INTRODUCTION
Rotator cuff tears are common shoulder injuries and a significant source of pain and disability. In the general population, 8-26% of patients suffer from full thickness tears8, and 13-37% suffer from partial-thickness tears6, 11-13. Despite the high incidence of rotator cuff tears, objective data to guide clinical decision-making for their treatment has not been established. Ideally, surgery should be indicated for tears with a high risk of progression, for which non-surgical management will eventually lead to larger, less manageable tears. However, in the absence of data that allows for reliable prediction of the risk of tear propagation, most patients suffering from rotator cuff tears undergo an initial period of nonoperative treatment in an effort to alleviate symptoms and restore range of motion19. Failure of nonoperative treatment leads to surgical intervention6, 19. This delay in surgery is common, despite the well-documented correlation between the success of repair with duration of symptoms or size of the tear2, 9, 14. In addition, relief in symptoms and improvement in function following nonoperative treatment does not indicate a decrease in risk of tear propagation, and potential propagation during nonoperative management may result in a less surgically manageable tear.
To date, tear size has been influential in considering surgical intervention. For instance, partial-thickness tears that extend through more than 50% of the thickness of the tendon, regardless of tear location, are typically surgically repaired10, 22, 26, 27. This criteria assumes that tear size is the most critical factor in predicting the likelihood of tear propagation, without accounting for the complex loading environment resulting from the inhomogeneity of the tendon. It is likely that some large tears are less likely to progress and may not require surgery, while some small tears are more likely to progress and would benefit from early surgical treatment.
Other parameters, such as tissue strain, that more directly reflect the complex loading environment of the supraspinatus tendon may be more useful to investigate as criteria for surgical decision-making than tear size. For example, several studies have investigated the effect of a supraspinatus tendon tear on the remaining intact portion of the tendon to better understand the change in supraspinatus tendon loading environment.3, 24. Tissue strain was used to evaluate the load bearing capacity of the remaining intact portion of the tendon in order to infer the risk of tear propagation. As expected, significant changes in tendon strain resulted following surgical introduction of a rotator cuff tear. Bey et al. measured local maximum principal strain and found it to increase with the introduction of an insertion site supraspinatus tendon tear. Reilly et al. found general increases in tensile strains with a full-thickness tear, joint-side partial-thickness tear and intratendinous delamination. While this data is promising, other aspects of the complex loading environment and tissue architecture of the rotator cuff must also be considered when assessing the likelihood of tear propagation. For instance, contact between the humeral head and the torn supraspinatus tendon has been shown to decrease the effect of a supraspinatus tendon tear on tendon strain at certain joint positions3. Additionally, interactions between the complex network of tendons and other soft connective tissues that provide the glenohumeral joint with its wide range of motion and stability further complicate the ability to predict the likelihood of tear propagation. Therefore, other rotator cuff structures, including the other rotator cuff tendons, may impact the likelihood of supraspinatus tear propagation and reciprocally be impacted by the existence of a supraspinatus tendon tear.
We propose that the infraspinatus tendon may be subjected to increased strains in the presence of a supraspinatus tendon tear. In such an environment, the infraspinatus tendon may protect the supraspinatus tendon from further injury. Therefore, the objective of this study is to investigate whether a mechanical interaction exists between the intact infraspinatus tendon and the torn supraspinatus tendon that would cause increased strains in the infraspinatus tendon when the supraspinatus tendon is subjected to conditions of high strain. We hypothesize that increasing the tear size and the load in the supraspinatus tendon will each result in an increase in maximum and a decrease in minimum principal strain in the infraspinatus tendon. This potential strain shielding effect of the infraspinatus would represent a mechanism by which the risk of tear progression in the supraspinatus is decreased. Additionally, a repetitive change in strain in the infraspinatus tendon associated with increased strain levels in the supraspinatus tendon may have an effect on the load bearing capacity of the infraspinatus tendon.
MATERIALS AND METHODS
Four healthy, fresh-frozen cadaveric shoulders (average age 63 ± 11.55 yrs, 2 left and 2 right shoulders) were carefully dissected free of soft tissue, retaining only the proximal humerus, supraspinatus and infraspinatus tendons. Specimens were considered healthy if they exhibited no visible degeneration, fraying of fibers, or abnormal pathology. Specimens with grossly visible rotator cuff tears or fraying of the bursal or articular surface were excluded from the study. The proximal end of the infraspinatus tendon was sutured with a Krakow stitch using heavy suture material to allow for tendon loading. Prior to mechanical testing, the specimens were maintained in a physiologic bath of phosphate buffered saline (PBS).
The bursal side of both the supraspinatus and the infraspinatus tendons was air-brushed with black paint to create a fine speckled texture for subsequent texture correlation strain analysis using Vic2D (Version 4.4.1, Correlated Solutions Inc., Columbia, SC). This commercial software utilizes a texture correlation algorithm5 to determine displacements of pixels in undeformed and deformed image pairs.
