Abstract
A novel generation of silk-based brain implants engineered to release adenosine was recently shown to provide robust seizure suppression in kindled rats. As a first step to develop stem cell-coated silk-based 3D-scaffolds for the therapeutic long-term delivery of adenosine we engineered human mesenchymal stem cells (hMSCs) to release adenosine. Here we demonstrate reduction of chronic seizures in a mouse model of CA3-selective epileptogenesis after infrahippocampal transplantation of adenosine-releasing hMSCs.
Keywords: epilepsy, adenosine, human mesenchymal stem cells, cell therapy, grafting, silk
1. Introduction
Adenosine is an endogenous anticonvulsant with efficacy in pharmacoresistant epilepsy (Gouder et al., 2003). To avoid peripheral side effects of systemic adenosine, focal adenosine augmentation therapies have been evaluated and demonstrated to provide therapeutic benefit in rodent models of induced and chronic seizures (Boison, 2007a). To develop a therapeutic system compatible with future clinical application we recently developed a new generation of silk-based polymers engineered to release adenosine (Wilz et al., 2008). Infrahippocampal implants of these polymers provided robust seizure suppression in the rat kindling model. Seizure suppression correlated with the dose/release profile of adenosine (Wilz et al., 2008).
Transplantation of stem cells as a novel approach to epilepsy therapy has received much attention, recently (Boison, 2007b; Loscher et al., 2008; Raedt et al., 2007; Shetty and Hattiangady, 2007). Stem cells are considered for reconstruction of the epileptic hippocampus and for the delivery of trophic and/or anticonvulsant compounds. Human mesenchymal stem cells (hMSCs) that hold promise as potential autologous patient-derived brain-implants have already successfully been used in models of neuronal cell loss, including stroke and Parkinson’s disease (e.g. Kim et al., 2009; Ohtaki et al., 2008), but have not yet been used in models of epilepsy. To achieve better control of stem cell location within the brain and to allow for surgical retrieval – an important safety-consideration – a combination of stem cells with suitable biopolymers might offer a promising alternative.
To develop a silk-based stem cell-coated 3D-scaffold for the therapeutic long-term delivery of adenosine we recently engineered hMSCs to release a therapeutically active dose of adenosine (Ren et al., 2007) and demonstrated that silk supports the release of adenosine from stem cells (Uebersax et al., 2006). As a first step in the development of therapeutic hMSC/silk-scaffolds we conducted a proof-of-feasibility study to demonstrate that adenosine-releasing hMSC-derived brain implants can suppress chronic seizures in a post status epilepticus model. For these studies we used a novel mouse model of CA3-selective epileptogenesis (Li et al., 2008). This model is based on the intraamygdaloid injection of the excitotoxin kainic acid (KA) and induces acute seizures resulting in ipsilateral CA3-selective neuronal cell loss. Within 12 days after KA-injection astrogliosis, upregulation of the adenosine-removing enzyme adenosine kinase (ADK), and spontaneous electrographic subclinical seizures coincide, all restricted to the ipsilateral CA3 (Li et al., 2007a); these seizures are focal in nature and do not have a behavioral correlate. Three weeks after KA-injection spontaneous seizure patterns are robust and reproducible and present as 4.3 ± 1.5 CA3-selective electrographic seizures per hour with each seizure lasting 17.5 ± 5.8 seconds (Li et al., 2008). We consider this model ideal for the following reasons: (i) This model affords the unique opportunity to study epileptogenesis and seizure development in a highly restricted area within a largely intact brain environment. Focal adenosine deficiency causes seizures in this model. Thus, this model is highly suited to study focal cell-based adenosine augmentation therapies. (ii) This model allows studying three different parameters: acute seizures and acute injury, epileptogenesis, and expression of chronic seizures. (iii) This model is highly reproducible and yields a high seizure frequency (around 4 seizures per hour) within a relatively short time frame (stable seizure patterns are fully established three weeks after KA-injection). Thus, this model is highly suited for the rapid screening of novel neuroprotective, antiepileptogenic, and/or anti-ictogenic strategies.
