Abstract
In 40% of children with symptomatic idiopathic dilated cardiomyopathy (IDC), medical therapy fails within 2 years of diagnosis. Strong evidence-based therapies are not available for these children, and how evidence-based therapies for adults with IDC should be applied to children is unclear. Using data from the National Heart, Lung, and Blood Institute’s Pediatric Cardiomyopathy Registry, we compared practice patterns of initial therapies for children with IDC diagnosed between 1990 and 1995 (n=350) and between 2000 and 2006 (n=219). At diagnosis, 73% had symptomatic heart failure, and 7% had one or more family members with IDC. Anti-heart failure medications were most commonly prescribed initially. Anti-heart failure medication use was similar across both time periods (84% and 87%, respectively), as was angiotensin-converting enzyme inhibitor (ACEI) use (66% and 70%, respectively). These medications were used more commonly in children with greater left ventricular dilation and poorer left ventricular fractional shortening and functional class (P<0.001). Beta-blocker use ranged from 4% to 18% over the two time periods. Treatments for pediatric IDC have changed little over the past 25 years. Anti-heart failure medications remain the most common treatment, and they are often given to children with asymptomatic left ventricular dysfunction. Children with asymptomatic left ventricular dysfunction are often not offered ACEIs without echocardiographic evidence of advanced disease. Therapeutic clinical trials are strongly indicated because practice variation is substantial and medical outcomes in these children have not improved in the past several decades.
Keywords: Pediatrics, Cardiomyopathy, Heart Failure, Anti-heart failure Therapy
In the absence of evidence-based standards, the clinical treatment of children with idiopathic dilated cardiomyopathy (IDC) and heart failure (HF) varies widely. We examined the medical therapies offered at presentation to children with IDC who were enrolled in the retrospective arm of the PCMR database to identify and characterize treatment patterns in managing childhood HF. To examine management trends over time, we compared the rates of pharmacological therapy to a later cohort from the prospective arm of the registry. 1-11
METHODS
The purpose of the PCMR is to identify the epidemiologic characteristics and clinical course of selected cardiomyopathies in children and to promote the development of cause-specific prevention and treatment strategies. The design of the PCMR is described elsewhere.12 The current analysis is based on the retrospective cohort of the PCMR, for which detailed therapeutic data were obtained by standardized chart abstraction on 920 children with cardiomyopathy who presented to a pediatric cardiologist between January 1, 1990, and December 31, 1995. Data were collected at 38 sites in the United States (818 children) and at 1 site in Canada (102 children). A comparison cohort diagnosed between 2000 and 2006 was also analyzed to determine rates of anti-heart failure medication, ACE inhibitor (ACEI), and beta-blocker use. The window for baseline registry data was 1 month after the initial diagnosis of cardiomyopathy; subsequent data were collected annually.
The institutional review board or ethics committee at each participating PCMR site reviewed and approved the protocol. Because there was no direct patient contact and no procurement of medical materials other than written records, written informed consent from individual patients or surrogates was not required.
Children were eligible for inclusion in the registry if they were 18 years old or younger and were diagnosed with cardiomyopathy on or after January 1, 1990. Idiopathic dilated cardiomyopathy was diagnosed from echocardiographic evidence, including at least two left ventricular measurements (decreased fractional shortening, decreased posterior wall thickness, or increased end-diastolic dimension) exceeding 2 standard deviations for age (fractional shortening) or for body surface area (other measurements), a pathological diagnosis of cardiomyopathy at autopsy or endomyocardial biopsy, or other evidence of clinical cardiomyopathy, as provided by the cardiologist. The 14 clinical exclusion criteria included the presence of a congenital heart defect not associated with a malformation syndrome, endocrine disease associated with myocardial injury, chronic arrhythmia, pulmonary or immunologic disease, and chemotherapy-associated cardiac disease.12
This report is based on children with pure (not mixed type) DC for whom the cause was unknown at presentation (n=325) or who also had at least one family member with DC (n=25). For the purpose of this report, this entire cohort of 350 children is referred to as having IDC.
