Summary
Clustering of αvβ3 integrin after interaction with the RGD-like integrin-binding sequence present in neuronal Thy-1 triggers formation of focal adhesions and stress fibers in astrocytes via RhoA activation. A putative heparin-binding domain is present in Thy-1, raising the possibility that this membrane protein stimulates astrocyte adhesion via engagement of an integrin and the proteoglycan syndecan-4. Indeed, heparin, heparitinase treatment and mutation of the Thy-1 heparin-binding site each inhibited Thy-1-induced RhoA activation, as well as formation of focal adhesions and stress fibers in DI TNC1 astrocytes. These responses required both syndecan-4 binding and signaling, as evidenced by silencing syndecan-4 expression and by overexpressing a syndecan-4 mutant lacking the intracellular domain, respectively. Furthermore, lack of RhoA activation and astrocyte responses in the presence of a PKC inhibitor or a dominant-negative form of PKCα implicated PKCα and RhoA activation in these events. Therefore, combined interaction of the astrocyte αvβ3-integrin–syndecan-4 receptor pair with Thy-1, promotes adhesion to the underlying matrix via PKCα- and RhoA-dependent pathways. Importantly, signaling events triggered by such receptor cooperation are shown here to be the consequence of cell-cell rather than cell-matrix interactions. These observations are likely to be of widespread biological relevance because Thy-1–integrin binding is reportedly relevant to melanoma invasion, monocyte transmigration through endothelial cells and host defense mechanisms.
Keywords: Thy-1, Syndecan-4, Cell adhesion, Astrocytes
Introduction
Interactions of cells with the extracellular matrix (ECM) are primarily mediated by integrins and profoundly influence cell behavior (Hynes, 2002). Following initial attachment, cell spreading might occur, depending on the cell type and the additional signals received. Many processes including proliferation, migration and survival require both cell adhesion and spreading (Hood and Cheresh, 2002; Reddig and Juliano, 2005; Schwartz and Assoian, 2001). The latter event involves reorganization of the actin cytoskeleton and the formation of new and stronger integrin-substrate adhesions, referred to as focal adhesions (FAs) (Small et al., 1999). These macromolecular complexes, composed of transmembrane adhesion receptors, intracellular cytoplasmic structural proteins and signal transduction molecules are linked to actin-containing microfilament bundles termed stress fibers (SFs) (Burridge and Chrzanowska-Wodnicka, 1996).
In some instances, FA formation requires two independent adhesion receptor-mediated signals. When cells are plated on fibronectin, one of these signals is generated via interaction of integrins with the RGD-containing cell-binding domain of fibronectin. The second signal stems from binding of cell-surface heparan sulfate proteoglycans (HSPGs) to the heparin-binding domains (HBD) of fibronectin (Bloom et al., 1999; Woods et al., 1986). Widespread cooperation between different integrin family members and syndecans has been reported. However, in all cases, receptor stimulation is the consequence of engagement by an ECM protein such as fibronectin, vitronectin or laminin (reviewed by Morgan et al., 2007).
Essential signaling events initiated by syndecan-4 in cell-to-matrix adhesion can be bypassed in fibroblasts adhering to the cell-binding domain of fibronectin by either adding the protein kinase C (PKC) activator phorbol 12-myristate 13-acetate (Woods and Couchman, 1992), or stimulating directly the small GTPase RhoA with lysophosphatidic acid (Saoncella et al., 1999). Furthermore, FA assembly through syndecan-4 clustering is sensitive to RhoA inhibition by C3 transferase (Saoncella et al., 1999). Taken together, these data implicate both PKCα and RhoA-dependent pathways(s) downstream of syndecan-4. Indeed, it was recently shown that syndecan-4, PKCα and RhoA activation participate in a linear pathway that promotes adhesion to the cell matrix in mouse embryonic fibroblasts (Dovas et al., 2006).
Several reports support the idea that cell surface HSPGs mediate binding to cell matrix proteins, soluble proteins, viral and bacterial proteins. HSPGs also act as important cofactors for cytokines, chemokines and growth factors (Kirkpatrick and Selleck, 2007; Mahalingam et al., 2007; Park et al., 2000; Swertfeger and Hui, 2001). However, their role in signaling triggered by cell-cell adhesion is poorly documented.
Findings from this laboratory have implicated Thy-1 membrane glycoprotein (Thy-1) – a small glycosyl phosphatidylinositol-anchored protein and member of the immunoglobulin superfamily that is abundantly expressed on the neuronal surface – in binding to and clustering of αvβ3 integrin on astrocytes, as well as triggering the assembly of FAs and SFs (Hermosilla et al., 2008; Leyton et al., 2001) through the activation of RhoA and ROCK (Avalos et al., 2004; Avalos et al., 2002). These events require αvβ3 integrin in order to respond to Thy-1 stimulation, since mutation of the Thy-1-integrin-binding motif (RLD to RLE) prevents its ability to rapidly induce FA and SF formation in these cells (Hermosilla et al., 2008; Leyton et al., 2001). However, neuron-to-astrocyte adhesion is only partially inhibited by recombinant Thy-1-Fc protein, antibodies to αvβ3 integrin or RGD-like peptides, suggesting that receptors other than αvβ3 integrin might be implicated in the association of Thy-1 with astrocytes (Hermosilla et al., 2008; Leyton et al., 2001). Interestingly, direct Thy-1 interaction with sulfated glycans, as well as the functional consequences of Thy-1-dependent thymo-epithelial cell adhesion have been reported (Hueber et al., 1992). Thus, we hypothesized that Thy-1 increases cell-to-matrix adhesion in astrocytes by engaging, via cell-cell contact, not only αvβ3 integrin but also syndecan-4 in a cooperative interaction.
Our findings indicate that Thy-1 interaction with both receptors can activate the autophosphorylation of FAK in cells in suspension. Furthermore, Thy-1 interaction with its receptors on the dorsal surface of matrix-bound astrocytes is required to trigger activation of RhoA in a PKCα-dependent manner. These signaling events increased cell adhesion by inducing FAs and SF formation. Thus, combined interaction of the astrocytic αvβ3 integrin-syndecan-4 receptor pair with a protein present on the surface of a different cell promotes astrocyte signaling events, as well as adhesion to the underlying matrix.
There have been several reports of the formation of FAs and cell contraction requiring α5β1 integrin and syndecan-4 interaction with ECM proteins (Saoncella et al., 1999; Woods et al., 2000). However, the study reported here constitutes the first evidence that αvβ3 integrin also requires cooperation with syndecan-4 to lead to FA and SF formation. This is important given that β1 and β3 reportedly have different effects on RhoA activity (Bass et al., 2008; Danen et al., 2002). Additionally, a cluster of positive charges present in Thy-1 sequence is shown here to be important in Thy-1 interaction with proteoglycans.
Since the interaction between Thy-1 and integrins has been shown to mediate cell-cell adhesion in several systems (reviewed by Barker and Hagood, 2008; Rege and Hagood, 2006) the findings reported here are likely to be relevant not only to neuron-astrocyte communication, but also to melanoma invasion, monocyte transmigration through endothelial cells and host defense mechanisms.
Results
Heparin inhibits Thy-1-stimulated RhoA activity in astrocytes
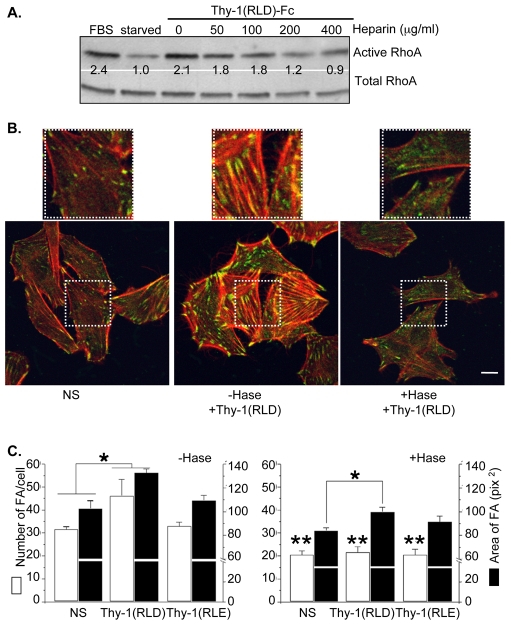
Thy-1 has been reported to interact directly with sulfated glycans, such as heparin, which inhibits adhesion between thymocytes and thymic epithelial cells (Hueber et al., 1992). If the cellular response triggered by Thy-1 in astrocytes also depends on a HSPG, such as syndecan-4, then sulfated glycans should compete with the Thy-1 region that interacts with syndecan-4. Thus, upon confirming the presence of both syndecan-4 mRNA and protein in the rat astrocyte cell line used in these studies (supplementary material Fig. S1), the effect of heparin on Thy-1-stimulated RhoA activity in astrocytes was investigated. Thy-1-Fc-beads [Thy-1(RLD)-Fc bound to protein-A–Sepharose beads], previously incubated with increasing concentrations of heparin (50-400 μg/ml) were used to stimulate astrocytes. RhoA activity was then measured using the affinity precipitation assay. As expected, Thy-1-stimulated RhoA activity in serum-starved astrocytes was reduced by heparin treatment in a dose-dependent manner (Fig. 1A). These results implicated a putative Thy-1 heparin-binding domain (HBD) in both binding to syndecan-4 and activating RhoA.
Fig. 1.