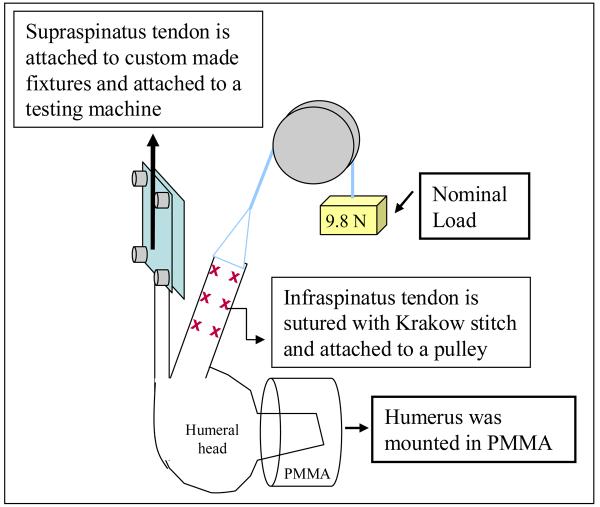
The humerus was secured in a custom fixture with Poly(methyl methacrylate) (PMMA), then mounted in custom grips for mechanical testing. The supraspinatus tendon was attached to a testing machine (5543, Instron, Norwood, MA) to allow for controlled loading (Figure 1). During supraspinatus tendon loading, the infraspinatus tendon was attached to a pulley system with a nominal, constant load of 9.8 N via the free suture ends of the Krakow stitch passed through the tendon (Figure 1). This load level was chosen as it was sufficiently large enough to overcome the friction in the experimental setup and to ensure that the tendon was taught. The joint was positioned such that the direction of loading of both tendons was along their physiologically neutral position, defined by a 0° humeral abduction, rotation and flexion angle.
Figure 1.
Schematic representation of experimental setup. Two cameras were used in this study, with one camera optimally positioned to capture deformations in the insertion site of the supraspinatus tendon and the other camera optimally positioned to capture deformations in the insertion site of the infraspinatus tendon.
Preliminary experiments were conducted to determine all parameters of the loading protocol. Parameters were chosen based on repeatability of the strain results. The supraspinatus tendon loading protocol consisted of preconditioning followed by a constant ramp to 90N at a strain rate of 0.1% of the length of the tendon. Briefly, the tendon was pre-loaded to a load approximately 0.5% of the ultimate load. The tendon was then preconditioned, by cyclically loading it between 0.5% and 1% of ultimate load, 10 times at a rate approximately equal to 0.1% times the length of the tendon. After preconditioning, the load corresponding to 0.5% of ultimate load was applied and held for 5 minutes. A ramp to 90N was then applied at a rate approximately equal to 0.1% times the length of the tendon. A maximum load of 90N was chosen as it is approximately 15% of ultimate load for this tendon, and represents a large but physiologically reasonable load15, 16. The loading protocol was first applied to the intact supraspinatus tendon. An anterior full-thickness tear through 33% of the width of the tendon was then surgically created in the supraspinatus tendon insertion site (sharp removal of the anterior 33% portion of the tendon off its footprint on the greater tuberosity), and the loading protocol was repeated again. Finally, the tear was extended further posteriorly to create a 66% width (sharp removal of the anterior 66% portion of the tendon off its footprint on the greater tuberosity) full thickness tear and the loading protocol was repeated once again. Tendons were preloaded and preconditioned between each of the tear conditions and were generously moistened with PBS after completion of each loading protocol.
One camera was optimally positioned to capture deformations in the insertion site of the supraspinatus tendon and another camera was optimally positioned to capture deformations in the insertion site of the infraspinatus tendon. The image capture program (custom written in labview) that controls each of the 2 cameras was synchronized with the software to control Instron loading (Merlin software), allowing images corresponding to the loads of interest to be easily isolated for analysis. Digital images were taken of the insertion site of both the supraspinatus and the infraspinatus tendons at 1 second intervals beginning at the end of preconditioning until the end of the ramp to 90N.
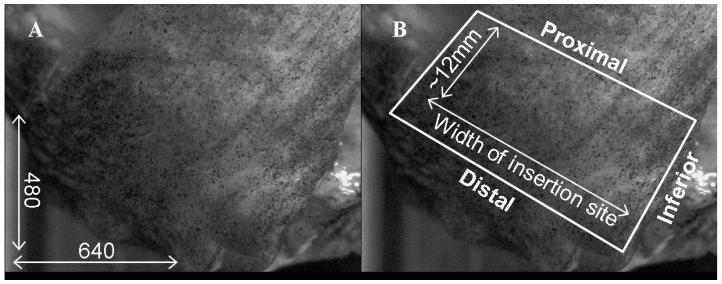
For each tear level (intact, 33% tear, 66% tear), images at 5N, 30N, 60N and 90N of supraspinatus tendon loads were evaluated for the infraspinatus and supraspinatus tendons. A region of interest was outlined to mark the insertion site of the infraspinatus tendon for digital images that corresponded to a nominally loaded (corresponding to a 5N load) supraspinatus tendon (figure 2). The region of interest was defined by a polygon with a distal edge along the distal border of the insertion site and a proximal edge approximately 12mm proximal to the distal edge. The superior and inferior edges of the region of interest were defined by the anatomical superior and inferior borders of the tendon. Similarly, a polygon marked the region of interest in the supraspinatus tendon as the consistently intact posterior 1/3 of the supraspinatus insertion site.
