Abstract
The mechanisms that contribute to the extinction of previously acquired memories are not well understood. These processes, often referred to as inhibitory learning, are thought to be parallel learning mechanisms that require a reacquisition of new information and suppression of previously acquired experiences in order to adapt to novel situations. Using newly generated metabotropic glutamate receptor 5 (mGluR5) knock-out mice, we investigated the role of mGluR5 in the acquisition and reversal of an associative conditioned task and a spatial reference task. We found that acquisition of fear conditioning is partially impaired in mice lacking mGluR5. More markedly, we found that extinction of both contextual and auditory fear was completely abolished in mGluR5 knock-out mice. In the Morris Water Maze test (MWM), mGluR5 knock-out mice exhibited mild deficits in the rate of acquisition of the regular water maze task, but again had significant deficits in the reversal task, despite overall spatial memory being intact. Together, these results demonstrate that mGluR5 is critical to the function of neural circuits that are required for inhibitory learning mechanisms, and suggest that targeting metabotropic receptors may be useful in treating psychiatric disorders in which aversive memories are inappropriately retained.
Introduction
Metabotropic glutamate receptors (mGluRs) modulate neural activity via their linkage to various intracellular cascades (Nakanishi, 1992). The eight individual mammalian mGluRs can be subdivided into three groups based on their sequence homologies and physiological activities. mGluR5 belongs to the Group I mGluRs and is coupled to inositol phosphate/Ca2+ signal transduction pathway (Abe et al., 1992). mGluR5 has been demonstrated to have important roles in several forms of synaptic plasticity (Lu et al., 1997; Jia et al., 1998; Huber et al., 2000; Bikbaev et al., 2008) and learning behaviors (Lu et al., 1997; Chiamulera et al., 2001; Balschun and Wetzel, 2002), and has been suggested as a potential therapeutic target in several neurological disorders (Brody et al., 2004; Slassi et al., 2005; Marino and Conn, 2006; Dölen et al., 2007). mGluR5 is expressed throughout the CNS including in the hippocampus and lateral nucleus of the amygdala (Abe et al., 1992), which are both structures central to learning and memory mechanisms. Previous studies have demonstrated a role for mGluR5 in two very different forms of learning associated with the hippocampus and the amygdala. Systemic injection (Schulz et al., 2001) or local amygdala perfusion (Rodrigues et al., 2002) of mGluR5 antagonists can disrupt the acquisition of the fear response, and contextual fear conditioning is impaired in mice deficient for mGluR5 (Lu et al., 1997). Similarly mGluR5 knock-out mice show deficits in the acquisition of hippocampal dependent learning (Lu et al., 1997). Thus, while there is significant evidence that mGluR5 is involved in the acquisition of new memories and synaptic plasticity mechanisms, the role of mGluR5 in one important aspect of learning is unknown: the reversal or extinction of a previously acquired task, an important adaptive process that corrects for an altered environmental or situation (Bouton, 1993). These learning mechanisms are important to retasking, and are particularly relevant to anxiety disorders in humans such as phobias and post-traumatic stress disorder (Barad, 2005). The mechanisms and molecules involved in these adaptive learning processes, which are often termed inhibitory learning (Bouton and Bolles, 1979; Bouton, 1993; Barad, 2005; Myers and Davis, 2007), are not fully understood, although both ionotropic and metabotropic glutamate receptors have been implicated (Walker et al., 2002; Callaerts-Vegh et al., 2006; Kim et al., 2007; Fendt et al., 2008).
In this study, we tested whether mGluR5 plays a role in inhibitory learning processes using newly generated mGluR5 knock-out mice (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Toward this end, we tested mGluR5 knock-out mice in the acquisition and reversal of an associative conditioned task and a spatial reference task. In the classic pavlovian fear conditioning test, we found that mGluR5 null mice were impaired in the acquisition of fear conditioning, confirming a role for this receptor in neural circuits required for this form of learning (Lu et al., 1997; Rodrigues et al., 2002). Even more strikingly we observed a complete deficit in the ability of mGluR5 knock-out mice to extinguish the fear association in both a tone-cued or context-cued test. In the MWM test, mGluR5 knock-out mice exhibited mild deficits in the rate of acquisition of the regular water maze task. However, when the task was reversed and mice were compelled to learn a new location for the escape platform, mGluR5 knock-out mice performed poorly in this novel situation. Together these findings demonstrate a significant role for mGluR5 in adaptive processes that underlie inhibitory learning.
Materials and Methods
All experiments were approved by the Institutional Animal Care and Use Committees of the Salk Institute for Biological Studies.
Generation of mGluR5 knock-out mice.
Standard gene targeting techniques were used to generate mutant floxed mGluR5 mice (supplemental Fig. 1, available at www.jneurosci.org as supplemental material). Genomic DNA for mGluR5 was cloned from a phage library of 129 SVJ mouse genomic DNA fragments (Stratagene). A cassette containing a neomycin resistance (neo) gene, flanked by loxP sites, under the control of the phosphoglycerol kinase (PGK) promoter was introduced into the intron 720 bp downstream of exon 7 (supplemental Fig. 1a, available at www.jneurosci.org as supplemental material). R1 embryonic stem cells (Nagy et al., 1993) were electroporated with the linearized targeting construct, maintained under G418 positive selection, and screened by Southern blot analysis for homologous recombination (supplemental Fig. 1b, available at www.jneurosci.org as supplemental material). Chimeric animals produced by injection of these cells into C57BL/6 blastocysts were bred with C57BL/6 mice, and germ-line transmission of the mutation was assessed by PCR and Southern blot. The neo cassette was removed in mice by crossing with transgenic mice with Cre recombinase under control of the protamine promoter (O'Gorman et al., 1997) to produce the floxed mice mGluR5loxP/loxP (supplemental Fig. 1c–e, available at www.jneurosci.org as supplemental material). Finally mGluR5loxP/loxP mice homozygous for the conditional allele were crossed to protamine-cre mice to generate the mGluR5 knock-out mice (mGluR5del/del).
Same sex littermates were housed 2–5 per cage, and maintained at 22°C, with a 12 h light/dark cycle. mGluR5loxP/loxP and mGluR5del/del mice used in this study were produced by heterozygous breeding of mGluR5loxP/del, or heterozygous breeding of mGluR5loxP/+ and mGluR5del/+. Age (2- to 4-month-old) and gender-matched littermates were used for behavioral studies.
Fear conditioning.
An automated video tracking system was used to monitor mice in the fear conditioning paradigm (Med Associates). Samples were collected at the rate of 30 frames/s. A freezing event was registered only when activity was below motion threshold (20 arbitrary units) for at least 0.5 s. Mice were handled daily for 1 week before the test and were transferred to a holding place adjacent to the testing room for 20 min to 1 h on the day of the test. The training on day 0 (d0) lasted for 6 min. Each mouse was subjected to either training paradigm A, that consisted of 3 min of baseline monitoring, followed by 3 pairs of 20 s tone (85 db, 2900 Hz) coterminated with 1 s footshock (0.7 mA) given at 1 min intervals (Fig. 1 a), or paradigm B, which was similar to the paradigm A except that the 1 s footshock was presented without the tone (Fig. 1 b).
Figure 1.
