Abstract
A general feature of stem cells is the ability to routinely proliferate in order to build, maintain, and repair organ systems. Accordingly, embryonic and germline, as well as some adult stem cells, produce the telomerase enzyme at various levels of expression. Our results show that, while muscle is a largely post-mitotic tissue, the muscle stem cells (satellite cells) that maintain this biological system throughout adult life do indeed display robust telomerase activity. Conversely, primary myoblasts (the immediate progeny of satellite cells) quickly and dramatically down-regulate telomerase activity. This work thus suggests that satellite cells, and early transient myoblasts, may be more promising therapeutic candidates for regenerative medicine than traditionally utilized myoblast cultures.
Muscle atrophy accompanies human aging, and satellite cells endogenous to aged muscle can be triggered to regenerate old tissue by exogenous molecular cues. Therefore, we also examined whether these aged muscle stem cells would produce tissue that is “young” with respect to telomere maintenance. Interestingly, this work shows that the telomerase activity in muscle stem cells is largely retained into old age wintin inbred “long” telomere mice and in wild-derived short telomere mouse strains, and that age-specific telomere shortening is undetectable in the old differentiated muscle fibers of either strain. Summarily, this work establishes that young and old muscle stem cells, but not necessarily their progeny, myoblasts, are likely to produce tissue with normal telomere maintenance when used in molecular and regenerative medicine approaches for tissue repair.
Keywords: aging, muscle, satellite cell, myoblast, telomerase, telomere, tissue engineering
Introduction
Satellite cells exist, in vivo, as a quiescent cellular subset located between the basal lamina and sarcolemma of mature muscle fibers. Upon injury, satellite cells are activated and begin to proliferate. A portion of the satellite cells return to quiescence [1] while the rest differentiate into myoblasts, which migrate to the injury site and differentiate further into multinucleated myotubes (or myofibers), hence repairing the injury.
There has been speculation that muscle telomeres may become dysfunctional with age [2], as do the telomeres of other, more proliferative tissues, e.g., blood lineages and intestines [3-6]. Considering the previous reports that human muscle progenitors are mortal in ex vivo cell culture [7], the general conclusion in the field was that telomerase activity does not play a role in skeletal muscle maintenance and repair. Telomerase has not been well studied in satellite cells or in primary myoblasts, while the immortalized long-term line of mouse myogenic progenitors, C2C12, is known to have high telomerase activity [8].
Interestingly, there is little difference between the proliferative capacity of human muscle progenitor cells grown in culture, which were derived from young adults and very old donors [9]. There is, however, a tremendous decline in the ability of aged humans and animals to repair and maintain skeletal muscle in vivo [10]. This argues that aging causes a defect in myogenesis that is unrelated to telomere state. Such an argument is further substantiated by the ability of the aged satellite cells to be rejuvenated in the young extrinsic milieu [11] and by specific molecular cues [12, 13].
Muscle is a tissue that is impacted by many congenital neurodegenerative disorders, and by age-related acquired myopathies. Tissue engineering approaches, such as myoblast transplantation, in the context of synthetic scaffolds, have therefore been proposed as possible treatments for muscle degeneration in diseases such as Duchenne muscular dystrophy (DMD) [14-16]. A major hurdle for treatment of muscular disorders using transplanted myoblasts has been the survival, proliferation, and efficient differentiation of transplanted cells in vivo. One experiment was to use matrix metalloproteinase to partially degrade the extracellular matrix (ECM) around the site of transplantation, in order to facilitate myoblast migration within the treated muscle [17]. Another approach has been to try to build muscle tissue in vitro on an aligned collagen matrix, which could then be grafted onto dysfunctional muscle [18]. It has also been shown that mouse primary myoblasts exhibit higher rates of proliferation in biodegradable gels than in nonbiodegradable materials [19]. Additionally, freshly isolated rat myoblasts, expanded in a 3D fibrin matrix for 7 days, were capable of fusing with and/or forming myofibers in vivo [20].
While significant progress has been made in selecting and optimizing biomaterials, much less work has been done on clarifying the best source of cells to be used in the tissue engineering of skeletal muscle, and virtually all studies were performed with myoblasts (which are the only muscle progenitor that can be expanded ex-vivo) (reviewed in [21]).
Considering that tissue engineering methods are dependent on the in vitro and in vivo expansion and/or manipulation of cells, it is important to establish the long-term genomic stability and telomere maintenance of the cellular components of the engineered tissues.
In this regard, comparative analysis of telomerase activity between the satellite cells and myoblasts was performed in this work. As compared to mice, there is only a partial understanding of the cellular and molecular determinants of human myogenesis. Thus, we used a genetically and environmentally controlled mouse model of myogenesis in order to generate data on the dynamics of telomerase activity in muscle stem and progenitor cells. Purification of myofiber-associated cells, and dissection of myogenic lineage progression, in regenerating adult skeletal muscle have allowed us to distinguish between quiescent satellite cells, asymmetrically dividing activated satellite cells, and transiently existing myoblasts based on distinct genetic markers and functional properties of these cell populations [22-24]. This previously published work has, for the first time, enabled the comparative study of telomerase activity in myogenic stem cells and in their more differentiated progeny, myoblasts; both of which are capable of proliferation and are necessary for muscle repair [10]. While it is believed by many that, in contrast to human cells, most cycling mouse cells possess telomerase activity, there is little support in the literature for this conclusion (one exception being the comparison between telomerase negative fused mouse myotubes and telomerase positive immortalized mouse C2C12 cell line [8]). We therefore took advantage of the current state of the art to analyze the phenomenon of telomerase regulation during myogenic lineage progression: from stem to progenitor cells.
Here, we used two strains of mice: one with long telomeres and another with short telomeres, and measured the telomerase activity as a function of differentiation (stem to progenitor cell) and as a function of stem cell aging (2-3 month old mice, equivalent to 20-30 year old humans, and 20-24 month old mice, equivalent to 70-80 year old humans).
Our data suggest that true stem cells (young or old), but not cultured primary progenitor cells, will produce tissue with normal telomeres in transplantation-based or molecular medicine-based methods for regenerative medicine. Such conclusions are consistent with the limited data obtained using human myoblast cultures [9], and support the relevance of this work for understanding telomere regulation during regeneration of human muscle and for human tissue engineering approaches.
