Abstract
Cellular superoxide radicals (O2−) are mostly generated during mitochondrial oxygen metabolism. O2− serves as the raw material for many reactive oxygen species (ROS) members like H2O2 and OH.− radicals following its catalysis by superoxide dismutase (SOD) enzymes and also by autocatalysis (autodismutation) reactions. Mitochondrial ROS generation could have serious implications on degenerative diseases. In model systems overproduction of mitochondrial O2− resulting from the loss of SOD2 function leads to movement disorders and drastic reduction in life span in vertebrates and invertebrates alike. With the help of a mitochondrial SOD2 loss-of-function mutant, Sod2n283, we measured the sensitivity of muscles and neurons to ROS attack. Neural outputs from flight motor neurons and sensory neurons were unchanged in Sod2n283 and the entire neural circuitry between the giant fiber (GF) and the dorsal longitudinal muscles (DLM) showed no overt defect due to elevated ROS. Such insensitivity of neurons to mitochondrial superoxides was further established through neuronal expression of SOD2, which failed to improve survival or locomotive ability of Sod2n283. On the other hand, ultrastructural analysis of Sod2n283 muscles revealed fewer mitochondria and reduced muscle ATP production. By targeting the SOD2 expression to the muscle we demonstrate that the early mortality phenotype of Sod2n283 can be ameliorated along with signs of improved mobility. In summary, muscles appear to be more sensitive to superoxide attack relative to the neurons and such overt phenotypes observed in SOD2-deficient animals can be directly attributed to the muscle.
BETWEEN Drosophila, mouse, and human, the enzymatic antioxidant defense system shares similar organization both structurally (Landis and Tower 2005) and functionally. Besides having a good degree of homology (Duttaroy et al. 1994; Landis and Tower 2005), other significant similarities include the presence of a single copy of Sod1 and Sod2 genes in each with no degree of functional complementation between these enzymes (Copin et al. 2000). While vertebrates have developed additional antioxidant defense enzymes such as glutathione peroxidase (Gpx) and extracellular superoxide dismutase (EcSOD or Sod3), neither Gpx nor an active SOD3 has been demonstrated in Drosophila, although a Sod3-like sequence has been identified (Landis and Tower 2005). Complete loss of SOD2 function is fatally injurious for both mice and Drosophila (Li et al. 1995; Lebovitz et al. 1996; Kirby et al. 2002; Duttaroy et al. 2003). The severe phenotypic effects of SOD2 loss of function have been attributed to elevated DNA damage and protein carbonylation (Golden and Melov 2001). SOD2 loss of function has also been attributed to “free radical attack” or “oxidative insult” on mitochondria where obvious mitochondrial damage was apparent from the inactivation of mitochondrial Fe-S cluster enzymes aconitase and succinate dehydrogenase (Melov et al. 1999; Kirby et al. 2002; Paul et al. 2007). Furthermore, impairment of cellular signaling, specifically those induced by reactive oxygen species (ROS) (Klotz 2005), might also play a very significant role in the early mortality effects of SOD2-deficient flies as indicated recently (Wicks et al. 2009).
Sod2 null mice with damaged mitochondria display a number of pathologies including cardiomyopathy (Li et al. 1995), neurodegeneration, and seizures (Melov et al. 1998). Drosophila mutants of mitochondrial dysfunction are also claimed to be associated with neurodegeneration (Kretzschmar et al. 1997; Min and Benzer 1997, 1999; Rogina et al. 1997; Palladino et al. 2002, 2003; Celotto et al. 2006). In addition to the neurons, muscles are important targets for oxidative modification (Choksi and Papaconstantinou 2008; Choksi et al. 2008). Aerobic muscles with high mitochondrial content and high myoglobin levels, for example, show a significant increase in oxidative modification of all electron transport chain proteins compared to muscles with fewer mitochondria and less myoglobin (anaerobic muscle) (Choksi and Papaconstantinou 2008; Choksi et al. 2008). Mice lacking the Cu-ZnSOD enzyme suffer from a rapid loss of skeletal muscle mass, resembling an accelerated sarcopenia (Jackson 2006; Muller et al. 2006). We therefore set out to measure the impact of heightened superoxide concentration on neurons and muscles of Sod2n283 flies that are devoid of SOD2, the principal scavenger of superoxide radicals in mitochondria (Duttaroy et al. 2003; Belton et al. 2006).
MATERIALS AND METHODS
Fly stocks and genetic crosses:
Sod2n283 is a bona fide null mutant for the Drosophila Sod2 gene as described previously (Duttaroy et al. 2003; Belton et al. 2006). KG06854R is a precise excision revertant stock of a P-element insertion in the Sod2 gene and therefore used as a control in all experiments because it shares a similar genetic background with Sod2n283 (Paul et al. 2007). MHC (myosin heavy chain)-Gal4, Mef-2 (myocyte enhancer factor-2) GAL4, Tub (tubulin)-Gal4, Elav (embryonic lethal abnormal vision)-Gal4, and UAS-lacZ flies were obtained from the Drosophila Stock Center in Bloomington, Indiana. The UAS-SOD2 line is a gift from John Phillips of University of Guelph, Ontario, Canada. For the purpose of rescue, Sod2n283/CyO; UAS-SOD2 flies were crossed with Sod2n283/CyO; GAL4 driver stocks and straight wing flies were selected for all subsequent measurements. To combine the Mef-2-GAL4 and Elav -Gal4 drivers together on the third chromosome, we first tagged these chromosomes with taxi (tx;96A) and multiple wing hairs (mwh;61F), two terminal markers on the third chromosome, respectively. Recombination was carried out between the tx mef2-GAL4 and Elav-GAL4 mwh chromosome, and recombinants carrying the dual insertion mef2-GAL4 Elav-GAL4 were selected on the basis of these two recessive markers appearing together in the same fly.
All fly cultures were maintained in a standard Drosophila culture medium recipe obtained from the Bloomington Drosophila Stock Center (http://flystocks.bio.indiana.edu/Fly).