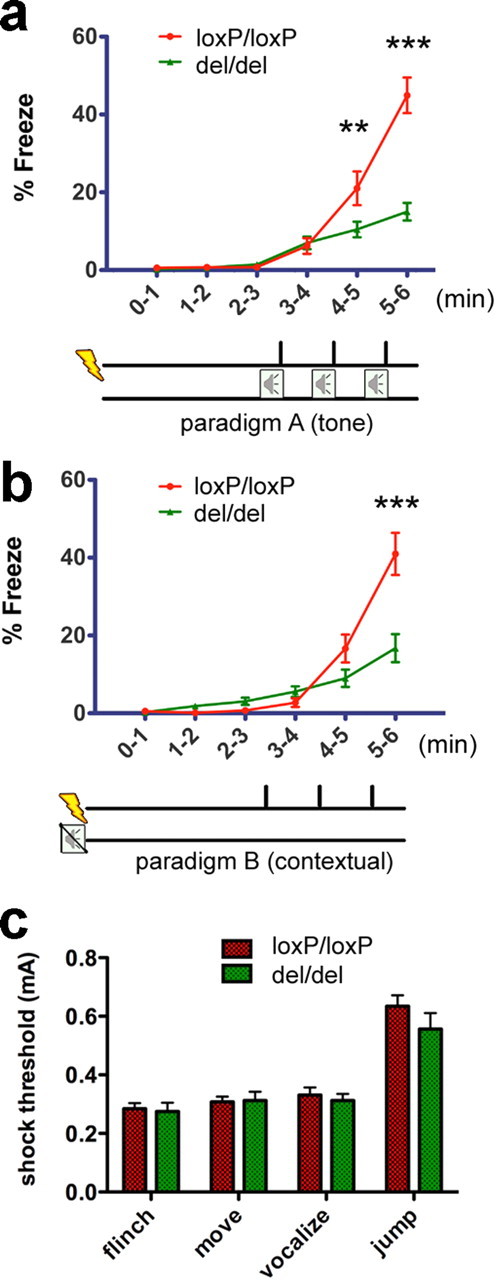
Deficits in fear acquisition in mice lacking mGluR5. a, Fear acquisition by three footshocks paired with tones. mGluR5del/del mice (n = 28) froze significantly less than mGluR5loxP/loxP(n = 27) (F (1,106) = 13.14, p = 0.0006 for genotype; F (2,106) = 33.93, p < 0.0001 for training–genotype interaction; Bonferroni post-tests for genotype, p < 0.05 at 4–5 min, p < 0.0001 at 5–6 min). b, Fear acquisition by three footshocks. There was less freezing in mGluR5del/del mice (n = 20) than mGluR5loxP/loxPmice (n = 25) (F (1,86) = 6.31, p = 0.0158 for genotype; F (2,86) = 14.48, p < 0.0001 for training–genotype interaction; Bonferroni post-tests for genotype, p < 0.0001 at 5–6 min). c, Acute pain threshold to footshock was not altered in mGluR5 null mice. We measured the minimal currents required to elicit four stereotyped reactions against footshock: flinch, move, vocalization, and jump. mGluR5del/del mice (n = 8) and mGluR5loxP/loxP mice (n = 13) showed the same pain sensitivity to an increasing electric footshock (t test, p > 0.05 for each reactions between genotypes). Data are presented as SEM (**p < 0.01,***p < 0.001 for Bonferroni post-tests).
For contextual memory testing each mouse was returned to the same chamber with the exact contextual settings, but without tone and footshock and monitored for 6 min. Chambers were cleaned with isopropanol between each set of mice (Fig. 2 a, d1, d15). For the extinction of contextual memory the same procedure was repeated daily for 10 consecutive days (Fig. 3).
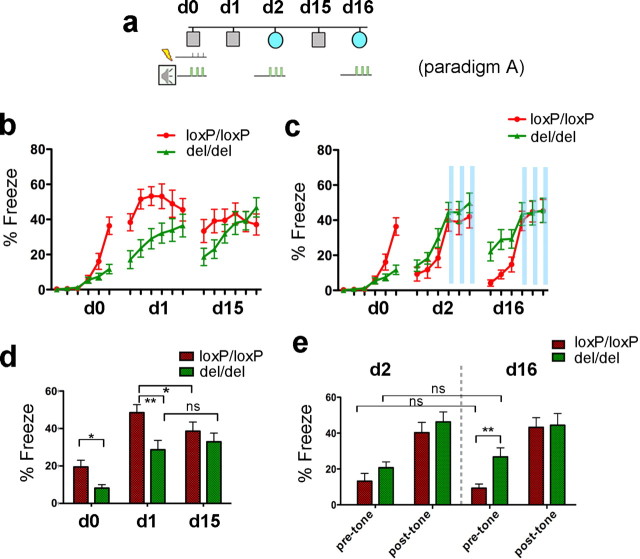
Figure 2.
Effects of deleting mGluR5 on for both contextual and tone-cued fear conditioning. a, The experimental paradigm. Mice were trained at d0 by three footshocks paired with tones in context 1 (marked by gray box), retuned to the same context at d1 and d15 for 6 min without tone presentation. At d2 and d16, mice were put into different context (marked by light blue circle) for 6 min, with three pairs of 20 s tone presentations of 1 min intervals starting at 3 min. b, After fear acquisition at d0, context-cued tests were conducted at d1 and d15, respectively. Freezing was scored at 1 min intervals (mGluR5del/del, n = 17; mGluR5loxP/loxP, n = 18). c, Auditory fear conditioning tests were conducted at d2 and d16, respectively. Freezing was scored at 1 min intervals (mGluR5del/del, n = 17; mGluR5loxP/loxP, n = 18). Time periods for tone presentations were highlighted with blue bars. d, Freezing in (b) was scored at 6 min intervals. mGluR5del/del mice exhibited <50% postshock freezing (d0) compared with mGluR5loxP/loxP mice (p = 0.0104, t test). In the first context test (d1) performed 24 h after training, mGluR5del/del mice showed a 40% reduction in the context-cued freezing time (p = 0.0047, t test). The ratios of postshock freezing in d0 versus contextual freezing in d1 were comparable between the two groups (0.465 ± 0.1154 and 0.3830 ± 0.1123 for mGluR5loxP/loxP and mGluR5del/del respectively; p > 0.05, t test). Contextual fear in the second test (d15) was slightly reduced in mGluR5loxP/loxP mice (d1 vs d15; paired t test, p = 0.047), but was slightly increased in mGluR5del/ldel mice (d1 vs d15; paired t test, p > 0.05). e, Freezing in (c) was scored at 3 min intervals (for pretone fear and post-tone fear, respectively). Pretone freezing in the second auditory fear conditioning test (d16) was 2.5 times greater in mGluR5del/del mice than the control group (p = 0.0028 t test). A significant difference was not detected between other genotype comparisons. Pretone freezing was reduced in mGluR5loxP/loxP mice but was elevated in mGluR5del/del mice (d2 vs d16), but the differences were not significant. Data are presented as SEM (*p < 0.05, **p < 0.01, for t tests).
Figure 3.
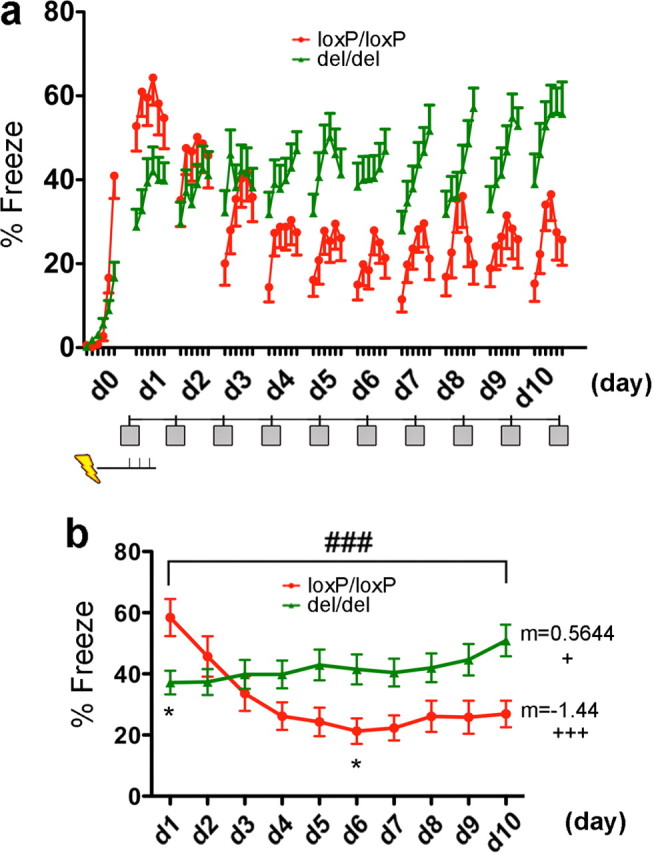
Contextual fear extinction was abolished in mGluR5-deficient mice. a, Extinction of contextual fear. Mice [mGluR5del/del, n = 20 (d1–d9), n = 14 (d1–d10); mGluR5loxP/loxP, n = 25 (d1–d7), n = 15, (d1–d10)] were trained in d0 with three footshocks and returned to the same context (marked by gray box) up to 10 consecutive days for 6 min each day. Freezing was analyzed at 1 min intervals. During extinction training (d1–d10), mGLuR5loxP/loxP mice generally showed bell-shaped time–response with highest freezing occurring at 3–4 min, whereas mGluR5del/del mice showed a trend of increase freezing toward the end of 6 min training session. b, Freezing in a was scored at 6 min intervals. mGluR5del/del mice displayed no extinction over the trials (nonmatching two-way ANOVA, F (1,394) = 21.26, p < 0.0001 for genotype; F9, 394 = 4.22, p < 0.0001 for day–genotype interaction; Bonferroni post-tests for genotype, *p < 0.05 at d1 and d6). For the mGluR5loxP/loxP group, one-way ANOVA detected significant effect of training (F (9,210) = 6.644, p < 0.0001). Post-test further detected significant trend of fear extinction (slope = −1.44, p < 0.0001). For the mGluR5del/del group, effect of training was not significant by one-way ANOVA (F (9,184) = 0.61, p > 0.05). However, post-test for liner trend detected a significant positive trend (slope = 0.57, p = 0.036). Data are presented as SEM (*p < 0.05, for Bonferroni post-tests; ### p < 0.001 for two-way ANOVA). (Linear slope is denoted by “m”; + p < 0.05 and +++ p < 0.001 for ANOVA post-test for linear trend). ns, Not significant.
