Abstract
Iron homeostasis in fungi is regulated at the transcriptional level by two different mechanisms. It is mediated by a conserved GATA-type repressor in most fungi except in the yeast Saccharomyces cerevisiae, where it is controlled by the transcription activators Aft1 and Aft2. These activators are encoded by the paralogous genes AFT1 and AFT2, which result from the whole-genome duplication. Here, we explore regulation of iron homeostasis in the yeast Kluyveromyces lactis that diverged from S. cerevisiae before this event. We identify an ortholog of AFT1/AFT2, designated KlAFT, whose deletion leads to the inability to grow under iron limitation. We show with quantitative real-time PCR analysis that KlAft activates the transcription of all homologs of the Aft1-target genes involved in the iron transport at the cell surface in response to iron limitation. However, homologs of Aft2-specific target genes encoding intracellular iron transporters are regulated neither by KlAft nor by iron. Both bioinformatic and DNA binding and transcription analyses demonstrate that KlAft activates iron-responsive gene expression through the PuCACCC Aft-type sequence. Thus, K. lactis is the first documented species with a positive iron-transcriptional control mediated by only one copy of the Aft-type regulator. This indicates that this function was acquired before the whole-genome duplication and was then diversified into two regulators in S. cerevisiae.
IRON is an essential nutrient. However, despite its abundance in the earth's crust, iron assimilation poses two significant challenges for most organisms. First, the bioavailability of iron is extremely low in aerobic environments because it is mostly present as highly insoluble ferric hydroxides. Second, iron accumulation may be cytotoxic (Halliwell and Gutteridge 1984). Therefore, organisms have developed tightly regulated systems for the acquisition and utilization of iron (Hentze et al. 2004). In fungi, two opposite modes of iron-dependent regulation of transcription have been described. The first is mediated by a Zn-finger GATA-type factor that functions by repressing the transcription of genes involved in iron assimilation under iron-replete conditions (Voisard et al. 1993). This negative regulatory mechanism of iron homeostasis is conserved in most fungi (Haas et al. 2008). The second iron-regulatory pathway has been characterized only in the yeast Saccharomyces cerevisiae. It is mediated by two transcription factors, Aft1 and Aft2, which activate gene expression under iron limitation (Yamaguchi-Iwai et al. 1995; Blaiseau et al. 2001; Rutherford et al. 2001).
Aft1 and Aft2 are encoded by the AFT1/AFT2 paralogous genes, which arose from a single ancestral gene after the whole-genome duplication (WGD) event (Wolfe and Shields 1997; Kellis et al. 2004). Aft1 and Aft2 have overlapping but not redundant functions (Blaiseau et al. 2001; Rutherford et al. 2001, 2003; Courel et al. 2005). Strains with single deletions for either AFT1 or AFT2 exhibit clear phenotypic differences: the aft2Δ strain displays no mutant phenotype whereas the aft1Δ strain grows poorly in low-iron conditions. However, consistent with the functional similarity of Aft1 and Aft2, the double aft1Δaft2Δ mutant is more sensitive to iron deprivation than a single aft1Δ mutant (Blaiseau et al. 2001; Rutherford et al. 2001). Aft1 activates the transcription of all the genes involved in iron acquisition at the cell surface. These include genes that are involved in both the reductive and the siderophore-mediated high-affinity iron transport systems, such as FET3, FTR1, ATX1, CCC2, and FRE1-2 and ARN1-4, FIT1-3, and FRE3, respectively (Philpott and Protchenko 2008). In addition to genes involved in iron uptake, Aft1 activates the transcription of genes involved in metabolic adaptation to conditions of low iron. Such genes include CTH1 and CTH2, which encode conserved mRNA-binding protein involved in the post-transcriptional control of iron homeostasis (Shakoury-Elizeh et al. 2004; Puig et al. 2005, 2008), and HMX1, which encodes a yeast heme oxygenase (Protchenko and Philpott 2003; Shakoury-Elizeh et al. 2004). Aft2 controls the transcription of some of the Aft1 target genes (e.g., FTR1, CTH1, and CTH2) (Courel et al. 2005; Puig et al. 2005, 2008) but it also activates the transcription of genes that are not Aft1 target genes, including SMF3 and MRS4, involved in vacuolar and mitochondrial iron transport, respectively (Rutherford et al. 2003; Courel et al. 2005). Promoter sequence examination and in vivo DNA binding analyses showed that Aft1 and Aft2 recognize similar, but distinct, DNA sequences: TGCACCC and PuCACCC, respectively (Courel et al. 2005). These various observations suggest that Aft1 and Aft2 have become specialized during evolution.
S. cerevisiae serves as a paradigm for iron transport and regulation in hemiascomycete yeasts (Kosman 2003). However, the mechanisms underlying the regulation of the high-affinity iron uptake systems appear to be strikingly different between S. cerevisiae and other hemiascomycete species such as the human yeast pathogen Candida albicans or the methylotrophic yeast Pichia pastoris. The iron-responsive regulator characterized in these other yeasts is the GATA-type transcription repressor conserved in most fungi (Lan et al. 2004; Miele et al. 2007). To elucidate the mechanisms regulating iron homeostasis in hemiascomycetes, we investigated iron regulation in Kluyveromyces lactis. K. lactis is Crabtree-negative and displays a respiratory lifestyle, which is more typical of eukaryotic organisms than the S. cerevisiae fermentative lifestyle (Merico et al. 2007). K. lactis is phylogenetically close to S. cerevisiae but a major difference between K. lactis and S. cerevisiae is that the former diverged before the WGD and the latter diverged after this event (Fitzpatrick et al. 2006). Consequently, K. lactis exhibits much less overall gene redundancy, facilitating genetic studies of metabolic and regulatory pathways and allowing a better understanding of their evolution. In this study, we identify the KlAFT gene as the K. lactis ortholog of AFT1/AFT2. A combination of bioinformatics and experimental analyses allowed us to demonstrate that KlAft regulates iron-regulated genes through an Aft-type DNA-binding sequence. This indicates that the Aft iron-regulatory function was acquired before the WGD. Moreover, consistent with specialization of Aft1 and Aft2 after the WGD, we show that KlAft regulates the homologs of Aft1-target genes but not those of genes regulated only by Aft2.
MATERIALS AND METHODS
K. lactis and S. cerevisiae strains:
The K. lactis strains used in this study were PM6-7A (MATa uraA1-1 adeT-600), MW270-7B (MATa uraA1-1 leu2 metA1-1), MLK53 (MATa uraA1-1 adeT-600 Klaft∷KanMX4), and MLK131 (MATa uraA1-1 adeT-600 lac4∷URA3). The MLK53 KlaftΔ mutant was constructed by one-step gene deletion, integrating kanMX4 at the KlAFT locus in PM6-7A, as described in Wach (1996). The primers used for the KlaftΔ∷kanMX4 cassette were P5′ KlAFT 5′-GATTATTCTCGCTCTCTGTA-3′, P5′L KlAFT 5′-GGGATCCGTCGACCTGCAGCGTACGCATTCAGAAAATAGACAAAATCTC-3′, P3′L KlAFT 5′-AAACGAGCTCGAATTCATCGATGATATGAGATTTTAGCAGTGGAAAAAAGTCT-3′, and P3′ KlAFT 5′-CAAAGTCATTCCCGTTCTGT-3′. Kanamycin-resistant clones were selected on YPD plates containing 200 μg/ml of G418. Among 52 G418R transformants, only one (MLK43) displayed a typical aftΔ mutant phenotype, a growth deficiency on YPD plates supplemented with 200 μM bathophenantholine disulfonic acid (BPS). PCR and meiotic analyses confirmed that the KlAFT gene was disrupted by kanMX4 in MLK43 and that the aftΔ phenotype was genetically linked to G418 resistance. The MLK131 lac4Δ mutant was constructed as described in Neil et al. (2007). The S. cerevisiae strains used in this study were BY4742 (MATα; his3Δ1; leu2Δ0; lys2Δ0; ura3Δ0), Y14438 (MATα; his3Δ1; leu2Δ0; lys2Δ0; ura3Δ0; aft1∷kanMX4), and SCMC01 (MATα; his3Δ1; leu2Δ0; lys2Δ0; ura3Δ0; aft1∷kanMX4; aft2∷kanMX4).
