Abstract
Background
Exposure to airborne fungi has been associated with increased airway hyperreactivity and asthma prevalence.
Objective
To investigate the association between common indoor fungi and airway hyperreactivity measured by peak expiratory flow variability in asthmatic children.
Methods
Children 6 to 12 years of age (n = 225) with a physician diagnosis of asthma were enrolled in the study to have their peak expiratory flow recorded twice daily during a 2-week period. Genus-specific, quantitative, in-home airborne mold concentrations were measured. Logistic regression models were used to examine the relationship between a mean peak expiratory flow variability greater than 18.5% (75th percentile) and any mold in the home (total mold, Cladosporium, Penicillium, Aspergillus, and Alternaria).
Results
Mold was detected in 93% of the homes. The most common molds were Cladosporium in 72% and Penicillium in 42% of the samples. Controlling for sex, ethnicity, age, and winter season of sampling, Penicillium measured in the home was associated with a mean peak expiratory flow variability greater than 18.5% (odds ratio, 2.4; 95% confidence interval, 1.2–4.8). Greater peak expiratory flow variability was not associated with total mold or other mold measured in the home.
Conclusion
Exposure to airborne Penicillium is associated with increased peak expiratory flow variability in asthmatic children.
Introduction
For epidemiologic studies that evaluate asthma, peak expiratory flow (PEF) monitoring permits multiple measurements each day for a prolonged period and thus can measure day to day variations in lung function. Previous studies1–6 have demonstrated associations between fungal exposure and respiratory symptoms in asthmatic patients.
It has been suggested that PEF variability (PEFV) better reflects the dynamic nature of asthma and may help avert impending asthma exacerbations.7–9 The National Asthma Education and Prevention Program Expert Panel 3 Report8 and the 2006 Global Initiative for Asthma guidelines7 have suggested the use of home PEF monitoring as an important tool in measuring trends in asthma control over time. PEF monitoring was reinforced as 1 element in the standard of care by the National Asthma Education and Prevention Program Expert Panel Report 3 2007 guidelines for patients with moderate to severe persistent asthma and any patient with a history of severe exacerbations. In this study we examine whether measured indoor airborne mold concentrations are associated with PEFV in asthmatic children.
Methods
The study population consisted of 322 children with physician-diagnosed asthma. These children were enrolled in a cross-sectional study that involved a 2-week trial of in-home PEF monitoring as a part of a larger study of 466 children that evaluated environmental determinants of childhood asthma severity.5,10 Homes were located in Connecticut and Western Massachusetts. Of the 322 individuals in the present study, 307 (95%) met our age criterion of 6 to 12 years and the American Thoracic Society–recommended cutoffs for PEF of 60 to 400 L/min.11 Complete data, defined as twice-daily PEF measurements, were available for 1 to 14 days for 225 patients (70%). The Human Investigation Committee of Yale University School of Medicine, New Haven, Connecticut, approved this study. All respondents (mothers of study participants) gave informed consent, and children 7 years or older gave assent before participation.
In-home, genus-specific, quantitative airborne mold samples were collected and analyzed as previously described.10,12 Mold samples were collected from the main living area of the child's home at the enrollment interview. Culturable airborne propagules were collected using a Burkhard portable air sampler (Burkhard Manufacturing Co, Richmansworth, England) in combination with dichloran–18% glycerol agar. The sampling period was 1 minute with an airflow rate of 20 L/min. After exposure, culture plates were incubated at 25° for 7 to 10 days, after which the resulting colonies were counted. Fungi were identified to the genus level by colony morphologic analysis or microscopic examination of the spore structure. Air levels were recorded as colony-forming units per cubic meter of air sampled in the home for each genus. This approach measures the air concentration of viable mold spores but does not measure air concentrations of other fungal products. Mold spore air concentrations were categorized into 4 levels according to the Commission of European Communities report13: 0 CFU/m3, undetectable; 1 to 499 CFU/m3, low; 500 to 999 CFU/m3, moderate; and more than 1,000 CFU/m3, high.