For testing auditory fear (Figs. 2 a, d2, d16; 4), the conditioning chamber was altered by covering the grid floor and the three sides with patterned plastic boards, and the top with colored paper. New visual cues were provided on the inside wall of the insulating box and on the walls of the testing room. The conditioning chamber was also scented with Windex and vanilla solution. Windex was used for cleaning after each testing. The testing lasted 6 min for each session. For the experiments presented in Figure 2 (d2 and d16) each mouse was placed into the chamber for 3 min and three sets of 20 s tones were presented at 1 min intervals. For the extinction of auditory fear conditioning each mouse was put into the chamber for 3 min before the 3 min tone was presented. The same procedure was repeated daily for 16 consecutive days (Fig. 4).
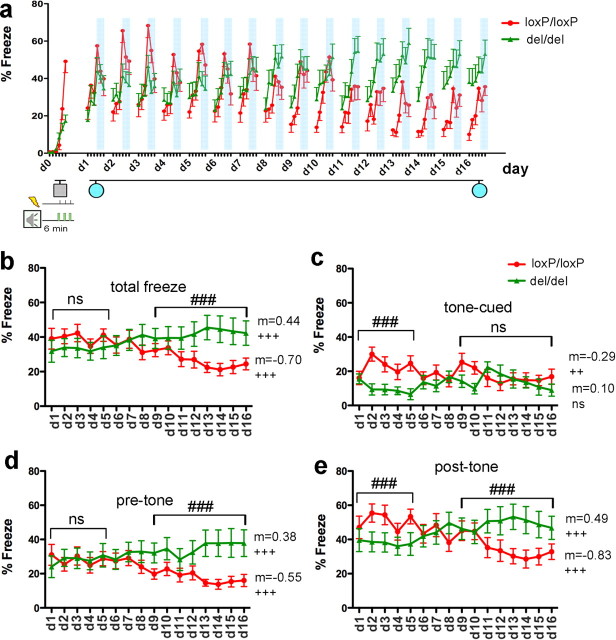
Figure 4.
Extinction of auditory fear conditioning was abolished in mGluR5-deficient mice. a, Extinction of auditory fear conditioning. Mice were trained with three footshocks paired with tones at d0 in one context (gray square) and subsequently returned to a different context (blue circles) for 16 consecutive days. Extinction training on each day lasted 6 min, with one tone of 3 min presented during the final 3 min. Freezing was scored at 1 min intervals to compare within-session fear expression profiles between the two genotype groups (mGluR5del/del n = 14; mGluR5loxP/loxP n = 16). Time periods for the tone presentations were highlighted with blue bars (each represents 3 min). Gray square marked for the context for fear acquisition. Blue circles indicate the neutral context where extinction trainings were conducted. b, Total freezing in each day analyzed in 6 min block. Initially, mGluR5del/del mice (n = 14) showed slightly less freezing (F (1,140) = 3.86, p = 0.0513, d1–d5), but this freezing behavior was not diminished during the course of repeated tone presentations. In contrast, mGluR5loxP/loxP mice (n = 16) showed extinction of auditory fear conditioning upon repeated daily exposure, resulting in less freezing in the second half of testing (F (1, 224) = 32.64, p < 0.001, d9–d16). Overall, day–genotype interaction was significant (F (15,420) = 7.52, p < 0.0001). Post-test detected significant trend of fear extinction in mGluR5loxP/loxP mice (slope = −0.70, p < 0.0001) and significant trend of increase in fear in mGluR5del/del mice (slope = 0.44, p < 0.0001). c, Extinction of tone-cued freezing. At the onset, tone-cued freezing was less in mGluR5del/del mice (F (1,140) = 29.57, p < 0.0001, d1–d15). Near the completion of the test, tone-cued freezing amounts were similar between the two groups (F (1,224) = 2.16, p = 0.14, d9–d16). Two-way ANOVA showed a significant day–genotype interaction from d1 to d16 (F (15,420) = 2.80, p = 0.0004). Post-test for linear trend detected significant trend of decrease of fear in mGluR5loxP/loxP mice (slope = −0.29, p < 0.0031) but not in mGluR5del/del mice (slope = 0.11, p = 0.23). d, Pretone freezing analyzed in 3 min block. Although both groups displayed similar amounts of freezing in the early and middle sessions of training (d1–d9), mGluR5del/del mice froze more in the late sessions (d9–d16) (F1, 224 = 40.15, p < 0.0001). Two-way ANOVA showed a significant day–genotype interaction from d1 to d16 (F (15,420) = 4.13, p < 0.0001). Post-test for linear trend detected significant trend of fear reduction in mGluR5loxP/loxP mice (slope = −0.55, p < 0.0001) and a significant trend of fear increase in mGluR5del/del mice (slope = 0.38, p < 0.0001). e, Post-tone freezing analyzed in 3 min block. Initially, post-tone freezing was less in mGluR5del/del mice (F (1,140) = 12.87, p = 0.0005, d1–d15). However, mGluR5del/del mice froze more in the end (F (1,224) = 20.2, p < 0.0001, d9–d16). Two-way ANOVA showed a significant day–genotype interaction from d1 to d16 (F (15,420) = 7.50, p < 0.0001). A significant trend of fear reduction in mGluR5del/del mice (m = −0.83, p < 0.0001) and a significant trend of fear increase in mGluR5del/del mice (m = 0.49, p < 0.0001) were detected. Data are presented as SEM (### p < 0.001 for two-way ANOVA). (Linear slope is denoted by “m”; ++ p < 0.01 and +++ p < 0.001 for ANOVA post-test for linear trend). ns, Not significant.
Morris water maze.
The Morris Water Maze experiments were conducted as previously described with some modifications (van Praag et al., 2005; Zhang et al., 2008). A white plastic water tank of 120 cm diameter was filled with water at room temperature. The water was made opaque with white nontoxic Crayola washable paint. A transparent platform (8 ×13 cm) was submerged 1 cm below the surface of opaque water. An automated video tracking system (Ethovision; Noldus Information Technology) was used to record the swim path, velocity and time taken to reach the platform (latency) or the time spent in each zone. The water maze procedure consisted of three phases: (1) visible platform training; (2) hidden platform training and probe test 1; and (3) reversed platform and probe test 2.
Mice were first trained to find the visible platform for 3 d (three trials per day). A thin black plastic brick (8 × 13 × 1.5 cm) was placed above the transparent platform and a flag was installed 10–15 cm above the surface, allowing mice to visualize the location of the platform. Mice were released from the Southwest (SW) quadrant for all trials. The platform was rotated from the Northeast (NE) to Northwest (NW) to Southeast (SE) quadrant for each trial. Upon release, each mouse was allowed a maximum of 60 s to find the visible platform. Mice that failed to find the platform within 60 s were placed onto the platform. The mouse was allowed to remain on the platform for 15 s after each trial.
Hidden platform training was conducted one day after the completion of the visible platform training. Mice were trained for 7 d (three trials per day) to find the submerged platform at a fixed position (center of NE quadrant) without any visible local cues. Distal cues in the testing room, such as a computer desk and patterned cardboard on a white wall, were provided as spatial references. Each trial lasted either until the mouse found the platform, or for 60 s. Starting points were changed every trial. Mice were allowed to rest on the platform for 15 s after each trial. The first probe trial was administered 24 h after the last trial of the hidden platform training. During the probe trial the mouse was allowed to swim for 60 s without the platform in the tank.
For the reverse platform training, the hidden platform was moved from the NE quadrant to the center of the SW quadrant without changing any distal visual cues. Mice were then trained to find this new platform location for 4 d (three trials per day). Day 1 of reverse training was conducted 2–3 h after the first probe test. Starting points were changed every trial. The second probe test was performed 7 d after the final training.
Statistical analysis.
Statistical analyses were conducted with Graphpad Prism. For the multiple trial experiments including fear extinction tests and MWM tests, Two-way repeated-measures ANOVA was conducted to assess the effects of both genotype and sessions/trials. Bonferroni post hoc tests were conducted to compare genotype effects at individual session/trial (Figs. 3 b, 5 a,b, 6 a–c). Repeated measures one-way ANOVA were also conducted to assess the effects of training blocks/trials within the same genotype group. Dunnett's multiple comparison post-test was also performed to compare the means at individual time points to the control (first trial/block) (Fig. 6 a,b). Differences between two means presented in Figure 2 d–e were assessed with t tests. Post-test for linear trend was performed to determine whether there is an increasing/deceasing trend in fear response during fear extinction tests (Figs. 3, 4). Data are presented as mean ± SEM. Differences were considered significant if p values were < 0.05.
