Abstract
The initiation of replication in bacteria is regulated via the initiator protein DnaA. ATP-bound DnaA binds to multiple sequences at the origin of replication, oriC, unwinding the DNA and promoting the binding of DnaB helicase. From an Escherichia coli mutant highly perturbed for replication control, obgE∷Tn5-EZ seqAΔ, we isolated multiple spontaneous suppressor mutants with enhanced growth and viability. These suppressors suppressed the replication control defects of mutants in seqA alone and genetically mapped to the essential dnaA replication initiator gene. DNA sequence analysis of four independent isolates revealed an identical deletion of the DnaA-coding region at a repeated hexanucleotide sequence, causing a loss of 25 amino acids in domain II of the DnaA protein. Previous work has established no function for this region of protein, and deletions in the region, unlike other domains of the DnaA protein, do not produce lethality. Flow cytometric analysis established that this allele, dnaAΔ96-120, ameliorated the over-replication phenotype of seqA mutants and reduced the DNA content of wild-type strains; virtually identical effects were produced by loss of the DnaA-positive regulatory protein DiaA. DiaA binds to multiple DnaA subunits and is thought to promote cooperative DnaA binding to weak affinity DNA sites through interactions with DnaA in domains I and/or II. The dnaAΔ96-120 mutation did not affect DiaA binding in pull-down assays, and we propose that domain II, like DiaA, is required to promote optimal DnaB recruitment to oriC.
REPLICATION initiation in bacteria is controlled by a number of factors that regulate the activity of the the AAA+ DnaA protein (reviewed in Messer 2002; Leonard and Grimwade 2005; Kaguni 2006; Katayama 2008), the functional and structural equivalent of eukaryotic Cdc6/Orc proteins. DnaA binds to several high-affinity binding sites, known as DnaA boxes, near the origin of replication, oriC, in Escherichia coli. In the presence of ATP, DnaA binding extends to several other sites by cooperative interactions, ultimately leading to the melting of an AT-rich region in oriC. The replicative helicase, DnaB, escorted by DnaC, is then recruited to this site, through interactions with the DnaA protein. Once the helicase is assembled on DNA, other factors including the DNA polymerase holoenzyme, processivity clamp, and primase bind to establish bidirectional replication forks emanating from oriC.
A number of regulatory systems impinge on replication initiation via DnaA. One of these involves SeqA, a protein that binds cooperatively to GATC sites found in abundance near the origin. SeqA's binding is stronger when such sites are hemi-methylated by DNA adenine methylase (Dam), a situation that occurs transiently after these sequences are replicated. The binding of SeqA “sequesters” the origin (hence its name) and prevents it from accessing Dam and becoming fully methylated for up to one-third of the cell cycle (Campbell and Kleckner 1990; Lu et al. 1994; von Freiesleben et al. 2000). This sequestration establishes an “eclipse” period, a time at which binding of DnaA and reinitiation is actively prevented. In seqA mutants, cells initiate replication more frequently and more asynchronously than wild-type cells.
A second system controls initiation capacity by altering the levels of ATP-bound DnaA protein. A protein homologous to DnaA (Hda, for homolog of DnaA) binds the processivity clamp, β, and DnaA, promoting hydrolysis of ATP (Kato and Katayama 2001). Mutants in hda are somewhat inviable and show over-replication, particularly when DnaA levels are elevated (Kato and Katayama 2001; Camara et al. 2005; Riber et al. 2006; Fujimitsu et al. 2008).
In addition to these negative regulators, the DiaA protein positively regulates replication initiation. Mutants in diaA were isolated as suppressors of mutants in dnaA that over-initiate replication. By itself, loss of diaA is not lethal but modestly reduces replication initiation frequency and average DNA content per cell and alters the timing and synchrony of initiation (Ishida et al. 2004). DiaA forms a tetramer and directly interacts with multiple DnaA molecules and in vitro recruits DnaA to sites in oriC to stimulate open complex formation (Keyamura et al. 2007). It has been proposed that DnaA cooperative binding, especially to low-affinity sites dependent on the ATP-bound form of DnaA, may be promoted by DiaA.
We became interested in SeqA while studying factors that promoted survival to chronic exposure to low levels of replication inhibitors (Sutera and Lovett 2006). Mutants in seqA and dam were sensitive to such agents, such as hydroxyurea and azidothymidine; this sensitivity was exacerbated under fast-growth conditions during which E. coli has multiple ongoing replication cycles. The sensitivity of seqA mutants to fork damage could be suppressed by two mutations in dnaA that reduced replication initiation efficiency. This study concluded that convergence of an unrestrained replication fork onto the site of previous damage was the basis of this sensitivity.
Another mutant similarly sensitive to fork inhibitors was in the conserved GTPase, obgE (Foti et al. 2005). Hypomorphic alleles of obgE caused by C-terminal insertion of a Tn5-EZ transposon or mutation in the GTPase motif caused sensitivity to replication inhibitors. Moreover, these obgE alleles caused more inviability in combination with recA and recB, mutations that block double-strand break repair, especially when confronted with agents that slow or block DNA replication fork progression. We concluded that forks are more vulnerable to breakage or collapse in the obgE mutants. We also noted effects of obgE on replication initiation: in minimal medium, cells defective in obgE or overexpressing obgE had asynchronously initiated more replication forks than wild-type cells, as deduced by flow cytometry.
Combining mutations in seqA with obgE caused synergistic effects on cell viability and DNA damage sensitivity (Foti et al. 2005). A double mutant in seqA and obgE formed extremely small colonies on rich medium and was much more sensitive, relative to either single mutant, to DNA damage. The phenotype of seqA obgE double mutants was unstable, and we observed the formation of large-colony suppressor variants that arose spontaneously. In this study, we characterize these suppressor mutations and show them to be caused by a single nonlethal deletion in domain II of the replication initiator protein DnaA. The phenotypes caused by this allele are consistent with reduction of replication initiation, properties that are shared by the loss of the positive regulatory protein DiaA. This work therefore establishes a role for domain II in the regulation of replication initiation, potentially in conjunction with DiaA.