2. Methods
2.1. Stem cells
Human mesenchymal stem cells (hMSCs) were engineered to release adenosine using a lentivirus co-expressing emerald green fluorescent protein (EmGFP) and miRNA directed against the major adenosine-removing enzyme adenosine kinase (ADK). The resulting ADK-knockdown cells H239 were used in the present study. Production and characterization of the cells has been described in detail (Ren et al., 2007).
2.2. Epilepsy model and cell transplantation
All experiments were conducted in an AAALAC-accredited facility adhering to protocols approved by Legacy’s Institutional Animal Care and Use Committee adhering to NIH regulations and guidelines on the humane use of animals in research. We used a mouse model of CA3-selective epileptogenesis that has been fully described (Li et al., 2008). In this model CA3-selective spontaneous recurrent seizures develop 3 weeks after intraamygdaloid injection of kainic acid (KA). Briefly, under full anesthesia, 12 adult male C57BL/6 mice received unilateral stereotaxic microinjection of KA (0.3μg in 0.2μl) into the basolateral amygdala nucleus based on stereotactic coordinates relative to bregma: AP=−0.94mm, ML=−2.85mm, DV=−3.75mm. Status epilepticus following the KA-injection was terminated after 30min with lorazepam (6mg/kg, i.v.). 24h after KA-injection 6 animals were treated with 50,000 H239 cells that were slowly injected in a volume of 2.5μl of culture medium (unilateral injection of DMEM, ipsilateral to KA-injection) using a drill hole above the left hippocampus and a single diagonal injection tract spanning from coordinate (AP +1.6; ML +1.2, DV 0.0) to coordinate (AP −2.8; ML −1.75; DV − 4.0), thus depositing the cells within the infrahippocampal fissure. The remaining 6 animals received corresponding sham treatments (injection of 2.5μl culture medium using the same stereotactic coordinates). All animals, including the sham controls received daily immunosuppression with cyclosporine A (15 mg/kg, i.p.) initiated 2d prior to cell transplantation and maintained throughout the course of this study.
2.3. Seizure monitoring
Three weeks after transplantation all animals were equipped with bipolar EEG recording electrodes implanted into the CA3 ipsilateral to the cell graft/KA-injection. As reference a monopolar electrode was placed onto the cortex as described (Li et al., 2008). One day after electrode implantation mice were placed in Plexiglas cages where they could move freely. Electrical brain activity was monitored using the Nervus EEG Recording System connected with a Nervus magnus 32/8 Amplifier and filtered (high-pass filter 0.3 Hz cutoff, low-pass 100 Hz). Each EEG file was analyzed manually by an observer not aware of the animal’s identities. EEG-seizure activity was defined as high-amplitude rhythmic discharges that clearly represented a new pattern of tracing (repetitive spikes, spike-and-wave discharges, and slow waves) that lasted at least 5 s. Epileptic events occurring with an interval less than 5 s without the EEG returning to baseline were defined as belonging to the same seizure. All animals were subjected 16 h of continuous EEG monitoring. After the injection of the adenosine A1 receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX, 1mg/kg in 20% DMSO/saline, i.p.) an additional 8 h of EEG recordings were added.
2.4. Histology
After completion of EEG-monitoring, the animals were transcardially perfused with 4% paraformaldehyde in phosphate buffer (0.15 M. pH 7.4). The brains were postfixed in the same fixative for 6 h and cryoprotected before being cut into 40-μm coronal sections that were mounted on slides using 4′,6-diamidino-2-phenylindole (DAPI) –containing mounting medium. Using a Leica fluorescence microscope, graft-derived cells were identified by green EmGFP-derived fluorescence.
2.5. Statistical analysis
Statistical variability is indicated as ±SD and data were analyzed using one-way ANOVA with Student-Newman-Keuls test.