Heparin and heparitinase treatments inhibit Thy-1-stimulated responses in astrocytes. (A) DI TNC1 cells were serum starved for approximately 16 hours and then stimulated for 2 minutes with 3% fetal bovine serum (FBS, positive control) or 15 minutes with Thy-1-Fc-beads that had been previously treated with heparin (50-400 μg/ml) or left untreated for 30 minutes at 4°C. The cells were then lysed and affinity-precipitated active RhoA or total RhoA from whole cell lysates were visualized by immunoblotting with anti-RhoA monoclonal antibody. A representative western blot is shown. The values indicated were averaged from three independent experiments and indicate the fold increase in RhoA activity normalized to total protein. (B) DI TNC1 astrocytes were treated with 0.5 mU of heparitinase (+Hase) for 3 hours at 37°C in serum-free medium, or left untreated (–Hase) for the same amount of time in serum-free medium. The cells were then stimulated for 10 minutes with Thy-1(RLD)-Fc-beads. Non-stimulated cells (NS) were used as controls. After the different treatments, cells were washed, fixed and permeabilized. Focal adhesions (FA) were stained with an anti-paxillin monoclonal antibody followed by Alexa Fluor 488 anti-mouse IgG (green). Stress fibers (SFs) were stained with Rhodamine-conjugated phalloidin (red) and were visualized by confocal microscopy. Scale bar: 15 μm. White squares indicate the areas shown at a higher magnification in the top row. (C) The average number of FAs per cell ± s.e.m. (white bars), as well as the average area per FA ± s.e.m. (black bars), obtained after treating the cells with medium (NS), Thy-1(RLD)-Fc-beads or Thy-1(RLE)-Fc-beads, were determined for at least 50 cells in each experimental condition using the ImageJ program and the function `Analyze particles'. The values shown were obtained by averaging data from at least three independent experiments. Non-parametric Mann-Whitney analysis was used to compare the data. Significant differences are indicated between NS and stimulated cells treated (+Hase) and non-treated (–Hase) with heparitinase (*P<0.05). Significant differences are indicated in the average number of FAs per cell (white bars) between cells in the presence (+Hase) or the absence (–Hase) of heparitinase treatment (**P<0.05).
Heparitinase inhibits focal-adhesion and stress-fiber formation stimulated by Thy-1 in astrocytes
Thy-1 and syndecan-4 interaction was also evaluated by treating astrocytes with heparitinase before stimulating the cells with Thy-1-Fc-beads. The effect of this treatment on FA and SF formation was then studied by indirect immunofluorescence. Astrocytes were treated with Thy-1-Fc-beads prepared with either wild-type protein (containing the RLD motif), or protein containing the RLE mutation. RLE-containing Thy-1 does not bind to the recombinant αvβ3-Fc fusion protein (Hermosilla et al., 2008). Furthermore, Thy-1(RLE)-Fc neither supports astrocyte adhesion nor stimulates FA and SF formation (Avalos et al., 2002; Leyton et al., 2001).
In heparitinase-treated astrocytes there were not only fewer Thy-1-stimulated FAs in number but they were also smaller. In addition, SFs were thinner and less robust than those formed in cells not digested with heparitinase (Fig. 1B). In the absence of heparitinase treatment, Thy-1(RLD)-Fc-, but not Thy-1(RLE)-Fc-beads, stimulated the formation of FAs within 10 minutes, leading to significantly increased numbers of FAs per cell (Fig. 1C, –Hase, white bars). Heparitinase treatment blocked stimulation by Thy-1-Fc-beads and significantly decreased basal levels of FA formation in all experimental groups compared to untreated cells (Fig. 1C, +Hase, compared with NS in –Hase, white bars). The average area of FAs was also quantified for each experimental condition. FAs increased in size upon stimulation with Thy-1(RLD)-Fc-beads, whereas no significant effect was seen with Thy-1(RLE)-Fc-beads (Fig. 1C; –Hase, black bars). Interestingly, a small but significant difference was still observed in heparitinase-treated cells following Thy-1(RLD)-Fc stimulation (Fig. 1C, +Hase, black bars).
Thy-1 stimulation of cells in suspension activates signaling in astrocytes
To demonstrate that the results obtained were due to direct stimulation of signaling pathways in astrocytes by Thy-1, rather than alterations in cell-matrix interactions, cells were stimulated in suspension culture with Thy-1-Fc. In these experiments, FAK autophosphorylation on tyrosine 397 was employed as a read-out. Additionally, to avoid effects of the Sepharose beads, Thy-1 was complexed to protein A in an equimolar ratio. This alternative mode of stimulation is as efficient as bead-immobilized Thy-1 (N.M. and L.L., unpublished data). The results obtained by immunoblotting using phospho-specific antibodies show that Thy-1 induced FAK phosphorylation on Y397 after 10 minutes and that the increase in autophosphorylation became statistically significant after 15-20 minutes. The effect of heparin was also tested in this assay by incubating the Thy-1-Fc-protein-A complex with heparin before its addition to the cells. A statistically significant reduction in FAK phosphorylation by heparin was only apparent after 20 minutes of incubation (Fig. 2). These results suggest that Thy-1 directly engages integrin-dependent pathways that activate FAK autophosphorylation. Furthermore, this initial signaling event appears to be regulated subsequently by the Thy-1-syndecan-4 interaction.
Fig. 2.
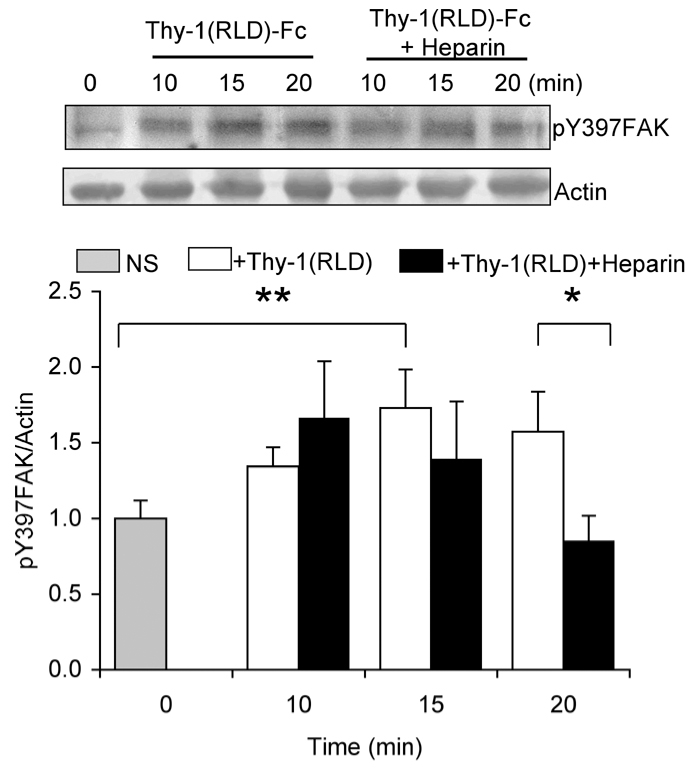
Thy-1 stimulates FAK autophosphorylation of cells in suspension. Astrocytes in suspension (2×105 cells) were treated for the indicated period of time with Thy-1(RLD)-Fc complexed to protein A (4:0.4 μg/tube), pre-incubated or not with heparin (200 μg/ml). Then, cells were rinsed with cold PBS and lysed in Laemmli buffer. Total protein extracts from DI TNC1 astrocytes were separated by SDS-PAGE and analyzed by western blotting with anti-pY397FAK antibodies. Tyrosine phosphorylated FAK levels were quantified by scanning densitometric analysis of western blots and normalized to actin (graph). Phosphorylated Y397FAK levels in astrocytes treated with either Thy-1(RLD)-Fc (white bars) or heparin-treated Thy-1(RLD)-Fc (black bars) at different time points were compared with those in the non-stimulated control (gray bar). Results shown are the means ± s.e.m. of data from three independent experiments. Non-parametric Mann-Whitney analysis was used to compare the data. Statistically significant differences are indicated (*P<0.05, **P<0.01).
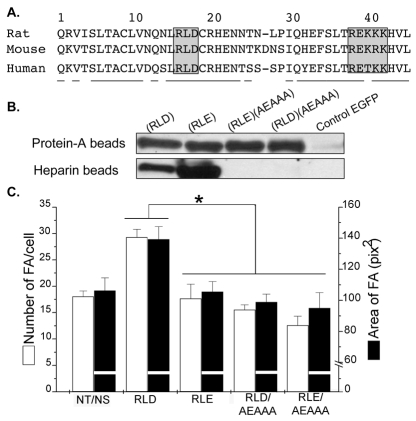
Thy-1 possesses a heparin binding domain involved in focal adhesion formation
A putative but as yet unidentified HBD has been reported to exist in Thy-1 that inhibits thymo-epithelial cell adhesion (Hueber et al., 1992). Here, a [x37BxxBBBx44] region was detected in the primary amino acid sequence of Thy-1, where B are basic amino acids and x represents any amino acid for residues 37-44 (Fig. 3A). To determine whether this is the putative HBD required for Thy-1-induced FA and SF formation, the basic motif R38EKRK42 of Thy-1 was mutated to A38EAAA42, and the proteins were expressed as Fc fusion molecules. Thy-1-Fc proteins, with or without mutation in the integrin-binding site (RLD) and/or the HBD (REKRK) were all recovered by precipitation with protein-A–Sepharose beads. Thy-1-Fc proteins containing an intact HBD, but not those with the mutated HBD, could also be precipitated by heparin-Sepharose, strongly suggesting that the basic motif REKRK was the sequence required for Thy-1 interaction with heparan sulfate side chains of proteoglycans (Fig. 3B).
Fig. 3.