MATERIALS AND METHODS
Bacterial strains and media:
All strains used in this study (Table 1) are derivations of E. coli K12 and isogenic to MG1655 (Bachmann 1996). P1 vira transduction was used to construct strains (Miller 1992). Two types of liquid media were used for growth: Luria–Bertani (LB) medium (Miller 1992) or 56/2 minimal medium (Willetts et al. 1969) with plate media containing 1.5% w/v of agar. Antibiotics were used at final concentration of 100 μg/ml ampicillin, 60 μg/ml kanamycin, 15 μg/ml tetracycline, and 15 μg/ml chloramphenicol.
TABLE 1.
E. coli K-12 strains and plasmids used in this study
| Strain (STL) or plasmid (pSTL) | Relevant genotype | Source or derivation |
|---|---|---|
| MG1655 | rph-1 | E. coli Genetic Stock Center (Bachmann 1996) |
| CAG18433 | asnB3057∷Tn10 rph-1 | E. coli Genetic Stock Center (Singer et al. 1989; Nichols et al. 1998) |
| CAG12072 | sfsB203∷Tn10 rph-1 | E. coli Genetic Stock Center (Singer et al. 1989; Nichols et al. 1998) |
| CAG18499 | zid-501∷ Tn10 rph-1 | E. coli Genetic Stock Center (Singer et al. 1989; Nichols et al. 1998) |
| STL7222 | seqAΔ∷[FRT cat] rph-1 | Sutera and Lovett (2006) |
| STL7742 | obgE∷Tn5-EZ rph-1 | Foti et al. (2005) |
| STL9122 | obgE∷Tn5-EZ seqAΔ∷[FRT cat] rph-1 | Cmr transductant P1 STL7222 × STL 7742 |
| STL10421 | dnaA (oss-1a) obgE∷Tn5-EZ seqAΔ∷[FRT cat] rph-1 | Large-colony isolate of STL9122 |
| STL10422 | dnaAΔ96-120 (oss-2a) obgE∷Tn5-EZ seqAΔ∷[FRT cat] rph-1 | Large-colony isolate of STL9122 |
| STL10539 | zid-502∷Tn10 obgE∷Tn5-EZ seqAΔ∷[FRT cat] rph-1 | Tcr transductant P1 CAG18499 × STL10420, small colony |
| STL10543 | zid-502∷Tn10 obgE∷Tn5-EZ seqAΔ∷[FRT cat] rph-1 | Tcr transductant P1 CAG18499 × STL10421, small colony |
| STL12648 | zid-502∷Tn10 dnaAΔ96-120 (oss-1a) obgE∷Tn5-EZ seqAΔ∷[FRT cat] rph-1 | Tcr transductant P1 CAG18499 × STL10420, large colony |
| STL12692 | dnaAΔ96-120zid-501∷Tn10 F- rph-1 | Tcr transductant P1 STL12648 × MG1655 |
| STL12697 | seqAΔ∷[FRT cat] dnaAΔ96-120zid-501∷Tn10 rph-1 | Tcr transductant P1 STL12648 × STL7222 |
| STL12846 | diaAΔ∷[FRT kan] rph-1 | Kmr transductant P1 JW3118 (Baba et al. 2006) × MG1655 |
| STL12848 | seqAΔ∷[FRT cat] diaAΔ∷[FRT kan] rph-1 | Kmr Cmr transductant P1 JW3118 (Baba et al. 2006) × STL12692 |
| STL12851 | dnaAΔ96-120zid-501∷Tn10 diaAΔ∷[FRT kan] rph-1 | Tcr Kmr transductant P1 STL12842 × STL12692 |
| STL12854 | seqAΔ∷[FRT cat] dnaAΔ96-120zid-501∷Tn10 diaAΔ∷[FRT kan] rph-1 | Tcr Kmr Cmr transductant P1 STL12842 × STL12692 |
| pET104.1DEST | amp cat BioEaseTM tag | Invitrogen |
| pDONOR201 | kan cat ccdB | Invitrogen |
| pSTL375 | kan diaA+ | diaA+ in pDONR201 |
| pSTL376 | amp diaA+ BioEaseTM tag | diaA+ in pET104.1DEST |
| pSTL377 | cat 6xHis dnaA+ | Kitagawa et al. (2005) |
| pSTL378 | cat 6xHis dnaAΔ96-120 | Mutant of pSTL377 (this study) |
Genetically mapped and sequenced and identified as dnaAΔ96-120. Km, kanamycin; Tc, tetracycline; Cm, chloramphenicol.
Plasmids:
Plasmid isolation was done using the GeneElute plasmid miniprep kit (Sigma Life Science). Plasmid transformations were performed by electroporation (Dower et al. 1988). Plasmid pSTL377 (His6-DnaA+) was derived from the ASKA collection (Kitagawa et al. 2005), and a comparable plasmid expressing His6- dnaAΔ96-120 was constructed as follows. PCR was performed using pSTL377 as a template with primers reverse pCA24NΔdnaA 5′-gacgttgctc gtcactgccg cttgtggcgt ttgcgtcacc-3′ and forward pCA24NΔdnaA 5′-aacgtcccgg ccccggcaga accgacctat cgttctaacg-3′. The PCR fragment was cleaned using the Qiaquick PCR purification kit (Qiagen), ligated using T4 DNA ligase (New England Biolabs), and transformed into MG1655. The resulting construct, pSTL378, causes loss of a BsgI site, which was confirmed by restriction digest using BsgI (New England Biolabs). Plasmids expressing DiaA as a biotin-binding domain fusion protein were constructed using the site-specific recombination of Gateway Cloning Technology (Invitrogen). PCR was performed on MG1655 using diaAB1F 5′-ggggacaag tttgtacaaa aaagcaggct tccaagaaag aattaaagct-3′ and diaAB2 5′-ggggaccact ttgtacaaga aagctgggtc ttaatcatcctgg tgagg-3′ to produce a diaA+ fragment with attB1 and attB2 regions. The diaA+ fragment was subjected to a restriction digest with DpnI and cleaned using the Qiaquick PCR purification kit to separate the diaA+ PCR product from the template. The cleaned diaA+ PCR product was cloned into the vector pDONR201 via the BP reaction as described in the Gateway Cloning Technology manual (Invitrogen). The resulting plasmid, pSTL375, was used in the LR reaction with BioEase destination vector pET104.1DEST (Invitrogen), a vector for an N-terminal fusion to the Klebsiella pneumoniae oxalacetate decarboxylase α-subunit to which biotin is covalently bound in vivo. This generated pSTL376, expressing the biotin-binding domain (BBD) as an N-terminal fusion to DiaA under T7 promoter control.