Materials and Methods
Animals
C57.BL6 (C57 Black 6) is an inbred strain of laboratory mice commonly used for muscle and other biological studies. Young male C57.BL6 mice were provided by Harland and old C57.BL6 mice by the National Institute on Aging. M. m. castaneus (Cast/Ei) is a wild derived strain of mouse provided by Jackson Labs. Mice (all male) were housed singly and old mice were aged in our animal facility.
Isolation of Satellite Cells and CD45 MACS Purification
Satellite cells were purified as described [11, 25], with some modifications. Briefly, mice were hindlimb injured by cardiotoxin injection into the gastrocnemius, quadricep, and hamstring muscle groups. Three days after injury mice were euthanized and dissected. Collected muscle was digested for 1.5 hr in a collagenase II - Dulbecco’s Modified Eagle Medium (DMEM) solution (300U/ml) at 37°. Myofibers were released by mechanical dissociation, collected, and washed in media by gravity sedimentation. Myofibers were then shredded by syringing through a 20G needle and passed through a 40u cell strainer (Fisher). In some cases, after shredding, an additional collagenase/dispase digestion was performed (30 min 37°). Cells were washed once in mPBS (0.5% BSA, 2mM EDTA in Phosphate Buffered Saline) and then resuspended in 90 ul mPBS. 10μl of CD45 coated magnetic beads (Miltenyi) were added to cells and incubated on ice for 30 min. Cells were washed once in mPBS and then resuspended in 1.5 ml mPBS. Cells were then passed through the MS column, collected, and counted on a hemocytometer.
Collection of Migrated Myoblasts
Fibers were purified from bulk skeletal muscle as above, except that only uninjured (resting) muscle was used. Fibers were then cultured in suspension (in ECM coated plates) in 40 volumes myoblast growth media (Hams F-10 with 20% FBS and 5ng per ml of bFGF (basic Fibroblast Growth Factor)). After 5 days myoblasts had migrated out of the fibers and adhered to the ECM coated plate. Fibers were discarded and myoblasts were collected and counted.
Flow Cytometry
CD45 positive and negative fractions of myofiber associated cells were purified as above. Each fraction (of unfixed cells) was then labeled with mouse anti-mouse CD45.2 antibody (Biolegend) and a cocktail of rat anti-mouse antibodies composed of anti Ly-6G/Ly-6C (GR-1), anti CD45R/B220, anti CD3, and anti CD11b (Mac1) (BD Pharmingen). Secondary antibodies were goat anti-mouse PE (Caltag) and donkey anti-rat 488 (Molecular Probes). 5,000 events per sample were counted using a Beckman-Coulter Epics XL FACs machine and quantified using the Expo 32 ADC analysis software.
Myogenic potential functional assay
Purified satellite cells were plated on chamber slides for 48 hours in Hams F-10 with 20% FBS and 5ng per ml of bFGF. Cells were forced to differentiate by replacing this mitogenic growth medium with DMEM containing 2% horse serum for 48 hours. Newly-formed myotubes were stained with anti-eMyHC (Vector Labs) and counterstained with Hoechst nuclear stain. Secondary antibody was goat anti-mouse 546 (Molecular Probes). Images were visualized and captured using a Zeiss Axio Imager A1 and monochrome Axiocam MRm camera.
Telomerase activity and telomere length determination
The Roche Telomerase PCR ELISA kit was used, as recommended by the manufacturer, to quantify the relative telomerase activity from 2 x 105 cells per sample. Cells were counted, lysed, and RT-PCT was performed (standardized by cell number) on each sample. Results for each data point described in Figure 3 were derived from the indicated number of independent experiments starting from satellite cell isolation from separate mice; and each independent experiment consists of three averaged ELISAs that followed three independent TRAP reactions (amplified for 27 PCR cycles).
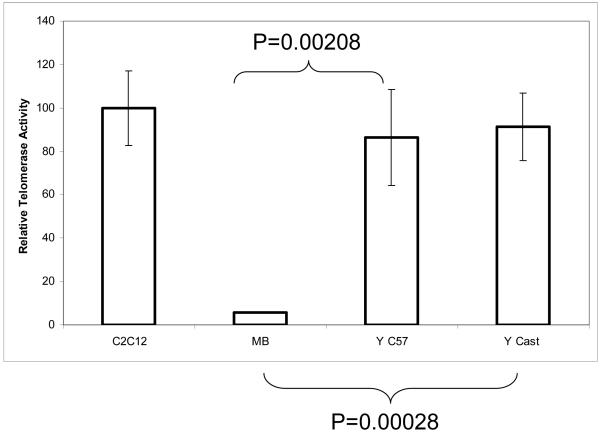
Figure 3.
Muscle stem cells from both long and short telomere strains possess robust telomerase activity. Telomerase activity determined as above by TRAP assay is expressed as a percentage of the positive control (C2C12 cells). C2C12 N=22, MB N=7, Y C57 (young) N=13, Y Cast/EiJ (young “short telomere”) N=8. Error bars represent the standard error of the mean.
Telomere length was determined by telomere restriction fragment length analysis (TRFL). Briefly, muscle was collected and fibers isolated by dissociation and gravity sedimentation as above. DNA was purified using the DNeasy tissue kit (Qiagen) and non-telomeric DNA digested with HinF1 and RSA1. ∼1μg DNA was separated on a 0.65% TBE-agarose gel and separated by gel electrophoresis. Gel was blotted using basic (0.4N NaOH) capillary transfer and telomere signal detected using the non-radioactive TeloTAGGG Telomere Length Assay kit (Roche).
Statistical analysis
Error bars were generated by calculating the Standard Error (SE) of each independent experiment. Each independent data point was generated from 3 averaged TRAP reactions. P values were calculated using the Student’s T Test.
