Abstract
Wound healing studies, which have chiefly examined dermal tissues, have reported a female advantage in healing rates. Recently our laboratory demonstrated women heal mucosal wounds more slowly than men. We hypothesized sex hormones influence wound healing rates, possibly through their modulating effects on inflammation. This study involved 329 younger subjects aged 18-43 (165 women, 164 men) and 93 older subjects aged 50-88 (60 women, 33 men). A 3.5mm diameter wound was created on the hard oral palate and videographed daily to assess wound closure. Blood collected at the time of wounding was used to assess circulating testosterone, progesterone and estradiol levels, and in vitro cytokine production in response to LPS. No strong associations were observed between healing times and estradiol or progesterone levels. However, in younger subjects, lower testosterone levels related to faster wound closure. Conversely, in older women higher testosterone levels related to 1) lower inflammatory responses; and 2) faster healing times. No such relationships were seen in older men, or in women taking oral contraceptives or hormone replacement therapy [HRT]. Older women (50-54 years) not yet experiencing menopause healed similarly to younger women and dissimilarly from age-matched post-menopausal women. This suggests that the deleterious effects of aging on wound healing occur secondary to the effects of menopause. Supporting this, there was evidence in post-menopausal women that HRT augmented wound closure. Overall, this study suggests that human mucosal healing rates are modulated by testosterone levels. Based upon when between-group differences were observed, testosterone may impact upon the proliferative phase of healing which involves immune processes such as re-epithelialization and angiogenesis.
Keywords: Testosterone, estradiol, progesterone, follicular, luteal, menopause, inflammation, cytokines, aging, menstrual cycle
Introduction
To date, wound healing studies have chiefly examined dermal wounds and reported a female advantage in healing rates (Ashcroft et al., 1997; Ashcroft and Mills, 2002; Jorgensen et al., 2002; Shimizu et al., 2004; Gilliver and Ashcroft, 2007). Conversely, when observing oral mucosal wounds our laboratory has found a male advantage in healing rates (Engeland et al., 2006). In addition, mucosal wound healing after oral surgical procedures has been associated with greater complications and longer recovery times in women (Conrad et al., 1999; Phillips et al., 2003; Benediktsdottir et al., 2004; Adeyemo et al., 2006). Thus, gender advantages in wound healing appear to be tissue specific.
Sex hormones, specifically estrogens and progesterone, play a role in mucosal inflammation as demonstrated in both gingivitis (Ashcroft et al., 1999) and periodontal disease (Mascarenhas et al., 2003), suggesting they are mechanistically related to mucosal wound healing. However, this association has not been verified. Importantly, the sexual dimorphism observed in dermal healing rates has been linked to the modulating effects of sex hormones on healing processes, specifically on inflammation (Ashcroft et al., 1997; Ashcroft and Mills, 2002; Gilliver and Ashcroft, 2007). Overall, androgens generally lengthen, whereas estrogens shorten, healing times in skin (for recent reviews see Gilliver et al., 2007; Marucha and Engeland, 2007).
Compared to dermal tissue, mucosal tissue heals much faster with less inflammation and scarring (Lee and Eun, 1999; Szpaderska et al., 2003; Heikkinen, 2006). This suggests that the level of inflammation needed for optimal healing is lower in mucosal tissue. Females mount higher cellular, humoral and inflammatory responses (Schuurs and Verheul, 1990; Miller and Hunt, 1996; Zuk and McKean, 1996; Giglio et al., 1994), have higher levels of circulating antibodies (Giglio et al., 1994; Miller and Hunt, 1996) and a greater ability to clear bacteria than males (Krzych et al., 1981; Miller and Hunt, 1996; Engeland et al., 2003) (for review see Bouman et al., 2005). These enhanced immune responses in females have been primarily attributed to differences in levels of circulating sex hormones (Gaillard and Spinedi, 1998; Lahita, 2000), and in particular the lack of circulating androgens (Bilbo and Nelson, 2001). Sex hormones can influence healing by modulating inflammation, which may explain the observed reversal in the gender advantage for healing between dermal and mucosal tissues. Testosterone generally has immunosuppressive and anti-inflammatory properties (McCruden and Stimson, 1991; Giglio et al., 1994; Wichmann et al., 1997; Savita and Rai, 1998), although there is evidence that testosterone promotes inflammation in dermal wound healing (Ashcroft and Mills, 2002; Ashcroft et al., 2003a).
Estrogens have been shown to generally have anti-inflammatory effects (Ashcroft et al., 1999; Ashcroft and Ashworth, 2003; Mascarenhas et al., 2003) whereas progesterone may promote inflammation (Leslie and Dubey, 1994; Cannon and St. Pierre, 1997; Cannon, 1998; Bouman et al., 2001a, 2001b, 2005; Mascarenhas et al., 2003). In line with these findings, it has been shown that women exhibit higher inflammatory responses during the luteal phase (characterized by high progesterone levels) compared to the follicular phase (characterized by high estrogen levels) of the menstrual cycle (Bouman et al., 2001a, 2001b; Leslie and Dubey, 2004; Cannon and St Pierre, 2007; O'Brien et al., 2007). Although beyond the scope of this paper, it is important to note that that both estrogens and progesterone have complex interactions with immunity and may either inhibit or activate the immune system depending which immune responses are being observed (for reviews see Beagley and Gockel, 2003; Cutolo et al., 2002, 2006; Bird et al., 2008).
The gender differences in mucosal healing rates previously reported by this laboratory (Engeland et al., 2006) encouraged us to look into the role of sex hormones in mucosal wound healing. The current study determined circulating sex hormone levels from three past human wound healing studies using available blood samples. Testosterone levels were ascertained for all subjects since this hormone is the principal androgen in men, and is produced by the ovaries and adrenals in women. Estradiol and progesterone levels were only determined in naturally cycling young women, as the use of oral contraceptives (OCs) alters the production of these endogenous hormones (Chabbert et al., 1998). Also, after menopause the predominant estrogen becomes estrone, rendering estradiol a poor measure of biological function in post-menopausal women.
