Abstract
Background
Atopic dermatitis (AD) patients are prone to disseminated viral skin infections and therefore are not vaccinated against smallpox due to potential complications. Macrophage inflammatory protein (MIP)-3α is a C-C chemokine expressed by keratinocytes that exhibits anti-microbial activity against bacteria and fungi; however its role in anti-viral innate immunity is unknown.
Objective
Evaluate the level of MIP-3α in AD skin and its role in the innate immune response to vaccinia virus (VV).
Methods
MIP-3α levels were evaluated using real-time RT-PCR, immunodot-blot, and immunohistochemistry. The anti-viral activity of MIP-3α was determined using a standard viral plaque assay.
Results
MIP-3α gene expression was significantly (p<0.01) decreased in AD skin (0.21 ± 0.05 ng MIP-3α/ng GAPDH) compared to psoriasis skin (0.67 ± 0.13). This was confirmed at the protein level using immunohistochemistry. We further demonstrate that Th2 cytokines down-regulate MIP-3α expression. The importance of MIP-3α in the innate immune response against VV was established by first demonstrating that MIP-3α exhibits activity against VV. Secondly, VV replication was significantly increased (p<0.01) in keratinocytes treated with an antibody to neutralize MIP-3α.
Conclusions
The current study demonstrates that MIP-3α exhibits anti-viral activity against VV and the importance of MIP-3α in the innate immune response against VV. Additionally, AD skin is deficient in MIP-3α, due, in part, to the over-expression of Th2 cytokines in AD skin.
Clinical Implications
MIP-3α deficiency in AD skin contributes to their increased propensity towards eczema vaccinatum. Increasing MIP-3α or neutralizing Th2 cytokines could prevent adverse reactions in AD patients following smallpox vaccination.
Keywords: Atopic dermatitis, chemokines, innate immunity, vaccinia virus, anti-microbial peptides
Introduction
Atopic dermatitis (AD) is a chronic inflammatory skin disorder that affects approximately 17% of children and is associated with significant disruption in the quality of life.1,2 Recent studies have shown that the Th2 cytokine milieu in AD skin down-regulates several innate immune response genes3-6 thereby pre-disposing patients to recurrent bacterial and viral skin infections.1 Currently, the Center for Disease Control and Prevention recommends that AD patients refrain from smallpox vaccination due to potential complications of disseminated viral infection including eczema vaccinatum.7 This represents a significant challenge to any mass vaccination campaign using the currently available Wyeth Strain of vaccinia virus (VV).
Chemokines are a family of small proteins (5 -- 20 kDa) with a crucial role in innate and adaptive immunity.8 In recent years, chemokines have been shown to exhibit anti-bacterial activity and therefore may serve as a new class of anti-microbial molecules.9 In particular, macrophage inflammatory protein (MIP-) 3α (CCL20) is a C-C chemokine constitutively expressed by keratinocytes in the epidermis.10 It is additionally expressed by endothelial cells, monocytes, and fibroblasts following stimulation11 and neutrophils in response to microbial pathogens.12 MIP-3α is an important chemokine responsible for the epidermal recruitment of Langerhans cells through its interaction with the C-C chemokine receptor 6 (CCR6).11 This characteristic is an important step in the development of cutaneous immunity.
Using gene chip microarray technology, our laboratory previously found that MIP-3α expression is decreased in lesional skin from AD patients, as compared to psoriasis.4 In the current study, we set forth to confirm this observation using more quantitative methods with increased sensitivity and reliability at both the gene and protein level as well as to study its modulation by Th2 cytokines. Additionally, we examined whether physiological levels of MIP-3α protein exhibit activity against VV and may therefore contribute to the increased propensity of AD patients toward eczema vaccinatum.
Materials and Methods
Subjects
Study participants included 12 healthy controls (mean age: 24.7 years), eighteen patients with AD (mean age: 34.1 years; 10 – 60% skin involvement) and fourteen patients with psoriasis (mean age: 46.7 years; 15 – 40% skin involvement). None of the patients had previously received any systemic immunosuppressive drugs such as corticosteroids or cyclosporine and all patients were asked to refrain from use of topical corticosteroids or calcineurin inhibitor for more than one week prior to enrollment. These studies were conducted according to the Declaration of the Helsinki Guidelines and were approved by the Institutional Review Board at National Jewish Medical and Research Center in Denver. All subjects gave written informed consent prior to enrollment.
Four 2 mm skin biopsies were collected from enrolled subjects and immediately submerged in 1 ml of Tri-Reagent (Molecular Research Center, Inc., Cincinnati, OH) and frozen at -80°C for future RNA and protein isolation or immediately submerged in 1 ml of 10% buffered formalin for immunohistochemistry.