Figure 2.
The region of interest for strain analysis in the infraspinatus tendon is shown in white. (A) The image resolution was approximately 0.05mm/pixel. (B) The distal, superior and inferior borders are defined by the natural edges of the tendon, and the proximal border is approximately 12mm proximal to the distal edge.
Using Vic2D, a digital grid of nodes was fitted in the region of interest that satisfied criteria determined from a preliminary parametric study that demonstrated a plateau in sensitivity of strain measurements to small changes in parametric values or region of interest. This software uses a pattern matching algorithm to calculate displacements of the nodes between the 5N load and each of the 30N, 60N and 90N loads. The nodal points were used to form finite elements. The deformation of these finite elements was used to calculate the two-dimensional Lagrangian finite strain tensor (εxx, εyy, and εxy) and the principal strain components. Average maximum and minimum principal strains were determined for the entire insertion site of the infraspinatus and the posterior 1/3 of the insertion site in the supraspinatus tendon. Evaluating the principal strain components provides a simple and more complete understanding of the loading environment of the tendon by eliminating the shear strain component through a coordinate rotation that causes its contribution to be absorbed into the tensile (maximum principal strain) and compressive (minimum principal strain) orthogonal components. In this case, tensile and compressive deformation is completely isolated by average maximum and minimum principal strain respectively.
The insertion site of the infraspinatus and supraspinatus tendons is not planar but rather slightly curved due to the curvature of the humeral head. However, the region of interest is small (as shown in figure 2) minimizing the effect of the curvature and allowing the use of a planar estimate. Additionally, the position of the tendon with respect to the humeral head is unaltered across all testing conditions allowing the negligible effect of the curvature of the humeral head to equally impact all tested conditions and therefore have minimal effect on the relationships being evaluated. Despite this negligible effect, for mechanical rigor, from this point forward, we will term our “strain” measurements as “apparent strain”.
A power analysis was conducted prior to initiation of experiments that indicated that based on the expected variability in the data and the repeated measures design, a total of 4 specimens were required to achieve a power of 80 with an alpha level of 0.05 with a medium effect size (0.5-1.0). Due to the repeated measures design, each specimen contributes to the data in every cell, and the necessary 4 specimens per cell, resulted in a total of 36 data points. In contrast, a non-repeated measures design, where data from each specimen appears in a single cell would have required 36 total specimens (4 × 9) to acquire 36 total data points. For data analysis, two-way ANOVAs with repeated measures were conducted to evaluate the effect of supraspinatus tendon tear size and load on both infraspinatus and supraspinatus tendon strain. If a lack of a statistically significant interaction between the effect of full-thickness tear size and load was found, the main effects (load and tear size) were subsequently independently evaluated. Significance was set at p ≤ 0.05 (denoted by a *) and a trend at p ≤ 0.1 (denoted by #). Post hoc Bonferroni tests were conducted to determine significant pairs.
RESULTS
In general, increase in loading or full-thickness tear size in the supraspinatus tendon caused an increase in maximum and a decrease in minimum apparent average principal strain in both the supraspinatus and infraspinatus tendons. A statistical interaction between supraspinatus tendon-tear size and load was found for strain in the posterior third of the supraspinatus tendon. However, no interaction was found between supraspinatus tendon full-thickness tear size and load for average apparent maximum or minimum principal strain in the infraspinatus tendon.
As supraspinatus tendon load was increased from 30N to 60N to 90N, infraspinatus tendon average apparent maximum principal strain increased, and average apparent minimum principal strain decreased. Similarly, as supraspinatus tendon tear size was extended from intact and 33% width to 66% width anterior full thickness tear, infraspinatus tendon average apparent maximum principal strain increased and average apparent minimum principal strain decreased.
Effect of supraspinatus tendon load on infraspinatus tendon strain
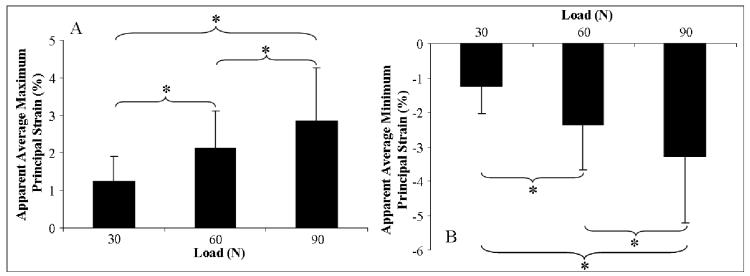
Since there was no significant statistical interaction between supraspinatus tendon loading and tear-size for average maximum and minimum infraspinatus tendon strain, the effect of load was evaluated independent of tear size. Increasing supraspinatus tendon loading caused a significant and progressive increase in the average apparent maximum principal strain (figure 3A) and decrease in the average apparent minimum principal strain (figure 3B) in the infraspinatus tendon. More specifically, a change in supraspinatus tendon load from 30N to 60N and 90N and from 60N to 90N resulted in a respective increase in apparent average maximum principal strain of 72.2%, 131.0% and a 42.4% and a respective decrease in apparent average minimum principal strain of 86.8%, 159.4% and 48.8%. For both principal strain components, significant differences were found between all of the loading conditions and are denoted in Table I.