3. Results and Discussion
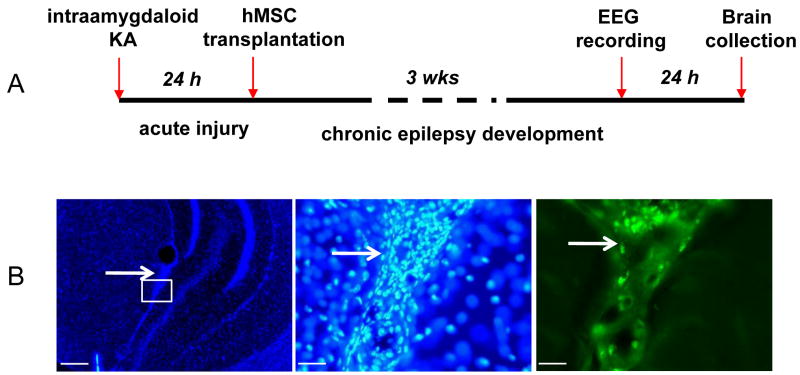
As outlined in Fig. 1 hMSCs (H239 cells) were grafted into the infrahippocampal fissure of mice 24 h after intraamygdaloid injection of KA, i.e. after the initial epileptogenesis precipitating injury had occurred. This is a viable strategy, since the location of the KA injection (amygdala) is distinct from the location of the cells (infrahippocampal fissure), thus avoiding possible interference between both procedures. When analyzed three weeks after grafting, all animals displayed dense clusters of EmGFP-positive cells located within the infrahippocampal fissure (Fig. 1). Grafted cells were restricted to the ipsilateral infrahippocampal fissure and not found elsewhere. Thus, these types of infrahippocampal grafts are ideally located to support the ipsilateral CA3 through paracrine augmentation of adenosine.
Figure 1.
Scheme of experimental design (A) and histology of graft recipients (B). (B, left) Representative DAPI stained coronal brain section 3 weeks after transplantation of H239 hMSCs. Composite fluorescence image at lower magnification showing general graft morphology and location within the infrahippocampal cleft (arrow). (B, middle) Fluorescence image of the same graft (boxed area indicated in left panel) at higher resolution. Note the high density of intact graft-derived nuclei (arrow). (B right) Same image as (B middle) viewed under an EmGFP specific filter. Scale bars: B left: 100μm, B middle and right: 12.5 μm.
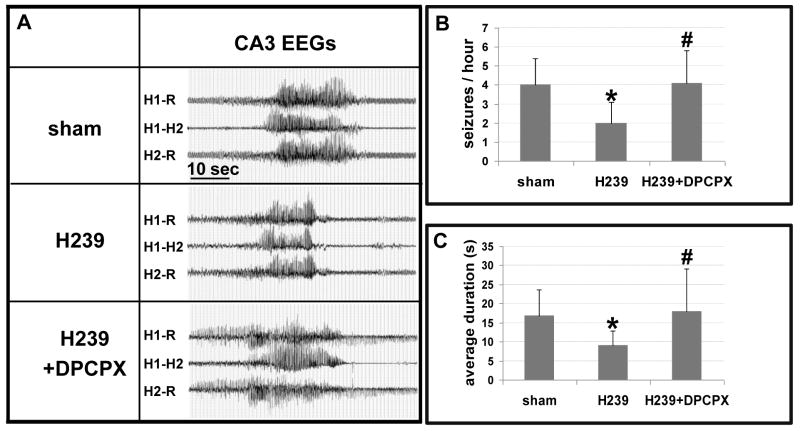
Seizure recordings performed three weeks after KA-injection revealed typical spontaneous seizures in the CA3 of all sham-treated animals at a frequency of 4.0 ± 1.4 seizures per hour and an average duration of 16.8 ± 6.9 seconds (63 seizures in 16 h amounting to a total seizure time of 17.7 min; duration range of individual seizures: 5 – 55 sec), data in agreement with the seizure characteristics of this model (Li et al., 2008). Likewise, seizure patterns were not altered in recipients of wild-type mouse (4.0 ± 1.3 seizures per hour at 16.8 ± 5.5 sec, n = 6, Li et al., 2008) or human (4.0 ± 1.4 seizures per hour at 16.9 ± 5.3 sec, n = 6, Ren et al., unpublished data) embryonic stem cell-derived neural precursor cells. In contrast, seizure intensity was significantly reduced in recipients of H239 cells (2.0 ± 1.1 seizures per hour at 9.1 ± 3.7 sec; P<0.001) (Fig. 2) (31 seizures in 16 h amounting to a total seizure time of 4.8 min; duration range of individual seizures: 5 – 22 sec). Seizure protection could be reversed after the injection of the selective A1R antagonist DPCPX (4.1 ± 1.7 seizures per hour at 18.0 ± 11.1 sec, P>0.05; 32 seizures in 8 h amounting to a total seizure time of 9.7 min; duration range of individual seizures: 5 – 69 sec), indicating that the reduction of seizure-intensity by H239 grafts was due to paracrine augmentation of adenosine, exerting seizure suppression via activation of A1 receptors.