Virus source and culture
The Wyeth strain of VV was obtained from the Centers for Disease Control and Prevention (Atlanta, GA). VV was cultured in HeLa S3 (American Type Culture Collection, Manassas, VA; CCK-2.2) human adenocarcinoma cells as previously described.13 This yielded infectious virions in the form of intracellular mature virions.14
Keratinocyte cell cultures
Primary human keratinocytes were routinely grown in serum-free keratinocyte growth medium (EpiLife®, Cascade Biologics, Portland, OR), supplemented with 1% human keratinocyte growth supplement V2 (Cascade Biologics), 0.06 mM calcium chloride and 1% of antibiotics (penicillin/streptomycin). To study the effects of Th2 cytokines on MIP-3α and VV gene expression, primary keratinocytes were seeded at 2 × 105 cells per well and differentiated in the presence of 1.3 mM CaCl2 for five days. Keratinocytes were then incubated with 50 ng/ml of interleukin (IL)-4 (R&D Systems; Minneapolis, MN) and 50 ng/ml of IL-13 (R&D Systems) for 24 hours. After 24 hours, fresh cytokines were added to the culture and cells were then infected with VV (0.1 pfu/cell) for an additional 24 hours. Following the final 24 hour incubation, media was removed and RNA isolated from keratinocytes using RNeasy kits according to the manufacturer's guidelines (Qiagen, Valencia, CA) for real-time RT-PCR.
The HaCaT human keratinocyte cell line was grown in Dulbecco's Modified Eagle's Medium (Cellgro, Herndon, VA), supplemented with 10% fetal calf serum (FCS; Gemini Bio Products, Calabasas, CA) and 1% of the following: penicillin/streptomycin, L-glutamine, minimal essential medium (MEM) with non-essential amino acids (GIBCO, Grand Island, New York), and MEM vitamin solution (GIBCO). Once confluent, cells were seeded in 24-well plates at 2 × 105 cells per well and cultured for 24 hours to allow adherence. To examine the direct effect of MIP-3α on VV gene expression, HaCaT cells were infected with 0.1 pfu of VV per cell in the presence and absence of either 1 μg/ml anti-MIP-3α (R&D Systems) or isotype control (mouse IgG1). Following a 24 hour incubation, media was removed and RNA isolated from keratinocytes according to the manufacturer's guidelines (Qiagen).
RNA preparation and analysis
Total RNA was isolated from skin biopsies by chloroform:phenol extraction and isopropanol precipitation according to the manufacturer's guidelines (Sigma Chemical Co., St Louis, Missouri). RNeasy Mini Kits (Qiagen) were used to isolate RNA from cell cultures and to further purify RNA from skin biopsies. One microgram of RNA was reverse transcribed in a 20 μl reaction containing Random Primers (Invitrogen, Carlsbad, CA), RNase Inhibitor (Invitrogen), and Superscript II enzyme (Invitrogen) for 60 minutes at 42°C and then 70°C for 15 minutes. Real-time PCR was performed and analyzed by the dual-labeled fluorigenic probe method using an ABI Prism 7000 sequence detector (Applied Biosystems, Foster City, CA) as previously described.5 Primers and probe for human GAPDH and MIP-3α were purchased from Applied Biosystems. The primer sequences that were used to assay for the vaccinia gene transcripts are: Forward, 5′-GCCAATGAGGGTTCGAGTTC-3′ and Reverse, 5′-CAACATCCCGTCGTTCATCA-3′. This region of the genome encodes a subunit of a DNA-directed RNA polymerase expressed within two hours of viral entry.15 Relative expression levels were calculated by the relative standard curve method as outlined in the manufacturer's technical bulletin (Applied Biosystems). A standard curve was generated using the fluorescent data from ten-fold serial dilutions of total RNA of the highest expression sample. To allow for comparisons between samples and groups, quantities of all targets in test samples were normalized to the corresponding GAPDH levels in the skin biopsies and cultured keratinocytes and expressed as target gene normalized to GAPDH.
Immunohistochemistry for MIP -3α
Paraffin-embedded tissues were cut into 5 μm sections and placed on frosted microscope slides. Through a series of toluene and ethanol washes, slides were de-paraffinized and re-hydrated. Slides were then immersed in a 10 mM Citrate solution (pH 6.0) and microwaved for 16 minutes to retrieve masked antigens. Slides were washed in PBS and treated with a 0.2% Triton solution for 30 minutes at room temperature. Endogenous peroxidase was blocked with 5% H2O2 in PBS for 30 minutes followed by a universal block (Dako, Inc, Mississauga, Ontario) for 30 minutes at room temperature. Slides were stained with a monoclonal mouse anti-human antibody directed against MIP-3α (1:25 dilution; R&D Systems) overnight at 4°C. Slides were then washed and incubated for 45 minutes with a biotinylated rabbit anti-mouse Ig antibody (1:100 dilution; Dako, Inc.). The antigen-antibody complex was detected using the avidin-biotin peroxidase complex (ABC) method. Diamino benzidine (Dako, Inc.) was used to visualize the antibody specific staining. Slides were counterstained with Hematoxylin Gill II. Antibody specificity was determined by replacing the primary antibody with an isotype-matched control.
