Abstract
Objective
Scleroderma (SSc) is characterized by a unique widespread vascular disease that can lead to severe digital ischemia, pulmonary arterial hypertension (PAH) or other organ dysfunction. Microthrombotic events and pro-coagulable factors such as anti-beta 2 glycoprotein 1 (B2GPI) or anti-cardiolipin (aCL) antibodies may be implicated in the development of these manifestations. This study investigated whether anti-B2GPI and aCL antibodies are correlated with macrovascular disease including ischemic digital loss and PAH in SSc patients.
Methods
75 SSc patients with history of ischemic digital loss and 75 matched SSc controls were evaluated. Anti-centromere (ACA), anti-B2GPI and aCL antibodies were tested and clinical associations measured using conditional and simple logistic regression models.
Results
Anti-B2GPI, but not aCL, were significantly more frequent (p=0.01) in digital loss patients, with IgA isotype showing the strongest association (OR 4.0). After adjusting for demographics, disease type, smoking and ACA, anti-B2GPI were significantly associated with active digital ischemia (OR 9.4), echocardiographic evidence for PAH (OR 4.8), and mortality (OR 2.9). ACA positivity was associated with history of digital loss (OR 3.8), but not with PAH or mortality. History of digital loss was strongly associated with increased mortality (OR 12.5).
Conclusion
Anti-B2GPI antibodies are significantly associated with macrovascular disease in SSc and independently predict mortality. It is unclear whether they play a pathogenic role or simply reveal the presence of underlying endothelial injury. The use of anti-B2GPI antibodies as a biomarker of vascular disease in SSc should be further explored.
Systemic sclerosis (SSc; scleroderma) is a multisystem disease characterized by immune activation, tissue fibrosis and underlying vascular disease (1). These pathogenetic hallmarks are closely associated and likely interact to ultimately determine the clinical phenotype expressed in scleroderma patients. Microvascular disease is universally present and is characterized by structural damage (obliterative vasculopathy) as well as functional disturbances (secondary Raynaud’s phenomenon). A distinct subset of SSc patients presents with episodes of progressive digital ischemia sometimes resulting in severe outcomes such as digital gangrene and amputation. This clinical presentation is usually associated with narrowing and occlusion of the ulnar (less frequently radial), palmar and digital arteries (2). Medium and large-size arteries (macrovascular disease) can be affected in the lower extremities as well (3–5). Pulmonary arterial hypertension (PAH) also develops with evidence of a progressive vascular disease with luminal narrowing and intimal thickening of medium-size pulmonary arteries. Other macrovascular manifestations such as coronary artery disease and cerebral vascular ischemia are incompletely studied in SSc; nevertheless, current data suggest that they are not more prevalent in SSc patients than in the general population (3, 6).
Angiographic and pathologic studies indicate that the vascular disease in SSc is characterized by progressive obliteration of affected arteries with defective angiogenesis and vasculogenesis resulting in extensive disease and inadequate collateral circulation. Endothelial dysfunction, vascular smooth muscle cell activation and intimal hyperplasia characterize the SSc vasculopathy (7). The occurrence of (micro) thrombotic events has also been linked to the onset of SSc ischemic complications. Although autoantibodies directed against the endothelial surface are detected in SSc and their presence is associated with severe digital ischemia, no specific autoantigenic determinant has been consistently characterized (8).
Anti-phospholipid antibodies (aPL) are immunoglobulins associated with recurrent thrombo-embolic events in primary antiphospholipid syndrome (APS) and systemic lupus erythematosus (SLE). They are directed against negatively charged phospholipid-binding proteins mostly involved in blood coagulation. Clinical manifestations of APS were initially linked with the presence of anti-cardiolipin antibodies (aCL) and lupus anticoagulant (9). Subsequently, β2 glycoprotein I (B2GPI) has been identified as the major target antigen for aCL or LAC. Anti-B2GPI antibodies (anti-B2GPI) are now included in the classification criteria for APS (10). Although the causal association between anti-B2GPI antibodies and thrombotic events has been demonstrated in APS and SLE, their significance in SSc and their relationship with the severity of clinical manifestations has not been fully addressed. The prevalence of anti-B2GPI in SSc ranges between 5% and 41% of patients (11–18). A similar prevalence is reported in SSc for aCL (12 to 45%) (12–14, 16–20). In none of these reports was the presence of aPL associated with the clinical manifestations typically seen in APS, such as recurrent arterial and venous thrombosis or pregnancy losses. Moreover, most of these studies, partly due to the low prevalence of aPL in SSc, did not fully address or could not find a correlation with any specific clinical manifestations. However, two studies reported an association between overall aPL positivity or combined aCL/anti-B2GPI positivity and PAH, digital ischemia or severe Raynaud’s phenomenon (11, 18). Interestingly, we encountered several scleroderma patients with recent episodes of critical digital ischemia or digital loss who also tested positive for anti-B2GPI.
In the present study, we investigated whether SSc patients with a history of digital loss have a higher prevalence of anti-B2GPI and whether the presence of these autoantibodies is associated with other clinical features of vascular disease, including active digital ischemia and pulmonary hypertension, as well as mortality.
PATIENTS AND METHODS
Patients
Seventy five SSc patients with history of ischemic digital loss were identified in the Johns Hopkins Scleroderma Center database and matched with 75 SSc controls by age, gender, race and disease subtype. Subjects with traumatic or post-infectious digital loss were excluded from the study. All the patients met the American College of Rheumatology criteria for SSc, and were classified as having diffuse (dcSSC) or limited (lcSSc) systemic sclerosis on the basis of the extent of their skin involvement (21, 22). The study was approved by the Johns Hopkins Institutional Review Board and written consent was obtained from all participants.
