Abstract
In previous work, we established interference-based cut-points to differentiate moderate from mild, and severe from moderate levels of severity for 16 symptoms as reported by cancer patients undergoing chemotherapy. This work examines how consistent the differentiation is over time. If the levels of severity successfully differentiate interference scores over time, then anchor-based categories can be developed to evaluate patients’ responses to the intervention. To test the differentiation of the interference scores by the three severity categories, data from two symptom management trials were used. Five hundred ninety-nine patients were queried at each of the six contacts that occurred over eight weeks as to the severity of each of 16 symptoms on the scale from 0 (not present) to 10 and the extent to which each symptom interfered with enjoyment of life, relationships with others, general daily activities and emotions. Longitudinal models that related interference scores to severity levels of symptoms were used. Differences among adjusted mean interference scores for mild, moderate and severe levels were tested at each contact. Differences among interference-based severity categories were consistent over time and clinically important, and thus can be used to anchor changes in symptom severity.
Keywords: Anchor-based responses, levels of severity, measurement of symptom severity change
Introduction
This research team drew on work by Cleeland and colleagues [1–3], who incorporated the assessments of both severity and interference of symptoms with general activity, mood, enjoyment of life, social relationships, walking ability and normal work into their instruments (Brief Pain Inventory, Brief Fatigue Inventory and M. D. Anderson Symptom Inventory). Cut-points separating mild from moderate and moderate from severe levels of pain [4–7] and fatigue [2] have been identified based on the increases in the levels of interference associated with successive increases in severity. This methodology has been extended to 16 cancer-related symptoms reported by patients undergoing chemotherapy [8]. The resulting interference-based cut-points differed by symptom. For depression, the cut-point between moderate and severe was between 3 and 4 (3 marking moderate depression, and 4 severe); for pain, fatigue, weakness, cough, and difficulty remembering, the cut-point was between 4 and 5; and for the remaining symptoms, cut-points at 6 or 7 separated moderate from severe. The cut-points were obtained using data from two trials of symptom management interventions (n=588) at the initial contact prior to the delivery of any intervention strategies [8].
This paper extends prior work of this team by examining the performance of cut-points over time, across six contacts covering an eight-week symptom management intervention. The 16 targeted symptoms described in previous work [8], including alopecia, anxiety, poor appetite, constipation, cough, depression, diarrhea, dry mouth, dyspnea, fatigue, nausea/vomiting, pain, peripheral neuropathy, difficulty remembering, sleep disturbances, and weakness, were assessed in this study. We seek to determine if the cut-points provide consistent differentiation among the levels of interference due to symptoms as patients receive interventions to address symptoms that reach a pre-specified severity threshold. From a cognitive perspective, this assumes that patients maintain a consistent association between the severity of a symptom and the interference it imposes on dimensions of their daily lives. Further, these associations will persist even as patients report lower (or higher) levels of severity over the six contacts. If these associations are consistent over time and interference scores are consistently different for mild, moderate and severe levels of symptoms, then we may anchor patients’ responses to symptom management interventions to these levels (mild, moderate, severe). For example, a response to an intervention might be defined as an improvement from severe or moderate to mild or from severe to moderate, and non-response as remaining moderate or worsening from moderate to severe over time. If important differences in interference scores among these categories persist over time, then they could be used in clinical trials of symptom management to capture meaningful improvement or deterioration.
The research question that guides this work is: Can the cut-points that separate mild, moderate and severe levels of symptoms established at the initial contact consistently differentiate the interference scores for each of 16 cancer-related symptoms across six contacts covering eight weeks?
Review of the Literature
In this research, we seek to determine if, while receiving strategies to manage their symptoms, patients continue over time to differentiate among levels of interference that define severity based cut-points. Response shifts are a major threat to this argument. As patients implement strategies that lower the severity of their symptoms, they may “re-calibrate” their definitions of severity, interference, or both. For example, patients may report declines in severity but continue to associate these new levels of severity with the same or increased interference. Standard investigations of response shifts compare post-test with retrospective “then” assessments. Retrospective assessments are likely to be adjusted by current patient perceptions. In this research, we follow patients’ responses to interventions, compare their reports of interference at each subsequent observation where symptoms are rated as severe moderate or mild, and then seek to determine if interference scores consistently differentiate severe from moderate and moderate from mild levels of severity. If so, the integrity of the interference-based severity cut-points are preserved over time and can be used to measure patients responses to these intervention strategies [9].
Patient reports of the extent to which pain interferes with dimensions of their daily lives have become an accepted metric for differentiating severe from moderate and moderate from mild severity. Different analytical techniques have been used to assess the magnitude of the differences in interference scores as a means of separating pain severity ratings into mild moderate and severe [2, 6–8]. The important analytical distinctions centered on whether interference measures were treated as separate items or combined into a summary scale and if their distributions were skewed or assumed to be normally distributed.
Less information is available regarding the validity of the measures of reductions in pain intensity in response to interventions. Clinically significant reductions in severity have been evaluated in different ways, including a 20–50% reduction in severity over baseline [10–11]; difference scores based on patient report of pain relief attributed to a drug or procedure; a reduction in two or more points on a 10-point scale, or a 33% and 50% reduction [9,12]. A limitation of measures using percent change in symptom severity is that a reduction from a 9 to a 6 may be much less important than from a 7 to a 4. Miaskowski et al. separated patients assigned to the experimental intervention into responders with a > 30% reduction in the mean of average and worst pain and non-responders [13]. While no differences in interference with daily activities, mood and enjoyment of life were reported among non-responders, significant reductions were found among the responders. This subgroup analysis suggested that a reduction in pain severity is associated with lowered interference and, by implication, non-responders continued to report higher levels of interference. This research seeks to determine if interference scores continue over time to differentiate cut-points used to define severity categories. If so, then these categories may be used to define cognitively meaningful change/responses to interventions directed toward managing symptom severity.
Methods
The data used to test the differentiation of interference by the cut-points for separating severity of 16 symptoms into mild, moderate, and severe are taken from two large randomized trials designed to test alternative strategies for the management of cancer symptoms for patients receiving chemotherapy. Each trial employed a nurse-administered cognitive behavioral intervention in one arm. In the first trial, the nurse arm was paired against an automated voice response (AVR) system. In the second trial, the nurse arm was paired against a non-nurse coach. Patients in both trials received six contacts over eight weeks. Careful analyses of both trials revealed no differences in summed symptom severity scores at the 10-week endpoint (post-intervention) [14], and significant reduction in symptom severity from baseline to 10 weeks in all four arms. The effect sizes for decrease in symptom severity were 0.57 and 0.73 in two nurse arms, 0.66 in the AVR arm, and 0.69 in the coach arm. Given comparable moderate to large impacts of the trial arms on symptom severity, we decided to combine all data available at each of the six intervention contacts covering eight weeks. Evidence of comparability regarding the impact of all interventions on symptom severity is provided in Table 1, confirming our decision to combine data from the two trials for this analysis.
Table 1.