The objective of this study was to determine the relationships between sex hormones and mucosal wound healing rates in several comparison groups: younger and older men, naturally cycling women versus women taking OCs, naturally cycling women in the follicular phase versus the luteal phase of the menstrual cycle, and post-menopausal women with and without hormone replacement therapy (HRT). Due to previously observed gender differences, we hypothesized that sex hormone levels would be predictive of wound healing rates, possibly through the modulation of inflammatory responses.
2. Methods
2.1. Participants
This study involved 329 younger subjects aged 18-43 years (165 women, 164 men) and 93 older subjects aged 50-88 years (60 women, 33 men). All individuals gave written informed consent and received monetary compensation for their participation. Questionnaires were used to determine demographics, health history and behaviors, and current medication use. Participants were excluded only if they had an oral disease needing emergency intervention or medical problems that would make them a high surgical risk (e.g., unstable angina or an infectious disease such as hepatitis, tuberculosis, or AIDS). All of the wounding was performed by a licensed periodontist (P.T.M). All of the procedures were carried out in The Ohio State University and the analysis of the data was carried out at the University Of Illinois College Of Dentistry and met with institutional review board and ethics committee approval at both institutions.
2.2. Wound placement
Subjects arrived and were seated in the dental clinic between 9:30 AM and 10:30 AM. Wounds were created between the first and second molar approximately 3 mm from the marginal gingival. The site was anesthetized with 2% lidocaine. The wound area was outlined using a 3.5 mm tissue punch. A scalpel was used to remove the surface epithelium and superficial connective tissue to create a wound with a uniform depth of 1.5 mm. No dressing was used over the wound site. Participants were encouraged to resume their normal oral hygiene procedures but refrain from using alcohol-based mouth wash. A longitudinal wound (1×5×1.5 mm) was placed anterior to this first wound, and a 2×5×1.5 mm biopsy of this second wound was obtained at either 6h or 24h post-wounding. Gene expression for inflammatory mediators was determined from all tissues obtained (0h, 6h, 24h) using real-time PCR.
2.3. Wound size assessment
Wounds were videographed at 24h intervals for 7 days after wounding. A standard 6 mm label was placed around the wound to account for variations in magnification and angulation. These images were transferred to a Macintosh computer, blind coded and measured for area. The same person measured all the wounds. All values were expressed as a ratio of the wound to the standard label. These values were then expressed as a ratio to the original wound size. This is an objective measure of wound closure which has been used extensively in dermal wound studies in humans (Kiecolt-Glaser, 1995) and animals (Padgett et al., 1998), and in mucosal wound studies in humans (Bosch et al., 2007; Engeland et al., 2006)
2.4. Sex hormone assessment
Blood was drawn at the time of wounding, as well as 15 and 30 min post-wounding, via catheter. A total of 5 ml blood was drawn in injectable EDTA-coated tubes and blood plasma was used to measure sex hormones levels. Enzyme immunoassays (EIAs) were performed on available blood samples using commercial kits to determine plasma testosterone and estradiol levels (ALPCO Diagnostics, Salem, NH), as well as progesterone levels (Immuno-Biological Laboratories, Inc. IBL America, Minneapolis, MN). Standard protocols provided with the kits were followed. Testosterone levels were measured for all subjects. Estradiol and progesterone levels were measured only in naturally cycling young women.
2.5. Determining the stage of the menstrual cycle
Due to the sharp rise in progesterone levels shortly after ovulation, progesterone concentration can be used to predict the stage of the menstrual cycle (Hampson and Young, 2008). The United States National Institutes of Health reported in 2004 that normal cycling women produce 0.2-1.4 ng/ml of progesterone in the follicular phase and 4.0-25 ng/ml in the luteal phase. Based on this report, values of 4.0 ng/ml or higher were designated to the luteal phase and values below 1.4 ng/ml were designated to the follicular phase. The phase of the menstrual cycle for all subjects was further validated by self-report obtained at the time of wounding. By using this method we were able to determine the phase of the menstrual cycle in the majority of subjects. Of 99 naturally cycling young women, three subjects had progesterone levels between 1.4-4.0 ng/ml and two subjects had self-reports which did not agree with their hormone levels. These five subjects were excluded from the analyses. Overall, 79 subjects were determined to be in the follicular phase and 15 subjects in the luteal phase of the menstrual cycle.
2.6. Assessment of inflammation
Inflammatory responses in blood were determined by incubating 250 μl whole blood overnight at 37°C with 1 μg/ml lipopolysaccharide (LPS) (derived from E. coli serotype 055:B5, lot 42K4044, Sigma Chemical, St. Louis MO). Blood was then frozen at −80°C. Protein expressions were determined with Multiplex Bead Immunoassays following the manufacturer's protocol (Invitrogen, Carlsbad CA) and using the Bio-Plex Array System (Bio-Rad Laboratories, Hercules CA).
2.7. Statistical analyses
Data were analyzed using a mixed-design analysis of covariance (ANCOVA) which covaried for age, and treated wound size for 7 days post-wounding as a within-subjects factor, and the various groups as between-subjects factors. Spearman's rho (r) analysis was used to assess correlations between variables. Median splits, based on lower and higher testosterone levels, were performed as follow-up analyses to some correlations. All hypothesis tests were 2-tailed and used α=.05 to determine significance. Data were analyzed using SPSS 14.0 (SPSS Inc., Chicago IL).
3. Results
3.1. Sex hormones levels
Hormone levels in all subgroups are shown in Tables 1 and 2. Groups with different hormonal profiles included 1) younger and older men; 2) naturally cycling women versus women taking OCs; 3) naturally cycling women in the follicular phase versus the luteal phase; and 4) post-menopausal women taking HRT or not taking HRT. In addition, six women were identified who were in the older group (50+ years) but still cycling (none were taking OCs). The median age of menopause is 51 years of age (Hampson and Young, 2008). These women were 50-54 years of age and were likely perimenopausal (i.e., within the 2-8 year time period before menopause begins). However, perimenopause is characterized by anovulatory cycles which were not assessed for. For the purpose of this study, these women were termed as being “close to menopause”.
Table 1.