Clinical phenotyping
Detailed demographic data including age, gender, ethnicity, education, smoking status and clinical information about disease duration (calculated from the date of onset of first non-Raynaud’s symptom), scleroderma subtype, specific organ involvement and autoantibody status were obtained from each patient at the time of their visit. Ischemic digital loss was defined as amputation of a portion or the entire finger or toe following an irreversible ischemic event at any time in their disease course. Raynaud’s activity and presence of digital ischemia was determined using a previously published severity score: 0-No Raynaud’s, 1-Raynaud’s with/without vasodilator required, 2-Digital Pitting Scars, 3-Digital Tip Ulcerations, 4-Digital Gangrene (23). Active digital ischemia was defined as a Raynaud’s severity score ≥ 3. Skin involvement was scored according to the modified Rodnan skin score (mRSS, range 0–51) (24). Internal organ involvement was assessed using previously published criteria (23). Pulmonary involvement was determined by abnormal pulmonary function tests (PFTs) including measures of the absolute as well as percent (%) of predicted values for race, gender and age of the forced vital capacity (FVC) and single-breath carbon monoxide diffusing capacity (DLCO) according to the American Thoracic Society recommendations (25). For the purpose of this study, evidence for pulmonary arterial hypertension (PAH) was considered present if the estimated right ventricular systolic pressure (eRVSP) by Doppler echocardiography (ECHO) was ≥ 40 mmHg in two separate tests and there was no overt clinical evidence of congestive heart failure, thrombo-embolic disease or severe pulmonary interstitial fibrosis (FVC<50%). This assumption has been supported and confirmed by other studies (26). In addition, although data from right heart catheterization were limited in our dataset, 16 out of 18 SSc patients with a measured mean pulmonary artery (PA) pressure >25 mmHg diagnostic of PAH, also showed an eRVSP >40 mmHg at the echocardiogram. Heart, gastrointestinal, renal or musculoskeletal involvement was considered present when the relative Medsger severity score was ≥ 1 (23). Evidence of sicca complex was determined by clinical criteria. Medical records of each patient were also reviewed to identify previous manifestations of APS, including venous or arterial thrombosis. Information about pregnancy morbidity, thrombocytopenia or livedo reticularis were not consistently available and therefore omitted from the analysis.
ELISA assays
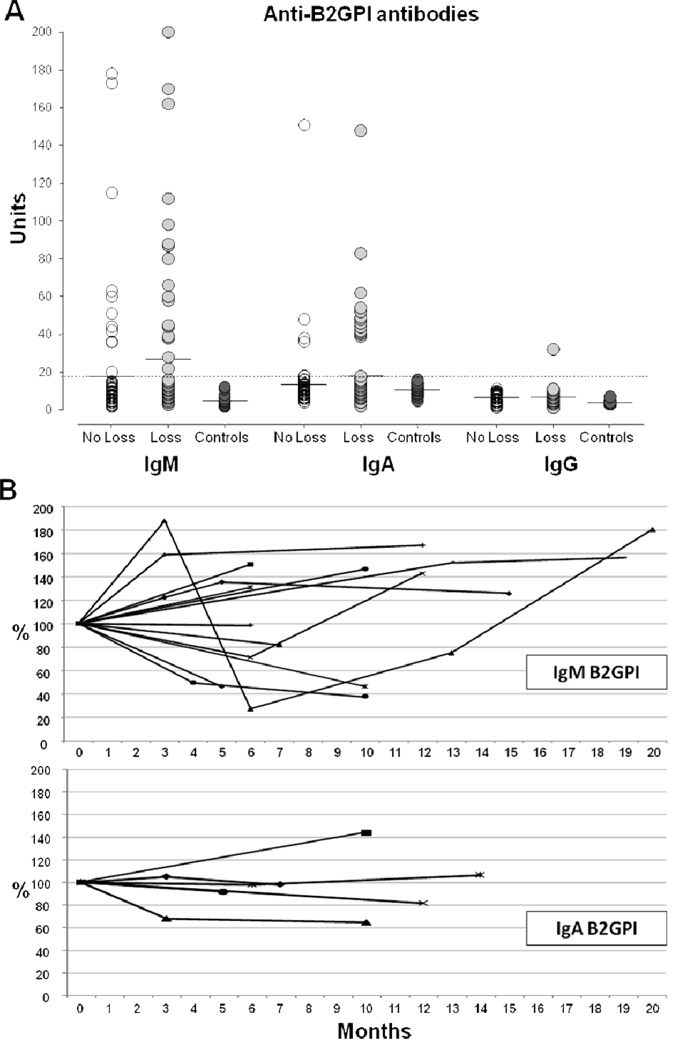
Serum samples were previously obtained during routine clinical patient visits at the Johns Hopkins Scleroderma Center and stored at −80°C. Levels of anti-B2GPI and aCL antibodies (IgM, IgA, IgG isotypes) were quantitated using commercially available enzyme-linked immunosorbent assay (ELISA) kits (QUANTA Lite™ Mircowell ELISA, INOVA Diagnostics Inc., San Diego, CA), following manufacturer’s instructions. Results were converted into units using the standard calibrators provided. Samples with autoantibody values more than 20 units were considered positive for all anti-B2GPI isotypes. This cut-off value for the assays was previously established by the manufacturer after testing sera from healthy controls and using the 95th percentile of the obtained control values. The precision and reproducibility for the IgA and IgM anti-B2GPI assays were assessed at 3 levels of antibody activity (negative, low and high positive) using patients’ sera. The intra- and inter-assay coefficients of variation (CV) were calculated after running each sample 5 times on 5 consecutive days. These data are summarized in Table 1. To further confirm the specificity of the assays, sera from 20 healthy donors were tested for anti-B2GPI (IgA, IgM and IgG), resulting negative as shown in Figure 1.
Table 1.
Summary of IgA and IgM anti-B2GPI assay precision.
| Samples | Intra-assay mean (SD), CV§ |
Inter-assay mean, SD, CV§ |
Overall mean, SD, CV§ |
|---|---|---|---|
| Anti-B2GPI IgA | |||
| negative | 6.1 (0.2), 2.9 | 5.8 (0.2), 3 | 5.9 (0.5), 7.9 |
| lower + | 29 (0.8), 2.7 | 31.8 (2.7), 8.4 | 31.8 (2.6), 8.1 |
| higher + | 125 (1.4), 1.1 | 127 (3.3), 2.6 | 127 (4.9), 3.9 |
| Anti-B2GPI IgM | |||
| negative | 3.5 (0.2), 6.3 | 3.9 (0.4), 10.2 | 3.8 (0.4), 11.6 |
| lower + | 33.8 (1.1), 3.4 | 33 (2.7), 8.3 | 33 (2.9), 8.8 |
| higher + | 216.3 (4.2), 1.9 | 214.9 (10), 4.7 | 214.9 (10.8), 5.1 |
Mean values ± SD are expressed in units; the coefficient of variation (CV) as percentage. Each sample (negative, low and high positive) for IgA and IgM anti-B2GPI was run five times on five consecutive days.
Figure 1.
A, Serum levels of IgM, IgA and IgG anti-B2GPI antibodies in SSc patients with (n=75) or without (n=75) digital loss and healthy controls (n=20). The horizontal bars indicate the mean values. The dotted line shows the cut-off value. B, Relative variation of IgM and IgA anti-B2GPI antibody titers from baseline (defined as 100%) over time (months). Each line represents a study patient for whom serial serum samples were available (IgM n=16 and IgA n =6).