Results
Comparison of telomerase activity in primary myoblasts and long-term immortalized C2C12 cells
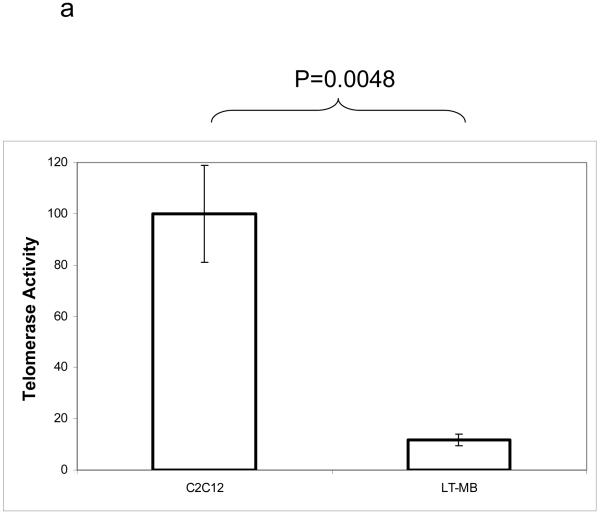
Based on the fact that human primary myoblasts senesce in culture [7], one would assume that human myoblasts maintain negligible telomerase activity. Primary mouse myoblasts telomeres have not, however, been studied and to our knowledge the only population of mouse myoblasts which have been examined are the immortal C2C12 line [8]. Some have assumed that this implies that cycling human and mouse cells are fundamentally different and that, in contrast to human cells, cycling mouse cells express telomerase. To detect telomerase activity, we utilized the TRAP DIG-ELISA method, which is based on the traditional RT PCR TRAP assay but yields more quantifiable data than resolving the signal by gel electrophoresis. We found that analogous to human cells [7, 26], long-term cultured mouse myoblasts also lack telomerase activity. These results establish that mouse and human proliferative muscle progenitor cells are similar, with respect to the absence of their telomerase activity, which is down-regulated during adult myogenesis in both species (Figure 1A). Human foreskin fibroblasts (BJ cells) were used as a negative control in initial experiments and showed a similar basal level of signal as long-term myoblasts (data not shown). In agreement with previous work [8], immortalized C2C12 cells do possess high levels of telomerase activity, which is in contrast to primary myoblasts (Figure 1A).
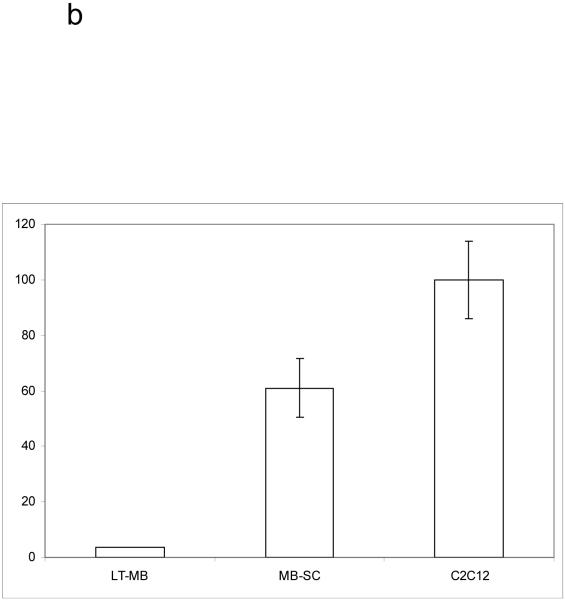
Figure 1.
Partially differentiated muscle progenitors are telomerase negative. A. Cultured primary myoblasts were lysed and relative telomerase activity determined by DIG-TRAP. Error bars represent standard error of the mean. Telomerase activity expressed as a percent of the positive control (C2C12 cell activity raw value set to 100%). C2C12 N=22. Long term cultured myoblasts (LT-MB) N=7. B. Myofibers were isolated from resting hindlimb muscle and cultured in myoblast growth media for 5 days. After 5 days myogenic progenitor cells had migrated out of the fibers and attached to the plate (MB-SC). Cells were collected and telomerase activity determined as described. Shown are the means and standard deviations of 3 TRAP reactions.
To confirm that our purified myoblasts maintain the stem-like ability to self-renew (at least for some period of time) in vitro, we used our mouse model of adult myogenesis. We collected myoblasts, which were produced by satellite cells and migrated out of freshly purified muscle fibers within 5 days post-muscle explantation, and adhered to ECM coated plates. Fibers were collected from uninjured (resting) muscle and incubated in myoblast growth media for 5 days. This mimics injury ex-vivo and routinely yields primary myoblast cultures [27]. At 5 days post-explantation, these primary myoblasts were collected and subjected to TRAP assay analysis as above. Cells were compared to long-term cultured primary myoblasts (several months in vitro) and to immortalized C2C12 cells. In contrast to their long-term cultured counterparts, these early myoblasts displayed significant telomerase activity (Figure 1b), even after the beginning steps of differentiation and past the point at which they had committed to fusion-competence [28-30]. In vivo, the amount of time in which the transient population of myoblasts exists corresponds well with the retention of telomerase activity in the cells (∼5 days).
Purification of Muscle Satellite Cells
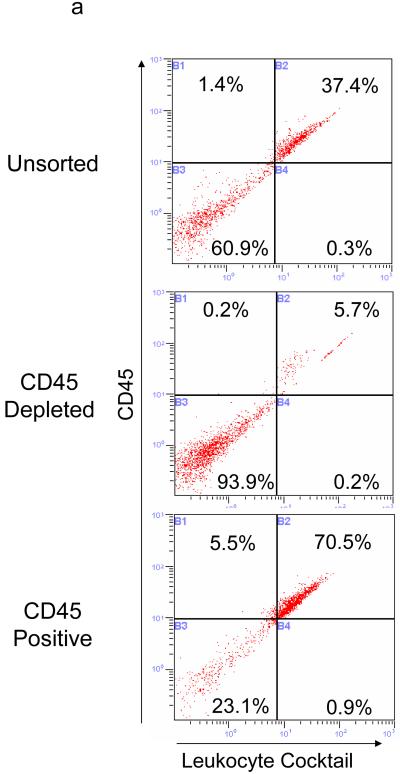
Next, we endeavored to assay telomerase activity in a highly pure population of mouse muscle stem cells (satellite cells) using a simple modification of a previously established procedure [11, 25]. Myofiber-associated cells were first purified away from myofibers and muscle interstitial cells using enzymatic and mechanical dissociation procedures, as previously described [11, 25]. Then, CD45+ cells were depleted from the pool of myofiber-associated cells using magnetic-activated cell sorting (MACS) with magnetic beads coated with anti-CD45 antibody. This negative selection was verified by flow cytometry (Figure 2A). Flow cytometry analysis was based on immuno-detection with a pan-leukocyte antibody cocktail, designed to detect T-cells (anti-CD3), B-cells (anti-B220), macrophages (anti-MAC1) and granulocytes (anti-Gr1). The CD45+ cell fraction (negatively selected away from our satellite cell preparations) expressed, as expected, leukocyte markers, and the efficiency of purification of myofiber-associated cells away from CD45+ leukocytes by MACS was readily confirmed (Figure 2A).