Data for the PCMR were collected through on-site abstraction of patient records by a trained outreach team or research staff at the participating site. After patient identification, study personnel confirmed eligibility and enrollment through chart review and assigned a unique study identifier to ensure confidentiality. Supplemental information included clinical history, procedures, outcomes, family history, results of laboratory studies, and therapies administered. In addition to demographic and echocardiographic characteristics, functional classifications based on the New York Heart Association13 classification or, for younger children, the Canadian Consensus (Ross) classification14 plus the objective classification were derived from data in the chart (New York Heart Association classification was used if possible, then Ross, then objective). Children with asymptomatic left ventricular dysfunction were classified as functional Class I. Anti-heart failure therapy was defined as the use of digoxin, a diuretic, or both.
Left ventricular end-diastolic dimension and end-diastolic posterior wall thickness, as well as end-diastolic septal thickness were expressed as Z-scores relative to the distribution of these measurements to body surface area in normal children15, and left ventricular fractional shortening was expressed as the Z-score relative to age.16 Z-scores are the number of standard deviations each value lies from the mean value of healthy children of similar body surface area or age. Body surface area was calculated from height and weight.17
Summary statistics are presented as means and standard deviations or as medians and interquartile ranges. Echocardiographic Z-scores were compared by therapy status with t tests. The proportions of children receiving a given therapy were compared by cause of cardiomyopathy with chi-square tests and by functional class and by year of diagnosis with a Mantel-Haenszel test for linear trend. Crude and adjusted therapy rates by center were compared using univariate and multivariate logistic regression. Candidate predictors used in multivariate logistic regression models for therapy included age at diagnosis, center, presence of HF symptoms, cause of IDC, and echocardiographic Z-scores.
Alpha was set at 0.05, and all tests were two-sided. The SAS statistical software package (version 9.1, Statistical Analysis System Corp., Cary, NC) was used for analysis.
RESULTS
The PCMR enrolled 920 children with cardiomyopathy diagnosed between 1990 and 1995, of which of 350 had pure idiopathic IDC or familial isolated IDC (Table 1). Echocardiographic findings from the month of presentation were consistent with IDC. Use of selected medications in this patient group was compared to that in a group of 219 children with pure IDC diagnosed between 2000 and 2006 for whom medication data, other than anti-heart failure therapy, was collected. Anti-heart failure therapy data for children diagnosed between 2000 and 2006 were collected for all IDC cases (N=462) in the prospective cohort. All results below are based on the earlier cohort diagnosed between 1990 and 1995, unless otherwise noted.
Table 1.
Demographic Characteristics and Clinical Status at Presentation of 350 Children with Idiopathic Dilated Cardiomyopathy Diagnosed between 1990 and 1995
| Patient Characteristic | Value |
|---|---|
| Male, N (%) | 180 (51) |
| Mean (SD) Age, y | 4.9 (6) |
| Median Age, y | 1.5 |
| Age distribution, N (%) | |
| < 1 y | 152 (43) |
| 1 to < 6, y | 81 (23) |
| 6 to < 12, y | 48 (14) |
| 12 to 18, y | 69 (20) |
| Race/Ethnicity, N (%) | |
| White | 205 (59) |
| Black | 82 (23) |
| Hispanic | 40 (11) |
| Other | 1 (0.3) |
| Unknown | 5 (1) |
| Congestive Heart Failure, N (%) | 256 (73) |
| Functional Class*, N (%) | |
| Class I | 100 (28) |
| Class II | 47 (13) |
| Class III | 86 (25) |
| Class IV | 111 (32) |
| Unknown | 6 (2) |
| Mean (SD) Echocardiographic Left Ventricular Z-scores† | |
| End Systolic Dimension (N=220) | 6.29 (2.90) |
| End Diastolic Dimension (N=256) | 4.46 (2.72) |
| Fractional Shortening (N=276) | -8.85 (3.58) |
| End-diastolic Posterior Wall Thickness (N=199) | -0.43 (2.39) |
| End-diastolic Septal Wall Thickness (N=177) | -0.78 (2.02) |
Functional class is a composite hierarchical variable based on data in the medical record denoting New York Heart Association congestive heart failure class, Canadian Consensus (Ross) heart failure class for children, or Objective heart class. Children without congestive heart failure symptoms at diagnosis were classified as Class I.