Statistical analysis
All variables were examined and transformed when a non-normal distribution was evident. The dependence of digital loss (response variable) on the risk factors considered (explanatory variables) was evaluated by a conditional logistic regression model. The association between autoantibody (anti-B2GPI and aCL) status (dichotomous) and disease characteristics or outcome was estimated using an unadjusted conditional logistic regression for digital loss or adjusted logistic regression models for digital ischemia, eRVSP and mortality. Age, disease duration, skin score, PFTs and eRVSP, together with Raynaud’s, gastrointestinal and lung severity scores were treated as continuous variables. The other sociodemographic or disease characteristics and the autoantibody status were included in the models as dichotomous or categorical variables. Significance was tested using the regression coefficients, and the association between risk factors and outcome was expressed as odds ratio (OR) and the corresponding 95% confidence interval (95% CI) or as a p-value (significant when less or equal to 0.05). Differences between anti-B2GPI isotype levels in patients with or without digital loss were evaluated using the rank-sum test. Statistical analyses were performed with Stata version 10 software (Stata Corporation, College Station, TX).
RESULTS
Association between history of digital loss and disease characteristics of SSc
The sociodemographic and disease characteristics of SSc patients with digital loss and matched controls (age, gender, race and disease subtype) are summarized on Table 2. There was no statistically significant difference between the two groups in terms of disease duration, presence of sicca symptoms, gastrointestinal and kidney involvement. In the digital loss group the skin score, albeit overall low, was higher (p=0.012) and the lung severity score was worse (p=0.003), corresponding also to a significantly lower DLCO (p=0.002) and a trend for a higher eRVSP. In the digital loss group anti-centromere antibodies (ACA), but not anti-SCL70, were more prevalent (p=0.003). Significant differences were detected in the two groups in terms of Raynaud’s severity score (p<0.001), presence of active digital ischemia (p<0.001), median survival from diagnosis (14.5 vs. 30.5 years; p<0.001) and mortality (48% vs. 11%; p<0.001). The positive associations between history of digital loss and disease characteristics of SSc are also reported in Table 2. Not surprisingly, past or current smoking (OR 2.2, 95% CI 1.0–4.7; OR 2.8, 95% CI 1.1–7.2, respectively), more severe Raynaud’s phenomenon (OR 3.4, 95% CI 2.0–5.8) and active digital ischemia (OR 6.00, 95% CI 2.5–14.2) were significantly associated with history of digital loss. Importantly, we estimated that the odds of death are 12.5 times greater in the digital loss group compared to controls (OR 12.5, 95% CI 3.0–52.8), and this was independent from the disease duration. No significant history of arterial or venous thrombosis was identified by chart review in both groups.
Table 2.
Sociodemographic and disease characteristics of SSc patients according to history of digital loss*
| Variable | Digital Loss (n=75) |
No Digital Loss (n=75) |
P§ | OR (95% CI)§ |
|---|---|---|---|---|
| Age, years (range 22–86) | 52.5 ± 13.6 | 53.1 ± 12.5 | NA | |
| Female, % | 76 | 87 | NA | |
| Race/Ethnicity, % | ||||
| White | 71 | 75 | ||
| Black | 24 | 23 | NA | |
| Other | 5 | 2 | ||
| Smoking, % | ||||
| Never | 37 | 60 | 0.023 | |
| Past | 35 | 24 | 2.2 (1.0, 4.7) | |
| Current | 28 | 16 | 2.8 (1.1, 7.2) | |
| SSc type, % | ||||
| Limited | 83 | 85 | NA | |
| Diffuse | 17 | 15 | ||
| Disease duration, years (range 0.1–36.6)† | 10.4 ± 7.9 | 9.6 ± 7.6 | 0.536 | 1.0 (0.9–1.1) |
| Rodnan’s skin score, modified (0–51) | 8.0 ± 8.7 | 5.3 ± 6.8 | 0.012 | 1.1 (1.0–1.2) |
| Gastrointestinal severity score (0–4) | 1.1 ± 0.9 | 1.4 ± 0.9 | 0.084 | 0.7 (05–1.1) |
| Kidney involvement, % | 12 | 11 | 0.796 | 1.1 (0.4–3.1) |
| Sicca complex, % | 48 | 56 | 0.277 | 0.7 (0.3–1.4) |
| Lung severity score (0–4) | 1.8 ± 1.6 | 1.2 ± 1.2 | 0.003 | 1.5 (1.1, 2.0) |
| Pulmonary function†† | ||||
| FVC, % predicted | 82.1 ± 19.2 | 86.1 ± 18.0 | 0.227 | 0.99 (0.97, 1.01) |
| DLCO, % predicted | 61.5 ± 17.6 | 71.9 ± 20.2 | 0.002 | 0.97 (0.96, 0.99) |
| Elevated eRVSP (>40 mmHg), % | 43 | 32 | 0.168 | 1.6 (0.8, 3.2) |
| Raynaud’s Phenomenon severity score (0–4) | 2.6 ± 0.8 | 1.5 ± 0.8 | < 0.001 | 3.4 (2.0, 5.8) |
| Active digital ischemia, % | ||||
| (Raynaud’s Severity Score ε3) | 55 | 15 | < 0.001 | 6.0 (2.5, 14.2) |
| Mortality | ||||
| Deceased, % | 48 | 11 | < 0.001 | 12.5 (3.0, 52.8) |
| Survival from SSc diagnosis (median, years)¶ | 14.5 | 30.5 | < 0.001 | |
| Autoantibodies, % | ||||
| Anti-SCL70 | 11 | 8 | 0.526 | 1.5 (0.4, 5.3) |
| Anti-centromere | 63 | 41 | 0.003 | 3.3 (1.4, 7.7) |
Values are the mean ± SD unless indicated otherwise. The range is given with each variable group, where applicable. SSc = systemic sclerosis; FVC = forced vital capacity; DLCO = diffusing capacity for carbon monoxide; eRVSP = estimated right ventricular systolic pressure.
Results are based on conditional logistic regression model for digital loss as a function of the sociodemographic or disease characteristics.
Time from first non-Raynaud’s symptom
Odds ratios of digital loss are per unit increase in the continuous predictor.
Survival from diagnosis was calculated using Kaplan-Meier survival estimates.
Anti-phospholipid (aPL) antibody profiles
The prevalence of anti-B2GPI and aCL antibodies and their isotypes in cases and controls is shown in Table 3. Overall, anti-B2GPI (p=0.017) but not aCL (p=0.08) were significantly more frequent in patients with digital loss. In this group, anti-B2GPI were detected in 36% of patients compared to 19% of controls. The IgM and IgA (p=0.016) anti-B2GPI isotypes were individually more represented, while IgG was detected in only one patient. The distribution and combination of the different isotypes did not differ between the two groups. Interestingly, in the majority of anti-B2GPI positive SSc patients the IgM isotype was present alone. The prevalence of the IgA isotype was increased among anti-B2GPI positive African-American patients compared to Caucasian (50% vs. 40%), but the difference was not statistically significant (p=0.63). The analytical values for anti-B2GPI and aCL antibodies in study subjects and healthy controls are reported in Figure 1A. Positive autoantibody isotypes displayed a moderate to high titer, but their mean levels did not differ significantly between patients with or without digital loss. A small number of subjects had serial serum samples available. We found that all the anti-B2GPI positive patients do remain positive over time. In particular, the titers of the IgA anti-B2GPI seem to be more stable, while the IgM exhibit a greater titer fluctuation (Figure 1B). In addition, the IgM do not undergo isotype class switching. We observed only one seroconversion.