Characteristics of Patients Who Participated in the Two Studies at Baseline (Intake) and 10 Weeks (Post-Intervention).
| Characteristics | Trial 1 |
Trial 2 |
||||||
|---|---|---|---|---|---|---|---|---|
| N | % | Severity at Baseline Mean (SD) | Severity at 10 Weeks Mean (SD) | n | % | Severity at Baseline Mean (SD) | Severity at 10 Weeks Mean (SD) | |
| Patients’ Age | ||||||||
| 25 – 44 | 50 | 13.0 | 35.5 (20.0) | 22.6 (22.0) | 31 | 14.5 | 39.6 (19.3) | 21.5 (17.5) |
| 45 – 54 | 100 | 26.0 | 41.3 (22.7) | 22.3 (19.8) | 57 | 26.6 | 46.7 (25.7) | 27.4 (22.5) |
| 55 – 64 | 129 | 33.5 | 35.2 (21.8) | 21.7 (16.9) | 76 | 35.5 | 36.5 (21.3) | 18.8 (16.6) |
| 65 – 74 | 62 | 16.1 | 29.5 (21.1) | 18.5 (13.4) | 37 | 17.3 | 33.7 (20.1) | 17.7 (17.3) |
| 75 + | 44 | 11.4 | 29.1 (22.8) | 21.3 (15.7) | 13 | 6.1 | 26.5 (25.8) | 20.9 (22.9) |
|
| ||||||||
| Gender | ||||||||
| Male | 120 | 31.2 | 31.3 (23.9) | 21.0 (18.3) | 61 | 28.5 | 33.9 (22.8) | 18.5 (15.1) |
| Female | 265 | 68.8 | 37.0 (21.1) | 21.6 (17.7) | 153 | 71.5 | 40.5 (22.7) | 22.8 (20.6) |
|
| ||||||||
| Cancer Site | ||||||||
| Breast | 135 | 35.1 | 39.0 (20.9) | 22.3 (17.4) | 78 | 36.5 | 39.8 (22.4) | 24.2 (21.8) |
| Colon | 40 | 10.4 | 28.6 (18.0) | 20.2 (18.4) | 28 | 13.1 | 31.5 (24.2) | 16.8 (11.6) |
| Lung | 87 | 22.6 | 35.4 (24.0) | 21.4 (18.1) | 39 | 18.2 | 41.3 (23.3) | 21.3 (19.5) |
| Other | 123 | 31.9 | 33.0 (22.8) | 20.9 (18.0) | 69 | 32.2 | 38.6 (22.6) | 20.2 (17.9) |
|
| ||||||||
| Cancer Stage | ||||||||
| Early | 112 | 29.4 | 36.8 (19.2) | 22.4 (19.2) | 66 | 31.0 | 38.6 (21.2) | 25.0 (21.7) |
| Late | 269 | 70.6 | 34.6 (23.4) | 21.1 (17.2) | 147 | 69.0 | 38.6 (23.7) | 19.5 (17.5) |
|
| ||||||||
| Intervention Arm | ||||||||
| Nurse | 198 | 51.4 | 34.9 (22.1) | 22.1 (19.3) | 100 | 46.7 | 36.8 (20.6) | 21.4 (18.5) |
| AVR or Coach | 187 | 48.6 | 35.6 (22.3) | 20.7 (16.0) | 114 | 53.3 | 40.2 (24.7) | 21.5 (19.7) |
SD = standard deviation; AVR = automated voice response.
Sample
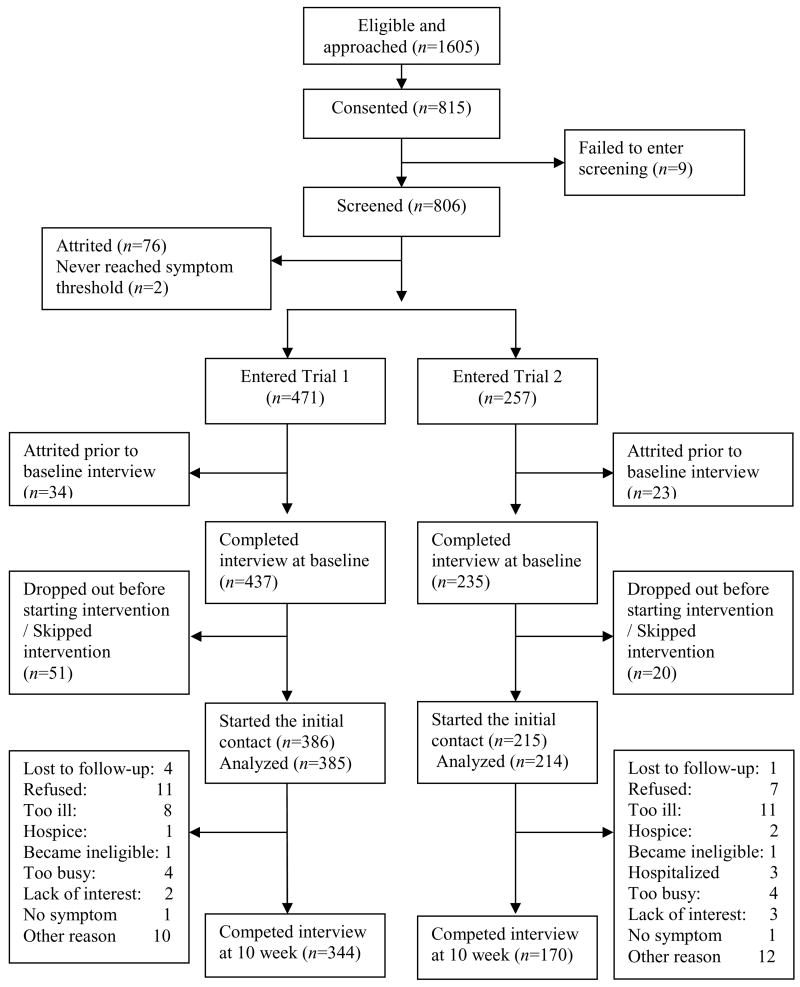
All locations participating in the accrual of subjects for these trials approved consent forms designed to protect and inform eligible patients wishing to participate in one of these two trials. This research was approved and accrual occurred from two comprehensive cancer centers, one community cancer oncology program, and six hospital affiliated community oncology centers. Nurses in the clinical trials offices implemented the recruitment protocol. Eligible patients met the following requirements: 1) 21 years of age or older, 2) a diagnosis of a solid tumor cancer or non-Hodgkin’s lymphoma, 3) undergoing a course of chemotherapy, 4) speak and read English, and 5) have a touchtone telephone. If patients had a family member (a caregiver) who was assisting them, willing to participate, and who signed a consent form, then they were included. To assure that all patients met minimum symptom severity criteria and could benefit from the intervention, they were screened for symptom severity using M. D. Anderson symptom inventory [3]. Twice weekly calls were made for up to six weeks. Since clinical guidelines suggest monitoring for symptom rated between 1and 3 on a 0–10 scale [15], patients scoring 2 or higher on severity of at least one symptom (range 0–10) at any contact were entered into trial. Patients never reaching a 2 were sent a letter thanking them for participation. Patients without a caregiver were enrolled in a second trial comparing the nurse administered intervention with the AVR system. Patients with caregivers who scored a 2 or higher on both pain and fatigue or a 3 or higher on pain or fatigue were entered into the trial 2 where the nurse administered arm was compared with the coach. Figure 1 summarizes the flow of patients beginning with eligibility and through the arms of both trials including attrition as it occurred after the initial contact point. The data for this analysis were taken from the six intervention contacts covering eight weeks. Only data collected from patients in both trials were used. In trial 1, in addition to the interventions delivered to patients in both arms, interventions were also delivered to caregivers in both arms. No data collected from caregivers were used as proxy for patient data in the analysis.