Mutation of the heparin binding domain of Thy-1 renders the protein unable to bind heparin and to promote focal-adhesion formation. (A) Partial sequences (amino acids 1-45) of rat, mouse and human Thy-1. Underlined amino acid residues are almost identical for all three species. Gray boxes indicate the integrin-binding sequence (RLD) and the putative heparin-binding domain (REKRK) present in Thy-1. Mutations in both integrin- and heparin-binding domains were obtained by the double-PCR technique to obtain four different forms of the secreted Thy-1-Fc molecules by transiently transfecting HEK293T cells: wild-type Thy-1(RLD), integrin-binding-site-mutated Thy-1(RLE), double-mutated Thy-1(RLE)(AEAAA), HBD-mutated Thy-1(RLD)(AEAAA). (B) The aformentioned wild-type and mutated Thy-1-Fc proteins were precipitated from supernatants with protein-A-Sepharose or heparin-Sepharose, and analyzed by western blotting using anti-human IgG (Fc specific)-HRP. Additionally, the supernatant of cells transfected with pEGFP-C1 alone was used as a control. (C) The effect of these proteins on FA formation was tested and evaluated by immunofluorescence as indicated in Fig. 1C. White bars, the number of focal adhesions per cell; black bars, the area of focal adhesions. Values shown are mean ± s.e.m. of data from at least three different experiments. Non-parametric Mann-Whitney analysis was used to compare the data. Statistically significant differences are indicated (*P<0.05).
Protein-A beads coated with equivalent amounts of the four different Thy-1-Fc proteins, or with the supernatant of mock-transfected cells, were used in the astrocyte stimulation assay. The only protein able to stimulate astrocytes was the wild-type Thy-1-Fc, whereas cells treated with beads coated with Thy-1 mutated in the integrin-binding site, in the HBD, or in both were indistinguishable from non-treated cells (Fig. 3C). It is noteworthy, that wild-type Thy-1-Fc was equally active whether it was loaded on beads directly from supernatants of transfected cells (Fig. 3C) or as purified protein (see below) and that cells treated with the control beads behaved as non-stimulated astrocytes (data not shown). Taken together, these results confirm that both the integrin-binding site and the HBD of Thy-1 are required to stimulate astrocyte adhesion.
Expression of a syndecan-4 siRNA interferes with focal-adhesion and stress-fiber formation stimulated by Thy-1 in astrocytes
To more rigorously explore the role of syndecan-4 on FA and SF formation, we employed small interference RNA (siRNA) to knock down endogenous syndecan-4 protein expression in astrocytes. siRNA constructs designed to match non-conserved 21 nucleotide sequences within the syndecan-4 mRNA were transfected into DI TNC1 astrocytes. Semi-quantitative RT-PCR was employed to detect levels of syndecan-4 message in cDNA derived from astrocytes transfected with different combinations of siRNA. A substantial decrease in syndecan-4 mRNA levels was observed in cells treated with all combinations of siRNA as compared to control siRNA treated cells (Fig. 4A). Note that decreased syndecan-4 mRNA levels correlated with increased residual amounts of syndecan-4-specific primers in the PCR reaction mixture (lower band in Fig. 4A).
Fig. 4.
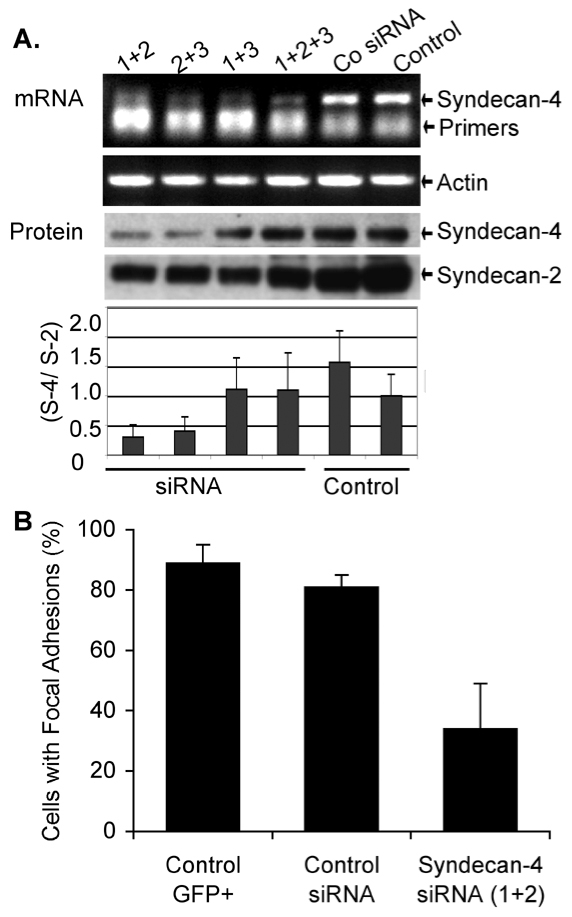
Silencing of syndecan-4 with siRNA transfections decreases focal-adhesion and stress-fiber formation stimulated by Thy-1. Syndecan-4 silencing was performed by co-transfecting pEGFP-C1 with different siRNA directed against syndecan-4. (A) Expression levels of syndecan-4 and actin mRNA or syndecan-4 and syndecan-2 protein, in either non-transfected cells (Control) or cells transfected with siRNA control (Co siRNA) or different mixes of syndecan-4-specific siRNA, were measured by semi-quantitative RT-PCR or western blots, respectively. Bands obtained for syndecan-4 (S-4) and syndecan-2 (S-2) by western blot were quantified by densitometry and results are presented as average ratio ± s.e.m. between values obtained (S-4/S-2) from three independent experiments. (B) Astrocytes were co-transfected with pEGFP-C1 (Control GFP+) and either control siRNA or siRNA (1+2). After allowing GFP expression, cells were stimulated with Thy-1-Fc-beads for 10 minutes. The graph shows the percentage of GFP-positive cells that were stimulated by Thy-1 under each condition. Cells were scored as stimulated when elongated focal adhesions were present and there was at least a 1.5-fold increase in the number of focal adhesions per cell. Data were obtained from three independent experiments. Values mean ± s.e.m. calculated from counting at least 100 GFP-positive cells per condition.
To test whether low levels of mRNA correlated with decreased protein expression, syndecan-4 was detected by western blot analysis in lysates from transfected cells. Lysates were previously treated with heparitinase to liberate the core proteins of all syndecans. A readily detectable decrease (>50%) in syndecan-4 protein levels was also observed in syndecan-4 siRNA-treated cells (mix 1+2 or 2+3), as compared to either untreated or control siRNA-treated cells (Fig. 4A, protein blot and graph). Taken together, these results demonstrated that the specific siRNA combinations (mix 1+2 or 2+3) can efficiently and specifically reduce the amount of syndecan-4 protein in transfected astrocytes.
Next, we studied the requirement for syndecan-4 in Thy-1-induced FA and SF formation in astrocytes. Syndecan-4 siRNA (mix 1+2) was co-transfected with EGFP-containing plasmid to identify those cells that had incorporated the probes. Cells were stimulated as before with Thy-1-Fc-beads or with neuronal CAD cells which express high levels of Thy-1 on their surface. Previous reports indicate that these cells induce FA and SF formation in astrocytes exclusively via Thy-1-αvβ3 integrin interaction (Hermosilla et al., 2008). GFP-positive astrocytes co-transfected with syndecan-4 siRNA did not respond as well to either CAD cells or to Thy-1-Fc-beads when compared to those transfected with the control siRNA or pEGFP-C1 only (not shown). These observations were verified by quantifying the number of GFP-transfected cells that contained Thy-1-stimulated FAs. Those cells that contained elongated FAs and showed at least a 1.5-fold increase in the number of FAs compared to non-stimulated cells were scored as GFP-positive cells with FAs. More than 80% of GFP-expressing cells co-transfected (81±4%) or not (89±6%) with control siRNA responded to Thy-1-Fc-beads by forming more and larger FAs. However, a smaller percentage of GFP-positive cells co-transfected with siRNA for syndecan-4 (34±15%) were stimulated by Thy-1-Fc-beads (Fig. 4B). Similar results, obtained when using CAD cells to stimulate astrocytes (not shown), suggest that neuronal Thy-1 requires the presence of syndecan-4 to induce a cellular response in astrocytes.
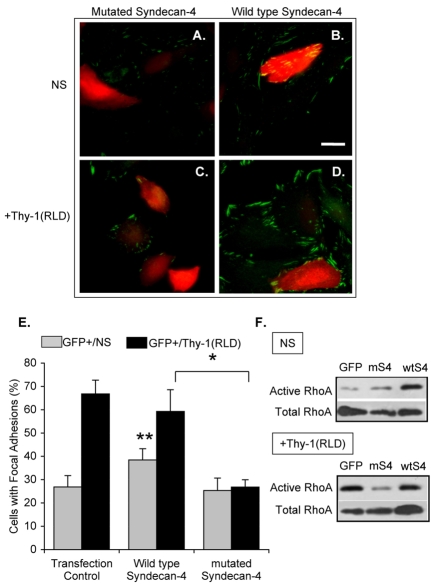
Expression of a syndecan-4 mutant interferes with focal-adhesion formation stimulated by Thy-1 in astrocytes
Syndecan-4-dependent signaling relies on the variable region located in its cytoplasmic domain (reviewed by Couchman and Woods, 1999). To demonstrate that Thy-1-stimulated FA and SF formation requires not only binding to syndecan-4, but also syndecan-4 signaling, astrocytes were transiently co-transfected with full-length syndecan-4 [(wt)syndecan-4] or a mutant of syndecan-4 lacking the cytoplasmic domain [(m)syndecan-4] (Keum et al., 2004) and pEGFP-C1. Transfections were corroborated by microscopy or cell cytometry to detect GFP-positive cells (not shown), and by RT-PCR to confirm the presence of the respective mRNA (supplementary material Fig. S2). In the non-transfected cells, present in all transfection conditions (GFP negative cells), a similar number of cells with FAs was observed in the absence of stimulation (not shown). Expression of (wt)syndecan-4, under non-stimulated conditions, increased the number of cells that had FAs with respect to cells transfected with EGFP only or with (m)syndecan-4 (Fig. 5A,B and Fig. 5E, gray bars). Accordingly, RhoA activity was also increased (compare GFP with wtS4 lanes in Fig. 5F under non-stimulated conditions). However, introduction of (wt)syndecan-4 affected neither FA formation induced by Thy-1 (Fig. 5D; Fig. 5E, black bars) nor RhoA activity [compare GFP with wtS4 lanes in Fig. 5F, +Thy-1(RLD)]. By contrast, (m)syndecan-4-expressing cells lost responsiveness to Thy-1 stimulation (compare Fig. 5C and D), since fewer cells with FAs were detectable (Fig. 5E, black bars). In agreement with the previous results, obtained by silencing syndecan-4, transfection with (m)syndecan-4 decreased the percentage of GFP-positive cells responsive to Thy-1 to 27±3%. However, around 60% of the GFP-positive cells were stimulated by Thy-1 treatment in cells transfected with (wt)syndecan-4 (59±9%) or pEGFP-C1 vector (67±6%; Fig. 5E, black bars). As expected, levels of active RhoA did not change following Thy-1 treatment of cells transfected with (m)syndecan-4 lacking signaling properties (Fig. 5F).