Plasmids construction:
Genomic DNA from K. lactis strain MW270-7B was used as a template for PCR to amplify a 4400-bp fragment containing KlAFT with both upstream and downstream sequences. Genomic DNA from S. cerevisiae strain BY4742 was used as a template for PCR to amplify 3757- and 2860-bp fragments containing AFT1 and AFT2 with both upstream and downstream sequences, respectively. The KlAFT, AFT1, and AFT2 PCR products were first inserted into the vector pCR-2.1-TOPO (Invitrogen, Carlsbad, CA) and then transferred into the low-copy-number K. lactis vector pCXJ18 (Chen 1996), yielding the pCXJ18-KlAFT, pCXJ18-AFT1, and pCXJ18-AFT2 plasmids. The PCR products were also ligated into the S. cerevisiae/K. lactis shuttle vector pCXJ22 (Chen 1996), yielding the pCXJ22-KlAFT, pCXJ22-AFT1, and pCXJ22-AFT2 plasmids. The plasmid pCXJ22-KlAFT-13Myc carrying 13 tandem copies of the c-myc-encoded Myc epitope at the very carboxy terminus of the KlAft protein was constructed by in vivo recombination in S. cerevisiae. The Myc epitope tag for KlAFT was amplified from the template pFA6a-13Myc-HIS3MX6 as described previously (Longtine et al. 1998). The primers used were 5′-CCACAAATGCTTTGGGATGAACCTCACGGCTTTTTTCAACGGATCCCCGGGTTAATTAA-3′and 5′-GTGTTGTACTAAATGAAAGACTTTTTTCCACTGCTAAAATCGAATTCGAGCTCGTTTAAAC-3′. The KLAFT-13Myc PCR product was transformed into a SCMC01 aft1Δaft2Δ mutant containing pCXJ22-KlAFT. The plasmids contained in His+ transformants were rescued in Escherichia coli for molecular analysis. The plasmid pXW3, a K. lactis URA3 multicopy vector carrying the promoterless lacZ operon (Chen et al. 1992), was used to construct a transcriptional KLLA0E14652g-lacZ fusion. The 590-bp fragment of KLLA0E14652g (from −585 to +5 with respect to the start codon) was amplified by PCR and inserted between the BamHI and HindIII sites of pXW3, yielding pKLLA0E14652g-WT-lacZ. The primers used were 5′-CTCAAGCTTGCTTGAATCCTAGTTCATC-3′ and 5′-TCGAAGCTTTCCATTGATTAATGTTCTAGATCG-3′. pKLLA0E14652g-WT-lacZ was used as a PCR template for the QuikChange mutagenesis kit (Stratagene, La Jolla, CA) according to the manufacturer's instructions. The primers used were 5′-GTTTAAAGTGGGAAGTCTTGATCTGGGAAGTTTTTATCCGTTGTC-3′ and its complement for M1 substitutions, 5′-GAAATCGTCATTTCACAGGGAACTATTTTGCTTTTTTTCTAGTAG-3′ and its complement for M3 substitutions, and 5′-GGAAGATTGGAAAAAAAAAATGACACCCAAGAAATATTTGGTTAC-3′ and its complement for M4 substitutions. Nucleotides that deviate from the KLLA0E14652g sequence are underlined. All PCR-generated sequences were confirmed by DNA sequencing. All yeast transformations were performed by the lithium acetate method.
Media, growth conditions, and plate assays:
Rich YPD medium contained 1% yeast extract, 2% peptone, and 2% glucose. Minimal YNB medium contained 0.67% yeast nitrogen base (Difco, Detroit), 0.5% ammonium sulfate, 2% glucose, and the required amino acids and bases. Iron- and copper-limiting yeast nitrogen base medium (Bio101 minus iron and copper) contained 0.17% yeast nitrogen base without iron and copper (Bio101 no. 4027-122), 0.5% ammonium sulfate, 2% glucose, and the required amino acids and bases. For RNA isolation, the wild-type and KlaftΔ mutant cells were pregrown at 30° in Bio101 minus iron and copper supplemented with 1 μm ferric ammonium sulfate. For comparison of KlAFT and KlaftΔ, the wild-type and KlaftΔ mutant cultures were then grown in Bio101 minus iron and copper. For comparison of KlAFT with and without Fe, the wild-type cultures were then grown in Bio101 minus iron and copper with (+Fe) or without (−Fe) 100 μm ferric ammonium sulfate. All the cultures were grown exponentially to an OD600 = 1 and total RNA was then extracted. For β-galactosidase assays, cultures were grown in Bio101 minus iron and copper with (+Fe) or without (−Fe) 100 μm ferric ammonium sulfate. For plate assays, cells were pregrown on YPD medium supplemented with 100 μm ferric ammonium sulfate. Cells were suspended in water at 2 × 106 cells/ml, plated in serial 10-fold dilutions onto YPD agar plates with or without 200 μm BPS, and incubated at 30° for 3–4 days prior to imaging.
RNA isolation and quantitative real-time PCR analysis:
For analyses of mRNA abundance, total RNA was extracted by the hot phenol method and reverse transcribed using AMV Reverse Transcriptase (Roche Applied Science) following the manufacturer's instructions. The amounts of resulting cDNA were evaluated by quantitative real-time PCR with the Mx3000P system (Stratagene, La Jolla, CA) and normalized to the K. lactis KLLA0D05357g gene, a putative ortholog of the S. cerevisiae ACT1 gene. Values reported represent the average of two independent experiments, each performed in duplicate. Standard deviations were <10%. Primers for quantitative real-time PCR (see supporting information, Table S1) were designed with the Primer3 program.
β-Galactosidase assay:
β-Galactosidase was assayed using o-nitrophenyl-d-galactopyranoside as described previously (Guarente 1983).
Electrophoretic mobility shift assays:
The cell extracts were prepared as described previously (Kuras et al. 1996) with the following modifications. Strains were grown at 30° in 250 ml of minimal YNB medium supplemented to meet the auxotrophic requirements, until the optical density at 600 nm was ≈1.5. The following procedures were performed at 4° with ice-cold buffer. Cells were harvested by centrifugation, and the cell pellet was washed with 15 ml of extraction buffer (100 mm Tris, pH 87.5, 1 mm EDTA, 10 mm MgCl2, 10% glycerol, 10 mm β-mercaptoethanol, and 1 mm PMSF). After centrifugation, the cell pellet was resuspended in 1 ml of extraction buffer and the cells were disrupted in a “One Shot” Cell Disrupter (Constant Systems LDT, Daventry, UK) at a pressure of 2 kbar. The resulting lysate was cleared by a first centrifugation for 30 min at 12,000 × g and a second for 20 min at 13,000 × g. Aliquots were stored at −80°. For the mobility shift assays, the binding reaction mixtures (20 μl) contained 25 mm HEPES, pH 7.6, 60 mm KCI, 7.5% glycerol, 0.1 mm EDTA, 1 mm dithiothreitol, 5 mm MgCl2. Totals of 20–40 μg of cell extract and 0.5–0.75 μg of poly(dIdC)2 were used. DNA probes were prepared by PCR amplification. For KLLA0E14652g, the primers 5′-TTCATCATCTAGTAGTGAAG-3′ and 5′-CTTGCATTAAGATCTAACC-3′ were used to produce a DNA fragment containing the −530 ACACCC sequence and the primers 5′-AAAGGGCCTTTCGTG-3′ and 5′-ACTTTAAACTTAATAGTAACC-3′ to produce a DNA fragment containing the −288 ACACCC sequence. For KLLA0E26400g, the primers 5′-AAAGGGCCTTTCGTG-3′ and 5′-ACTTTAAACTTAATAGTAACC-3′ were used to produce a fragment containing the −571 ACACCC sequence. Probes were P-end labeled with [γ-32P]dATP. Approximately 10,000 cpm of probe (1.7 ng) was used in each binding mixture. Samples were incubated for 30 min at room temperature. Competition assays were performed by adding double-stranded DNA fragments made with unlabeled oligonucleotides to the reaction mixtures for another 30 min. To test the specificity of the complex formation with respect to the ACACCC element, competition experiments were performed with 5, 10, and 15 pmol of oligonucleotides centered on wild-type ACACCC (WT) or mutated ACAGGG (M) sequences. The oligonucleotides 5′-AAAAATGACACCCAAGAAAT-3′ and 5′-TCATTTCACACCCAACTATT-3′ were used for the −530 ACACCC and −288 ACACCC sequences of KLLA0E14652g, respectively, and the oligonucleotide 5′-GTAAAAAGAAATCGTCATTTCACACCCAACTATTTTGCTTTTTTTCTAGTAG-3′ for the −571 ACACCC sequence of KLLA0E26400g. To test the specificity of the complex formation with respect to the nucleotide type at position +1 of the −571 ACACCC sequence of KLLA0E26400g, competition experiments were performed with 25 pmol of the oligonucleotide 5′-GTAAAAAGAAATCGTCATTTCXCACCCAACTATTTTGCTTTTTTTCTAGTAG-3′ with X = A, C, G, or T. Samples were loaded onto a 5% polyacrylamide gel in 0.25× TBE (22 mm Tris, pH 8.3, 22 mm boric acid, 0.6 mm EDTA) and electrophoresed at 15 V/cm at 7°. Gels were preelectrophoresed for 1 hr at 7.5 V/cm at 7°. Gels were run for 4 hr, dried, and autoradiographed for 16 hr with an intensifying screen.
Genome and protein sequences, coding sequence annotations, detection of orthology, and homology:
Data on S. cerevisiae (strain S288C) and Kluyveromyces. lactis (strain CLIB210; release 93) were taken from the Saccharomyces Genome Database (SGD) (www.yeastgenome.org) and the Genolevures Consortium site (Sherman et al. 2009), respectively. Protein sequences were downloaded from http://www.genolevures.org for Candida glabrata, Debaryomyces hansenii, Kluyveromyces thermotolerans, Saccharomyces kluyveri, Yarrowia lipolytica, and Zygosaccharomyces rouxii; from http://wolfe.gen.tcd.ie/ygob/ for Saccharomyces bayanus, Saccharomyces castellii, Kluyveromyces waltii, and Kluyveromyces. polysporus; from http://www.ebi.ac.uk/integr8 for Ustilago maydis, Neurospora crassa, Schizosaccharomyces pombe, and Ashbya gossypii; from http://www.candidagenome.org for C. albicans; from http://genome.jgi-psf.org for P. stipitis; and from http://www.broad.mit.edu for Aspergillus nidulans.
