Abstract
Both during and after a period of iron deficiency (ID), iron-dependent neural processes are affected, which raises the potential concern that the anemia commonly experienced by many growing infants could have a protracted effect on the developing brain. To further investigate the effects of ID on the immature brain, 49 infant rhesus monkeys were evaluated across the first year of life. The mothers, and subsequently the infants after weaning, were maintained on a standardized diet containing 180 mg/kg of iron and were not provided other iron-rich foods as treats or supplements. As the infants grew, they were all screened with hematological tests, which documented that 16 (33.3%) became markedly ID between 4-to-8 months of age. During this anemic period and subsequently at one year of age, cerebrospinal fluid (CSF) specimens were collected to compare monoamine activity in the ID and iron-sufficient infants. Monoamine neurotransmitters and metabolite levels were normal at 4 and 8 months of age, but by one year the formerly anemic monkeys had significantly lower dopamine and significantly higher norepinephrine levels. These findings indicate that ID can affect the developmental trajectory of these two important neurotransmitter systems, which are associated with emotionality and behavioral performance, and further that the impact in the young monkey was most evident during the period of recovery.
Keywords: Iron deficiency, anemia, cerebrospinal fluid, dopamine, norepinephrine, monkey, infancy
INTRODUCTION
The need for iron to sustain growth during infancy is substantial and many babies become iron deficient (ID) during the nursing period before they are able to consume the extra iron available in solid foods (Dallman, 1986 Yip, 1989). This transient ID during the period of rapid infant growth has been described in developing rodents, farm animals, monkeys, and children (Allen, 1997; Weinberg, Dallman, & Levine, 1980; Zeterstrom, 2004). During the 1980s, ID still occurred in approximately 30% of children in the US, but today the overall prevalence has been reduced to 3-4% through the use of fortified formulas and cereals (Lonnerdal & Dewey, 1995; Looker, Dallman, Carroll, Gunter, & Johnson, 1997; Oski, 1992). The need to obtain iron from dietary sources is compounded if the infant is born with low stores of iron in the form of ferritin and hemoglobin, or in the other smaller but critical reserves found in iron-sulfur complexes and cytochromes (Rao & Georgieff, 2001; Sherriff, Emond, Bell, & Golding, 2001). Several prenatal factors, including maternal anemia during pregnancy or a premature birth, can also increase the likelihood of an infant depleting its iron stores and being challenged by the limited amount of iron in breast milk (Rao & Georgieff, 2002).
Despite extensive information about this ‘early anemia of childhood’ going back over 8 decades (Mackay, 1928), the significance for clinical practice has remained unclear. The very fact that ID is so common across species has contributed to some believing that a transient decline in iron-related hematology does not threaten normal development and is not a major concern for pediatricians. In addition, it was once believed that the brain had preferential access to the available dietary iron as it was partitioned between various body tissues. More recently, the traditional notion that the brain is spared from iron depletion even when there is a blood profile indicative of anemia has been qualified (Beard & Connor, 1993; Bradbury, 1997; Siimes, 1990). Several studies have found that a history of ID may be associated with impaired learning abilities in children at school age (Lozoff, Jimenz, Hagne, Mollen & Wolf, 2000; Lozoff, Beard, Connor, Felt, Georgieff, & Schallert, 2006a). Moreover, both animal and human studies have repeatedly demonstrated that ID can influence neural processes related to attention, sensory processing, and cognitive functioning (Algarin, Peirano, Garrid, Pizarro, & Lozoff, 2003; Siddappa, Rao, Wobken, Casperson, Leibold, Connor, & Georgieff, 2003).
When brain iron concentrations are low, the synthesis and release of iron-dependent monoamine neurotransmitters is altered and there is an impairment in the essential iron-dependent oligodendrocyte processes required to support myelination (Beard, Wiesinger, & Connor, 2003; Ortiz, Pasquini, Thompson, Felt, Butkus, Beard & Connor, 2004). This association between iron status and DA activity and D2 receptors has been found consistently (Youdim, Ben-Sachar, & Yehuda, 1989). The primary goal of the following study was to investigate the potential effect of ID on monoamine activity in the primate, because the prior research had all been done on rodent species. In addition, it was not clear when the alterations in monoamine levels would be most evident, because the timing and severity of the ID determines the extent of these neural deficits across development, due to the fact that the brain requirements for iron vary by age and region (Beard, Connor, & Jones, 1993; Beard, Felt, Schallert, Burhas, Connor, & Georgieff, 2006).
Two reports in veterinary journals had already documented that ID is a common occurrence in infants at primate facilities, even when not induced by an experimental manipulations (Bicknese, George, Hird, Paul-Murphy, Anderson, & Roberts, 1993; Kriete, Champoux & Suomi, 1995). A subset of young monkeys routinely becomes anemic, and their hematological profile will follow a predictable course with ID emerging between 4-8 months of age and then resolving after weaning with the consumption of solid foods. Our laboratory had also reported previously that the likelihood of an infant monkey becoming anemic was increased if its mother was ID prior to conception or if she experienced stress during her pregnancy (Lubach & Coe, 2006; Coe, Lubach & Shirtcliff, 2007). To create a naturalistic model without having to manipulate the infants postnatally, a diet was provided with adequate iron for the gravid female, but not fortified with the extra iron levels now recommended to prevent ID in the mother and infant (Beard, 2000; Bothwell, 2000; Milman, Bergholt, Erisken, Berg, Graudel, Pedersen, & Hertz, 2005). CSF specimens were then collected across the first year of life to determine the consequences of anemia for brain monoamine activity. Our a priori hypothesis was that the ID infants would have lower DA levels. Effects on serotonin (5HT) and NE were not as easy to predict, although one can find reports in the older pediatric literature of increased NE in the plasma and urine of anemic children (Voorhees, Stuart, Stockman, & Oski, 1975; Webb, Krill, Oski, & Tsou, 1982). In addition, when women are ID, they have higher plasma NE, which can be corrected with iron treatment, suggesting that it too is affected by iron biology (Borel, Smith, Derr, & Beard, 1991).