All slides were coded before evaluation to ensure subject anonymity and that the evaluator was unaware of the disease diagnosis in each slide. Images were collected at 200X magnification and the intensity of the immunostaining scored on a scale from 0 to 8, where 0 indicated no staining, 1 = 0 – 12.5% staining, 2 = 12.5 – 25%, with a stepwise increase by 12.5% to 8 = 100%.
Immuno-dot Blot
Immuno-dot blot was performed with a Bio-Dot Microfiltration Apparatus from Bio-Rad (Hercules, CA). Protein was isolated from skin biopsies by acetone precipitation and washed using 0.3M guanidine hydrochloride in 95% ethanol with 2.5% glycerol according to the manufacturer's guideline (Molecular Research Center, Inc). Fifty micrograms of total protein were loaded in a final volume of 200 μl of Tris buffered saline with 0.5% Tween-20 (TBST) and allowed to bind to the nitrocellulose membrane by gravity filtration. The membrane was then blocked in 5% milk in TBST for 30 minutes at room temperature. After blocking, the membrane was washed once with TBST to remove residual blocking solution and then incubated overnight at 4°C with anti-MIP-3α (R&D Systems) at 2 μg/ml in TBST. The blot was washed three times with TBST for five minutes and then incubated with anti-mouse HRP conjugated secondary antibody (Amersham Biosciences, UK) at a dilution of 1:10,000 in TSBT for one hour at room temperature. Blots were washed three times, five minutes each with TBST and then developed with ECL Western Blotting Detection Reagents (Amersham Biosciences, UK) according to the manufacturer's protocol. The intensity of MIP-3α proteins was determined by digital image analysis using NIH image 1.61 software program. Specificity of the antibody was determined by replacing the primary antibody with a mouse IgG1 isotype control (Southern Biotechnology, Birmingham, AL).
In order to make comparisons between study subject populations, a standard curve was generated for MIP-3α protein using recombinant human MIP-3α protein (R&D Systems). The concentration of MIP-3α in the original skin-biopsy sample was then estimated by dividing the experimentally determined mass of MIP-3α from the skin-biopsy sample by the epidermal volume. The epidermal volume was estimated assuming an epidermal thickness of 0.1 mm; thus, the volume of epidermis in a 2-mm punch-biopsy specimen was 3.14×10-1 mm3.
Anti-viral assays
BS-C-1 (American Type Culture Collection: CCL-26), African green monkey kidney cells (2 × 105/well), were seeded in 24-well tissue culture plates in MEM-10% FCS and penicillin/streptomycin and allowed to grow overnight before the supernatant was removed and replaced with MEM-2.5% FCS for virus incubation. BS-C-1 cells were used for the quantitative estimates because they present uniform plaques.16 HeLa S3 cells are routinely used for preparations of virus stock because they give consistently high yields of virus but, due to their rounded morphology, do not present uniform plaques.
MIP-3α (R&D systems) was diluted to the proper concentrations in 0.01X tryptic soy broth containing 10 mM sodium phosphate buffer, pH 7.4. Virus was diluted in the same buffer and then added to the MIP-3α. The MIP-3α/virus mixture was incubated for 24 hours at 37°C and then 20 μl was added to the BS-C-1 cells in 0.5 ml of MEM-2.5% FCS and allowed to infect for 48 hours for plaque development. For the plaque assay, the medium was removed, and wells were overlaid with 0.5 ml of 10% buffered formalin and allowed to fix for five minutes at room temperature. The formalin was removed, and 0.5 ml of 0.1% crystal violet in PBS was added to the wells for five minutes at room temperature. Wells were then aspirated and air-dried for visualization of plaques. VV was diluted for this assay to generate 50 – 80 plaques in wells with VV alone.
Statistical analysis
Statistical analysis was conducted using Graph Pad Prism, version 4.03 (San Diego, CA). Data were analyzed by a one-way ANOVA and significant differences between exposure groups were determined by a Tukey-Kramer test.17
Results
MIP-3α levels in normal subjects, AD and psoriasis skin
Skin biopsies were collected from non-inflamed skin of normal subjects, as well as the inflamed skin lesions of AD and psoriasis patients. MIP-3α gene expression was evaluated using real-time RT-PCR and normalized to the endogenous control, GAPDH, for all samples to allow comparisons between samples and patient populations. As shown in Figure 1, MIP-3α gene expression was significantly higher in lesional skin from psoriasis patients (mean: 0.67 ± 0.13 ng, MIP-3α/ng GAPDH) as compared to lesional AD skin (mean: 0.21 ± 0.05; p < 0.01), uninvolved AD skin (0.19 ± 0.05; p<0.01), or normal skin (mean: 0.20 ± 0.07; p < 0.01). Uninvolved skin from psoriasis patients exhibited higher expression of MIP-3a (0.33 ± 0.13) as compared to uninvolved AD skin, lesional AD skin, and normal skin; however these differences did not reach statistical significance. Immunohistochemical staining confirmed increased MIP-3α levels in the skin of psoriasis, as compared to AD, patients (Figure 2A). The composite data for MIP-3α immunostaining in all samples are shown in Figure 2B. The intensity of MIP-3α staining in skin from psoriasis patients was significantly higher than that for normal (p<0.001) and AD (p<0.001) skin. Additionally, the greatest staining intensity was seen in keratinocytes.