Electroretinogram:
The electroretinogram (ERG) records the difference in voltage between the surface of the Drosophila compound eye and a reference point, typically the thorax. Each unanesthetized fly was aspirated from a vial and placed inside a cut micropipette tip, so that only the head and a small area of the thorax protruded out of the tip. Tungsten electrodes (A-M Systems, Everett, WA), coated with conducting electrode cream (Signa Creme; Parker Lab, Fairfield, NJ), were applied to the eye and the thorax at the points of recording. The electrodes fed into a digital amplifier and the output was split between a digital oscilloscope and an analog-to-digital converter (National Instruments, Austin, TX). The flies were dark adapted for 10 min before each recording. A bright light was delivered to the fly by a fiber-optic guide from a 150-W xenon arc lamp to an intensity of 3 mW/cm2. The flies were stimulated with an electronic shutter, which generated a train of six light pulses for a period of 3 sec. ERG recordings started 10 sec prior to the light stimulus and ended 10 sec after the stimulus for a total of 23 sec duration. An 11-sec interstimulus interval was allowed between each train of light pulses. Data acquisition was done at 250 Hz (every 4 msec) and combined from all the responses using Lab View software (National Instruments) to yield an average ERG trace. For quantification, a baseline voltage was obtained by averaging the values from 35 sample points immediately before the lights-on. The amplitude of the lights-on transient was calculated as the difference between the highest voltage reached and the baseline voltage. The receptor wave potential was calculated as the difference between the baseline value and the last point before lights-off. The off transient was calculated as the difference between this last voltage and the lowest recorded voltage reached after lights-off. The time to return to 50% of baseline potential was measured in seconds and was used as an indicator of membrane excitability. For any given genotype and age group, four to seven flies were used per assay.
Giant fiber assay:
Intracellular recordings from muscles of adult flies were obtained following Tanouye and Wyman (1980) and Gorczyca and Hall (1984). The giant fibers (GFs) were activated extracellularly with brain stimulation by two etched tungsten electrodes, one placed through each eye, by giving pulses of 40–60 V for 0.03 msec using a Grass S44 stimulator (Grass Instruments, Quincy, MA). For direct extracellular stimulation of the motor neurons, the electrodes were placed into the thoracic ganglion. A tungsten electrode placed in the abdominal cavity served as a ground. Glass electrodes pulled to a resistance of 40–60 MΩ were filled with saline and were driven through the cuticle into the dorsal longitudinal muscle (DLM) fibers and intracellular recordings were amplified using a Getting 5A amplifier (Getting Instruments, Iowa City, IA). Signals were stored on a PC with pCLAMP software and a DMA interface board (Axon Instruments, Foster City, CA). Analysis was performed on a PC using pCLAMP and Excel software (Microsoft, Redmond, WA).
For each animal, the biggest amplitude of the DLM response was measured for brain or thoracic stimulation. We also evaluated the reliability of GF–peripheral synapsing interneuron (PSI)–DLM circuitry by testing its ability to follow multiple stimuli at high frequencies. Each animal was given 10 trains of 10 pulses from a Grass S48 stimulator at 100 Hz and the total amount of responses was counted.
Electron microscopy:
Tissue preparation was done according to Wolff (2000). Briefly, brain and thoraxes were dissected, fixed (2% glutaraldehyde/2.5% formaldehyde in 0.1 m cacodylate; Ted Pella, Redding, CA), and washed (0.1 m cacodylate for 30 min at room temperature). Postfixation was carried out with 2% osmium tetroxide in 0.1 m cacodylate buffer for 2 hr, and samples were dehydrated in ethanol (30–100%) and incubated in 100% propylene oxide. Sample perfusion was done with increasing concentrations of Epon 812 resin (EM Sciences, Fort Washington, PA) in propylene oxide (plastic resin:propylene oxide). Finally, the samples were incubated in 100% plastic resin overnight at room temperature. Ultrathin (80 nm) sections were stained with uranyl acetate/lead citrate (Leica EM stain; Leica, Wetzlar, Germany) and air dried overnight. Samples were viewed using a Jeol (Peabody, MA) JEM-1210 transmission electron microscope. Individual mesh grids are 205 μm in diameter. Statistical analysis was done by counting all the mitochondria under five of these 205-μm openings in each brain and thoracic section.
ATP measurement:
ATP measurements were done as described previously (Fergestad et al. 2006), with minor modifications. ATP was quantitated using the Promega (Madison, WI) ENLITEN ATP Assay kit. Luminescence was recorded using the Modulus Single Tube Luminometer (Turner Biosystems, Sunnyvale, CA). ATP extraction was done using eight flies (four males and four females) of each genotype. Flies were homogenized in 2.5% trichloroacetic acid for 30 sec on ice. The homogenate was centrifuged at 13,000 rpm for 10 min at 4°, and 100 μl of the supernatant was removed and neutralized with 20 μl of 4 m Tris–HCl, pH 7.4; the supernatant was then diluted 1:1000 using ATP-free water. The assay mix consisted of 80 μl of the 1:1000 dilution along with 20 μl luminescent reagent. ATP concentrations were calculated on the basis of an ATP standard curve and corrected against total protein as determined by Bio-Rad Protein Assay (Bio-Rad Laboratories, Hercules, CA).
Western blotting and histochemical analyses:
Western analysis with Drosophila SOD2 (Stressgen, San Diego) and actin (Abcam, Cambridge, MA) antibody was done essentially as described previously in Paul et al. (2007). For β-galactosidase staining cryosections were stained with X-gal substrate (Wolff 2000).
Measurement of vertical climbing ability:
Flies were collected within 3 hr of eclosion. An empty vial marked at 4 and 8 cm was used, where the unanesthetized flies were transferred and the vial was plugged. The vial was inverted (plug side down) and tapped a few times. This action allowed the flies to drop on the plug and instinctively climb upward (negative geotaxis). After 20 sec, the number of flies that climbed above the 4-cm mark was counted. Twenty flies were used from each genotype and five trials were performed per experiment.
Aconitase assay:
Mitochondrial and cytosolic aconitase activities were assayed. Approximately 30 adult flies were homogenized in 200 μl of extraction buffer [0.6 mm MnCl2, 2 mm citric acid, 50 mm Tris–HCl, pH 8.0 containing complete protease inhibitor cocktail from Roche (Indianapolis)]. The extract was sonicated for 15 sec to rupture the mitochondria and centrifuged at 4° at 13,000 × g for 10 min. Protein concentration was determined using a standard Bio-Rad assay. Aliquots were electrophoresed in 20 mm potassium phosphate (pH 7.8) and 3.6 mm citric acid running buffer on Sepraphore III membranes (Pall) for 15 min at 210 V. Aconitase activity was detected chromogenically by incubating the membrane in 100 mm potassium phosphate, (pH 6.5), 1 mm NADP+, 25 mm MgCl2, 2 mm cis-aconitic acid, 0.5 mg/ml 2,3-bis-(2-methoxy-4-nitro-5-sulfenyl)-2H-tetrazolium-5carboxanilide disodium salt (MTT), 0.3 mm phenazine methosulfate, and 5 units/ml of isocitrate dehydrogenase.