Testosterone levels (mean ± SEM)
| Group | N | Testosterone (ng/ml) | Age |
|---|---|---|---|
| Young men | 164 | 5.81 ± 0.17 | 22.5 ± 0.3 |
| Older men | 33 | 4.19 ± 0.53 | 60.5 ± 1.6 |
| Young women (no OCs) | 94 | 1.82 ± 0.18 | 22.4 ± 0.5 |
| Young women (OCs) | 66 | 1.45 ± 0.21 | 23.2 ± 0.5 |
| Young women (follicular) | 79 | 1.89 ± 0.21 | 22.0 ± 0.5 |
| Young women (luteal) | 15 | 1.45 ± 0.14 | 24.8 ± 1.8 |
| Older women (no HRT) | 39 | 0.92 ± 0.13 | 61.0 ± 1.6 |
| Older women (HRT) | 15 | 0.83 ± 0.15 | 62.1 ± 2.2 |
| Perimenopausal women | 6 | 1.24 ± 0.26 | 51.5 ± 0.6 |
Table 2.
Progesterone and estradiol levels in naturally cycling young women across the menstrual cycle (mean ± SEM)
| Phase | N | Progesterone (ng/ml) | Estradiol (pg/ml) |
|---|---|---|---|
| Follicular | 79 | 0.49 ± 0.10 | 116.82 ± 8.71 |
| Luteal | 15 | 8.98 ± 1.10 | 138.40 ± 13.31 |
Testosterone levels were higher in younger compared to older men (p<0.001). Similarly, testosterone levels were higher in younger versus older women, regardless of either OC or HRT use (p<0.001). Among naturally cycling women, all three hormones were assessed and the only significant finding was the expected difference in progesterone levels between follicular and luteal phases of the menstrual cycle (p<0.001).
3.2. Testosterone in men
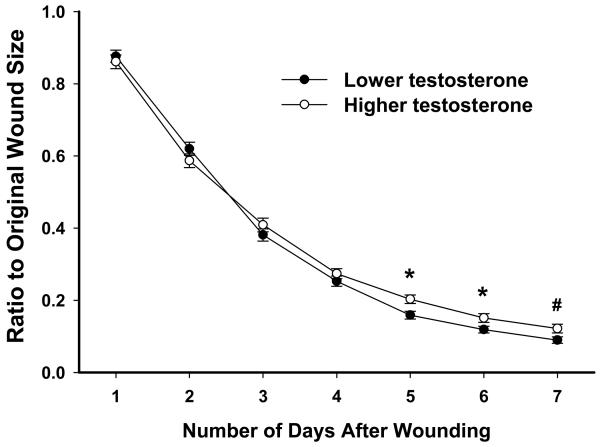
In young men, significant relationships between testosterone levels and wound closure were evident on days 5 and 6 (rs=0.212, p<.01; rs=0.144, p=.066), with lower levels of testosterone being related to smaller wound sizes. To further explore this relationship, median splits were performed based on testosterone levels to divide young men into lower and higher testosterone groups (lower mean: 4.20±.08 ng/ml, n=83; higher mean: 7.46±.21 ng/ml, n=81). Individuals with higher testosterone levels showed significantly delayed wound healing on days 5-6 (p<.05) compared to those with lower testosterone levels (Fig. 1). Among older men, no relationship was found between testosterone levels and healing rates (not shown).
Fig 1.
Young men with higher testosterone levels exhibited delayed wound closure on days 5-7 compared to young men with lower testosterone levels (based on median split). No such differences were seen in older men. Error bars represent standard error of the mean (SEM). * p<0.05, # p<.10
3.3. Testosterone in young women
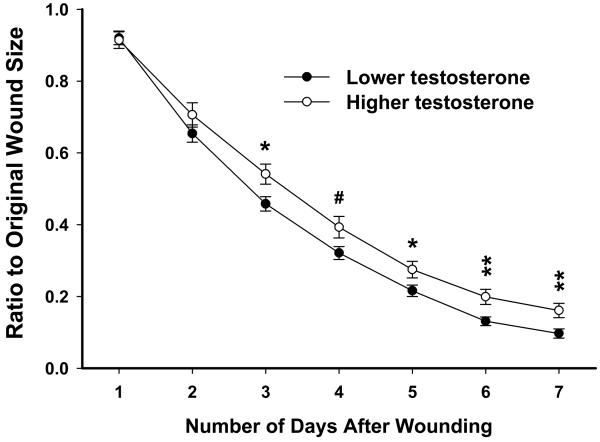
Similar to young men, in naturally cycling young women there were significant relationships between testosterone levels and wound sizes on days 3, 6 and 7 (rs= 0.214 to 0.245, p<.05), with lower levels of testosterone being related to smaller wound sizes. These individuals were divided into higher and lower testosterone groups (lower mean: 1.03±.05 ng/ml, n=48; higher mean: 2.64±.31 ng/ml, n=46). ANCOVA revealed there was a main effect of Hormone Level (F(1,89)=4.85, p<.05). Naturally cycling young women with higher testosterone levels showed delayed wound healing on days 3 and 5-7 (p<.05 or better) compared to the lower testosterone group (Fig. 2). Similar effects of testosterone were apparent within each phase of the menstrual cycle (not shown). Interestingly, women taking OCs exhibited no relationship between testosterone levels and wound closure (not shown).
Fig 2.