Figure 3.
A) Average maximum principal strain in the infraspinatus tendon significantly increases with increasing supraspinatus tendon loading. B) Average minimum principal strain in the infraspinatus tendon significantly increases with increasing supraspinatus tendon loading
Table I.
Statistical analysis of the effect of supraspinatus tendon load on infraspinatus tendon strain. Shown P values are corrected for multiple comparisons.
| Average Maximum Principal Strain (%) |
Average Minimum Principal Strain (%) |
||||||
|---|---|---|---|---|---|---|---|
| 30 N | 60 N | 90 N | 30 N | 60 N | 90 N | ||
| 30 N | 30 N | ||||||
| 60 N | 0.000 | 60 N | 0.001 | ||||
| 90 N | 0.001 | 0.015 | 90 N | 0.001 | 0.003 | ||
Effect of supraspinatus tendon tear-size on infraspinatus tendon strain
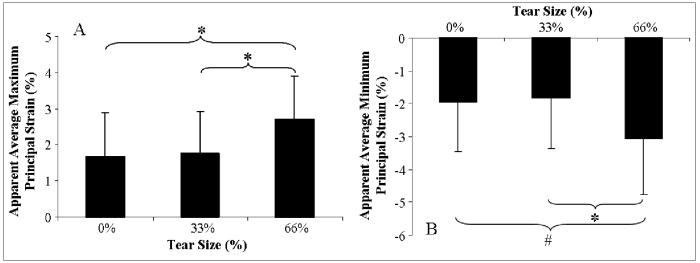
Since there was no significant statistical interaction between supraspinatus tendon loading and tear-size for average maximum and minimum infraspinatus tendon strain, the effect of tear was evaluated independent of load level. No significant differences were found in the infraspinatus tendon for either of the principal strain components in extending from an intact supraspinatus tendon to a 33% width supraspinatus tear. Increasing supraspinatus tendon tear size from 33% to 66% of the width of the tendon caused a significant increase in the average apparent maximum principal strain (figure 4A) and decrease in the average apparent minimum principal strain (figure 4B) in the infraspinatus tendon when compared to both the intact and 33% full-thickness tear cases. Significant differences were found in the average apparent maximum principal strain in the infraspinatus tendon resulting from a 66% width, full-thickness supraspinatus tendon tear compared with the average apparent maximum principal strain from both the intact (difference of 62.0%) and 33% width, full-thickness supraspinatus tendon tear (difference of 53.1%). Similarly, a trend and a statistically significant difference were found in the average apparent minimum principal strain in the infraspinatus tendon resulting from a 66% width, full-thickness supraspinatus tear compared with the intact (difference of 57.2%) and 33% width, full-thickness supraspinatus tear (difference of 67.3%), respectively (see Table II for p values).
Figure 4.
A) Average maximum principal strain in the infraspinatus tendon significantly increases with increasing supraspinatus tendon tear size. B) Average minimum principal strain in the infraspinatus tendon significantly increases with increasing supraspinatus tendon tear size.
Table II.
Statistical analysis of the effect of supraspinatus tendon tear-size (anterior-side full-thickness tears) on infraspinatus tendon strain. Shown P values are corrected for multiple comparisons.
| Average Maximum Principal Strain (%) |
Average Minimum Principal Strain (%) |
||||||
|---|---|---|---|---|---|---|---|
| Intact | 33% | 66% | Intact | 33% | 66% | ||
| Intact | Intact | ||||||
| 33% | 0.92 | 33% | 0.33 | ||||
| 66% | 0.01 | 0.004 | 66% | 0.06 | 0.05 | ||
Effect of supraspinatus tendon load on strain in the posterior third of the supraspinatus tendon
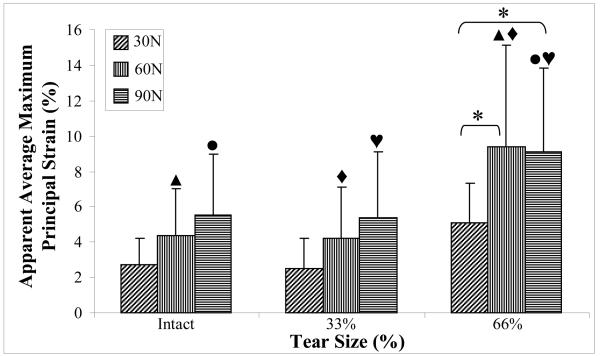
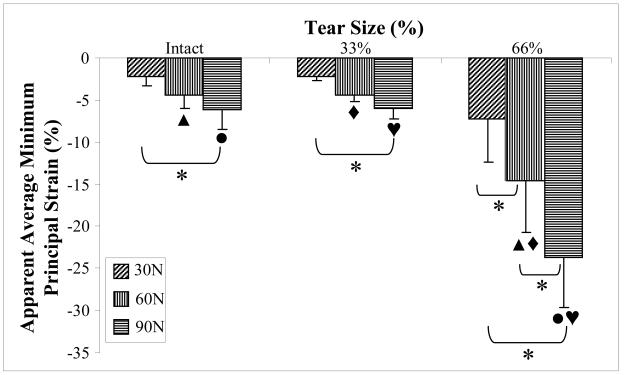
In general, increasing load in the supraspinatus tendon caused an increase and a decrease in apparent average maximum and minimum principal strain respectively in the supraspinatus tendon. This was most consistently observed for the 66% full-thickness tear condition where average maximum principal strain at 30N load was significantly lower than at 60N and 90N (difference of 85.3% and 79.5%, respectively). There was no significant effect of load on supraspinatus tendon apparent average maximum principal strain at the intact or 33% anterior full-thickness tear conditions. There was also no significant difference between apparent average maximum principal strain in the supraspinatus tendon between 60N and 90N load for any tear size (figure 5). For all tear conditions, average apparent minimum principal strain was significantly higher at 30N than at 90N. In the 66% anterior full-thickness tear only, average apparent minimum principal strain was significantly lower at 90N than at 60N, which was lower than average apparent minimum principal strain at 30N (difference of 62.3% and 104.1%) (figure 6).