Table 3.
Frequency and isotype distribution of anti-B2GPI and aCL antibodies in SSc patients with digital loss and controls
| Digital Loss | |||
|---|---|---|---|
| Anti-B2GPI | Cases (n=75) | Controls (n=75) | P§ |
| Any Positive, % (n) | 36 (27) | 19 (14) | 0.017 |
| IgM | 27 (20) | 15 (11) | 0.08 |
| IgA | 17 (13) | 5 (4) | 0.016 |
| IgG | 1 (1) | 0 (0) | NA |
| Isotype distribution | |||
| IgM only | 17 (13) | 12 (9) | 0.356 |
| IgA only | 9 (7) | 3 (2) | 0.086 |
| IgG only | 0 | 0 | NA |
| IgM+IgA | 7 (5) | 3 (2) | 0.246 |
| IgM+IgA+IgG | 1 (1) | 0 | NA |
| Anti-cardiolipin | |||
| Any positive % (n) | 24 (18) | 13 (10) | 0.08 |
| IgM | 19 (14) | 12 (9) | 0.248 |
| IgA | 4 (3) | 0 (0) | NA |
| IgG | 9 (7) | 3 (2) | 0.097 |
| Isotype distribution | |||
| IgM only | 13 (10) | 11 (8) | 0.615 |
| IgA only | 1(1) | 0 | NA |
| IgG only | 4(3) | 1(1) | NA |
| IgM+IgA | 0 | 0 | NA |
| IgM+IgG | 3 (2) | 1 (1) | NA |
| IgM+IgA+IgG | 3 (2) | 0 | NA |
P-values were calculated using a conditional logistic regression model
Association of aPL antibodies with SSc disease characteristics
Both cases and controls were utilized to assess the association between presence of aPL antibodies and SSc disease characteristics. With the exception of older age in anti-B2GPI positive subjects (55.1±15.9 vs. 52±11.7 years, p=0.008), sociodemographic variables and general disease characteristics were similar in the two groups for both autoantibodies (data not shown). Table 4 summarizes the associations of positive anti-B2GPI and aCL antibody testing with digital loss (conditional logistic regression) and digital ischemia, eRVSP (≥ 40 mm/Hg) or mortality (logistic regression models). Anti-B2GPI exhibited a significant association with digital loss (OR 2.4, 95% CI 1.1–5.3) and other features of macrovascular disease as well as mortality in SSc patients. In particular, this association was maintained after adjusting for age, gender, race, disease type, smoking, digital loss and anti-centromere status, indicating that anti-B2GPI antibodies are independently associated with the presence of active digital ischemia (OR 9.4, 95% CI 3.5–25.4), elevated eRVSP (OR 4.8, 95% CI 1.0–11.4) and mortality (OR 2.9, 95% CI 1.1–7.7). Positive trends but no statistically significant associations were estimated according to aCL status. Using the adjusted logistic regression model, data were also analyzed to identify other predictors of higher mortality (beyond history digital loss or anti-B2GPI status). Comparing alive vs. deceased patients, a significant association was found with current smoking status (OR 4.6, 95% CI 1.6–13.6, p=0.005), active digital ischemia (OR 5.8, 95% CI 2.3–14.9; p<0.001) and skin score (9.6±10.6 vs. 5.2±6.3 mRSS, p=0.005). Disease duration, anti-SCL70, anti-centromere, elevated eRVSP and the other sociodemographic or disease characteristics were comparable in the two groups.
Table 4.
Associations of positive anti-B2GPI and aCL antibody testing with clinical features of macrovascular disease and mortality in SSc patients
| Digital Loss | Cases (n=75) |
Controls (n=75) |
OR (95% CI)§ | OR (95%CI)¶ |
|---|---|---|---|---|
| Anti-B2GPI | ||||
| Any Positive % (n) | 36 (27) | 19 (14) | 2.4 (1.1, 5.3) | |
| IgM | 27 (20) | 15 (11) | 2.0 (0.9, 4.5) | |
| IgA | 17 (13) | 5 (4) | 4.0 (1.1, 14.2) | |
| IgG | 1 (1) | 0 (0) | NA | |
| Anti-cardiolipin | ||||
| Any positive % (n) | 24 (18) | 13 (10) | 2.1 (0.9, 5.3) | |
| IgM | 19 (14) | 12 (9) | 1.7 (0.7, 4.4) | |
| IgA | 4 (3) | 0 (0) | NA | |
| IgG | 9 (7) | 3 (2) | 6.0 (0.7, 49.8) | |
| Active Digital Ischemia | Active (n=52) |
Inactive (n=98) |
||
| Anti-B2GPI | ||||
| Any Positive % (n) | 50 (26) | 15 (15) | 5.5 (2.6, 12.0) | 9.4 (3.5, 25.4) |
| IgM | 38 (20) | 11 (11) | 4.9 (2.1, 11.4) | 13.9 (4.4, 43.5) |
| IgA | 23 (12) | 5 (5) | 5.6 (1.8, 16.9) | 4.5 (1.4, 15.3) |
| IgG | 0 (0) | 1 (1) | NA | NA |
| Anti-cardiolipin | ||||
| Any % (n) | 21 (11) | 17 (17) | 1.3 (0.5, 3.0) | 1.2 (0.5, 3.3) |
| IgM | 19 (10) | 13 (13) | 1.6 (0.6, 3.8) | 1.7 (0.6, 4.9) |
| IgA | 6 (3) | 0 (0) | NA | NA |
| IgG | 6 (3) | 6 (6) | 0.9 (0.2, 3.9) | 0.5 (0.1, 2.8) |
| eRVSP | >40mmHg (n=56) |
<40 mmHg (n=94) |
||
| Anti-B2GPI | ||||
| Any Positive % (n) | 48 (27) | 15 (14) | 5.3 (2.5, 11.5) | 4.8 (1.0, 11.4) |
| IgM | 38 (21) | 11 (10) | 5.0 (2.2, 11.8) | 5.4 (2.1, 14.4) |
| IgA | 21 (12) | 5 (5) | 4.9 (1.6, 14.6) | 4.6 (1.3, 15.7) |
| IgG | 2 (1) | 0 (0) | NA | NA |
| Anti-cardiolipin | ||||
| Any Positive % (n) | 27 (15) | 14 (13) | 2.3 (1.0, 5.2) | 1.7 (0.7, 4.1) |
| IgM | 21 (12) | 12 (11) | 2.1 (0.8, 5.0) | 1.6 (0.6, 4.2) |
| IgA | 5 (3) | 0 (0) | NA | NA |
| IgG | 13 (7) | 2 (2) | 6.6 (1.3, 32.9) | 4.1 (0.7, 22.4) |
| Status | Dead (n=39) |
Alive (n=98) |
||
| Anti-B2GPI | ||||
| Any Positive % (n) | 43 (17) | 23 (23) | 2.5 (1.1, 5.5) | 2.9 (1.1, 7.7) |
| IgM | 30 (12) | 18 (17) | 2.0 (0.86, 4.7) | 3.1 (1.1, 9.2) |
| IgA | 23 (9) | 7 (7) | 3.9 (1.3, 10.9) | 2.8 (0.8, 9.6) |
| IgG | 0 (0) | 1 (1) | NA | NA |
| Anti-cardiolipin | ||||
| Any % (n) | 25 (10) | 14 (14) | 2.0 (0.8, 5.0) | 2.2 (0.8, 6.3) |
| IgM | 23 (9) | 11 (11) | 2.3 (0.8, 6.0) | 2.8 (0.9, 8.6) |
| IgA | 8 (3) | 0 (0) | NA | NA |
| IgG | 8 (3) | 5 (5) | 1.5 (0.3, 6.5) | 1.1 (0.2, 6.8) |
Based on an unadjusted conditional logistic regression (for Digital Loss) or logistic regression models for Digital Ischemia, eRVSP and Mortality.