In naturally cycling young women, individuals with higher testosterone levels exhibited delayed wound closure on days 3-7 compared to individuals with lower testosterone levels (based on median split). These effects were not seen in women taking OCs. Error bars represent SEM. * p<.05, ** p<0.01, # p<0.10
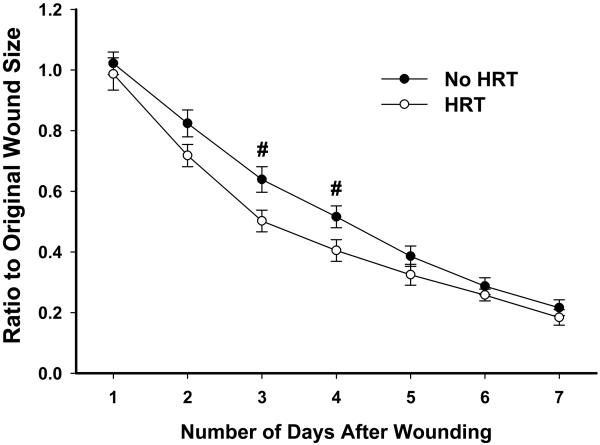
3.4. Testosterone in older women
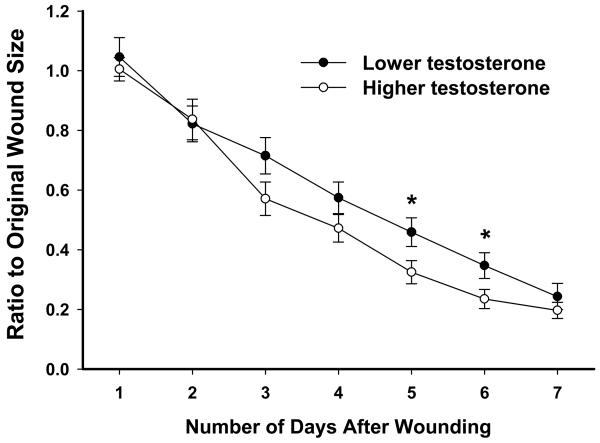
Post-menopausal women were divided into two groups based on whether they were taking HRT or not. Older women taking HRT showed a trend towards faster healing on days 3 and 4 compared to those not taking HRT (Fig 3). Among older women not taking HRT there were significant negative correlations between testosterone levels and wound sizes on days 5 and 6 (rs=−0.391, p<.05; rs=−0.332, p<.05), with higher levels of testosterone being related to smaller wound sizes. Dividing these women into lower and higher testosterone groups (lower mean: .43±.04 ng/ml, n=20; higher mean: 1.44±.22 ng/ml, n=19) revealed that individuals with lower testosterone levels exhibited delayed healing on days 5 and 6 (p<.05) compared to the higher testosterone group (Fig 4). Within either group, there was no relationship between testosterone levels and age. Among older women taking HRT, no relationships were found between wound sizes and testosterone levels (not shown).
Fig 3.
In post-menopausal women, those taking HRT exhibited a trend for faster wound closure compared to those not taking HRT. Error bars represent SEM. # p<.10
Fig 4.
In post-menopausal women not taking HRT, those with higher testosterone levels exhibited faster wound closure on days 5-6 compared to those with lower testosterone levels (based on median split). Error bars represent SEM. * p<.05
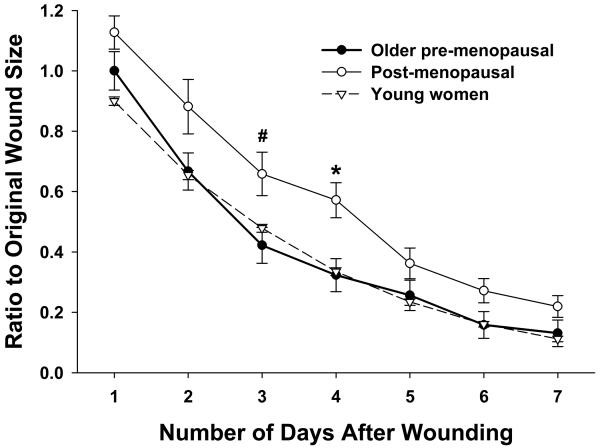
Six naturally cycling older women (50-54 years of age) participated in this study. The patterns of healing between naturally cycling young women and these women were similar, and group wound sizes did not differ at any time point. Interestingly, overall wound closure occurred significantly faster in these six women compared to age-matched (50-55 years of age; n=13) post-menopausal women not taking HRT (F(1,17)=4.93, p<.05, n=13) (Fig. 5). As these observed healing differences were not due to age, this suggests that older women heal mucosal wounds similarly to younger women until the onset of menopause.
Fig 5.
Older women who were pre-menopausal (50-54 years; n=6) showed a pattern of healing similar to that of young women (n=160) and dissimilar to that of age-matched (50-55 years) post-menopausal women not taking HRT (n=13). This suggests that age is not a negative factor on wound healing in women until menopause begins. Error bars represent SEM. * p<.05, # p<.10
3.5. Estradiol and progesterone in young naturally cycling women
The relationships between estradiol, progesterone and wound healing were investigated in naturally cycling women. No relationships were found between wound healing rates and either estradiol levels or estradiol/progesterone ratios (not shown). Progesterone levels significantly correlated with wound sizes on days 6 and 7 (rs=0.220, p<.05; rs=0.214, p<.05). A median split based on progesterone levels showed that women with higher progesterone exhibited slower healing on day 6 (p<.05) (not shown). This effect was strongest during the follicular phase. However, given that healing differences did not occur between the phases of the menstrual cycle which are inherent to large differences in progesterone levels, this relationship was viewed to be less biologically significant in the context of wound healing than that of testosterone.
3.6. Sex hormones and inflammation
LPS-stimulated blood was examined for cytokine production associated with inflammation (i.e., IL-1α, IL-1β, IL-1ra, IL-6, TNF-α). Significant negative correlations were found between testosterone levels and IL-1α, IL-1β and IL-1ra levels in post-menopausal women not taking HRT (rs=−0.338 to −0.370; p<.05; correlations with IL-6 and TNF-α were negative [−.232, −.236] but non-significant). Thus, higher testosterone levels related to lower inflammatory responses to immune challenge in these women (see Table 3 for cytokine levels). No other correlations were evident between sex hormones and cytokine levels in any subgroup.
Table 3.
LPS-stimulated plasma levels of cytokines in post-menopausal women not taking HRT (ng/ml).
| Testosterone | IL-1α | IL-1β | IL-1ra | IL-6 | TNF-α |
|---|---|---|---|---|---|
| Lower (n=20) | 14.80±1.32 | 83.32±7.88 | 105.36±5.36 | 243.88±13.96 | 66.12±12.60 |
| Higher (n=19) | 12.32±1.44 | 67.68±7.20 | 97.16±8.72 | 249.20±29.52 | 51.68±13.28 |
Discussion
This study demonstrates a relationship between circulating testosterone levels and mucosal wound healing. In both young men and naturally cycling young women lower testosterone levels related to faster wound closure later in the healing process. Conversely in post-menopausal women not taking HRT, higher testosterone levels related to faster healing times. Previous studies have shown the importance of testosterone on dermal wound healing (Ashcroft and Mills, 2002; Ashcroft et al., 2003a; Gilliver et al., 2003) and the modulatory effects of this hormone on immune responses (Cutolo et al., 2002; Palaszynski et al., 2004). Generally, lower testosterone levels have been related to faster healing times. The present findings, in young adults, are in concordance with these studies. Although there are studies demonstrating beneficial effects of estrogens on dermal healing in both animals (Ashcroft et al., 1997, 2003a, 2003b) and humans (Ashcroft et al., 1997, 1999), no strong relationships were found between estradiol or progesterone levels and mucosal healing in this study. Also, no differences in mucosal healing were observed between the follicular and luteal phases of the menstrual cycle.