Figure 5.
Apparent average maximum principal strain increases with increasing supraspinatus tendon tear size and load. A common symbol above any two bars is indicative of a significantly difference between the pair of bars for the effect of tear size.
Figure 6.
Apparent average minimum principal strain decreases with increasing supraspinatus tendon tear size and load. A common symbol above any tow bars is indicative of a significant difference between the pair of bars for the effect of tear size.
Effect of supraspinatus tendon tear on strain in the posterior third of the supraspinatus tendon
There was no significant difference between apparent average maximum or minimum principal strain in the supraspinatus tendon between the intact and the 33% anterior full-thickness tear condition at all loads evaluated. Additionally, no significant difference between any supraspinatus tear condition for average apparent maximum or minimum principal strain was found at 30N of supraspinatus tendon loading. At 60N of supraspinatus tendon load, average apparent maximum principal strain in the supraspinatus tendon was significantly lower for intact and 33% anterior full-thickness tear conditions (difference of 114.1% and 123.7%, respectively) than for the 66% anterior full-thickness tear case (figure 5). At this load, the average apparent minimum principal strain in the supraspinatus tendon was significantly higher for intact and 33% anterior full-thickness tear conditions (difference of 238.4% and 234.1%, respectively) than for the 66% anterior full-thickness tear case (figure 6). Similarly, at 90N of supraspinatus tendon load, average apparent maximum principal strain in the supraspinatus tendon was significantly lower for intact and 33% anterior full-thickness tear conditions (difference of 64.8% and 70.4%, respectively) than for the 66% anterior full-thickness tear case (figure 5). At this load, the average apparent minimum principal strain in the supraspinatus tendon was significantly higher for intact and 33% anterior full-thickness tear conditions (290.2% and 297.7%, respectively) than for the 66% anterior full-thickness tear case (figure 6).
DISCUSSION
Our results support the hypothesis that (1) as supraspinatus tendon loading increases, there is an increase in average apparent maximum and a decrease in the average apparent minimum principal strain in the infraspinatus tendon, and (2) as supraspinatus tendon tear size is extended to 66% of the width, there is an increase in average apparent maximum and a decrease in average apparent minimum principal strain in the infraspinatus tendon, although not progressive. There was no significant increase in maximum or decrease in minimum principal strain in the infraspinatus tendon as supraspinatus tendon tear size was increased from intact to 33% of tendon width, however. The increase in infraspinatus tendon strain that occurs when the supraspinatus tendon is subjected to conditions that will result in significant increases in its strain implies a potential strain shielding role of the infraspinatus tendon. Since it is assumed that an increase in local strain correlates with an increased risk of rotator cuff tear propagation, in effect, the infraspinatus tendon may play a role in minimizing the risk of supraspinatus tendon tear propagation.
Unexpectedly, increase in tear size resulted in increase in infraspinatus tendon strain only for the 66% width, anterior full-thickness supraspinatus tendon tear in comparison to the intact and 33% width, anterior full-thickness supraspinatus tendon tear. There was no effect on infraspinatus tendon strain with introduction of a 33% width, anterior full thickness tear in comparison with the intact tendon case. These results are particularly interesting since the supraspinatus and infraspinatus tendons are confluent in the posterior 30-50% of the tendon20, a region that was torn only for the 66% width, anterior full-thickness tear case. These results imply that the effect of a small tear (that does not compromise the confluent region between the supraspinatus and infraspinatus tendons) may be very localized, eliminating its effect on infraspinatus tendon strain.
We expect that since infraspinatus tendon loading occurs secondary to supraspinatus loading, loading in the infraspinatus tendon more closely mimics transverse tendon loading. Several studies have evaluated the mechanical properties of tendons30 and ligaments23 loaded transverse to the direction of the collagen fibers and concluded that the modulus of tissue loaded in the direction of length of the fibers was more than an order of magnitude higher than that of specimens loaded along the transverse direction. This supports the much larger strain values obtained from this loading configuration in association with smaller applied loads in this study. In addition, the strain measurement technique used in this study allowed for high resolution measurements. Much of the reported data on tendon and ligament strain measurements utilize techniques that provide an average strain over a relatively large portion of the tissue. Typically, tendon and ligament strain under loading along the long axis of the tendon is investigated by recording the position of stain lines with a video camera28. These measurements are known to result in lower strain values than in higher resolution strain measurement techniques that measure strain more locally,17 such as the technique used in this study.