Because all 3 monoamines--DA, NE, and 5HT-- show marked age-related changes across the first year of life in the monkey, and are known to be sensitive to both experimental manipulations of diet and the early rearing environment (Goldman-Rackic & MacBrown, 1982; Grimes, Cameron, & Fernstrom, 2000; Higley, Suomi, & Linnoila, 1992; Maestripieri, Higley, Lindell, Newman, McCormack, & Sanchez, 2006), the specific question was whether the monoamine activity would be most affected during or subsequent to the period of ID Several reports in rodent models had indicated the effects of ID on the brain linger after the anemia has resolved, and there may even be a compensatory response following recovery (Dallman and Spirito, 1977). Severe ID generally lowers DA concentrations and reduces transporter and receptor levels in the rat brain, but a more moderate ID that is corrected in early lactation causes an up-regulation of DA activity in association with increased transferrin receptors and iron transporters in the brain (Beard, Felt, Schallert, Burhas, Connor, & Georgieff, 2006; Felt, Beard, Schallert, Shao, Aldridge, Connor, Georgieff, & Lozoff, 2006). Alternatively, when the ID is delayed until after weaning, there is a reduction of tyrosine hydroxylase, the rate-limiting enzyme critical for monoamine synthesis, and a general decrease in tissue DA. Simultaneously, in the latter case, the synthesis and/or release of NE appears to be enhanced, as indicated by increased brain NE responses to an infusion of L-DOPA in ID rats (Bianco, Wiesinger, Earley, Jones, & Beard, 2008).
This evidence in the rat for bidirectional effects depending upon the time of onset and severity of ID indicated a need to more systematically assess normative development in the infant monkey. Because primates have relatively high concentrations of the transport proteins that deliver iron to the monoamine projection areas (Huang, Ong, & Connor, 2004), it was also possible that the neural effects would be lessened. We also analyzed for the possible occurrence of sex differences in the prevalence of ID and its effect on brain functioning because so many papers on mice and rats had reported that males and females respond differentially to ID (Burhans, Dailey, Wiesinger, Murray-Kolb, Jones & Beard, 2006; Morse Beard, Azar, & Jones, 1999; Erikson, Jones, Hess, Zhang, & Beard, 2001).
METHODS
Subjects
Forty-nine infant rhesus macaques (Macaca mulatta) were evaluated under standardized housing conditions in a large breeding colony at the Harlow Primate Laboratory, where it was possible to carefully control the monkeys’ diet and rearing environment (Price, Coe & Hyde, 1999). The large number of infants available for this project also allowed us to consider whether the sex of the infant influenced the likelihood of developing ID. All husbandry and experimental procedures were approved by the institutional Animal Care and Use Committee (ACUC).
Breeding, diet, and housing conditions
The mothers were multiparous adult females between 5-15 years of age, and each was time-mated with a single adult male. Twelve males served as sires, each fathering between 1-7 offspring; thus many offspring had half-siblings on the paternal side. There was no effect of paternity on either the likelihood of becoming ID or any evidence of heritable effects on the infants’ monoamine levels (data not shown). Thirty-seven different females generated the infants for the project. Twelve adult females were bred twice and contributed more than one infant to this data set across two years (6 produced iron-sufficient infants in both years, 4 had ID infants in both years and 2 had one IS and one ID infant). While suggestive of a possible heritable maternal factor, the female cohort was too genetically diverse to assess the contribution of pedigree to the risk for ID.
All monkeys were fed a standard diet with an iron concentration adequate for a nonpregnant animal (180 mg/kg biscuit, PMI International. St. Louis, MO), but not specifically fortified to provide extra iron for a gravid female (Lubach & Coe, 2006). A fixed number of 10 biscuits (each approximately 25g) was given in the early morning and supplemented with 2-3 more biscuits in the late afternoon. Iron-rich treats and food supplements, such as raisins and peanuts, were not permitted in these housing rooms.
Following the natural deliveries at term, the infant was breast-fed and reared by its mother as an individual dyad in order to control the diet and to facilitate the rapid collection of blood and CSF specimens. At 6-7 months of age, they were weaned from the mothers into small social groups, each comprised of 2-4 animals. Thus, the monoamine assessments were conducted while all animals lived in similar housing conditions. The light/dark schedule was 14:10, with lights on at 0600. Samples were always collected in the morning between 0930-1100.
Infant Assessment
At 2-month intervals, small blood samples (1-3 ml) were collected for hematological testing. The infant was briefly removed from the mother, and blood collected by either saphenous or femoral venipuncture while it has held manually. A Complete Blood Count (CBC) was performed by a clinical laboratory knowledgeable about monkey specimens (Meriter Laboratory, Madison, WI). RBC values at 4, 6 and 8 months of age were used to designate the infants as iron-sufficient (IS) or ID. Normal ranges for MCV, Hct and Hgb for the rhesus monkey are known, allowing diagnostic cutoffs to be used for this determination of anemia (Bicknese et al., 1993; Lubach & Coe, 2006). The iron dependency of the anemia was further confirmed by quantifying the zinc protoporphyrin/heme ratio (μmole ZPP/mole heme) on a hematofluorometer (Aviv Biomedical, Lakewood, NJ), a good index of ID erythropoiesis in young infants (Winzerling & Kling, 2001; Baumann-Blackmore, Goetz, Blohowiak, Zaka, & Kling, 2008).
CSF samples were collected three times at 4, 8 and 12 months of age. The infant was briefly sedated with ketamine hydrochloride (15 mg/kg, i.m.), and 1 ml of CSF collected by cervical puncture with a small 25-gauge needle. Specimens were collected within 10-15 min of the initial removal from the cage and ketamine sedation. The proximity of the collection site to the hindbrain enabled us to obtain a more accurate reflection of brain monoamine activity, because of the known gradient in monoamine levels lower in the spinal cord (Ruckeusch & Sutra, 1984). CSF was placed immediately on wet ice, spun in a refrigerated centrifuge at 2000g to remove any trace RBC, and frozen in an ultracold freezer at −60 °C until analysis. In keeping with the concerns expressed by others about the potentially confounding effects of blood contamination on monoamine values, only pristine CSF specimens were used in the assays (Anderson Bennett, Weld, Pushkas, Ocame, & Higley, (2002). Many previous papers have documented the appropriateness of these collection and storage methods for assessing monoamine concentrations (e.g., Langlais, Bird, & McEntee, 1982).