The finding that lower testosterone levels relate to faster healing times in young men and women is in line with work by Ashcroft's group which has shown testosterone to be detrimental to healing rates in murine skin. For instance, castrated male mice were found to heal dermal wounds faster and with less inflammation than sham-operated controls and androgen treatment reversed this effect (Ashcroft and Mills, 2002; Ashcroft et al., 2003a; Gilliver et al., 2003). This may have to do with the ability to clear bacteria which, in male mice, is similarly increased following castration and reversed with testosterone replacement (Schuurs and Verheul, 1990). Interestingly, we have found in mucosal tissues that men heal faster than women (Engeland et al., 2006). This was associated with higher tissue inflammation in women than in men at 6h post-wounding, and testosterone levels were not related to this response (unpublished observations).
In mucosal tissues the inflammatory phase of wound healing typically subsides within the first 24h. However, in young adults the observed differences in wound sizes between higher and lower testosterone groups occurred during later time points in this study (i.e., from day 3 onwards in young women, from day 5 onwards in young men). Also, no relationships were observed between testosterone levels and LPS-induced inflammatory responses in these two groups. This suggests that testosterone affects mucosal healing at a later time point in the healing process than during the inflammatory phase. The proliferative phase of mucosal healing, which occurs 1-7 days post-wounding and involves re-epithelialization, encompasses these later time points. Testosterone reduces levels of IL-6, which is mitogenic to keratinocytes and appears necessary for re-epithelialization to occur in a timely manner (Ashcroft and Mills, 2002). For instance, IL-6 knockout mice healed dermal wounds three times slower than wild-type controls due to delayed re-epithelialization (Ashcroft and Mills, 2002). It is important to note that metabolites of testosterone and other androgens (e.g., DHEA) can also affect wound healing. For instance, it has recently been reported in male rats that 5α-dihydrotestosterone (DHT), a testosterone metabolite, retards dermal wound closure by slowing the migration of epidermal keratinocytes (Gilliver et al., in press). Thus, modulation of re-epithelialization is a possible mechanism through which testosterone and/or its metabolite DHT may have influenced healing in young adults in this study. In future studies, the effects of testosterone should be re-examined with a specific focus on the proliferative phase of healing and its associated mechanistic processes, such as re-epithelialization and angiogenesis.
Testosterone levels begin to reliably drop in men after the age of 40. Since aging negatively influences both testosterone levels (present observations) and healing rates (Engeland et al., 2006), the relationship seen in younger men between lower testosterone levels and faster mucosal healing may have become obscured with age. In addition, free testosterone is the component which declines in men the most with age. As this study assessed total testosterone, the determination of relationships to testosterone levels in older men may have lacked in sensitivity. Indeed, in older men, no relationship was observed between testosterone levels and mucosal wound healing.
When examining wound healing mechanisms, it is important to differentiate between women using synthetic hormones (e.g., OCs) from those who are not. The relationship between testosterone levels and wound closure was only evident when women were not taking OCs or HRT, suggesting this relationship was altered by the use of exogenous hormones. Whether this was due to direct effects of these synthetic hormones on wound healing or to indirect effects through their modulation of endogenous hormone levels could not be determined. It is known that the synthetic hormones contained in these treatments modulate the levels and the effects of endogenously produced hormones (Chabbert et al., 1998). Although the differences were non-significant, mean testosterone levels in OC and HRT groups were lower than non-OC and non-HRT groups, suggesting that endogenous testosterone levels were down-regulated in individuals taking synthetic hormones in this study.
After menopause, both the elasticity and the strength of the skin are diminished due to collagen loss and reduced capillary blood flow (Ashcroft et al., 1997, 2003b; Ashcroft and Ashworth, 2003; Raine-Fenning et al., 2003). Not surprisingly, impaired dermal healing following menopause has been well documented. Dermal wounds in post-menopausal women are characterized by increased neutrophil influx and protease production, decreased phagocytosis, and excessive inflammation (Ashcroft and Ashworth, 2003). The end result is delayed re-epithelialization, reduced collagen deposition and slower healing (Ashcroft et al., 1997a). In animals, ovariectomized mice have longer healing times than sham-operated controls, and the healing deficits seen in these mice are qualitatively similar to those listed above in post-menopausal women (Ashcroft and Ashworth, 2003; Ashcroft et al., 2003a). Moreover, these impairments can be reversed by estrogen replacement (Ashcroft et al., 2003a). This suggests that the deficits in mucosal wound healing which have been reported in older women (Engeland et al., 2006) involve post-menopausal changes.
In this study, the healing pattern of older women not yet menopausal was similar to that of young women and dissimilar from that of post-menopausal women of the same age. This suggests that: 1) aging in women may not be a negative factor on wound healing until menopause begins; and 2) menopause, rather than age, may serve as the better indicator of risk for impaired healing in oral surgical procedures in women. This may also explain why healing rates in this study seemed to improve when women used HRT. These data are in line with previous reports that HRT is beneficial to dermal healing in post-menopausal women (Ashcroft et al., 1997), and extend these findings to oral mucosal healing.
Among post-menopausal women not taking HRT, the relationship between testosterone levels and wound closure rates was opposite to that observed in young naturally cycling women. Specifically, post-menopausal women with higher testosterone levels exhibited faster mucosal healing, independent of age. In addition, lower inflammatory responses in blood were associated with higher testosterone levels. Importantly, lower inflammation in mucosal tissues has been related to faster healing (Szpaderska et al., 2003; unpublished observations). Thus, a putative mechanism by which testosterone may benefit wound closure in post-menopausal women involves its inhibitory effects on inflammation. Indeed, after menopause women exhibit higher inflammation (i.e., spontaneous increases in pro-inflammatory cytokines) (Bouman et al., 2005; Kovacs, 2005; for review see Pfeilschifter et al., 2002), which may explain the reversal in association between testosterone levels and wound closure in this subgroup.