To find orthologs of Aft1/Aft2 iron-regulated genes in the K. lactis genome, we used the Yeast Gene Order Browser (YGOB) (Byrne and Wolfe 2006), which considers both sequence similarity and genomic context (synteny). This also allowed identification of S. cerevisiae ohnologs, corresponding to paralogous genes that remained as duplicates after the whole-genome duplication. When no K. lactis ortholog was assigned to a S. cerevisiae gene in YGOB, we chose, when existing, the reciprocal best hit using BLASTP (Altschul et al. 1997). Reciprocal BLASTP searches between all S. cerevisiae and K. lactis proteins were performed using the scoring matrix BLOSUM62, a lower coverage limit of 50%, and a score cutoff of 50 bits. When orthologous relations were too difficult to infer, for the siderophore transporter- and metalloreductase-encoding gene families, the K. lactis homologs were selected using BLASTP with an arbitrary E-value cutoff of 1e-50. The other genomes were searched for homologs of Aft-type activator encoding genes by similarity with the Pfam (Finn et al. 2008) transcription factor Aft domain (PF08731), using the Conserved Domain Database (Marchler-Bauer et al. 2009) and RPS-BLAST, a variant of the Psi-BLAST algorithm (Altschul et al. 1997), with 1e-6 as the E-value cutoff. We also searched for iron-responsive Zn-finger GATA-type transcription repressor homologs in the genomes. All known repressors have three conserved domains that distinguish them from all other fungal GATA factors: two zinc fingers separated by a conserved intervening cysteine-rich region (Haas 2003). We used previous alignments of each of the three conserved domains (Lan et al. 2004) to build position-specific scoring matrices (PSSM) with the MEME program (Bailey and Elkan 1994). The PSSM obtained for the 28 amino acid residues of the cysteine-rich domain was the most specific of the repressor family (data not shown) and it was used with the MAST program (Bailey and Gribskov 1998) and 1e-6 as the E-value cutoff to identify the proteins with the corresponding motif in proteomes.
Identification and enrichment of regulatory patterns:
The 23 homologs of the Aft1- and Aft2-regulated S. cerevisiae genes identified in the K. lactis genome were considered as a regulon, possibly regulated by KlAft. We analyzed the sequences between coordinates −1 and −800, upstream from the start of the coding sequence (CDS) of the 23 K. lactis genes. We used two different approaches to find elements in this set of sequences. First, the oligo-analysis (words) pattern discovery program (van Helden et al. 1998), from the regulatory sequence analysis tools (RSAT) website, was used to detect overrepresented oligonucleotides within our set of sequences. All regions upstream from K. lactis CDS, allowing overlap with upstream CDS, were used as the background model. The significance of overrepresentation of an oligonucleotide was based on the observed frequency in the sequences of the background model, our sequence set size, and a binomial formula. This program was used for words of between six and eight nucleotides. Second, the MEME program (Bailey and Elkan 1994) was used to find highly conserved elements in our set of sequences. This program was used with the anr model, allowing zero or multiple occurrences of an element per sequence. In this program, the statistical significance of an element is based on its log-likelihood ratio, its width and number of occurrences, the background nucleotide frequencies, and the size of the training set. Here, the background model used was the letter frequencies in the set itself. For each identified element, the number of genes in the K. lactis genome, with at least one occurrence of this element in the upstream sequence of its promoter, was computed using regular expressions in a Python script.
RESULTS
The K. lactis genome contains an ortholog of S.cerevisiae AFT1/AFT2:
We searched the K. lactis genome for orthologs of genes coding for known transcriptional regulators of iron homeostasis. We were unable to identify a gene encoding a GATA-type iron transcription repressor in K. lactis by searching for the conserved domain of this repressor family (see materials and methods). However, analysis of the K. lactis genome with the Yeast Gene Order Browser (Byrne and Wolfe 2005) revealed one region on chromosome D that contains the KlAFT gene (ORF KLLA0D03256g), an ortholog of AFT1 and AFT2. KlAft, the predicted product of KlAFT, is very similar in sequence to Aft1 (70% identity) and Aft2 (58% identity) between residues 86 and 325, gaps excluded (Figure 1 and File S1). This homologous region includes the Aft1 and Aft2 N-terminal DNA binding domains and the Cys-Asp-Cys element that confers iron sensitivity (Yamaguchi-Iwai et al. 1995; Rutherford et al. 2001). This region contains two other conserved Cys residues, which have been suggested in recent sequence comparison studies to participate in a zinc finger domain with two conserved His residues (Figure 1 and Babu et al. 2006). Interestingly, KlAft and Aft1, but not Aft2, have additional segments of 72 and 49 aa, respectively, in the region between these two conserved Cys residues. The N-terminal region of KlAft contains also two conserved leucine residues found in a nuclear export signal (NES)-like sequence. These residues have been demonstrated to be critical for the iron-dependent export of Aft1 from the nucleus to the cytoplasm (Yamaguchi-Iwai et al. 2002). The C-terminal end sequences of KlAft and Aft1 contain a glutamine-rich region (57.8% of residues 723–767 in KlAft and 34.1% of residues 617–657 in Aft1 are Gln). This glutamine-rich region is not present in Aft2, this protein being shorter (416 amino acids) than Aft1 or KlAft (690 and 823 amino acids, respectively). The high degree of identity of KlAft with Aft1/Aft2 in the N-terminal region, notably the conservation of the Cys-Asp-Cys and NES-like motifs, led us to test the possibility that KlAft is an iron transcription regulator.
Figure 1.—
Sequence comparison of KlAft, Aft1, and Aft2. The amino acid sequences of KlAft, Aft1, and Aft2 were aligned using the Clustalw program (Larkin et al. 2007). This alignment was then manually refined with the Seaview program (Galtier et al. 1996). The NES-like sequence of Aft1 (Yamaguchi-Iwai et al. 2002) and the homologous regions in Aft2 and KlAft are boxed. The conserved leucines critical for nuclear export of Aft1 are in boldface type. Conserved cysteine and histidine residues predicted to participate in a Zn-finger domain (Babu et al. 2006) are in boldface type. Conserved cysteines involved in the CDC element are in boldface type and this element is boxed. Glutamine-rich regions were detected with the ScanProsite tool (De Castro et al. 2006).
The KlaftΔ deletion mutant is unable to grow under low-iron conditions:
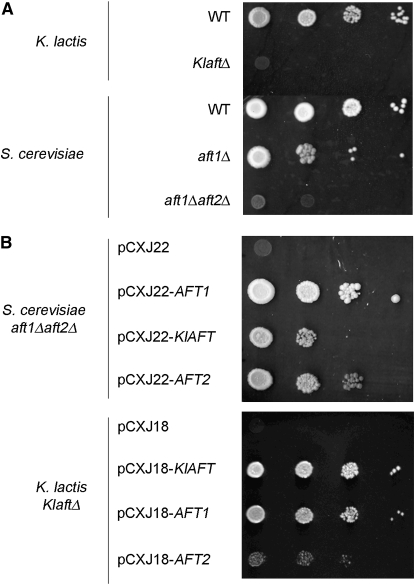
To determine wether KlAft plays a role in iron homeostasis, we deleted the KlAFT gene from the PM6-7A K. lactis wild-type strain and compared the growth of this mutant to that of the isogenic wild-type strain grown under low-iron conditions. Growth was completely abolished in the KlaftΔ mutant under iron-deficient conditions (Figure 2A). The growth of the KlaftΔ mutant was also compared with the growth of the S. cerevisiae strains with either a single deletion of AFT1 or the double deletion of both AFT1 and AFT2. The aft1Δaft2Δ strain exhibits a more severe mutant phenotype than the aft1Δ strain under low-iron conditions (Blaiseau et al. 2001; Rutherford et al. 2001). The KlaftΔ mutant phenotype is similar to that of the aft1Δaft2Δ mutant (Figure 2A). These findings showed that KlAft is essential for the growth of K. lactis under low-iron conditions. Next, we performed cross-complementation experiments. The S. cerevisiae aft1Δaft2Δ mutant was transformed with plasmids containing the KlAFT, the AFT1, or the AFT2 gene (Figure 2B, top). While the growth defect of the aft1Δaft2Δ strain under low-iron conditions was totally reversed with a plasmid containing AFT1, it was only partially suppressed with plasmids containing either KlAFT or AFT2. The same results were obtained with a single aft1Δ deleted mutant (data not shown). In contrast with the results described above in S. cerevisiae, the growth defect of the K. lactis KlaftΔ mutant was totally suppressed with a plasmid containing AFT1 (Figure 2B, bottom).
Figure 2.—
Growth of the KlaftΔ, aft1Δ, and aft1Δaft2Δ mutants under low-iron conditions and cross-complementation analysis. (A) K. lactis and S. cerevisiae wild-type, KlaftΔ, aft1Δ, and aft1Δaft2Δ cells were suspended in water and plated onto rich medium YPD agar plates with BPS (200 μm). (B) The aft1Δaft2Δ cells harboring the S. cerevisiae low-copy number plasmid pCXJ22 (empty vector) or derived plasmids pCXJ22-AFT1, pCXJ22-KlAFT, and pCXJ22-AFT2 (top) and the KlaftΔ cells harboring the K. lactis low-copy number plasmid pCXJ18 (empty vector) or derived plasmids pCXJ18-KlAFT, pCXJ18-AFT1, or pCXJ18-AFT2 (bottom) were suspended in water and plated onto rich medium YPD agar plates with BPS (200 μm).