Echocardiographic Z-scores are corrected for body surface area (end-diastolic and endsystolic dimension, and end-diastolic posterior and septal wall thicknesses) or for age (fractional shortening). The Z-score represents the number of standard deviations from the mean of healthy children of similar body surface area or age. All mean Z-scores significantly differ (P<0.01) from normal (Z=0).
Practice variation by center was examined using the eight largest centers in terms of number of IDC cases (range, 15 to 58 per center). After accounting for differences in disease severity (left ventricular fractional shortening Z-score) in the center populations, center-specific rates of anti-heart failure therapeutic use were similar (P=0.07). However, ACEI use differed significantly among centers, with center-specific rates ranging from 46% to 89%. Anti-arrhythmic use also varied significantly, with center-specific rates ranging from 13% to 54%, as did carnitine supplementation (4% to 48%). Differences by center persisted for ACEI use (P=0.04), anti-arrhythmic use (P=0.01), and carnitine supplementation (P=0.007), even after adjustment for fractional shortening Z-score.
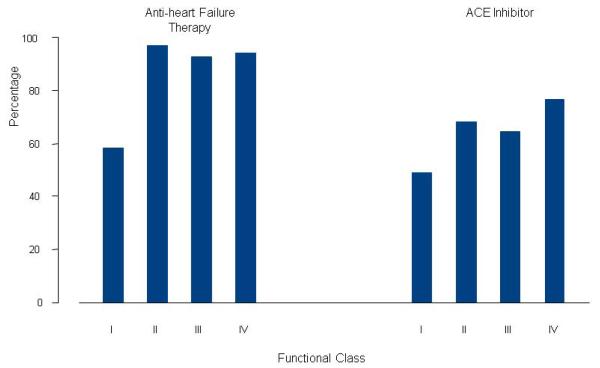
Anti-heart failure therapy at diagnosis was the most commonly reported intervention for all children, being reported in 84% (Table 2). Anti-heart failure administration differed by functional class (Figure 1), being administered to 60% of asymptomatic (Class I) children and to 93% of children in Class II or higher (P<0.001). Anti-heart failure agents were also prescribed more frequently in children with echocardiographic evidence of more advanced HF (Table 3). Multivariate modeling (N=272) indicated that HF (odds ratio, 6.5, 95% confidence interval 3.0 to 14.0, P<0.001) and left ventricular fractional shortening Z-score (odds ratio, 0.8, 95% confidence interval 0.7 to 0.9, P<0.001) were independently associated with anti-heart failure use. Anti-heart failure therapy use was similar in the earlier and later cohorts (84% and 87%, respectively).
Table 2.
Initial Management of 350 Children with Idiopathic Cardiomyopathy (All rates are for children diagnosed between n 1990 and 1995, unless otherwise noted)
| Therapy | % |
|---|---|
| Anti-heart failure | |
| 1990 to 1995 | 84 |
| 2000 to 2006‡ | 87 |
| ACE Inhibitor | |
| 1990 to 1995 | 66 |
| 2000 to 2006‡ | 70 |
| Anti-arrhythmic | 40 |
| Beta-adrenergic Antagonist | 5 |
| Calcium Channel Antagonist | 2 |
| Other Medication | 52 |
| Carnitine Supplementation | 18 |
| Diet Modification | 14 |
| Intra-aortic balloon pump | 2 |
| ECMO§ | 2 |
| Ventricular Assist Device | 1 |
| Pacemaker | 1 |
| Heart Transplant | 5 |
| Other Procedures | 7 |
Chi-square test
N for 2000 to 2006 diagnoses is 462 for anti-heart failuretherapy and 219 for ACE inhibition therapy.
ECMO: extracorporeal membrane oxygenation
Figure 1.
Anti-heart failure and ACE inhibitor use by functional class at diagnosis in 350 children with idiopathic dilated cardiomyopathy. (P <0.001 for the association between each therapy and functional class). See Table 1 footnote for definition of functional class.
Table 3.