High Pressure Liquid Chromatography (HPLC)
CSF specimens were thawed on ice and passed through centrifuge tube filters (0.2 um) (Spin-X Costar, Corning Inc, Corning, NY). Aliquots (10 μl) were loaded into an ESA 542 (ESA Inc, Chelmsford, MA) refrigerated autosampler maintained at 4 °C. They were then injected on to an ESA MD-150 narrow-bore HPLC column 150 × 2 mm (ESA Inc., Chelmsford, MA) for separation followed by detection with an ESA 5014B microdialysis cell (E1:-175mV, E2:+300 mV) (ESA Coulochem III, ESA Inc., Chelmsford, MA). A guard cell (ESA 5020) placed in line before the injection loop was set at a potential of +350 mV. The mobile phase consisted of 75 mM sodium phosphate monobasic (EMD Chemical, Gibbstown, NJ), 1.7 mM 1-octanesulfonic acid (EMD Chemical, Gibbstown, NJ), 25 μM ethylenediaminetetracetic acid (Acros, NJ), 10% acetonitrile (EMD Chemical, Gibbstown, NJ), and 0.01% triethylamine (Sigma Aldrich, St. Louis, MO) in a volume of 1 L (pH 3.0). The neurotransmitter and metabolite peak areas were integrated using EZ Chrom Elite software (Scientific Software Inc., Pleasanton, CA) and quantified against known standards of NE (ESA Inc., Chelmsford, MA), DA (ESA Inc., Chelmsford, MA), 3,4-Dihydroxyphenylacetic acid (DOPAC) (Sigma Aldrich, St. Louis, MO), and homovanillic acid (HVA) (Sigma Aldrich, St. Louis, MO). The relative standard deviation (RSD) between injections with these procedures is 0.5% and the RSD between days for the calibration curve is 5%. Data across assays were normalized to one standard curve.
Statistical Analyses
Differences in the hematological profiles between the IS and ID groups were tested with mixed model, analyses of variance (ANOVA). Age at assessment was entered as a repeated measure to verify that the infants designated as ID had experienced a period of clinical anemia and underwent a hematological recovery by 12 months of age. Additional ANOVAs were then performed to assess potential differences in monoamine and metabolite concentrations between the two groups of monkeys, specifically during the period of ID (4-8 months) and after recovery (12 months). After finding a large maturational age effect on DA and 5HT levels across the first year of life, which is commonly seen in young monkeys and children (Higley et al., 1992; Langlais, Walsh, Bird, & Levy, 1985; Maestripieri, et al., 2006) the CSF results at 12 months of age were assessed in separate post hoc ANOVAs from the data obtained at the 4 and 8 month taps. Because of the marked differential response of males and females to ID reported in rodents, all analyses considered the possible effect of sex difference, by including it as a between subjects factor in the ANOVAs.
RESULTS
Infant Status and Hematology
All 49 infants were delivered vaginally without human assistance, and reared naturally by their mothers. Despite their overall healthy status, the hematological status of 16 (33.3%) declined markedly in a manner indicative of a growth-related ID. There was not a significant difference in the prevalence of ID in males and females, and based on the hematological tests, the extent of ID was similar (ID: 8 male, 8 female; IS: 22 male, 11 female). All 16 infants categorized to the ID group met diagnostic criteria for anemia on at least 2 hematological measures between 4-to-8 months of age. At 6 months of age, the mean MCV of 57.5 fL in the ID group was below the standard cutoff of 60 fL (Fig. 1). Similarly, their Hgb levels averaged 10.7 (0.3) g/dL, below the typical criteria for anemia of 12.0 g/dL, and significantly below the mean 13.4 (1.6) value of the IS infants (F[1,46] = 52.37, p < .0001). The iron dependency of these changes in the RBC was also supported by finding a significantly elevated mean ZPP/heme ratio in the ID infants as compared to the IS animals (104.1 vs, 48.6 μM; F[1,45] = 18.68, p < .0001). Despite the marked hematological differences at this developmental stage, there was a progressive recovery once they began to exclusively consume solid food after weaning from the mother. By 12 months of age, the hematological values were similar in the two groups (Fig. 1).
Fig. 1.
Hematological profiles of healthy (n = 33) and iron-deficient (ID, n = 16) infant rhesus monkeys at 6 months of age, and subsequent to the anemic monkeys recovery at 12 months of age. Raw data and mean (+SD) are shown. Note that the designation of ID required both a low MCV and Hgb value simultaneously (below 60 fL and 12 g/dL, respectively). ID infants were classified as anemic based on meeting this criteria at 4, 6 or 8 months of age. At the 6-month time point, the group differences between IS and ID animals were statistically significant. There was also significant interaction between Iron Status and Age because the hematological profile of the ID animals rebounded by12 months of age.
CSF Monoamine Levels
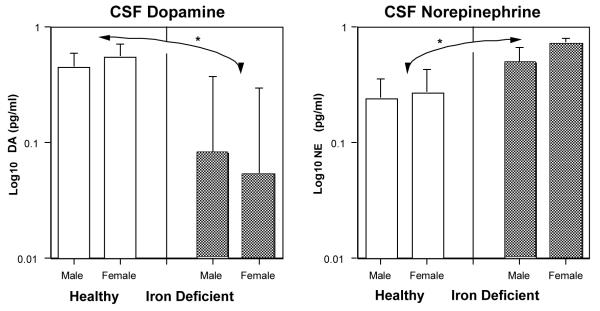
Neither the monoamine neurotransmitter nor metabolite concentrations differed between ID and IS infants at 4 and 8 months of age (see Table 1). While there were age-related changes in monoamine activity during this 4-month period, the overall levels in the ID infants appeared normal at this time. However, at 12 months of age, subsequent to the hematological recovery, the CSF values of the previously ID monkeys were noticeably different (Fig. 2). Their DA concentrations in CSF were significantly lower and NE concentrations significantly higher than the levels found in the monkeys that had been IS throughout the entire first year of life (F[1,45] = 4.38, p < .042; F[1,45] = 5.37, p < .026, respectively). For the monoamine metabolites, only age-related changes were evident; there were no significant differences in any metabolite between ID and IS monkeys. At one year of age, the lower DA levels in the formerly anemic monkeys resulted in a downward skewing of the neurotransmitter/metabolite ratio, but this trend did not reach significance (mean DA/HVA ratio = 0.01 versus 0.016 in the IS animals, F[1,47] = 2.95, p <.093)
Table 1.