Odds ratio with 95% confidence interval adjusted for age, gender, race, disease type, smoking and centromere status.
Finally, the relationship between anti-B2GPI and anti-centromere antibodies (ACA) was explored. Forty nine patients were positive for ACA only, while 29 subjects had both antibodies simultaneously. As summarized in Table 5, the presence of anti-B2GPI carried, independently from ACA status, a significant association with higher risk for active digital ischemia, elevated eRVSP suggestive of PAH and mortality (OR 16.4, 95% CI 3.4–80.5, p<0.001; OR 7.9, 2.6–24.4, p=0.002 and OR 2.9, 95%CI 1.1–10.1, p=0.004 respectively).
Table 5.
Association between digital loss, digital ischemia, eRVSP and mortality and the presence of anti-B2GPI and anti-centromere antibodies.
| ACA + (n=78) |
ACA and B2GPI+ (n = 29) |
ACA+ only (n = 49) |
ACA and B2GPI+ vs. ACA+ only |
||
|---|---|---|---|---|---|
| Outcome | % (n) | OR (95% CI)§ | % | % | OR (95% CI)§ |
| Digital Loss | 60 (47) | 3.28 (1.4, 7.7) | 66 (19) | 57 (28) | 1.5 (0.5, 4.8) |
| Digital Ischemia | 35 (27) | 1.15 (0.3, 3.9) | 59 (17) | 20 (10) | 16.4 (3.4, 80.5) |
| High eRVSP | 42 (33) | 1.3 (0.4, 3.8) | 69 (20) | 27 (13) | 7.9 (2.6, 24.4) |
| Mortality | 28 (22) | 0.4 (0.1, 1.3) | 39 (11) | 22 (10) | 2.9 (1.1, 10.1) |
ORs for digital loss were estimated using conditional logistic regression. ORs for digital ischemia, eRVSP and mortality were estimated using logistic regression adjusting for age, gender, race, disease type, digital loss and smoking status. ACA = anti-centromere antibodies
Discussion
This investigation addresses the association between presence of aPL and vascular disease in a large well characterized cohort of SSc patients. Our study shows that anti-B2GPI antibodies are more prevalent in SSc patients with digital loss and are significantly associated with features of macrovascular disease including active digital ischemia and echocardiographic evidence of PAH. Also, we report for the first time that positivity to anti-B2GPI is independently associated with higher mortality.
We found that patients with history of ischemic digital loss have, independently from disease duration, a substantially increased risk of death (OR 12.5), together with worse lung severity scores and a lower DLCO. Based on these associations, it is possible that pulmonary vascular disease may be in part responsible for the worse outcome. In fact, at the time of sample testing, the estimated right ventricular systolic pressure was overall higher in digital loss patients. Smoking (current or past) was also more prevalent in the digital loss group and may have contributed as well to a more severe vasculopathy.
We confirmed the known association between anti-centromere antibodies and vascular disease in SSc, and in particular with severe digital ischemia and digital loss (27, 28). However, in accordance with previous studies, we found that anti-centromere status did not independently predict higher mortality in SSc patients (29).
Anti-B2GPI antibodies were significantly more frequent in digital loss patients compared to controls (36% vs. 19%, p=0.017). We detected almost exclusively IgM and IgA isotypes, alone or in combination. Similarly to published data on patients with APS, the IgA isotype was more prevalent in anti-B2GPI positive African-American subjects, although the difference was not statistically significant (30). Unlike previous studies, all the anti-B2GPI isotypes, including IgA, were measured in our SSc patients (11–18). In several subjects, IgA resulted the only isotype present and at relatively high titers. The exclusion of measuring IgA by other investigators may have been driven by some older literature suggesting that screening for IgA anti-B2GPI is not helpful for the diagnosis of APS in SLE (31, 32). This issue remains slightly controversial, with more recent publications reporting a significant association between IgA aPL and thrombosis (33, 34). Importantly, anti-B2GPI antibodies and in particular the IgA isotype have been associated with higher risk for other non connective tissue disorders-related vascular conditions, including peripheral vascular disease (PVD), cerebral ischemia and myocardial infarction (MI) (35–38). Franck et al. reported that the IgA isotype of aCL and anti-B2GPI antibodies was associated with peripheral arterial disease with an adjusted OR 12.1 (95% CI 5.8–30) (34). The same group and other authors showed that IgA anti-B2GPI antibodies were significantly more frequent in patients with ischemic stroke (36). Meroni et al. found that IgM and IgG anti-B2GPI antibodies (IgA not tested) carry a higher risk for MI in young premenopausal women independently from underlying atherosclerotic disease (37). Other investigators detected higher anti-B2GPI levels in patients with acute coronary syndrome compared to controls (14.4% vs. 2%), with particular significance for the IgA isotype (38). All these reports consistently linked different clinical manifestations of larger vessel disease with the detection of higher levels of anti-B2GPI, in particular of the IgA isotype. Our study extends these findings to vascular manifestations associated with SSc and suggests that anti-B2GPI represents an independent biomarker in SSc macrovascular disease.