In this study, the effect of anterior full-thickness supraspinatus tendon tears was evaluated because of their higher incidence than posterior-side tears. The results provide insight regarding the rotator cuff loading environment associated with this common clinical tear, but do not explain the role of the infraspinatus tendon in posterior side tears. Interestingly, the role of the infraspinatus tendon may differ in this situation. We expect that an interaction between the supraspinatus tendon and the infraspinatus tendon depends on the type and location of the supraspinatus tear. Minagawa et al. described the anatomy of the rotator cuff and noted that the supraspinatus and infraspinatus tendons interdigitate in the posterior half of the supraspinatus tendon20. The anterior tears evaluated in this study preserved the entire (for the case of the 33% tears) or some (for the case of the 66% tears) of the confluent region between the supraspinatus and infraspinatus tendons, thereby preserving the interaction between the two tendons. We expect that tears that compromise the entire confluent region, such as posterior-side supraspinatus tears, may cause a disruption in the interaction between the supraspinatus and infraspinatus tendons, leading to minimal or no changes in infraspinatus tendon strain with significant increases in supraspinatus tendon loading or tear size. Therefore, the location of a supraspinatus tendon tear is expected to greatly impact the interaction between it and the infraspinatus tendon.
The thickness of a supraspinatus tendon tear is also likely to impact the interaction of the supraspinatus and infraspinatus tendons. Five-axial plane layers have been used to describe the structure of the rotator cuff through the depth of the critical zone7. The supraspinatus and infraspinatus tendons interdigitate in the third layer only. Therefore, partial thickness tears that retain an intact third layer are likely to result in a greater interaction between the supraspinatus and infraspinatus tendons than tears that compromise the third layer. Future studies will evaluate the rotator cuff loading environment associated with partial thickness supraspinatus tendon tears.
The effect of tear size and loading was evaluated at a glenohumeral abduction angle of 0° in the current study. Previous studies have shown that glenohumeral abduction angle impacts the measured strain in the supraspinatus tendon4, 21. The interaction of the humeral head with the supraspinatus tendon was speculated to explain the increase in strain in the posterior third of the supraspinatus tendon at joint positions at which such interactions are minimized. We anticipate that the amount of humeral head strain shielding due to changes in abduction angle will impact the interaction between the supraspinatus and infraspinatus tendons. Therefore, future studies will investigate the effect of joint position on supraspinatus and infraspinatus tendon interactions in the rotator cuff.
Previous studies have also investigated the effect of supraspinatus tendon tears on the load bearing capacity of the remaining intact portion of the tendon in an effort to predict the likelihood of tear propagation3, 24, 25. Several of these models evaluated the excised supraspinatus tendon (typically retaining the attachment to the humeral head), assuming that an understanding of the likelihood of tear propagation can be obtained from evaluating the isolated torn tendon. However, since our results showed significant strain changes in the infraspinatus tendon with conditions that cause an increase in strain in the supraspinatus tendon, it can be expected that the load bearing capacity of the supraspinatus tendon differs in a more complete rotator cuff model in comparison with the isolated tendon model. Therefore, the inclusion of the infraspinatus tendon in models evaluating supraspinatus tendon loading more accurately mimics the in-vivo loading environment.
The tears investigated in this experiment were surgically introduced in cadaveric supraspinatus tendons that exhibited no visible tears, degeneration, fraying of tendon fibers, or abnormal pathology prior to surgery. Therefore, factors present in chronic rotator cuff tears, such as tissue degeneration and remodeling due to injury and repair, were not incorporated in the current model. Chronically degenerated tissue may exhibit higher strains under loading than healthy tissue, due to diminished mechanical properties. Additionally, retraction of the supraspinatus tendon in a chronic tear may result in traction of the suprascapular nerve1, 18. Tethering of this nerve may lead to atrophy and loss of function of the infraspinatus tendon, potentially compromising its ability to share loads with the torn supraspinatus tendon. While the current study does not explicitly address factors present in chronic rotator cuff tears, our results have implications for clinical management of supraspinatus tendon tears. In approaching clinical treatment of the torn supraspinatus tendon, the functional capability of the infraspinatus should be evaluated. Clinical evaluation demonstrating preserved anatomical confluence between the infraspinatus and supraspinatus tendons implies a potential strain shielding role of the infraspinatus tendon. In chronic situations, the higher supraspinatus tendon strains associated with a chronic tear are expected to elicit an even greater strain shielding role from the infraspinatus tendon.
To our knowledge, mechanical interactions between rotator cuff tendons have not been previously evaluated. We have shown that a mechanical interaction between the supraspinatus and infraspinatus tendons exists. The long term effects of this interaction on the supraspinatus and infraspinatus tendons needs further evaluation. For instance, supraspinatus tendon tears often progress in size and may potentially progress into multiple tendon tears, implying a change in the loading environment and an increase in susceptibility to tear propagation in other intact rotator cuff tendons29, 31. These results suggest that the effects of the interaction between the supraspinatus and infraspinatus tendons may be detrimental to the infraspinatus tendon over time. In contrast, the interaction between the torn supraspinatus tendon and the intact infraspinatus may provide relief to the straining supraspinatus tendon and shield it from further propagation. Therefore, it is possible that the interaction between the two tendons could be associated with both positive and negative long-term outcomes and should be further evaluated.