Mean monoamine (pg/ml) and metabolite (ng/ml) concentrations in the CSF of iron-sufficient (IS) and iron-deficient (ID) monkeys at 4, 8 and 12 months of age.
| Period of ID Anemia | Post-Recovery | |||
|---|---|---|---|---|
| 4 | 8 | 12 | ||
| DA | IS | 6.4 (.7) | 9.5 (1.1) | 5.4 (.7) |
| ID | 7.0 (1.0) | 9.8 (1.7) | 3.3 (1.1) * | |
| NE | IS | 3.8 (.9) | 5.0 (1.2) | 2.8 (.4) |
| ID | 1.8 (1.0) | 6.1 (1.5) | 5.1 (.8) * | |
| 5HT | IS | 2.1 (1.8) | 1.1 (.7) | 1.0 (.1) |
| ID | 2.8 (2.3) | 0.3 (.1) | 1.0 (.2) | |
| Epi | IS | 1.8 (1.1) | 2.8 (1.3) | 0.7 (.1) |
| ID | 5.8 (4.6) | 0.8 (.3) | 0.8 (.1) | |
| HVA | IS | 193 (12) | 180 (15) | 350 (14) |
| ID | 181 (9) | 165 (19) | 350 (17) | |
| DOPAC | IS | 13 (2) | 11 (1) | 13 (1) |
| ID | 13 (2) | 11 (2) | 14 (1) | |
| 5HIAA | IS | 345 (19) | 281 (9) | 306 (12) |
| ID | 331 (29) | 282 (23) | 325 (19) | |
|
|
||||
p < .05, difference between IS and ID at 12 months of age. DA and HVA levels changed significantly with age, and there was also an interaction between iron status and age-related changes in Epi.
Fig. 2.
CSF monoamine concentrations in yearling monkeys that had been iron-sufficient or -deficient at 6 months of age. In both male and female monkeys, the levels of DA were significantly lower and NE significantly higher subsequent to recovery from ID. Note the logarithmic scale visually accentuates the variance estimates (error bars) for the lower DA values in ID monkeys relative to the actual magnitude of the mean difference from the IS condition.
DISCUSSION
This research on infant monkeys has confirmed previous studies on developing mice and rats, indicating that a period of ID can affect the maturation of the monoamine systems (Beard, 2001; Kwik-Uribe, Gietzen, German, Golub, & Keen, 2000). However, in the young primate, the effects were not apparent during the period of anemia, but subsequent to hematological recovery. The delayed impact is similar to some aspects of both the acute and chronic models of ID in the rat. Our finding of lower DA concentrations in CSF concurs with the effect seen after uncorrected ID and also when the ID is first induced post-weaning (Beard, Chen, Connor, & Jones 1994). However, the lack of a difference during the actual period of anemia is more similar to the abbreviated ID paradigm in which rat pups receive iron supplementation during early lactation to correct the deficiency. In that paradigm the rats may even show a compensatory up-regulation of iron transport into the CNS and subsequently an enhanced production of DA (Beard et al., 2006; Felt et al., 2006). Whether the compensatory response occurs depends upon the severity of the ID. If striatal brain iron falls 30-40% below normal, the up-regulation does not occur, but if the terminal field iron concentrations decrease just 10-20% of normal, then there is a resilient capacity for a compensatory response (Beard et al., 2006).
The ID in the current study occurred relatively late in the nursing stage, during the time when monkey mother begins to naturally wean her infants between 4-6 months of age. The developmental timing and the actual hematological values found in our study are very similar to prior reports on rhesus monkeys in two other colonies (Bicknese et al., 1993; Kriete et al., 1995). We had previously identified several risk factors that increase the likelihood of an infant monkey becoming ID, including if its mother was ID prior to conception or if it grew rapidly after birth (Coe et al., 2007; Lubach & Coe, 2006). The anemia then emerges as the infant’s growth-related iron needs begin to exceed the stores available at birth and the iron present in breast milk. The 33% prevalence of infants meeting criteria for anemia by weaning age is in keeping with the prior surveys. In contrast to the frequent finding of marked sex differences in mice and rats, it was of interest that the likelihood of anemia was similar in both males and females, as was the impact of ID on their CSF monoamine levels. The similarity of the brain response in males and females may also be because our infants were still prepubertal, and thus too young for gonadal hormones to affect monoamine activity (Becker, 1999).
The dissociation in the timing of the blood and brain effects, and the quicker hematological recovery after the infants began to eat solid food, probably reflects how iron is partitioned between various tissue compartments, with the red blood cells showing a more rapid repletion than the brain. On a daily basis it is believed that the erythron mass comprises approximately 70-80% of the cell and tissue need for iron. In the ID individual, there may be a further prioritizing of iron to bone marrow, liver, spleen, and red blood cells, which may be placing the brain needs for iron in jeopardy. Our finding of a delayed but sustained effect on the CNS also concurs with a prior proteomic analysis of CSF from a different group of ID monkeys, which indicated that their iron regulatory proteins were increased and the intrathecal proteome disturbed for at least 4 months after the period of anemia had ended (Geguchadze, Coe, Lubach, Clardy, Beard, & Connor, 2008).
The developmental effects of ID in the monkey are not always so protracted. At another primate facility, young infants were challenged by just a delimited period of low dietary iron during fetal life or in early infancy (Golub, Hogref, & Germann, 2007). In those rearing conditions, brain monoamine activity was not overtly affected later during the first year of life. When their results are considered with ours, it suggests that a more sustained and marked anemia may be required to evoke lasting changes in monoamine levels. Perhaps what is most remarkable is that the growing infants of so many mammalian species are challenged in this manner to meet their iron needs while nursing and transitioning to solid foods. These findings led the National Research Council to recommend markedly increasing the iron fortification of commercial diets for rodents and primates. In fact, the current iron concentration at >350 mg/kg in many primate chows is so high that a new husbandry concern may be the provision of an iron excess, which can create different health concerns through a stimulation of oxidative metabolism and an accumulation of iron in the brain (Andrews, 2008).