Previous studies on the prevalence and significance of anti-B2GPI in SSc are limited (11–18). None of these investigations found evidence of APS clinical features in anti-B2GPI positive patients. Only two studies showed a significant association between aPL and SSc vascular disease manifestations such as isolated PAH, peripheral ischemia, and digital pitting (11, 18).
While we found that aCL were more frequent in digital loss patients (24% vs. 13%), the association did not reach statistical significance (p=0.08). Similarly, Herrick et al. found no significant association between presence of IgM and IgG aCL (IgA were not studied) and severe ischemia or amputation in SSc patients (28). Also, consistent with the majority of previous reports, we did not detect any association between aCL and clinical features of APS or macrovascular disease in our cohort. In contrast, after correcting for confounders or disease modifiers, we showed a strong, statistically significant association between anti-B2GPI positivity and SSc manifestations of macrovascular disease such as digital loss, active digital ischemia and echocardiographic evidence of PAH.
The independent association of anti-B2GPI positivity with mortality (OR 2.9, 95% CI 1.1–7.7) is another important finding emerging from our study. We speculate that anti-B2GPI antibodies identify SSc patients with persistent and widespread vascular disease. Our data supports the possibility that the detection of these autoantibodies in patients with active digital ischemia or PAH may indicate sustained and progressive endothelial/vascular damage and predict a worse outcome. Prospective studies are underway to address this initial observation.
How the immune response against B2GPI is generated, and what role these anti- B2GPI antibodies play in SSc vascular manifestations, is no t known. B2GPI is an abundant plasma protein which can be expressed on the surface of endothelial cells and has been found in the intima of the arterial wall as well as within atherosclerotic plaques (39, 40). The formation of complexes with oxidized low-density lipoprotein (oxLDL) or anionic phospholipids has been shown to modify and increase its immunogenicity, facilitating antigen presentation by macrophages and dendritic cells (41). B2GPI-reactive T cells have been identified in the peripheral blood and within atherosclerotic plaques, further confirming that this protein can become immunogenic under certain circumstances (42, 43). On the other hand, anti-B2GPI antibodies are present also in younger SSc patients without overt evidence of atherosclerosis, and can be found in association with acute vascular events, suggesting that other mechanisms such as ongoing perturbation of endothelium homeostasis may be relevant for their generation.
The dominant anti-IgM/IgA B2GPI response detected in SSc sera represents a clear distinction from the anti-B2GPI specificities usually observed in patients with APS and SLE, typically characterized by a substantial IgG isotype class switch (31). This was not observed in the analysis conducted on serial serum samples from several IgM anti-B2GPI positive patients. If confirmed, this interesting finding may suggest that the generation of anti-B2GPI in SSc patients occurs through a T cell independent mechanism. A molecular mimicry between microbial pathogens and B2GPI epitopes has also been identified by some authors (44). Many of these agents (i.e. H. pylori, adenovirus, etc.) are involved in mucosal infections and may trigger an immune response biased towards production of anti-B2GPI IgA. The influence of transforming growth factor beta (TGF-β) should also be considered. This cytokine exerts profibrotic effects in SSc and can promote IgA class switching and plasma cell IgA secretion (45, 46).
Anti-B2GPI antibodies can determine a pro-thrombotic status through many mechanisms, for example by modifying the functional properties of thrombin and protein C (47, 48). They also have the ability to bind to negatively charged phospholipids on the surface of activated or apoptotic cells, promoting pro-inflammatory responses (49). Whether anti-B2GPI antibodies exert some pathogenic role in SSc patients by means of their anti-endothelial and pro-coagulable properties, or are merely an epiphenomenon of the ongoing vascular damage/dysfunction, still remains to be defined. Autoantibodies directed against other proteins (i.e. annexin V) binding to negatively charged phospholipids and interfering with the coagulation cascade have also been detected in SSc (50). However, their role in the pathogenesis of SSc vascular manifestations has never been confirmed.
Strengths of the present study include the relatively large number of patients, their excellent clinical phenotyping, the use of commercial ELISA kits widely used in hospital clinical laboratories and a careful statistical approach, allowing us to properly interrogate the clinical significance of aCL and anti-B2GPI antibodies in SSc. There are some limitations. The case-control and cross-sectional study design does not account for variation of serum levels of aPL overtime. Lupus anticoagulant testing was not performed due to the limited availability of plasma samples. Data regarding the use of platelet antiaggregants and anticoagulants are not included in the study. Such therapies may have influenced the long term survival in our patients.
In conclusion, our investigation showed a significant association between anti-B2GPI antibodies and clinical features of severe vascular disease in SSc including active digital ischemia, digital loss and echocardiographic evidence for PAH. An independent association with higher mortality was also demonstrated. These findings indicate that anti-B2GPI should be further investigated as a reproducible biomarker of vascular disease and a predictor of clinical outcomes in SSc.
Acknowledgements
This work was supported by grants from the Scleroderma Research Foundation (FB, FW) and the National Institutes of Health AR-055667-02 (FB), AR053503 (AR) and AR44684 (LCR). The authors thank Brandon Boring and David Hines for their valuable technical assistance.
Contributor Information
Francesco Boin, Division of Rheumatology, Johns Hopkins University School of Medicine.
Stefano Franchini, Division of General Medicine, University Vita-Salute San Raffaele.
Elizabeth Colantuoni, Anesthesiology and, Critical Care Medicine, Johns Hopkins, University School of Medicine, Biostatistics, Bloomberg School of Public Health.
Antony Rosen, Director, Division of Rheumatology, Johns Hopkins University School of Medicine.
Fredrick M. Wigley, Director, Johns Hopkins Scleroderma Center, Johns Hopkins University School of, Medicine.
Livia Casciola-Rosen, Division of Rheumatology, Johns Hopkins University School of Medicine.