The Vitalograph recording PEF monitor was used (Vitalograph Ltd, Buckingham, Ireland). Families were instructed to help the asthmatic child make 3 serial PEF measurements twice daily (morning and evening) during a 2-week period beginning the day after the enrollment interview. Children were instructed to use the PEF meter in the morning after waking and before taking any asthma medication and again in the evening before retiring. The Vitalograph records the time and PEF value of each test. An acceptable test result was defined as the time to a PEF of greater than 40 milliseconds and less than 290 milliseconds from the start of the test. A test session was deemed complete if the highest PEF compared with the second highest PEF value for a minimum of 3 PEF tests was within 10%. The PEF data were recorded as liters per minute.
The enrollment interview was conducted by a trained research assistant and included the collection of sociodemographic and medical history information, including the mother's report of her child's allergies. Mothers were also given calendars on which to record their asthmatic children's daily symptoms (wheeze, persistent cough, shortness of breath, chest tightness, nocturnal symptoms) and medication use (short-acting inhalers and asthma controller medications, including systemic or inhaled corticosteroids, leukotriene modifiers, cromolyn sodium, long-acting β2-agonists, and theophylline).
The health outcome of interest was PEFV for each child and was expressed as a mean daily percentage for the monitoring period (ie, mean during all days of measurement): (daily maximum peak flow – daily minimum peak flow)/(mean of all peak flows). The exposures of interest were expressed as total colony-forming units of mold categorized into 4 levels according to the Commission of European Communities report13: 0 CFU/m3, undetectable; 1 to 499 CFU/m3, low; 500 to 999 CFU/m3, moderate; and more than 1,000 CFU/m3, high. All statistical analyses were conducted using SAS statistical software, version 9.1 (SAS Institute Inc, Cary, North Carolina). Unadjusted associations were examined with χ2 analyses. Logistic regression models were used to examine the relationship between a mean variability percentage of more than 18.5% (75th percentile) and any mold in the home, including total mold, Cladosporium, Penicillium, Aspergillus, or Alternaria, or other, defined as total mold – (Cladosporium + Penicillium + yeast). Separate models were examined for each mold, and all models were adjusted for sex, ethnicity, age, atopy, asthma medication use, and winter season of sampling.
Results
Study children participated in the 2-week PEF monitoring period for a mean (SD) of 6 (3) days. More than half (65%) of the individuals participated for 3 to 8 days; 11% contributed fewer than 3 days of data, 23% had 9 to 13 days, and less than 1% (0.9%) had a full 14 days of data. Unadjusted associations between personal characteristics and PEF measurements are given in Table 1. Girls, children with allergies, and children whose only medication was short-acting inhalers had significantly greater PEFV (Table 1). No significant associations were found between parental history of asthma (P = .65), parental history of allergy (P = .70), or season of sampling (P = .44) and mean PEFV of children in the study. A trend was seen toward higher PEFV in black children, but this finding was not statistically significant (P = .08).
Table 1.
Unadjusted Associations Between Personal Characteristics and Peak Expiratory Flow Variability (PEFV) Measurements for 225 Asthmatic Children in Connecticut and Western Massachusetts, 2000–2004
| Characteristics | No. (%) of children | PEFV, % | P valuea | |||
|---|---|---|---|---|---|---|
| 0–8.1 | 8.1–12.5 | 12.5–18.5 | ≥18.5 | |||
| Total patients in sample | 225 | 24.9 | 25.8 | 24.0 | 25.3 | |
| Sex | ||||||
| Male | 133 (59.1) | 22.6 | 33.1 | 22.6 | 21.8 | .03 |
| Female | 92 (40.9) | 28.3 | 15.2 | 26.1 | 30.4 | |
| Age, y | ||||||
| <8 | 93 (41.3) | 22.6 | 23.7 | 28.0 | 25.8 | .64 |
| ≥8 | 132 (58.7) | 26.5 | 27.3 | 21.2 | 25.0 | |
| Ethnicity | ||||||
| White | 160 (71.1) | 27.2 | 28.4 | 21.0 | 23.5 | .08 |