The possibility that a sustained period of ID will affect the developing brain should no longer be viewed as benign. In addition to effects on monoamine activity, many studies have documented a deleterious influence on myelination, including a lower synthesis of myelin lipids and phospholipids (Ortiz, et al., 2004) and a reduction in oligodendrocyte numbers and their metabolic support for myelination (Beard, Connor, & Jones, 1993; Morath & Mayer-Proschel, 2002). Even after iron concentrations in the rat normalize, the iron transport and management proteins in the brain continue to be changed into adulthood (Clardy, Wang, Zhao, Liu, Chase, Beard, Felt, & Connor, 2006). In addition, several papers have reported that ID affects the development of sensitive brain regions, including the hippocampus (DeUngria, Rao, Wobken, Luciana, Nelso, Georgieff, 2000; Rao et al., 1999; Jorgenson, Wobken, & Georgieff, 2003; Sanchez, Diaz-Hido, & Avila. 2002). Beyond just compromising the hippocampus, ID was found to reduce dendritogenesis and synaptogenesis in the iron-rich midbrain and cerebellar regions (Rao, DeUngria, Sullivan, Wu, Wobken, Nelson, & Georgieff, 1999).
While the finding of lower DA levels is in keeping with literature and confirmed our a priori hypothesis, the significantly higher NE levels post-anemia requires further explanation. The latter effect does appear to concur with older papers on changes in the peripheral physiology of anemic individuals. Increased NE was found in the blood and urine of anemic children, which was interpreted as indicative of greater sympathetic activity, perhaps to assist with thermoregulation in the face of a lower oxygen-carrying capacity by their red blood cells (Dillmann, Johnson, Martin, Mackler, & Finch, 1979; Voorhees et al. 1975; Webb et al., 1982). In addition, when rats are ID, they have an increased level of extracellular NE and higher NE turnover in their brains, as well as lower brain levels of MHPG, the primary NE metabolite, all of which could contribute to the diffusion of more NE into the intrathecal compartment (Burhans, Daily, Beard, Wiesinger, Murray-Kolb, Jones, & Beard, 2005; Erikson et al., 2001). It has also recently been suggested that the increased brain NE in ID rats could be due to elevated dopamine-beta-hydoxylase activity, which would explain their exaggerated NE response to L-DOPA (Bianco et al., 2008).
Nevertheless, it still may be parsimonious to attribute some of the increased NE, and the delayed manner in which the effect emerged, to the type of up-regulation of NE levels seen when young monkeys are reared away from their mothers. When infant monkeys are raised initially by humans in a nursery and then later housed with other infant monkeys (i.e., peer-reared), they have chronically elevated NE and MHPG in their CSF after they reach 6-24 months of age (Clarke, Hedker, Ebert, Schmidt, McKinney, & Kraemer, 1996; Higley et al., 1992). Moreover, this upward skewing is associated with a greater stress reactivity and emotionality. Thus, it could be that ID serves to create a neurobehavioral profile that has some features in common with the one induced by other disturbances of early rearing (Corapci, Tadan, & Lozoff, 2006; Perz, Hendricks, Beard, Murray-Kolb, Beg, Tomlinson, Irlam, Isaacs, Njengele, Sive, & Vernon-Feagans, 2005). In fact, it is even possible that an abnormal iron profile could contribute to some of these behavioral deficits in infant monkeys from disturbed backgrounds. For example, both maternal alcohol consumption and prenatal stress have been shown to compromise iron transfer to fetus (Connor, 1994; Coe et al., 2007). Both of these pregnancy conditions are also known to result in lower DA activity in the young rat and monkey (Roberts, Moore, DeJesus, Barnhart, Larson, Mukherjee, Nickles, Scueller, Shelton, & Schneider, 2004). The possibility that there are additional links between iron nutriture and neurobehavioral development underscores the importance of paying closer attention to the micronutrient needs of pregnant females as well as to the diets of immature animals and young children.
Acknowledgments
NOTES Supported by a Program-Project grant on Brain, Behavior and Early Iron Deficiency from the National Institute of Child Health and Development (P01 HD39386, P.I.: B. Lozoff). Additional support was provided by grants from the National Institute of Allergy and Infectious Diseases (AI46521, AI607517). The authors thank Ms. H. Crispen for her invaluable assistance with the animal husbandry and sample collection.