Figure 1.
Flowchart of patient accrual and retention for the two trials.
The Two Trials
Each trial compared separately the impact of a six contact eight-week intervention to lower the severity of symptoms for cancer patients undergoing chemotherapy. In Trial 1, the nurse arm was compared with an AVR system. For this system a pleasant female voice read each of the 16 symptoms and patients reported their severity using the telephone keypad. In the second trial the nurse intervention was compared with an intervention delivered by a non-nurse coach who monitored all symptoms. In both the coach and the AVR arms, when patients reported symptoms at a severity of 4 or higher they were referred to the Symptom Management Guide (SMG). This guide, written at an 8th grade level covered the causes, management strategies, when to call the oncologist and other sources for information. Each symptom was tabbed so as to be easily located. In each section of the SMG, causes of each symptom were described and strategies for managing the symptom presented.
Patients assigned to the nurse arms received cognitive behavioral interventions from cancer nurses using scripted protocols. Up to four strategies for each symptom supplemented with references to the SMG could be delivered. These strategies followed cognitive behavioral theory, where patients are assessed frequently, and interventions altered to assure that patients begin to acquire self-efficacious behaviors that can be sustained following the end of the formal intervention [16–18].
At each subsequent contact, assigned strategies were evaluated. For the nurse arms patients were asked for each symptom above threshold if the strategies proposed at the prior contact were tried, and, if tried, were they helpful in managing that symptom. If a strategy was not tried, was tried but not helpful, then the strategy was altered and a new one proposed. Successful strategies were retained and reinforced.
For patients assigned to the coach or AVR arms, when symptoms reached a threshold of 4 or higher, patients were referred to appropriate sections of the SMG. At subsequent contacts, patients were asked if they had tried the assigned interventions, and if they did, to rate how successful the intervention was in reducing the severity of that symptom. Once evaluations of the impact of interventions on symptoms reported above threshold at the last contact were completed, then patients were asked about the severity of each of the 16 symptoms at the current time. In both of these arms, data were entered into computer assisted documentation programs for storage and analysis.
Measures
All arms of the two trials employed identical assessment at intake interview, intervention contacts, and at ten week (post-intervention) interview. Demographic data were collected during intake interview. Site and stage of cancer were obtained from the patients’ medical records.
At each intervention contact, an 11-point scale was used to rate the severity of 16 symptoms. Patients were asked to rate the average severity of each symptom over the last week on a scale of 0 not present to 10 worst possible. For each symptom present in the past week (severity score >0), patients rated on an 11 point scale the extent to which that symptom interfered with their enjoyment of life, social relationships, general daily activities, emotions, and sleep. Four of the interference dimensions (general activity, emotions, enjoyment of life and social relationships) were taken from Brief Pain Inventory [1], Brief Fatigue Inventory [2], and M. D. Anderson Symptom Inventory [3]. The other two interference items used by Cleeland and colleagues [1–3], walking ability and normal work, were not assessed during intervention contacts to decrease the respondent burden. Interference with sleep was added as this was thought to be an important dimension based on reports from the literature. [19–21]
Development of the Interference Scale
Four of the five interference items were highly and positively correlated. Sleep was not correlated with the other four items and, after conducting an exploratory factor analysis, appeared to be tapping a separate dimension. Therefore sleep was removed from the total interference score. The remaining four items were submitted to exploratory factor analyses for each of the 16 symptoms. For each symptom the factor with the largest Eigenvalue explained between 70% and 90% of the total variance, and all items had approximately equal (high) loadings on this factor. Since loadings did not differ substantially in magnitude, a summed score across four items was used to measure each symptom’s interference with life.
Data Analysis
The optimal cut-points to separate mild from moderate and moderate from severe based on the interference scale at the initial contact were established for the both trials. [8] This analysis extends prior work by seeking to determine if the mean interference scores for the mild, moderate and severe categories remain consistently different across six contacts for each of the 16 symptoms. Descriptive statistics for symptom severity by trial and by various patient characteristics were obtained to ensure that data in two trials are comparable and can be combined for this analysis. To assess the longitudinal association between interference and the categories of severity established at the initial contact, the linear mixed effects (LME) models [22] with autoregressive covariance structure were employed. The LME model is a natural generalization of the classical analysis of repeated measures that allow for data missing at random and structured covariance matrices. By employing LME models in this analysis, we were able to include data from patients who completed at least two intervention contacts. Sixteen models (one for each symptom) were fit. The outcome in each model was the interference score (sum of 4 items as described above). The explanatory variables were the symptom severity categories (mild, moderate, severe), time (contact number), time by symptom severity category interaction, and the covariates used when the cut-points were developed [8]. Symptom severity category and the number of other symptoms were time varying covariates. Even though symptom-specific interference items were assessed (e.g., how much fatigue interfered with general activity), we adjusted for the possible effect of the number of other symptoms a patient was experiencing. Least square (LS) means [23] by the interaction terms were calculated, and differences by symptom severity category (mild, moderate, severe) tested at each of the six contacts. The LS means are also referred to as means of the outcome variable that are adjusted for other variables in the model or adjusted means. The LME models were fit using PROC MIXED in SAS version 9.1 [24].
Results
Figure 1 displays the number of patients who were approached, consented, and completed each stage of the two trials. Based on the information in their medical records, 1605 cancer patients were eligible for the trials and were approached by nurse recruiters, 815 signed informed consent forms, 806 were screened simultaneously for the two trials. Reasons patients declined participation included: being too busy or too stressed by their diagnosis and treatment to assume another obligation; identified but then missed by recruiters at subsequent visits; or unwilling to remain following infusions to have the study explained to them. Reasons for attrition during the intervention contacts are summarized on the flowchart. Refusing intervention (20.7%), becoming too ill (21.8%), and being too busy (9.2%) are main reasons for attrition after the initial contact. Those who completed screening and entered the trial had the mean summed mean severity of 33.4 for the 13 symptoms in M.D. Anderson symptom inventory [3] (standard deviation 18.8) at screening, and they did not differ from those who completed screening but dropped out prior to baseline interview (mean severity of 37.4 (standard deviation 24.4)). Two hundred fifty seven patients had a consented caregiver and entered trial 1, while 471 entered trial 2. Nine patients failed to enter screening, two never reached threshold in screening, and 76 patients dropped out prior to entry. Another 57 patients dropped out prior to baseline interview, leaving 234 patients completing the baseline interview and randomized in trial 1 and 437 patients randomized in trial 2.
Table 1 presents the sociodemographic and clinical characteristics of the two samples along with symptom severity at the intake and 10-week (post-intervention) interviews for each of the selected characteristics. The distributions of patient and disease characteristics and the mean of symptom severity confirm the similarities of the two samples and support our decision to combine them for the purposes of the investigation of stability of cut-points over time.
The eigenvalues and the loadings of the four interference items on the first factor are listed in Table 2, supporting our decision to use a single summed score to reflect each symptom’s interference with daily life. Table 3 presents the least square (LS) means of interference scores and their standard errors for mild, moderate, and severe categories of each of the 16 symptoms across six contacts. Tests comparing the adjusted mean interference scores among mild, moderate, and severe categories resulted in rejecting the null hypothesis of equality of means with most P-values being less than 0.001 (Table 3).
Table 2.