| Black | 28 (12.4) | 7.1 | 25.0 | 28.6 | 39.3 | |
| Hispanic | 35 (15.6) | 28.6 | 14.3 | 34.3 | 22.9 | |
| Other | 2 (0.8) | |||||
| Allergiesb | ||||||
| Yes | 149 (66.8) | 20.1 | 30.2 | 19.5 | 30.2 | .003 |
| No | 74 (33.2) | 33.8 | 17.6 | 32.4 | 16.2 | |
| Parental history of asthma | ||||||
| Mother only | 54 (24.9) | 25.9 | 29.6 | 24.1 | 20.4 | .65 |
| Father only | 37 (17.0) | 16.2 | 29.7 | 27.0 | 27.0 | |
| Both | 12 (5.5) | 16.7 | 33.3 | 8.3 | 41.7 | |
| Neither | 114 (52.5) | 29.8 | 22.8 | 23.7 | 23.7 | |
| Parental history of allergy | ||||||
| Mother only | 70 (32.3) | 27.1 | 22.9 | 22.9 | 27.1 | .70 |
| Father only | 41 (18.9) | 29.3 | 31.7 | 17.1 | 22.0 | |
| Both | 69 (31.8) | 26.1 | 27.5 | 21.7 | 24.6 | |
| Neither | 37 (17.0) | 18.9 | 21.6 | 37.8 | 21.6 | |
| Season of sampling | ||||||
| Winter | 45 (20.0) | 20.0 | 33.3 | 13.3 | 33.3 | .44 |
| Spring | 39 (17.3) | 23.1 | 25.6 | 23.1 | 28.2 | |
| Summer | 66 (29.3) | 30.3 | 22.7 | 22.7 | 24.2 | |
| Fall | 75 (33.3) | 24.0 | 24.0 | 32.0 | 20.0 | |
| Symptomsc | ||||||
| None | 150 (66.7) | 27.3 | 22.7 | 25.3 | 24.7 | .48 |
| Daytime only | 39 (17.3) | 15.4 | 28.2 | 25.6 | 30.8 | |
| Day and night | 36 (16.0) | 25.0 | 36.1 | 16.7 | 22.2 | |
| Medication usec | ||||||
| None | 112 (49.8) | 27.7 | 18.8 | 27.7 | 25.9 | .03 |
| Short-acting inhaler only | 28 (12.4) | 14.3 | 17.9 | 32.1 | 35.7 | |
| Controller medication | 85 (37.8) | 24.7 | 37.6 | 16.5 | 21.2 | |
P values from χ2 tests.
Mother's report of allergies from enrollment interview.
Symptoms and medication use recorded daily during peak expiratory flow monitoring period. Symptoms include wheeze, persistent cough, chest tightness, shortness of breath, or night symptoms. Controller medications include systemic or inhaled corticosteroids, leukotriene inhibitors, cromolyn sodium, long-acting β2-agonists, and theophylline.
Mold was detected in 93% of all homes (Table 2). The most frequent molds identified in the homes were Cladosporium (72% of home samples), Penicillium (42%), Aspergillus (38%), and Alternaria (15%). Although Aspergillus (38%) and Alternaria (14%) were identified, neither was available in adequate quantities to allow further analysis. Both of these common fungi are classified under “other” in this analysis. Fungal concentrations were categorized into quartiles of colony-forming units per cubic meter as previously described (Fig 1).13 Distributions of total mold and Cladosporium were much less skewed than distributions for the other molds that tended to be detected or not (Fig 1). Unadjusted associations between the highest quartile of PEFV (≥18.5%) and any mold in the home (ie, measurements >0 CFU/m3) are shown in Figure 2. Penicillium was the only mold significantly associated with PEFV (P = .04). In logistic regression models, controlling for sex, ethnicity, age, atopy, asthma medication use, and winter season of sampling, any Penicillium measured in the home was associated with a mean variability of more than 18.5% (odds ratio, 2.42; 95% confidence interval, 1.23–4.76) (Table 3). There was also a tendency for children exposed to any Alternaria to be twice as likely to have the highest PEFV (odds ratio, 2.05; 95% confidence interval, 0.81–5.20).
Table 2.
Odds Ratios (OR) and 95% Confidence Intervals (CIs) From Logistic Regression Models for Any Mold and a Mean Peak Expiratory Flow Variability Percentage of 18.5% or More for 225 Asthmatic Children in Connecticut and Western Massachusetts, 2000–2004a
| Measured mold | OR (95% CI) |
|---|---|
| Total mold | |
| Undetectable | 1.0 [Reference] |
| >0 CFU/m3 | 1.48 (0.32–6.87) |
| Cladosporium | |
| Undetectable | 1.0 [Reference] |
| >0 CFU/m3 | 0.82 (0.37–1.85) |
| Penicillium | |
| Undetectable | 1.0 [Reference] |
| >0 CFU/m3 | 2.39 (1.19–4.81) |
| Aspergillus | |
| Undetectable | 1.0 [Reference] |
| >0 CFU/m3 | 0.82 (0.40–1.68) |
| Alternaria | |
| Undetectable | 1.0 [Reference] |
| >0 CFU/m3 | 2.05 (0.81–5.20) |
Separate models were run for each type of mold. All models were controlled for sex, ethnicity, age, atopy, asthma medication use, and winter season of sampling.