RESULTS
Sensory neuron output in Sod2n283:
We reported earlier that after eclosion from the pupal case Sod2 null (Sod2n283) adult flies survive up to 24 hr maximum (Duttaroy et al. 2003; Belton et al. 2006). A rapid decline in vertical climbing behavior and movement is apparent in them within hours after eclosion (Piazza et al. 2009). Soon they drop on the media, lie on their sides, and gradually lose the capacity to show any movement 10–16 hr posteclosion (supporting information, Figure S1). Although it is customary to consider flies in this state as dead, our results below bring this thought into question.
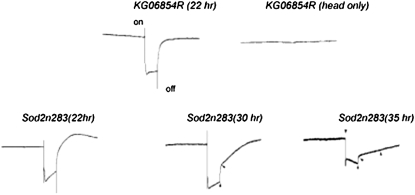
We first traced synaptic transmission in the visual system by monitoring sensory neuron outputs in Sod2n283 flies. The ERG measures synaptic transmission between retinal photoreceptor neurons and laminar monopolar neurons (Curtin et al. 2002). A light pulse of a few seconds prompts the cation-selective channels in the fly's photoreceptor (PR) cells to open, thus initiating the synaptic transmission. A typical ERG trace, therefore, begins with a spike (“on transient”) representing depolarization of PR neurons followed by the duration of the stimulus and the stimulus ends with a brief negative trace (“off transient”), followed by repolarization (Figure 1).
Figure 1.—
Synaptic transmission in the visual system of Sodn283. ERG traces of KG06854R and Sod2n283 (average of six traces from three replicates) show photoreceptor neurons in Sod2n283 flies respond normally to bright light flashes around 22 hr posteclosion although they were considered as dead. Around 30 hr posteclosion a delay in membrane repolarization began with loss of the “off” transient. By 35 hr both “on” and off transients were lost. “Receptor wave” potential also diminished by 35 hr. To mimic the response of a dead fly to bright light stimulus heads of KG06854R were separated from the rest of body and recorded. ERG response disappears following the separation of the head.
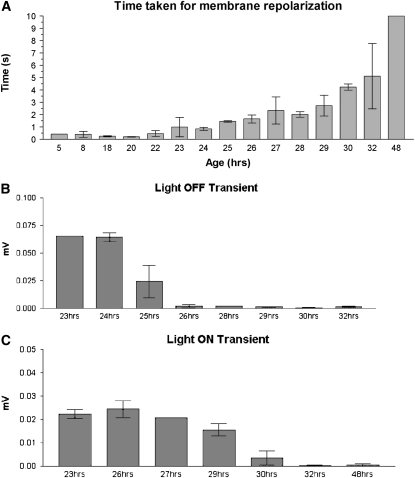
ERG traces of KG06854R (control) and Sod2n283 remain indistinguishable with both “on” and “off” transients present (Figure 1). Rapid repolarization to the prestimulus baseline continues to occur in Sod2n283 even 22 hr posteclosion (Figure 1). Thus, the ERG traces observed in Sod2n283 flies appear completely normal as they responded normally to an external stimulus such as bright light 28–34 hr posteclosion (Figure 1). Sod2n283 flies demonstrated measurable on and off transients, suggesting synaptic transmission at the primary optic neuropil was still functioning (Figure 2). As progressively older flies were recorded, the delayed repolarization phenomenon worsened to the point where at ∼30 hr the off transient disappeared or was negligibly small (Figure 2, A–C). After 30 hr the depolarization potential of the “receptor wave” (during the period of the light pulse) also diminished, perhaps due to decreased numbers of “live” photoreceptor neurons participating in the event. Synaptic transmission events continued for 34–36 hr, after which the trace became flat and resembled the ERG trace of a dead fly. Incidentally, the ERG response of a dead fly is mimicked here simply by separating the head from the body, which leads to immediate disappearance of light response (Figure 1). The ERG results therefore suggest that cessation of movement in Sod2n283 is not due to generalized disappearance of synaptic transmission in all neurons as photoreceptor neurons are responding normally. These results confirm that sensory neurons are not immediately affected by mitochondrial superoxide attack until long after the onset of loss of movement, which suggests that these two events are possibly independent of each other.
Figure 2.—
Membrane repolarization declines with time. (A) A time course study of membrane repolarization revealed repolarization action potential increased with time. A small number of flies responded in the last two time points. (B and C) The “off transient” dropped faster than the “on transient,” which suggests with increased time, depolarized neurons had more difficulty going back to the ground state.
Motor neuron outputs in Sod2n283:
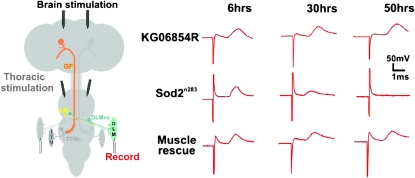
The giant fiber system of the fly is an adult neuronal circuit that mediates escape responses and allows one to determine the functional connection between identified neurons in the CNS as well as motor neuron output (Allen et al. 2006). Electrophysiological recordings from thoracic or brain stimulation at different time points were obtained from the giant fiber system of Sod2n283 mutants and control flies (KG06854R) to determine the presence of a response and the amplitude of the response in DLM. This allowed us to test for the status of the neuromuscular junction (DLM motor neuron, DLMn) as well as the entire circuitry between the GF and the DLM, respectively (Figure 3).
Figure 3.—
Restoration of SOD2 improves the function of the neuromuscular junction. Left, schematic of the giant fiber system. For simplicity only the left giant fiber (GF) circuitry has been highlighted in color. The GF (shown in orange) soma is in the brain and the axon makes a synaptic connection to the peripheral synapsing interneuron (PSI) (in yellow) and the tergo-trochanteral motor neuron (TTMn) (in dark gray) in the second thoracic neuromere. The PSI synapses onto the dorsal longitudinal motor neurons (DLMn) (in green), which drive the flight muscles (DLM). The schematic also depicts the stimulus and recording arrangement. For simplicity only recordings from one side of the GF circuitry have been depicted but recordings were done from both muscles on both sides. Two methods of stimulation are illustrated: (1) brain stimulation was used to activate the GF, which then activates the flight motor neurons (DLMn's) via the PSI, and (2) thoracic stimulation was used to bypass the GF and excite the DLMn's directly. Right, intracellular recordings of the DLMs from homozygous KG06845R (top), Sod2n283 (middle), and muscle rescue flies (bottom) taken at 6, 30, and 50 hr after eclosion. The MHC-Gal4 driver was used to express UAS-SOD2 in the muscles of the Sod2 mutant background.
We found no difference between the two genotypes within the first 22 hr posteclosion (Table 1). Approximately 30 hr posteclosion, however, the response amplitude in some Sod2n283 flies was reduced while in others no response was detected after stimulation of the brain or the thorax (Table 1, Figure 3). Around 50 hr posteclosion DLM responses were completely absent in all tested Sod2 mutants, while the responses in control animals (KG06854R) were normal. Interestingly, these data support the fact that the flight motor neuron outputs remain completely normal at a time (24 hr after eclosion) when movement or any other activity has completely ceased. However, motor neuron output becomes compromised several hours later and is possibly exacerbated by dehydration and starvation.