KlAft activates the transcription of iron-regulated genes:
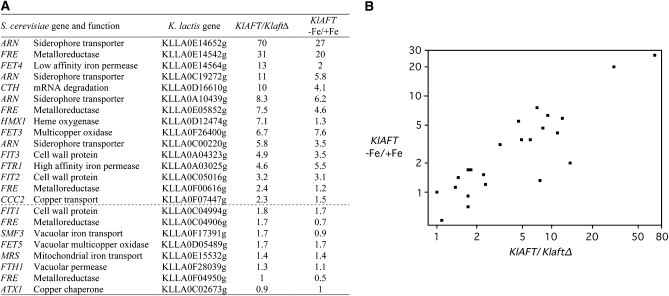
We performed transcription analyses to determine whether KlAft functions as a regulator of genes involved in iron homeostasis. We identified a set of 23 K. lactis homologs of the S. cerevisiae genes involved in iron homeostasis and regulated by Aft1 and Aft2 (Rutherford et al. 2003; Shakoury-Elizeh et al. 2004; Courel et al. 2005). A putative K. lactis ortholog was assigned to most S. cerevisiae Aft1/Aft2 iron-regulated genes. For the genes belonging to the iron-siderophore transporter (ARN) or the metalloreductase (FRE) families, we could not identify orthologous genes but did identify homologous genes (Table 1). For each of the 23 K. lactis genes selected, we used quantitative real-time PCR to assay their mRNAs in wild-type and the KlaftΔ mutant strains, both grown in a low-iron medium. We also compared mRNA levels in the wild-type strain grown in iron-depleted or iron-replete media. The mRNAs of 15 of the 23 genes analyzed were 2.3- to 70-fold more abundant in the wild-type strain than in the KlaftΔ mutant; for 12 of these genes the mRNAs were 2- to 27-fold more abundant in iron-depleted than in iron-replete conditions (Figure 3A). As shown in Figure 3B, the transcription profiles obtained for the two comparisons were strongly correlated. The group of 12 genes activated under iron-deprivation conditions in a KlAft-dependent manner includes all homologs of the S. cerevisiae genes encoding iron transporters at the plasma membrane and the ortholog of the post-transcription iron regulators CTH1 and CTH2. In contrast, there was no difference or little difference (<2-fold change) in mRNA abundance between the wild-type strain and the KlaftΔ mutant or between iron-depleted and iron-replete conditions for 8 genes. This group of genes included all the orthologs of genes involved in the vacuolar or mitochondrial iron transport (SMF3, FET5, FTH1, and MRS4), orthologs of ATX1 and FIT1, and two homologs of the FRE metalloreductase-encoding genes. These results indicated that KlAft activates the transcription of homologs of genes specifically involved in cell surface iron transport under iron limitation, but not the transcription of genes encoding intracellular iron transporters.
TABLE 1.
Putative K. lactis orthologs or homologs of the S. cerevisiae Aft1/Aft2 iron-regulated genes
| S. cerevisiae gene | ORF | Function | K. lactis gene | % identity/% similarity |
|---|---|---|---|---|
| FET3 | YMR058W | Multicopper oxidase | KLLA0F26400gS | 66/82 |
| FTR1 | YER145C | High-affinity iron permease | KLLA0A03025g | 68/85 |
| CCC2 | YDR270W | Copper transport into vesicles | KLLA0F07447g | 52/71 |
| ATX1 | YNL259C | Copper chaperone | KLLA0C02673gS | 71/82 |
| FIT1 | YDR534C | Cell wall proteins | KLLA0C04994g | 36/56 |
| FIT2 | YOR382W | KLLA0C05016g | 57/71 | |
| FIT3 | YOR383C | KLLA0A04323g | 53/72 | |
| FET4 | YMR319C | Low-affinity iron permease | KLLA0E14564g | 55/70 |
| CTH1 | YDR151C | mRNA degradation | KLLA0D16610gS | 43/59 |
| CTH2 | YLR136C | 49/64 | ||
| HMX1 | YLR205C | Heme oxygenase | KLLA0D12474gS | 61/78 |
| FET5 | YFL041W | Vacuolar multicopper oxidase | KLLA0D05489gS | 60/77 |
| FTH1 | YBR207W | Vacuolar permease | KLLA0F28039g | 66/79 |
| SMF3a | YLR034C | Vacuolar iron transporter | KLLA0F17391gS | 73/85 |
| MRS3 | YJL133W | Mitochondrial iron transport | KLLA0E15532gS | 71/85 |
| MRS4a | YKR052C | 71/86 | ||
| ARN1 | YHL040C | Siderophore transporters | KLLA0A10439g | 51–64/71–80 |
| ARN2/TAF1 | YHL047C | KLLA0E14652g | ||
| ARN3/SIT1 | YEL065W | KLLA0C19272g | ||
| ARN4/ENB1 | YOL158C | KLLA0C00220g | ||
| FRE1 | YLR214W | Metalloreductases | KLLA0F04950g | 28–44/51–63 |
| FRE2 | YKL220C | KLLA0E14542g | ||
| FRE3 | YOR381W | KLLA0E05852g | ||
| FRE4 | YNR060W | KLLA0F00616g | ||
| FRE5 | YOR384W | KLLA0C04906g | ||
| FRE6 | YLL051C |
The first three columns contain the S. cerevisiae gene names, ORF names, and the associated protein functions according to the S. cerevisiae Genome Database. When possible, for each of the S. cerevisiae genes, a putative ortholog in the K. lactis genome is inferred (see materials and methods and File S1). If the putative ortholog is syntenic with the S. cerevisiae gene, there is a superscript s at the end of the K. lactis gene name. For each S. cerevisiae/K. lactis gene pair, the identity and similarity between the two genes are indicated. They were computed with the high scoring pairs of BLASTP, gaps excluded. Paralog pairs are indicated one above the other and underlined.
Aft2-specific target genes (Courel et al. 2005).
Figure 3.—
KlAft- and iron-dependent expression of K. lactis homologs of S. cerevisiae Aft1/Aft2 iron-regulated genes. For the KlAFT/KlaftΔ comparison, the wild-type and KlaftΔ mutant cultures were grown in Bio101 minus iron and copper. For the KlAFT (−Fe/+Fe) comparison, wild-type cultures were grown in Bio101 minus iron and copper with (+Fe) or without (−Fe) ferric ammonium sulfate (100 μm). Expression of K. lactis genes was assessed by quantitative real-time PCR. The values shown are the KlAFT/KlaftΔ and the KlAFT (−Fe/+Fe) ratios calculated as the means of two independent experiments, each performed in duplicate. Standard deviations were <10%. (A) The first column contains the corresponding S. cerevisiae homolog gene or family name and the associated protein function. The second column contains the ORF name for the K. lactis genes analyzed. The group of genes for which the mRNA is at least twice as abundant in the wild type as in the KlaftΔ mutant is underlined with a dashed line. (B) −Fe/+Fe in the wild-type strain (y-axis on a logarithmic scale) is plotted against KlAFT/KlaftΔ (x-axis on a logarithmic scale). The transcription profiles obtained for the two comparisons were correlated with Spearman's rho coefficient = 0.83 and P-value <0.0001.
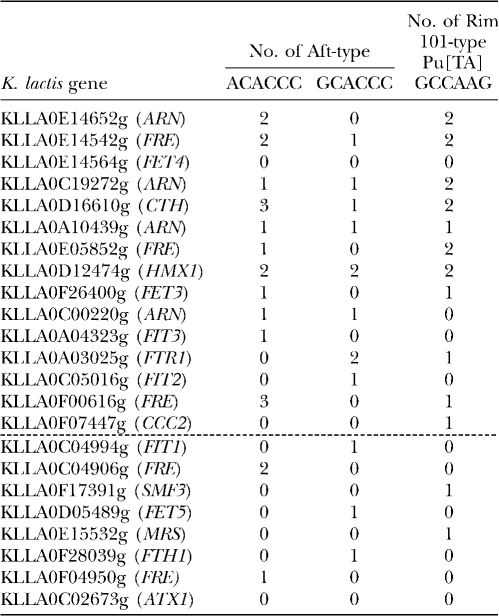
Sequence analyses were performed to identify potential regulatory sequences between the start codon and 800 bp upstream from the 23 analyzed genes. Two consensus sequences, PuCACCC and Pu[TA]GCCAAG, were identified independently by different approaches using the MEME (Bailey and Elkan 1994) and the RSAT (van Helden et al. 1998) programs. The PuCACCC sequence corresponds to the core Aft1/Aft2 DNA-binding sequence (Yamaguchi-Iwai et al. 1996; Rutherford et al. 2003; Courel et al. 2005). The Pu[TA]GCCAAG sequence was predicted by the MYBS program (Tsai et al. 2007) to be a DNA-binding site for PacC/Rim101, a C2H2 Zn finger transcription factor involved in the alkaline pH response in fungi (Penalva and Arst 2004; Penalva et al. 2008). Of the 23 genes that were analyzed, 18 contained at least one copy of the PuCACCC sequence and 13 contained at least one copy of the Pu[TA]GCCAAG sequence in either orientation (Table 2 and Table S2). Thus, PuCACCC was present in 78% and Pu[TA]GCCAAG was present in 57% of the 5′ regions upstream from the analyzed genes; in comparison, these sequences are found in 22.5 and 9.8%, respectively, of the 5′ regions upstream from all open reading frames in the K. lactis genome. We then examined the PuCACCC and Pu[TA]GCCAAG sequences in the 5′ regions upstream from the 23 analyzed genes and the effect of KlAft on their transcription. The frequency of the PuCACCC Aft-type sequence among the 15 genes exhibiting higher mRNAs levels (at least twofold) in the wild-type than in the KlaftΔ mutant strain was 2.5 times higher (P-value <0.05; chi-square test) than that among the 8 other genes. The ACACCC sequence was 1.8 times more frequent than the GCACCC sequence (Table 2). The putative PacC/Rim101 DNA-binding sequence Pu[TA]GCCAAG was 4.8 times more frequent (P-value <0.02; chi-square test) among the 15 KlAft-regulated genes than among the 8 other genes (Table 2). These results suggest that the PuCACCC and/or Pu[TA]GCCAAG sequences are potential iron-regulatory elements.