Figure 1.
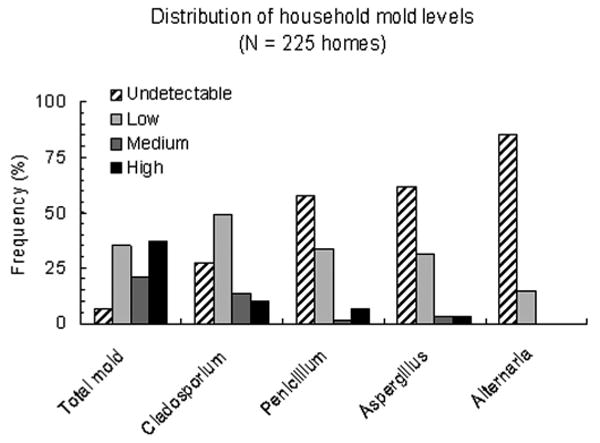
Levels of mold measured in the Connecticut and Western Massachusetts homes of the study participants (N = 225) from 2000 to 2004. Undetectable, 0 CFU/m3; low, 1 to 499 CFU/m3; medium, 500 to 599 CFU/m3; and high, 1,000 CFU/m3 or higher. Molds identified most frequently included Cladosporium, Penicillium, Aspergillus, and Alternaria.
Figure 2.
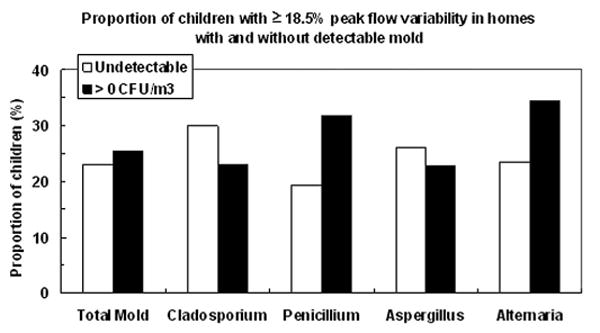
The proportion of children in the highest quartile of peak expiratory flow variability (≥18.5%) was significantly greater in homes with any detectable level of Penicillium (ie, levels >0 CFU/m3) compared with homes where none was detected (P = .04, χ2 test). Proportions were not statistically significantly different for detectable vs undetectable levels of total mold or any other mold.
Discussion
To our knowledge, this study is the largest to evaluate the association between directly measured indoor home fungi and PEFV. Our data suggest that exposure to Penicillium is associated with PEFV in asthmatic children. Exposure to indoor fungal allergens is important because of both the high percentage of time spent indoors14 and data suggesting the importance of fungal antigens across a variety of allergic diseases, including chronic rhinosinusitis,15 atopic conjunctivitis,16 and atopic dermatitis.17 Most studies published to date are cross-sectional and do not establish a causal role for fungi in allergic disease.
Outdoor exposures to a variety of fungi, including Alternaria,18–23 Aspergillus and Cladosporium,24 and Penicillium,3,23,25 have been associated with an increased risk for asthma. Although some studies have evaluated the association between outdoor fungal allergens and the effect on PEFV in children with asthma,26 few studies have examined quantitative indoor fungal exposure in asthmatic children with PEFV.
Andriessen et al27 reported that PEFV in atopic children correlated with parental report of observed but not measured mold in the home. As part of the comprehensive Childhood Asthma Management Program study, Nelson et al19 showed that asthmatic children with skin prick test sensitivity to Alternaria had increased bronchial hyperresponsiveness to methacholine challenge but not decreased lung function by spirometry compared with asthmatic children without skin prick test sensitivity. In our study, the fact that an association was seen between Penicillium and PEFV but not Cladosporium or other molds suggests a specific effect of Penicillium on airways not explained by the total number of mold spores in the home alone.