REFERENCES
- Algarin C, Peirano P, Garrido M, Pizarro F, Lozoff B. Iron deficiency anemia in infancy: Long-lasting effects on auditory and visual systems functioning. Pediatric Research. 2003;53:217–223. doi: 10.1203/01.PDR.0000047657.23156.55. [DOI] [PubMed] [Google Scholar]
- Allen LH. Pregnancy and iron deficiency: Unresolved issues. Nutrition Review. 1997;55:91–101. doi: 10.1111/j.1753-4887.1997.tb06460.x. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Bennett AJ, Weld KP, Pushkas JG, Ocame DM, Higley JD. Serotonin in cisternal cerebrospinal fluid of rhesus monkeys. Basal levels and effects of setraline administration. Psychopharmacologica. 2002;16:95–99. doi: 10.1007/s00213-002-1034-1. [DOI] [PubMed] [Google Scholar]
- Andrews NC. Forging a field: the golden age of iron biology. Blood. 2008;112:219–230. doi: 10.1182/blood-2007-12-077388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman-Blackmore NL, Goetz G, Blohowiak SE, Zaka O, Kling PJ. Cord blood zinc protoporphyrin/heme ratio in minority neonates at risk for iron deficiency. The Journal of Pediatrics. 2008;153:133–126. doi: 10.1016/j.jpeds.2008.01.032. [DOI] [PubMed] [Google Scholar]
- Beard JL. Effectiveness and strategies of iron supplementation during pregnancy. American Journal of Clinical Nutrition. 2000;71:1288S–1294S. doi: 10.1093/ajcn/71.5.1288s. [DOI] [PubMed] [Google Scholar]
- Beard JL. Iron biology in immune function, muscle metabolism, and neuronal functioning. Journal of Nutrition. 2001;131:568–580. doi: 10.1093/jn/131.2.568S. [DOI] [PubMed] [Google Scholar]
- Beard JL, Chen Q, Connor J, Jones BC. Altered monamine metabolism in caudate-putamen of iron-deficient rats. Pharmacology, Biochemistry and Behavior. 1994;48:621–624. doi: 10.1016/0091-3057(94)90323-9. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR. Iron status and neural functioning. Annual Review of Nutrition. 1993;23:41–58. doi: 10.1146/annurev.nutr.23.020102.075739. [DOI] [PubMed] [Google Scholar]
- Beard JL, Connor JR, Jones BC. Iron in the brain. Nutrition Review. 1993;51:157–170. doi: 10.1111/j.1753-4887.1993.tb03096.x. [DOI] [PubMed] [Google Scholar]
- Beard JL, Felt BT, Schallert T, Burhas M, Connor JR, Georgieff MK. Moderate iron deficiency in infancy: Biology and behavior in young rats. Behavioral Brain Research. 2006;170:224–232. doi: 10.1016/j.bbr.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Beard JL, Wiesinger JA, Connor JR. Pre- and postweaning iron deficiency alters myelination in Sprague-Dawley rats. Developmental Neuroscience. 2003;25:308–315. doi: 10.1159/000073507. [DOI] [PubMed] [Google Scholar]
- Becker JB. Gender differences in dopaminergic function in striatum and nucleus accumbens. Pharmacology, Biochemistry and Behavior. 1999;64(4):803–812. doi: 10.1016/s0091-3057(99)00168-9. [DOI] [PubMed] [Google Scholar]
- Bianco LE, Wiesinger J, Earley CJ, Jones BC, Beard JL. Iron deficiency alters dopamine uptake and response to L-DOPA injection in Sprague-Dawley rats. Journal of Neurochemistry. 2008;106:205–215. doi: 10.1111/j.1471-4159.2008.05358.x. [DOI] [PubMed] [Google Scholar]
- Bicknese EJ, George JW, Hird DW, Paul-Murphy J, Anderson JA, Roberts JR. Prevalence and risk factors for iron deficiency in anemia in weanling rhesus macaques. Laboratory Animal Science. 1993;43:434–438. [PubMed] [Google Scholar]
- Borel MJ, Smith SM, Derr J, Beard JL. Day-to-day variation in iron status indices in healthy men and women. American Journal of Clinical Nutrition. 1991;54:729–735. doi: 10.1093/ajcn/54.4.729. [DOI] [PubMed] [Google Scholar]
- Bothwell TH. Iron requirements in pregnancy and strategies to meet them. American Journal of Clinical Nutrition. 2000;72:257S–264S. doi: 10.1093/ajcn/72.1.257S. [DOI] [PubMed] [Google Scholar]
- Bradbury MWB. Transport of iron in the blood-brain-cerebrospinal fluid system. Journal of Neurochemistry. 1997;69:443–454. doi: 10.1046/j.1471-4159.1997.69020443.x. [DOI] [PubMed] [Google Scholar]
- Burhans MS, Dailey C, Beard Z, Wiesinger J, Murray-Kolb L, Jones BC, Beard JL. Iron deficiency: differential effects on monoamine transporters. Nutrition Neuroscience. 2005;8:31–38. doi: 10.1080/10284150500047070. [DOI] [PubMed] [Google Scholar]
- Burhans MS, Daily C, Wiesinger J, Murray-Kolb LE, Jones BC, Beard JL. Iron deficiency affects acoustical starte response and latency, but not prepulse inhibition in young adult rats. Physiology and Behavior. 2006;87(5):917–924. doi: 10.1016/j.physbeh.2006.02.014. [DOI] [PubMed] [Google Scholar]
- Clardy SL, Wang X, Zhao W, Liu W, Chase GA, Beard JL, Felt BT, Connor JR. Acute and chronic effects of developmental iron deficiency on mRNA expression patterns in the brain. Journal of Neural Transmission Supplementum. 2006;71:173–196. doi: 10.1007/978-3-211-33328-0_19. [DOI] [PubMed] [Google Scholar]
- Clarke AS, Hedker DR, Ebert MH, Schmidt DE, McKinney WT, Kraemer GW. Rearing experience and biogenic amine activity in infant rhesus monkeys. Biological Psychiatry. 1996;40:338–352. doi: 10.1016/0006-3223(95)00663-X. [DOI] [PubMed] [Google Scholar]
- Coe CL, Lubach GR, Shirtcliff EA. Maternal stress during pregnancy predisposes for iron deficiency in infant monkeys impacting innate immunity. Pediatric Research. 2007;61(5):520–524. doi: 10.1203/pdr.0b013e318045be53. [DOI] [PubMed] [Google Scholar]
- Connor JR. Iron acquisition and expression of iron regulatory proteins in the developing brain: manipulation by ethanol exposure, iron deprivation and cellular dysfunction. Developmental Neuroscience. 1994;16:233–247. doi: 10.1159/000112115. [DOI] [PubMed] [Google Scholar]