TABLE 2.
Aft- and PacC/Rim101-type elements identified in the upstream sequences of the K. lactis orthologs or homologs of S. cerevisiae Aft1/Aft2 iron-regulated genes
Two elements were identified using the MEME element finder in sequences located between the coordinates −1 and −800 upstream from the start of the CDS of the 23 K. lactis genes listed in Table 1. The K. lactis genes are sorted as indicated in Figure 3A and the gene name of the putative S. cerevisiae ortholog or homolog is indicated in parentheses. The group of genes for which the mRNA is at least twice as abundant in the wild type as in the KlaftΔ mutant is underlined with a dashed line. The second and third columns contain the numbers of Aft-type ACACCC and GCACCC sequences found in the 5′-upstream region of each gene. The fourth column contains the number of Pu[TA]GCCAAG PacC/Rim101-type sequences (Table S2).
The ACACCC sequence is an iron-responsive activating sequence in K.lactis:
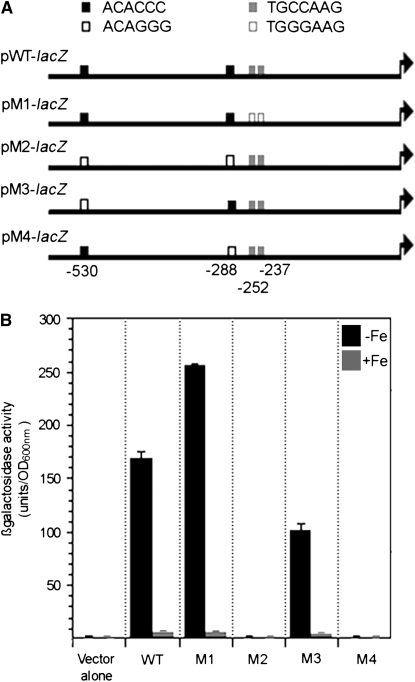
To test this prediction, we performed transcription analysis with a fusion of the 5′-upstream region of KLLA0E14652g with the lacZ reporter gene. KLLA0E14652g is a homolog of the siderophore transporter family of genes. It exhibits the highest sensitivity to iron, its mRNAs being 27-fold more abundant in iron-depleted than in iron-replete conditions (Figure 3A). There are two ACACCC sequences of the Aft type (positions −530 and −288) and two TGCCAAG sequences of the PacC/Rim101 type (positions −252 and −237) within the 800-bp 5′ region of KLLA0E14652g (Table 2 and Table S2). To test if the ACACCC and/or TGCCAAG sequences are involved in the iron-regulated expression of KLLA0E14652g, we constructed plasmids containing KLLA0E14652–lacZ fusions from the wild-type and mutated versions of these sequences (Figure 4A). The plasmids were used to transform the MLK131 strain isogenic to PM6-A and inactivated for the K. lactis LAC4 gene coding for β-galactosidase. β-Galactosidase activity was 30-fold higher with pWT-lacZ in iron-depleted than in iron-replete conditions, indicating that it retains fully iron-regulated expression (Figure 4B). In pM1-lacZ the two PacC/Rim101-type TGCCAAG sequences were changed to TGGGAAG sequences; β-galactosidase activity was strongly induced from this plasmid in iron-depleted conditions. Interestingly, the mutations introduced conferred a slight increase of β-galactosidase activity (1.5-fold) under iron-limitation conditions (Figure 4B). Unlike with pM1-lacZ, no β-galactosidase activity was detected with pM2-lacZ in which the two Aft-type ACACCC sequences were changed to the sequence ACAGGG. These results indicate that ACACCC but not TGCCAAG sequences are required for activation of KLLA0E14652g transcription in our experimental conditions. To study the role of the −530 ACACCC and −288 ACACCC sequences, each was individually changed to the sequence ACAGGG, yielding the plasmids pM3-lacZ and pM4-lacZ, respectively (Figure 4A): under iron-limitation conditions pM4-lacZ expressed no β-galactosidase activity whereas expression from pM3-lacZ was only 1.7-fold lower (Figure 4B). These results indicate that the −288 ACACCC sequence is required for activation of KLLA0E14652g transcription in iron-depleted conditions. The decreased β-galactosidase activity observed with a mutated version of the −530 ACACCC sequence suggests that this sequence may amplify the induction mediated by the −288 ACACCC sequence. The identification of ACACCC as an iron-responsive activating sequence and the conservation of the N-terminal DNA-binding domain in KlAft and Aft1/Aft2 strongly suggest that KlAft binds to the PuCACCC sequence found as a consensus in the 5′ region upstream from KlAft and iron-regulated genes.
Figure 4.—
Mutational analysis of the KLLA0E14652g promoter. (A) pKLLA0E14652g-WT-lacZ contains the upstream region of the KLLA0E14652g gene (from −585 to +5 with respect to the start codon) inserted into the promoterless lacZ operon of pXW3 (Chen et al. 1992). pKLLA0E14652g-M1-lacZ is identical to pKLLA0E14652g-WT-lacZ, except that the dinucleotide CC in the TGCCAAG PacC/Rim101-type sequences at positions −249/−248 and −234/−233 (solid gray boxes) was replaced by the dinucleotide GG (open gray boxes). pKLLA0E14652g-M2-lacZ is identical to pKLLA0E14652g-WT-lacZ, except that the trinucleotide CCC in the ACACCC Aft-type sequences at positions −527/−525 and −285/−283 (solid black boxes) was replaced by the trinucleotide GGG (open black boxes). pKLLA0E14652g-M3-lacZ and pKLLA0E14652g-M4-lacZ contain only one CCC to GGG substitution in the Aft-type sequences at positions −527/−525 and −285/−283, respectively. (B) The MLK131 lac4Δ mutant harboring pKLLA0E14652g-WT-lacZ, pKLLA0E14652g-M1-lacZ, pKLLA0E14652g-M2-lacZ, pKLLA0E14652g-M3-lacZ, and pKLLA0E14652g-M4-lacZ (WT, M1, M2, M3, and M4, respectively) was grown exponentially in iron-depleted (−Fe) or iron-replete (+Fe) medium (see materials and methods). Errors bars represent the standard deviations (<10%) for assays performed with at least three independent transformants.
KlAft binds to the PuCACCC Aft-type sequence:
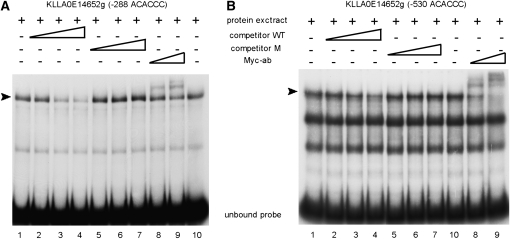
We performed electrophoretic mobility shift assays with probes corresponding to promoter regions of KlAft-regulated genes and extracts from KlaftΔ cells expressing the KlAft-13Myc fusion protein, which was shown to be fully functional (Figure S1). First, we used a probe with the sequence of nucleotides −382 to −251 of KLLA0E14652g and containing the −288 ACACCC sequence required for its transcription (see above). Mobility shift assays showed a prominent complex of high mobility and a second complex of lower electrophoretic mobility (Figure 5A). To test the specificity of the complex formation, we performed competition assays with addition of unlabeled oligomers centered on wild-type ACACCC (WT) or mutated ACAGGG (M) sequences. The high-mobility complex was specifically displaced by the addition of excess wild-type but not mutated oligomers. Therefore, the high-mobility complex was formed by specific interactions with the ACACCC sequence. To determine whether KlAft-13Myc is present in the complexes formed, we performed assays with addition of anti-Myc antibodies (Figure 5A). Addition of anti-Myc antibodies overshifted the higher mobility complex, in a dose-dependent manner, but had no effect on the lower-mobility complex. This demonstrates that KlAft-13Myc is a component of the higher-mobility complex formed with the −288 ACACCC sequence. Next, electrophoretic mobility shift assays were performed with a probe corresponding to nucleotides −570 to −429 of KLLA0E14652g and containing the −530 ACACCC sequence involved in but not required for activation of transcription under conditions of iron limitation (Figure 4). Mobility shift assays showed three mobility complexes with high-intensity signals (Figure 5B). The highest-mobility complex was the only complex to be specifically displaced by the addition of excess wild-type ACACCC oligomers. Addition of anti-Myc antibodies overshifted the higher-mobility complex, indicating that it contained KlAft-13Myc. This shows that KlAft binds to the −530 ACACCC sequence of the KLLA0E14652g promoter. These results indicate that KlAft is able to bind to both ACACCC sequences identified in the KLLA0E14652g promoter.