Eigenvalues (λ), Percent of Variation Explained by the First Factor, and Factor Loadings for the Four Interference Items for Each Symptom at Contact 1
| Alopecia λ = 3.06 (76 %) | Anxiety λ = 3.14 (78 %) | Poor Appetite λ = 3.01 (75 %) | Constipation λ = 3.14 (78 %) | |
|---|---|---|---|---|
| Enjoyment of life | 0.9156 | 0.9144 | 0.8603 | 0.9059 |
| Relationship with others | 0.8707 | 0.8688 | 0.8501 | 0.8111 |
| General daily activities | 0.8777 | 0.8664 | 0.8767 | 0.9175 |
| Emotions | 0.8315 | 0.8932 | 0.8824 | 0.9037 |
|
| ||||
| Cough λ = 3.14 (78 %) | Depression λ = 3.26 (81 %) | Diarrhea λ = 3.12 (78 %) | Dry Mouth λ = 3.00 (75 %) | |
|
| ||||
| Enjoyment of life | 0.9040 | 0.9333 | 0.9137 | 0.8770 |
| Relationship with others | 0.8341 | 0.8703 | 0.8074 | 0.8436 |
| General daily activities | 0.9051 | 0.9087 | 0.9070 | 0.8761 |
| Emotions | 0.9016 | 0.8979 | 0.9022 | 0.8685 |
|
| ||||
| Dyspnea λ = 3.04 (76 %) | Fatigue λ = 3.15 (79 %) | Sleep Disturbance λ = 3.23 (81 %) | Nausea/Vomiting λ = 3.32 (83 %) | |
|
| ||||
| Enjoyment of life | 0.9066 | 0.9108 | 0.9102 | 0.9268 |
| Relationship with others | 0.8135 | 0.8683 | 0.8698 | 0.8662 |
| General daily activities | 0.8692 | 0.8912 | 0.9074 | 0.9328 |
| Emotions | 0.8935 | 0.8790 | 0.9077 | 0.9170 |
|
| ||||
| Pain λ = 3.29 (82 %) | Peripheral Neuropathy λ = 2.99 (75 %) | Difficulty Remembering λ = 3.23 (81 %) | Weakness λ = 3.11 (78 %) | |
|
| ||||
| Enjoyment of life | 0.9187 | 0.9102 | 0.9147 | 0.9151 |
| Relationship with others | 0.8872 | 0.7676 | 0.8877 | 0.8404 |
| General daily activities | 0.9152 | 0.8906 | 0.8982 | 0.8834 |
| Emotions | 0.9064 | 0.8823 | 0.8946 | 0.8897 |
Table 3.
Estimated Mean Interference Score Across Six Intervention Contacts by Categories of Symptom Severity (Mild, Moderate, Severe) and P-Values for Tests of the Differences in Interference Scores for Mild, Moderate and Severe Categories at Each Contact
| Symptom | Level | Contact 1 |
Contact 2 |
Contact 3 |
Contact 4 |
Contact 5 |
Contact 6 |
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SD) | n | Mean (SD) | N | Mean (SD) | n | Mean (SD) | n | Mean (SD) | n | Mean (SD) | ||
| Alopecia | Mild 1 | 54 | 1.9 (0.8) | 41 | 2.4 (0.9) | 31 | 1.9 (1.0) | 33 | 1.7 (1.0) | 23 | 1.6 (1.2) | 19 | 2.6 (1.2) |
| Moderate 2–5 | 101 | 5.2 (0.6) | 79 | 4.4 (0.6) | 65 | 4.2 (0.7) | 59 | 3.0 (0.7) | 61 | 3.1 (0.7) | 61 | 3.5 (0.7) | |
| Severe 6–10 | 112 | 8.3 (0.6) | 40 | 8.6 (0.9) | 30 | 7.7 (1.0) | 19 | 8.2 (1.3) | 18 | 8.3 (1.3) | 16 | 11.6 (1.5) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | 0.0004 | 0.0002 | 0.0005 | <0.0001 | |||||||
|
| |||||||||||||
| Anxiety | Mild 1–3 | 172 | 7.2 (0.5) | 127 | 6.5 (0.5) | 100 | 5.5 (0.6) | 116 | 6.5 (0.6) | 90 | 6.3 (0.6) | 96 | 5.3 (0.6) |
| Moderate 4–5 | 97 | 13.1 (0.6) | 52 | 12.1 (0.8) | 48 | 12.1 (0.8) | 36 | 11.7 (1.0) | 45 | 11.2 (0.9) | 28 | 11.9 (1.1) | |
| Severe 6–10 | 69 | 21.6 (0.7) | 34 | 19.6 (1.0) | 36 | 20.2 (1.0) | 21 | 20.0 (1.3) | 23 | 22.1 (1.3) | 24 | 18.8 (1.2) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Poor Appetite | Mild 1–3 | 119 | 3.6 (0.6) | 111 | 3.5 (0.6) | 90 | 3.5 (6.4) | 94 | 3.8 (0.6) | 74 | 3.5 (0.7) | 75 | 3.5 (0.7) |
| Moderate 4–6 | 116 | 8.4 (0.6) | 97 | 8.7 (0.6) | 64 | 8.8 (0.7) | 65 | 6.6 (0.7) | 59 | 7.2 (0.8) | 46 | 7.6 (0.9) | |
| Severe 7–10 | 71 | 14.8 (0.7) | 20 | 12.8 (1.3) | 33 | 13.4 (1.0) | 17 | 12.1 (1.4) | 25 | 14.0 (1.2) | 21 | 12.4 (1.3) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Constipation | Mild 1–3 | 106 | 2.8 (0.6) | 60 | 2.8 (0.7) | 55 | 3.0 (0.8) | 48 | 3.1 (0.8) | 46 | 2.3 (0.8) | 44 | 3.0 (0.8) |
| Moderate 4–6 | 67 | 6.3 (0.7) | 41 | 7.7 (0.9) | 42 | 6.7 (0.9) | 27 | 6.4 (1.0) | 24 | 5.9 (1.1) | 23 | 9.3 (1.2) | |
| Severe 7–10 | 43 | 14.7 (0.9) | 13 | 15.0 (1.5) | 16 | 10.9 (1.4) | 17 | 14.8 (1.4) | 12 | 16.0 (1.6) | 16 | 15.4 (1.4) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Cough | Mild 1–2 | 107 | 1.1 (0.5) | 74 | 1.5 (0.6) | 55 | 2.2 (0.6) | 55 | 1.9 (0.6) | 48 | 2.4 (0.7) | 50 | 2.4 (0.7) |
| Moderate 3–4 | 60 | 4.4 (0.6) | 44 | 4.9 (0.7) | 33 | 4.5 (0.8) | 41 | 4.6 (0.7) | 36 | 4.6 (0.8) | 32 | 4.6 (0.8) | |
| Severe 5–10 | 50 | 10.5 (0.7) | 34 | 10.9 (0.8) | 22 | 9.3 (1.0) | 10 | 14.8 (1.4) | 16 | 10.9 (1.2) | 20 | 10.1 (1.1) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Depression | Mild 1 | 46 | 4.1 (0.9) | 43 | 3.6 (0.9) | 25 | 6.9 (1.2) | 18 | 6.4 (1.4) | 18 | 5.3 (1.4) | 29 | 6.2 (1.1) |
| Moderate 2–3 | 106 | 8.2 (0.6) | 62 | 9.0 (0.8) | 66 | 7.1 (0.7) | 61 | 7.2 (0.8) | 48 | 7.9 (0.9) | 40 | 9.2 (0.9) | |