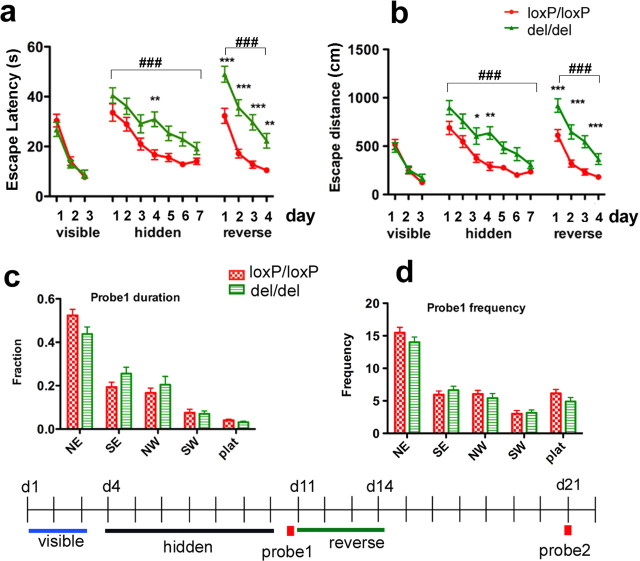
Figure 5.
Deleting mGluR5 impaired performance in the MWM. The water maze procedure consisted of three phases: (1) visible platform training, d1–d3; (2) hidden platform training and probe test 1, d4–d11; and (3) reversed platform and probe test 2, d11–d21. The experimental paradigm is illustrated at the bottom of the figure. a, b, Escape times (a) and path lengths (b) taken to reach the platform. Escape times and path lengths were analyzed by training block (3 trials per day). In the visible platform test, both groups learned the task, as indicated by incremental reductions in escape times (F (2,72) = 82.31, p < 0.0001) and distances (F (2,72) = 53.91, p < 0.0001) during training. There was no significant difference in either escape times (F (1,72) = 0.36, p = 0.5499) or path lengths (F (1,72) = 0.07, p = 0.7951) between genotype groups (mGluR5del/del, n = 19; mGluR5loxP/loxP n = 19) in the visible platform test (left block). Mice were then trained to find a hidden platform during the next seven consecutive days (3 trials per day). mGluR5del/del mice consistently showed a longer escape latencies and path lengths (middle block) over the training blocks. Repeated-measure two-way ANOVA showed a significant effect of genotype (F (1,216) = 15.49, p = 0.0004) and day (F (6,216) = 21.59, p < 0.0001) but not of the genotype–day interaction (F (6, 216) = 0.83 p = 0.5494) on escape latency. Bonferroni post-tests detected significant differences at hidden day 4, **p < 0.01. Similarly, there were significant effects of genotype (F (1,216) = 23.99, p < 0.0001) and day (F (6,216) = 26.44, p < 0.0001) but not genotype–day interaction (F (6,216) = 1.17, p = 0.3236) on escape length (Bonferroni post-tests for genotype, *p < 0.05 hidden day 3, **p < 0.01, hidden 4). A probe test was conducted 24 h after the completion of regular platform training (c, d). Immediately after the first probe test, the platform was removed to the opposite quadrant in the pool and mice were trained at three trials per day for another 4 d in this reversed setting. Although both groups acquired the task after 4 d training, mGluR5del/del mice demonstrated longer escape time (Fig. 5 a, right block) (F (3,108) = 42.38, p < 0.0001 for day; F (1,108) = 45.68, p < 0.0001 for genotype; F (3,108) = 0.80, p = 0.4978 for day–genotype interaction; Bonferroni post-tests, p < 0.001 for reverse day 1 and day 3, p < 0.01 for reverse day 4), and path lengths (Fig. 5 b, right block) (F (3,108) = 36.59, p < 0.0001 for day; F (1,108) = 35.52, p < 0.0001 for genotype; F (3,108) = 0.90, p = 0.4418 for day–genotype interaction; Bonferroni post-tests, p < 0.001 for reverse day 1, day 2, and day 3, p > 0.05 for reverse day 4). c, d, Performances in the first probe test. The first probe test was conducted 24 h after the completion of regular platform training. Analysis of the time spent in the four quadrants (c) revealed a significant effect of quadrant (F (4, 144) = 86.20, p < 0.0001). mGluR5loxP/loxP mice spent slightly more time in the target quadrant (NE) and platform zone than mGluR5del/del. However, the genotype–quadrant interaction was not significant (F (4, 144) = 2.24, p = 0.0678). Further analysis of times entered in each testing zone (d) also revealed a significant effect of quadrant (F (4, 144) = 125.02, p < 0.0001). Entries to NE quadrant and platform zone were slightly higher in mGluR5loxP/loxP mice compared with mGluR5del/del mice, but the genotype–quadrant interaction was not significant (F (4, 144) = 1.33, p = 0.2615). Data are presented as SEM (*p < 0.05, **p < 0.01,***p < 0.001 for Bonferroni posttests; ### p < 0.001 for two-way ANOVA).
Figure 6.
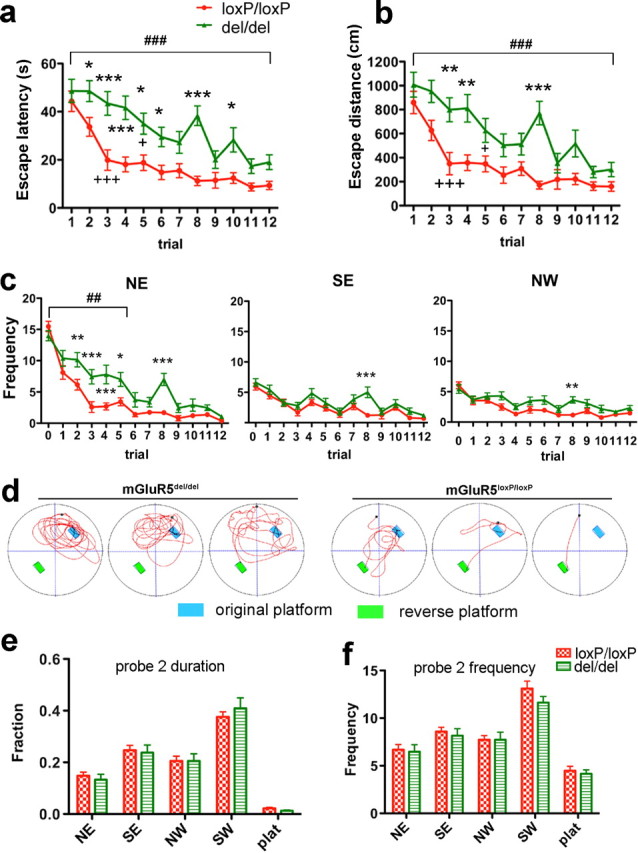
Ablation of mGluR5 caused a significant deficit in performance of the reversal task of the MWM. a, b, Trial-by-trial analysis of escape times (a) and path lengths (b) taken to reach the hidden platform at a reversed position. Two-way ANOVA analysis of escape duration showed significant effect for genotype group (F (1,396) = 45.68, p < 0.0001), trial (F (11,396) = 18.39, p < 0.0001) and trial–genotype interaction (F (11,396) = 1.99, p = 0.0284). Dunnett's multiple comparison test comparing to trial #1 showed that the improvement in mGluR5loxP/loxP mice reached significant difference at trial #3 (+++ p < 0.001), The improvement in mGluR5del/del group became significant on fifth trial (+ p < 0.05). Two-way ANOVA analysis of path lengths also showed significant effect for genotype group (F (1,396) = 35.52, p < 0.0001), trial (F (11, 395) = 15.85, p < 0.0001) and trial–genotype interaction (F (11,396) = 1.95, p = 0.0322). Dunnett's multiple comparison test showed that the improvement reached significant difference on trial #3 (+++ p < 0.001) for mGluR5del/del mice and fifth trial (+ p < 0.05) for mGluR5loxP/loxP mice, respectively. Bonferroni posttests used to compare individual points between genotype groups detected significant differences in escape latency and distance as early as trial 2 and trial 3 (mGluR5del/del, n = 19; mGluR5loxP/loxP, n = 19). c, Throughout training, mGluR5del/del mice entered the NE quadrant (previous target location) more often then the control group (F (1,180) = 11.70, p = 0.0016, for genotype, trial 0–5; F (5,180) = 3.88, p = 0.0023, for genotype–trial interaction, trial 0–5) (left). Meanwhile, entries to SE quadrant (trial 0–5, genotype effect: F (1,180) = 2.90, p = 0.097, genotype–trial interaction: F (5,180) = 0.45, p = 0.8164) and NW quadrant (F (1,180) = 4.29, p = 0.0455 for genotype; F (5,180) = 1.44, p = 0.2116 for genotype–trial interaction) were much lower for the mGluR5del/del mice, and were more comparable to the control group. Trial 0 represents frequencies entered in each testing zone during probe test 1 from the same group of animals (mGluR5del/del, n = 19; mGluR5loxP/loxP, n = 19). Due to an unknown reason, mGluR5del/del mice performed poorly in trial 8. They had much longer escape latency (Fig. 6 a) and distance (Fig. 6 b) than the control group. Their longer search in trial 8 also resulted in much higher number of entries into all quadrants compared with the control group. d, Representative trajectories taken from trial 2, demonstrating that mGluR5del/del mice continued to return to the previous target location while control mice more rapidly found the new location. e, f, Second probe test performed 7 d after reversed platform training. Both groups remembered the last target location (SW) (F (4,144) = 59.61, p < 0.0001 for time spent in the different quadrants; F (4,144) = 52.54, p < 0.0001 for number of entries). There was no significant genotype–quadrant interaction for time spent (F (4,144) = 0.29, p = 0.8815) (e) and entry numbers (F (4, 144) = 0.50, p = 0.7369) (f). Data are presented as SEM (*p < 0.05, **p < 0.01, ***p < 0.001 for Bonferroni posttests; ## p < 0.01, ### p < 0.001 for two-way ANOVA; + p < 0.05, +++ p < 0.001 for Dunnett's multiple comparison test).