In summary, this study demonstrates that an interaction between the infraspinatus tendon and the strained supraspinatus tendon exists, leading to increases in infraspinatus tendon strain. For a small supraspinatus tendon tear, the mechanical interaction between the supraspinatus and infraspinatus tendon did not result in increases in infraspinatus tendon strain. Therefore, when considering treatment and risk of progression of a supraspinatus tendon tear, not only the load bearing capacity of the remaining intact portion of the tendon must be assessed, but evaluation of the adjacent infraspinatus tendon and the efficiency of the interaction between the two tendons must also be considered.
Contributor Information
Nelly Andarawis-Puri, McKay Orthopaedic Research Laboratory University of Pennsylvania.
Eric T. Ricchetti, McKay Orthopaedic Research Laboratory University of Pennsylvania.
Louis J. Soslowsky, McKay Orthopaedic Research Laboratory University of Pennsylvania, 424 Stemmler Hall, Philadelphia, PA
REFERENCES
- 1.Albritton MJ, Graham RD, Richards RS, 2nd, Basamania CJ. An anatomic study of the effects on the suprascapular nerve due to retraction of the supraspinatus muscle after a rotator cuff tear. J Shoulder Elbow Surg. 2003 Sep-Oct;12(5):497–500. doi: 10.1016/s1058-2746(03)00182-4. [DOI] [PubMed] [Google Scholar]
- 2.Bassett RW, Cofield RH. Acute tears of the rotator cuff. The timing of surgical repair. Clin Orthop Relat Res. 1983 May;(175):18–24. [PubMed] [Google Scholar]
- 3.Bey MJ, Ramsey ML, Soslowsky LJ. Intratendinous strain fields of the supraspinatus tendon: effect of a surgically created articular-surface rotator cuff tear. J Shoulder Elbow Surg. 2002 Nov-Dec;11(6):562–569. doi: 10.1067/mse.2002.126767. [DOI] [PubMed] [Google Scholar]
- 4.Bey MJ, Song HK, Wehrli FW, Soslowsky LJ. Intratendinous strain fields of the intact supraspinatus tendon: the effect of glenohumeral joint position and tendon region. J Orthop Res. 2002 Jul;20(4):869–874. doi: 10.1016/S0736-0266(01)00177-2. [DOI] [PubMed] [Google Scholar]
- 5.Bey MJ, Song HK, Wehrli FW, Soslowsky LJ. A noncontact, nondestructive method for quantifying intratissue deformations and strains. J Biomech Eng. 2002 Apr;124(2):253–258. doi: 10.1115/1.1449917. [DOI] [PubMed] [Google Scholar]
- 6.Breazeale NM, Craig EV. Partial-thickness rotator cuff tears. Pathogenesis and treatment. Orthop Clin North Am. 1997 Apr;28(2):145–155. doi: 10.1016/s0030-5898(05)70275-3. [DOI] [PubMed] [Google Scholar]
- 7.Clark JM, Harryman DT., 2nd Tendons, ligaments, and capsule of the rotator cuff. Gross and microscopic anatomy. J Bone Joint Surg Am. 1992 Jun;74(5):713–725. [PubMed] [Google Scholar]
- 8.Cotton RE, Rideout DF. Tears Of The Humeral Rotator Cuff; A Radiological And Pathological Necropsy Survey. J Bone Joint Surg Br. 1964 May;46:314–328. [PubMed] [Google Scholar]
- 9.Feng S, Guo S, Nobuhara K, Hashimoto J, Mimori K. Prognostic indicators for outcome following rotator cuff tear repair. J Orthop Surg (Hong Kong) 2003 Dec;11(2):110–116. doi: 10.1177/230949900301100202. [DOI] [PubMed] [Google Scholar]
- 10.Flatow EL, Altchek DW, Gartsman GM, et al. The rotator cuff. Commentary. Orthop Clin North Am. 1997 Apr;28(2):277–294. doi: 10.1016/s0030-5898(05)70286-8. [DOI] [PubMed] [Google Scholar]
- 11.Fukuda H. Partial-thickness rotator cuff tears: a modern view on Codman's classic. J Shoulder Elbow Surg. 2000 Mar-Apr;9(2):163–168. [PubMed] [Google Scholar]
- 12.Fukuda H, Hamada K, Nakajima T, Tomonaga A. Pathology and pathogenesis of the intratendinous tearing of the rotator cuff viewed from en bloc histologic sections. Clin Orthop Relat Res. 1994 Jul;(304):60–67. [PubMed] [Google Scholar]
- 13.Fukuda H, Hamada K, Yamanaka K. Pathology and pathogenesis of bursal-side rotator cuff tears viewed from en bloc histologic sections. Clin Orthop Relat Res. 1990 May;(254):75–80. [PubMed] [Google Scholar]