Figure 5.—
DNA binding of KlAft to the −288 ACACCC (A) and −530 ACACCC (B) sequences of the KLLA0E14652g promoter. Gel-shift assays were carried out with extracts from KlaftΔ (MLK53) cells expressing the KlAft-13Myc fusion protein. The radiolabeled probes used to perform the gel-shift assays correspond to the sequences of positions −382 to −251 and −570 to −429 of KLLA0E14652g (A and B, respectively). The arrowhead indicates the KlAft-containing complexes. Competitive assays were performed with excess oligonucleotide centered on the wild-type (competitor WT) ACACCC sequence (lanes 2–4) and mutant (competitor M) ACAGGG sequence (lanes 5–7). Where indicated, 1 and 2 μl of a monoclonal antibody raised against the Myc epitope were added (lanes 8 and 9, respectively).
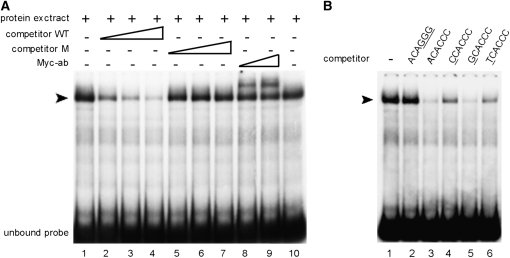
The Aft-type consensus identified in the KlAft-regulated promoters is the PuCACCC element including the ACACCC and GCACCC sequences (Table 2 and Table S2). This suggests that KlAft–DNA binding activity exhibits a preference for a purine rather than a pyrimidine nucleotide upstream from the CACCC core sequence. To test this prediction and to characterize the DNA binding specificity of KlAft, we performed further DNA binding experiments with a facilitating promoter region. We chose the 5′ region of KLLA0E26400g, the K. lactis ortholog of the canonical Aft1-regulated FET3 gene. KLLA0E26400g is clearly regulated by KlAft and iron (Figure 3A) and contains one single copy of the Aft-type consensus element: the −571 ACACCC sequence (Table 2). First, we demonstrated that KlAft binds to the −571 ACACCC sequence by using a probe corresponding to nucleotides −660 to −502 of KLLA0E26400g. A prominent complex of high mobility was specifically displaced by the addition of excess wild-type oligomers (Figure 6A). Moreover, addition of anti-Myc antibodies resulted in a dose-dependent supershift of the high-mobility complex, indicating the presence of KlAft in the complex formed with the −571 ACACCC sequence. Then, we performed competition assays with unlabeled oligomers differing by one nucleotide (A, C, G, or T) at position +1 of the −571 ACACCC sequence. Addition of oligonucleotides with either A or G decreased the complex formation more strongly than those with either C or T (Figure 6B). This demonstrates, in perfect agreement with bioinformatic analysis, that KlAft binds preferentially to a purine rather than a pyrimidine nucleotide upstream from the core CACCC sequence.
Figure 6.—
DNA binding of KlAft to the −571 ACACCC sequence of the KLLA0E26400g promoter (A) and effect of the +1 nucleotide of the ACACCC sequence on the formation of the KlAft-DNA complex (B). Gel-shift assays were carried out with extracts from KlaftΔ (MLK53) cells expressing the KlAft-13Myc fusion protein. The radiolabeled probes used to perform the gel-shift assays correspond to the sequences of positions −660 to −502 of KLLA0E26400g. The arrowhead indicates the KlAft-containing complexes. (A) Competitive assays were performed with oligonucleotides centered on the wild-type (competitor WT) ACACCC sequence (lanes 2–4) and mutant (competitor M) ACAGGG sequence (lanes 5–7). Where indicated, 1 and 2 μl of a monoclonal antibody raised against the Myc epitope were added (lanes 8 and 9, respectively). (B) Competition assay with various unlabeled oligonucleotides centered on the wild-type −571 ACACCC. Nucleotides that deviate from the KLLA0E14652g promoter sequence are underlined.
DISCUSSION
In this study, we show that KlAft, the ortholog of Aft1/Aft2, mediates iron homeostasis regulation in K. lactis: (1) deletion of the KlAFT gene abolished the ability of cells to grow under iron-limitation conditions; and (2) most homologs of Aft1-regulated genes were upregulated in iron-depleted conditions and markedly downregulated in the KlaftΔ mutant cells.
In addition, complementation experiments indicated that the iron-dependent growth deficiency of the KlaftΔ mutant is totally suppressed by AFT1. Interestingly, the reciprocal complementation experiment showed that KlAFT is not able to reverse totally the growth deficiency phenotype of the S. cerevisiae aft1Δaft2Δ mutant. Several nonexclusive hypotheses may explain this partial complementation by KlAFT. This may be due to some defaults in expression/stability/localization of the KlAft protein in S. cerevisiae. Alternatively, this may reflect some differences in the mechanisms of transcriptional activation of KlAft and Aft1. Indeed, these mechanisms could have evolved since K. lactis and S. cerevisiae diverged from their common ancestor.
The genes encoding homologs of the S. cerevisiae reductive and nonreductive high-affinity iron transporters at the plasma membrane (FET3, FTR1, and ARN1-4) are strongly regulated by both KlAft and iron (by at least 4.6- and 3.5-fold, respectively). All of them contain between one and three copies of the PuCACCC Aft-type element in their 5′-upstream region. Moreover, gel shift experiments demonstrate that KlAft binds to the Aft-type elements in the promoter regions of KLLA0E14652g and KLLA0E26400g that belong to this group of genes. These results indicate that KlAft regulates directly the homologs of high-affinity iron-transporter genes in K. lactis. In contrast, KLLA0E14564g, the ortholog of the FET4 gene that encodes a low-affinity iron and zinc transporter, is clearly regulated by KlAft (by 13-fold) but weakly so by iron (by 2-fold). Moreover, its promoter does not contain any copies of the PuCACCC sequence. These data suggest that KLLA0E14564g is not a direct target gene of KlAft and that it may be regulated by other transcription factors associated with the KlAft-mediated pathway. In S. cerevisiae, FET4 is controlled by zinc- and oxygen-responsive regulators in addition to Aft1 (Jensen and Culotta 2002; Waters and Eide 2002). Similarly, in K. lactis, KLLA0E14564g might be under the control of numerous environmental regulatory pathways that might be disturbed by KlAFT deletion.
Previous computer analyses identified the TGCACCC sequence as a consensus in the promoters of the Aft1-regulated genes (Rutherford et al. 2003; Courel et al. 2005). DNA-binding experiments and transcription analyses with promoter variants of FET3 confirmed that Aft1 is specific for the TGCACCC sequence (Yamaguchi-Iwai et al. 1996; Courel et al. 2005). Here in K. lactis, we identify the more simple PuCACCC sequence as a consensus in KlAft-regulated promoters. Only 4 of 28 sequences identified (14%) conformed entirely to the TGCACCC sequence; the other sequences differ by one or two nucleotides at the 5′ end of the TGCACCC sequence (Table S2). Moreover, electrophoretic mobility shift assays confirm that KlAft does not exhibit preferential DNA binding to the TGCACCC sequence: KlAft is able to bind to the GACACCC and CACACCC sequences of the KLLA0E14652g promoter and to the TACACCC sequence of KLLA0E26400g promoter. These bioinformatic and experimental analyses reveal that homolog genes are controlled by KlAft and Aft1 through similar but distinct iron-regulatory sequences. Interestingly, Aft2 has the same PuCACCC DNA binding sequence as KlAft (Courel et al. 2005). The presence of the PuCACCC sequence in both the K. lactis and the S. cerevisiae lineages suggests that it corresponds to an ancestral Aft-type element. This presumably subsequently evolved toward the more precise TGCACCC sequence in the case of the Aft1-regulatory function.
In addition to the PuCACCC sequence, computer analysis identified the sequence Pu[TA]GCCAAG as another consensus in the promoters of K. lactis iron-regulated genes. This sequence contains the core DNA binding site GCCAAG of the PacC/Rim101 pH-responsive transcription factor conserved in fungi (Penalva and Arst 2004; Penalva et al. 2008). PacC/Rim101 has been shown to be required in alkaline environments for the activation of genes involved in iron homeostasis (Lamb et al. 2001; Bensen et al. 2004; Eisendle et al. 2004). In A. nidulans and C. albicans, PacC/Rim101 directly activates the alkaline pH-induced genes whereas in S. cerevisiae Rim101 acts in an indirect manner through repression of the transcription repressor Nrg1 (Penalva and Arst 2004). Here, in K. lactis, the presence of the GCCAAG sequence in the 5′ region of most iron-regulated genes and its absence from that of KLLA0F18524g, the K. lactis ortholog of NRG1 (data not shown), suggests that the ortholog of Rim101 (Bussereau et al. 2006) may directly drive the expression of most iron-regulated genes. Further investigations are needed to confirm this prediction.