TABLE 1.
Muscle response in Sod2n283 following brain and thoracic stimulation
| Genotype | DLM response | 2–6 hr | 22–25 hr | 30–34 hr | 50–55 hr |
|---|---|---|---|---|---|
| KG06854R | n | 16 | 22 | ND | 18 |
| DLM NMJ present (%) | 100 | 100 | ND | 100 | |
| DLM NMJ amplitude (mV) | 51 ± 2.7 | 65 ± 1.5 | ND | 67 ± 2.3 | |
| GF-DLM present (%)a | 100 | 100 | ND | 100 | |
| GF-DLM 100Hz (%)b | 98.3 ± 0.9 | 98.6 ± 0.5 | ND | 94.6 ± 2.3 | |
| Sod2n283 | n | 20 | 16 | 18 | 20 |
| DLM NMJ present (%) | 100 | 100 | 75 | 0 | |
| DLM NMJ Amplitude (mV) | 57 ± 3.2 | 56.8 ± 4.2 | 27 ± 6.1 | 0 ± 0 | |
| GF-DLM present (%)a | 100 | 100 | 67 | NA | |
| GF-DLM 100Hz (%)b | 93.6 ± 2.1 | 81.6 ± 6.3 | 78.3 ± 8.1 | NA | |
| Sod2n283; UAS-Sod2/MHC-Gal4 | n | 12 | 10 | 18 | 24 |
| DLM NMJ present (%) | 100 | 100 | 100 | 95.8 | |
| DLM NMJ Amplitude (mV) | 55.6 ± 3.2 | 59.1 ± 5.3 | 45.2 ± 4.1 | 46.8 ± 4.1 | |
| GF-DLM present (%)a | 100 | 100 | 77 | 81 | |
| GF-DLM 100Hz (%)b | 90.2 ± 3.1 | 90.3 ± 3.1 | 71.2 ± 6.7 | 49.4 ± 6.1 |
ND, not determined; NA, not applicable.
The presence of the GF-DLM pathway by brain stimulation was determined only in the subset of animals that showed a DLM muscles response by thoracic stimulation.
The ability of the GF-DLM pathway to follow stimulus 1:1 at 100 Hz was determined only in the subset of animals that showed a DLM response upon brain stimulation.
Restoration of SOD2 in muscles but not neurons ameliorates the Sod2n283 phenotypes:
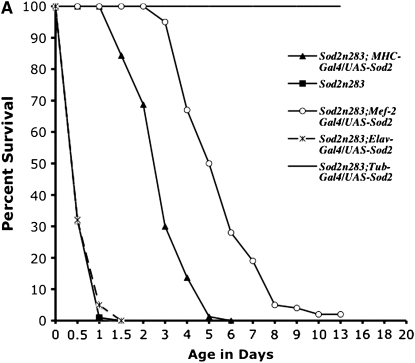
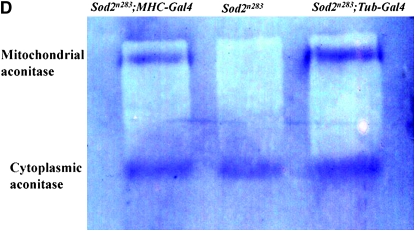
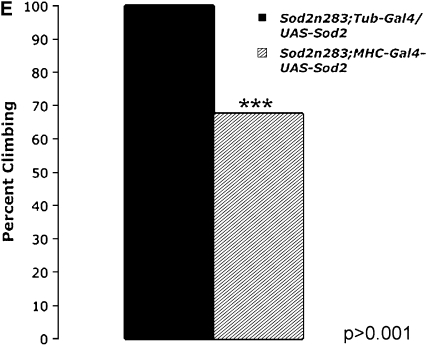
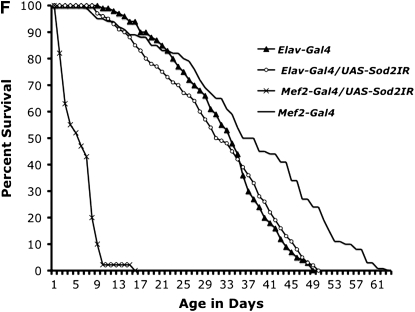
Neuron-specific expression of SOD2 using a panneural driver line Elav-GAL4 fails to improve either survival or movement of Sod2n283 phenotypes (Figure 4A). In contrast, restoration of SOD2 expression in muscles only with MHC-GAL4 (Figure 4, B and C) reduces the early mortality phenotype of Sod2n283 homozygotes so these flies now live up to 6 days with no deaths occurring during the first 24 hr (Figure 4A). We tried a second muscle-specific driver Mef-2-GAL4, which was slightly more efficient than MHC-GAL4 since Sod2n283; Mef-2-GAL4/UAS-SOD2 flies now live up to 8 days (Figure 4A). The difference can be attributed to Mef-2 expression starting earlier than MHC during development. Improved survival is associated with better functionality of the mitochondria as evident from restoration of mitochondrial aconitase activity in the thoracic muscles of Sod2n283; MHC-GAL4/UAS-SOD2 flies (Figure 4D). In terms of their mobility, muscle expression of SOD2 improves the vertical climbing defect quite dramatically until the first 24 hr of measurement (Figure 4E). Could it be that combined expression of SOD2 in neurons and muscles will have an additive effect on the survival of Sod2n283? We tested this possibility by combining Elav-GAL4 and Mef2-GAL4 together on the same chromosome and tried to rescue the Sod2n283 survival, but we see no additive effect (not shown). Finally, the increased muscle requirement of SOD2 protection is further evident through tissue-specific diminution of SOD2 with the help of a Sod2IR (Sod2 RNAi) transgene (Kirby et al. 2002). Activation of a Sod2 IR with a mef2-GAL4 driver shortens the life span of flies significantly, whereas the Elav-GAL4 driver alone imposes no such threats to the life span (Figure 4F). Therefore, Sod2IR data further reinforce our observation that the early behavioral phenotype loss of motility leading to death is due to loss of muscle function and not a defect in the neurons.