Results
To test whether mGluR5-deficient mice demonstrate memory or learning impairments we performed two learning and memory tasks: pavlovian fear conditioning and the Morris Water Maze (MWM) test. Pavlovian fear conditioning is a task in which a fear response to a neutral conditioned stimulus (CS) is learned when the CS is repeatedly paired with an aversive unconditioned stimulus (US) (LeDoux, 2000). In these experiments, the CS was either a context-specific environment or a loud tone (85 db, 2900 Hz) that was coupled to the US (footshock, 0.7 mA). To determine whether ablation of mGluR5 affects acute acquisition of fear, animals were presented with three pairings of the CS and US (Fig. 1 a, paradigm A). mGluR5loxP/loxP mice displayed immediate postshock freezing that progressively increased after repeated presentations of CS-US. mGluR5del/del mice froze after footshocks, but the increment in freezing behavior was significantly lower in comparison with the control group upon repeated trials (Fig. 1 a). mGluR5del/del mice were also impaired in a second training paradigm in which mice were given three footshocks without the presentation of the tone (Fig. 1 b, paradigm B). Thus, acquisition of fear is impaired in mice lacking mGluR5. This impairment is unlikely to be caused by reduced pain sensitivity to electric footshock because mGluR5del/del mice showed the same pain response as mGluR5loxP/loxP mice (Fig. 1 c).
Next, we tested whether mGluR5del/del mice retained contextual cued and auditory fear memories. In the first context test performed 24 h after training (d1) in paradigm A, mGluR5del/del mice showed a significant reduction in the context-cued freezing time (Fig. 2 b, in d1). The fractions of context-cued freezing were 0.49 ± 0.04 and 0.29 ± 0.05 for mGluR5loxP/loxP mice and mGluR5del/del mice respectively (Fig. 2 d) (p = 0.0047, t test). However, the ratio of freezing during acquisition (d0) to d1 was comparable between the two groups (0.47 ± 0.12 and 0.38 ± 0.11 for mGluR5loxP/loxP and mGluR5del/del respectively; p > 0.05, t test), suggesting that although mGluR5del/del mice are impaired in the initial fear acquisition, they retain the ability to express the once-memorized fear response. In contrast to this context-specific deficit, we found that 24 h later (d2) when mice were subjected to the CS tone-cued test, both groups showed similar pretone freezing (Fig. 2 c,e; 0–3 min in d2) and post-tone freezing (Fig. 2 c,e; 4–6 min in d2). Therefore mGluR5del/del mice are able to remember the association between the CS and US 2 d after training. To determine the retention of these memories at later time points, mice were tested again after 2 weeks (d15 and d16). Contextual fear (d15) was slightly reduced in mGluR5loxP/loxP mice (paired t test, p = 0.044) but was slightly increased in mGluR5del/ldel mice (paired t test, p > 0.05) (Fig. 2 b,d, compare d1, d15). In the second CS tone-cued test conducted in d16, both groups displayed similar post-tone freezing (Fig. 2 c,e in d16). Noticeably, however, pretone freezing (0–3 min) was reduced in mGluR5loxP/loxP mice but was elevated in mGluR5del/del mice, although these differences were not significant (Fig. 2 e, compare d2, d16). Pretone freezing in the second auditory fear memory test was 2.5 times greater in mGluR5del/del mice than the control group (Fig. 2 e, d16) (p = 0.0028, t test), suggesting that mGluR5del/del mice became less discriminative to the fear environment compared with the control group after training.
A previously learned aversive association can be suppressed or extinguished by repeated presentation of the CS in the absence of the US. With this training the animal learns that the CS no longer predicts the US such that the fear response is “inhibited.” The most widely accepted theory for the neural basis of extinction is that it is a parallel process distinct from fear acquisition and is often termed inhibitory learning (Bouton and Bolles, 1979; Bouton, 1993; Barad, 2005; Myers and Davis, 2007). This is a distinct mechanism from when extinction is performed very shortly after acquisition of the fear association, when it may be a reversal or unlearning process (Myers et al., 2006). To determine whether inhibitory learning requires mGluR5 we performed fear extinction studies. In the first experiment, mice were trained with paradigm B in which they learn to associate the footshock with a context. Mice were then returned to the same context everyday without footshock for 10 consecutive days (Fig. 3 a). Freezing in mGluR5loxP/loxP mice decreased during the extinction training, as revealed by one-way ANOVA (F (9,210) = 5.644, p < 0.0001) (Fig. 3 b). Dunnett's multiple comparison test revealed a significant difference between day 1 and all subsequent days. In addition, the post-test for linear trend revealed a significant decreasing trend in freezing during the period of extinction training in mGluR5loxpP/loxP mice (slope = −1.438, p < 0.0001). In contrast to this, fear extinction in mGluR5del/del mice was completely abolished (Fig. 3 a). One-way ANOVA comparison did not detect any effects of extinction training for mGluR5del/del mice (F (9,184) = 0.7882, p > 0.05) (Fig. 3 b). In fact, mGluR5del/del mice exhibited a slightly increased amount of freezing on d10 (51 ± 5.2%) than on the first day (d1) (37 ± 3.9%), although the difference did not reach significance (by Dunnett's post-tests analysis). The post-test for linear trend revealed a significant increasing trend in freezing for mGluR5del/del mice (slope = 0.5644, p = 0.0036). These experiments are the first to demonstrate a substantial role for mGluR5 in contextual fear extinction.
Next, to determine whether a similar deficit might be observed in extinction of auditory fear, we performed a separate series of experiments. Mice were first trained in paradigm A (tone-cued test), and were then tested in a different context without footshock but with tone presentation for 16 consecutive days. There was a clear difference between mGluR5loxP/loxP and mGluR5del/del animals in the extinction of both pretone and post-tone fear (Fig. 4 a). To better assess fear behaviors, freezing was analyzed in four categories: total freezing (0–6 min) (Fig. 4 b), pretone freezing (0–3 min) (Fig. 4 d), post-tone freezing (4–6 min) (Fig. 4 e) and tone-cued freezing (post-tone freezing minus pretone freezing) (Fig. 4 c). Although initially mGluR5del/del mice showed less post-tone freezing than control mice (Fig. 4 e), this freezing behavior did not diminish during the course of repeated daily exposure to the tone. The control group showed a clear reduction in post-tone freezing which was significant between days 9 and 16 of extinction training (Fig. 4 e). Both groups displayed similar amount of pretone freezing between days 1 and 9 of training (Fig. 4 d). However as training progressed there was a steady reduction of pretone freezing in mGluR5loxP/loxP mice whereas mGluR5del/del mice continued to display the same degree, or increased, pretone freezing during the late stages of extinction training (Fig. 4 d). To further examine the freezing response as a more direct result of the tone cue, we subtracted pretone freezing from post-tone freezing and presented it as tone-cued fear in Figure 4 c. At the onset (d1–d5), tone-cued freezing was lower in mGluR5del/del mice compared with the control group, which displayed the most robust freezing on the second and third day (Fig. 4 c). However, tone-cued freezing was progressively reduced in mGluR5loxP/loxP group, while it was maintained at a similar level throughout the test in mGluR5del/del mice. Near completion of the test (d9–d16), tone-cued freezing was no longer different between the two genotypes groups (Fig. 4 c). Overall, there was a significant trend in the decrease of the fear curve in mGluR5loxP/loxP mice (slope = −0.29, p < 0.0031), but not in mGluR5del/del mice (slope = 0.10, p = 0.23) (Fig. 4 c). Finally, comparing total freezing revealed the opposite trends between mGluR5loxP/loxP mice and the mGluR5del/del mice: no difference in the early part of the test, and significant differences later (Fig. 4 b). Together these data demonstrate that mice lacking mGluR5 were not able to extinguish tone-cued fear (Fig. 4 c) and became less able to discriminate the fear environment (Fig. 4 d) during the course of extinction training. Notably, even the extinction of pretone freezing in the control group was much slower compared with the extinction of context-cued freezing shown in Figure 3; although it might be expected that pretone freezing should stay at relatively low levels when the mice were exposed to a neutral context. A likely explanation for this is that during the extinction training, the mice learned to associate the neutral environment to tone, which they had previously associated to the US (footshocks). In support of this idea, extinction of pretone freezing correlated very well with the extinction of post-tone freezing for both genotype groups, suggesting that the original neutral environment had become another CS for fear.