Sequence confirmation:
The following primer sets were used to confirm the sequence of dnaAΔ96-120: DnaAseq1111r 5′-tattgtcgatggtgaccagtttttc-3′, dnaAseq991f 5′-tctaacgtacgtgagctggaagggg-3′, dnaAseq811r 5′-caacgccgttgatctctttcggata-3′, dnaAseq451r 5′-ccgccacctggcgagccgccgcgcg-3′, and dnaAseq31f 5′-gcccgattgcaggatgagttaccag-3′. PCR of ΔdnaAΔ96-120 was performed by following the amplification method outlined in the GoTaq Hot Start with Green Buffer with the addition of adding 0.25 units of the high-fidelity DNA polymerase Phusion (New England BioLabs). Primers used in the amplification were 61545dnaAGWN1 5′-GGGGACAGTT TGTACAAAAA AGCAGGCTTC TATCGACTTT TGTTCGAGTG GAGT-3′ and 61546dnaAGWN2 5′-GGGGACCACT TTGTACAA GAAAGCTGGT CAAATTTCAT AGGTTTACGA TGACAA-3′. Primers DCattL1S 5′-TCGCGTTAACGCTAGCATGGATCTC-3′ and DCattL2S 5′-GTAACATCAG AGATTTTGAG ACAC-3′ were used to confirm the sequence of pSTL375.
Phenotypic assays:
Multiple growth curves were obtained for the strains of interest grown in parallel, using aerated flask cultures and measurement for OD600 with a BioRad SmartSpec3000 spectrophotometer or in multiwell dishes using a BioScreen C (Oy Growth Curves AB). Values were averaged for each time point from at least three cultures, allowing the determination of doubling times for both 25° and 37°. Plating efficiency of cultures was determined on LB medium supplemented with AZT (Sigma) at 25 or 50 ng/ml (Foti et al. 2005).
Flow cytometry was used to measure the direct DNA content per cell and was performed using the PicoGreen staining method previously described (Ferullo et al. 2009). “Run-out” conditions included the addition of 300 μg/ml rifampicin and 3.2 μg/ml cephalexin to cultures grown to an OD600 of 0.2. Cultures were allowed to grow for an additional 2.5 hr before analysis by flow cytometry using a Becton-Dickinson FACSCalibur instrument with a 488-nm argon laser. Median DNA content was derived using FlowJo software version 6.4.7 from Treestar.
Pull-down assays for DnaA and DiaA protein binding:
Plasmid pSTL376, carrying BBD fusion to DiaA under control of the T7 promoter, was transformed into BL21(DE3) for protein expression. Likewise strain MG1655 was transformed with plasmid pSTL377 (expressing His-tagged DnaA+) or pSTL378 (His-tagged DnaAΔ), whose expression was controlled under the lac promoter. Strains were grown up to an OD600 of 0.6 and then were induced by adding 1 mm IPTG. The cultures were allowed to express for a total of 2 hr of shaking at 37°. Each culture was then spun down at 4000 rpm at 20° and resuspended 1:100 in Tris–sucrose buffer (50 mm Tris 7.5 10% sucrose) and frozen at −20°. Crude cell extracts were prepared as described (Viswanathan and Lovett 1999) except cells were spun at 17,000 × g for 15 min.
The binding assay consisted of immobilization of BBD-DiaA on steptavidin beads, followed by application of extracts of His6-DnaA- or His6-DnaAΔ96-120-expressing cells; bound protein was detected by Western blotting using an antibody to the His-tag moiety. One hundred microliters of a 50% slurry of Novagen streptavidin agarose beads were equilibrated through three washes with 500 μl of Blank Lysis Buffer [1 mm dithiothreitol (DTT), 200 mm NaCl, 10% w/v ultrapure sucrose, 5 mm Tris, pH 7.5] for each reaction at 4° with recovery of beads by microcentrifugation at 4900 rpm. An equal volume of a cleared lysate of BBD-DiaA strain was added and rotated for 1 hr at 4°. Beads were then gently washed three times using 500 μl of high-salt wash buffer (50 mm Tris, pH 7.5, 1 m NaCl) and reequilibrated in low-salt wash buffer (50 mm Tris, pH 7.5, 150 mm NaCl). For the binding reactions, 90 μl of His-tagged DnaA or DnaAΔ cleared lysate was added to each 90 μl of BBD-DiaA-streptavidin beads and rotated for 1 hr at 4°. Samples were washed three times using 500 μl of low-salt wash buffer. The bound protein was eluted by resuspension of 10 μl of beads in 10 μl of 2× FSB [4% sodium dodecyl sulfate (SDS), 200 mm DTT, 120 mm Tris, pH 6.8, 0.002% bromophenol blue, 10% glycerol]. Additionally, 10 μl of each His6-DnaA+ or His6-DnaAΔ96-120 lysate was resuspended in 10 μl of 2× FSB to assay similarly to determine the amount of input protein for the binding reactions. As a control, mock-treated BBD-DiaA beads in which His6-DnaA was not applied were also analyzed. Proteins were resolved by SDS–PAGE on 12% acrylamide gels and blotted to PVDF membranes with a mini trans-blot electrophoretic transfer cell (Biorad), according to the procedures recommended by the manufacturer. The detection of His-tagged DnaA proteins followed the QIAexpress detection and assay protocol (Qiagen), using a primary Penta-His antibody, a secondary mouse IgG antibody horseradish-peroxidase conjugant (GeneTex) and detection by SuperSignal West Pico chemiluminescence (Thermo Scientific).
RESULTS
Genetic analysis of spontaneous suppressor mutations:
Plating of seqAΔ∷cat obgE∷Tn5-EZ mutant strains on LB medium at 37° (Foti et al. 2005) showed that this strain is extremely poor growing and forms very small colonies. (This strain carries a complete deletion of the seqA gene and a C-terminal Tn5 insertion in obgE that does not completely disrupt its essential function but yields sensitivity to replication inhibitors). However, obvious fast-growing variants were readily apparent in the population, indicating that growth under these conditions selects strongly for such suppressors. We designated these suppressors as “oss,” for obgE seqA suppressors, and saved several such isolates from independent cultures. Whereas seqA obgE strains had a doubling time of 150 min in LB at 37°, oss derivatives had doubling times ranging from 60 to 80 min (Table 2); wild-type strains under these conditions doubled every 19 min. Flow cytometry showed that the DNA content of the oss-suppressed strains was markedly reduced relative to nonsuppressed derivatives, suggesting that the suppressor mutations reduce the efficiency of DNA replication initiation (Table 2).