| Severe 4–10 | 107 | 17.3 (0.6) | 59 | 15.7 (0.8) | 46 | 16.6 (0.9) | 45 | 15.4 (0.9) | 42 | 16.3 (0.9) | 42 | 16.1 (1.0) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Diarrhea | Mild 1–3 | 78 | 2.9 (0.7) | 63 | 2.5 (0.7) | 57 | 3.1 (0.8) | 53 | 3.9 (0.8) | 38 | 1.6 (0.9) | 39 | 2.4 (0.9) |
| Moderate 4–5 | 32 | 7.6 (1.0) | 25 | 5.5 (1.1) | 17 | 6.7 (1.4) | 17 | 4.8 (1.3) | 26 | 8.2 (1.1) | 21 | 8.7 (1.2) | |
| Severe 6–10 | 28 | 18.2 (1.1) | 11 | 13.5 (1.7) | 9 | 16.5 (1.8) | 11 | 15.9 (1.7) | 7 | 12.0 (2.1) | 13 | 16.4 (1.6) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Dry Mouth | Mild 1–4 | 192 | 1.1 (0.3) | 165 | 1.3 (0.3) | 133 | 1.6 (0.3) | 137 | 1.6 (0.3) | 121 | 1.6 (0.3) | 114 | 1.3 (0.4) |
| Moderate 5–6 | 98 | 4.5 (0.4) | 54 | 3.8 (0.5) | 50 | 4.7 (0.5) | 34 | 4.6 (0.6) | 33 | 4.2 (0.7) | 44 | 3.6 (0.6) | |
| Severe 9–10 | 14 | 11.8 (1.0) | 7 | 14.6 (1.4) | 5 | 12.9 (1.7) | 6 | 13.6 (1.5) | 6 | 14.0 (1.6) | 1 | 19.6 (3.9) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Dyspnea | Mild 1–2 | 68 | 3.2 (0.7) | 48 | 2.8 (0.8) | 58 | 3.7 (0.7) | 48 | 4.0 (0.8) | 41 | 4.0 (0.9) | 41 | 4.5 (0.9) |
| Moderate 3–6 | 123 | 9.7 (0.5) | 89 | 8.7 (0.6) | 77 | 8.7 (0.7) | 74 | 8.5 (0.7) | 63 | 9.4 (0.7) | 71 | 7.4 (0.7) | |
| Severe 7–10 | 24 | 22.7 (1.2) | 11 | 15.7 (1.6) | 7 | 18.6 (2.1) | 6 | 16.0 (2.4) | 14 | 18.9 (1.5) | 11 | 17.2 (1.7) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Fatigue | Mild 1 | 34 | 3.8 (1.1) | 49 | 5.1 (0.9) | 44 | 4.6 (0.9) | 39 | 4.2 (1.0) | 32 | 4.7 (1.0) | 39 | 4.6 (1.0) |
| Moderate 2–4 | 227 | 8.5 (0.4) | 208 | 7.2 (0.4) | 212 | 7.2 (0.4) | 198 | 7.5 (0.4) | 192 | 7.6 (0.4) | 209 | 6.7 (0.4) | |
| Severe 5–10 | 268 | 16.6 (0.4) | 149 | 15.0 (0.5) | 132 | 15.2 (0.5) | 123 | 15.1 (0.5) | 105 | 14.8 (0.6) | 113 | 15.5 (0.6) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Sleep Disturbance | Mild 1–3 | 124 | 3.6 (0.5) | 129 | 3.7 (0.5) | 117 | 3.8 (0.5) | 103 | 3.8 (0.5) | 100 | 3.9 (0.5) | 79 | 3.7 (0.6) |
| Moderate 4–6 | 126 | 8.4 (0.5) | 78 | 8.5 (0.6) | 67 | 7.9 (0.6) | 70 | 8.1 (0.6) | 55 | 7.6 (0.7) | 57 | 7.5 (0.7) | |
| Severe 7–10 | 83 | 14.1 (0.6) | 29 | 11.6 (0.9) | 23 | 12.7 (1.0) | 14 | 12.3 (1.3) | 24 | 12.5 (1.0) | 27 | 13.4 (1.0) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Nausea/Vomiting | Mild 1–3 | 98 | 4.9 (0.7) | 74 | 4.8 (0.7) | 79 | 5.1 (0.7) | 66 | 4.9 (0.8) | 49 | 4.4 (0.9) | 44 | 5.1 (0.9) |
| Moderate 4–6 | 51 | 13.5 (0.9) | 40 | 10.0 (1.0) | 28 | 12.5 (1.2) | 23 | 12.4 (1.3) | 33 | 11.7 (1.1) | 33 | 10.8 (1.1) | |
| Severe 7–10 | 27 | 24.1 (1.2) | 14 | 20.9 (1.7) | 8 | 10.8 (2.2) | 15 | 25.9 (1.6) | 11 | 21.9 (1.9) | 4 | 21.8 (3.1) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Pain | Mild 1 | 28 | 3.0 (1.3) | 50 | 2.3 (1.0) | 36 | 5.1 (1.1) | 32 | 4.3 (1.2) | 34 | 4.7 (1.2) | 20 | 5.9 (1.5) |
| Moderate 2–4 | 119 | 8.7 (0.6) | 97 | 7.4 (0.7) | 91 | 8.5 (0.7) | 95 | 8.1 (0.7) | 87 | 8.5 (0.7) | 94 | 8.3 (0.7) | |
| Severe 5–10 | 108 | 18.7 (0.7) | 53 | 14.8 (0.9) | 47 | 16.7 (1.0) | 48 | 16.7 (1.0) | 44 | 13.5 (1.0) | 51 | 16.7 (1.0) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Peripheral Neuropathy | Mild 1–3 | 131 | 1.5 (0.4) | 114 | 2.4 (0.4) | 109 | 2.5 (0.4) | 123 | 2.5 (0.4) | 105 | 2.4 (0.4) | 111 | 2.7 (0.4) |
| Moderate 4–7 | 82 | 6.6 (0.5) | 62 | 4.6 (0.6) | 64 | 5.4 (0.6) | 58 | 6.1 (0.6) | 70 | 6.1 (0.5) | 64 | 6.5 (0.6) | |
| Severe 5–10 | 22 | 13.4 (1.0) | 8 | 14.3 (1.5) | 13 | 9.6 (1.2) | 10 | 12.3 (1.4) | 8 | 20.5 (1.6) | 10 | 20.2 (1.5) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Difficulty Remembering | Mild 1 | 38 | 1.5 (0.8) | 49 | 1.7 (0.7) | 53 | 1.7 (0.6) | 43 | 1.6 (0.7) | 35 | 2.1 (0.7) | 31 | 1.6 (0.8) |
| Moderate 2–4 | 149 | 3.4 (0.4) | 93 | 3.8 (0.5) | 87 | 3.7 (0.5) | 76 | 4.3 (0.5) | 63 | 5.0 (0.6) | 78 | 4.0 (0.5) | |
| Severe 5–10 | 69 | 11.0 (0.6) | 24 | 11.0 (0.9) | 25 | 13.4 (0.9) | 15 | 11.5 (1.1) | 32 | 11.0 (0.8) | 23 | 12.9 (1.0) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
|
| |||||||||||||
| Weakness | Mild 1–2 | 92 | 4.5 (0.6) | 70 | 3.6 (0.7) | 60 | 4.6 (0.7) | 74 | 4.2 (0.7) | 64 | 4.3 (0.7) | 66 | 5.1 (0.7) |
| Moderate 3–4 | 114 | 7.1 (0.5) | 96 | 8.0 (0.6) | 79 | 9.4 (0.6) | 82 | 8.5 (0.6) | 73 | 7.7 (0.7) | 64 | 7.5 (0.7) | |
| Severe 5–10 | 146 | 16.1 (0.5) | 74 | 14.8 (0.7) | 65 | 14.3 (0.7) | 61 | 13.4 (0.7) | 64 | 15.6 (0.7) | 59 | 14.9 (0.8) | |
|
| |||||||||||||
| P-value | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | <0.0001 | |||||||
SD = standard deviation.