References
- 1.Wigley FM, Hummers LK. Clinical features of systemic sclerosis. In: Hochberg MC, Silman AJ, Smolen JS, Weinblatt ME, Weisman MH, editors. RHEUMATOLOGY. 3rd edition. Mosby: St. Louis, MO; 2003. pp. 1463–1480. [Google Scholar]
- 2.Allanore Y, Seror R, Chevrot A, Kahan A, Drape JL. Hand vascular involvement assessed by magnetic resonance angiography in systemic sclerosis. Arthritis Rheum. 2007;56:2747–2754. doi: 10.1002/art.22734. [DOI] [PubMed] [Google Scholar]
- 3.Youssef P, Brama T, Englert H, Bertouch J. Limited scleroderma is associated with increased prevalence of macrovascular disease. J Rheumatol. 1995;22:469–472. [PubMed] [Google Scholar]
- 4.Hettema ME, Bootsma H, Kallenberg CG. Macrovascular disease and atherosclerosis in SSc. Rheumatology (Oxford) 2008;47:578–583. doi: 10.1093/rheumatology/ken078. [DOI] [PubMed] [Google Scholar]
- 5.Dick EA, Aviv R, Francis I, Hamilton G, Baker D, Black C, et al. Catheter angiography and angioplasty in patients with scleroderma. Br J Radiol. 2001;74:1091–1096. doi: 10.1259/bjr.74.888.741091. [DOI] [PubMed] [Google Scholar]
- 6.Akram MR, Handler CE, Williams M, Carulli MT, Andron M, Black CM, et al. Angiographically proven coronary artery disease in scleroderma. Rheumatology (Oxford) 2006;45:1395–1398. doi: 10.1093/rheumatology/kel120. [DOI] [PubMed] [Google Scholar]
- 7.Wigley FM. Vascular Disease in Scleroderma. Clin Rev Allergy Immunol. 2008 Dec 9; doi: 10.1007/s12016-008-8106-x. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 8.Negi VS, Tripathy NK, Misra R, Nityanand S. Antiendothelial cell antibodies in scleroderma correlate with severe digital ischemia and pulmonary arterial hypertension. J Rheumatol. 1998;25:462–466. [PubMed] [Google Scholar]
- 9.Hughes GR. The anticardiolipin syndrome. Clin Exp Rheumatol. 1985;3:285–286. [PubMed] [Google Scholar]
- 10.Matsuura E, Igarashi Y, Yasuda T, Triplett DA, Koike T. Anticardiolipin antibodies recognize beta 2-glycoprotein I structure altered by interacting with an oxygen modified solid phase surface. J Exp Med. 1994;179:457–462. doi: 10.1084/jem.179.2.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ihn H, Sato S, Fujimoto M, Kikuchi K, Igarashi A, Soma Y, et al. Measurement of anticardiolipin antibodies by ELISA using beta 2-glycoprotein I (beta 2-GPI) in systemic sclerosis. Clin Exp Immunol. 1996;105:475–479. doi: 10.1046/j.1365-2249.1996.d01-774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenroth L, Fritzler M, Lonzetti L, Senecal JL. Antibodies to beta2 glycoprotein I and cardiolipin in SSc. Ann Rheum Dis. 2002;61:183–184. doi: 10.1136/ard.61.2.183-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antonioli CM, Danieli E, Airo P, Cattaneo R, Tincani A. More on anticardiolipin and antibeta2 glycoprotein I in systemic sclerosis. Ann Rheum Dis. 2003;62:589–590. doi: 10.1136/ard.62.6.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assous N, Allanore Y, Batteux F, Meune C, Toulon P, Weill B, et al. Prevalence of antiphospholipid antibodies in systemic sclerosis and association with primitive pulmonary arterial hypertension and endothelial injury. Clin Exp Rheumatol. 2005;23:199–204. [PubMed] [Google Scholar]
- 15.Parodi A, Drosera M, Barbieri L, Rebora A. Antiphospholipid antibody system in systemic sclerosis. Rheumatology (Oxford) 2001;40:111–112. doi: 10.1093/rheumatology/40.1.111. [DOI] [PubMed] [Google Scholar]
- 16.Sanna G, Bertolaccini ML, Mameli A, Hughes GR, Khamashta MA, Mathieu A. Antiphospholipid antibodies in patients with scleroderma: prevalence and clinical significance. Ann Rheum Dis. 2005;64:1795–1796. doi: 10.1136/ard.2005.038430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sulik A, Kowal-Bielecka O, Domyslawska I, Chwiecko J, Sierakowski S. The prevalence and clinical significance of antiphospholipid antibodies in the patients with systemic sclerosis--preliminary report. Rocz Akad Med Bialymst. 2005;50 Suppl 1:228–231. [PubMed] [Google Scholar]
- 18.Marie I, Jouen F, Hellot MF, Levesque H. Anticardiolipin and anti-beta2 glycoprotein I antibodies and lupus-like anticoagulant: prevalence and significance in systemic sclerosis. Br J Dermatol. 2008;158:141–144. doi: 10.1111/j.1365-2133.2007.08309.x. [DOI] [PubMed] [Google Scholar]
- 19.Merkel PA, Chang Y, Pierangeli SS, Convery K, Harris EN, Polisson RP. The prevalence and clinical associations of anticardiolipin antibodies in a large inception cohort of patients with connective tissue diseases. Am J Med. 1996;101:576–583. doi: 10.1016/s0002-9343(96)00335-x. [DOI] [PubMed] [Google Scholar]
- 20.Pope JE, Thompson A. The frequency and significance of anticardiolipin antibodies in scleroderma. J Rheumatol. 2000;27:1450–1452. [PubMed] [Google Scholar]
- 21.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23:581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 22.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28:1573–1576. [PubMed] [Google Scholar]
- 23.Medsger TA, Jr, Silman AJ, Steen VD, Black CM, Akesson A, Bacon PA, et al. A disease severity scale for systemic sclerosis: development and testing. J Rheumatol. 1999;26:2159–2167. [PubMed] [Google Scholar]
- 24.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22:1281–1285. [PubMed] [Google Scholar]
- 25.American Thoracic Society. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1991;144:1202–1218. doi: 10.1164/ajrccm/144.5.1202. [DOI] [PubMed] [Google Scholar]
- 26.Denton CP, Cailes JB, Phillips GD, Wells AU, Black CM, Bois RM. Comparison of Doppler echocardiography and right heart catheterization to assess pulmonary hypertension in systemic sclerosis. Br J Rheumatol. 1997;36:239–243. doi: 10.1093/rheumatology/36.2.239. [DOI] [PubMed] [Google Scholar]
- 27.Wigley FM, Wise RA, Miller R, Needleman BW, Spence RJ. Anticentromere antibody as a predictor of digital ischemic loss in patients with systemic sclerosis. Arthritis Rheum. 1992;35:688–693. doi: 10.1002/art.1780350614. [DOI] [PubMed] [Google Scholar]
