistent with the hypothesis that radiation-induced immunogenic tumor cell death as well as its induction of danger signals contribute to generate an in situ vaccine (20, 21) (22). While the response generated is not sufficient to be therapeutically significant, additional immunotherapeutic interventions might enable it to result in meaningful anti-tumor immunity.
Clinically, radiotherapy is usually given in multiple fractions to identify a compromise that achieves tumor control while enabling repair of damage to normal tissues within the field. Modern technologies enable better visualization and targeting of tumors, with selective concentration of dose distributions to achieve a therapeutic advantage (23). Our data indicate that a large single dose of 20 Gy was as effective as the two fractionation regimens of 8 Gy × 3 and 6 Gy × 5 at controlling the growth of the irradiated tumor (Figure 2). However, the degree to which radiation by itself achieved local tumor control did not predict its ability to synergize with CTLA-4 blockade. Addition of 9H10 mAb to mice receiving 20 Gy did not significantly improve the response, whereas a dramatic improvement in control of both primary and secondary tumors was seen when 9H10 was administered to mice treated with either of the two fractionated radiotherapy regimens tested (Figure 2). Importantly, the regimen of 8 Gy × 3 was superior to 6 Gy × 5 in induction of the abscopal effect and of tumor-specific T cells (Figures 4 and 5 C), suggesting that a specific therapeutic window exists for the optimal use of fractionated radiotherapy in combination with CTLA-4 blockade.
Employing the B16 mouse melanoma model Lugade et al. have shown that a single dose of 15 Gy irradiation resulted in priming of tumor-specific T cells in the draining lymph node that was at least comparable to that achieved after a regimen of 3 Gy × 5 fractions (3). We have previously shown in the 4T1 mouse breast cancer model that, although two fractions were better, a single dose of 12 Gy did also synergize with CTLA-4 blockade and induce anti-tumor CD8 cells capable of inhibiting lung micro-metastases (13). It is conceivable that single dose radiotherapy could promote cross-priming, but that the magnitude of the elicited immune response resulted insufficient at controlling “bulky” palpable tumors such as the tumors outside the radiation field in the current study.
The mechanisms underlying our findings that single dose and fractionated radiation differ in their ability to synergize with CTLA-4 blockade warrant further investigation. Interestingly, a recent report analyzing gene expression profiles of breast, prostate and glioma tumor cells exposed to single dose (10 Gy) versus fractionated (2 Gy × 5) radiation showed marked differences in the molecular response of these cells to the two regimens both in vitro and in vivo (24). Among the genes selectively induced by fractionated radiation in all three tumor cell lines were several IFN-related genes, including STAT1, but their role in promoting inflammation versus radio-resistance remains to be clarified (24).
Antibodies targeting immunomodulatory molecules on T cells to induce or enhance anti-tumor immunity are entering in the clinic. Among them, two CTLA-4 blocking mAbs (ipilimumab and tremelimumab) are at more advanced stage of testing and have shown some promising results (25). CTLA-4 blockade has activity as single treatment in melanoma, but the rate of complete response, disease control and overall survival was improved when given together with a cytotoxic agent (26). No data is currently available on the clinical use of radiotherapy with CTLA-4 blockade, whilst local radiation has been tested in combination with other immunotherapies (27-29). Results of these studies are consistent with pre-clinical predictions and support the hypothesis that local radiation can synergize with immunotherapy to promote anti-tumor immunity (1).
The data presented indicate that, in tumors that are refractory to treatment with CTLA-4 blockade alone, the combination with radiotherapy to one tumor site can induce systemic tumor control and in some cases complete regression. Importantly, the dose-fractionation of radiation can determine the overall efficacy of the combination treatment, an invaluable information in designing the clinical translation of this work.
Acknowledgments
We thank the personnel of NYU Cancer Institute Flow Cytometry and Experimental Pathology Histopathology core facilities, and of the Department of Radiation Oncology for expert assistance.
Grant support: NIH R01 CA113851, Research Scholar award RSG-05-145-01-LIB from the American Cancer Society, and The Chemotherapy Foundation (S. Demaria). Department of Defense Center of Excellence Award BC030282, and The Breast Cancer Research Foundation (S. C. Formenti). MZD is supported by Molecular Oncology and Immunology Training Grant T32 CA009161-33-34. NYU Cancer Institute is supported by NIH 5P30CA016087-27.