Further, the examination of the magnitude of the differences between moderate and mild and severe and moderate categories shows large differences except for a few symptom categories (e.g., mild versus moderate depression at contacts 3 and 4 and moderate versus severe vomiting at contact 3), where smaller magnitudes of difference could have occurred by chance alone or could be attributed to small numbers of cases (listed in Table 3).
At each contact the number of cases decline over time for two reasons: 1) patients received interventions to manage their symptoms and, therefore, symptoms declined in severity or resolve; and 2) not all patients completed all contacts: some patients were too sick, some skipped contacts yet completed later assessments, and some dropped out of the trials. Another reason explaining declines in symptom counts for alopecia is that once patients lost hair, they did not report this symptom the following week as they were not losing any more hair. So, for this symptom, the fact that patients did not report a symptom does not mean their hair re-grew.
While the absolute numbers reporting each symptom at each contact decline, when patients who report each symptom (severity >0) are considered, the proportions of the patients reporting each severity level do not differ much across six contacts. The largest differences are among patients reporting severe levels of each symptom. The decline in counts for the severe category is greatest between the first and second contact and then the counts decline slowly from contact 3 through 6. Table 4 lists the counts for cases falling into mild, moderate and severe categories at contact 1, transitions to mild, moderate and severe categories at contact 2, and the number and percent not reporting a symptom, skipping a contact, or stopping an intervention between the first and second contact. Note that the counts for mild, moderate and severe categories at contact 2 listed in Table 3 are larger than those in Table 4 because counts in Table 3 include all patients who reported a symptom at a given contact, while Table 4 tracks patients who had the symptom present at contact 1. Around 10% of patients who reported their symptoms at contact 1 skipped contact 2 and were retained during the rest of contacts. Percent of symptom cases lost due to patients stopping the intervention are very small in the mild category and are mostly less than 10% even in the severe category. Thus the association between the interference and the severity level appears not to be affected by attrition.
Table 4.
Numbers and Percents of Patients By Levels of Severity and Attrition Between Contacts 1 and 2
| Symptom | Severity Category at Contact 1 | Number of Cases | Severity Level at Contact 2 |
|||||
|---|---|---|---|---|---|---|---|---|
| Mild n (%) | Moderate n (%) | Severe n (%) | Did Not Report Symptoman (%) | Skipped Contact n (%) | Stopped Interventions n (%) | |||
| Alopecia | Mild | 54 | 12 (22.2) | 6 (11.1) | 3 (5.6) | 25 (46.3) | 7 (13.0) | 1 (1.8) |
| Moderate | 101 | 10 (9.9) | 34 (33.7) | 9 (8.9) | 35 (34.6) | 13 (12.9) | 0 (0.0) | |
| Severe | 112 | 6 (5.4) | 30 (26.8) | 25 (22.3) | 35 (31.2) | 8 (7.1) | 8 (7.1) | |
|
| ||||||||
| Anxiety | Mild | 172 | 57 (33.1) | 11 (6.4) | 6 (3.5) | 73 (42.4) | 15 (8.7) | 10 (5.8) |
| Moderate | 97 | 36 (37.1) | 16 (16.5) | 12 (12.4) | 24 (24.7) | 4 (4.1) | 5 (5.1) | |
| Severe | 69 | 12 (17.4) | 14 (20.3) | 13 (18.8) | 11 (15.9) | 11 (15.9) | 8 (11.6) | |
|
| ||||||||
| Poor Appetite | Mild | 119 | 36 (30.3) | 22 (18.5) | 1 (0.8) | 45 (37.8) | 8 (6.7) | 7 (5.9) |
| Moderate | 116 | 33 (28.5) | 36 (31.0) | 6 (5.2) | 27 (23.3) | 8 (6.9) | 6 (5.2) | |
| Severe | 71 | 9 (12.7) | 23 (32.4) | 9 (12.7) | 15 (21.1) | 8 (11.3) | 7 (9.9) | |
|
| ||||||||
| Constipation | Mild | 106 | 26 (24.5) | 5 (4.7) | 1 (0.9) | 62 (58.5) | 7 (6.6) | 5 (4.7) |
| Moderate | 67 | 15 (22.4) | 13 (19.4) | 2 (3.0) | 25 (37.3) | 10 (14.9) | 2 (3.0) | |
| Severe | 43 | 4 (9.3) | 9 (20.9) | 7 (16.3) | 16 (37.2) | 4 (9.3) | 3 (7.0) | |
|
| ||||||||
| Cough | Mild | 107 | 30 (28.0) | 8 (7.5) | 5 (4.7) | 46 (43.0) | 9 (8.4) | 8 (7.5) |
| Moderate | 60 | 10 (16.7) | 16 (26.7) | 9 (15.0) | 17 (28.3) | 4 (6.7) | 4 (6.7) | |
| Severe | 50 | 5 (10.0) | 9 (18.0) | 18 (36.0) | 12 (24.0) | 4 (8.0) | 2 (4.0) | |
|
| ||||||||
| Depression | Mild | 46 | 6 (13.0) | 7 (15.2) | 0 (0.0) | 25 (54.3) | 7 (15.2) | 1 (2.2) |
| Moderate | 106 | 16 (15.1) | 27 (25.5) | 9 (8.5) | 39 (36.8) | 8 (7.5) | 7 (6.6) | |
| Severe | 107 | 8 (7.5) | 12 (11.2) | 34 (31.8) | 31 (29.0) | 10 (9.3) | 11 (10.3) | |
|
| ||||||||
| Diarrhea | Mild | 78 | 27 (34.6) | 6 (7.7) | 1 (1.3) | 36 (46.1) | 5 (6.4) | 3 (3.8) |
| Moderate | 32 | 11 (34.4) | 4 (12.5) | 2 (6.2) | 11 (34.4) | 3 (9.4) | 1 (3.1) | |
| Severe | 28 | 4 (14.3) | 4 (14.3) | 1 (3.6) | 14 (50.0) | 3 (10.7) | 2 (7.1) | |
|
| ||||||||
| Dry Mouth | Mild | 192 | 86 (44.8) | 14 (7.3) | 1 (0.5) | 64 (33.3) | 16 (8.3) | 11 (5.7) |
| Moderate | 98 | 31 (31.6) | 31 (31.6) | 1 (1.0) | 16 (16.3) | 12 (12.2) | 7 (7.1) | |
| Severe | 14 | 4 (28.6) | 3 (21.4) | 4 (28.6) | 0 (0.0) | 1 (7.1) | 2 (14.3) | |
|
| ||||||||
| Dyspnea | Mild | 68 | 13 (19.1) | 17 (25.0) | 0 (0.0) | 33 (48.5) | 2 (2.9) | 3 (4.4) |
| Moderate | 123 | 18 (14.6) | 48 (39.0) | 5 (4.0) | 34 (27.6) | 13 (10.6) | 5 (4.1) | |
| Severe | 24 | 2 (8.3) | 8 (33.3) | 5 (20.8) | 5 (20.8) | 2 (8.3) | 2 (8.3) | |
|
| ||||||||
| Fatigue | Mild | 34 | 10 (29.4) | 11 (32.3) | 2 (5.9) | 6 (17.6) | 3 (8.8) | 2 (5.9) |
| Moderate | 227 | 27 (11.9) | 98 (43.2) | 32 (14.1) | 36 (15.9) | 21 (9.2) | 13 (5.7) | |
| Severe | 268 | 8 (3.0) | 82 (30.6) | 107 (39.9) | 25 (9.3) | 26 (9.7) | 19 (7.1) | |
|
| ||||||||
| Sleep Disturbance | Mild | 124 | 45 (36.3) | 12 (9.7) | 3 (2.4) | 49 (39.5) | 9 (7.3) | 6 (4.8) |
| Moderate | 126 | 45 (35.7) | 26 (20.6) | 9 (7.1) | 34 (27.0) | 9 (7.1) | 3 (2.4) | |
| Severe | 83 | 13 (15.7) | 22 (26.5) | 14 (16.9) | 15 (18.1) | 10 (12.0) | 8 (9.6) | |
|
| ||||||||
| Nausea/Vomiting | Mild | 98 | 29 (29.6) | 12 (12.2) | 1 (1.0) | 44 (44.9) | 7 (7.1) | 4 (4.1) |