Fig. 5.
Transfection of astrocytes with a syndecan-4 mutant lacking the cytoplasmic domain decreases Thy-1-stimulated focal-adhesion formation. Cells were transiently co-transfected with EGFP-containing plasmid and either truncated (A,C) or wild-type (B,D) syndecan-4. DI TNC1 astrocytes were then left unstimulated (NS; A,B) or were stimulated with Thy-1(RLD)-Fc-beads [+Thy-1(RLD); C,D]. Samples were analyzed by immunofluorescence using mouse anti-paxillin antibodies followed by Cy3-labeled anti-mouse IgG. Red and green colors were inverted to improve visualization of focal adhesions; therefore, in images obtained using confocal microscopy, focal adhesions are in green in both non-transfected (no color) and GFP-transfected (red) cells. Scale bar: 25 μm. (E) Quantification of cells with focal adhesions within the transfected cell population in each transfection condition (GFP+) is shown for non-stimulated (gray bars) and Thy-1-stimulated cells (black bars). Cells with focal adhesions were evaluated as indicated in Fig. 4. Values were obtained by evaluating at least 100 GFP-positive cells per condition from three independent experiments. Statistically significant differences are indicated between values obtained for wild-type syndecan-4 with either transfected control (**P<0.05) or mutated syndecan-4 (*P<0.05). (F) Transfected DI TNC1 cells were serum-starved overnight and then stimulated or not (NS) for 15 minutes with Thy-1-Fc-beads [+Thy-1(RLD)]. The cells were then lysed and active RhoA was affinity-precipitated with RBD-GST-beads. RhoA was visualized by immunoblotting as indicated in Fig. 1A. A representative result from three independent experiments is shown.
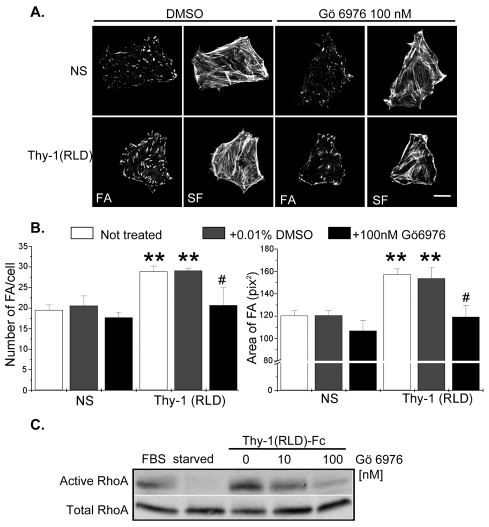
PKCα inhibition decreases focal-adhesion and stress-fiber formation and RhoA activity stimulated by Thy-1 in astrocytes
Since syndecan-4-dependent signal transduction involves the formation of a ternary complex with PtdIns(4,5)P2 and PKCα, as well as PKCα activation (Couchman, 2003), we studied the participation of PKCα in Thy-1-mediated responses. To this end, astrocytes were treated with the inhibitor Gö 6976, which, at low concentrations is highly selective for the PKC isoforms α, β and γ (Martiny-Baron et al., 1993). Cells stimulated with Thy-1(RLD)-Fc-beads contained a significantly greater number of FAs per cell and FAs of larger average size, than non-stimulated cells (Fig. 6B, white bars). Treatment with vehicle (0.01% DMSO) did not affect FA and SF formation in either non-stimulated cells or cells stimulated with Thy-1(RLD)-Fc-beads (left panels in Fig. 6A and gray bars in B). Treatment with Gö 6976 (100 nM) significantly decreased the number and size of FAs in Thy-1-stimulated astrocytes (right panels in Fig. 6A and black bars in B), compared with cells treated with vehicle.
Fig. 6.
Pharmacological inhibition of PKCα reduces the formation of focal adhesions and stress fibers, as well as RhoA activity in astrocytes. (A) DI TNC1 cells were treated with 100 nM Gö 6976, left untreated or treated with 0.01% DMSO (vehicle) for 30 minutes at 37°C in serum-free medium and were then either stimulated with Thy-1(RLD)-Fc-beads for 10 minutes or not stimulated (NS). Samples were analyzed by immunofluorescence as described in Fig. 1B. Scale bar: 25 μm. (B) The number of FAs per cell and the average area of FAs were determined as for Fig. 1C. Values shown are mean ± s.e.m. of data from at least three different experiments. Significant differences are indicated (**P<0.05) between NS cells and cells stimulated with Thy-1(RLD)-Fc-beads, for the indicated conditions (–DMSO, white bars; + DMSO, gray bars). A significant difference (#P<0.05) is indicated between cells treated or not with Gö 6976 (black bars versus gray bars) in Thy-1(RLD)-Fc-stimulated cells. (C) Astrocytes were serum-starved and then treated with either 10 nM or 100 nM Gö 6976 before stimulating with Thy-1(RLD)-Fc-beads for 15 minutes. Active RhoA levels were determined as described in Fig. 1A. Controls included starved cells and cells stimulated with 3% fetal bovine serum (FBS) for 2 minutes. Results are representative of two independent experiments.
The effect of PKCα inhibition with Gö 6976 on Thy-1-stimulated RhoA activity was also evaluated. Astrocytes were serum-starved and then treated with either 10 nM or 100 nM inhibitor, before being stimulated with Thy-1(RLD)-Fc-beads. As expected, Gö 6976 decreased, in a concentration-dependent manner, RhoA activity stimulated by Thy-1 in astrocytes (Fig. 6C).
Dominant-negative PKCα inhibits focal-adhesion and stress-fiber formation as well as RhoA activity stimulated by Thy-1 in astrocytes
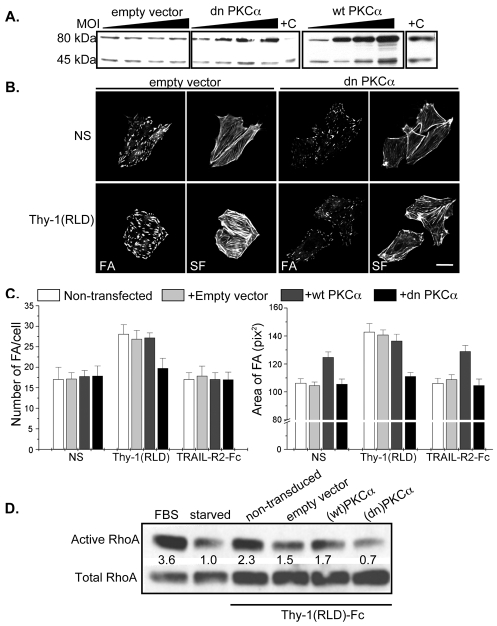
To further evaluate the participation of PKCα in Thy-1-triggered responses, astrocytes were transduced with adenoviral vectors encoding wild-type (wt) or dominant-negative (dn) PKCα and their effect on RhoA activity, as well as FA and SF formation was evaluated. In order to determine the multiplicity of infection (MOI) required to achieve optimal transduction efficiency, astrocytes were transduced with increasing MOI (1-10,000) of adenoviral vectors encoding β-galactosidase or GFP and 24 hours later, the respective parameter (β-galactosidase activity or the number of green versus non-green cells) was measured. According to results obtained, astrocytes required a MOI of 1000 for 100% transduction efficiency (data not shown).
Therefore, astrocytes were transduced with adenovirus containing empty vector (negative control), (wt)PKCα or (dn)PKCα. PKCα expression was evaluated at three different MOI by western blot analysis. As expected, PKCα protein levels increased using MOI up to 1000, both in (wt)- and (dn)PKCα-transduced cells, but not in cells transduced with empty vector (Fig. 7A). Densitometric analysis indicated that PKCα protein levels increased approximately threefold in (wt)PKCα- and (dn)PKCα-transduced cells using a MOI 1000 (not shown).
Fig. 7.