Our results are consistent with the findings of other studies3,23,25 that suggest an association between Penicillium and asthma morbidity. We have previously reported that exposure to high household levels of Penicillium is associated with increased respiratory symptoms in infants at risk of developing asthma.5 The present study shows that Penicillium exposure continues to be important into childhood. Otitis media in the first 6 months of life among the infants at risk for asthma was associated with day care outside the home and birth during the summer or fall season but not with household air concentrations of mold after controlling for potential cofounders.28
Bush and Portnoy29 suggest that a strong relationship exists between exposure and sensitivity to certain fungi and the development of allergic disease. Although remediation efforts have been shown to have a positive effect on health for other allergens such as house dust mite,29–31 there are limited studies looking at efforts to reduce or eliminate household fungi. Bernstein et al32 showed that UV irradiation of household air in the central air handling system decreased airway hyperresponsiveness as evidenced by lower PEFV and an improvement in some asthma symptoms in a small cohort (n = 19) of mold-sensitized asthmatic children.
One limitation of our study is that compliance with PEF monitoring instructions was poor. This limitation is common in studies that involve PEF monitoring.32–34 However, despite the poor compliance rates, we were able to identify an association between Penicillium and the lung function of children. This may actually underestimate the true effect of Penicillium and other fungi on the airway hyperresponsiveness of asthmatic patients. Another limitation is that a single in-home measurement early in life was used to represent mold exposure during a 2-week period. Seasonal variability among fungi biomass34,35 and genus-specific fungal propagules35–37 have been described previously. However, PEF measurements were taken and completed within 2 weeks of the mold measurement, making it likely that mold measurements taken at the beginning of the monitoring period were representative of mold levels present throughout.
Although difficult to achieve, avoidance of known allergens is the cornerstone of therapy for much of allergic disease. Much remains to be understood about the relationships between fungal inhalation exposure, immune response, asthma causation, and asthma triggering. These data suggest the important association of Penicillium exposure with greater PEFV in asthmatic children.
Acknowledgments
Funding Sources: This work was supported by grants ES07456 and ES05410 from the National Institute of Environmental Health Sciences, National Institutes of Health.
Footnotes
Disclosures: Authors have nothing to disclose.
References
- 1.Damp Indoor Spaces and Health. Washington, DC: National Academies Press; 2004. [PubMed] [Google Scholar]
- 2.Targonski PV, Persky VW, Ramekrishnan V. Effect of environmental molds on risk of death from asthma during the pollen season. J Allergy Clin Immunol. 1995;95(5 pt 1):955–961. doi: 10.1016/s0091-6749(95)70095-1. [DOI] [PubMed] [Google Scholar]
- 3.Turyk M, Curtis L, Scheff P, et al. Environmental allergens and asthma morbidity in low-income children. J Asthma. 2006;43:453–457. doi: 10.1080/02770900600758333. [DOI] [PubMed] [Google Scholar]
- 4.Atkinson RW, Strachan DP, Anderson HR, Hajat S, Emberlin J. Temporal associations between daily counts of fungal spores and asthma exacerbations. Occup Environ Med. 2006;63:580–590. doi: 10.1136/oem.2005.024448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gent JF, Ren P, Belanger K, et al. Levels of household mold associated with respiratory symptoms in the first year of life in a cohort at risk for asthma. Environ Health Perspect. 2002;110:A781–A786. doi: 10.1289/ehp.021100781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zock JP, Jarvis D, Luczynska C, Sunyer J, Burney P. Housing characteristics, reported mold exposure, and asthma in the European Community Respiratory Health Survey. J Allergy Clin Immunol. 2002;110:285–292. doi: 10.1067/mai.2002.126383. [DOI] [PubMed] [Google Scholar]
- 7.Global Strategy for Asthma Management and Prevention. Global Initiative for Asthma (GINA) [July 15, 2008];2006 www.ginasthma.org.