Figure 2.
Representative EEGs recorded from the ipsilateral CA3 three weeks after injury. (A) Recordings from a montage using a bipolar electrode inserted into injured CA3 (H1; H2) and a cortical ground/reference electrode (R). H1-R and H2-R traces show hippocampus/cortical recordings whereas trace H1–H2 shows intrahippocampal recordings (i.e. between the two tips of the bipolar electrode). Sham treated controls were compared with recipients of H239 hMSCs before (middle) or after (bottom) injection of DPCPX (1 mg/kg, i.p.). (B, C) Average number of seizures per hour and average seizure duration in respective treatment groups. Data are based on n = 6 animals for each group and 16 h of continuous EEG monitoring. Data are presented as ±SD and were analyzed using one-way ANOVA compared to sham. *P<0.001, #P>0.05 compared to sham control.
In conclusion, using infrahippocampal implants of hMSCs engineered to release adenosine, we demonstrate a significant reduction of seizure intensity in a mouse model of focal CA3-selective spontaneous seizures. While adenosine-releasing embryonic stem cell-derived brain implants were previously shown to suppress kindling epileptogenesis (Li et al., 2007b), the data presented here constitute the first use of adenosine releasing hMSCs in a model of focal spontaneous seizures. It remains to be determined, whether adenosine-releasing hMSCs are effective in different models of epilepsy. Although not directly verified for wild-type hMSCs here, wild-type mouse or human embryonic stem cell-derived implants did not affect the development of spontaneous CA3 seizures. hMSCs have been reported to modulate immune-functions in immune-competent animals (Kim et al., 2009; Ohtaki et al., 2008); these modulatory effects of hMSCs could theoretically contribute to a reduction of seizure intensity. These effects are, however, unlikely to contribute to the present results for the following reasons: (i) The experiments reported here were done under constant immune-suppression; we have previously demonstrated that immune-suppression does not affect the development of epilepsy in this model (Li et al., 2008). (ii) Wild-type embryonic stem cell derived brain implants that displayed an endogenous neuroprotective effect in a stroke model (Pignataro et al., 2007), did not influence seizure parameters in our model of CA3-selective epileptogenesis (Li et al., 2008). (iii) Although the contribution of host-derived adenosine cannot be completely excluded, seizure activity in recipients of adenosine releasing hMSCs could be reconstituted after the injection of DPCPX (Fig. 2).
Our data are of significance for two reasons:
Demonstration of therapeutic efficacy of a human stem cell line in suppressing spontaneous seizures in a post status epilepticus model. This is of importance in regard to future engineering of patient identical hMSCs for the therapeutic delivery of adenosine in an autologous transplantation approach.
These data, in combination with our previous report (Wilz et al., 2008) support the feasibility to use hMSCs engineered to release adenosine in silk-based 3D-scaffolds as a novel therapeutic strategy to provide suppression of spontaneous seizures.
Recent data suggest that silk-based scaffolds are highly suitable for in vivo applications of at least 1 year (Wang et al., 2008). Future work will focus on combining adenosine-releasing hMSCs with silk-based 3D-scaffolds in an attempt to provide long-term seizure suppression in animal models of induced and chronic seizures. As a safety precaution – e.g. in case of tumor-formation – cells can be engineered to express a suicide gene, for example herpes simplex virus thymidine kinase (HSV-Tk). Using this strategy transplanted cells can effectively be eliminated with ganciclovir, if needed.