Figure 2.
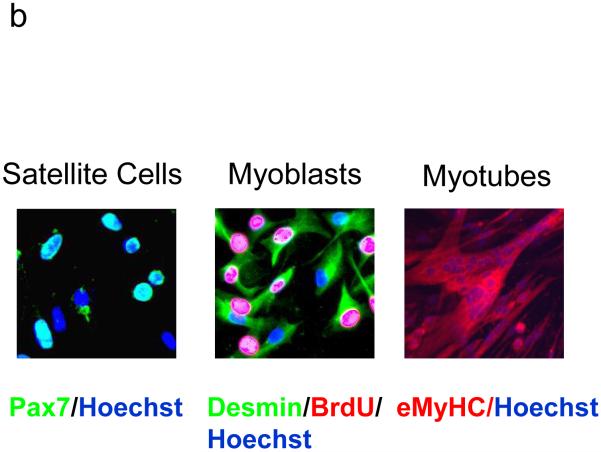
Isolation of satellite cells from mouse skeletal muscle (adapted from 11). (a) Flow cytometry analysis of myofiber associated cells before (top) and after CD45+ cell depletion (middle) or elution (bottom). Cells were labeled with anti-CD45 antibodies (Y-axis) and a pan-leukocyte cocktail (X-axis). (b) Myogenic potential of CD45 depleted satellite cells (right) is evident from the rapid and robust generation of eMyHC+ myotubes. Purified satellite cells were either immediately fixed and stained for Pax7 (green) and Hoecht dye (blue), cultured for 48 hours in growth media and labeled with Brdu (red)/Desmin (green)/Hoecht dye (blue) or cultured for an additional 48 hours in differentiation media after which the myogenic progeny of these cells was stained with Hoechst nuclear dye (blue) and eMyHC (red).
Satellite cells grown ex-vivo will spontaneously differentiate into Myf-5+ desmin+ myoblasts, which rapidly produce multinucleated myotubes expressing the muscle marker embryonic myosin heavy chain (eMyHC) in conventional differentiation-promoting medium [10, 31, 32]. As shown in Figure 2B, the purified myofiber-associated cells were overwhelmingly (∼95%) myogenic (based on Pax7 expression, reviewed in [33, 34]) and followed the in vitro myogenic lineage progression, as previously established (reviewed in [10]). The remaining ∼5% of cells displayed clear fibroblast morphology and likely represented fibroblast contamination of myofiber preparations.
These results demonstrate that highly enriched endogenous muscle stem cells capable of in vitro differentiation into myoblasts and myotubes, can be isolated solely based on their location beneath the basement lamina of myofibers, with the necessary depletion of CD45+ myofiber-associated leukocytes.
Telomerase Activity in Muscle Stem Cells
We next asked whether these purified adult stem cells have the theoretical ability to divide indefinitely in vivo and to maintain their telomeres with endogenous telomerase, as several other adult stem cell types, most embryonic and germ cells, and the vast majority of transformed cells possess [4, 35-44]. As shown in Figure 3, CD45- satellite cells purified from adult mouse skeletal muscle are highly telomerase positive, displaying levels of enzymatic activity similar to immortalized myoblasts (C2C12). The CD45+ leukocyte fraction with a few remaining “contaminating” satellite cells (Figure 2A) displayed significantly lower telomerase activity than CD45- cells (not shown), confirming that these cells do not generate false positive TRAP results.
Most laboratory strains of inbred mice (including C57.B6) have aberrantly long and unstable telomeres. Wild mice reportedly have “normal” telomeres as do some more “recently” derived inbred strains [45]. The Cast/Ei strain of mouse has been well characterized to have short telomeres that respond dramatically to telomerase loss and haploinsufficiency [46]. We therefore sought to confirm that the herein discovered phenomena were generally applicable and were not due to atypically long telomeres of the C57.B6 strain. Endogenous telomerase activity was assayed in satellite cells derived from C57.B6 and the Cast/Ei strains using the same TRAP DIG-ELISA assay as described above in Figure 1. The results of these experiments definitively show that, unlike their more differentiated progeny (long-term myoblasts), freshly isolated mouse muscle stem cells from both long and short telomere animals reproducibly and robustly manifest endogenous telomerase activity at levels comparable to that of our positive control (the immortalized mouse myoblast cell line C2C12, which is capable of growth in soft agar, forming tumors in immuno-deficient mice, and have been shown to be strongly telomerase positive [8, 47-49], and MJ Conboy, personal communication) (Figure 3). Therefore, muscle stem cells represent a better cell source for engineered skeletal muscle than the long-term primary myoblasts.
Time Course of Telomerase Inactivation
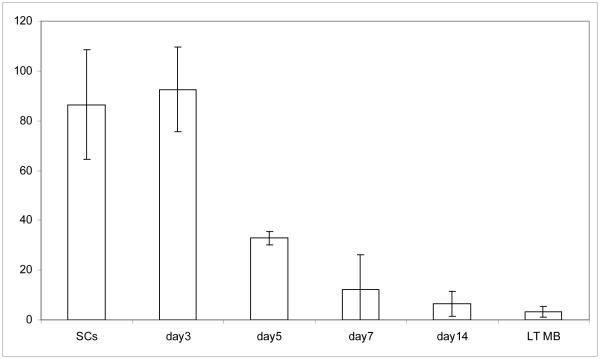
In order to investigate the possibility that telomere maintenance remains optimal in freshly migrated transient myoblasts (which likely more closely reflect the in vivo situation), as compared to myoblasts that have been grown in culture for an extended period of time (weeks to months), we endeavored to perform a time course experiment. A mouse model of muscle injury and regeneration was used to analyze telomerase activity in muscle stem cells activated for their myogenic responses in vivo and in the progeny of these stem cells during 3-14 days of culture. It is known in the field that primary mouse myoblasts will grow ex-vivo for a short time (∼7 days), then cease dividing (“crash”) for a number of days, after which some cells are selected for by their culture conditions to resume normal growth at least, for several months. Thus, we analyzed the telomerase activity in freshly isolated muscle stem cells and in their progeny after 3, 5, 7, or 14 days in vitro. As shown in Figure 4, and in agreement with results shown above, myoblasts initially maintained a high level of telomerase activity, but quickly lost it as they approached and entered the “crash.” In these experiments, we found myogenic differentiation and myogenic lineage marker expression to be the same (e.g. Pax7, Myf5 and desmin), regardless of the different levels of telomerase activity observed with time in these cultured cells. Interestingly, our experiments with numerous primary myoblast lines established that the cells never regain their ability to maintain telomeres unless they are clonally selected for immortalization (as in the case of the immortalized C2C12 cell line [8, 50]). Thus, long-term cultured myoblasts, which display no detectable telomerase activity, are fundamentally different from transient myoblasts (which continue to display telomerase activity for several days after activation).