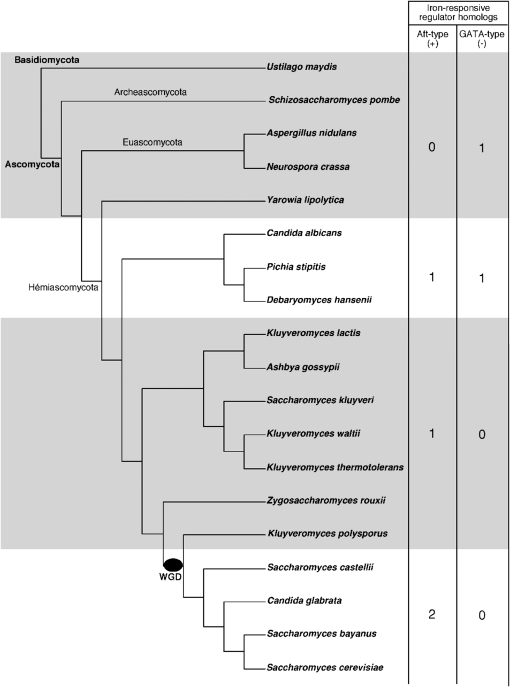
In most fungi, the iron-regulatory pathway is under the control of a conserved Zn-finger GATA-type transcription repressor (Haas et al. 2008). Because it is widespread in fungi, this negative mode of iron-dependent regulation of transcription may be an ancestral mechanism of regulation. In the yeast K. lactis that diverged before the WGD, we did not find any ortholog of the iron-responsive GATA-binding transcription repressor (see materials and methods and Figure 7). On the contrary, we show that, in K. lactis, iron homeostasis is regulated by KlAft, a transcription activator ortholog of the S. cerevisiae Aft1/Aft2 iron-responsive activators. This indicates that the Aft-type iron-regulatory function was acquired before the WGD event. We identified also an ortholog of Aft1/Aft2 in all pre-WGD hemiascomycetes analyzed, except in the species Y. lipolytica (Figure 7). In contrast, and as described previously (Haas et al. 2008), we could not identify any ortholog of Aft1/2 in fungi other than hemiascomycetes. Thus, these results indicate that a transition from negative to positive regulation occurred in the hemiascomycete lineage before the WGD. To date, only the negative or the positive regulatory mechanism, but not both, has been identified in any one species, raising the question of the existence of species with both regulatory systems. Interestingly, the C. albicans genome contains an ortholog of AFT1/AFT2 in addition to the SFU1 gene encoding the iron repressor (Haas 2003; Lan et al. 2004). We also identified an ortholog of AFT1/AFT2 in D. hansenii and P. stipitis species belonging to the same clade as C. albicans (Figure 7). Characterization of one of these AFT1/AFT2 orthologous genes as an iron-responsive transcription activator would be valuable to improve our understanding of the iron-regulatory mechanisms in fungi.
Figure 7.—
Evolution of iron-responsive transcription regulators in ascomycota. The species tree of ascomycota with the basidiomycota Ustilago maydis as the outgroup was adapted from Fitzpatrick et al. (2006) and Souciet et al. (2009). The S. castellii location in the tree (outgroup to the clade containing C. glabrata and S.cerevisiae) is supported by shared rearrangement data (Gordon et al. 2009). The whole-genome duplication is indicated with a black oval. The numbers in the table correspond to the number of homologs of iron-responsive transcription regulator-encoding genes per genome. The Aft-type proteins were identified by similarity with the Pfam transcription factor Aft domain (PF08731) and the GATA-type proteins were identified with the cysteine-rich domain of the Zn-finger GATA-type repressors (see materials and methods and File S2 and File S3).
S. cerevisiae, a yeast that underwent whole-genome duplication, has two well-studied Aft-type iron-responsive transcriptional activator genes: AFT1 and AFT2. They correspond to a duplicated gene pair that was created by the WGD. All post-WGD yeasts analyzed also have two members of the Aft family, except K. polysporus, the most divergent from S. cerevisiae (Figure 7). We showed in a previous study that Aft1 and Aft2 display functional specialization in the control of iron homeostasis in S. cerevisiae. Aft1 specifically activates the transcription of genes involved in cell-surface uptake systems, whereas Aft2 but not Aft1 directly activates the transcription of genes involved in vacuolar and mitochondrial iron transport, such as SMF3 and MRS4 (Courel et al. 2005). The results of transcription analysis reported herein indicate that KlAft activates the transcription of most homologs of the Aft1-target genes but not those of specifically Aft2-target genes encoding intracellular iron transporters. Additionally, SMF3 and MRS4 orthologs did not have the PuCACCC sequence in their promoter regions. These results suggest that KlAft and Aft1 kept the ancestral function of the Aft-type regulator family, which was the regulation of genes specifically involved in cell surface iron transport. Thus, and in line with the theory proposed by Ohno (1970), it is tempting to speculate that the WGD event, by creating two copies of the AFT1/AFT2 genes, favored the emergence of a new iron-regulatory function in the Aft-type regulator family. This new function, encoded by AFT2, appears to be specialized in the iron-dependent transcriptional control of genes involved in vacuolar and mitochondrial transport.
Acknowledgments
We thank Eduardo Rocha, Ingrid Lafontaine, Guillaume Achaz, Pierre Netter, and Micheline Wésolowski-Louvel for generous help and suggestions. This work was supported by a grant from the Groupement des Entreprises Françaises dans la Lutte contre le Cancer.
Supporting information is available online at http://www.genetics.org/cgi/content/full/genetics.109.104364/DC1.
References
- Altschul, S. F., T. L. Madden, A. A. Schaffer, J. Zhang, Z. Zhang et al., 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babu, M. M., L. M. Iyer, S. Balaji and L. Aravind, 2006. The natural history of the WRKY-GCM1 zinc fingers and the relationship between transcription factors and transposons. Nucleic Acids Res. 34: 6505–6520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey, T. L., and C. Elkan, 1994. Fitting a mixture model by expectation maximization to discover motifs in biopolymers. Proc. Int. Conf. Intell. Syst. Mol. Biol. 2: 28–36. [PubMed] [Google Scholar]
- Bailey, T. L., and M. Gribskov, 1998. Combining evidence using p-values: application to sequence homology searches. Bioinformatics 14: 48–54. [DOI] [PubMed] [Google Scholar]
- Bensen, E. S., S. J. Martin, M. Li, J. Berman and D. A. Davis, 2004. Transcriptional profiling in Candida albicans reveals new adaptive responses to extracellular pH and functions for Rim101p. Mol. Microbiol. 54: 1335–1351. [DOI] [PubMed] [Google Scholar]
- Blaiseau, P. L., E. Lesuisse and J. M. Camadro, 2001. Aft2p, a novel iron-regulated transcription activator that modulates, with Aft1p, intracellular iron use and resistance to oxidative stress in yeast. J. Biol. Chem. 276: 34221–34226. [DOI] [PubMed] [Google Scholar]
- Bussereau, F., S. Casaregola, J. F. Lafay and M. Bolotin-Fukuhara, 2006. The Kluyveromyces lactis repertoire of transcriptional regulators. FEMS Yeast Res. 6: 325–335. [DOI] [PubMed] [Google Scholar]
- Byrne, K. P., and K. H. Wolfe, 2005. The Yeast Gene Order Browser: combining curated homology and syntenic context reveals gene fate in polyploid species. Genome Res. 15: 1456–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne, K. P., and K. H. Wolfe, 2006. Visualizing syntenic relationships among the hemiascomycetes with the Yeast Gene Order Browser. Nucleic Acids Res. 34: D452–D455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, X. J., 1996. Low- and high-copy-number shuttle vectors for replication in the budding yeast Kluyveromyces lactis. Gene 172: 131–136. [DOI] [PubMed] [Google Scholar]
- Chen, X. J., M. Wesolowski-Louvel and H. Fukuhara, 1992. Glucose transport in the yeast Kluyveromyces lactis. II. Transcriptional regulation of the glucose transporter gene RAG1. Mol. Gen. Genet. 233: 97–105. [DOI] [PubMed] [Google Scholar]
- Courel, M., S. Lallet, J. M. Camadro and P. L. Blaiseau, 2005. Direct activation of genes involved in intracellular iron use by the yeast iron-responsive transcription factor Aft2 without its paralog Aft1. Mol. Cell. Biol. 25: 6760–6771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Castro, E., C. J. Sigrist, A. Gattiker, V. Bulliard, P. S. Langendijk-Genevaux et al., 2006. ScanProsite: detection of PROSITE signature matches and ProRule-associated functional and structural residues in proteins. Nucleic Acids Res. 34: W362–W365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisendle, M., H. Oberegger, R. Buttinger, P. Illmer and H. Haas, 2004. Biosynthesis and uptake of siderophores is controlled by the PacC-mediated ambient-pH regulatory system in Aspergillus nidulans. Eukaryot. Cell 3: 561–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finn, R. D., J. Tate, J. Mistry, P. C. Coggill, S. J. Sammut et al., 2008. The Pfam protein families database. Nucleic Acids Res. 36: D281–D288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick, D. A., M. E. Logue, J. E. Stajich and G. Butler, 2006. A fungal phylogeny based on 42 complete genomes derived from supertree and combined gene analysis. BMC Evol. Biol. 6: 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galtier, N., M. Gouy and C. Gautier, 1996. SEAVIEW and PHYLO_WIN: two graphic tools for sequence alignment and molecular phylogeny. Comput. Appl. Biosci. 12: 543–548. [DOI] [PubMed] [Google Scholar]