The expression of a dominant-negative mutant of PKCα reduces the formation of focal adhesions and stress fibers, as well as RhoA activity in astrocytes. (A) DI TNC1 cells were transduced with adenoviral vectors for wild type (wt) and dominant-negative (dn)PKCα, and empty vector using MOIs of 0, 300, 600 and 1000. After 24 hours, astrocytes were lysed and samples were analyzed by immunoblotting with anti-PKCα antibodies. Rat brain extract was used as a positive control (+C) for PKCα. Bands of 80 and 45 kDa correspond to full-length and the catalytic region of PKCα, respectively. (B) Astrocytes were transduced with empty or (dn)PKCα adenoviral vectors using a MOI of 1000. After 24 hours, cells were seeded on coverslips and, the next day, stimulated with Thy-1(RLD)-Fc-beads, or left unstimulated (NS). Focal adhesions and stress fibers were visualized by immunofluorescence as detailed in Fig. 1B. Scale bar: 25 μm. (C) Astrocytes were transduced with empty vector (light gray bars), (wt)PKCα (dark gray bars), (dn)PKCα (black bars) as in B or were not transduced (white bars). Cells were then stimulated with Thy-1(RLD)-Fc-beads, treated with TRAIL-R2-Fc-beads (negative control) or not stimulated (NS). For each experimental condition, the number of FAs per cell and the average size of FAs were determined as for Fig. 1C. Values shown are mean ± s.d. of one representative experiment performed in duplicate. (D) Astrocytes were transduced with the different adenoviral vectors described in C and RhoA activity was measured as in Fig. 1A. Results are representative of two independent experiments.
After transducing the astrocytes with adenoviral empty vector, cells treated with Thy-1(RLD)-Fc-beads formed FAs as expected (compare lower with upper panels in Fig. 7B). When transducing the cells with (dn)PKCα adenoviral vector, Thy-1 no longer stimulated these astrocytes and a reduction in the number and size of FAs was observed when compared with cells transduced with empty vector (lower panels in Fig. 7B). Transduction with (dn)PKCα also decreased SF formation in cells stimulated with Thy-1(RLD)-Fc beads, whereby a reduction in the thickness of these structures was apparent (Fig. 7B).
Quantification of FAs per cell in each of the above conditions, as well as in non-transduced cells and cells transduced with (wt)PKCα indicates that only those cells over-expressing (dn)PKCα could not be stimulated with Thy-1(RLD)-Fc-beads (black bars in Fig. 7C). In non-transduced cells (white bars), cells transduced with empty vector (light gray bars) or with (wt)PKCα (dark gray bars), stimulation with Thy-1-Fc-beads increased the number of FAs per cell and the average size of FAs, compared with the corresponding controls (NS or TRAIL-R2-Fc beads; Fig. 7C). Interestingly, overexpression of (wt)PKCα did not alter the number of FAs per cell, but did alter the size in both non-stimulated and TRAIL-R2-Fc-treated cells (Fig. 7C).
Finally, RhoA activation in cells overexpressing the different forms of PKCα was analyzed. Even though the adenoviral transducing procedure decreased RhoA activity in all cases, the inhibitory effect of (dn)PKCα on RhoA activity upon Thy-1 stimulation was more pronounced than when using (wt)PKCα or empty vector (Fig. 7D).
Discussion
In this study, we have shown that Thy-1 functions in cell-cell signaling via dual interaction with αvβ3 integrin and syndecan-4. Engagement of these two receptors leads to FA and SF formation in astrocytes, increasing their adhesion to the underlying matrix. To date, the following cooperative interactions of integrins and syndecans with the extracellular matrix have been reported to increase adhesion: (1) α5β1 integrin and syndecan-4 with fibronectin (Bloom et al., 1999; Woods et al., 1986); (2) αvβ3 integrin, αvβ5 integrin and syndecan-1 with vitronectin (Beauvais et al., 2004; McQuade et al., 2006), and (3) α2β1 integrin, α6β4 integrin and syndecans with laminin (Hozumi et al., 2006; Ogawa et al., 2007). These examples underscore the fact that cooperation between these types of receptors has so far been viewed as a matrix-rather than a cell-initiated signaling process. Interestingly, despite the distinct nature of the ligand implicated in this case, namely Thy-1, similar signaling molecules are activated downstream, including PKCα and RhoA. Additionally, since β1 and β3 reportedly activate RhoA in distinct manners upon binding to fibronectin (Danen et al., 2002), signaling pathways triggered by Thy-1 engagement of αvβ3 integrin and/or syndecan-4 were not necessarily expected to be identical to those triggered by ECM proteins.
Heparin competed with syndecan-4 for Thy-1-HBD, thereby reducing Thy-1-mediated RhoA activation (Fig. 1A). Studies performed in other cell types have demonstrated that heparin also decreases FA and SF formation by binding to the HBD of fibronectin (Bloom et al., 1999; Midwood et al., 2004; Mostafavi-Pour et al., 2003). Thus, the effect of heparin on RhoA activity reported here suggests that Thy-1 regulates this GTPase activity through syndecan-4. Additionally, heparin also inhibited FAK autophosphorylation at 20 minutes, but not at 15 minutes after Thy-1 stimulation of cells in suspension. Since FAK autophosphorylation on Y397 is triggered by Thy-1 via engagement of αvβ3 integrin (Leyton et al., 2001) and syndecan-4 is reported to regulate phosphorylation of FAK on this tyrosine residue (Wilcox-Adelman et al., 2002), these results implicate both integrin and syndecan-4 in this signaling response. Moreover, they confirm the interpretation that heparin is exerting its effect by impeding interactions that require the HBD of Thy-1, rather than by disrupting constitutive cell-matrix interactions.
Heparitinase treatment to digest heparan sulfate chains present on the surface of astrocytes, not only blocked the number of FAs in astrocytes stimulated by Thy-1-Fc-beads, but also decreased the basal number of FAs in non-stimulated cells (Fig. 1C, white bars). These observations most probably indicate that heparitinase has additional effects on the interactions of cells with their underlying matrix. Even though the number of FAs was not elevated by stimulation with Thy-1-Fc-beads, the size of these structures increased significantly (Fig. 1C, black bars), indicating that Thy-1-induced responses were not completely prevented by heparitinase treatment. This residual signaling may be due to incomplete heparitinase digestion or direct signaling mediated by either Thy-1 and integrin or Thy-1 and syndecan-4(core) interaction. The latter has been previously reported for fibronectin interaction with syndecan-4 (Echtermeyer et al., 1999).
In contrast to cells stimulated with Thy-1(RLD)-Fc-beads, stimulation with Thy-1(RLE)-Fc-beads did not significantly increase the number of FAs (Fig. 1C, Fig. 3C). This mutation hampers Thy-1 interaction with integrin αvβ3 but does not affect the putative HBD of the molecule. Therefore, the results suggest that Thy-1 interaction with syndecan-4 alone cannot induce the formation of FAs and SFs. Additionally, no effect was observed on FA formation when treating astrocytes with either the single [Thy-1(RLD)(AEAAA)-Fc] or double [Thy-1(RLE)(AEAAA)-Fc] HBD mutant immobilized on beads (Fig. 3C). Intriguingly, the single mutant containing intact RLD sequence did not induce changes in astrocyte adhesion indicating that, under the conditions used, both the integrin-binding site and the HBD of Thy-1 are required to stimulate FA formation in astrocytes (see below).
Syndecan-4 involvement in Thy-1-induced cellular responses was corroborated by silencing expression of the protein with siRNA. Thy-1 stimulation was not observed in syndecan-4 knockdown cells (Fig. 4). Likewise, overexpression of a mutant lacking the intracellular portion of syndecan-4, precluded FA and SF formation as well as the increase in RhoA activity induced by Thy-1 (Fig. 5), indicating not only that syndecan-4 engagement is required, but also that the signaling cascade emanating from the cytoplasmic domain of this receptor is essential. We propose that in the absence of syndecan-4 binding and signaling, FAs are unstable and undergo rapid turnover. However, in the presence of syndecan-4, astrocyte stimulation with only the RLD peptide bound to microspheres appears as effective as with the whole Thy-1 molecule (Hermosilla et al., 2008). Presumably, this may be explained by the fact that in those reported experiments, cells were attached to their own matrix and, therefore, syndecan-4 was already engaged by ECM proteins in stabilizing focal adhesions.
A minimum lateral spacing of 58 nm between nanogold-anchored RGD peptides is reported to be crucial for induction of FA formation (Arnold et al., 2004; Koo et al., 2002; Maheshwari et al., 2000). However, ligand density for both integrin and syndecan-4 have been shown to be crucial for FA formation, whereby each receptor appears to play a unique role in generating the response (Bass et al., 2007). These controversial findings indicate that composition and strength of the primary signal are important in determining the final cellular outcome. Thus, results reported here using the single mutant for the HBD of Thy-1-Fc [Thy-1(RLD)(AEAAA)-Fc] which, despite containing the integrin-binding site did not induce FA formation, may indicate that either higher amounts of the mutated Thy-1 are required to trigger the responses or that, as indicated above, both binding sites (for integrin and heparin) are necessary to control the dynamics of the adhesive response. Interestingly, the consensus sequences that reportedly act as heparin-binding regions were not detected in the Thy-1 primary sequence (Hueber et al., 1992). Here, we report that the mutation of a cationic motif completely abolished Thy-1 binding to heparin (Fig. 3B), indicating that this motif is required for Thy-1 interaction with heparin.
Cell spreading and the formation of FAs and SFs through syndecan-4, not only depend on Rho, but also on PKCα activation in some cell types (Dovas et al., 2006; Koo et al., 2006; Woods and Couchman, 1992). Thus, participation of PKCα in Thy-1-stimulated responses in astrocytes was studied. Indeed, results presented here (Figs 6 and 7) suggest that RhoA activation via syndecan-4 in astrocytes requires PKCα activation. However, they do not shed light on whether PKCα activation depends directly or exclusively on syndecan-4 engagement. A possible substrate for PKCα is Rho-GDI, whereby Rho-GDI phosphorylation leads to dissociation from Rho, which in turn permits RhoA activation (Mehta et al., 2001; Pan et al., 2005). Thus, the possibility that Rho-GDI functions as an intermediate between PKCα and RhoA in this syndecan-4-dependent pathway is currently being explored.