| Moderate | 51 | 11 (21.6) | 9 (17.6) | 4 (7.8) | 20 (39.2) | 4 (7.8) | 3 (5.9) | |
| Severe | 27 | 6 (22.2) | 3 (11.1) | 4 (14.8) | 7 (25.9) | 4 (14.8) | 3 (11.1) | |
|
| ||||||||
| Pain | Mild | 28 | 8 (28.6) | 4 (14.3) | 0 (0.0) | 13 (46.4) | 2 (7.1) | 1 (3.6) |
| Moderate | 119 | 15 (12.6) | 41 (34.5) | 11 (9.2) | 32 (26.9) | 15 (12.6) | 5 (4.2) | |
| Severe | 108 | 10 (9.3) | 26 (24.1) | 32 (29.6) | 15 (13.9) | 13 (12.0) | 12 (11.1) | |
|
| ||||||||
| Peripheral Neuropathy | Mild | 131 | 66 (50.4) | 11 (8.4) | 3 (2.3) | 32 (24.4) | 12 (9.2) | 7 (5.3) |
| Moderate | 82 | 22 (26.8) | 38 (46.3) | 1 (1.2) | 6 (7.3) | 10 (12.2) | 5 (6.1) | |
| Severe | 22 | 1 (4.5) | 8 (36.4) | 4 (18.2) | 1 (4.5) | 5 (22.7) | 2 (9.1) | |
|
| ||||||||
| Difficulty Remembering | Mild | 38 | 10 (26.3) | 5 (13.2) | 0 (0.0) | 19 (50.0) | 4 (10.5) | 0 (0.0) |
| Moderate | 149 | 23 (15.4) | 52 (34.9) | 7 (4.7) | 44 (29.5) | 16 (10.7) | 7 (4.7) | |
| Severe | 69 | 5 (7.3) | 21 (30.4) | 15 (21.7) | 10 (14.5) | 8 (11.6) | 8 (11.6) | |
|
| ||||||||
| Weakness | Mild | 92 | 24 (26.1) | 13 (14.1) | 3 (3.3) | 39 (43.4) | 11 (12.0) | 2 (2.2) |
| Moderate | 114 | 22 (19.3) | 36 (31.6) | 13 (11.4) | 28 (24.6) | 9 (7.9) | 6 (5.3) | |
| Severe | 146 | 10 (6.8) | 37 (25.3) | 46 (31.5) | 24 (16.4) | 15 (10.3) | 13 (8.9) | |
Patient not reporting a symptom means that patient did not report a symptom in the past 7 days at contact 2.
Discussion
This research builds upon and extends our earlier work [8]. In that paper, we identified interference-based severity cut-points for 16 cancer-related symptoms prior to the introduction of intervention strategies designed to lower the severity of each symptom. Severity cut-points were based on the largest differences in interference scores between consecutive levels of severity. The findings indicated rather clearly that moderate levels of severity could be differentiated from mild and severe levels from moderate based on differences in the ways patients assigned interference scores to each symptom. The interference scores were based on a psychometrically sound scale consisting of four items.
In this research, we sought to determine if the interference scores were consistently different over time for the three severity categories defined by the cut-points. The data presented here support that contention. First, the hypotheses of equality among adjusted means of interference scores for mild, moderate, and severe cut-points were rejected for the 16 symptoms at all contacts. While some variations occurred, they probably occurred due to small cell size or by chance. Thus, the cut-points consistently differentiate the interference scores over time as patients are receiving interventions to manage symptoms.
Most importantly, the evidence provided here indicates a strong and sustained relationship between interference scores and their corresponding symptom severity categories. Thus, we observed that patients retained consistently different interference scores corresponding to mild, moderate, and severe categories at each observation. Patients’ symptom severities were lowered over time, and the interference scores were, for the most part, significantly different at each observation; therefore, it appears that patients adjusted their interference scores to the level of symptom severity they reported. Together, these data offer evidence against response shifts in interference over the course of the six contacts. Thus, we conclude that patients appear able to associate interference scores with severity categories and to modify those scores as symptoms improve, resolve, or worsen.
If the consistent differences among mild, moderate and severe categories are accepted, then we can begin to argue that these interference-based severity cut-points that are tailored to each symptom can be used to develop response categories to measure the impact of intervention strategies and to classify patients as responders, or non-responders. Patients who move from severe to moderate or moderate to mild from symptom onset to the last contact completed can be classified as responders, whereas those who remain at moderate or severe level or deteriorate from moderate to severe can be classified as non-responders. These response categories are anchored on differences in symptom interference rather than on absolute or relative decline in symptom severity. Other approaches to defining response such as a decrease in severity of 30% or more [10] classify as responders include patients who improved from 3 to 2 as well as those whose severity declines from 9 to 6. Using mild, moderate and severe cut-points tested in this paper, most symptoms that move from severe to mild achieve a close to 50% reduction and would represent clinically important differences that oncologists would view as meaningful. Reductions from severe to moderate are of note, and meet the 30% reduction in most cases and can be of clinical importance as well. Reductions from moderate to mild, for the most part, may be less important clinically. For example, reduction in fatigue from 3 (moderate) to 1 (mild) may not be considered important by oncologists, since fatigue of severity 3 would not be considered serious enough to alter the treatment dosing or schedule. However, from a quality of life perspective, a reduction of 3 to 1 on fatigue severity corresponds to a decrease in the limitations of daily activities caused by fatigue and substantial improvement in physical function [15]. From a clinical perspective these categories for selected symptoms could be compared with the National Cancer Institute’s Common Terminology Criteria for Adverse Events. As patient reported outcomes become part of the clinical care system, it will be important to determine how our classification system and reported changes correspond to these existing clinically defined categories for assessing symptoms as levels of toxicity. Currently, approaches to patient reported toxicities are underway. Clinical utility will come as interference-based severity measures are calibrated to existing toxicity grading for symptoms [25].