- 8.Expert Panel Report 3: Guidelines for the Diagnosis and Management of Asthma. Bethesda, MD: National Asthma Education and Prevention Program, National Institutes of Health; 2007. National Asthma Education and Prevention Program; p. 4051. Report 07. [Google Scholar]
- 9.Lebowitz MD, Krzyzanowski M, Quackenboss JJ, O'Rourke MK. Diurnal variation of PEF and its use in epidemiological studies. Eur Respir J. 1997;24:49s–56s. [PubMed] [Google Scholar]
- 10.Leaderer BP, Belanger K, Triche E, et al. Dust mite, cockroach, cat, and dog allergen concentrations in homes of asthmatic children in the northeastern United States: impact of socioeconomic factors and population density. Environ Health Perspect. 2002;110:419–425. doi: 10.1289/ehp.02110419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med. 1995;152:1107–1136. doi: 10.1164/ajrccm.152.3.7663792. [DOI] [PubMed] [Google Scholar]
- 12.Ren P, Jankun TM, Belanger K, Bracken MB, Leaderer BP. The relation between fungal propagules in indoor air and home characteristics. Allergy. 2001;56:419–424. doi: 10.1034/j.1398-9995.2001.056005419.x. [DOI] [PubMed] [Google Scholar]
- 13.Commission of the European Communities. Biological Particles in Indoor Environments. Luxembourg, Sweden: Commission of the European Communities; 1994. Report 12. [Google Scholar]
- 14.Leech JA, Nelson WC, Burnett RT, Aaron S, Raizenne ME. It's about time: a comparison of Canadian and American time-activity patterns. Expo Anal Environ Epidemiol. 2002;12:427–432. doi: 10.1038/sj.jea.7500244. [DOI] [PubMed] [Google Scholar]
- 15.Ponikau JU, Sherris DA, Weaver A, Kita H. Treatment of chronic rhinosinusitis with intranasal amphotericin B: a randomized, placebo-controlled, double-blind pilot trial. J Allergy Clin Immunol. 2005;115:125–131. doi: 10.1016/j.jaci.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 16.Kari O. Atopic conjunctivitis: a cytologic examination. Acta Ophthalmol. 1988;66:381–386. doi: 10.1111/j.1755-3768.1988.tb04027.x. [DOI] [PubMed] [Google Scholar]
- 17.Scalabrin DM, Bavbek S, Perzanowski MS, Wilson BB, Platts-Mills TA, Wheatley LM. Use of specific IgE in assessing the relevance of fungal and dust mite allergens to atopic dermatitis: a comparison with asthmatic and nonasthmatic control subjects. J Allergy Clin Immunol. 1999;104:1273–1279. doi: 10.1016/s0091-6749(99)70024-2. [DOI] [PubMed] [Google Scholar]
- 18.Salo PM, Arbes SJ, Sever M, et al. Exposure to Alternaria alternata in US homes is associated with asthma symptoms. J Allergy Clin Immunol. 2006;118:892–898. doi: 10.1016/j.jaci.2006.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nelson HS, Szefler SJ, Jacobs J, Huss K, Shapiro G, Sternberg AL. The relationships among environmental allergen sensitization, allergen exposure, pulmonary function, and bronchial hyperresponsiveness in the Childhood Asthma Management Program. J Allergy Clin Immunol. 1999;104(4 pt 1):775–785. doi: 10.1016/s0091-6749(99)70287-3. [DOI] [PubMed] [Google Scholar]
- 20.Bush RK, Prochnau JJ. Alternaria-induced asthma. J Allergy Clin Immunol. 2004;113:227–234. doi: 10.1016/j.jaci.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 21.Delfino RJ, Zeiger RS, Seltzer JM, et al. The effect of outdoor fungal spore concentrations on daily asthma severity. Environ Health Perspect. 1997;105:622–635. doi: 10.1289/ehp.97105622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barnes C, Tuck J, Simon S, Pacheco F, Hu F, Portnoy J. Allergenic materials in the house dust of allergy clinic patients. Ann Allergy Asthma Immunol. 2001;86:517–523. doi: 10.1016/S1081-1206(10)62899-2. [DOI] [PubMed] [Google Scholar]