- 14.Habernek H, Schmid L, Frauenschuh E. Five year results of rotator cuff repair. Br J Sports Med. 1999 Dec;33(6):430–433. doi: 10.1136/bjsm.33.6.430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Halder A, Zobitz ME, Schultz F, An KN. Mechanical properties of the posterior rotator cuff. Clin Biomech (Bristol, Avon) 2000 Jul;15(6):456–462. doi: 10.1016/s0268-0033(99)00095-9. [DOI] [PubMed] [Google Scholar]
- 16.Itoi E, Berglund LJ, Grabowski JJ, et al. Tensile properties of the supraspinatus tendon. J Orthop Res. 1995 Jul;13(4):578–584. doi: 10.1002/jor.1100130413. [DOI] [PubMed] [Google Scholar]
- 17.Malicky DM, Soslowsky LJ, Kuhn JE, et al. Total strain fields of the antero-inferior shoulder capsule under subluxation: a stereoradiogrammetric study. J Biomech Eng. 2001 Oct;123(5):425–431. doi: 10.1115/1.1394197. [DOI] [PubMed] [Google Scholar]
- 18.Mallon WJ, Wilson RJ, Basamania CJ. The association of suprascapular neuropathy with massive rotator cuff tears: a preliminary report. J Shoulder Elbow Surg. 2006 Jul-Aug;15(4):395–398. doi: 10.1016/j.jse.2005.10.019. [DOI] [PubMed] [Google Scholar]
- 19.Matava MJ, Purcell DB, Rudzki JR. Partial-thickness rotator cuff tears. Am J Sports Med. 2005 Sep;33(9):1405–1417. doi: 10.1177/0363546505280213. [DOI] [PubMed] [Google Scholar]
- 20.Minagawa H, Itoi E, Konno N, et al. Humeral attachment of the supraspinatus and infraspinatus tendons: an anatomic study. Arthroscopy. 1998 Apr;14(3):302–306. doi: 10.1016/s0749-8063(98)70147-1. [DOI] [PubMed] [Google Scholar]
- 21.Muraki T, Aoki M, Uchiyama E, Murakami G, Miyamoto S. The effect of arm position on stretching of the supraspinatus, infraspinatus, and posterior portion of deltoid muscles: a cadaveric study. Clin Biomech (Bristol, Avon) 2006 Jun;21(5):474–480. doi: 10.1016/j.clinbiomech.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 22.Peterson CA, 2nd, Altchek DW. Arthroscopic treatment of rotator cuff disorders. Clin Sports Med. 1996 Oct;15(4):715–736. [PubMed] [Google Scholar]
- 23.Quapp KM, Weiss JA. Material characterization of human medial collateral ligament. J Biomech Eng. 1998 Dec;120(6):757–763. doi: 10.1115/1.2834890. [DOI] [PubMed] [Google Scholar]
- 24.Reilly P, Amis AA, Wallace AL, Emery RJ. Supraspinatus tears: propagation and strain alteration. J Shoulder Elbow Surg. 2003 Mar-Apr;12(2):134–138. doi: 10.1067/mse.2003.7. [DOI] [PubMed] [Google Scholar]
- 25.Sano H, Wakabayashi I, Itoi E. Stress distribution in the supraspinatus tendon with partial-thickness tears: An analysis using two-dimensional finite element model. J Shoulder Elbow Surg. 2006 Jan-Feb;15(1):100–105. doi: 10.1016/j.jse.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the management of significant partial-thickness tears of the rotator cuff. Orthop Clin North Am. 1997 Jan;28(1):79–82. doi: 10.1016/s0030-5898(05)70266-2. [DOI] [PubMed] [Google Scholar]
- 27.Weber SC. Arthroscopic debridement and acromioplasty versus mini-open repair in the treatment of significant partial-thickness rotator cuff tears. Arthroscopy. 1999 Mar;15(2):126–131. doi: 10.1053/ar.1999.v15.0150121. [DOI] [PubMed] [Google Scholar]
- 28.Woo SL, Gomez MA, Seguchi Y, Endo CM, Akeson WH. Measurement of mechanical properties of ligament substance from a bone-ligament-bone preparation. J Orthop Res. 1983;1(1):22–29. doi: 10.1002/jor.1100010104. [DOI] [PubMed] [Google Scholar]
- 29.Yamaguchi K, Tetro AM, Blam O, Evanoff BA, Teefey SA, Middleton WD. Natural history of asymptomatic rotator cuff tears: a longitudinal analysis of asymptomatic tears detected sonographically. J Shoulder Elbow Surg. 2001 May-Jun;10(3):199–203. doi: 10.1067/mse.2001.113086. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto E, Hayashi K, Yamamoto N. Effects of stress shielding on the transverse mechanical properties of rabbit patellar tendons. J Biomech Eng. 2000 Dec;122(6):608–614. doi: 10.1115/1.1319660. [DOI] [PubMed] [Google Scholar]
- 31.Zingg PO, Jost B, Sukthankar A, Buhler M, Pfirrmann CW, Gerber C. Clinical and structural outcomes of nonoperative management of massive rotator cuff tears. J Bone Joint Surg Am. 2007 Sep;89(9):1928–1934. doi: 10.2106/JBJS.F.01073. [DOI] [PubMed] [Google Scholar]