Figure 4.—
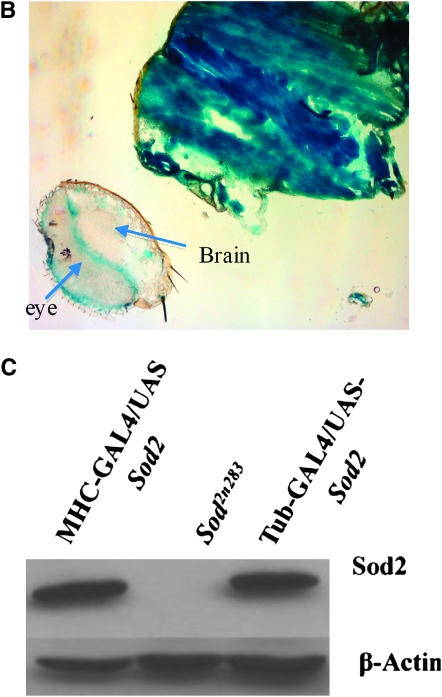
SOD2 protection is more effective in the muscle than in neurons. (A) Panneural expression of SOD2 with the Elav-GAL4 driver could not improve the survival of Sod2n283. However, expression of SOD2 in the muscle with MHC-GAL4 and Mef-2-GAL4 ameliorates the reduced survival phenotype with maximum life span extended up to 6 and 8 days, respectively. (B) Saggital section through an MHC-GAL4;UAS-LacZ fly demonstrates the specificity of the MHC promoter to drive lacZ expression in muscles only. The majority of the fly thorax is composed of flight muscle; therefore, an abundance of lacZ activity was observed. LacZ staining was observed in a minute amount of muscle surrounding the eye (arrow). Brain and retina are mostly neuronal; these two structures therefore remained unlabeled. (C) Protein was extracted from the muscle-rich thorax and immunoblotting was performed. The MHC-GAL4 driver restored SOD2 expression in muscles of Sod2n283 flies almost to the same level as Tub-GAL4, an ubiquitous driver. (D) The mitochondrial aconitase Fe-S cluster enzyme is inactivated in Sod2n283 because of ROS. Mitochondrial aconitase activity was restored in muscles of Sod2n283 flies when SOD2 activity was reinstated to the muscles. (E) Selective expression of SOD2 in the muscle helped Sod2n283 flies regain locomotive ability. After 24 hr of eclosion ∼83% of Sod2n283 flies were able to climb vertically up >4 cm. P < 0.001. (F) Selective reduction of SOD2 with a UAS-SOD2 RNAi in muscles and neurons shows differential effect on survival. Muscle-specific suppression of SOD2 causes severe reduction in life span, whereas suppression of SOD2 activity in neurons does not affect their survival.
Restoration of SOD2 in the muscle reestablishes the function of the neuromuscular junction:
The improved survival and mobility observed in Sod2 null flies expressing SOD2 in the muscles only raises the possibility that SOD2 function is necessary in neuromuscular junctions compared to the neurons per se. We therefore tested the giant fiber system in Sod2n283; MHC-Gal4/UAS-SOD2 flies. Conforming with the mobility data, we found rescue of the DLM response in almost all specimens that were tested at 50 hr posteclosion although the average amplitude size was slightly smaller compared to that in control animals (Table 1, Figure 3). These findings demonstrate that SOD2 is required in the muscle for proper functioning of the neuromuscular junction (NMJ).
The ability to restore NMJ function up to 55 hr posteclosion enables us to test for ROS sensitivity of central synapses, which are involved in mediating the DLM response upon brain stimulation (Figure 3). Hence, we tested for the presence of a DLM response upon brain stimulation. The reliability of the central synapses between the GF and the PSI as well as between the PSI and the DLMn was tested by their ability to follow 10 stimuli given at 100 Hz in specimens that lack muscle expression of SOD2 (Sod2n283) and compared with that in Sod2n283; MHC-Gal4/UAS-SOD2 flies. We found that the GF–PSI–DLMn circuit was present in most Sod2 mutants 81% of the time, even 50 hr posteclosion. However, their reliability was compromised compared to control specimens (Table 1). The mutants were able to follow stimuli only 1 to 1 in 49.4% of the cases, but KG06854R flies followed stimuli reliably in 94.6% of the cases. These findings suggest that the tested neurons were less sensitive to the lack of SOD2 than the DLM. In summary, the findings in the giant fiber system are in accord with the notion that muscle—as opposed to neuronal—dysfunction is a principal causal factor underlying the whole-organism phenotypes arising from deficiency for SOD2. However, the inability to completely rescue the phenotype is likely due to the action of ROS on other tissues including neurons with a later onset than the rapid effect on muscle function.
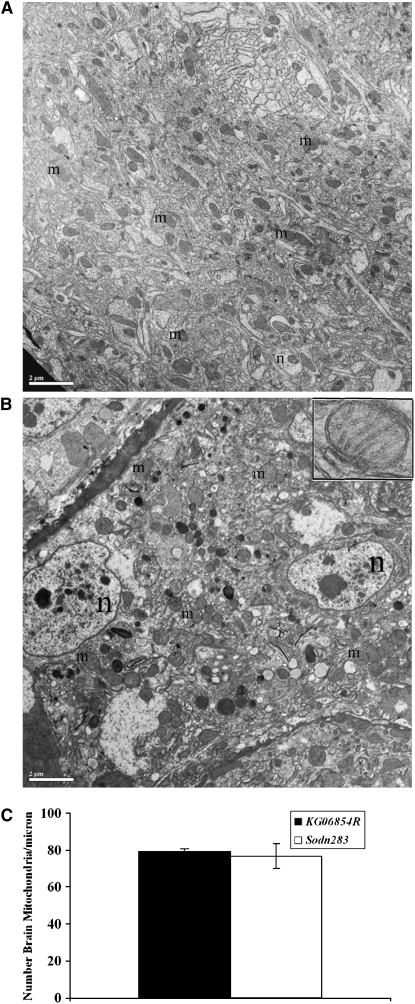
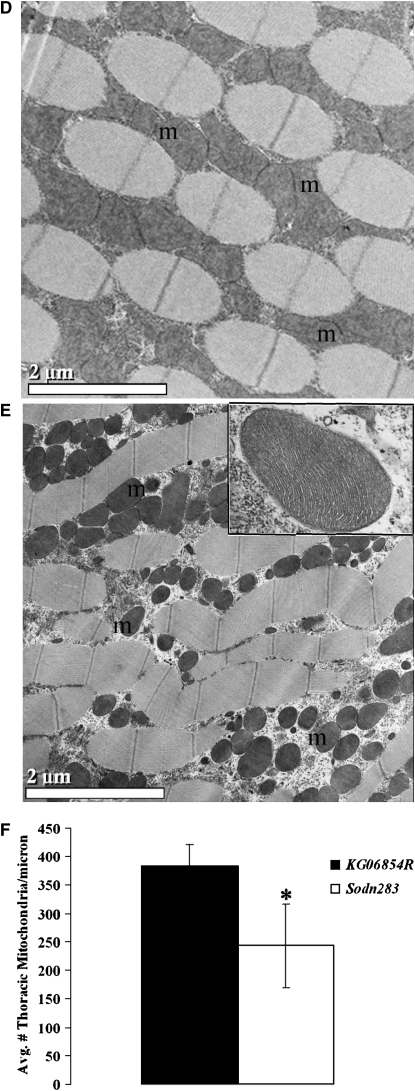
Mitochondrial defects are apparent in SOD2n283 muscles, but not in neurons:
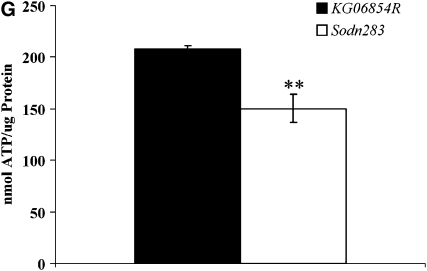
SOD2 protection is essential for mitochondrial health (Melov et al. 1999). To assess the effects of diminished ROS scavenging on mitochondria, ultrastructural analysis was performed on skeletal muscles and CNS neurons in Sod2n283 and KG06854R control specimens. While neuronal mitochondria show no noticeable differences in shape, size, or numbers compared to the control (Figure 5, A–C), we found fewer skeletal muscle mitochondria in Sod2n283 ∼24 hr posteclosion compared to the control (Figure 5, D and E). When we determined the number of mitochondria in Sod2n283 muscles, we found a 40% reduction because muscle mitochondria are rapidly degrading in this mutant (Figure 5F). This rapid reduction in mitochondrial density in muscles is reflected in reduced ATP content in their mitochondria (Figure 5G), which is the likely cause for the accelerated decline in behavioral performances of Sod2n283 flies by affecting their ability to contract their muscles properly.