TABLE 2.
SeqA ObgE suppressors and their growth phenotypes in LB at 37°
| Strain | Description | Doubling timea (min) | Median DNA content (a.u.) | Colony size | Temperature effects | AZT survival (25 ng/ml) |
|---|---|---|---|---|---|---|
| MG1655 | Wild type | 19 | ND | Large | — | R (1) |
| STL9122 | seqA obgE | 150 | 143 | Tiny | cs | S (0.01) |
| STL10421 | oss-1 seqA obgE | 86 | 67 | Small | — | R (1) |
| STL10422 | oss-2 seqA obgE | 86 | 72 | Small | — | R (1) |
| STL10539 | P1 dnaA+ × oss-1 seqA obgE | ND | 132 | Tiny | cs | S (0.01) |
| STL10543 | P1 dnaA+ × oss-2 seqA obgE | ND | 135 | Tiny | cs | S (0.01) |
Doubling time determined with aerobic flask-grown cultures. ND, not determined; cs, cold sensitive for growth; R, resistant with plating efficiency of 1; S, sensitive with plating efficiency of 0.01.
To characterize the suppressor mutations genetically, we established conditions under which selection for suppressors would be diminished. This allowed us to perform genetic crosses under nonselective conditions, after which the presence of the suppressor was deduced by plating under selective conditions. Mutants in seqA have been reported to be somewhat cold sensitive for growth and more inviable on rich growth medium (Lu et al. 1994; von Freiesleben et al. 1994). On LB medium, we found that the double seqA obgE mutant was cold sensitive for growth, with very poor colony formation at temperatures <34° (data not shown), whereas the suppressed seqA obgE oss strains formed colonies even at 34°. On minimal medium all strains formed colonies, although growth of the seqA obgE strain was stronger at temperatures >34°. Therefore, growth on minimal medium at 37°–42° relieved selection for suppressors, but the presence of suppressors could easily be determined subsequently at 25°–30° on LB medium, where only seqA obgE oss strains could form robust colonies.
In the first cross, we tested the effect of oss suppressor mutations on seqAΔ∷cat or obgE∷Tn5-EZ alone. We introduced seqA+ or obgE+ by cotransduction with a linked Tn10 insertion in asnB (CAG18433) or sfsB (CAG12072), respectively, in two independent oss derivatives. Selection for recombinants was on minimal tetracycline medium at 37°; seqA+ was scored by chloramphenicol sensitivity and obgE+ was scored by kanamycin sensitivity. Strains of genotype seqAΔ∷cat oss formed larger colonies at low temperatures than comparable isogenic seqAΔ∷cat strains. In contrast, obgE∷Tn5-EZ colonies looked similar whether they carried oss or not (data not shown). These results suggest that the oss mutations specifically suppress the seqA deficiency in these strains, which was confirmed and documented by the backcrosses described below.
A mutation in dnaA is necessary and sufficient for suppression of seqA:
Because dnaA and seqA mutations are mutually suppressive (Lu et al. 1994; von Freiesleben et al. 1994; Sutera and Lovett 2006), we performed genetic crosses to establish whether two independently isolated oss mutations mapped near dnaA. We crossed seqA obgE oss strains with a selectable marker, zid-501∷Tn10, 86% cotransducible with dnaA. P1 transductions were performed, selecting tetracycline resistance from P1 grown on the zid-501∷Tn10 strain (strain CAG18499) and recipients carrying seqA obgE oss-1 or seqA obgE oss-2, with selection on minimal medium with tetracycline at 42°. Isolates from these crosses showed that most transductants became cold sensitive on LB medium at 30°, indicating loss of suppression (Table 2) and inheritance of oss+. Flow cytometry confirmed that DNA content in these nonsuppressed transduced derivatives was greater than the oss-suppressed original strains. This cross suggests that the region near dnaA is necessary for seqA suppression and that the spontaneous suppressor mutations could potentially be alleles of this gene.
To determine whether the suppressors resulted from mutations within the dnaA gene, we PCR amplified this locus from four independent suppressed isolates and subjected it to DNA sequence determination. As controls, four nonsuppressed transductants from the cross above were also amplified and sequenced. The nonsuppressed transductants gave the wild-type dnaA sequence. Each of the four oss-suppressed strains carried the identical mutation, a 75-bp deletion of the dnaA coding region (Figure 1). This deletion was in-frame, such that 25 residues from amino acids 96 to 120 would be removed from the protein in nonessential domain II of the DnaA, with other regions intact.
Figure 1.—
Map of the DnaA protein and location of the suppressor allele. The DNA sequence of the dnaAΔ96-120, oss suppressor mutation relative to the functional domains of the DnaA protein is shown.
To test whether the dnaA mutation was sufficient to suppress seqA, we moved the dnaAΔ96-120 mutation into strains carrying seqA, using transduction with a linked zid-501∷Tn10 tetracycline-resistance marker. These transductants were found to be cold resistant for growth on LB, in contrast to the cold sensitivity for the original seqA strain (Table 3, Figure 2), confirming that dnaAΔ96-120 alone can suppress seqA.
TABLE 3.
SeqA suppressors and their phenotypes, with growth in LB at 25° and 37°
| Doubling timea (min)
|
Median DNA content (a.u.)
|
AZT survival (50 ng/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| Strain | Description | 25° | 37° | 25° | 37° | 25° | 37° |
| MG1655 | Wild type | 105 | 51 | 74 | 101 | 0.80 | 0.18 |
| STL7222 | seqAΔ | 353 | 125 | 140 | 305 | 0.00039 | 0.00038 |
| STL12692 | dnaAΔ96-120 | 118 | 47 | 60 | 60 | 0.71 | 0.58 |
| STL12697 | seqAΔ dnaAΔ96-120 | 127 | 56 | 89 | 85 | 0.44 | 0.0025 |
| STL12846 | diaAΔ | 117 | 54 | 64 | 58 | 0.83 | 0.54 |
| STL12848 | seqAΔ diaAΔ | 147 | 73 | 120 | 77 | 0.82 | 0.0024 |
| STL12851 | dnaAΔ96-120diaAΔ | 109 | 51 | 56 | 68 | 0.91 | 0.55 |
| STL12854 | seqAΔ dnaAΔ96-120diaAΔ | 125 | 60 | 84 | 75 | 1.1 | 0.036 |
Doubling time determined with microtiter plate grown cultures. a.u., arbitrary units.