Figure 1.
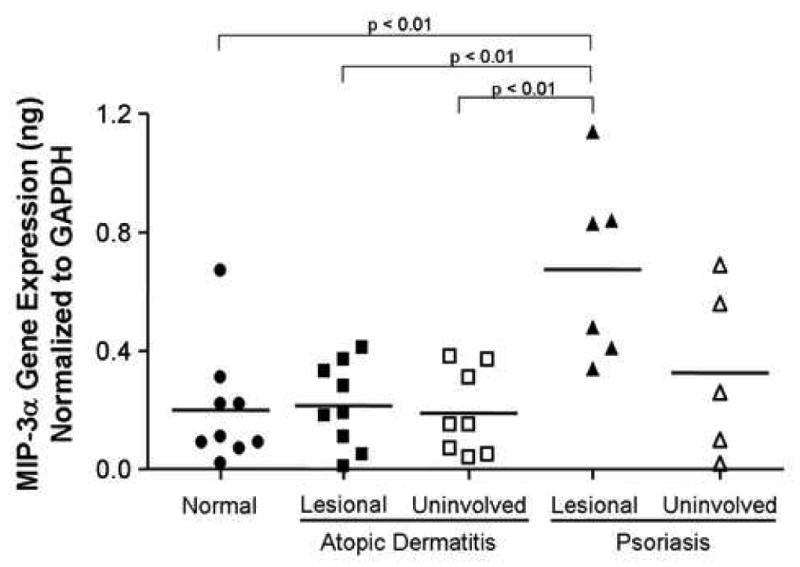
MIP-3α gene expression is decreased in AD, as compared to psoriasis, skin. RNA was isolated from the skin of nine normal subjects, nine AD, and six psoriasis patients and the levels of MIP-3α gene expression were evaluated by real-time RT-PCR as described in the methods section. Data are expressed as the mean ± SEM.
Figure 2.
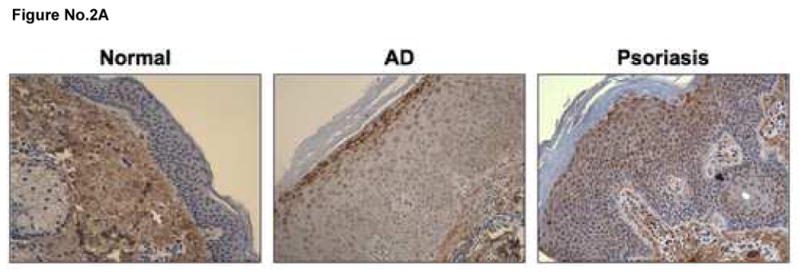

MIP-3α protein expression is decreased in AD, as compared to psoriasis, skin. A. Paraffin embedded skin biopsies were cut into 5 μm sections and stained for MIP-3α. Representative images are shown at 200X magnification evaluated from six AD, six psoriasis and six normal subjects. B. The intensity of the immunostaining was visually scored on a scale from 0 to 8, where 0 indicated no staining, 1 = 0 – 12.5% staining, 2 = 12.5 – 25%, with a stepwise increase by 12.5% to 8 = 100. *indicates statistical significance of p<0.05.
Quantitation of Physiological MIP-3α Protein Levels
Cultured keratinocytes have been shown to secrete greater than 2 ng/ml of MIP-3α following stimulation,18 however the concentration of MIP-3α within the skin is not known. Therefore skin biopsies were collected from the non-inflamed skin of normal subjects, as well as inflammatory lesions of AD and psoriasis patients, then evaluated for MIP-3α protein expression using an immuno-dot blot assay. As shown in Figure 3, MIP-3α protein expression was significantly higher in skin lesions from psoriasis (mean: 104.20 ± 11.97 μM) as compared to AD skin (mean: 44.49 ± 9.15 μM; p<0.01) or skin from normal subjects (mean: 39.51 ± 6.81 μM; p < 0.001).
Figure 3.

MIP-3α is induced by VV and down-regulated by Th2 cytokines. Primary keratinocytes were stimulated with 0.1 pfu/cell of VV in the presence and absence of IL-4 (50 ng/ml) and IL-13 (50 ng/ml) for 24 hours. MIP-3α gene expression was evaluated by real-time RT-PCR. Data are expressed as the mean ± SEM with an n=6.