Figure 5.—
Ultrastructural analysis of neurons and muscles of Sod2n283. (A and B) KG06854R and Sod2n283 brains 24 hr posteclosion show no difference in mitochondrial number, size, or structure (m, mitochondria; n, nucleus). The inset shows intact mitochondria with cristae. (C) Brain sections of KG06854R and Sod2n283 show no differences in mitochondrial number, P = 0.8. (D and E) Micrographs of the dorsal longitudinal muscles (DLM). Control DLMs (D) are packed with mitochondria while Sod2n283 DLMs (E) show fewer mitochondria. The inset shows no abnormality in mitochondrial structure. Bars (A, B, D, and E), 2 μm. (F) Counting mitochondrial numbers confirmed significantly less mitochondria are carried by Sod2n283 DLMs, P > 0.05. (G) Reduction in mitochondrial numbers is associated with less production of ATP in Sod2n283 flies; N = 5, **P < 0.001. Solid bars, KG06854R; open bars, Sod2n283.
DISCUSSION
In the absence of SOD2 function incremental production of mitochondrial ROS can happen due to the exposure of cellular substrates like Fe-S clusters to superoxides. This process, known as autodismutation, will cause an increase in cellular H2O2 content (Buettner et al. 2006) albeit at a much slower rate (1.3 × 105 mol−1 liter sec−1) because autodismutation of O2− follows a second-order reaction (Durot et al. 2005). On the other hand, SOD2-mediated catalysis of superoxide to H2O2 happens much more rapidly following a first-order reaction (2.3 × 109 mol−1 liter sec−1) (Turrens 2003; Murphy 2009). Hydrogen peroxides thus produced from both these reactions can exit the mitochondrion to activate the H2O2 signal transduction pathways or can be converted to hydroxyl radicals. Since our data show that motor neuron outputs in the giant fiber system depict no immediate changes between Sod2n283 and the KG06854R control, we therefore propose that depolarization of neurons and synaptic transmission are not as much influenced by the O2− radicals, particularly when other ROS metabolites (H2O2 and OH.−) are formed inadequately, and thus the functionality of neurons remains unchanged for a long period of time.
Our claim on superoxide radicals being weak effectors of neural function can be further justified by comparing Sod2n283 with mutants of the electron transport chain (ETC). In both cases superoxide radicals are produced in excess due to incomplete reduction of oxygen molecules; however, ETC mutant effects are much more potent than those of Sod2n283. Loss of complex I and IV enzymes causes preadult lethality and a drastic tissue phenotype in cell clones such as roughness of the eye resulting from the lack of cell proliferation during development (Owusu-Ansah et al. 2008). Conversely, no developmental defects or delays are apparent in SOD2n283 mutant animals (A. Duttaroy, S. Mukherjee and R. Forde, unpublished results), which die after turning into adults. ETC mutants are capable of generating all kinds of ROS metabolites from superoxides at a much faster rate because their SOD system is intact. Finally, severity of the ETC mutant effects can be reduced through overexpression of SOD (Owusu-Ansah et al. 2008). All this points to the fact that collective action of all ROS metabolites is important for the degenerative phenotype to develop.
Assuming that superoxide itself is not enough to affect the functionality of the neurons when SOD is absent, it might also explain our earlier claim that precocious neurodegeneration was observed in a Sod2wk/Sod2n283 (Sod2 weak/null) situation where SOD2 activity has been reduced to ∼22% (Paul et al. 2007). It is entirely possible that such a basal level of SOD2 activity was sufficient to convert enough superoxides into H2O2 and hydroxyl radicals and thereby cause neurodegeneration. Alternately, degeneration per se is a progressive event. Sod2wk/Sod2n283 flies live shorter than the wild type but their average life span is 30 days, which is much longer than that of Sod2n283. One major hindrance of the Sod2n283 model is its short life span, which makes it a poor candidate for late onset neurodegeneration studies; however, as it stands now it can provide a wealth of information on the effectiveness of specific ROS metabolite(s) on muscular and neural function.
Could selective loss of dopaminergic neurons be the underlying reason for the locomotion defect observed in Sod2n283 (Piazza et al. 2009)? In the Drosophila parkin mutant selective loss of dopaminergic neurons is associated with climbing defects (Cha et al. 2005; Whitworth et al. 2005). We have two reasons to argue against such a proposition: (1) panneural expression of SOD2 includes the dopaminergic neurons, yet it failed to rescue the locomotion defect in Sod2n283, and (2) the Drosophila mutant of the human PARK2 homolog showed impaired locomotion but loss of tyrosine hydroxylase (TH)-positive neurons was not observed (Pesah et al. 2004). This led us to believe that loss of TH-positive neurons may not be the answer to reduced locomotion ability in Sod2n283.