Figure 4.
Gradual loss of telomerase activity during stem cell to progenitor cell transition in culture. Myofiber-associated satellite cells were isolated 3 days after muscle injury and their in vitro differentiation into myogenic progenitor cells were performed, as in [25] and Methods. Cells were collected for TRAP assay either immediately after isolation from muscle (SCs) or after in vitro differentiation for the specified number of days and telomerase activity was determined as described. Shown are representative means and standard deviations of TRAP reactions preformed in triplicate. Similar data was obtained in 3 independent experiments.
Maintenance of Muscle Telomeres in Aged Tissue
The data described above implies that mouse muscle stem cells are immortal in vivo as far as telomere-dependent genomic stability is concerned. The possibility existed, however, that satellite cells endogenous to old skeletal muscle might lose their telomerase activity with age, allowing telomeres to erode in old skeletal muscle. If this were true, then aged autologous satellite cells would be inferior cell sources for tissue engineering of skeletal muscle.
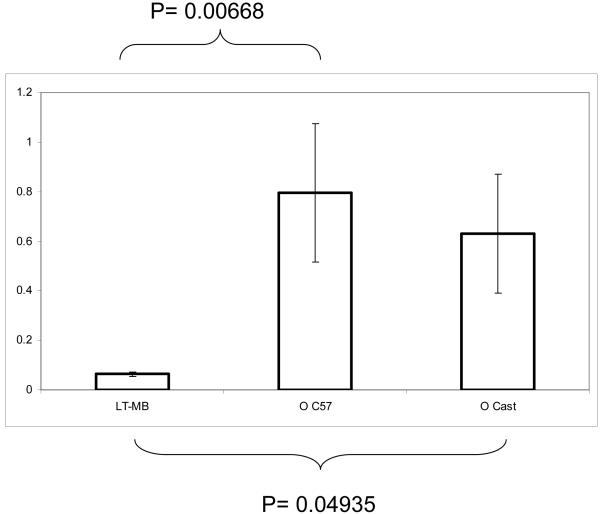
We therefore also purified muscle stem cells from young (2-3 mo old) and old (22-24 mo old) C57.B6 and Cast/EiJ mice 2 days post muscle injury using the procedure described above in Figure 2. The TRAP DIG-ELISA assay was then performed on these freshly isolated cells, using the same negative and positive controls as above. Remarkably, in both strains of mice, old satellite cells were found to maintain their telomerase activity, albeit perhaps not as well as satellite cells derived from young mice, but much better than primary myoblasts (Figure 5). Robust telomerase activity in both strains and the persistence of such activity at all ages suggests that, during tissue maintenance and repair, muscle stem cells have the ability to maintain telomeres in their differentiated progeny throughout their lifespan and even in old animals.
Figure 5.
Satellite cells from old animals maintain their intrinsic telomerase activity. Telomerase activity from satellite cells isolated from old (22-24 mo N=6) c57.B6 mice or old Cast/EiJ N=6 compared to long-term myoblast culture. Data from old animals and LT-MB expressed as a percentage of the pooled activity from the SCs of young animals. Shown are means and standard errors of multiple independent experiments. Statistically significant presence of telomerase activity was detected in satellite cells isolated from old mice, as compared to the differentiated progenitor cells: primary myoblasts.
To examine this directly, we analyzed telomere length in the DNA of C57.B6 and Cast/EiJ myofibers collected from young and old animals. While cells expressing telomerase generally have healthy telomeres, the possibility exists that there could be telomerase-independent muscle telomere dysfunction in differentiated muscle. Additionally, we detected a small trend toward an age-specific decline in stem cell telomerase activity in both studied strains; and hence, we sought to establish whether such decline translates into telomere shortening in the differentiated progeny of muscle stem cells: mature myofibers. Myofibers were dissociated from the hindlimb muscles of old and young animals as previously described [25]. DNA was isolated, and TRFL assays performed, using conventional techniques [51].
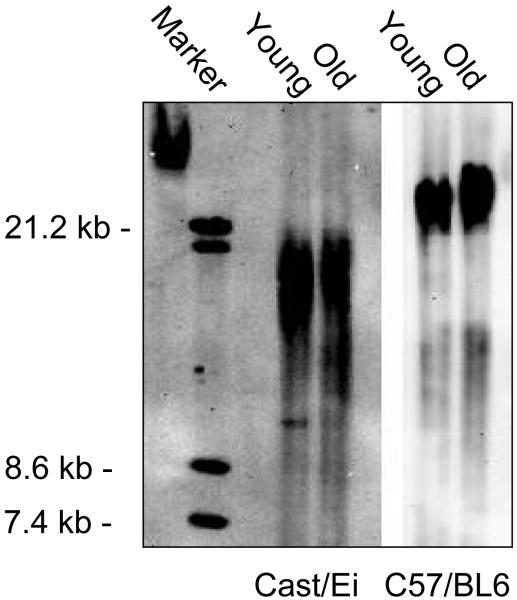
As shown in Figure 6, telomeres from not only the “long” telomere C57.B6 mice but also from Cast/EiJ “short” telomere mice failed to display detectable reduction in telomere length with age. In complete agreement with the previously published data, and hence confirming the validity of our experimental systems, myofibers isolated from Cast/EiJ mice displayed shorter telomeres as compared to C57.B6-derived myofibers (Figure 6).