The ubiquitous distribution of syndecan-4 is generally associated with low levels of expression (Kim et al., 1994; Yoneda and Couchman, 2003). Therefore, syndecan-4 function might be more relevant in situations where its expression increases. Reportedly, syndecan-4 is upregulated during wound healing and localizes to wounded areas (Cizmeci-Smith et al., 1997; Gallo et al., 1996; Gallo et al., 1994). Additionally, although syndecan-4 knockout mice are healthy, delays in tissue repair mechanisms and impaired angiogenesis have been observed (Echtermeyer et al., 2001). There is also evidence for increased syndecan expression in reactive astrocytes upon brain injury (Iseki et al., 2002). Syndecans could, via interaction with Thy-1 in neurons, enhance astrocyte contraction, wound healing and scar formation. In doing so, an environment that prevents neuronal regeneration would be created.
However, Thy-1-integrin binding is also important to interactions between melanomas or monocytes and/or neutrophils and activated endothelial cells (Saalbach et al., 2005; Wetzel et al., 2004; Wetzel et al., 2006). In the latter case, Thy-1 promotes strong adhesion of the cells to the endothelium to favor subsequent migration across this cell layer. Endothelial cells are activated in inflammatory processes. Therefore, it is possible that the cells interacting with them, such as melanomas and monocytes, upregulate syndecan-4 under these conditions. Thus, these syndecan-4-dependent interactions are likely to be relevant to a variety of pathological conditions. In summary, it is highly probable that the dual interaction of Thy-1 with integrins and syndecan-4 will also be important for these other events that require cell-cell interactions.
Materials and Methods
Cells, recombinant proteins and reagents
The rat astrocytic cell line DI TNC1 (ATCC CRL-2005) was used in these studies and these cells were maintained as described previously (Hermosilla et al., 2008). Human embryonic kidney 293T cells (HEK293T, ATCC CRL-11268) were grown according to ATCC guidelines. Cells were maintained in a humidified atmosphere of 5% CO2 and 37°C.
Reagents used were heparin and heparitinase III (EC 4.2.2.8, Sigma), siPORT Amine transfection reagent (Ambion), Fugene HD transfection reagent (Roche) and Lipofectamine Plus (Invitrogen). The recombinant proteins Thy-1(RLD)-Fc, Thy-1(RLE)-Fc and TRAIL-R2-Fc were obtained as previously reported (Leyton et al., 2001) and coupled to protein-A–Sepharose beads (Sigma) for cell stimulation. Additionally, the putative Thy-1 heparin-binding domain REKRK was mutated to AEAAA by a conventional, PCR-based technique to obtain the mutants [Thy-1(RLD)(AEAAA)-Fc and Thy-1(RLE)(AEAAA)-Fc]. The fusion proteins were expressed by transient transfection of HEK293T cells using Lipofectamine Plus according to the manufacturer's instructions. Recombinant proteins were purified from the supernatants (4 ml) using protein-A–Sepharose beads (80 μl of 50% slurry) and employed for immunoblotting and cell stimulation experiments. GST-RBD fusion protein employed in the affinity precipitation assay was obtained as previously described (Avalos et al., 2004). DNAs were purified using a Qiagen Plasmid Purification Kit. Full-length syndecan-4 cDNA (Syn 4W) and a mutant cDNA lacking the cytoplasmic domain (Syn 4R) were kindly provided by Eok-Soo Oh (Department of Life Sciences, Ewha Womans University, Seoul, South Korea) (Keum et al., 2004). Adenoviral vectors for expression of wild-type and dominant-negative PKCα (which contains a mutation in the ATP-binding region, K368R) were from Seven Hills Bioreagents (Cincinnati Children's Hospital Medical Center, Cincinnati, OH). These vectors were generated and characterized as described previously (Braz et al., 2002; Matsumoto et al., 2001; Ohba et al., 1998) using the AdEasy adenoviral vector system (He et al., 1998).
Rhodamine-conjugated phalloidin (Sigma) and the antibodies mouse anti-vinculin (Sigma) or anti-paxillin monoclonal antibody (Transduction Laboratories), goat anti-mouse Alexa Fluor 488 polyclonal antibodies (Molecular Probes) and Cy3-labeled anti-mouse IgG (Bio-Rad) were used in immunofluorescence experiments. Antibodies used for western blots were mouse anti-Δ-heparan sulfate (3G10, Seikagaku), mouse anti-RhoA monoclonal antibody (Santa Cruz Biotechnology), rabbit anti-pY397FAK polyclonal antibody (Upstate Biotechnology), horseradish peroxidase (HRP)-coupled goat anti-mouse or anti-rabbit IgG polyclonal antibodies (Bio-Rad) and anti-human IgG (Fc specific)-HRP (Jackson ImmunoResearch). Specifically bound HRP-conjugated antibodies were visualized using the ChemiLucent Detection System Kit (Chemicon International). The BCA reagent (Pierce Chemical) was used to determine the protein concentration. All other reagents used for immunofluorescence, affinity precipitation assays and western blots were from Sigma or of the highest grade available.
Digestion with heparitinase
Cells grown to confluency in 10-cm plates were lysed with 1 ml of cold buffer containing 50 mM Tris-HCl pH 7.4, 100 mM NaCl, 0.5% Triton X-100 and protease inhibitors (10 μg/ml benzamidine, 2 μg/ml antipain, 1 μg/ml leupeptin), as well as the phosphatase inhibitors (1 mM sodium orthovanadate and 0.5 mM sodium fluoride). Lysates were centrifuged at 13,000 g for 5 minutes at 4°C and the protein concentration in supernatants was measured using the BCA method. Each sample (45 μg) was digested for 3 hours at 37°C with 0.3 mU of heparitinase resuspended in 10 μl of buffer: 20 mM Tris-HCl pH 7.4 containing 50 mM NaCl and 2 mM CaCl2. As a control, undigested samples were also prepared by incubating them only with 10 μl of the digestion buffer mentioned. Samples were then boiled for 5 minutes in Laemmli buffer (Laemmli, 1970). Proteins were separated by SDS-PAGE on 8% gels, transferred onto PVDF membranes (Millipore), blocked in 5% non-fat milk solution in PBS plus 0.05% Tween 20 and probed with anti-Δ-heparan sulfate antibodies followed by incubation with HRP-conjugated secondary antibodies.
RhoA activity assay (affinity precipitation)
RhoA activity was measured using the affinity precipitation assay described previously (Avalos et al., 2004). Astrocytes were grown in 6-cm plates and, after serum deprivation, were stimulated for 15 minutes with 20 μg of Thy-1(RLD)-Fc bound to 20 μl of protein-A–Sepharose beads (Thy-1-beads). Thy-1-beads were previously incubated or not with different concentrations of heparin (50-400 μg/ml) for 30 minutes at 4°C. In other experiments, cells were incubated for 30 minutes with the PKC inhibitor Gö 6976 (10 nM or 100 nM) or vehicle (0.01% DMSO) in serum-free medium, before being stimulated with Thy-1-beads. Astrocytes grown to 40% confluency in 6-cm dishes were co-transfected with wild-type or mutated syndecan-4 and pEGFP-C1 DNA (10:1) using Fugene HD transfection reagent according to the manufacturer's instructions. Astrocytes were also transduced in suspension with adenoviral vectors encoding wild-type, dominant-negative PKCα or empty vector (MOI = 1000). Following transduction (24 hours), astrocytes were serum-starved for the assay. The densitometric analysis was performed as reported previously (Avalos et al., 2004).
Western blotting
Astrocytes (2×105 cells) suspended in 400 μl of serum-free medium were incubated for 30 minutes prior to stimulation with Thy-1(RLD)-Fc complexed to protein A. Complexes were formed by incubating Thy-1(RLD)-Fc:protein A (10:1) in serum-free medium for 1 hour at 4°C on a rotating wheel. Then, 4 μg of Thy-1(RLD)-Fc complexed to 0.4 μg of protein A were added to each tube. To test the heparin effect in this assay, complexes were incubated for an additional 30 minutes with heparin (200 μg/ml) before adding them to the cells. Thy-1(RLD)-Fc–protein-A complexes were incubated at 37°C for 10, 15 or 20 minutes. Cell stimulation was stopped by quickly washing the cells with cold PBS and the cells were solubilized in reducing Laemmli buffer containing protease inhibitor cocktail and tyrosine phosphatase inhibitors, as indicated previously. Proteins were separated by SDS-PAGE in 10% minigels and electrotransferred to nitrocellulose. The immunoblot was probed with an anti-phospho-specific antibody to Y397FAK, which was detected with a secondary antibody conjugated to HRP. The densitometric analysis was as reported previously (Avalos et al., 2004).
Mutated Thy-1 proteins present in supernatants of transiently transfected HEK293T cells were recovered with protein A beads (10 μl of beads per ml of supernatant) by incubating them at 4°C for 1 hour on a rotating wheel. The heparin binding capacity of the mutated Thy-1 proteins was tested in a similar manner but using 10 μl of heparin-Sepharose beads. Upon incubation, both protein-A beads and heparin beads were washed twice with cold PBS before solubilization of bound proteins with reducing Laemmli buffer containing protease and phosphatase inhibitors, as indicated above. Solubilized proteins were separated by SDS-PAGE in 10% minigels and electrotransferred to nitrocellulose. Immunoblots were probed with anti-human IgG (Fc specific)-HRP and binding was revealed using the chemiluminescent detection system kit.