- 28.Herrick AL, Heaney M, Hollis S, Jayson MI. Anticardiolipin, anticentromere and anti-Scl-70 antibodies in patients with systemic sclerosis and severe digital ischaemia. Ann Rheum Dis. 1994;53:540–542. doi: 10.1136/ard.53.8.540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Steen VD, Powell DL, Medsger TA., Jr Clinical correlations and prognosis based on serum autoantibodies in patients with systemic sclerosis. Arthritis Rheum. 1988;31:196–203. doi: 10.1002/art.1780310207. [DOI] [PubMed] [Google Scholar]
- 30.Diri E, Cucurull E, Gharavi AE, Kapoor D, Mendez EA, Scopelitis E, et al. Antiphospholipid (Hughes') syndrome in African-Americans: IgA aCL and abeta2 glycoprotein-I is the most frequent isotype. Lupus. 1999;8(4):263–268. doi: 10.1191/096120399678847812. [DOI] [PubMed] [Google Scholar]
- 31.Bertolaccini ML, Atsumi T, Escudero Contreras A, Khamashta MA, Hughes GR. The value of IgA antiphospholipid testing for diagnosis of antiphospholipid (Hughes) syndrome in systemic lupus erythematosus. J Rheumatol. 2001;28:2637–2643. [PubMed] [Google Scholar]
- 32.Danowski A, Kickler TS, Petri M. Anti-beta2-glycoprotein I: prevalence, clinical correlations, and importance of persistent positivity in patients with antiphospholipid syndrome and systemic lupus erythematosus. J Rheumatol. 2006;33:1775–1779. [PubMed] [Google Scholar]
- 33.Lopez LR, Dier KJ, Lopez D, Merrill JT, Fink CA. Anti-beta 2-glycoprotein I and antiphosphatidylserine antibodies are predictors of arterial thrombosis in patients with antiphospholipid syndrome. Am J Clin Pathol. 2004;121:142–149. doi: 10.1309/YVQ6-PX76-XMYM-3J29. [DOI] [PubMed] [Google Scholar]
- 34.Shen Y, Lee R, Frenkel E, Sarode R. IgA antiphospholipid antibodies are an independent risk factor for thromboses. Lupus. 2008;17:996–1003. doi: 10.1177/0961203308093460. [DOI] [PubMed] [Google Scholar]
- 35.Franck M, Staub HL, Petracco JB, Norman GL, Lassen AJ, Schiavo N, et al. Autoantibodies to the atheroma component beta2-glycoprotein I and risk of symptomatic peripheral artery disease. Angiology. 2007;58:295–302. doi: 10.1177/0003319707302493. [DOI] [PubMed] [Google Scholar]
- 36.Kahles T, Humpich M, Steinmetz H, Sitzer M, Lindhoff-Last E. Phosphatidylserine IgG and beta-2-glycoprotein I IgA antibodies may be a risk factor for ischaemic stroke. Rheumatology (Oxford) 2005;44:1161–1165. doi: 10.1093/rheumatology/keh698. [DOI] [PubMed] [Google Scholar]
- 37.Meroni PL, Peyvandi F, Foco L, Bernardinelli L, Fetiveau R, Mannucci PM, et al. Anti-beta 2 glycoprotein I antibodies and the risk of myocardial infarction in young premenopausal women. J Thromb Haemost. 2007;5:2421–2428. doi: 10.1111/j.1538-7836.2007.02763.x. [DOI] [PubMed] [Google Scholar]
- 38.Veres K, Lakos G, Kerenyi A, Szekanecz Z, Szegedi G, Shoenfeld Y, et al. Antiphospholipid antibodies in acute coronary syndrome. Lupus. 2004;13:423–427. doi: 10.1191/0961203304lu1011oa. [DOI] [PubMed] [Google Scholar]
- 39.Caronti B, Calderaro C, Alessandri C, Conti F, Tinghino R, Palladini G, et al. Beta2-glycoprotein I (beta2-GPI) mRNA is expressed by several cell types involved in antiphospholipid syndrome-related tissue damage. Clin Exp Immunol. 1999;115:214–219. doi: 10.1046/j.1365-2249.1999.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yla-Herttuala S, Palinski W, Rosenfeld ME, Parthasarathy S, Carew TE, Butler S, et al. Evidence for the presence of oxidatively modified low density lipoprotein in atherosclerotic lesions of rabbit and man. J Clin Invest. 1989;84:1086–1095. doi: 10.1172/JCI114271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hasunuma Y, Matsuura E, Makita Z, Katahira T, Nishi S, Koike T. Involvement of beta 2-glycoprotein I and anticardiolipin antibodies in oxidatively modified low-density lipoprotein uptake by macrophages. Clin Exp Immunol. 1997;107:569–573. doi: 10.1046/j.1365-2249.1997.d01-948.x. [DOI] [PubMed] [Google Scholar]
- 42.Ito H, Matsushita S, Tokano Y, Nishimura H, Tanaka Y, Fujisao S, et al. Analysis of T cell responses to the beta 2-glycoprotein I-derived peptide library in patients with anti-beta 2-glycoprotein I antibody-associated autoimmunity. Hum Immunol. 2000;61:366–377. doi: 10.1016/s0198-8859(99)00184-6. [DOI] [PubMed] [Google Scholar]
- 43.Hattori N, Kuwana M, Kaburaki J, Mimori T, Ikeda Y, Kawakami Y. T cells that are autoreactive to beta2-glycoprotein I in patients with antiphospholipid syndrome and healthy individuals. Arthritis Rheum. 2000;43:65–75. doi: 10.1002/1529-0131(200001)43:1<65::AID-ANR9>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 44.Blank M, Krause I, Fridkin M, Keller N, Kopolovic J, Goldberg I, et al. Bacterial induction of autoantibodies to beta2-glycoprotein-I accounts for the infectious etiology of antiphospholipid syndrome. J Clin Invest. 2002;109:797–804. doi: 10.1172/JCI12337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Varga J, Abraham D. Systemic sclerosis: a prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA. Transforming growth factor-beta regulation of immune responses. Annu Rev Immunol. 2006;24:99–146. doi: 10.1146/annurev.immunol.24.021605.090737. [DOI] [PubMed] [Google Scholar]
- 47.Rahgozar S, Yang Q, Giannakopoulos B, Yan X, Miyakis S, Krilis SA. Beta2-glycoprotein I binds thrombin via exosite I and exosite II: anti-beta2-glycoprotein I antibodies potentiate the inhibitory effect of beta2-glycoprotein I on thrombin-mediated factor XIa generation. Arthritis Rheum. 2007;56:605–613. doi: 10.1002/art.22367. [DOI] [PubMed] [Google Scholar]
- 48.Ieko M, Ichikawa K, Triplett DA, Matsuura E, Atsumi T, Sawada K, et al. Beta2-glycoprotein I is necessary to inhibit protein C activity by monoclonal anticardiolipin antibodies. Arthritis Rheum. 1999;42:167–174. doi: 10.1002/1529-0131(199901)42:1<167::AID-ANR20>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 49.Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood. 2005 Mar 1;105(5):1964–1969. doi: 10.1182/blood-2004-05-1708. [DOI] [PubMed] [Google Scholar]
- 50.Sugiura K, Muro Y. Anti-annexin V antibodies and digital ischemia in patients with scleroderma. J Rheumatol. 1999;26:2168–2172. [PubMed] [Google Scholar]