Left Ventricular Echocardiographic Z-Scores in 350 Children with and without Anti-heart failure and ACE Inhibition Therapy at Diagnosis of Cardiomyopathy (1990-1995)
| Echocardiographic Z-score* | Therapy Mean (SD) |
No Therapy Mean (SD) |
P |
|---|---|---|---|
| Anti-heart failure Therapy | |||
| End-diastolic dimension | 4.88 (2.52) | 2.66 (2.91) | <0.001 |
| End-systolic dimension | 6.80 (2.50) | 3.69 (3.43) | <0.001 |
| Fractional shortening | -9.49 (2.96) | -5.71 (4.70) | <0.001 |
| End-diastolic posterior wall thickness | -0.35 (2.49) | -0.82 (1.51) | 0.16 |
| End-diastolic septal wall thickness | -0.72 (2.11) | -1.19 (1.19) | 0.11 |
| ACE Inhibitor Therapy | |||
| End-diastolic dimension | 5.06 (2.56) | 3.48 (2.73) | <0.001 |
| End-systolic dimension | 6.92 (2.53) | 5.17 (3.26) | <0.001 |
| Fractional shortening | -9.38 (3.00) | -7.80 (4.35) | 0.002 |
| End-diastolic posterior wall thickness | -0.23 (2.55) | -0.93 (1.81) | 0.028 |
| End-diastolic septal wall thickness | -0.66 (2.13) | -1.14 (1.70) | 0.13 |
Echocardiographic Z-scores are corrected for body surface area (end-diastolic and end-systolic dimension, and end-diastolic posterior and septal wall thickness and left ventricular mass) or corrected for age (fractional shortening). All mean Z-scores significantly differ (P<0.05) from normal (Z=0) except for end-diastolic posterior wall thickness in the therapy group.
The second most frequently reported therapy, ACEI, was prescribed for 66% of children during the first month of diagnosis and for 74% within the first year. At presentation, ACEI administration was more common (P<0.001) in children with more advanced HF, as evidenced by larger left ventricular dimension and lower fractional shortening Z-scores (Table 3) and in those with a worse functional class, where 77% of those in Class IV received an ACEI (Figure 1). Multivariate modeling (N=249) indicated that left ventricular end-diastolic dimension Z-score (odds ratio, 1.3; 95% confidence interval, 1.1 to 1.4, P<0.001) was independently correlated with of ACEI use.
Beta-adrenergic blockade was generally not employed in the earlier cohort, being prescribed for 4% of IDC cases at presentation and 6% within the first year. The rate of ACEI administration at diagnosis did not change significantly over the 6-year (1990 to 1995) diagnostic period (65% vs. 69%). In contrast, beta-adrenergic blockade use was higher in the later cohort (18%). This difference may be the result, in part, of the fact that some children in the later cohort from several PCMR centers were enrolled in a clinical trial of a beta-blocker.18
Dietary modification (mainly salt restriction) was infrequently reported (14%), but nevertheless was associated with functional class (P=0.01), being used in 5% of Class I children and in 14% to 19% of symptomatic (Class II through IV) children. Carnitine supplementation was prescribed in 18%. As noted earlier, use of this therapy varied by center.
Calcium channel blockers (2%) and pacemakers or automatic implanted cardiac defibrillators (1%) were generally not used as initial therapy. Mechanical support was instituted in 17 children (5%) in the first 30 days after presentation. Mechanical support was provided using extracorporeal membrane oxygenation in 8 children, intra-aortic balloon pumps in 6, and ventricular assist devices in 3. Within a month after diagnosis, 17 children (5%) underwent cardiac transplantation.