Indirect immunofluorescence
Astrocytes (3×104 cells) were seeded on sterile 12-mm coverslips in 24-well plates in complete RPMI medium 12 hours before experiments were initiated. Then, astrocytes were incubated either with 0.5 mU of heparitinase for 3 hours in 400 μl of serum-free medium or with the inhibitor Gö 6976 (100 nM) or corresponding vehicle (0.01% DMSO) in serum-free medium for 30 minutes at 37°C. Astrocytes were also transiently co-transfected with EGFP containing plasmid and syndecan-4 siRNA using siPORT Amine transfection reagent, or with EGFP- and wild-type or truncated syndecan-4-containing plasmids using Lipofectamine Plus reagent, following the manufacturer's instructions. In other experiments, astrocytes were grown in 6-cm plates and, after 24 hours, were transduced with the adenoviral vectors for wild-type or dominant-negative PKCα or with empty vectors. After an additional 24 hours, transduced cells were seeded on coverslips and analyzed the next day.
Astrocytes were stimulated with 2 μg of either Thy-1(RLD)-Fc-beads, Thy-1(RLE)-Fc-beads or TRAIL-R2-Fc-beads for 10 minutes, or left unstimulated (NS). Alternatively, astrocytes were stimulated with neuronal CAD cells as reported (Hermosilla et al., 2008) or with the Thy-1-Fc mutants coupled to protein-A–Sepharose beads (20 μl) obtained by incubating the beads with supernatants of transiently transfected HEK293T cells. Immunofluorescence experiments were performed as described previously (Avalos et al., 2004). Cells were stained with anti-paxillin or anti-vinculin antibodies followed by secondary antibodies coupled to Alexa Fluor 488 or Cy3. Filamentous actin was stained with phalloidin conjugated to Rhodamine. Fluorophores were visualized by confocal microscopy in a Carl Zeiss LSM-Pascal 5 confocal microscope and the number of FAs per cell was quantified and analyzed for statistical significance as described previously (Avalos et al., 2004). In cases where only the GFP-positive cell population was analyzed (siRNA and truncated syndecan-4 experiments), the percentage of GFP-positive cells with augmented FA formation was estimated. In general, cells were considered as stimulated when at least a 1.5-fold increase in the number of FAs was detectable compared to the non-stimulated control cells.
Silencing of syndecan-4
Three pre-designed sense and antisense siRNA oligonucleotides for syndecan-4 were obtained from Ambion. The target sequences were exon 3 (referred to as oligo 1), exon 3,4 (oligo 2) and exon 4 (oligo 3). Silencing was evaluated for all possible siRNA combinations (1+2, 1+3, 2+3 and 1+2+3) by RT-PCR and optimal silencing conditions were confirmed by western blotting with 3G10 antibody after digestion with heparitinase. Silencer Negative Control #1 siRNA (Ambion) was used as a control for specificity.
Supplementary Material
Supplementary material available online at http://jcs.biologists.org/cgi/content/full/122/19/3462/DC1
This work was supported by the following awards: Fogarty International Center-NIH grant #1R03TW006024-1 and 1R03TW007810-01A1 (to K.B. and L.L.), FONDECYT grants #1040390 and #1070699 (to L.L.), #3050037 and #11070116 (to J.C.T.), NIH grant GM-29860 (to K.B.), FONDAP #15010006 (to A.F.G.Q. and S.L.), ICGEB grant CRP/CH100-05 (to A.F.G.Q.), PhD fellowships from CONICYT (to A.M.A., A.D.V. and R.H.-M.), Grants of the Swiss National Science Foundation (to P.S.). The authors kindly acknowledge the helpful discussions and experimental support provided by Enrique Brandan's group (Centro Fondap de Regulación Celular y Patología, P. Universidad Católica de Chile, Santiago, Chile). Deposited in PMC for release after 12 months.
References
- Arnold, M., Cavalcanti-Adam, E. A., Glass, R., Blummel, J., Eck, W., Kantlehner, M., Kessler, H. and Spatz, J. P. (2004). Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem 5, 383-388. [DOI] [PubMed] [Google Scholar]
- Avalos, A. M., Labra, C. V., Quest, A. F. and Leyton, L. (2002). Signaling triggered by Thy-1 interaction with β3 integrin on astrocytes is an essential step towards unraveling neuronal Thy-1 function. Biol. Res. 35, 231-238. [DOI] [PubMed] [Google Scholar]
- Avalos, A. M., Arthur, W. T., Schneider, P., Quest, A. F., Burridge, K. and Leyton, L. (2004). Aggregation of integrins and RhoA activation are required for Thy-1-induced morphological changes in astrocytes. J. Biol. Chem. 279, 39139-39145. [DOI] [PubMed] [Google Scholar]
- Barker, T. H. and Hagood, J. S. (2008). Getting a grip on Thy-1 signaling. Biochim. Biophys. Acta 1793, 921-923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, M. D., Morgan, M. R. and Humphries, M. J. (2007). Integrins and syndecan-4 make distinct, but critical, contributions to adhesion contact formation. Eur. Phys. J. E Soft Matter 3, 372-376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bass, M. D., Morgan, M. R., Roach, K. A., Settleman, J., Goryachev, A. B. and Humphries, M. J. (2008). p190RhoGAP is the convergence point of adhesion signals from α5β1 integrin and syndecan-4. J. Cell Biol. 181, 1013-1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauvais, D. M., Burbach, B. J. and Rapraeger, A. C. (2004). The syndecan-1 ectodomain regulates αVβ3 integrin activity in human mammary carcinoma cells. J. Cell Biol. 167, 171-181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom, L., Ingham, K. C. and Hynes, R. O. (1999). Fibronectin regulates assembly of actin filaments and focal contacts in cultured cells via the heparin-binding site in repeat III13. Mol. Biol. Cell 10, 1521-1536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braz, J. C., Bueno, O. F., De Windt, L. J. and Molkentin, J. D. (2002). PKCα regulates the hypertrophic growth of cardiomyocytes through extracellular signal-regulated kinase1/2 (ERK1/2). J. Cell Biol. 156, 905-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burridge, K. and Chrzanowska-Wodnicka, M. (1996). Focal adhesions, contractility, and signaling. Annu. Rev. Cell Dev. Biol. 12, 463-518. [DOI] [PubMed] [Google Scholar]
- Cizmeci-Smith, G., Langan, E., Youkey, J., Showalter, L. J. and Carey, D. J. (1997). Syndecan-4 is a primary-response gene induced by basic fibroblast growth factor and arterial injury in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 17, 172-180. [DOI] [PubMed] [Google Scholar]
- Couchman, J. R. (2003). Syndecans: proteoglycan regulators of cell-surface microdomains? Nat. Rev. Mol. Cell Biol. 4, 926-937. [DOI] [PubMed] [Google Scholar]
- Couchman, J. R. and Woods, A. (1999). Syndecan-4 and integrins: combinatorial signaling in cell adhesion. J. Cell Sci. 112, 3415-3420. [DOI] [PubMed] [Google Scholar]
- Danen, E. H., Sonneveld, P., Brakebusch, C., Fassler, R. and Sonnenberg, A. (2002). The fibronectin-binding integrins α5β1 and αVβ3 differentially modulate RhoA-GTP loading, organization of cell matrix adhesions, and fibronectin fibrillogenesis. J. Cell Biol. 159, 1071-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dovas, A., Yoneda, A. and Couchman, J. R. (2006). PKCα-dependent activation of RhoA by syndecan-4 during focal adhesion formation. J. Cell Sci. 119, 2837-2846. [DOI] [PubMed] [Google Scholar]
- Echtermeyer, F., Baciu, P. C., Saoncella, S., Ge, Y. and Goetinck, P. F. (1999). Syndecan-4 core protein is sufficient for the assembly of focal adhesions and actin stress fibers. J. Cell Sci. 112, 3433-3441. [DOI] [PubMed] [Google Scholar]
- Echtermeyer, F., Streit, M., Wilcox-Adelman, S., Saoncella, S., Denhez, F., Detmar, M. and Goetinck, P. (2001). Delayed wound repair and impaired angiogenesis in mice lacking syndecan-4. J. Clin. Invest. 107, R9-R14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, R. L., Ono, M., Povsic, T., Page, C., Eriksson, E., Klagsbrun, M. and Bernfield, M. (1994). Syndecans, cell surface heparan sulfate proteoglycans, are induced by a proline-rich antimicrobial peptide from wounds. Proc. Natl. Acad. Sci. USA 91, 11035-11039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallo, R., Kim, C., Kokenyesi, R., Adzick, N. S. and Bernfield, M. (1996). Syndecans-1 and -4 are induced during wound repair of neonatal but not fetal skin. J. Invest. Dermatol. 107, 676-683. [DOI] [PubMed] [Google Scholar]
- He, T. C., Zhou, S., da Costa, L. T., Yu, J., Kinzler, K. W. and Vogelstein, B. (1998). A simplified system for generating recombinant adenoviruses. Proc. Natl. Acad. Sci. USA 95, 2509-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermosilla, T., Munoz, D., Herrera-Molina, R., Valdivia, A., Munoz, N., Nham, S. U., Schneider, P., Burridge, K., Quest, A. F. and Leyton, L. (2008). Direct Thy-1/αVβ3 integrin interaction mediates neuron to astrocyte communication. Biochim. Biophys. Acta 1783, 1111-1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood, J. and Cheresh, D. (2002). Role of integrins in cell invasion and migration. Nat. Rev. Cancer 2, 91-100. [DOI] [PubMed] [Google Scholar]