Figure 6.
Telomere length does not decrease in differentiated muscle cells with age. Telomeric DNA was isolated from purified myofibers derived from young and old Cast/EiJ and C57.B6 mice. Telomere length was determined by telomere restriction fragment length analysis (TRFL), using the non-radioactive TeloTAGGG Telomere Length Assay kit, as described in the Methods. Similar results were obtained in two independent experiments.
Thus, with age there is no decline in telomere length in the myonuclei of differentiated myofibers, likely due to the continuing presence of telomerase activity in aged satellite cells. Accordingly, autologous old muscle stem cells are likely to be suitable for regenerative medicine in aged skeletal muscle.
Conclusions
The results of this work are the first to reliably establish that muscle stem cells and transient myoblasts possess high telomerase activity, in contrast to primary myoblasts. These findings confer upon satellite cells the status of potentially immortal adult stem cells that are capable of indefinite tissue maintenance and repair. This work also demonstrates that the cell-fate dependent down-regulation of telomerase activity upon differentiation from stem into progenitor cell occurs during adult myogenesis both in mice and in humans [9]. Thus, the previous notion of high telomerase activity in proliferative mouse cells does not seem to be true in regard to the myogenic lineage.
The emerging picture of the temporal pattern of telomerase expression seems to be that satellite cells maintain telomerase expression in vivo, but quickly lose this activity during differentiation into myogenic progenitor cells in vitro (Figure 4) and, as expected, after fusion into myotubes [8]. Very interestingly, the association with myofibers promotes the maintenance of telomerase activity in transient myoblasts (Figures 1b and 4), suggesting that it is not simply the culture conditions that cause the decline in telomerase activity and that this process can be regulated by the muscle stem cell niche (differentiated myofibers).
The loss of telomerase activity in vitro coincides closely with the fusion of proliferating myoblasts into de-novo post-mitotic myotubes in vivo, i.e., at 5-7 days post-injury (Figure 4 and [25, 52]). Therefore, there might also be a “cell-autonomous” regulation (intrinsic “memory”), by which telomerase activity is rapidly extinguished in vitro at a very similar time when it is no longer necessary in vivo. The correlation between telomerase activity and genetic markers of myogenic differentiation will be interesting to investigate in the future, and such work might point toward novel methods for the isolation of cells that are optimal for engineering of skeletal muscle.
One interesting possibility is that the dramatic decline in telomerase activity in proliferating progenitor cells protects against cancers and ensures that these cells generate terminally post-mitotic progeny. In this regard, re-acquisition of telomerase activity in C2C12 cells does correlate positively with the oncogenic properties of these cells [47, 48]. Since telomerase itself is insufficient to immortalize myoblasts [53, 54], other mutation(s) must be occurring in such immortalized populations. Accordingly, the presence of telomerase activity in a given cell does not signify the suitability of such cells for regenerative tissue engineering; and in contrast, only stem cells which naturally display telomerase activity would seem to be the best candidates for cell-based therapies. It has been proposed that exogenous telomerase (and other genes) be used in myoblasts as a method to make myoblasts more viable for transplantation and bioengineering purposes [26, 53, 55]. It would seem, however, that there are drawbacks and potential dangers of oncogenic transformations in this approach. Satellite cells and early transient myoblasts, which maintain telomerase for several days and then down-regulate such activity, may be more amenable and safe for transplantation purposes than long-term passaged myoblasts (which express high levels of exogenous telomerase and other proto-oncogenes). Our results also demonstrate that satellite cells endogenous to old skeletal muscle do not lose telomerase activity, even though such muscle stem cells fail to be activated in response to injury. This fact, in conjunction with the previously established ability to rescue the proliferative and regenerative capacity of aged satellite cells by their exposure to young extrinsic cues [11, 12], strongly suggests that satellite cells residing in old skeletal muscle have the intrinsic capacity not only to repair muscle but also to produce “young” tissue with normal telomeres and to self-renew. The direct evidence for such a conclusion is provided in our data establishing that the length of telomeres does not decline with age in muscle fibers isolated from two distinct strains of mice (Figure 6). This is consistent with previous studies of telomere length maintenance in various mouse tissues [8, 56]. Tissues which lack telomerase and/or proliferate significantly tend to shorten with age, while less proliferative tissues and those expressing telomerase tend to be maintained. Muscle is a dynamic tissue which is periodically replenished by satellite cells. Thus, if satellite cell telomeres were shortening one would expect that to be reflected in shorter myofiber telomeres. Also, if myofiber telomeres were shortening by non-replicative means (such as by oxidative damage) or end-resection by endonucleases, then replenishment of the myonuclei by satellite cells would partially mask that affect.
This work suggests that mouse muscle telomeres are maintained by virtue of telomerase activity, specifically in the undifferentiated satellite cell. The loss of telomerase activity in mouse myoblasts is quite dramatic; and we would expect that the consequences of this lack of telomere maintenance would be even more dramatic in large, long-lived species with short telomeres, such as humans. In support of this notion, after satellite cell exhaustion there is telomere shortening in bulk skeletal muscle from DMD patients [57].
Summarily, our results establish that regulation of telomerase activity in muscle progenitor cells is similar in mice and humans, and further reveal that such activity rapidly declines upon differentiation of stem into progenitor cells, but remains relatively high in old muscle stem cells. Additionally, our works emphasizes the importance of selecting optimal cell sources for the engineering of skeletal muscle, which may succeed where long-term myoblast therapies have failed.
Supplementary Material
Acknowledgments
We would like to thank Jennifer Rhodes for technical assistance. This work was supported by the Ellison’s Medical Foundation, the NIH/NIA, and the Stem Cell Research Foundation grants to I.M.C. M.S.O. participated in the design and execution of the experiments and the writing of the manuscript; Morgan Carlson contributed the data for the left and center panels of figure 2b, and to manuscript revision; I.M.C. participated in the development of the concepts, in the design and execution of experiments, and in the writing of the manuscript.