Several limitations of this research should be noted. First, the consent rate out of those approached was around 50%, which may limit the ability to generalize findings. Second, most patients experienced multiple symptoms yet they were asked to attribute interference to each symptom. While analytically we adjusted for that by including the number of symptoms as a covariate in the statistical models, such adjustment may not fully account for how much of the interference is attributed to, for example, pain versus fatigue.
In conclusion, this research demonstrates that patients are able to differentiate among interference scores defining mild, moderate, and severe levels of symptoms over time. As a result, these categories may be used to define symptom specific anchor-based measures of response to symptom management strategies.
Acknowledgments
This research was supported by National Cancer Institute Grant # RO1 CA030724 (Automated Telephone Monitoring for Symptom Management, C.W. Given, PI, B. Given, Co-PI) and National Cancer Institute Grant #RO1 CA79280 (Family Home Care for Cancer: A Community Based Model, B. Given, PI, C.W. Given Co-PI).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Cleeland CS. Measurement of pain by subjective report. In: Chapman CR, Loeser JD, editors. Advances in pain research and therapy, vol. 12. Issues in pain measurement. New York: Raven Press; 1989. pp. 391–403. [Google Scholar]
- 2.Mendoza TR, Wang XS, Cleeland CS, et al. The rapid assessment of fatigue severity in cancer patients. Cancer. 1999;85(5):1186–1196. doi: 10.1002/(sici)1097-0142(19990301)85:5<1186::aid-cncr24>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- 3.Cleeland C, Mendoza T, Wang X, et al. Assessing symptom distress in cancer patients. Cancer. 2000;89:1634–1646. doi: 10.1002/1097-0142(20001001)89:7<1634::aid-cncr29>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 4.Serlin RC, Mendoza TR, Nakamura Y, et al. When is cancer pain mild, moderate, or severe? Grading pain severity by its interference with function. Pain. 1995;61:277–284. doi: 10.1016/0304-3959(94)00178-H. [DOI] [PubMed] [Google Scholar]
- 5.Palos GR, Mendoza TR, Mobley GM, et al. Asking the community about cut-points used to describe mild, moderate, and severe pain. J Pain. 2006;7(1):49–56. doi: 10.1016/j.jpain.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 6.Paul SM, Zelman DC, Smith M, et al. Categorizing the severity of cancer pain: further exploration of the establishment of cutpoints. Pain. 2005;113:37–44. doi: 10.1016/j.pain.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 7.Zelman DC, Dukes E, Brandenburg N, Bostrom A, Gore M. Identification of cut-points for mild, moderate and severe pain due to diabetic peripheral neuropathy. Pain. 2005;115(1–2):29–36. doi: 10.1016/j.pain.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 8.Given B, Given CW, Sikorskii A, et al. Establishing mild, moderate and severe scores for cancer-related symptoms: how consistent and clinically meaningful are interference based severity cut-points? J Pain Symptom Manage. 2008;35(2):126–135. doi: 10.1016/j.jpainsymman.2007.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz C, Bode R, Repucci N, Becker J, Sprangers A. The clinical significance of adaptation to changing health: a meta-analysis of response shifts. Qual Life Res. 2006;15:1533–1550. doi: 10.1007/s11136-006-0025-9. [DOI] [PubMed] [Google Scholar]
- 10.Farrar JT, Portenoy RK, Berlin JA, et al. Defining the clinically important differences in pain outcome measures. Pain. 2000;88:287–294. doi: 10.1016/S0304-3959(00)00339-0. [DOI] [PubMed] [Google Scholar]
- 11.Dionne RA, Bartoshuk L, Mogil J, et al. Individual responder analyses for pain: does one pain scale fit all? Trends Pharmacol Sci. 2005;26(3):125–130. doi: 10.1016/j.tips.2005.01.009. [DOI] [PubMed] [Google Scholar]
- 12.Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of chances in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain. 2001;94:149–158. doi: 10.1016/S0304-3959(01)00349-9. [DOI] [PubMed] [Google Scholar]
- 13.Miaskowski C, Dodd M, West C, et al. The use of responder analysis to identify differences in patient outcomes following a self-care intervention to improve cancer pain management. Pain. 2007;129:55–63. doi: 10.1016/j.pain.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sikorskii A, Given C, Given B. Symptom management for cancer patients: A trial comparing two multimodal interventions. J Pain Symptom Manage. 2007;34(3):253–264. doi: 10.1016/j.jpainsymman.2006.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.National Comprehensive Cancer Network. [Accessed September 28, 2007];Clinical practice guidelines in oncology. 2006 Available at: http://www.nccn.org/professionals/physician_gls/PDF/fatigue.pdf.
- 16.Barsky AJ, Ahern D. Cognitive behavior therapy for hypochondriasis. JAMA. 2004;291:1464–1470. doi: 10.1001/jama.291.12.1464. [DOI] [PubMed] [Google Scholar]
- 17.McGinn L, Sanderson W. What allows cognitive behavioral therapy to be brief: overview, efficacy and crucial factors facilitating brief treatment. Psychol Sci Pract. 2001;8:23–73. [Google Scholar]
- 18.Dobson K, Dozios D. Handbook of cognitive behavioral therapies. New York: Guilford Press; 2001. Historical and philosophical bases of cognitive behavioral therapy; pp. 3–39. [Google Scholar]
- 19.Miaskowski C, Lee K. Pain, fatigue, and sleep disturbances in oncology outpatients receiving radiation therapy for bone metastasis: a pilot study. J Pain Symptom Manage. 1999;17(5):320–332. doi: 10.1016/s0885-3924(99)00008-1. [DOI] [PubMed] [Google Scholar]
- 20.Anderson K, Getto C, Mendoza T, et al. Fatigue and sleep disturbance in patients with cancer, patients with clinical depression, and community-dwelling adults. J Pain Symptom Manage. 2003;25(4):307–318. doi: 10.1016/s0885-3924(02)00682-6. [DOI] [PubMed] [Google Scholar]
- 21.Berger A. Patterns of fatigue and activity and rest during adjuvant breast cancer chemotherapy. Oncol Nurs Forum. 1998;25(1):51–61. [PubMed] [Google Scholar]
- 22.McCullagh P, Nelder J. Generalized linear models. London: Chapman and Hall; 1989. [Google Scholar]
- 23.Searle SR, Speed F, Miliken GA. Population marginal means in the linear model: an alternative to least squares means. Am Stat. 1980;34:216–221. [Google Scholar]
- 24.SAS Institute Inc. SAS software, version 9 of the SAS System for Windows. Cary, NC: SAS Institute Inc.; 2002–2003. [Google Scholar]
- 25.Basch E, Iasonos A, Bartz A. Long-term toxicity monitoring via electronic patient reported outcomes in patients receiving chemotherapy. J Clin Oncol. 2007;25:5374–5380. doi: 10.1200/JCO.2007.11.2243. [DOI] [PubMed] [Google Scholar]