Induction and Regulation of MIP-3α
Previously, MIP-3α has been shown to be induced in keratinocytes by pro-inflammatory cytokines.18 Therefore we investigated whether VV would induce the expression of MIP-3α in primary keratinocytes. MIP-3α expression was significantly elevated (p<0.01) in keratinocytes infected with 0.1 pfu of VV per cell (0.34 ± 0.04 ng MIP-3α/ng GAPDH) as compared to media alone (0.21 ± 0.01) (Figure 4). IL-4 and IL-13 have previously been shown to be elevated in the skin of AD patients.5,19,20 Pre-incubation of keratinocytes with IL-4 and IL-13 prior to VV infection in these studies significantly reduced the expression of MIP-3α, (0.24 ± 0.03; p<0.05) as compared to infection with VV alone. IL-4 and IL-13 have previously been shown by our laboratory to augment VV replication in keratinocytes.21
Figure 4.
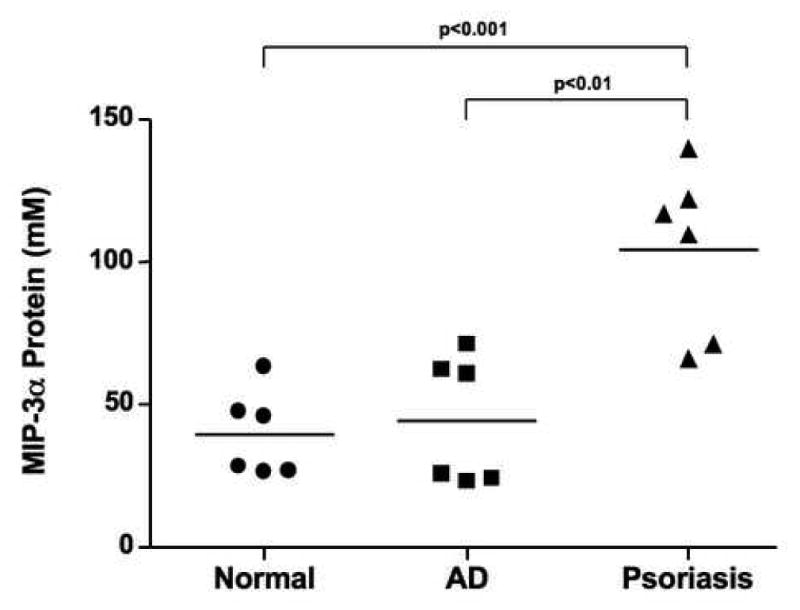
Quantification of MIP-3α protein levels in skin biopsies from normal subjects, lesional AD skin, and lesional psoriasis skin. Protein was isolated from the skin of six normal subjects, six AD, and six psoriasis patients and the levels of MIP-3α protein were evaluated by immuno-dot blot. Data are expressed as the mean ± SEM
Anti-viral Activity of MIP-3α Against VV
After identifying the physiological concentrations of MIP-3α reached in psoriasis, a skin disease not associated with recurrent infection, we used a viral plaque assay to determine whether these concentrations of MIP-3α exhibit anti-viral activity against VV. As shown in Figure 5, pre-incubation of VV with MIP-3α resulted in a concentration-dependent reduction in plaque formation. Significantly fewer plaques were observed upon pre-incubation of VV with 25 μM (32 ± 3 plaques; p<0.01) and 50 μM (21 ± 1 plaques; p<0.01) of MIP-3α as compared to VV alone (73 ± 3 plaques).
Figure 5.
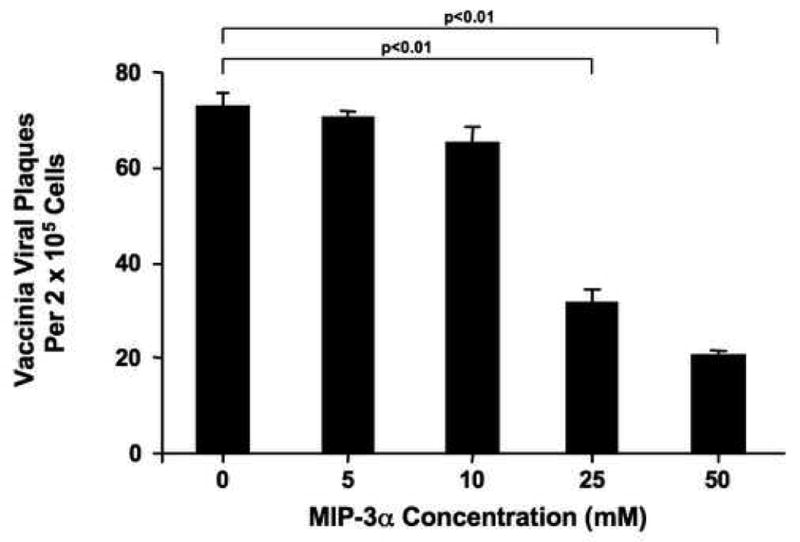
MIP-3α exhibits anti-viral activity against VV. VV was incubated with varying concentrations of MIP-3α for 24 hours and then evaluated using a standard viral plaque assay. Data are expressed as the mean ± SEM with an n=6.