We also conducted a control experiment to examine whether presentation of the 85 db 2900 Hz tone alone, without footshock, could cause freezing in mice. We found that an 85 db tone lasting for 3 min induced little freezing in mice (<3% in both groups, see supplemental Fig. 3, available at www.jneurosci.org as supplemental material). Therefore the influence of this freezing in our experiments is small, and unlikely to confound our conclusions.
In the next series of experiments we determined whether mGluR5del/del mice demonstrated a deficit in spatial learning in the standard MWM test. A previous study had found that mGluR5 null mice had a robust deficit in performance in this test (Lu et al., 1997). In contrast we found only a mild impairment in performance of mGluR5del/del mice in the MWM. During the first 3 experimental days, mice were trained to find a visible platform, and both groups performed equally on this task, suggesting that mutant and control mice could visualize the platform and swim equally effectively (Fig. 5 a,b, left). During the next seven experimental days, mice were trained to find a hidden platform (three trials per day) (Fig. 5 a,b, middle). Although both groups acquired the task during training, mGluR5del/del mice consistently showed longer escape latencies and path lengths over the training blocks. These disparities were not caused by differences in swim speed. In fact, mGluR5del/del mice swam slightly faster during hidden platform training (supplemental Fig. 2, available at www.jneurosci.org as supplemental material). We next performed a probe trial in which the platform was removed from the learned location. The first probe test conducted 24 h after the initial training demonstrated that both groups remembered the location of the platform (Fig. 5 c,d). mGluR5loxP/loxP slightly outperformed mGluR5del/del by spending more time in the target quadrant (NE) and the platform zone (Fig. 5 c). However, the genotype-by-quadrant interaction was not significant. mGluR5loxP/loxP also made slightly more entries to the target quadrant and the platform zone than mGluR5del/del (Fig. 5 d), but again, the genotype-by-quadrant interaction was not significant.
In the final test we performed a reversal of the hidden platform in the MWM. Immediately after probe test 1, the platform was moved to the opposite quadrant location (SW) and mice were trained for 4 d (three trials per day) in this reversed setting. mGluR5del/del mice showed significantly longer escape times (Fig. 5 a, right) and path lengths (Fig. 5 b, right) over the training blocks. When data were analyzed by trials, the group differences were more evident in early training, with significant differences between the genotypes occurring in both latency and distance as early as the second and third trial (Fig. 6 a,b). While mGluR5del/del mice continued to return to the previous target location, the control cohort more rapidly found the platform in the new location (Fig. 6 d, representative tracks taken from trial 2). Throughout this training, mGluR5del/del mice entered the NE (previous target location) more often then the control group (Fig. 6 c). Meanwhile, the number of entries into the SE and NW (no platform zone) quadrants were much lower for mGluR5del/del mice, and were comparable between mGluR5del/del and mGluR5loxP/loxP mice (Fig. 6 c). These results demonstrate that a persistent search in the previous target zone resulted in a significant delay for mGluR5del/del mice to find the new target location. The second probe test in which the platform was again removed was conducted after a further 7 d and revealed no differences in performance between groups (Fig. 6 e,f). Thus, spatial memory was intact in mice lacking mGluR5, whereas mice had a clear deficit in the reversal task. These experiments further support a role of mGluR5 in inhibitory learning.
Discussion
Previous work has highlighted a role for mGluR5 in learning and memory, and in various forms of synaptic plasticity. In the present study we generated a novel strain of mGluR5 mutant mice to test the role of mGluR5 in two common learning paradigms, fear conditioning and the MWM. We found that mGluR5 plays a role in the initial steps of memory acquisition rather than memory storage and retrieval. mGluR5-deficient mice were defective in the acquisition of fear conditioning. However, they retained the ability to express the once-memorized fear response (Fig. 2). Similarly, mGluR5-deficient mice displayed mild impairments in the acquisition of the standard of version of the MWM, but were able to remember the location of the hidden platform during the probe test (Fig. 5). Together these findings suggest that mGluR5 mutant mice are defective in the acquisition, rather than the retention and retrieval of the memories. These findings are consistent with prior studies which have demonstrated a role for mGluR5 in the acquisition of fear and spatial memories (Schulz et al., 2001; Rodrigues et al., 2002).
Our study is not the first to generate mGluR5 knock-out mice. The mice used here were generated as conditional allele mutations to eventually allow ablation of mGluR5 in a spatial and temporally restricted pattern. In a prior study a conventional global knock-out allele of mGluR5 was generated (Lu et al., 1997). In behavioral test these mice were impaired in contextual fear conditioning and acquisition of the standard MWM task in agreement with our finding with mGluR5del/del mice. In contrast to the prior study, in our experiments we found only mild impairments in the acquisition of spatial learning in the MWM whereas Lu et al., had reported more robust deficits in this form of learning. Notably, in both studies similar deficits were observed in acquisition during the early trial blocks. Knock-out mice displayed progressive learning during these early trial blocks, although their escape latencies were longer than the control groups. The main discrepancy between our findings and the prior study arise in the late training blocks. In the previous study, mGluR5 knock-out mice were unable to improve their performance further in the MWM task in late trial blocks. Their escape latencies leveled at 35–40 s throughout the rest of the training [Lu et al. (1997), their Fig. 5]. In our experiments, our knock-out mice had a steady increase in learning throughout all the training blocks. At the end of training, knock-out mice showed similar escape latencies as control group (Fig. 5). While it is unclear how these discrepancies might arise they may be due to differences in experimental protocols or strain differences in the mice used in each study.
The primary novel finding in our study is that mGluR5-deficient mice are impaired in the extinction or reversal of learning. We used two inhibitory learning paradigms to assess a role for mGluR5 in these adaptive learning processes. In the first we found that mGluR5-deficient mice were completely unable to extinguish a previously acquired fear response (Figs. 3–4). In the second we found that mGluR5-deficient mice were impaired in their ability to relearn a reversed platform location after they had previously learned the task (Figs. 5–6).
The genetic components that contribute to fear extinction are not fully understood although a number of molecules have been implicated (Falls et al., 1992; Lin et al., 2001, 2003; Lu et al., 2001; Cain et al., 2002, 2004; Marsicano et al., 2002; Wang et al., 2004; Chen et al., 2005; Ponnusamy et al., 2005; Callaerts-Vegh et al., 2006; Chhatwal et al., 2006; Davis et al., 2006; Kamprath et al., 2006; Burgos-Robles et al., 2007; Kim et al., 2007; Sananbenesi et al., 2007; Sotres-Bayon et al., 2007; Fendt et al., 2008; Hefner et al., 2008) (for a review, see Myers and Davis, 2007). Among these are two members of metabotropic glutamate receptor family: mGluR1 (Kim et al., 2007) and mGluR7 (Callaerts-Vegh et al., 2006; Fendt et al., 2008). Thus, with the results of our study, it appears that mGluRs play a central role in fear extinction.
We also found a previously unknown role for mGluR5 in the reversal task of the water maze. Interestingly, we found that mGluR5 knock-out mice were more impaired in the reversal spatial learning task, than in the initial acquisition of spatial learning task of the regular water maze. Thus, mGluR5 knock-out mice provide yet another example that acquisition of standard spatial learning, and reversal spatial learning can be dissociated by genetic or pharmacological manipulation (Hawasli et al., 2007; Duffy et al., 2008).