Figure 2.—
Colony morphology of isogenic dnaA, diaA, and seqA derivatives on LB medium. (Top) Growth at 25°; (bottom) growth at 37°.
We tested the ability of the dnaAΔ96-120 allele to suppress other phenotypes associated with seqA. The doubling time of the dnaAΔ96-120 seqA strain was substantially diminished relative to dnaA+ seqA strains (Table 3); this growth effect was also reflected in colony size on LB medium (Figure 2). Flow cytometry showed that the median DNA content of seqA strains is reduced by dnaAΔ96-120, suggesting that it partially reverses the over-replication phenotype of seqA (Figure 3, Table 3). The dnaAΔ96-120 allele also suppressed the DNA damage sensitivity of the seqA strain, for UV irradiation (data not shown) and to the replication chain terminator, azidothymidine (Table 3). The suppression of seqA sensitivity by dnaAΔ96-120 at 25° was virtually complete; at higher temperatures such as 37°, the suppression of AZT sensitivity was incomplete, indicating that the dnaAΔ96-120 suppressive effects were somewhat temperature sensitive.
Figure 3.—
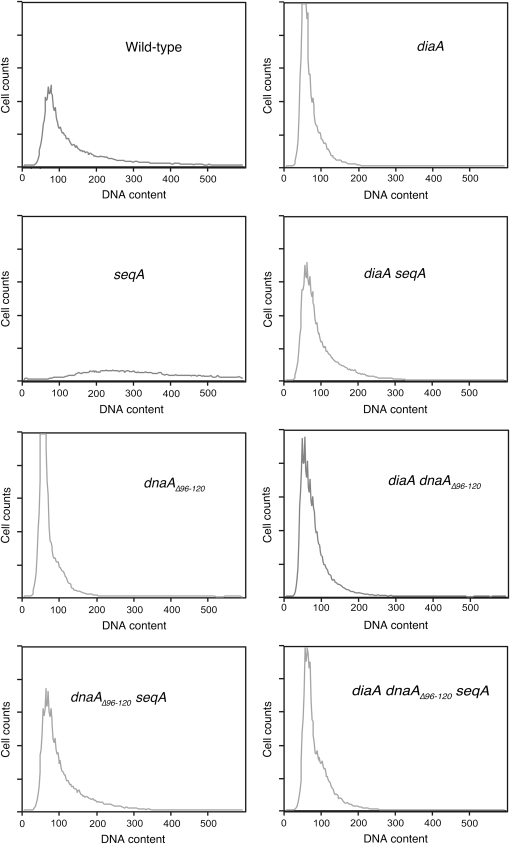
Flow cytometric analysis of DNA content in dnaA, diaA, and seqA derivatives. PicoGreen staining histograms for cultures of the following strains were grown at 37° in LB medium: wild-type MG1655, seqAΔ STL7222, dnaAΔ96-120 STL12692, dnaAΔ96-120 seqAΔ STL12697, diaAΔ STL12846, diaAΔ seqAΔ STL12848, diaAΔ dnaAΔ96-120 STL12851, and diaAΔ seqAΔ STL12854.
To determine if dnaAΔ96-120 had effects in the absence of seqA mutations, the dnaAΔ96-120 allele was crossed into seqA+ strains. Such derivatives had wild-type colony morphology (Figure 2) and resistance to AZT (Table 3). Median DNA content in the dnaAΔ96-120 single mutant was also somewhat less than wild-type strains, confirming that this mutation may reduce initiation efficiency (Table 3, Figure 3). We treated cultures with rifampicin to block replication initiation and cephalexin to block division (so-called “replication run-out conditions”); cells complete all replication forks and assume an integer DNA content, indicative, after flow cytometry, of the number of origins at the time of treatment. Whereas most wild-type strains assumed a 4N and 8N content, dnaAΔ96-120 strains exhibited a lower DNA content after run-out, primarily as 4N (Figure 4). This suggests that dnaAΔ96-120 strains have intrinsically reduced initiation capacity. We also noted a prominent 6N peak, suggesting that asynchronous replication is more common in this mutant background.
Figure 4.—
Flow cytometric analysis of DNA content in dnaA and diaA derivatives after “run-out” treatment with rifampicin and cephalexin. PicoGreen staining histograms for cultures of the following strains were grown at 37° in LB medium: wild-type MG1655, dnaAΔ96-120 STL12692, diaAΔ STL12846, and diaAΔ dnaAΔ96-120 STL12851.
Mutations in DiaA also suppress SeqA:
DiaA has been shown to be a positive regulator of DnaA function in replication initiation (Ishida et al. 2004). We wondered whether loss of diaA would likewise suppress seqA for growth defects, over-initiation, and sensitivity to AZT. In every respect, loss of DiaA mimicked the effects seen for the DnaAΔ mutation: it suppressed the cold sensitivity of seqA, as evident by colony size and doubling time, suppressed the AZT sensitivity, and reduced the over-replication phenotype as revealed by flow cytometry (Figure 3, Figure 4, Table 3). Curiously, like the dnaAΔ96-120 mutation, suppression of the AZT sensitivity of seqA mutants by loss of diaA was temperature sensitive, with incomplete suppression at 37°. As with dnaAΔ96-120 single mutants, diaA strains had a somewhat lower DNA content than wild-type strains, as evident in cycling and replication “run-out” cultures, indicating a reduction in replication initiation frequency (Table 3, Figure 3, Figure 4). Asynchronous replication, as evident by the 6N peak, was also observed. The double diaA dnaAΔ96-120 mutant had virtually identical phenotypic effects to either single mutant in all phenotypes except AZT sensitivity. At 25°, suppression of AZT sensitivity was complete; at 37°, suppression was diminished and the effects of the two mutations were somewhat additive on AZT survival, which was very low.