To further demonstrate the importance of MIP-3α, keratinocytes were pre-incubated with a MIP-3α neutralizing antibody or isotype control prior to VV infection. As shown in Figure 6, VV gene expression was significantly higher in keratinocytes pre-treated with anti-MIP-3α (mean: 13913 ± 1807 ng VV/ng GAPDH; p<0.01) as compared to the untreated keratinocytes (mean: 7800 ± 919). Keratinocytes treated with the isotype control antibody exhibited similar levels of VV gene expression as untreated keratinocytes.
Figure 6.
Neutralization of MIP-3α augments VV replication. HaCaT cells were stimulated with 0.1 pfu/cell of VV for 24 hours in the presence and absence of 1 μg/ml of anti-MIP-3α. VV gene expression was evaluated by real-time RT-PCR. Data are expressed as the mean ± SEM with an n=6.
Discussion
The epidermis is a keratinocyte rich region of the skin that provides a physical barrier against invading bacterial and viral pathogens. Keratinocytes in the epidermis migrate through the basal, spinous and granular regions to terminally differentiate into anucleate cells and form the stratum corneum.22 The stratum corneum is the skin's first line of defense against invading microbial pathogens. When the stratum corneum is compromised, further invasion by pathogens is prevented by the innate immune response, largely the production of antimicrobial peptides (AMPs), such as human beta defensins and cathelicidins, by keratinocytes. AMPs are an integral part of the innate immune response as they have been shown to be effective in killing bacterial20 and viral13 pathogens. Previously we have demonstrated that the AMPs human β-defensin (HBD)-2, HBD-3, and the cathelicidin LL-37 are deficient in AD skin.5,20 This deficiency may account, in part, for the increased susceptibility of AD patients to recurrent skin infections, however the role of additional molecules in the innate immune response, particularly against viral agents, such as VV, have not been well characterized.
MIP-3α is a C-C chemokine that plays an important role in the innate and adaptive immune response by exhibiting anti-bacterial activity and recruiting Langerhan's cells to the skin.9,11,23 Using gene chip microarrays, we previously observed elevated expression of MIP-3α in the lesional inflamed skin of psoriasis patients as compared to inflamed skin lesions from AD patients.4 We confirmed this observation in the current study using real-time RT-PCR and immunohistochemistry which have a higher degree of sensitivity and reproducibility. Levels of MIP-3α gene expression in inflamed AD skin lesions were approximately 50% lower than those in psoriasis skin lesions. This is most likely due to differences in the cytokine microenvironments in the skin. Psoriasis skin has been shown to over-express pro-inflammatory cytokines such as TNF-α, IL-6, IL-1, and IFN-γ5,24 which induce the expression of AMPs5,19,25,26 while negligible levels of TNF-α and IFN-γ have been shown in AD skin.5 Normal skin is unstimulated and therefore would not be expected to express increased levels of MIP-3α.
In the current study, we demonstrate for the first time that stimulation with VV induces MIP-3α chemokine expression in keratinocytes and therefore provides an added dimension to the innate anti-viral immune responses of this important cell type. However, pre-incubation of keratinocytes with IL-4 and IL-13, Th2 cytokines over-expressed in the AD skin,3,5,18,27 significantly inhibited the induction of MIP-3α. Interestingly, these cytokines have previously been shown to support VV replication.21 Combined with our current data, this could further explain why AD patients are susceptible to disseminated VV infection.
MIP-3α exhibits potent activity against bacteria and fungi,9,23 however its activity against viruses has not previously been studied. Based on our data suggesting a role for MIP-3α in the pathogenesis of VV infection, we investigated whether MIP-3α exhibits activity against VV. Our current study is the first to demonstrate that MIP-3α kills VV in a dose dependent manner. Since little is known about the anti-viral activity of MIP-3α, we further investigated what physiological levels of MIP-3α are found in AD versus psoriasis skin. Physiologic concentrations of MIP-3α found in psoriasis skin were significantly higher than those necessary to kill VV. In contrast, concentrations found in normal healthy skin and lesional AD skin were significantly lower than those found in psoriasis. Indeed, several of the AD patients enrolled in the current study possessed levels of MIP-3α concentration needed to achieve significant VV killing. This may explain why a subpopulation of AD patients is at greater risk of disseminated viral infection than the AD population as a whole. Normal skin is unstimulated, therefore it would not be expected to exhibit increased MIP-3α. The biological significance of these observations is further supported by our observation that neutralization of MIP-3α in keratinocytes, allows significantly greater VV replication (Figure 6). This further illustrates the importance of this chemokine in the innate immune response of the skin to VV. The exact mechanism by which MIP-3α exhibits its anti-viral activity is not known, however the high isoelectric point (pI>10.6) and specific distribution of positively charged residues suggests a direct interaction with the negatively charged viral envelope.8,9,23
The current study demonstrates that chemokines such as MIP-3α exhibits anti-viral activity against VV and further demonstrates a role for MIP-3α in controlling VV pathogenesis. Additionally, we demonstrate that insufficient expression and production of MIP-3α in AD skin increases the potential for disseminated VV infection following smallpox vaccination. Importantly, human keratinocytes have evolved to express a complex array of AMPs and chemokines to provide effective innate immune responses to viral invasion.