- Corapci F, Radan A, Lozoff B. Iron deficiency and mother-child interaction at 5 years. Journal of Developmental & Behavioral Pediatrics. 2006;27(5):371–378. doi: 10.1097/00004703-200610000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dallman PR. Iron deficiency in the weanling: a nutritional problem on the way to resolution. Acta Paediatrica Scandinavica Supplement. 1986;323:59–67. doi: 10.1111/j.1651-2227.1986.tb10351.x. [DOI] [PubMed] [Google Scholar]
- Dallman PR, Spirito RA. Brain iron in the rat: Extremely slow turnover in normal rats may explain long-lasting effects of iron deficiency. Journal of Nutrition. 1977;107:1075–1081. doi: 10.1093/jn/107.6.1075. [DOI] [PubMed] [Google Scholar]
- DeUngria M, Rao R, Wobken JD, Luciana M, Nelson CA, Georgieff MK. Perinatal iron deficiency decreases cytochrome c oxidase (CytOx) activity in selected regions of neonatal rat brain. Pediatric Research. 2000;48:169–176. doi: 10.1203/00006450-200008000-00009. [DOI] [PubMed] [Google Scholar]
- Dillmann E, Johnson G, Martin J, Mackler B, Finch C. Catecholamine elevation in iron deficiency. American Journal of Physiology. 1979;237:R297–R300. doi: 10.1152/ajpregu.1979.237.5.R297. [DOI] [PubMed] [Google Scholar]
- Erikson KM, Jones BC, Hess EJ, Zhang Q, Beard JL. Iron deficiency decreases dopamine D1 and D2 receptors in rat brain. Pharmacology, Biochemistry and Behavior. 2001;69:409–419. doi: 10.1016/s0091-3057(01)00563-9. [DOI] [PubMed] [Google Scholar]
- Felt BT, Beard JL, Schallert T, Shao J, Aldridge W, Connor JR, Georgieff M,K, Lozoff B. Persistent neurochemical and behavioral abnormalities in adulthood despite early iron supplementation for perinatal iron deficiency anemia in rats. Behavioural Brain Research. 2006;171:261–270. doi: 10.1016/j.bbr.2006.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geguchadze RN, Coe CL, Lubach GR, Clardy TW, Beard JW, Connor RJ. CSF proteomic analysis reveals persistent iron deficiency-induced alterations in nonhuman primate infants. Journal of Neurochemistry. 2008;105:127–136. doi: 10.1111/j.1471-4159.2007.05113.x. [DOI] [PubMed] [Google Scholar]
- Golub MS, Hogrefe CE, Germann SL. Iron deprivation during fetal development change the behavior of juvenile monkeys. Journal of Nutrition. 2007;137:979–984. doi: 10.1093/jn/137.4.979. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, MacBrown R. Postnatal development of monoamine content in the cerebral cortex of rhesus monkeys. Developmental Brain Research. 1982;4(3):339–349. doi: 10.1016/0165-3806(82)90146-8. [DOI] [PubMed] [Google Scholar]
- Grimes MA, Cameron JL, Fernstrom JD. Cerebrospinal fluid concentrations of tryptophan and 5-hydroxyindoleacetic acid in Macaca mulatta: Diurnal variations and response to chronic changes in dietary protein intake. Neurochemical Research. 2000;25:413–422. doi: 10.1023/a:1007557524370. [DOI] [PubMed] [Google Scholar]
- Higley JD, Suomi SJ, Linnoila M. A longitudinal assessment of CSF monoamine metabolite and plasma cortisol concentrations in young rhesus monkeys. Biological Psychiatry. 1992;32:127–145. doi: 10.1016/0006-3223(92)90016-s. [DOI] [PubMed] [Google Scholar]
- Huang E, Ong WY, Connor JR. Distribution of divalent metal transporter-1 in the monkey basal ganglia. Neuroscience. 2004;128:487–496. doi: 10.1016/j.neuroscience.2004.06.055. [DOI] [PubMed] [Google Scholar]
- Jorgenson LA, Wobken JD, Georgieff MK. Perinatal iron deficiency alters apical dendritic growth in hippocampal CA1 pyramidal neurons. Developmental Neuroscience. 2003;25:412–420. doi: 10.1159/000075667. [DOI] [PubMed] [Google Scholar]
- Kriete MF, Champoux M, Suomi SJ. Development of iron deficiency anemia in infant rhesus monkeys. Laboratory Animal Science. 1995;45:15–21. [PubMed] [Google Scholar]
- Kwik-Uribe CL, Gietzen D, German JB, Golub MS, Keen CL. Chronic Marginal iron intakes during early development in mice result in persistent changes in dopamine metabolism and myelin composition. Journal of Nutrition. 2000;130:2821–2830. doi: 10.1093/jn/130.11.2821. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Bird ED, McEntee WJ. Stability of monoamine metabolites in human cerebrospinal fluid. Annals of Neurology. 1982;12(1):48–51. doi: 10.1002/ana.410120109. [DOI] [PubMed] [Google Scholar]
- Langlais PJ, Walsh FX, Bird ED, Levy HL. Cerebrospinal fluid neurotransmitter metabolites in neurological normal infants and children. Pediatrics. 1985;75(3):580–586. [PubMed] [Google Scholar]
- Lonnerdal B, Dewey KG. Epidemiology of iron deficiency in infants and children. Annals Nestle. 1995;53:1–7. [Google Scholar]
- Looker AC, Dallman PR, Carroll MD, Gunter EW, Johnson CL. Prevalence of iron deficiency in the United States. Journal of the American Medical Association. 1997;277:973–976. doi: 10.1001/jama.1997.03540360041028. [DOI] [PubMed] [Google Scholar]
- Lozoff B, Beard JL, Connor JR, Felt BT, Georgieff MK, Schallert T. Long-lasting neural and behavioral effects of iron deficiency in infant. Nutrition Review. 2006a;64:S34–43. doi: 10.1301/nr.2006.may.S34-S43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lozoff B, Jimenez E, Hagen J, Mollen E, Wolf AW. Poorer behavioral and developmental outcome more than 10 years after treatment for iron deficiency in infancy. Pediatrics. 2000;105:852–858. doi: 10.1542/peds.105.4.e51. [DOI] [PubMed] [Google Scholar]
- Lubach GR, Coe CL. Preconception maternal iron status is a risk factor for iron deficiency in infant rhesus monkeys. Journal of Nutrition. 2006;136:2345–2349. doi: 10.1093/jn/136.9.2345. [DOI] [PubMed] [Google Scholar]