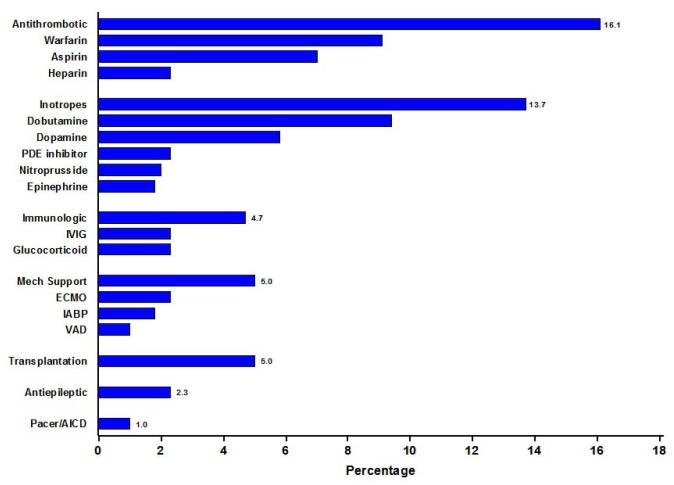
The PCMR specifically recorded the medical and surgical interventions as discussed above and as illustrated in Table 2. Medical records were further scrutinized for other medications. A majority (52%) of children received a variety of other medications during treatment of their cardiomyopathy (Figure 2). Anti-thrombotic agents were prescribed to 16% of the overall sample. Warfarin was the most widely used anticoagulant (9%), followed by aspirin and unfractionated heparin. Children treated with either warfarin or heparin were older at diagnosis (median 9.5 vs. 1.2 years) and more often had HF (91% vs.71%, P=0.003). However, anti-thrombotic use was independent of left ventricular size and function.
Figure 2.
Miscellaneous medical and surgical therapy reported for early treatment of idiopathic dilated cardiomyopathy in 350 children. PDE = phosphodiesterase; IVIG = intravenous immunoglobulin; ECMO = extracorporeal membrane oxygenation; IABP = intra-aortic balloon pump; VAD = ventricular assist device; AICD = automatic implanted cardiac defibrillator.
Children treated with intravenous inotropic infusions at presentation (16%) were more symptomatic than those not receiving them (HF was present in 86% vs. 71%, P<0.001). However, as was the case with anti-thrombotic use, children treated and those not treated with inotropic infusions did not differ with regard to their echocardiographic profiles. Immunomodulatory treatment was recorded in 5% of children and was reported as intravenous immunoglobulins, glucocorticoids, or both, in nearly equal proportions (Figure 2).
DISCUSSION
Childhood IDC is a rare but highly debilitating disease of multiple etiologies with profound morbidity and mortality.5, 8, 19 The disease most often affects very young children, and indeed, presentation during infancy was noted in 43% of the children studied here. The 1-year and 5-year rates for death or transplant for children with idiopathic IDC enrolled in the PCMR are 39% and 53%, respectively, illustrating the relative inadequacy of current medical therapy.5 In this study, we found little change in practice patterns over the past several decades. Our pharmacologic options have not greatly increased in the most recent decades. Among new options, ACEI use lags far behind expert recommendations.10
For adults with HF, numerous clinical trials have led to standardized practices that have improved the length and quality of life. Practice guidelines for treating IDC and HF in adults have been published jointly by the American College of Cardiology and the American Heart Association as evidenced-based standards of care.6 Pediatric HF experts have also examined potentially relevant adult and limited pediatric data to suggest management protocols for children with IDC and HF.10 In the absence of evidence-based pediatric studies, such guidelines cannot be considered as definitive standards of care, but nevertheless, they do represent a reasonable basis to compare management strategies over time. These recommendations for children begin by suggesting a thorough causative evaluation, including screening of all first-degree relatives. However, published data indicate that this evaluation rarely occurs.2,5
Angiotensin-converting enzyme inhibitor therapy is recommended for nearly all children with asymptomatic left ventricular dysfunction (unless they have a drug reaction), as well as with symptomatic HF, except in the initial management of decompensated disease.10,11 However, in our study, ACEI was only used in 53% of children with asymptomatic left ventricular dysfunction (functional Class I disease). However, we did identify increased left ventricular end-diastolic dimension Z-score at presentation as an independent correlate of ACEI use. It appears that many cardiologists wait until children have echocardiographic evidence of more advanced disease before initiating ACEI therapy. If recommendations for universal use had been followed, this association with markers of disease severity (increased left ventricular end-diastolic dimension Z-score at presentation) would probably not have been found.