References
- 1.Schultz E, Gibson MC, Champion T. Satellite cells are mitotically quiescent in mature mouse muscle: an EM and radioautographic study. J Exp Zool. 1978;206(3):451–6. doi: 10.1002/jez.1402060314. [DOI] [PubMed] [Google Scholar]
- 2.Collins M, et al. Athletes with exercise-associated fatigue have abnormally short muscle DNA telomeres. Med Sci Sports Exerc. 2003;35(9):1524–8. doi: 10.1249/01.MSS.0000084522.14168.49. [DOI] [PubMed] [Google Scholar]
- 3.Akbar AN, Vukmanovic-Stejic M. Telomerase in T lymphocytes: use it and lose it? J Immunol. 2007;178(11):6689–94. doi: 10.4049/jimmunol.178.11.6689. [DOI] [PubMed] [Google Scholar]
- 4.Morrison SJ, et al. Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity. 1996;5(3):207–16. doi: 10.1016/s1074-7613(00)80316-7. [DOI] [PubMed] [Google Scholar]
- 5.Leedham SJ, et al. Intestinal stem cells. J Cell Mol Med. 2005;9(1):11–24. doi: 10.1111/j.1582-4934.2005.tb00333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Effros RB, et al. The role of CD8+ T-cell replicative senescence in human aging. Immunol Rev. 2005;205:147–57. doi: 10.1111/j.0105-2896.2005.00259.x. [DOI] [PubMed] [Google Scholar]
- 7.Decary S, et al. Replicative potential and telomere length in human skeletal muscle: implications for satellite cell-mediated gene therapy. Hum Gene Ther. 1997;8(12):1429–38. doi: 10.1089/hum.1997.8.12-1429. [DOI] [PubMed] [Google Scholar]
- 8.Holt SE, Wright WE, Shay JW. Regulation of telomerase activity in immortal cell lines. Mol Cell Biol. 1996;16(6):2932–9. doi: 10.1128/mcb.16.6.2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wernig A, et al. On the regenerative capacity of human skeletal muscle. Artif Organs. 2005;29(3):192–8. doi: 10.1111/j.1525-1594.2005.29033.x. [DOI] [PubMed] [Google Scholar]
- 10.Wagers AJ, Conboy IM. Cellular and molecular signatures of muscle regeneration: current concepts and controversies in adult myogenesis. Cell. 2005;122(5):659–67. doi: 10.1016/j.cell.2005.08.021. [DOI] [PubMed] [Google Scholar]
- 11.Conboy IM, et al. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature. 2005;433(7027):760–4. doi: 10.1038/nature03260. [DOI] [PubMed] [Google Scholar]
- 12.Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007;6(3):371–82. doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldspink G. Loss of muscle strength during aging studied at the gene level. Rejuvenation Res. 2007;10(3):397–405. doi: 10.1089/rej.2007.0597. [DOI] [PubMed] [Google Scholar]
- 14.Negroni E, Butler-Browne GS, Mouly V. Myogenic stem cells: regeneration and cell therapy in human skeletal muscle. Pathol Biol (Paris) 2006;54(2):100–8. doi: 10.1016/j.patbio.2005.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Chakkalakal JV, et al. Molecular, cellular, and pharmacological therapies for Duchenne/Becker muscular dystrophies. Faseb J. 2005;19(8):880–91. doi: 10.1096/fj.04-1956rev. [DOI] [PubMed] [Google Scholar]
- 16.Partridge T. Myoblast transplantation. Neuromuscul Disord. 2002;12(Suppl 1):S3–6. doi: 10.1016/s0960-8966(02)00076-7. [DOI] [PubMed] [Google Scholar]
- 17.El Fahime E, et al. In vivo migration of transplanted myoblasts requires matrix metalloproteinase activity. Exp Cell Res. 2000;258(2):279–87. doi: 10.1006/excr.2000.4962. [DOI] [PubMed] [Google Scholar]
- 18.Yan W, et al. Tissue engineering of skeletal muscle. Tissue Eng. 2007;13(11):2781–90. doi: 10.1089/ten.2006.0408. [DOI] [PubMed] [Google Scholar]
- 19.Boontheekul T, et al. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007;13(7):1431–42. doi: 10.1089/ten.2006.0356. [DOI] [PubMed] [Google Scholar]
- 20.Beier JP, et al. Y chromosome detection of three-dimensional tissue-engineered skeletal muscle constructs in a syngeneic rat animal model. Cell Transplant. 2004;13(1):45–53. doi: 10.3727/000000004772664888. [DOI] [PubMed] [Google Scholar]
- 21.Peault B, et al. Stem and progenitor cells in skeletal muscle development, maintenance, and therapy. Mol Ther. 2007;15(5):867–77. doi: 10.1038/mt.sj.6300145. [DOI] [PubMed] [Google Scholar]
- 22.Conboy IM, Rando TA. The regulation of Notch signaling controls satellite cell activation and cell fate determination in postnatal myogenesis. Dev Cell. 2002;3(3):397–409. doi: 10.1016/s1534-5807(02)00254-x. [DOI] [PubMed] [Google Scholar]
- 23.Conboy MJ, Karasov AO, Rando TA. High incidence of non-random template strand segregation and asymmetric fate determination in dividing stem cells and their progeny. PLoS Biol. 2007;5(5):e102. doi: 10.1371/journal.pbio.0050102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Collins CA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell. 2005;122(2):289–301. doi: 10.1016/j.cell.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 25.Conboy IM, et al. Notch-mediated restoration of regenerative potential to aged muscle. Science. 2003;302(5650):1575–7. doi: 10.1126/science.1087573. [DOI] [PubMed] [Google Scholar]
- 26.Di Donna S, et al. Regenerative capacity of human satellite cells: the mitotic clock in cell transplantation. Neurol Sci. 2000;21(5 Suppl):S943–51. doi: 10.1007/s100720070008. [DOI] [PubMed] [Google Scholar]
- 27.Rosenblatt JD, et al. Culturing satellite cells from living single muscle fiber explants. In Vitro Cell Dev Biol Anim. 1995;31(10):773–9. doi: 10.1007/BF02634119. [DOI] [PubMed] [Google Scholar]
- 28.Schultz E, McCormick KM. Skeletal muscle satellite cells. Rev.Physiol Biochem.Pharmacol. 1994;123:213–257. doi: 10.1007/BFb0030904. [DOI] [PubMed] [Google Scholar]
- 29.Shi X, Garry DJ. Muscle stem cells in development, regeneration, and disease. Genes Dev. 2006;20(13):1692–1708. doi: 10.1101/gad.1419406. [DOI] [PubMed] [Google Scholar]