- Mackay HNM. Anaemia in infancy: its prevalence and prevention. Archives of Diseases in Childhood. 1928;3:117–146. doi: 10.1136/adc.3.15.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maestripieri D, Higley JD, Lindell SG, Newman TK, McCormack KM, Sanchez MM. Early maternal rejection affects the development of monoamine systems and adult abusive parenting in rhesus monkeys (Macaca mulatta) Behavioral Neuroscience. 2006;120:1017–1024. doi: 10.1037/0735-7044.120.5.1017. [DOI] [PubMed] [Google Scholar]
- Milman N, Bergholt T, Eriksen L, Beyg KE, Graudel N, Pedersen P, Hertz J. Iron prophylaxis during pregnancy-How much is needed: A randomized dose-response study of 20-80 mg ferrous iron daily in pregnant women. Acta Obstetricia Gynecologica Scandinavica. 2005;84:238–247. doi: 10.1111/j.0001-6349.2005.00610.x. [DOI] [PubMed] [Google Scholar]
- Morath DJ, Mayer-Proschel M. Iron modulates the differentiation of a Distinct population of glial precursor cells into oligodendrocytes. Developmental Biology. 2002;237:232–243. doi: 10.1006/dbio.2001.0352. [DOI] [PubMed] [Google Scholar]
- Morse AC, Beard JL, Azar MR, Jones B. Sex and genetics are important cofactors in assessing the impact of iron deficiency on the developing mouse brain. Nutrition Neuroscience. 1999;2:323–335. doi: 10.1080/1028415X.1999.11747287. [DOI] [PubMed] [Google Scholar]
- Ortiz E, Pasquini JM, Thompson K, Felt BT, Butkus G, Beard JL, Connor JR. Effect of manipulation of iron storage, transport, or availability on myelin composition and brain iron content in three different animal models. Journal of Neuroscience Research. 2004;77:681–689. doi: 10.1002/jnr.20207. [DOI] [PubMed] [Google Scholar]
- Oski FA. Iron deficiency in infancy and childhood. New England Journal of Medicine. 1993;329:190–193. doi: 10.1056/NEJM199307153290308. [DOI] [PubMed] [Google Scholar]
- Price KC, Coe CL, Hyde JS. Matrinlineal transmission of birth weight in the rhesus monkey across several generations. Obstetrics & Gynecology. 1999;94:129–134. doi: 10.1016/s0029-7844(99)00269-0. [DOI] [PubMed] [Google Scholar]
- Rao R, DeUngria M, Sullivan D, Wu P, Wobken JD, Nelson CA, Georgieff MK. Perinatal brain iron deficiency increases the vulnerability of rat hippocampus to hypoxic ischemic insult. Journal of Nutrition. 1999;129:199–206. doi: 10.1093/jn/129.1.199. [DOI] [PubMed] [Google Scholar]
- Rao R, Georgieff MK. Neonatal iron nutrition. Seminars in Neonatology. 2001;6:425–435. doi: 10.1053/siny.2001.0063. [DOI] [PubMed] [Google Scholar]
- Rao R, Georgieff MK. Perinatal aspects of iron metabolism. Acta Paediatrica Supplement. 2002;91:124–129. doi: 10.1111/j.1651-2227.2002.tb02917.x. [DOI] [PubMed] [Google Scholar]
- Roberts AD, Moore CF, DeJesus OT, Barnhart TE, Larson JA, Mukherjee J, Nickles RJ, Schueller MJ, Shelton SE, Schneider ML. Prenatal stress, moderate fetal alcohol and dopamine system function in rhesus monkeys. Neurotoxicology and Teratology. 2004;26:169–178. doi: 10.1016/j.ntt.2003.12.003. [DOI] [PubMed] [Google Scholar]
- Ruckeusch M, Sutra JF. On the significance of monoamines and their metabolites in the cerebrospinal fluid of the sheep. Journal of Physiology. 1984;348:457–469. doi: 10.1113/jphysiol.1984.sp015119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherriff A, Emond A, Bell JC, Golding J. Should infants be screened for anaemia? A prospective study investigating the relation between haemoglobin at 8, 12, and 18 months and development at 18 months. Archives of Diseases in Childhood. 2001;84:480–485. doi: 10.1136/adc.84.6.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddappa AM, Rao R, Wobken JD, Casperson K, Leibold EA, Connor JR, Georgieff MK. Iron deficiency alters iron regulatory protein and iron transport protein expression in the perinatal rat brain. Pediatric Research. 2003;53:800–807. doi: 10.1203/01.PDR.0000058922.67035.D5. [DOI] [PubMed] [Google Scholar]
- Siimes MA. Hematopoiesis and storage iron in infants. In: Lonnerdal B, editor. Iron Metabolism in Infants. FL CRC Press; Boca Raton: 1990. pp. 33–62. [Google Scholar]
- Voorhees ML, Stuart JA, Stockman JA, Oski FA. Iron deficiency and increased urinary norepinephrine excretion. Journal of Pediatrics. 1975;86:542–547. doi: 10.1016/s0022-3476(75)80144-2. [DOI] [PubMed] [Google Scholar]
- Webb TE, Krill CE, Oski FA, Tsou KC. Relationship of iron status to urinary norepinephrine in children 7-12 years of age. Journal of Pediatric Gastroenterology and Nutrition. 1982;1:207–209. doi: 10.1097/00005176-198201020-00009. [DOI] [PubMed] [Google Scholar]
- Weinberg J, Dallman PR, Levine S. Iron deficiency during early development in the rat: behavioral and physiologic consequences. Pharmacology Biochemistry and Behavior. 1980;12:493–502. doi: 10.1016/0091-3057(80)90179-3. [DOI] [PubMed] [Google Scholar]
- Winzerling JJ, Kling PJ. Iron-dependent erythropoiesis in premature infants measured by blood zinc protoporphoryin/heme. Journal of Pediatrics. 2001;139:134–136. doi: 10.1067/mpd.2001.115574. [DOI] [PubMed] [Google Scholar]
- Yip R. The changing characteristics of childhood iron nutritional status in the United States. In: Filer LR, editor. Dietary iron: Birth to two years. Raven Press; New York: 1989. pp. 38–56. [Google Scholar]
- Youdim MB, Beh-Shacher D, Yehuda S. Putative biological mechanisms of the effect of iron deficiency on brain biochemistry and behavior. American Journal of Clinical Nutrition. 1989;50:607–615. doi: 10.1093/ajcn/50.3.607. [DOI] [PubMed] [Google Scholar]
- Zetterstrom R. Iron deficiency and iron deficiency anaemia during infancy and childhood. Acta Paediatrica. 2004;93:436–439. doi: 10.1080/08035250410027535. [DOI] [PubMed] [Google Scholar]