Testosterone has strong anti-inflammatory properties (McCruden and Stimson, 1991; Giglio et al., 1994), which provides a potential mechanism by which inflammatory responses may have been reduced in these women. Intriguingly, in post-menopausal women, inflammatory responses in blood may serve as a predictor of oral healing rates. There is also evidence that testosterone directly promotes angiogenesis (Franck-Lissbrant et al., 1998; Sordello et al., 1998; Colombel et al., 2005). Impairments in angiogenesis and associated growth factor production have been shown to delay wound healing (Broadley et al., 1989; Ortega et al., 1998; Dovi et al., 2004), and decreased capillary blood flow occurs following menopause (Raine-Fenning et al., 2003). This suggests that angiogenesis may be especially important in post-menopausal women for normal healing to occur. Further studies are needed to more fully explore these relationships between testosterone, inflammation and mucosal tissue healing in women after menopause.
It is well accepted that delays in wound closure lead to increased risk of infection and poorer healing outcomes (Robson, 1997). For perspective, the delays in wound closure observed in the present study are about equal in magnitude to those seen: 1) in dermal wounds in animal models of diabetes vs. healthy controls (Langer et al., 2002; Herve Sroussi, personal communication); and 2) in human mucosal wounds due to the effects of age or depressive symptomology (Engeland et al., 2006; Bosch et al., 2006), all of which are known to negatively impact healing. Thus, the magnitude of the effects observed in this study may have clinical implications, especially if combined with other risk factors for slowed healing (e.g., stress) with which they might have additive or synergistic effects (for recent review see Engeland and Marucha, in press).
To sum, this study supports previous reports in mice that testosterone negatively impacts healing (Ashcroft and Mills, 2002; Ashcroft et al., 2003a; Gilliver et al., 2003), and extends these findings to human mucosal tissues but in young adults only. This relationship was not apparent in older men and it was opposite in older (post-menopausal) women. It is not known if a similar relationship exists between testosterone levels and dermal wound closure in older women. Given the similarities in findings between the present mucosal study and past dermal studies this seems quite possible.
Acknowledgements
The authors gratefully acknowledge April Logue, MS, for help in coordinating these studies and in scoring wound videographs. We also thank Dr. Elizabeth Hampson for valuable input concerning the assessment and interpretation of sex hormones in women. This study was supported by grants P01 AG-16321, P50 DE-13749 and RO1 DE-12792 from the National Institutes of Health, and by the UIC College of Dentistry.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Adeyemo WL, Ladeinde AL, Ogunlewe MO. Clinical evaluation of post-extraction site wound healing. J. Contemp. Dent. Pract. 2006;7:40–49. [PubMed] [Google Scholar]
- Ashcroft GS, Dodsworth J, van Boxtel E, Tarnuzzer RW, Horan MA, Schultz GS, Ferguson MW. Estrogen accelerates cutaneous wound healing associated with an increase in TGF-beta1 levels. Nat. Med. 1997;3:1209–1215. doi: 10.1038/nm1197-1209. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am. J. Pathol. 1999;155:1137–1146. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Mills SJ. Androgen receptor-mediated inhibition of cutaneous wound healing. J. Clin. Invest. 2002;110:615–624. doi: 10.1172/JCI15704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft GS, Ashworth JJ. Potential role of estrogens in wound healing. Am. J. Clin. Dermatol. 2003;4:737–743. doi: 10.2165/00128071-200304110-00002. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Mills SJ, Flanders KC, Lyakh LA, Anzano MA, Gilliver SC, Roberts AB. Role of Smad3 in the hormonal modulation of in vivo wound healing responses. Wound Repair Regen. 2003a;11:468–473. doi: 10.1046/j.1524-475x.2003.11614.x. [DOI] [PubMed] [Google Scholar]
- Ashcroft GS, Mills SJ, Lei K, Gibbons L, Jeong MJ, Taniguchi M, Burow M, Horan MA, Wahl SM, Nakayama T. Estrogen modulates cutaneous wound healing by downregulating macrophage migration inhibitory factor. J. Clin. Invest. 2003b;111:1309–1318. doi: 10.1172/JCI16288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beagley KW, Gockel CM. Regulation of innate and adaptive immunity by the female sex hormones oestradiol and progesterone. FEMS Immunol. Med. Microbiol. 2003;38:13–22. doi: 10.1016/S0928-8244(03)00202-5. [DOI] [PubMed] [Google Scholar]
- Benediktsdottir IS, Wenzel A, Petersen JK, Hintze H. Mandibular third molar removal: risk indicators for extended operation time, postoperative pain, and complications. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2004;97:438–446. doi: 10.1016/j.tripleo.2003.10.018. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Sex steroid hormones enhance immune function in male and female Siberian hamsters. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2001;280:R207–R213. doi: 10.1152/ajpregu.2001.280.1.R207. [DOI] [PubMed] [Google Scholar]
- Bird MD, Karavitis J, Kovacs EJ. Sex differences and estrogen modulation of the cellular immune response after injury. Cell Immunol. 2008;252:57–67. doi: 10.1016/j.cellimm.2007.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch JA, Engeland CG, Cacioppo JT, Marucha PT. Depressive symptoms predict mucosal wound healing. Psychosom. Med. 2007;69:597–605. doi: 10.1097/PSY.0b013e318148c682. [DOI] [PubMed] [Google Scholar]