Acknowledgments
This project was supported by grant R01NS058780 from the NINDS.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Boison D. Adenosine-based cell therapy approaches for pharmacoresistant epilepsies. Neurodegener Dis. 2007a;4:28–33. doi: 10.1159/000100356. [DOI] [PubMed] [Google Scholar]
- Boison D. Cell and gene therapies for refractory epilepsy. Current Neuropharmacology. 2007b;5:115–125. doi: 10.2174/157015907780866938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouder N, Fritschy JM, Boison D. Seizure suppression by adenosine A1 receptor activation in a mouse model of pharmacoresistant epilepsy. Epilepsia. 2003;44:877–885. doi: 10.1046/j.1528-1157.2003.03603.x. [DOI] [PubMed] [Google Scholar]
- Kim YJ, Park HJ, Lee G, Bang OY, Ahn YH, Joe E, Kim HO, Lee PH. Neuroprotective effects of human mesenchymal stem cells on dopaminergic neurons through anti-inflammatory action. Glia. 2009;57:13–23. doi: 10.1002/glia.20731. [DOI] [PubMed] [Google Scholar]
- Li T, Lan JQ, Fredholm BB, Simon RP, Boison D. Adenosine dysfunction in astrogliosis: cause for seizure generation? Neuron Glia Biology. 2007a;3:353–366. doi: 10.1017/S1740925X0800015X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Steinbeck JA, Lusardi T, Koch P, Lan JQ, Wilz A, Segschneider M, Simon RP, Brustle O, Boison D. Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain. 2007b;130:1276–1288. doi: 10.1093/brain/awm057. [DOI] [PubMed] [Google Scholar]
- Li T, Ren G, Lusardi T, Wilz A, Lan JQ, Iwasato T, Itohara S, Simon RP, Boison D. Adenosine kinase is a target for the prediction and prevention of epileptogenesis in mice. J Clin Inv. 2008;118:571–582. doi: 10.1172/JCI33737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscher W, Gernert M, Heinemann U. Cell and gene therapies in epilepsy - promising avenues or blind alleys? Trends in Neurosciences. 2008;31:62–73. doi: 10.1016/j.tins.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Ohtaki H, Ylostalo JH, Foraker JE, Robinson AP, Reger RL, Shioda S, Prockop DJ. Stem/progenitor cells from bone marrow decrease neuronal death in global ischemia by modulation of inflammatory/immune responses. Proc Natl Acad Sci U S A. 2008;105:14638–14643. doi: 10.1073/pnas.0803670105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pignataro G, Studer FE, Wilz A, Simon RP, Boison D. Neuroprotection in ischemic mouse brain induced by stem cell-derived brain implants. J Cereb Blood Flow Metab. 2007;27:919–927. doi: 10.1038/sj.jcbfm.9600422. [DOI] [PubMed] [Google Scholar]
- Raedt R, Van Dycke A, Vonck K, Boon P. Cell therapy in models for temporal lobe epilepsy. Seizure-European Journal of Epilepsy. 2007;16:565–578. doi: 10.1016/j.seizure.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Ren G, Li T, Lan JQ, Wilz A, Simon RP, Boison D. Lentiviral RNAi-induced downregulation of adenosine kinase in human mesenchymal stem cell grafts: a novel perspective for seizure control. Exp Neurol. 2007;208:26–37. doi: 10.1016/j.expneurol.2007.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty AK, Hattiangady B. Concise review: Prospects of stem cell therapy for temporal lobe epilepsy. Stem Cells. 2007;25:2396–2407. doi: 10.1634/stemcells.2007-0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uebersax L, Fedele DE, Schumacher C, Kaplan DL, Merkle HP, Boison D, Meinel L. The support of adenosine release from adenosine kinase deficient ES cells by silk substrates. Biomaterials. 2006;27:4599–4607. doi: 10.1016/j.biomaterials.2006.04.025. [DOI] [PubMed] [Google Scholar]
- Wang Y, Rudym DD, Walsh A, Abrahamsen L, Kim HJ, Kim HS, Kirker-Head C, Kaplan DL. In vivo degradation of three-dimensional silk fibroin scaffolds. Biomaterials. 2008;29:3415–3428. doi: 10.1016/j.biomaterials.2008.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilz A, Pritchard EM, Li T, Lan JQ, Kaplan DL, Boison D. Silk polymer-based adenosine release: Therapeutic potential for epilepsy. Biomaterials. 2008;29:3609–3616. doi: 10.1016/j.biomaterials.2008.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]