Diuretics are recommended in children with HF to achieve euvolemia and to minimize congestive symptoms. The addition of digoxin is then recommended if the child remains symptomatic. Digoxin is specifically not recommended for children with asymptomatic left ventricular dysfunction because its use has not been associated with increased survival in large adult trials. We found that anti-heart failure medications were often used in asymptomatic children. Although the rate of anti-heart failure use correlated positively with increase in functional class, 60% of children with asymptomatic left ventricular dysfunction (functional Class I) received these agents.
Beta-adrenergic blockade was generally not employed; only 5% of children received such therapy at presentation. Pending new pediatric-specific data, there are no current recommendations for the routine use of beta-adrenergic blockade in children with HF, other than to warn that it is not appropriate for treating end-stage, decompensated HF.10 Of note, 18% of children in the prospective cohort (diagnosed in 2000 and later) were treated with beta adrenergic blockade, often in the setting of an ongoing clinical trial.
Sixteen percent of children with newly diagnosed HF received concurrent warfarin or heparin, indicating a concern among practitioners for potential thromboembolic complications of childhood IDC. Few data exist to guide anti-coagulation in these children. Patient selection, risk stratification, and drug selection vary in the absence of evidence-derived data, making this a topic for further study.
This inventory of therapies offered to children enrolled in the PCMR has some limitations. First, children in the earlier cohort (1990-1995) were enrolled voluntarily by pediatric cardiologists across the United States and Canada, making it difficult to assure complete patient capture and to avoid potential selection bias. Most practitioners enrolled all of their cardiomyopathy patients, but total patient capture was neither assured nor controlled for. Nevertheless, the outcomes of the PCMR retrospective and prospective cohorts in children with IDC overlap, suggesting no marked bias.5
Second, the later cohort used to determine current therapies was derived from a subset of children for whom detailed therapeutic data had been collected. The ACEI data were available for 219 of 462 children in the data set. These data were included to assess usage in the last decade. The database structure, designed in 1995, did not allow for detailed analysis of all variables. For example, the coding of anti-heart failure therapy as the use of either digoxin or furosemide limited a closer examination of these medications. Lastly, observational registry data cannot determine the causal impact of any specific therapy on patient outcomes.
Acknowledgments
The authors would like to thank Meena Doshi, MS, for assistance with statistical analyses.
This work was supported by the National Heart Lung and Blood Institute (Department of Health and Human Services) Grant R01 HL53392 and the Children’s Cardiomyopathy Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Lipshultz SE, Sleeper LA, Towbin JA, Lowe AM, Orav EJ, Cox GF, Lurie PR, McCoy KL, McDonald MA, Messere JE, Colan SD. The incidence of pediatric cardiomyopathy in two regions of the United States. N Eng J Med. 2003;348:1647–1655. doi: 10.1056/NEJMoa021715. [DOI] [PubMed] [Google Scholar]
- 2.Cox GF, Sleeper LA, Lowe AM, Towbin JA, Colan SD, Orav EJ, Lurie PR, Messere JE, Wilkinson JD, Lipshultz SE. Factors associated with establishing a causal diagnosis for children with cardiomyopathy. Pediatrics. 2006;118:1519–1631. doi: 10.1542/peds.2006-0163. [DOI] [PubMed] [Google Scholar]
- 3.Lewis AB, Chabot M. Outcome of infants and children with dilated cardiomyopathy. Am J Cardiol. 1991;68:365–9. doi: 10.1016/0002-9149(91)90833-7. [DOI] [PubMed] [Google Scholar]
- 4.Akagi T, Benson LN, Lightfoot NE, Chin K, Wilson G, Freedom RM. Natural history of dilated cardiomyopathy in children. Am Heart J. 1991;121:1502–6. doi: 10.1016/0002-8703(91)90158-e. [DOI] [PubMed] [Google Scholar]