- Bouman A, Moes H, Heineman MJ, de Leij LF, Faas MM. The immune response during the luteal phase of the ovarian cycle: increasing sensitivity of human monocytes to endotoxin. Fertil. Steril. 2001a;76:555–559. doi: 10.1016/s0015-0282(01)01971-9. [DOI] [PubMed] [Google Scholar]
- Bouman A, Moes H, Heineman MJ, de Leij LF, Faas MM. Cytokine production by natural killer lymphocytes in follicular and luteal phase of the ovarian cycle in humans. Am. J. Reprod. Immunol. 2001b;45:130–134. doi: 10.1111/j.8755-8920.2001.450302.x. [DOI] [PubMed] [Google Scholar]
- Bouman A, Heineman MJ, Faas MM. Sex hormones and the immune response in humans. Hum. Reprod. Update. 2005;11:411–423. doi: 10.1093/humupd/dmi008. [DOI] [PubMed] [Google Scholar]
- Broadley KN, Aquino AM, Woodward SC, Buckley-Sturrock A, Sato Y, Rifkin DB, Davidson JM. Monospecific antibodies implicate basic fibroblast growth factor in normal wound repair. Lab. Invest. 1989;61:571–575. [PubMed] [Google Scholar]
- Cannon JG. Adaptive interactions between cytokines and the hypothalamic-pituitary-gonadal axis. Ann. N.Y. Acad. Sci. 1998;856:234–242. doi: 10.1111/j.1749-6632.1998.tb08330.x. [DOI] [PubMed] [Google Scholar]
- Cannon JG, Pierre BA. Gender differences in host defense mechanisms. J. Psychiatr. Res. 1997;31:99–113. doi: 10.1016/s0022-3956(96)00055-6. [DOI] [PubMed] [Google Scholar]
- Chabbert BN, Djakoure C, Maitre SC, Bouchard P. Regulation of the human menstrual cycle. Front Neuroendocrinol. 1998;19:151–186. doi: 10.1006/frne.1998.0167. [DOI] [PubMed] [Google Scholar]
- Colombel M, Filleur S, Fournier P, Merle C, Guglielmi J, Courtin A, Degeorges A, Serre CM, Bouvier R, Clezardin P, Cabon F. Androgens repress the expression of the angiogenesis inhibitor thrombospondin-1 in normal and neoplastic prostate. Cancer Res. 2005;65:300–308. [PubMed] [Google Scholar]
- Conrad SM, Blakey GH, Shugars DA, Marciani RD, Phillips C, White RP., Jr. Patients' perception of recovery after third molar surgery. J. Oral Maxillofac. Surg. 1999;57:1288–1294. doi: 10.1016/s0278-2391(99)90861-3. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Seriolo B, Villaggio B, Pizzorni C, Craviotto C, Sulli A. Androgens and estrogens modulate the immune and inflammatory responses in rheumatoid arthritis. Ann. N.Y. Acad. Sci. 2002;966:131–142. doi: 10.1111/j.1749-6632.2002.tb04210.x. [DOI] [PubMed] [Google Scholar]
- Cutolo M, Capellino S, Sulli A, Serioli B, Secchi ME, Villaggio B, Straub RH. Estrogens and autoimmune disease. Ann. N.Y. Acad. Sci. 2006;1089:538–547. doi: 10.1196/annals.1386.043. [DOI] [PubMed] [Google Scholar]
- Dovi JV, Szpaderska AM, DiPietro LA. Neutrophil function in the healing wound: adding insult to injury? Thromb. Haemost. 2004;92:275–280. doi: 10.1160/TH03-11-0720. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Kavaliers M, Ossenkopp KP. Sex differences in the effects of muramyl dipeptide and lipopolysaccharide on locomotor activity and the development of behavioral tolerance in rats. Pharmacol. Biochem. Behav. 2003;74:433–447. doi: 10.1016/s0091-3057(02)01024-9. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Bosch JA, Cacioppo JT, Marucha PT. Mucosal wound healing: the roles of age and sex. Arch. Surg. 2006;141:1193–1197. doi: 10.1001/archsurg.141.12.1193. [DOI] [PubMed] [Google Scholar]
- Engeland CG, Marucha PT. Stress and wound healing. In: Granstein RD, Luger TA, editors. Neuroimmunology of the skin: Basic science to clinical relevance. Springer-Verlag; New York: in press. [Google Scholar]
- Franck-Lissbrant I, Haggstrom S, Damber JE, Bergh A. Testosterone stimulates angiogenesis and vascular regrowth in the ventral prostate in castrated adult rats. Endocrinology. 1998;139:451–456. doi: 10.1210/endo.139.2.5683. [DOI] [PubMed] [Google Scholar]
- Gaillard RC, Spinedi E. Sex- and stress-steroids interactions and the immune system: evidence for a neuroendocrine-immunological sexual dimorphism. Domest. Anim. Endocrinol. 1998;15:345–352. doi: 10.1016/s0739-7240(98)00028-9. [DOI] [PubMed] [Google Scholar]
- Giglio T, Imro M, Filaci G, Scudeletti M, Puppo F, De Cecco L, Indiveri F, Costantini S. Immune cell circulating subsets are affected by gonadal function. Life Sci. 1994;54:1305–1312. doi: 10.1016/0024-3205(94)00508-7. [DOI] [PubMed] [Google Scholar]
- Gilliver SC, Wu F, Ashcroft GS. Regulatory roles of androgens in cutaneous wound healing. Thromb. Haemost. 2003;90:978–985. doi: 10.1160/TH03-05-0302. [DOI] [PubMed] [Google Scholar]
- Gilliver SC, Ashworth JJ, Ashcroft GS. The hormonal regulation of cutaneous wound healing. Clin. Dermatol. 2007;25:56–62. doi: 10.1016/j.clindermatol.2006.09.012. [DOI] [PubMed] [Google Scholar]
- Gilliver SC, Ruckshanthi JPD, Hardman MJ, Zeef LAH, Ashcroft GS. 5α-Dihydrotestosterone (DHT) retards wound closure by inhibiting re-epithelialization. J Pathol. doi: 10.1002/path.2444. in press. [DOI] [PubMed] [Google Scholar]
- Hampson E, Young EA. Methodological issues in the study of hormone-behavior relations in humans: Understanding and monitoring the menstrual cycle. In: Becker J, et al., editors. Sex differences in the brain: From genes to behavior. Oxford University Press; New York: 2008. pp. 63–78. [Google Scholar]
- Heikkinen J. Hormone therapy: maximizing the benefits. Gynecol. Endocrinol. 2006;22:160–162. doi: 10.1080/09513590600629183. [DOI] [PubMed] [Google Scholar]