Acknowledgments
The authors are indebted to the nursing staff in the General Clinical Research Center for the recruitment of patients and collection of specimens. Additionally the authors thank Rho, Inc. for their help in statistical analysis (NIH contract HHSN266200400033C) and Maureen Sandoval for her help in preparing this manuscript.
This work was supported in part by the Inje University Research grant (B.E.K); NIH grants NIH/NIAID contracts N01 AI40029 and N01 AI40030, AR41256, R21 AR051634-01, General Clinical Research Center grant MO1 RR00051 from the Division of Research Resources, the Edelstein Family Chair in Pediatric Allergy and Immunology, and the University of Colorado Cancer Center (D.Y.M.L)
Abreviations
- AD
Atopic dermatitis
- CCR6
C-C chemokine receptor 6
- EV
Eczema vaccinatum
- FCS
Fetal calf serum
- IL
Interleukin
- MEM
Minimum essential media
- MIP-3α
Macrophage inflammatory protein 3 alpha
- PBMC
Peripheral blood mononuclear cell
- TBST
Tris buffered saline with 0.05% Tween 20
- VV
Vaccinia virus
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Leung DYM, Boguniewicz M, Howell MD, Nomura I, Hamid QA. New insights into atopic dermatitis. J Clin Invest. 2004;113:651–7. doi: 10.1172/JCI21060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akdis CA, Akdis M, Bieber T, Bindslev-Jensen C, Boguniewicz M, Eigenmann P, et al. Diagnosis and treatment of atopic dermatitis in children and adults: European Academy of Allergology and Clinical Immunology/American Academy of Allergy, Asthma, and Immunology/PRACTALL Consensus Report. J Allergy Clin Immunol. 2006;118:152–69. doi: 10.1016/j.jaci.2006.03.045. [DOI] [PubMed] [Google Scholar]
- 3.Howell MD, Novak N, Bieber T, Pastore S, Girolomoni G, Boguniewicz M, et al. IL-10 down-regulates anti-microbial peptide expression in atopic dermatitis. J Invest Dermatol. 2005;125:738–45. doi: 10.1111/j.0022-202X.2005.23776.x. [DOI] [PubMed] [Google Scholar]
- 4.Nomura I, Gao B, Boguniewicz M, Darst MA, Travers JB, Leung DYM. Distinct patterns of gene expression in the skin lesions of atopic dermatitis and psoriasis: A gene microarray analysis. J Allergy Clin Immunol. 2004;112:1195–202. doi: 10.1016/j.jaci.2003.08.049. [DOI] [PubMed] [Google Scholar]
- 5.Nomura I, Goleva E, Howell MD, Hamid QA, Ong PY, Hall CF, et al. Cytokine milieu of atopic dermatitis, as compared to psoriasis, skin prevents induction of innate immune response genes. J Immunol. 2003;171:3262–9. doi: 10.4049/jimmunol.171.6.3262. [DOI] [PubMed] [Google Scholar]
- 6.McGirt LY, Beck LA. Innate immune defects in atopic dermatitis. J Allergy Clin Immunol. 2006;118:202–8. doi: 10.1016/j.jaci.2006.04.033. [DOI] [PubMed] [Google Scholar]
- 7.Rotz LD, Dotson DA, Damon IK, Becher JA. Vaccinia (smallpox) vaccine: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2001;50:1–25. [PubMed] [Google Scholar]
- 8.Esche C, Stellato C, Beck LA. Chemokines: Key players in innate and adaptive immunity. J Invest Dermatol. 2005;125:615–28. doi: 10.1111/j.0022-202X.2005.23841.x. [DOI] [PubMed] [Google Scholar]
- 9.Yang D, Chen Q, Hoover DM, Staley P, Tucker K, Lubkowski J, et al. Many chemokines including CCL20/MIP-3α display antimicrobial activity. J Leuk Biol. 2003;74:448–55. doi: 10.1189/jlb.0103024. [DOI] [PubMed] [Google Scholar]
- 10.Homey B, Dieu-Nosjean MC, Wiesenborn A, Massacrier C, Pin JJ, Oldham E, et al. Up-regulation of macrophage inflammatory protein-3α /CCL20 and CC chemokine receptor 6 in Psoriasis. Journal of Immunology. 2000;164:6621–32. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 11.Dieu-Nosjean MC, Massacrier C, Homey B, Vanbervliet B, Pin JJ, Vicari A, et al. Macrophage inflammatory protein-3α is expressed at inflammed epithelial surfaces and is the most potent chemokine known in attracting Langerhans cell precursors. J Exp Med. 2000;192:705–17. doi: 10.1084/jem.192.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akahoshi T, Sasahara T, Namai R, Matsui T, Watabe H, Kitasato H, et al. Production of macrophage inflammatory protein-3a (MIP-3α)(CCL20) and MIP-3β (CCL-19) by human peripheral blood neutrophils in response to microbial pathogens. Infect Immun. 2003;71:524–6. doi: 10.1128/IAI.71.1.524-526.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Howell MD, Jones JF, Kisich KO, Streib JE, Gallo RL, Leung DYM. Selective killing of vaccinia virus by LL-37: Implications for Eczema vaccinatum. J Immunol. 2004;172:1763–7. doi: 10.4049/jimmunol.172.3.1763. [DOI] [PubMed] [Google Scholar]