Footnotes
TRANSLATIONAL RELEVANCE
Therapeutics targeting immunomodulatory molecules to enhance anti-tumor immunity, such as the CTLA-4 inhibitory receptor on T cells, are being tested in clinical trials. When used as single agents in metastatic disease their activity is generally limited to a small fraction of patients, prompting testing in combination with other treatment modalities.
We have previously shown that local radiotherapy combined with anti-CTLA-4 antibody induces effective systemic anti-tumor responses (abscopal effect). Importantly, the preclinical definition of optimal dose and fractionation of radiotherapy when used in combination with anti-CTLA-4 antibody, is an important step to inform the correct design of a clinical trial that translates this experience to patients. Findings reported here indicate that the specific radiotherapy regimen employed is a critical determinant of the success of the combined treatment.
References
- 1.Demaria S, Bhardwaj N, McBride WH, Formenti SC. Combining radiotherapy and immunotherapy: a revived partnership. Int J Radiat Oncol Biol Phys. 2005;63:655–66. doi: 10.1016/j.ijrobp.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apetoh L, Ghiringhelli F, Tesniere A, et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med. 2007;13:1050–9. doi: 10.1038/nm1622. [DOI] [PubMed] [Google Scholar]
- 3.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 4.Demaria S, Formenti SC. Sensors of ionizing radiation effects on the immunological microenvironment of cancer. Int J Radiat Biol. 2007;83:819–25. doi: 10.1080/09553000701481816. [DOI] [PubMed] [Google Scholar]
- 5.Matsumura S, Wang B, Kawashima N, et al. Radiation-induced CXCL16 release by breast cancer cells attracts effector T cells. J Immunol. 2008;181:3099–107. doi: 10.4049/jimmunol.181.5.3099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ehlers G, Fridman M. Abscopal effect of radiation in papillary adenocarcinoma. Br J Radiol. 1973;46:220–2. doi: 10.1259/0007-1285-46-543-220. [DOI] [PubMed] [Google Scholar]
- 7.Robin HI, AuBuchon J, Varanasi VR, Weinstein AB. The abscopal effect: demonstration in lymphomatous involvement of kidneys. Med Pediat Oncol. 1981;9:473–6. doi: 10.1002/mpo.2950090510. [DOI] [PubMed] [Google Scholar]
- 8.Ohba K, Omagari K, Nakamura T, et al. Abscopal regression of hepatocellular carcinoma after radiotherapy for bone metastasis. Gut. 1998;43:575–7. doi: 10.1136/gut.43.4.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wersall PJ, Blomgren H, Pisa P, Lax I, Kalkner KM, Svedman C. Regression of non-irradiated metastases after extracranial stereotactic radiotherapy in metastatic renal cell carcinoma. Acta Oncol. 2006;45:493–7. doi: 10.1080/02841860600604611. [DOI] [PubMed] [Google Scholar]
- 10.Rosenberg SA, Sherry RM, Morton KE, et al. Tumor progression can occur despite the induction of very high levels of self/tumor antigen-specific CD8+ T cells in patients with melanoma. J Immunol. 2005;175:6169–76. doi: 10.4049/jimmunol.175.9.6169. [DOI] [PubMed] [Google Scholar]
- 11.Herber DL, Nagaraj S, Djeu JY, Gabrilovich DI. Mechanism and therapeutic reversal of immune suppression in cancer. Cancer Res. 2007;67:5067–9. doi: 10.1158/0008-5472.CAN-07-0897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Demaria S, Ng B, Devitt M-L, et al. Ionizing radiation inhibition of distant untreated tumors (abscopal effect) is immune mediated. Int J Radiat Oncol Biol Phys. 2004;58:862–70. doi: 10.1016/j.ijrobp.2003.09.012. [DOI] [PubMed] [Google Scholar]