- Gordon, J. L., K. P. Byrne and K. H. Wolfe, 2009. Additions, losses, and rearrangements on the evolutionary route from a reconstructed ancestor to the modern Saccharomyces cerevisiae genome. PLoS Genet. 5: e1000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guarente, L., 1983. Yeast promoters and lacZ fusions designed to study expression of cloned genes in yeast. Methods Enzymol. 101: 181–191. [DOI] [PubMed] [Google Scholar]
- Haas, H., 2003. Molecular genetics of fungal siderophore biosynthesis and uptake: the role of siderophores in iron uptake and storage. Appl. Microbiol. Biotechnol. 62: 316–330. [DOI] [PubMed] [Google Scholar]
- Haas, H., M. Eisendle and B. G. Turgeon, 2008. Siderophores in fungal physiology and virulence. Annu. Rev. Phytopathol. 46: 149–187. [DOI] [PubMed] [Google Scholar]
- Halliwell, B., and J. M. Gutteridge, 1984. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem. J. 219: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze, M. W., M. U. Muckenthaler and N. C. Andrews, 2004. Balancing acts: molecular control of mammalian iron metabolism. Cell 117: 285–297. [DOI] [PubMed] [Google Scholar]
- Jensen, L. T., and V. C. Culotta, 2002. Regulation of Saccharomyces cerevisiae FET4 by oxygen and iron. J. Mol. Biol. 318: 251–260. [DOI] [PubMed] [Google Scholar]
- Kellis, M., B. W. Birren and E. S. Lander, 2004. Proof and evolutionary analysis of ancient genome duplication in the yeast Saccharomyces cerevisiae. Nature 428: 617–624. [DOI] [PubMed] [Google Scholar]
- Kosman, D. J., 2003. Molecular mechanisms of iron uptake in fungi. Mol. Microbiol. 47: 1185–1197. [DOI] [PubMed] [Google Scholar]
- Kuras, L., H. Cherest, Y. Surdin-Kerjan and D. Thomas, 1996. A heteromeric complex containing the centromere binding factor 1 and two basic leucine zipper factors, Met4 and Met28, mediates the transcription activation of yeast sulfur metabolism. EMBO J. 15: 2519–2529. [PMC free article] [PubMed] [Google Scholar]
- Lamb, T. M., W. Xu, A. Diamond and A. P. Mitchell, 2001. Alkaline response genes of Saccharomyces cerevisiae and their relationship to the RIM101 pathway. J. Biol. Chem. 276: 1850–1856. [DOI] [PubMed] [Google Scholar]
- Lan, C. Y., G. Rodarte, L. A. Murillo, T. Jones, R. W. Davis et al., 2004. Regulatory networks affected by iron availability in Candida albicans. Mol. Microbiol. 53: 1451–1469. [DOI] [PubMed] [Google Scholar]
- Larkin, M. A., G. Blackshields, N. P. Brown, R. Chenna, P. A. McGettigan et al., 2007. Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948. [DOI] [PubMed] [Google Scholar]
- Longtine, M. S., A. McKenzie, 3rd, D. J. Demarini, N. G. Shah, A. Wach et al., 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast 14: 953–961. [DOI] [PubMed] [Google Scholar]
- Marchler-Bauer, A., J. B. Anderson, F. Chitsaz, M. K. Derbyshire, C. DeWeese-Scott et al., 2009. CDD: specific functional annotation with the Conserved Domain Database. Nucleic Acids Res. 37: D205–D210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merico, A., P. Sulo, J. Piskur and C. Compagno, 2007. Fermentative lifestyle in yeasts belonging to the Saccharomyces complex. FEBS J. 274: 976–989. [DOI] [PubMed] [Google Scholar]
- Miele, R., D. Barra and M. C. Bonaccorsi di Patti, 2007. A GATA-type transcription factor regulates expression of the high-affinity iron uptake system in the methylotrophic yeast Pichia pastoris. Arch. Biochem. Biophys. 465: 172–179. [DOI] [PubMed] [Google Scholar]
- Neil, H., M. Hnatova, M. Wesolowski-Louvel, A. Rycovska and M. Lemaire, 2007. Sck1 activator coordinates glucose transport and glycolysis and is controlled by Rag8 casein kinase I in Kluyveromyces lactis. Mol. Microbiol. 63: 1537–1548. [DOI] [PubMed] [Google Scholar]
- Ohno, S., 1970. Evolution by Gene Duplication. Springer-Verlag, Berlin/Heidelberg/New York.
- Penalva, M. A., and H. N. Arst, Jr., 2004. Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu. Rev. Microbiol. 58: 425–451. [DOI] [PubMed] [Google Scholar]
- Penalva, M. A., J. Tilburn, E. Bignell and H. N. Arst, Jr., 2008. Ambient pH gene regulation in fungi: making connections. Trends Microbiol. 16: 291–300. [DOI] [PubMed] [Google Scholar]
- Philpott, C. C., and O. Protchenko, 2008. Response to iron deprivation in Saccharomyces cerevisiae. Eukaryot. Cell 7: 20–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protchenko, O., and C. C. Philpott, 2003. Regulation of intracellular heme levels by HMX1, a homologue of heme oxygenase, in Saccharomyces cerevisiae. J. Biol. Chem. 278: 36582–36587. [DOI] [PubMed] [Google Scholar]
- Puig, S., E. Askeland and D. J. Thiele, 2005. Coordinated remodeling of cellular metabolism during iron deficiency through targeted mRNA degradation. Cell 120: 99–110. [DOI] [PubMed] [Google Scholar]
- Puig, S., S. V. Vergara and D. J. Thiele, 2008. Cooperation of two mRNA-binding proteins drives metabolic adaptation to iron deficiency. Cell Metab. 7: 555–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford, J. C., S. Jaron, E. Ray, P. O. Brown and D. R. Winge, 2001. A second iron-regulatory system in yeast independent of Aft1p. Proc. Natl. Acad. Sci. USA 98: 14322–14327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford, J. C., S. Jaron and D. R. Winge, 2003. Aft1p and Aft2p mediate iron-responsive gene expression in yeast through related promoter elements. J. Biol. Chem. 278: 27636–27643. [DOI] [PubMed] [Google Scholar]
- Shakoury-Elizeh, M., J. Tiedeman, J. Rashford, T. Ferea, J. Demeter et al., 2004. Transcriptional remodeling in response to iron deprivation in Saccharomyces cerevisiae. Mol. Biol. Cell 15: 1233–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman, D. J., T. Martin, M. Nikolski, C. Cayla, J. L. Souciet et al., 2009. Genolevures: protein families and synteny among complete hemiascomycetous yeast proteomes and genomes. Nucleic Acids Res. 37: D550–D554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souciet, J. L., B. Dujon, C. Gaillardin, M. Johnston, P. V. Baret et al., 2009. Comparative genomics of protoploid Saccharomycetaceae. Genome Res. (in press). [DOI] [PMC free article] [PubMed]
- Tsai, H. K., M. Y. Chou, C. H. Shih, G. T. Huang, T. H. Chang et al., 2007. MYBS: a comprehensive web server for mining transcription factor binding sites in yeast. Nucleic Acids Res. 35: W221–W226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Helden, J., B. Andre and J. Collado-Vides, 1998. Extracting regulatory sites from the upstream region of yeast genes by computational analysis of oligonucleotide frequencies. J. Mol. Biol. 281: 827–842. [DOI] [PubMed] [Google Scholar]
- Voisard, C., J. Wang, J. L. McEvoy, P. Xu and S. A. Leong, 1993. urbs1, a gene regulating siderophore biosynthesis in Ustilago maydis, encodes a protein similar to the erythroid transcription factor GATA-1. Mol. Cell. Biol. 13: 7091–7100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wach, A., 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12: 259–265. [DOI] [PubMed] [Google Scholar]
- Waters, B. M., and D. J. Eide, 2002. Combinatorial control of yeast FET4 gene expression by iron, zinc, and oxygen. J. Biol. Chem. 277: 33749–33757. [DOI] [PubMed] [Google Scholar]
- Wolfe, K. H., and D. C. Shields, 1997. Molecular evidence for an ancient duplication of the entire yeast genome. Nature 387: 708–713. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Iwai, Y., A. Dancis and R. D. Klausner, 1995. AFT1: a mediator of iron regulated transcriptional control in Saccharomyces cerevisiae. EMBO J. 14: 1231–1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai, Y., R. Stearman, A. Dancis and R. D. Klausner, 1996. Iron-regulated DNA binding by the AFT1 protein controls the iron regulon in yeast. EMBO J. 15: 3377–3384. [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi-Iwai, Y., R. Ueta, A. Fukunaka and R. Sasaki, 2002. Subcellular localization of Aft1 transcription factor responds to iron status in Saccharomyces cerevisiae. J. Biol. Chem. 277: 18914–18918. [DOI] [PubMed] [Google Scholar]