- 30.Carlson ME, Conboy IM. Loss of stem cell regenerative capacity within aged niches. Aging Cell. 2007 doi: 10.1111/j.1474-9726.2007.00286.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kelly R, et al. Myosin light chain 3F regulatory sequences confer regionalized cardiac and skeletal muscle expression in transgenic mice. J Cell Biol. 1995;129(2):383–96. doi: 10.1083/jcb.129.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dhawan J, Rando TA. Stem cells in postnatal myogenesis: molecular mechanisms of satellite cell quiescence, activation and replenishment. Trends Cell Biol. 2005;15(12):666–73. doi: 10.1016/j.tcb.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 33.Buckingham M. Skeletal muscle progenitor cells and the role of Pax genes. C R Biol. 2007;330(6-7):530–3. doi: 10.1016/j.crvi.2007.03.015. [DOI] [PubMed] [Google Scholar]
- 34.Collins CA. Satellite cell self-renewal. Curr Opin Pharmacol. 2006;6(3):301–6. doi: 10.1016/j.coph.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 35.Castro P, et al. Cellular senescence in the pathogenesis of benign prostatic hyperplasia. Prostate. 2003;55(1):30–8. doi: 10.1002/pros.10204. [DOI] [PubMed] [Google Scholar]
- 36.Eisenhauer KM, et al. Telomerase activity in female and male rat germ cells undergoing meiosis and in early embryos. Biol Reprod. 1997;56(5):1120–5. doi: 10.1095/biolreprod56.5.1120. [DOI] [PubMed] [Google Scholar]
- 37.Fujisawa M, et al. Telomerase activity in the testis of infertile patients with selected causes. Hum Reprod. 1998;13(6):1476–9. doi: 10.1093/humrep/13.6.1476. [DOI] [PubMed] [Google Scholar]
- 38.Gronthos S, et al. Molecular and cellular characterisation of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 2003;116(Pt 9):1827–35. doi: 10.1242/jcs.00369. [DOI] [PubMed] [Google Scholar]
- 39.Herrera E, Samper E, Blasco MA. Telomere shortening in mTR-/- embryos is associated with failure to close the neural tube. Embo J. 1999;18(5):1172–81. doi: 10.1093/emboj/18.5.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martin-Rivera L, et al. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc Natl Acad Sci U S A. 1998;95(18):10471–6. doi: 10.1073/pnas.95.18.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ravindranath N, et al. Loss of telomerase activity during male germ cell differentiation. Endocrinology. 1997;138(9):4026–9. doi: 10.1210/endo.138.9.5488. [DOI] [PubMed] [Google Scholar]
- 42.Thomson JA, et al. Embryonic stem cell lines derived from human blastocysts. Science. 1998;282(5391):1145–7. doi: 10.1126/science.282.5391.1145. [DOI] [PubMed] [Google Scholar]
- 43.Wright DL, et al. Characterization of telomerase activity in the human oocyte and preimplantation embryo. Mol Hum Reprod. 2001;7(10):947–55. doi: 10.1093/molehr/7.10.947. [DOI] [PubMed] [Google Scholar]
- 44.Yui J, Chiu CP, Lansdorp PM. Telomerase activity in candidate stem cells from fetal liver and adult bone marrow. Blood. 1998;91(9):3255–62. [PubMed] [Google Scholar]
- 45.Hemann MT, Greider CW. Wild-derived inbred mouse strains have short telomeres. Nucleic Acids Res. 2000;28(22):4474–8. doi: 10.1093/nar/28.22.4474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hathcock KS, et al. Haploinsufficiency of mTR results in defects in telomere elongation. Proc Natl Acad Sci U S A. 2002;99(6):3591–6. doi: 10.1073/pnas.012549799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rastinejad F, Blau HM. Genetic complementation reveals a novel regulatory role for 3′ untranslated regions in growth and differentiation. Cell. 1993;72(6):903–17. doi: 10.1016/0092-8674(93)90579-f. [DOI] [PubMed] [Google Scholar]
- 48.Rastinejad F, et al. Tumor suppression by RNA from the 3′ untranslated region of alpha-tropomyosin. Cell. 1993;75(6):1107–17. doi: 10.1016/0092-8674(93)90320-p. [DOI] [PubMed] [Google Scholar]
- 49.Taulli R, et al. Validation of met as a therapeutic target in alveolar and embryonal rhabdomyosarcoma. Cancer Res. 2006;66(9):4742–9. doi: 10.1158/0008-5472.CAN-05-4292. [DOI] [PubMed] [Google Scholar]
- 50.Yaffe D, Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977;270(5639):725–7. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]
- 51.Okabe J, et al. TRF1 is a critical trans-acting factor required for de novo telomere formation in human cells. Hum Mol Genet. 2000;9(18):2639–50. doi: 10.1093/hmg/9.18.2639. [DOI] [PubMed] [Google Scholar]
- 52.Robertson TA, et al. Fusion between myogenic cells in vivo: an ultrastructural study in regenerating murine skeletal muscle. J Struct Biol. 1990;105(1-3):170–82. doi: 10.1016/1047-8477(90)90111-o. [DOI] [PubMed] [Google Scholar]
- 53.Zhu CH, et al. Cellular senescence in human myoblasts is overcome by human telomerase reverse transcriptase and cyclin-dependent kinase 4: consequences in aging muscle and therapeutic strategies for muscular dystrophies. Aging Cell. 2007 doi: 10.1111/j.1474-9726.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 54.Di Donna S, et al. Telomerase can extend the proliferative capacity of human myoblasts, but does not lead to their immortalization. Mol Cancer Res. 2003;1(9):643–53. [PubMed] [Google Scholar]
- 55.Shay JW, Wright WE. Use of telomerase to create bioengineered tissues. Ann N Y Acad Sci. 2005;1057:479–91. doi: 10.1196/annals.1356.037. [DOI] [PubMed] [Google Scholar]
- 56.Coviello-McLaughlin GM, Prowse KR. Telomere length regulation during postnatal development and ageing in Mus spretus. Nucleic Acids Res. 1997;25(15):3051–8. doi: 10.1093/nar/25.15.3051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Decary S, et al. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscul Disord. 2000;10(2):113–20. doi: 10.1016/s0960-8966(99)00093-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.