- 14.Schmelz M, Sodeik B, Ericsson M, Wolffe EJ, Shida H, Hiller G, et al. Assembly of vaccinia virus: The second wrapping cisterna is derived from the trans golgi network. J Virol. 1994;68:130–47. doi: 10.1128/jvi.68.1.130-147.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amegadzie BY, Ahn BY, Moss B. Identification, sequence, and expression of the gene encoding a Mr 35,000 subunit of the vaccinia virus DNA-dependent RNA polymerase. J Biol Chem. 1991;266:13712–8. [PubMed] [Google Scholar]
- 16.Current Protocols in Molecular Biology. New York: John Wiley & Sons; 2002. [Google Scholar]
- 17.Tukey J. Exploratory data analysis. Reading, NY: Addison Wesley; 1977. [Google Scholar]
- 18.Nakayama T, Fujisawa R, Yamada H, Horikawa T, Kawasaki H, Hieshima K, et al. Inducible expression of a CC chemokine liver- and activation-regulated chemokine (LARC)/macrophage inflammatory protein (MIP)-3α /CCL20 by epidermal keratinocytes and its role in atopic dermatitis. Int Immunol. 2001;13:95–103. doi: 10.1093/intimm/13.1.95. [DOI] [PubMed] [Google Scholar]
- 19.Homey B, Steinhoff M, Ruzicka T, Leung DY. Cytokines and chemokines orchestrate atopic skin inflammation. J Allergy Clin Immunol. 2006 Jul;118(1):178–89. doi: 10.1016/j.jaci.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 20.Ong PY, Ohtake T, Brandt C, Strickland I, Boguniewicz M, Ganz T, et al. Endogenous antimicrobial peptides and skin infections in atopic dermatitis. N Engl J Med. 2002;347:1151–60. doi: 10.1056/NEJMoa021481. [DOI] [PubMed] [Google Scholar]
- 21.Howell MD, Gallo RL, Boguniewicz M, Jones JF, Wong C, Streib JE, et al. Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus. Immunity. 2006;24:341–8. doi: 10.1016/j.immuni.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 22.Candi E, Schmidt R, Melino G. The cornified envelope: A model of cell death in the skin. Nat Rev. 2005;6:328–40. doi: 10.1038/nrm1619. [DOI] [PubMed] [Google Scholar]
- 23.Hoover DM, Boulegue C, Yang D, Oppenheim JJ, Tucker K, Lu W, et al. The structure of human macrophage inflammatory protein-3α /CCL20. Linking antimicrobial and CC chemokine receptor-6-binding activities with human β-defensins. J Biol Chem. 2000;277:37647–54. doi: 10.1074/jbc.M203907200. [DOI] [PubMed] [Google Scholar]
- 24.Grossman RM, Krueger J, Yourish D, Granelli-Piperno A, Murphy DP, May LT, et al. Interleukin 6 is expressed in high levels in psoriatic skin and stimulates proliferation of cultured keratinocytes. PNAS. 1989;86:6367–71. doi: 10.1073/pnas.86.16.6367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Erdag G, Morgan JR. Interleukin-1a and interleukin-6 enhance the antibacterial properties of cultured composite keratinocyte grafts. Ann Surg. 2002;235:113–24. doi: 10.1097/00000658-200201000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu AY, Destoumieux D, Wong AV, Park CH, Valore EV, Liu L, et al. Human β-defensin-2 production in keratinocytes is regulated by interleukin-1, bacteria, and the state of differentiation. J Invest Dermatol. 2002;118:275–81. doi: 10.1046/j.0022-202x.2001.01651.x. [DOI] [PubMed] [Google Scholar]
- 27.Jeong CW, Ahn KS, Rho NK, Park YD, Lee DY, et al. Yang JM. Differential in vivo cytokine mRNA expression in lesional skin of intrinsic vs. extrinsic atopic dermatitis patients using semiquantitative RT-PCR. Clin Exp Allergy. 2003;33:1717–24. doi: 10.1111/j.1365-2222.2003.01782.x. [DOI] [PubMed] [Google Scholar]