Since selective reduction of mitochondrial ROS in neurons is not effective in improving the survival of Sod2n283, it signifies that another key tissue (or tissues) is more affected, which should be rejuvenated. Our data support that SOD2 expression in muscles alone ameliorates the early phenotypes of mobility loss in Sod2n283, indicating that muscles are probably the primary targets of mitochondrial superoxide radicals. Furthermore, ultrastructural analysis of muscles and CNS neurons from the same animals clearly demonstrates the tissue-specific selectivity of mitochondria to ROS damage. One reason may be the high demand for ATP in the muscle. Incidentally, muscle defects were noted in the parkin, VD-J1, and Pink-1 mutants (Elkon et al. 2002; Darios et al. 2003; Valente et al. 2004; Zhang et al. 2005; Poole et al. 2008), and muscle-specific expression of parkin rescues the vertical movement ability of the parkin loss-of-function mutant (Greene et al. 2003). Recently, skeletal muscles have been recognized as the primary targets for SOD1G93A mutant protein toxicity because SOD1G93A protein can cause muscle atrophy in transgenic mice (Dobrowolny et al. 2008). Although muscle is recognized as the primary target of ROS, muscle-specific SOD2 expression did not make the organism completely normal. We therefore believe that ROS protection is vital in other tissues as well, and to achieve natural life span all those critical tissues must be protected against ROS insult.
In conclusion, our results suggest a failure to inhibit mitochondrial ROS leads to rapid loss of mitochondrial activity in the muscles but not in neurons. The finding that normal neuronal transmission continues to happen long after the cessation of movement suggests that the loss of mitochondria in the muscle primarily affects muscle contractibility rather than synaptic connectivity.
Acknowledgments
The authors acknowledge Howard Nash for providing the facility for ERG analysis and John Phillips and William Eckberg for making insightful comments on the manuscript. This work was supported by National Institutes of Health grants U54NS039407 and AG025754-01 to A.D.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.103515/DC1.
References
- Allen, M. J., T. A. Godenschwege, M. A. Tanouye and P. Phelana, 2006. Making an escape: development and function of the Drosophila giant fibre system. Semin. Cell Dev. Biol. 17: 31–41. [DOI] [PubMed] [Google Scholar]
- Belton, A., A. Paul and A. Duttaroy, 2006. Deletions encompassing the manganese superoxide dismutase gene in the Drosophila melanogaster genome. Genome 49: 746–751. [DOI] [PubMed] [Google Scholar]
- Buettner, G. R., C. F. Ng, M. Wang, V. G. Rodgers and F. Q. Schafer, 2006. A new paradigm: manganese superoxide dismutase influences the production of H2O2 in cells and thereby their biological state. Free Radic. Biol. Med. 41: 1338–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Celotto, A. M., A. C. Frank, S. W. McGrath, T. Fergestad, W. A. Van Voorhies et al., 2006. Mitochondrial encephalomyopathy in Drosophila. J. Neurosci. 26: 810–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha, G. H., S. Kim, J. Park, E. Lee, M. Kim et al., 2005. Parkin negatively regulates JNK pathway in the dopaminergic neurons of Drosophila. Proc. Natl. Acad. Sci. USA 102: 10345–10350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choksi, K. B., and J. Papaconstantinou, 2008. Age-related alterations in oxidatively damaged proteins of mouse heart mitochondrial electron transport chain complexes. Free Radic. Biol. Med. 44: 1795–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choksi, K. B., J. E. Nuss, J. H. Deford and J. Papaconstantinou, 2008. Age-related alterations in oxidatively damaged proteins of mouse skeletal muscle mitochondrial electron transport chain complexes. Free Radic. Biol. Med. 45: 826–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Copin, J.-C., Y. Gaschea and P. H. Chan, 2000. Overexpression of copper/zinc superoxide dismutase does not prevent neonatal lethality in mutant mice that lack manganese superoxide dismutase. Free Radic. Biol. Med. 28: 1571–1576. [DOI] [PubMed] [Google Scholar]
- Curtin, K. D., Z. Zhang and R. J. Wyman, 2002. Gap junction proteins are not interchangeable in development of neural function in the Drosophila visual system. J. Cell Sci. 115: 3379–3388. [DOI] [PubMed] [Google Scholar]
- Darios, F., O. Corti, C. B. Lücking, C. Hampe, M. P. Muriel et al., 2003. Parkin prevents mitochondrial swelling and cytochrome c release in mitochondria-dependent cell death. Hum. Mol. Genet. 12: 517–526. [DOI] [PubMed] [Google Scholar]
- Dobrowolny, G., M. Aucello, E. Rizzuto, S. Beccafico, C. Mammucari et al., 2008. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 8: 425–436. [DOI] [PubMed] [Google Scholar]
- Durot, S., F. Lambert, J.-P. Renault and C. Policar, 2005. A pulse radiolysis study of catalytic superoxide radical dismutation by a manganese(II) complex with an N-tripodal ligand. Eur. J. Inorg. Chem. 14: 2789–2793. [Google Scholar]
- Duttaroy, A., R. Meidinger, K. Kirby, S. Carmichael, A. Hilliker et al., 1994. A manganese superoxide dismutase-encoding cDNA from Drosophila melanogaster. Gene 143: 223–225. [DOI] [PubMed] [Google Scholar]
- Duttaroy, A., A. Paul, M. Kundu and A. Belton, 2003. A Sod2 null mutation confers severely reduced adult life span in Drosophila. Genetics 165: 2295–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkon, H., J. Don, E. Melamed, I. Ziv, A. Shirvan et al., 2002. Mutant and wild-type alpha-synuclein interact with mitochondrial cytochrome c oxidase. J. Mol. Neurosci. 18: 229–238. [DOI] [PubMed] [Google Scholar]
- Fergestad, T., B. Bostwick and B. Ganetzky, 2006. Metabolic disruption in Drosophila bang-sensitive seizure mutants. Genetics 173: 1357–1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden, T. R., and S. Melov, 2001. Mitochondrial DNA mutations, oxidative stress, and aging. Mech. Aging Dev. 122: 1577–1589. [DOI] [PubMed] [Google Scholar]
- Gorczyca, M., and J. C. Hall, 1984. Identification of a cholinergic synapse in the giant fiber pathway of Drosophila using conditional mutation of acetylcholine synthesis. J. Neurogenet. 1: 289–313. [DOI] [PubMed] [Google Scholar]
- Greene, J. C., A. J. Whitworth, I. Kuo, L. A. Andrews, M. B. Feany et al., 2003. Mitochondrial pathology and apoptotic muscle degeneration in Drosophila parkin mutants. Proc. Natl. Acad. Sci. USA 100: 4078–4083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson, M. J., 2006. Lack of Cu-Zn SOD activity: a pointer to the mechanisms underlying age-related loss of muscle function, a commentary on “absence of CuZn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy”. Free Radic. Biol. Med. 40: 1900–1902. [DOI] [PubMed] [Google Scholar]
- Kirby, K., J. Hu, A. J. Hilliker and J. P. Phillips, 2002. RNA interference-mediated silencing of Sod2 in Drosophila leads to early adult-onset mortality and elevated endogenous oxidative stress. Proc. Natl. Acad. Sci. USA 99: 16162–16167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klotz, L.-O., 2005. Modulation of cellular signaling processes by reactive oxygen species, pp. 203–218 in The Handbook of Environmental Chemistry, edited by T. Grune. Springer, Berlin.