- 23.Licorish K, Novey HS, Kozak P, Fairshter RD, Wilson AF. Role of Alternaria and Penicillium spores in the pathogenesis of asthma. J Allergy Clin Immunol. 1985;76:819–825. doi: 10.1016/0091-6749(85)90755-9. [DOI] [PubMed] [Google Scholar]
- 24.Jaakkola MS, Ieromnimon A, Jaakkola JJ. Are atopy and specific IgE to mites and molds important for adult asthma? J Allergy Clin Immunol. 2006;117:642–648. doi: 10.1016/j.jaci.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Garrett MH, Rayment PR, Hooper MA, Abramson MJ, Hooper BM. Indoor airborne fungal spores, house dampness and associations with environmental factors and respiratory health in children. Clin Exp Allergy. 1998;28:459–467. doi: 10.1046/j.1365-2222.1998.00255.x. [DOI] [PubMed] [Google Scholar]
- 26.Higgins BG, Francis HC, Yates C, et al. Environmental exposure to air pollution and allergens and peak flow changes. Eur Respir J. 2000;16:61–66. doi: 10.1034/j.1399-3003.2000.16a11.x. [DOI] [PubMed] [Google Scholar]
- 27.Andriessen JW, Brunekreef B, Roemer W. Home dampness and respiratory health status in European children. Clin Exp Allergy. 1998;28:1191–1200. doi: 10.1046/j.1365-2222.1998.00322.x. [DOI] [PubMed] [Google Scholar]
- 28.Pettigrew MM, Gent JF, Triche EW, Belanger KD, Bracken MB, Leaderer BP. Association of early-onset otitis media in infants and exposure to household mould. Paediatr Perinat Epidemiol. 2004;18:441–447. doi: 10.1111/j.1365-3016.2004.00596.x. [DOI] [PubMed] [Google Scholar]
- 29.Bush RK, Portnoy JM. The role and abatement of fungal allergens in allergic diseases. J Allergy Clin Immunol. 2001;107(3 suppl):S430–S440. doi: 10.1067/mai.2001.113669. [DOI] [PubMed] [Google Scholar]
- 30.Arshad SH, Bateman B, Sadeghnejad A, Gant C, Matthews SM. Prevention of allergic disease during childhood by allergen avoidance: the Isle of Wight prevention study. J Allergy Clin Immunol. 2007;119:307–313. doi: 10.1016/j.jaci.2006.12.621. [DOI] [PubMed] [Google Scholar]
- 31.Becker A, Watson W, Ferguson A, Dimich-Ward H, Chan-Yeung M. The Canadian asthma primary prevention study: outcomes at 2 years of age. J Allergy Clin Immunol. 2004;113:650–656. doi: 10.1016/j.jaci.2004.01.754. [DOI] [PubMed] [Google Scholar]
- 32.Bernstein JA, Bobbitt RC, Levin L, et al. Health effects of ultraviolet irradiation in asthmatic children's homes. J Asthma. 2006;43:255–262. doi: 10.1080/02770900600616887. [DOI] [PubMed] [Google Scholar]
- 33.Verschelden P, Cartier A, L'Archeveque J, Trudeau C, Malo JL. Compliance with and accuracy of daily self-assessment of peak expiratory flows (PEF) in asthmatic subjects over a three month period. Eur Respir J. 1996;9:880–885. doi: 10.1183/09031936.96.09050880. [DOI] [PubMed] [Google Scholar]
- 34.Cote J, Cartier A, Malo JL, Rouleau M, Boulet LP. Compliance with peak expiratory flow monitoring in home management of asthma. Chest. 1998;113:968–972. doi: 10.1378/chest.113.4.968. [DOI] [PubMed] [Google Scholar]
- 35.Dharmage S, Bailey M, Raven J, et al. Mouldy houses influence symptoms of asthma among atopic individuals. Clin Exp Allergy. 2002;32:714–720. doi: 10.1046/j.1365-2222.2002.01371.x. [DOI] [PubMed] [Google Scholar]
- 36.Ren P, Jankun TM, Leaderer BP. Comparisons of seasonal fungal prevalence in indoor and outdoor air and in house dusts of dwellings in one Northeast American county. J Expo Anal Environ Epidemiol. 1999;9:560–568. doi: 10.1038/sj.jea.7500061. [DOI] [PubMed] [Google Scholar]
- 37.de Ana SG, Torres-Rodriguez JM, Ramirez EA, Garcia SM, Belmonte-Soler J. Seasonal distribution of Alternaria, Aspergillus, Cladosporium and Penicillium species isolated in homes of fungal allergic patients. J Investig Allergol Clin Immunol. 2006;16:357–363. [PubMed] [Google Scholar]