- 13.Demaria S, Kawashima N, Yang AM, et al. Immune-mediated inhibition of metastases following treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 14.Pilones KA, Kawashima N, Yang AM, Babb JS, Formenti SC, Demaria S. Invariant natural killer T cells regulate breast cancer response to radiation and CTLA-4 blockade. Clin Cancer Res. 2009 doi: 10.1158/1078-0432.CCR-08-1277. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rosato A, Santa SD, Zoso A, et al. The cytotoxic T-lymphocyte response against a poorly immunogenic mammary adenocarcinoma is focused on a single immunodominant class I epitope derived from the gp70 Env product of an endogenous retrovirus. Cancer Res. 2003;63:2158–63. [PubMed] [Google Scholar]
- 16.Thurnherr N, Deschner EE, Stonehill EH, Lipkin M. Induction of adenocarcinomas of the colon in mice by weekly injections of 1,2-dimethylhydrazine. Cancer Res. 1973;33:940–5. [PubMed] [Google Scholar]
- 17.Alexander-Miller MA, Burke K, Koszinowski UH, Hansen TH, Connolly JM. Alloreactive cytotoxic T lymphocytes generated in the presence of viral-derived peptides show exquisite peptide and MHC specificity. J Immunol. 1993;151:1–10. [PubMed] [Google Scholar]
- 18.Cavallo F, DiCarlo E, Butera M, et al. Immune events associated with the cure of established tumors and spontaneous metastases by local and systemic interleukin 12. Cancer Res. 1999;59:414–21. [PubMed] [Google Scholar]
- 19.Schaue D, Comin-Anduix B, Ribas A, et al. T-cell responses to survivin in cancer patients undergoing radiation therapy. Clin Cancer Res. 2008;14:4883–90. doi: 10.1158/1078-0432.CCR-07-4462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Obeid M, Panaretakis T, Joza N, et al. Calreticulin exposure is required for the immunogenicity of gamma-irradiation and UVC light-induced apoptosis. Cell Death Differ. 2007;14:1848–50. doi: 10.1038/sj.cdd.4402201. [DOI] [PubMed] [Google Scholar]
- 21.Formenti SC, Demaria S. Local control by radiotherapy: is that all there is? Breast Cancer Res. 2008;10:215. doi: 10.1186/bcr2160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Apetoh L, Ghiringhelli F, Tesniere A, et al. The interaction between HMGB1 and TLR4 dictates the outcome of anticancer chemotherapy and radiotherapy. Immunol Rev. 2007;220:47–59. doi: 10.1111/j.1600-065X.2007.00573.x. [DOI] [PubMed] [Google Scholar]
- 23.Verellen D, Ridder MD, Linthout N, Tournel K, Soete G, Storme G. Innovations in image-guided radiotherapy. Nat Rev Cancer. 2007;7:949–60. doi: 10.1038/nrc2288. [DOI] [PubMed] [Google Scholar]
- 24.Tsai MH, Cook JA, Chandramouli GV, et al. Gene expression profiling of breast, prostate, and glioma cells following single versus fractionated doses of radiation. Cancer Res. 2007;67:3845–52. doi: 10.1158/0008-5472.CAN-06-4250. [DOI] [PubMed] [Google Scholar]
- 25.Fong L, Small EJ. Anti-cytotoxic T-lymphocyte antigen-4 antibody: the first in an emerging class of immunomodulatory antibodies for cancer treatment. J Clin Oncol. 2008;26:5275–83. doi: 10.1200/JCO.2008.17.8954. [DOI] [PubMed] [Google Scholar]
- 26.Hersh EM, Weber JS, Powderly JD, et al. Disease control and long-term survival in chemotherapy-naive patients with advanced melanoma treated with ipilimumab (MDX-010) with or without dacarbazine. J Clin Oncol. 2008;26:485s. abst 9022. [Google Scholar]
- 27.Gulley JL, Arlen PM, Bastian N, et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin Cancer Res. 2005;11:3353–62. doi: 10.1158/1078-0432.CCR-04-2062. [DOI] [PubMed] [Google Scholar]
- 28.Chi KH, Liu SJ, Li CP, et al. Combination of conformal radiotherapy and intratumoral injection of adoptive dendritic cell immunotherapy in refractory hepatoma. J Immunother. 2005;28:129–35. doi: 10.1097/01.cji.0000154248.74383.5e. [DOI] [PubMed] [Google Scholar]
- 29.Lechleider RJ, Arlen PM, Tsang KY, et al. Safety and immunologic response of a viral vaccine to prostate-specific antigen in combination with radiation therapy when metronomic-dose interleukin 2 is used as an adjuvant. Clin Cancer Res. 2008;14:5284–91. doi: 10.1158/1078-0432.CCR-07-5162. [DOI] [PMC free article] [PubMed] [Google Scholar]