- 5.Towbin JA, Lowe AM, Colan SD, Sleeper LA, Orav EJ, Clunie S, Messere J, Cox GF, Lurie PR, Hsu D, Canter C, Wilkinson JD, Lipshultz SE. Incidence, causes, and outcomes of dilated cardiomyopathy in children. JAMA. 2006;296:1867–1876. doi: 10.1001/jama.296.15.1867. [DOI] [PubMed] [Google Scholar]
- 6.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B. ACC/AHA Guidelines update for the diagnosis and management of chronic heart failure in the adult: a report of the America College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2005;112:e154–235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 7.Levy D, Kenchaiah S, Larson MG, Benjamin EJ, Kupka MJ, Ho KK, Murabito JM, Vasan Rs. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 8.Bublik N, Alvarez JA, Lipshultz SE. Pediatric cardiomyopathy as a chronic disease: a perspective on comprehensive care programs. Prog Pediatr Cardiol. 2008;25:103–11. doi: 10.1016/j.ppedcard.2007.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ratnasamy C, Kinnamon DD, Lipshultz SE, Rusconi PG. Associations between neurohormonal and inflammatory activation and heart failure in children. Am Heart J. 2008;155:527–33. doi: 10.1016/j.ahj.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 10.Grenier MA, Fioravanti J, Truesdell SC, Mendelsohn AM, Vermilion RP, Lipshultz SE. Angiotensin-converting enzyme inhibitor therapy for ventricular dysfunction in infants, children and adolescents: a review. Prog Ped Card. 2000;12:91–111. doi: 10.1016/s1058-9813(00)00061-8. [DOI] [PubMed] [Google Scholar]
- 11.Rosenthal D, Chrisant MR, Edens E, Mahony L, Canter C, Colan S, Dubin A, Lamour J, Ross R, Shaddy R, Addonizio L, Beerman L, Berger S, Bernstein D, Blume E, Boucek M, Checchia P, Dipchand A, Drummond-Webb J, Fricker J, Friedman R, Hallowell S, Jaquiss R, Mital S, Pahl E, Pearce B, Rhodes L, Rotondo K, Rusconi P, Scheel J, Singh T Pal, Towbin J. International Society for Heart and Lung Transplantation: practice guidelines for the management of heart failure in children. J Heart Lung Transplant. 2004;23:1313–33. doi: 10.1016/j.healun.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 12.Grenier MA, Osganian SK, Cox FG, Towbin JA, Colan SD, Lurie PR, Sleeper LA, Orav EJ, Lipshultz SE. Design and implementation of the North American Pediatric Cardiomyopathy Registry. Am Heart J. 2000;139:S86–S95. doi: 10.1067/mhj.2000.103933. [DOI] [PubMed] [Google Scholar]
- 13.The Criteria Committee of the New York Heart Association . Nomenclature and Criteria for Diagnosis of Diseases of the Heart and Great Vessels. 9th ed Little, Brown & Co; Boston: 1994. pp. 253–6. [Google Scholar]
- 14.Ross RD, Bollinger RO, Pinsky WW. Grading the severity of congestive heart failure in infants. Pediatr Cardiol. 1992;13:72–5. doi: 10.1007/BF00798207. [DOI] [PubMed] [Google Scholar]
- 15.Sluysmans T, Colan SD. Theoretical and empirical derivation of cardiovascular allometric relationships in children. J Appl Physiol. 2005;99:445–7. doi: 10.1152/japplphysiol.01144.2004. [DOI] [PubMed] [Google Scholar]
- 16.Colan SD, Parness IA, Spevak PJ, Sanders SP. Developmental modulation of myocardial mechanics: age- and growth-related alterations in afterload and contractility. J Am Coll Cardiol. 1992;19:619–29. doi: 10.1016/s0735-1097(10)80282-7. [DOI] [PubMed] [Google Scholar]
- 17.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–6. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 18.Shaddy R, Boucek M, Hsu D, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY, Pediatric Carvedilol Study Group Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298:1171–1179. doi: 10.1001/jama.298.10.1171. [DOI] [PubMed] [Google Scholar]
- 19.Alvarez JA, Wilkinson JD, Lipshultz SE, the Pediatric Cardiomyopathy Registry Study Group Outcomes predictors for pediatric dilated cardiomyopathy: A systematic review. Prog Pediatr Cardiol. 2007;23:25–32. doi: 10.1016/j.ppedcard.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]