DnaAΔ96-120 mutants still bind DiaA:
DiaA binds multivalently to DnaA and is believed to promote cooperative binding of DnaA to lower affinity DNA sites by recruitment of DnaA. The site of DiaA binding in DnaA has been deduced to be in domain I and/or domain II (Ishida et al. 2004), the linker region affected by DnaAΔ96-120 (Figure 1).
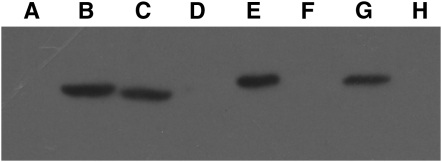
Because the dnaAΔ96-120 allele could potentially disrupt the DiaA-binding site of the protein, we determined, by pull-down experiments, whether the DnaAΔ96-120 protein retained the ability to interact with DiaA. DiaA was fused to the BBD, and the fusion protein was expressed in BL21 cells. A cell extract was applied to streptavidin beads to bind the DiaA fusion protein; these beads were washed under high-salt conditions to remove other bound proteins. We expressed His-tagged constructs of either DnaA+ or DnaAΔ96-120 in separate strains. Cell extracts from these were applied to the BBD-DiaA-streptavidin beads and washed with low-salt buffer, and the bound proteins were resolved by SDS–PAGE. The presence of DnaA in the samples was determined by Western blotting with His6 antibody. DnaA protein was detected in the DiaA-bound fraction equally for DnaA+ and for DnaAΔ96-120 (Figure 5, lanes E and G). As controls, we determined that levels of DnaA+ and DnaAΔ96-120 were comparable in the prebound extracts (Figure 5, lanes B and C); no signal was apparent for DiaA beads when the His6-DnaA extracts were omitted (Figure 5, lane D). DnaA did not detectably bind mock-treated beads with no loaded DiaA (Figure 5, lane H). These experiments indicated that there is no obvious defect in DiaA binding of the DnaAΔ96-120 protein.
Figure 5.—
Pull-down assays for binding of His6-DnaA derivatives to BBD-DiaA to stepavidin beads. Samples were analyzed by Western blotting with Anti-His6 antibody. (Lane A) Crude lysate BBD-DiaA alone. (Lane B) Crude lysate of His6-DnaA+ before pulldown. (Lane C) Crude lysate His6-DnaAΔ96-120 before pulldown. (Lane D) BBD-DiaA-streptavidin beads with no His-tagged DnaA added. (Lane E) BBD-DiaA-streptavidin beads with bound His6-DnaA+. (Lane F) Protein standard. (Lane G) BBD-DiaA-streptavidin beads with bound His6-DnaAΔ96-120. (Lane H) His6-DnaA+ added to streptavidin beads with no BBD-DiaA bound.
DISCUSSION
DnaA and replication control:
The DnaA initiator protein controls the timing of bacterial DNA replication. Although DnaA levels appear not to fluctuate during the cell cycle, the onset of replication is regulated by the ATP-bound state of the protein or its interaction with binding sites in oriC (reviewed in Messer 2002; Leonard and Grimwade 2005; Kaguni 2006; Katayama 2008). The SeqA protein sequesters the origin immediately after initiation due to its cooperative binding to hemi-methylated GATC sites, found in abundance near the origin (Campbell and Kleckner 1990; Lu et al. 1994; von Freiesleben et al. 2000). During this period, DnaA is bound only to its high-affinity sites in oriC (sites that bind DnaA in both ATP and ADP forms) and is precluded from binding to lower affinity sites (bound only by ATP-DnaA), whose occupancy is necessary for origin firing (Nievera et al. 2006). In seqA mutants, DnaA binds prematurely to low-affinity sites (Nievera et al. 2006), with the resulting asynchronous and premature initiation of replication.
Binding of DnaA to low-affinity sites in oriC normally requires cooperative interactions with DnaA-bound high-affinity sites and is aided by the DiaA protein. DiaA is a tetramer, each with capacity to bind DnaA, thereby aiding cooperative binding interactions and open complex formation at oriC by recruitment of multiple DnaA molecules (Keyamura et al. 2007). Mutations in diaA were isolated as suppressors of the over-initiation phenotype caused by dnaAcos and cause mild defects in replication initiation synchrony and timing (Ishida et al. 2004; Keyamura et al. 2007). Inactivation of diaA also causes poor inheritance of minichromosomes and enhances the lethality of certain conditionally lethal dnaA alleles. These results established DiaA as a positive regulator of replication initiation, but its connection to bacterial physiology has remained somewhat unclear.
A genetic system sensitive to replication control defects:
Our experiments capitalize on the role of initiation control in allowing cells to tolerate damage to the replication forks. In a screen for mutants sensitive to low levels of the replication inhibitors hydroxyurea and azidothymidine, we identified mutants in Dam and SeqA (Sutera and Lovett 2006). Our analysis suggested that the convergence of replication forks onto sites of DNA damage was responsible for this defect: SeqA restrains replication forks so that collision onto damage is minimized. Spontaneous damage to replication forks most likely explains the poor growth properties of seqA mutants, especially under growth conditions that support high initiation capacity.
Our genetic analysis also identified ObgE as a function required for survival of replication inhibition and suggested that ObgE may control replication fork stability, chromosome organization, or segregation (Foti et al. 2005, 2007). Viable hypomorphic mutants in obgE were synthetically lethal with those negating double-strand break repair (Foti et al. 2005) and have modest over-replication and asynchronous replication phenotypes.
A double mutant in obgE and seqA is highly inviable, much more so than the single mutants, and rapidly accumulates suppressor mutations (Foti et al. 2005), which we identify here as alleles of dnaA. Although we were initially surprised to find mutations arising at such high frequencies in an essential gene, our analysis suggests that they consist predominantly of deletions at short direct repeats in a nonessential region of the protein, domain II. Short repeated sequences act as hotspots for mutagenesis (Albertini et al. 1982), explaining the frequent nature of these spontaneous suppressor mutations.