The fundamental concept of inhibitory leaning, which is determined by the animal learning to suppress a prior learned response is broadly used to describe fear extinction studies (Bouton, 1993; Barad, 2005; Myers and Davis, 2007). The same concept of inhibitory learning has also been applied to reversal of spatial learning (Lattal and Abel, 2001; Varvel et al., 2005; Rossato et al., 2006; Duffy et al., 2008; Labrie et al., 2009). In the reversal task, mice learn that navigating to an area where they had previously learned a platform location is no longer an effective escape strategy. Consequently, mice able to perform this task return to this region with decreasing frequency during the training. Thus although fear extinction and reversal spatial learning are quite separate and distinct tasks they are linked as inhibitory learning tasks which require an active suppression of previously learned associations (Lattal and Abel, 2001; Barad, 2005; Myers and Davis, 2007). Our finding that mice lacking mGluR5 are impaired in both the fear extinction and reversal spatial learning, suggest that these two inhibitory learning paradigms may share common mechanistic properties which involve signaling through mGluR5. There are not many other examples of signaling molecules that have been demonstrated to have similar clear roles in multiple forms of inhibitory learning. However, one such molecule is the CB1 receptor (Marsicano et al., 2002; Varvel et al., 2005). CB1 knock-out mice have significant deficits in fear extinction (Marsicano et al., 2002) and in the reversed MWM task (Varvel et al., 2005) similar to the mGluR5-deficient mice. Interestingly, mGluR5 and CB1 receptors are linked together by their roles in synaptic plasticity. mGluR5 activation is required for endocannabinoid-mediated long-term depression of GABAergic synapses (eCB-LTD) in the hippocampus (Chevaleyre and Castillo, 2003) and depolarization-induced suppression of inhibition (DSI) in the basolateral amygdala (Zhu and Lovinger, 2005). In particular, the mechanisms of DSI in the basolateral amygdala require mGluR5-mediated release of endocannabinoids and activation of presynaptic CB1 receptors. Therefore it is interesting to speculate that a potential cellular basis for inhibitory learning might be the depression of inhibitory synapses in these brain regions. The findings of our study demonstrate a clear role for mGluR5 in fear extinction and reversal of a spatial task, suggesting that both forms of learning could be linked. However a definitive shared molecular link between these two forms of inhibitory learning will require further experimental clarification.
Neural circuits in several different brain regions play a role in fear extinction and reversal tasks. These include the basolateral nucleus of the amygdala (Falls et al., 1992; Walker et al., 2002), the prefrontal cortex (Quirk et al., 2000; Milad and Quirk, 2002; Burgos-Robles et al., 2007) and the hippocampus (Sananbenesi et al., 2007). mGluR5 is expressed in all these structures, and therefore whether or not mGluR5 signaling in all of these regions, or in specific locales are required for inhibitory learning, remains an open question. In future studies, the novel mice described here will enable us to ablate mGluR5 in specific brain regions to directly address this question, and will provide more detailed information of which circuits are involved in inhibitory learning. Reversal or extinction of previously acquired memories allows animals to adapt to a novel environment or situation. These mechanisms are perturbed in several neuropsychiatric disorders in which traumatic memories persist. Therefore mGluR5 provides a potential target for therapeutic intervention in processes of maladaptive learning.
Footnotes
This work was supported by grants from the National Institutes of Health–National Institute of Neurological Disorders and Stroke (5R01NS058894 to A.C. and NS28709 to S.F.H.). J.X. was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award. We thank Jelena Radulovic for comments on this manuscript.
References
- Abe T, Sugihara H, Nawa H, Shigemoto R, Mizuno N, Nakanishi S. Molecular characterization of a novel metabotropic glutamate receptor mGluR5 coupled to inositol phosphate/Ca2+ signal transduction. J Biol Chem. 1992;267:13361–13368. [PubMed] [Google Scholar]
- Balschun D, Wetzel W. Inhibition of mGluR5 blocks hippocampal LTP in vivo and spatial learning in rats. Pharmacol Biochem Behav. 2002;73:375–380. doi: 10.1016/s0091-3057(02)00847-x. [DOI] [PubMed] [Google Scholar]
- Barad M. Fear extinction in rodents: basic insight to clinical promise. Curr Opin Neurobiol. 2005;15:710–715. doi: 10.1016/j.conb.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Bikbaev A, Neyman S, Ngomba RT, Conn J, Nicoletti F, Manahan-Vaughan D. MGluR5 mediates the interaction between late-LTP, network activity, and learning. PLoS ONE. 2008;3:e2155. doi: 10.1371/journal.pone.0002155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouton ME. Context, time, and memory retrieval in the interference paradigms of Pavlovian learning. Psychol Bull. 1993;114:80–99. doi: 10.1037/0033-2909.114.1.80. [DOI] [PubMed] [Google Scholar]
- Bouton ME, Bolles RC. Role of conditioned contextual stimuli in reinstatement of extinguished fear. J Exp Psychol Anim Behav Process. 1979;5:368–378. doi: 10.1037//0097-7403.5.4.368. [DOI] [PubMed] [Google Scholar]
- Brody SA, Dulawa SC, Conquet F, Geyer MA. Assessment of a prepulse inhibition deficit in a mutant mouse lacking mGlu5 receptors. Mol Psychiatry. 2004;9:35–41. doi: 10.1038/sj.mp.4001404. [DOI] [PubMed] [Google Scholar]
- Burgos-Robles A, Vidal-Gonzalez I, Santini E, Quirk GJ. Consolidation of fear extinction requires NMDA receptor-dependent bursting in the ventromedial prefrontal cortex. Neuron. 2007;53:871–880. doi: 10.1016/j.neuron.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. L-type voltage-gated calcium channels are required for extinction, but not for acquisition or expression, of conditional fear in mice. J Neurosci. 2002;22:9113–9121. doi: 10.1523/JNEUROSCI.22-20-09113.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain CK, Blouin AM, Barad M. Adrenergic transmission facilitates extinction of conditional fear in mice. Learn Mem. 2004;11:179–187. doi: 10.1101/lm.71504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaerts-Vegh Z, Beckers T, Ball SM, Baeyens F, Callaerts PF, Cryan JF, Molnar E, D'Hooge R. Concomitant deficits in working memory and fear extinction are functionally dissociated from reduced anxiety in metabotropic glutamate receptor 7-deficient mice. J Neurosci. 2006;26:6573–6582. doi: 10.1523/JNEUROSCI.1497-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Garelick MG, Wang H, Lil V, Athos J, Storm DR. PI3 kinase signaling is required for retrieval and extinction of contextual memory. Nat Neurosci. 2005;8:925–931. doi: 10.1038/nn1482. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chhatwal JP, Stanek-Rattiner L, Davis M, Ressler KJ. Amygdala BDNF signaling is required for consolidation but not encoding of extinction. Nat Neurosci. 2006;9:870–872. doi: 10.1038/nn1718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiamulera C, Epping-Jordan MP, Zocchi A, Marcon C, Cottiny C, Tacconi S, Corsi M, Orzi F, Conquet F. Reinforcing and locomotor stimulant effects of cocaine are absent in mGluR5 null mutant mice. Nat Neurosci. 2001;4:873–874. doi: 10.1038/nn0901-873. [DOI] [PubMed] [Google Scholar]
- Davis M, Ressler K, Rothbaum BO, Richardson R. Effects of D-cycloserine on extinction: translation from preclinical to clinical work. Biol Psychiatry. 2006;60:369–375. doi: 10.1016/j.biopsych.2006.03.084. [DOI] [PubMed] [Google Scholar]
- Dölen G, Osterweil E, Rao BS, Smith GB, Auerbach BD, Chattarji S, Bear MF. Correction of fragile X syndrome in mice. Neuron. 2007;56:955–962. doi: 10.1016/j.neuron.2007.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffy S, Labrie V, Roder JC. D-serine augments NMDA-NR2B receptor-dependent hippocampal long-term depression and spatial reversal learning. Neuropsychopharmacology. 2008;33:1004–1018. doi: 10.1038/sj.npp.1301486. [DOI] [PubMed] [Google Scholar]