- Kretzschmar, D., G. Hasan, S. Sharma, M. Heisenberg and S. Benzer, 1997. The swiss cheese mutant causes glial hyperwrapping and brain degeneration in Drosophila. J. Neurosci. 17: 7425–7432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landis, G. N., and J. Tower, 2005. Superoxide dismutase evolution and life span regulation. Mech. Aging Dev. 126: 907–908. [DOI] [PubMed] [Google Scholar]
- Lebovitz, R. M., H. Zhang, H. Vogel, J. Cartwright, Jr., L. Dionne et al., 1996. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc. Natl. Acad. Sci. USA 93: 9782–9787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Y., T. T. Huang, E. J. Carlson, S. Melov, P. C. Ursell et al., 1995. Dilated cardiomyopathy and neonatal lethality in mutant mice lacking manganese superoxide dismutase. Nat. Genet. 11: 376–381. [DOI] [PubMed] [Google Scholar]
- Melov, S., J. A. Schneider, B. J. Day, D. Hinerfeld, P. Coskun et al., 1998. A novel neurological phenotype in mice lacking mitochondrial manganese superoxide dismutase. Nat. Genet. 18: 159–163. [DOI] [PubMed] [Google Scholar]
- Melov, S., P. Coskun, M. Patel, R. Tuinstra, B. Cottrell et al., 1999. Mitochondrial disease in superoxide dismutase 2 mutant mice. Proc. Natl. Acad. Sci. USA 96: 846–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, K. T., and S. Benzer, 1997. Wolbachia, normally a symbiont of Drosophila, can be virulent, causing degeneration and early death. Proc. Natl. Acad. Sci. USA 94: 70792–70796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min, K. T., and S. Benzer, 1999. Preventing neurodegeneration in the Drosophila mutant bubblegum. Science 284: 1985–1988. [DOI] [PubMed] [Google Scholar]
- Muller, F. L., W. Song, Y. Liu, A. Chaudhuri, S. Pieke-Dahl et al., 2006. Absence of Cu-Zn superoxide dismutase leads to elevated oxidative stress and acceleration of age-dependent skeletal muscle atrophy. Free Radic. Biol. Med. 40: 1993–2004. [DOI] [PubMed] [Google Scholar]
- Murphy, M. P., 2009. How mitochondria produce reactive oxygen species. Biochem. J. 417: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owusu-Ansah, E., A. Yavari, S. Mandal and U. Banerjee, 2008. Distinct mitochondrial retrograde signals control the G1-S cell cycle checkpoint. Nat. Genet. 40: 356–361. [DOI] [PubMed] [Google Scholar]
- Palladino, M., T. J. Hadley and B. Ganetzky, 2002. Temperature-sensitive paralytic mutants are enriched for those causing neurodegeneration in Drosophila. Genetics 161: 1197–1208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palladino, M. J., J. E. Bower, R. Kreber and B. Ganetzky, 2003. Neural dysfunction and neurodegeneration in Drosophila Na+/K+ ATPase alpha subunit mutants. J. Neurosci. 23: 1276–1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul, A., A. Belton, S. Nag, I. Martin, M. S. Grotewiel et al., 2007. Reduced mitochondrial SOD displays mortality characteristics reminiscent of natural aging. Mech. Ageing Dev. 128: 706–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesah, Y., T. Pham, H. Burgess, B. Middlebrooks, P. Verstreken et al., 2004. Drosophila parkin mutants have decreased mass and cell size and increased sensitivity to oxygen radical stress. Development 131: 2183–2194. [DOI] [PubMed] [Google Scholar]
- Piazza, N., M. Hayes, I. Martin, A. Duttaroy, M. Grotewiel et al., 2009. Multiple measures of functionality exhibit progressive decline in a parallel, stochastic fashion in Drosophila Sod2 null mutant. Biogerontology(in press). [DOI] [PMC free article] [PubMed]
- Poole, A. C., R. E. Thomas, L. A. Andrews, H. M. McBride, A. J. Whitworth et al., 2008. The PINK1/Parkin pathway regulates mitochondrial morphology. Proc. Natl. Acad. Sci. USA 105: 51638–51643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogina, B., S. Benzer and S. L. Helfand, 1997. Drosophila drop-dead mutations accelerate the time course of age-related markers. Proc. Natl. Acad. Sci. USA 94: 6303–6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanouye, M. A., and R. J. Wyman, 1980. Motor outputs of giant nerve fiber in Drosophila. J. Neurophysiol. 44: 405–421. [DOI] [PubMed] [Google Scholar]
- Turrens, J. F., 2003. Mitochondrial formation of reactive oxygen species. J. Physiol. 552: 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valente, E. M., P. M. Abou-Sleiman, V. Caputo, M. M. Muqit, K. Harvey et al., 2004. Hereditary early-onset Parkinson's disease caused by mutations in PINK1. Science 304: 1158–1160. [DOI] [PubMed] [Google Scholar]
- Whitworth, A. J., D. A. Theodore, J. C. Greene, H. Benes, P. D. Wes et al., 2005. Increased glutathione S-transferase activity rescues dopaminergic neuron loss in a Drosophila model of Parkinson's disease. Proc. Natl. Acad. Sci. USA 102: 28024–28029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wicks, S., N. Bain, A. Duttaroy, A. J. Hilliker and J. P. Phillips, 2009. Hypoxia rescues early mortality conferred by superoxide dismutase deficiency. Free Radic. Biol. Med. 46: 176–181. [DOI] [PubMed] [Google Scholar]
- Wolff, T., 2000. Histological techniques for the Drosophila eye part II: adult, pp. 238–243 in Drosophila Protocols, edited by W. Sullivan, M. Ashburner and R. S. Hawley. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Zhang, L., M. Shimoji, B. Thomas, D. J. Moore, S. W. Yu et al., 2005. Mitochondrial localization of the Parkinson's disease related protein DJ-1: implications for pathogenesis. Hum. Mol. Genet. 14: 2063–2073. [DOI] [PubMed] [Google Scholar]