The role of domain II in DnaA function:
Previous work has established the nonessential nature of domain II of DnaA, believed to be a flexible linker region between its oligomerization and DnaB-binding domain I and ATPase domain III of the protein. Domain II is variable in length and even absent among bacterial DnaAs (Messer et al. 1999; Erzberger et al. 2002) and is composed of residues 87–134 for E. coli DnaA, with our spontaneous deletion spanning amino acids 96–120. Although this domain can be deleted without loss of viability (Messer et al. 1999), our results suggest that this region does indeed have a function in E. coli and is required for optimal regulation of initiation.
For several phenotypes, the dnaAΔ96-120 allele strongly resembles loss of function in the positive regulatory protein DiaA. Both mutations substantially suppress the inviability and AZT-sensitive phenotypes conferred by loss of SeqA; both mutations appear to correct the seqA over-replication phenotype to the same extent, as revealed by excessive DNA content measured by flow cytometry. In otherwise wild-type strains, dnaAΔ96-120 and diaAΔ cause a modest reduction in DNA content per cell, consistent with a reduction in the efficiency, and cause initiation to become asynchronous, with firing of some sister origins in the absence of others, as evident by the 6N peaks in the flow cytometric analysis of DNA content after replication “run-out.” For the most part, the combination of dnaAΔ96-120 and diaAΔ produces phenotypes identical to either single mutant, suggesting genetic epistasis; this and the similarity of dnaAΔ96-120 and diaAΔ suggest that they work through a common mechanism. However, AZT sensitivity conferred by seqA is suppressed marginally better by double dnaAΔ96-120 diaAΔ mutations at 37°, confirming that they may have properties independent of each other. This could be because neither deletion of DnaA domain II nor of DiaA fully negates the activation of DnaA for replication initiation.
The domain II deletion in dnaAΔ96-120 does not alter the efficiency of DiaA binding, as detected by pull-down experiments, although we cannot rule out the possibility that binding is qualitatively different or affected by cellular conditions not recapitulated in these biochemical assays. Previous studies had implicated either domain I or II of DnaA as the site of DiaA binding (Ishida et al. 2004; Keyamura et al. 2007); our results narrow this to domain I and/or to regions at amino acids 87–95 or 120–134 of domain II.
DiaA regulation of DnaA:
The similarity of DiaA and DnaA domain II defects raises two possibilities. Domain II could be required for DiaA's function in the regulation of DnaA, although it does not appear to be required for binding. Alternatively, both DiaA and DnaA domain II could be independently required for a step in the activation of DnaA for replication initiation; for example, both could promote DnaB recruitment by formation of specific DnaA complexes at oriC.
Because of the ability of DiaA to bind multiple DnaA molecules, it has been assumed to promote DnaA binding to oriC by a recruitment mechanism, whereby occupancy of low-affinity sites, and subsequent activation of initiation, is enhanced by DiaA-bridged interaction to DnaA-bound high-affinity sites. It is also possible that conformational changes in the DnaA protein, which depend on the integrity of domain II, are also associated with this active configuration. Regions involved in DnaB binding include not only domain I of DnaA, most likely also the site of DiaA binding (see above), but also regions of domain III, aa 135–148, adjacent to the domain II linker (Seitz et al. 2000). Therefore the domain II linker could influence the geometry between two potential sites of DnaB recruitment. A model based on the nuclear magnetic resonance structure of domain I suggests that head-to-head dimerization of DnaA domain I could reveal a surface to unite DnaB-binding sites in domain I and III (Abe et al. 2007). Rotation around the domain II linker would be required for this domain I interaction on a scaffold of head-to-tail DnaA complexes mediated through domain III interactions, as is suggested by the crystal structure of domains III and IV (Erzberger et al. 2002, 2006).
Other more complex scenarios are possible. DnaB binding to domain I of DnaA may also function to modulate its interactions with DiaA, potentially releasing DiaA in a hand-off mechanism. Alternatively, the dnaAΔ96-120 mutation may influence interaction with other factors such as the architectural proteins FIS and IHF or change the cooperative nature of DnaA binding. Further characterization of the biochemical properties of this interesting DnaA mutant should clarify its genetic effects and the role of domain II in the regulation of replication initiation.
Acknowledgments
We thank Hirotada Mori, Barry Wanner, and the E. coli Genetic Stock Center for strains and plasmids. This work was supported by National Institutes of Health grants RO1 GM051753 and GM079510 and a Brandeis University Undergraduate Research Program fellowship to K.M.
References
- Abe, Y., T. Jo, Y. Matsuda, C. Matsunaga, T. Katayama et al., 2007. Structure and function of DnaA N-terminal domains: specific sites and mechanisms in inter-DnaA interaction and in DnaB helicase loading on oriC. J. Biol. Chem. 282: 17816–17827. [DOI] [PubMed] [Google Scholar]
- Albertini, A. M., M. Hofer, M. P. Calos and J. H. Miller, 1982. On the formation of spontaneous deletions: the importance of short sequence homologies in the generation of large deletions. Cell 29: 319–328. [DOI] [PubMed] [Google Scholar]
- Baba, T., T. Ara, M. Hasegawa, Y. Takai, Y. Okumura et al., 2006. Construction of Escherichia coli K-12 in-frame, single-gene knockout mutants: the Keio collection. Mol. Syst. Biol. 2: 2006.0008. [DOI] [PMC free article] [PubMed]
- Bachmann, B. J., 1996. Derivations and genotypes of some mutant derivatives of Escherichia coli K-12, pp. 2460–2488 in Escherichia coli and Salmonella: Cellular and Molecular Biology, edited by F. C. Neidhardt. ASM Press, Washington, DC.