- Jorgensen LN, Sorensen LT, Kallehave F, Vange J, Gottrup F. Premenopausal women deposit more collagen than men during healing of an experimental wound. Surgery. 2002;131:338–343. doi: 10.1067/msy.2002.119986. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Marucha PT, Malarkey WB, Mercado AM, Glaser R. Slowing of wound healing by psychological stress. Lancet. 1995;346:1194–1196. doi: 10.1016/s0140-6736(95)92899-5. [DOI] [PubMed] [Google Scholar]
- Kovacs EJ. Aging, traumatic injury, and estrogen treatment. Exp. Gerontol. 2005;40:549–555. doi: 10.1016/j.exger.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Krzych U, Strausser HR, Bressler JP, Goldstein AL. Effects of sex hormones on some T and B cell functions, evidenced by differential immune expression between male and female mice and cyclic pattern of immune responsiveness during the estrous cycle in female mice. Am. J Reprod. Immunol. 1981;1:73–77. doi: 10.1111/j.1600-0897.1981.tb00020.x. [DOI] [PubMed] [Google Scholar]
- Lahita R. Gender and the immune system. J. Gend. Specif. Med. 2000;3:19–22. [PubMed] [Google Scholar]
- Lee HG, Eun HC. Differences between fibroblasts cultured from oral mucosa and normal skin: implication to wound healing. J. Dermatol. Sci. 1999;21:176–182. doi: 10.1016/s0923-1811(99)00037-7. [DOI] [PubMed] [Google Scholar]
- Leslie CA, Dubey DP. Increased PGE2 from human monocytes isolated in the luteal phase of the menstrual cycle. Implications for immunity? Prostaglandins. 1994;47:41–54. doi: 10.1016/0090-6980(94)90073-6. [DOI] [PubMed] [Google Scholar]
- Marucha PT, Engeland CG. Stress, neuroendocrine hormones, and wound healing: Human models. In: Ader R, editor. Psychoneuroimmunology. Elsevier Inc.; San Diego: 2007. pp. 825–835. [Google Scholar]
- Mascarenhas P, Gapski R, Al Shammari K, Wang HL. Influence of sex hormones on the periodontium. J. Clin. Periodontol. 2003;30:671–681. doi: 10.1034/j.1600-051x.2003.00055.x. [DOI] [PubMed] [Google Scholar]
- McCruden AB, Stimson WH. Sex hormones and immune function. In: Ader R, editor. Psychoneuroimmunology. Academic Press; New York: 1991. pp. 475–493. [Google Scholar]
- Miller L, Hunt JS. Sex steroid hormones and macrophage function. Life Sci. 1996;59:1–14. doi: 10.1016/0024-3205(96)00122-1. [DOI] [PubMed] [Google Scholar]
- O'Brien SM, Fitzgerald P, Scully P, Landers A, Scott LV, Dinan TG. Impact of gender and menstrual cycle phase on plasma cytokine concentrations. Neuroimmunomodulation. 2007;14:84–90. doi: 10.1159/000107423. [DOI] [PubMed] [Google Scholar]
- Ortega S, Ittmann M, Tsang SH, Ehrlich M, Basilico C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc. Natl. Acad. Sci. U.S.A. 1998;95:5672–5677. doi: 10.1073/pnas.95.10.5672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett DA, Marucha PT, Sheridan JF. Restraint stress slows cutaneous wound healing in mice. Brain Behav. Immun. 1998;12:64–73. doi: 10.1006/brbi.1997.0512. [DOI] [PubMed] [Google Scholar]
- Palaszynski KM, Loo KK, Ashouri JF, Liu HB, Voskuhl RR. Androgens are protective in experimental autoimmune encephalomyelitis: implications for multiple sclerosis. J. Neuroimmunol. 2004;146:144–152. doi: 10.1016/j.jneuroim.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Pfeilschifter J, Koditz R, Pfohl M, Schatz H. Changes in proinflammatory cytokine activity after menopause. Endocr.Rev. 2002;23:90–119. doi: 10.1210/edrv.23.1.0456. [DOI] [PubMed] [Google Scholar]
- Phillips C, White RP, Jr., Shugars DA, Zhou X. Risk factors associated with prolonged recovery and delayed healing after third molar surgery. J. Oral Maxillofac. Surg. 2003;61:1436–1448. doi: 10.1016/j.joms.2003.08.003. [DOI] [PubMed] [Google Scholar]
- Raine-Fenning NJ, Brincat MP, Muscat-Baron Y. Skin aging and menopause: implications for treatment. Am. J. Clin. Dermatol. 2003;4:371–378. doi: 10.2165/00128071-200304060-00001. [DOI] [PubMed] [Google Scholar]
- Savita, Rai U. Sex steroid hormones modulate the activation of murine peritoneal macrophages: receptor mediated modulation. Comp. Biochem. Physiol. C. Pharmacol. Toxicol. Endocrinol. 1998;119:199–204. doi: 10.1016/s0742-8413(97)00207-7. [DOI] [PubMed] [Google Scholar]
- Schuurs AHWM, Verheul HAM. Effects of gender and sex steroids on the immune response. J. Steroid Biochem. 1990;35:157–172. doi: 10.1016/0022-4731(90)90270-3. [DOI] [PubMed] [Google Scholar]
- Shimizu T, Nishihira J, Watanabe H, Abe R, Honda A, Ishibashi T, Shimizu H. Macrophage migration inhibitory factor (MIF) is induced by thrombin and factor Xa in endothelial cells. J. Biol. Chem. 2004:13729–13737. doi: 10.1074/jbc.M400150200. [DOI] [PubMed] [Google Scholar]
- Sordello S, Bertrand N, Plouet J. Vascular endothelial growth factor is up-regulated in vitro and in vivo by androgens. Biochem. Biophys. Res. Commun. 1998;251:287–290. doi: 10.1006/bbrc.1998.9328. [DOI] [PubMed] [Google Scholar]
- Szpaderska AM, Zuckerman JD, DiPietro LA. Differential injury responses in oral mucosal and cutaneous wounds. J. Dent. Res. 2003;82:621–626. doi: 10.1177/154405910308200810. [DOI] [PubMed] [Google Scholar]
- Wichmann MW, Ayala A, Chaudry IH. Male sex steroids are responsible for depressing macrophage immune function after trauma-hemorrhage. Am. J. Physiol. 1997;273:C1335–C1340. doi: 10.1152/ajpcell.1997.273.4.C1335. [DOI] [PubMed] [Google Scholar]
- Zuk M, McKean KA. Sex differences in parasite infections: patterns and processes. Int. J. Parasitol. 1996;26:1009–1023. [PubMed] [Google Scholar]