- Falls WA, Miserendino MJ, Davis M. Extinction of fear-potentiated startle: blockade by infusion of an NMDA antagonist into the amygdala. J Neurosci. 1992;12:854–863. doi: 10.1523/JNEUROSCI.12-03-00854.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fendt M, Schmid S, Thakker DR, Jacobson LH, Yamamoto R, Mitsukawa K, Maier R, Natt F, Hüsken D, Kelly PH, McAllister KH, Hoyer D, van der Putten H, Cryan JF, Flor PJ. mGluR7 facilitates extinction of aversive memories and controls amygdala plasticity. Mol Psychiatry. 2008;13:970–979. doi: 10.1038/sj.mp.4002073. [DOI] [PubMed] [Google Scholar]
- Hawasli AH, Benavides DR, Nguyen C, Kansy JW, Hayashi K, Chambon P, Greengard P, Powell CM, Cooper DC, Bibb JA. Cyclin-dependent kinase 5 governs learning and synaptic plasticity via control of NMDAR degradation. Nat Neurosci. 2007;10:880–886. doi: 10.1038/nn1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson RM, Saksida LM, Bussey TJ, Singewald N, Holmes A. Impaired fear extinction learning and cortico-amygdala circuit abnormalities in a common genetic mouse strain. J Neurosci. 2008;28:8074–8085. doi: 10.1523/JNEUROSCI.4904-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huber KM, Kayser MS, Bear MF. Role for rapid dendritic protein synthesis in hippocampal mGluR-dependent long-term depression. Science. 2000;288:1254–1257. doi: 10.1126/science.288.5469.1254. [DOI] [PubMed] [Google Scholar]
- Jia Z, Lu Y, Henderson J, Taverna F, Romano C, Abramow-Newerly W, Wojtowicz JM, Roder J. Selective abolition of the NMDA component of long-term potentiation in mice lacking mGluR5. Learn Mem. 1998;5:331–343. [PMC free article] [PubMed] [Google Scholar]
- Kamprath K, Marsicano G, Tang J, Monory K, Bisogno T, Di Marzo V, Lutz B, Wotjak CT. Cannabinoid CB1 receptor mediates fear extinction via habituation-like processes. J Neurosci. 2006;26:6677–6686. doi: 10.1523/JNEUROSCI.0153-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee S, Park H, Song B, Hong I, Geum D, Shin K, Choi S. Blockade of amygdala metabotropic glutamate receptor subtype 1 impairs fear extinction. Biochem Biophys Res Commun. 2007;355:188–193. doi: 10.1016/j.bbrc.2007.01.125. [DOI] [PubMed] [Google Scholar]
- Labrie V, Duffy S, Wang W, Barger SW, Baker GB, Roder JC. Genetic inactivation of D-amino acid oxidase enhances extinction and reversal learning in mice. Learn Mem. 2009;16:28–37. doi: 10.1101/lm.1112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lattal KM, Abel T. Different requirements for protein synthesis in acquisition and extinction of spatial preferences and context-evoked fear. J Neurosci. 2001;21:5773–5780. doi: 10.1523/JNEUROSCI.21-15-05773.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeDoux JE. Emotion circuits in the brain. Annu Rev Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Lin CH, Lu KT, Leu TH, Chang WC, Gean PW. A role for the PI-3 kinase signaling pathway in fear conditioning and synaptic plasticity in the amygdala. Neuron. 2001;31:841–851. doi: 10.1016/s0896-6273(01)00433-0. [DOI] [PubMed] [Google Scholar]
- Lin CH, Yeh SH, Leu TH, Chang WC, Wang ST, Gean PW. Identification of calcineurin as a key signal in the extinction of fear memory. J Neurosci. 2003;23:1574–1579. doi: 10.1523/JNEUROSCI.23-05-01574.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu KT, Walker DL, Davis M. Mitogen-activated protein kinase cascade in the basolateral nucleus of amygdala is involved in extinction of fear-potentiated startle. J Neurosci. 2001;21:RC162. doi: 10.1523/JNEUROSCI.21-16-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Jia Z, Janus C, Henderson JT, Gerlai R, Wojtowicz JM, Roder JC. Mice lacking metabotropic glutamate receptor 5 show impaired learning and reduced CA1 long-term potentiation (LTP) but normal CA3 LTP. J Neurosci. 1997;17:5196–5205. doi: 10.1523/JNEUROSCI.17-13-05196.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino MJ, Conn PJ. Glutamate-based therapeutic approaches: allosteric modulators of metabotropic glutamate receptors. Curr Opin Pharmacol. 2006;6:98–102. doi: 10.1016/j.coph.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Myers KM, Davis M. Mechanisms of fear extinction. Mol Psychiatry. 2007;12:120–150. doi: 10.1038/sj.mp.4001939. [DOI] [PubMed] [Google Scholar]
- Myers KM, Ressler KJ, Davis M. Different mechanisms of fear extinction dependent on length of time since fear acquisition. Learn Mem. 2006;13:216–223. doi: 10.1101/lm.119806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder JC. Derivation of completely cell culture-derived mice from early-passage embryonic stem cells. Proc Natl Acad Sci U S A. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi S. Molecular diversity of glutamate receptors and implications for brain function. Science. 1992;258:597–603. doi: 10.1126/science.1329206. [DOI] [PubMed] [Google Scholar]
- O'Gorman S, Dagenais NA, Qian M, Marchuk Y. Protamine-Cre recombinase transgenes efficiently recombine target sequences in the male germ line of mice, but not in embryonic stem cells. Proc Natl Acad Sci U S A. 1997;94:14602–14607. doi: 10.1073/pnas.94.26.14602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponnusamy R, Nissim HA, Barad M. Systemic blockade of D2-like dopamine receptors facilitates extinction of conditioned fear in mice. Learn Mem. 2005;12:399–406. doi: 10.1101/lm.96605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirk GJ, Russo GK, Barron JL, Lebron K. The role of ventromedial prefrontal cortex in the recovery of extinguished fear. J Neurosci. 2000;20:6225–6231. doi: 10.1523/JNEUROSCI.20-16-06225.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigues SM, Bauer EP, Farb CR, Schafe GE, LeDoux JE. The group I metabotropic glutamate receptor mGluR5 is required for fear memory formation and long-term potentiation in the lateral amygdala. J Neurosci. 2002;22:5219–5229. doi: 10.1523/JNEUROSCI.22-12-05219.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossato JI, Bevilaqua LR, Medina JH, Izquierdo I, Cammarota M. Retrieval induces hippocampal-dependent reconsolidation of spatial memory. Learn Mem. 2006;13:431–440. doi: 10.1101/lm.315206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sananbenesi F, Fischer A, Wang X, Schrick C, Neve R, Radulovic J, Tsai LH. A hippocampal Cdk5 pathway regulates extinction of contextual fear. Nat Neurosci. 2007;10:1012–1019. doi: 10.1038/nn1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz B, Fendt M, Gasparini F, Lingenhöhl K, Kuhn R, Koch M. The metabotropic glutamate receptor antagonist 2-methyl-6-(phenylethynyl)-pyridine (MPEP) blocks fear conditioning in rats. Neuropharmacology. 2001;41:1–7. doi: 10.1016/s0028-3908(01)00036-3. [DOI] [PubMed] [Google Scholar]
- Slassi A, Isaac M, Edwards L, Minidis A, Wensbo D, Mattsson J, Nilsson K, Raboisson P, McLeod D, Stormann TM, Hammerland LG, Johnson E. Recent advances in non-competitive mGlu5 receptor antagonists and their potential therapeutic applications. Curr Top Med Chem. 2005;5:897–911. doi: 10.2174/1568026054750236. [DOI] [PubMed] [Google Scholar]
- Sotres-Bayon F, Bush DE, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology. 2007;32:1929–1940. doi: 10.1038/sj.npp.1301316. [DOI] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varvel SA, Anum EA, Lichtman AH. Disruption of CB(1) receptor signaling impairs extinction of spatial memory in mice. Psychopharmacology. 2005;179:863–872. doi: 10.1007/s00213-004-2121-2. [DOI] [PubMed] [Google Scholar]
- Walker DL, Ressler KJ, Lu KT, Davis M. Facilitation of conditioned fear extinction by systemic administration or intra-amygdala infusions of d-cycloserine as assessed with fear-potentiated startle in rats. J Neurosci. 2002;22:2343–2351. doi: 10.1523/JNEUROSCI.22-06-02343.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ferguson GD, Pineda VV, Cundiff PE, Storm DR. Overexpression of type-1 adenylyl cyclase in mouse forebrain enhances recognition memory and LTP. Nat Neurosci. 2004;7:635–642. doi: 10.1038/nn1248. [DOI] [PubMed] [Google Scholar]
- Zhang CL, Zou Y, He W, Gage FH, Evans RM. A role for adult TLX-positive neural stem cells in learning and behaviour. Nature. 2008;451:1004–1007. doi: 10.1038/nature06562. [DOI] [PubMed] [Google Scholar]
- Zhu PJ, Lovinger DM. Retrograde endocannabinoid signaling in a postsynaptic neuron/synaptic bouton preparation from basolateral amygdala. J Neurosci. 2005;25:6199–6207. doi: 10.1523/JNEUROSCI.1148-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]