- Hozumi, K., Suzuki, N., Nielsen, P. K., Nomizu, M. and Yamada, Y. (2006). Laminin α1 chain LG4 module promotes cell attachment through syndecans and cell spreading through integrin α2β1. J. Biol. Chem. 281, 32929-32940. [DOI] [PubMed] [Google Scholar]
- Hueber, A. O., Pierres, M. and He, H. T. (1992). Sulfated glycans directly interact with mouse Thy-1 and negatively regulate Thy-1 mediated adhesion of thymocytes to thymic epithelial cells. J. Immunol. 148, 3692-3699. [PubMed] [Google Scholar]
- Hynes, R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687. [DOI] [PubMed] [Google Scholar]
- Iseki, K., Hagino, S., Mori, T., Zhang, Y., Yokoya, S., Takaki, H., Tase, C., Murakawa, M. and Wanaka, A. (2002). Increased syndecan expression by pleiotrophin and FGF receptor-expressing astrocytes in injured brain tissue. Glia 39, 1-9. [DOI] [PubMed] [Google Scholar]
- Keum, E., Kim, Y., Kim, J., Kwon, S., Lim, Y., Han, I. and Oh, E. S. (2004). Syndecan-4 regulates localization, activity and stability of protein kinase Cα. Biochem. J. 378, 1007-1014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim, C. W., Goldberger, O. A., Gallo, R. L. and Bernfield, M. (1994). Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell 5, 797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick, C. A. and Selleck, S. B. (2007). Heparan sulfate proteoglycans at a glance. J. Cell Sci. 120, 1829-1832. [DOI] [PubMed] [Google Scholar]
- Koo, B. K., Jung, Y. S., Shin, J., Han, I., Mortier, E., Zimmermann, P., Whiteford, J. R., Couchman, J. R., Oh, E. S. and Lee, W. (2006). Structural basis of syndecan-4 phosphorylation as a molecular switch to regulate signaling. J. Mol. Biol. 355, 651-663. [DOI] [PubMed] [Google Scholar]
- Koo, L. Y., Irvine, D. J., Mayes, A. M., Lauffenburger, D. A. and Griffith, L. G. (2002). Co-regulation of cell adhesion by nanoscale RGD organization and mechanical stimulus. J. Cell Sci. 115, 1423-1433. [DOI] [PubMed] [Google Scholar]
- Laemmli, U. (1970). Cleavage of structural proteins during the assembly of bacteriophage T4. Nature 227, 680-681. [DOI] [PubMed] [Google Scholar]
- Leyton, L., Schneider, P., Labra, C. V., Rüegg, C., Hetz, C. A., Quest, A. F. G. and Bron, C. (2001). Thy-1 binds to the integrin β3 on astrocytes and triggers formation of focal contact sites. Curr. Biol. 11, 1028-1038. [DOI] [PubMed] [Google Scholar]
- Mahalingam, Y., Gallagher, J. T. and Couchman, J. R. (2007). Cellular adhesion responses to the heparin-binding (HepII) domain of fibronectin require heparan sulfate with specific properties. J. Biol. Chem. 282, 3221-3230. [DOI] [PubMed] [Google Scholar]
- Maheshwari, G., Brown, G., Lauffenburger, D. A., Wells, A. and Griffith, L. G. (2000). Cell adhesion and motility depend on nanoscale RGD clustering. J. Cell Sci. 113, 1677-1686. [DOI] [PubMed] [Google Scholar]
- Martiny-Baron, G., Kazanietz, M. G., Mischak, H., Blumberg, P. M., Kochs, G., Hug, H., Marme, D. and Schachtele, C. (1993). Selective inhibition of protein kinase C isozymes by the indolocarbazole Gö 6976. J. Biol. Chem. 268, 9194-9197. [PubMed] [Google Scholar]
- Matsumoto, M., Ogawa, W., Hino, Y., Furukawa, K., Ono, Y., Takahashi, M., Ohba, M., Kuroki, T. and Kasuga, M. (2001). Inhibition of insulin-induced activation of Akt by a kinase-deficient mutant of the epsilon isozyme of protein kinase C. J. Biol. Chem. 276, 14400-14406. [DOI] [PubMed] [Google Scholar]
- McQuade, K. J., Beauvais, D. M., Burbach, B. J. and Rapraeger, A. C. (2006). Syndecan-1 regulates αVβ5 integrin activity in B82L fibroblasts. J. Cell Sci. 119, 2445-2456. [DOI] [PubMed] [Google Scholar]
- Mehta, D., Rahman, A. and Malik, A. B. (2001). Protein kinase Cα signals rho-guanine nucleotide dissociation inhibitor phosphorylation and rho activation and regulates the endothelial cell barrier function. J. Biol. Chem. 276, 22614-22620. [DOI] [PubMed] [Google Scholar]
- Midwood, K. S., Valenick, L. V., Hsia, H. C. and Schwarzbauer, J. E. (2004). Coregulation of fibronectin signaling and matrix contraction by tenascin-C and syndecan-4. Mol. Biol. Cell 15, 5670-5677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, M. R., Humphries, M. J. and Bass, M. D. (2007). Synergistic control of cell adhesion by integrins and syndecans. Nat. Rev. Mol. Cell Biol. 8, 957-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafavi-Pour, Z., Askari, J. A., Parkinson, S. J., Parker, P. J., Ng, T. T. and Humphries, M. J. (2003). Integrin-specific signaling pathways controlling focal adhesion formation and cell migration. J. Cell Biol. 161, 155-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa, T., Tsubota, Y., Hashimoto, J., Kariya, Y. and Miyazaki, K. (2007). The short arm of laminin γ2 chain of laminin-5 (laminin-332) binds syndecan-1 and regulates cellular adhesion and migration by suppressing phosphorylation of integrin β4 chain. Mol. Biol. Cell 18, 1621-1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohba, M., Ishino, K., Kashiwagi, M., Kawabe, S., Chida, K., Huh, N. H. and Kuroki, T. (1998). Induction of differentiation in normal human keratinocytes by adenovirus-mediated introduction of the eta and delta isoforms of protein kinase C. Mol. Cell. Biol. 18, 5199-5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan, J., Singh, U. S., Takahashi, T., Oka, Y., Palm-Leis, A., Herbelin, B. S. and Baker, K. M. (2005). PKC mediates cyclic stretch-induced cardiac hypertrophy through Rho family GTPases and mitogen-activated protein kinases in cardiomyocytes. J. Cell Physiol. 202, 536-553. [DOI] [PubMed] [Google Scholar]
- Park, P. W., Reizes, O. and Bernfield, M. (2000). Cell surface heparan sulfate proteoglycans: selective regulators of ligand-receptor encounters. J. Biol. Chem. 275, 29923-29926. [DOI] [PubMed] [Google Scholar]
- Reddig, P. J. and Juliano, R. L. (2005). Clinging to life: cell to matrix adhesion and cell survival. Cancer Metastasis Rev. 24, 425-439. [DOI] [PubMed] [Google Scholar]
- Rege, T. A. and Hagood, J. S. (2006). Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 20, 1045-1054. [DOI] [PubMed] [Google Scholar]
- Saalbach, A., Wetzel, A., Haustein, U. F., Sticherling, M., Simon, J. C. and Anderegg, U. (2005). Interaction of human Thy-1 (CD 90) with the integrin αVβ3 (CD51/CD61): an important mechanism mediating melanoma cell adhesion to activated endothelium. Oncogene 24, 4710-4720. [DOI] [PubMed] [Google Scholar]
- Saoncella, S., Echtermeyer, F., Denhez, F., Nowlen, J. K., Mosher, D. F., Robinson, S. D., Hynes, R. O. and Goetinck, P. F. (1999). Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc. Natl. Acad. Sci. USA 96, 2805-2810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz, M. A. and Assoian, R. K. (2001). Integrins and cell proliferation: regulation of cyclin-dependent kinases via cytoplasmic signaling pathways. J. Cell Sci. 114, 2553-2560. [DOI] [PubMed] [Google Scholar]
- Small, J. V., Rottner, K. and Kaverina, I. (1999). Functional design in the actin cytoskeleton. Curr. Opin. Cell Biol. 11, 54-60. [DOI] [PubMed] [Google Scholar]
- Swertfeger, D. K. and Hui, D. Y. (2001). Apolipoprotein E receptor binding versus heparan sulfate proteoglycan binding in its regulation of smooth muscle cell migration and proliferation. J. Biol. Chem. 276, 25043-25048. [DOI] [PubMed] [Google Scholar]
- Wetzel, A., Chavakis, T., Preissner, K. T., Sticherling, M., Haustein, U. F., Anderegg, U. and Saalbach, A. (2004). Human Thy-1 (CD90) on activated endothelial cells is a counterreceptor for the leukocyte integrin Mac-1 (CD11b/CD18). J. Immunol. 172, 3850-3859. [DOI] [PubMed] [Google Scholar]
- Wetzel, A., Wetzig, T., Haustein, U. F., Sticherling, M., Anderegg, U., Simon, J. C. and Saalbach, A. (2006). Increased neutrophil adherence in psoriasis: role of the human endothelial cell receptor Thy-1 (CD90). J. Invest. Dermatol. 126, 441-452. [DOI] [PubMed] [Google Scholar]
- Wilcox-Adelman, S. A., Denhez, F. and Goetinck, P. F. (2002). Syndecan-4 modulates focal adhesion kinase phosphorylation. J. Biol. Chem. 277, 32970-32977. [DOI] [PubMed] [Google Scholar]
- Woods, A. and Couchman, J. R. (1992). Protein kinase C involvement in focal adhesion formation. J. Cell Sci. 101, 277-290. [DOI] [PubMed] [Google Scholar]
- Woods, A., Couchman, J. R., Johansson, S. and Hook, M. (1986). Adhesion and cytoskeletal organisation of fibroblasts in response to fibronectin fragments. EMBO J. 5, 665-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods, A., Longley, R. L., Tumova, S. and Couchman, J. R. (2000). Syndecan-4 binding to the high affinity heparin-binding domain of fibronectin drives focal adhesion formation in fibroblasts. Arch. Biochem. Biophys. 374, 66-72. [DOI] [PubMed] [Google Scholar]
- Yoneda, A. and Couchman, J. R. (2003). Regulation of cytoskeletal organization by syndecan transmembrane proteoglycans. Matrix Biol. 22, 25-33. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.