- Camara, J. E., A. M. Breier, T. Brendler, S. Austin, N. R. Cozzarelli et al., 2005. Hda inactivation of DnaA is the predominant mechanism preventing hyperinitiation of Escherichia coli DNA replication. EMBO Rep. 6: 736–741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell, J. L., and N. Kleckner, 1990. E. coli oriC and the dnaA gene promoter are sequestered from dam methyltransferase following the passage of the chromosomal replication fork. Cell 62: 967–979. [DOI] [PubMed] [Google Scholar]
- Dower, W. J., J. F. Miller and C. W. Ragsdale, 1988. High efficiency transformation of E. coli by high voltage electroporation. Nucleic Acids Res. 16: 6127–6145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzberger, J. P., M. M. Pirruccello and J. M. Berger, 2002. The structure of bacterial DnaA: implications for general mechanisms underlying DNA replication initiation. EMBO J. 21: 4763–4773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erzberger, J. P., M. L. Mott and J. M. Berger, 2006. Structural basis for ATP-dependent DnaA assembly and replication-origin remodeling. Nat. Struct. Mol. Biol. 13: 676–683. [DOI] [PubMed] [Google Scholar]
- Ferullo, D. J., D. L. Cooper, H. R. Moore and S. T. Lovett, 2009. Cell cycle synchronization of Escherichia coli using the stringent response, with fluorescence labeling assays for DNA content and replication. Methods 48: 8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foti, J. J., J. Schienda, V. A. Sutera, Jr. and S. T. Lovett, 2005. A bacterial G protein-mediated response to replication arrest. Mol. Cell 17: 549–560. [DOI] [PubMed] [Google Scholar]
- Foti, J. J., N. S. Persky, D. J. Ferullo and S. T. Lovett, 2007. Chromosome segregation control by Escherichia coli ObgE GTPase. Mol. Microbiol. 65: 569–581. [DOI] [PubMed] [Google Scholar]
- Fujimitsu, K., M. Su'etsugu, Y. Yamaguchi, K. Mazda, N. Fu et al., 2008. Modes of overinitiation, dnaA gene expression, and inhibition of cell division in a novel cold-sensitive hda mutant of Escherichia coli. J. Bacteriol. 190: 5368–5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishida, T., N. Akimitsu, T. Kashioka, M. Hatano, T. Kubota et al., 2004. DiaA, a novel DnaA-binding protein, ensures the timely initiation of Escherichia coli chromosome replication. J. Biol. Chem. 279: 45546–45555. [DOI] [PubMed] [Google Scholar]
- Kaguni, J. M., 2006. DnaA: controlling the initiation of bacterial DNA replication and more. Annu. Rev. Microbiol. 60: 351–371. [DOI] [PubMed] [Google Scholar]
- Katayama, T., 2008. Roles for the AAA+ motifs of DnaA in the initiation of DNA replication. Biochem. Soc. Trans. 36: 78. [DOI] [PubMed] [Google Scholar]
- Kato, J., and T. Katayama, 2001. Hda, a novel DnaA-related protein, regulates the replication cycle in Escherichia coli. EMBO J. 20: 4253–4262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyamura, K., N. Fujikawa, T. Ishida, S. Ozaki, M. Su'etsugu et al., 2007. The interaction of DiaA and DnaA regulates the replication cycle in E. coli by directly promoting ATP DnaA-specific initiation complexes. Genes Dev. 21: 2083–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitagawa, M., T. Ara, M. Arifuzzaman, T. Ioka-Nakamichi, E. Inamoto et al., 2005. Complete set of ORF clones of Escherichia coli ASKA library (a complete set of E. coli K-12 ORF archive): unique resources for biological research. DNA Res. 12: 291–299. [DOI] [PubMed] [Google Scholar]
- Leonard, A. C., and J. E. Grimwade, 2005. Building a bacterial orisome: emergence of new regulatory features for replication origin unwinding. Mol. Microbiol. 55: 978–985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, M., J. L. Campbell, E. Boye and N. Kleckner, 1994. SeqA: a negative modulator of replication initiation in E. coli. Cell 77: 413–426. [DOI] [PubMed] [Google Scholar]
- Messer, W., 2002. The bacterial replication initiator DnaA. DnaA and oriC, the bacterial mode to initiate DNA replication. FEMS Microbiol. Rev. 26: 355–374. [DOI] [PubMed] [Google Scholar]
- Messer, W., F. Blaesing, J. Majka, J. Nardmann, S. Schaper et al., 1999. Functional domains of DnaA proteins. Biochimie 81: 819–825. [DOI] [PubMed] [Google Scholar]
- Miller, J. H., 1992. A Short Course in Bacterial Genetics. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Nichols, B. P., O. Shafiq and V. Meiners, 1998. Sequence analysis of Tn10 insertion sites in a collection of Escherichia coli strains used for genetic mapping and strain construction. J. Bacteriol. 180: 6408–6411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nievera, C., J. J. Torgue, J. E. Grimwade and A. C. Leonard, 2006. SeqA blocking of DnaA-oriC interactions ensures staged assembly of the E. coli Pre-RC. Mol. Cell 24: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riber, L., J. A. Olsson, R. B. Jensen, O. Skovgaard, S. Dasgupta et al., 2006. Hda-mediated inactivation of the DnaA protein and dnaA gene autoregulation act in concert to ensure homeostatic maintenance of the Escherichia coli chromosome. Genes Dev. 20: 2121–2134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seitz, H., C. Weigel and W. Messer, 2000. The interaction domains of the DnaA and DnaB replication proteins of Escherichia coli. Mol. Microbiol. 37: 1270–1279. [DOI] [PubMed] [Google Scholar]
- Singer, M., T. A. Baker, G. Schnitzler, S. M. Deischel, M. Goel et al., 1989. A collection of strains containing genetically linked alternating antibiotic resistance elements for genetic mapping of Escherichia coli. Microbiol. Rev. 53: 1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutera, V. A., Jr., and S. T. Lovett, 2006. The role of replication initiation control in promoting survival of replication fork damage. Mol. Microbiol. 60: 229–239. [DOI] [PubMed] [Google Scholar]
- Viswanathan, M., and S. T. Lovett, 1999. Exonuclease X of Escherichia coli. A novel 3′-5′ DNase and DnaQ superfamily member involved in DNA repair. J. Biol. Chem. 274: 30094–30100. [DOI] [PubMed] [Google Scholar]
- von Freiesleben, U., K. V. Rasmussen and M. Schaechter, 1994. SeqA limits DnaA activity in replication from oriC in Escherichia coli. Mol. Microbiol. 14: 763–772. [DOI] [PubMed] [Google Scholar]
- von Freiesleben, U., M. A. Krekling, F. G. Hansen and A. Lobner-Olesen, 2000. The eclipse period of Escherichia coli. EMBO J. 19: 6240–6248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts, N. S., A. J. Clark and B. Low, 1969. Genetic location of certain mutations conferring recombination deficiency in Escherichia coli. J. Bacteriol. 97: 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]