Abstract
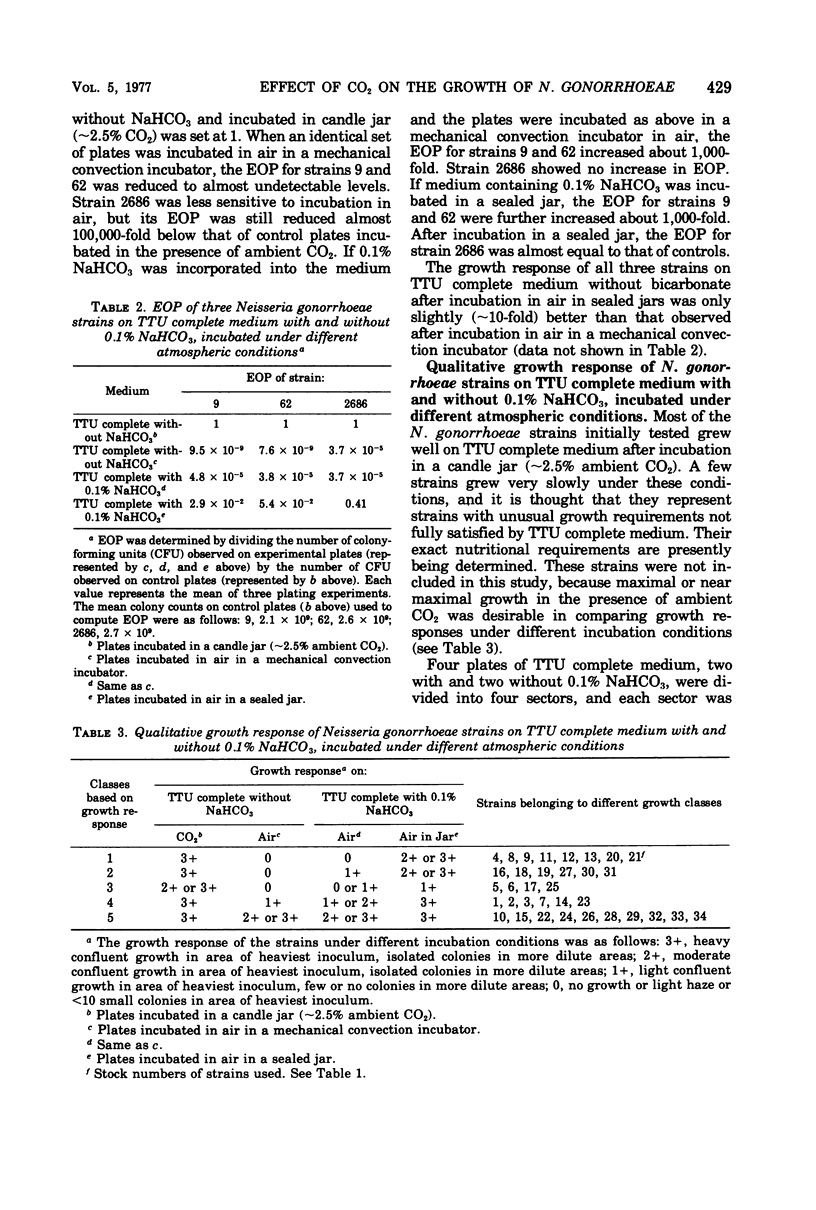
The quantitative and qualitative growth response of Neisseria gonorrhoeae strains was assessed under the following conditions: incubation in a candle jar (approximately 2.5% ambient CO2) on medium without bicarbonate, incubation in air on medium without bicarbonate, incubation in air on medium with bicarbonate, and incubation in air in a sealed jar on medium with bicarbonate. Incubation in the presence of ambient CO2 (candle jar) resulted in the highest plating efficiencies for the three laboratory strains 9, 62, and 2686. The addition of NaHCO3 to the medium enhanced the growth response in air of all three strains, particularly if incubation was carried out in a closed environment (sealed jar). The qualitative growth response of 34 clinical isolates and laboratory strains was assessed under the same conditions of incubation after the plating of an inoculum containing approximately 2 X 10(6) bacteria. The strains were divided into different classes based on their growth responses. About 40% of the strains grew as well on bicarbonate-containing medium incubated in air in sealed jars as they did on medium without bicarbonate incubated in a candle jar. Ten percent of the strains showed only slight growth on bicarbonate containing medium incubated in sealed jars and appeared to have an almost obligate requirement for ambient CO2. Twenty percent of the strains apparently had partially lost their requirement for gaseous C02 and showed slight growth in air on medium without bicarbonate and slight to moderate growth in air on medium containing NaHCO3.The remaining 30% seemed to have completely lost their requirement for gaseous C02 and/or the bicarbonate anion and grew almost as well in air on medium without bicarbonate as they did in either ambient C02 (candle jar) or on medium containing bicarbonate incubated in a sealed jar. These results suggest that N. gonorrhoeae strains may vary widely in their requirements for CO2 and/or the HCO3-anion. Incubation in the presence of ambient CO2 tends to maximize the growth response on solid medium of those strains, which require it for growth. The presence of ambient CO2 is particularly important if growth is to be obtained after the plating of small inocula. Medium conatining 0.1% NaHCO3, if incubated in a closed environment (sealed jar), apperas to be equivalent to medium without bicarbonate incubated in ambient CO2 in supporting the growth of some but not all strains of N. gonorrhoeae.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashworth J. M., Kornberg H. L. The anaplerotic fixation of carbon dioxide by Escherichia coli. Proc R Soc Lond B Biol Sci. 1966 Aug 16;165(999):179–188. doi: 10.1098/rspb.1966.0063. [DOI] [PubMed] [Google Scholar]
- Carifo K., Catlin B. W. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl Microbiol. 1973 Sep;26(3):223–230. doi: 10.1128/am.26.3.223-230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Cooper T. G., Tchen T. T., Wood H. G., Benedict C. R. The carboxylation of phosphoenolpyruvate and pyruvate. I. The active species of "CO2" utilized by phosphoenolpyruvate carboxykinase, carboxytransphosphorylase, and pyruvate carboxylase. J Biol Chem. 1968 Jul 25;243(14):3857–3863. [PubMed] [Google Scholar]
- Faur Y. C., Weisburd M. H., Wilson M. E. A new medium for the isolation of pathogenic Neisseria (NYC nmedium). 3. Performance as a culture and transport medium without addition of ambient carbon dioxide. Health Lab Sci. 1973 Apr;10(2):61–74. [PubMed] [Google Scholar]
- GRIFFIN P. J., RACKER E. The carbon dioxide requirement of Neisseria gonorrhoeae. J Bacteriol. 1956 Jun;71(6):717–721. doi: 10.1128/jb.71.6.717-721.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRIFFIN P. J., RIEDER S. V. A study on the growth requirements of Neisseria gonorrhoeae and its clinical application. Yale J Biol Med. 1957 Jun;29(6):613–621. [PMC free article] [PubMed] [Google Scholar]
- Higa A. I., Milrad de Forchetti S. R., Cazzulo J. J. CO2-fixing enzymes in Pseudomonas fluorescens. J Gen Microbiol. 1976 Mar;93(1):69–74. doi: 10.1099/00221287-93-1-69. [DOI] [PubMed] [Google Scholar]
- James-Holmquest A. N., Wende R. D., Mudd R. L., Williams R. P. Comparison of atmospheric conditions for culture of clinical specimens of Neisseria gonorrhoeae. Appl Microbiol. 1973 Oct;26(4):466–469. doi: 10.1128/am.26.4.466-469.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R. T., Talley R. S. Simplified complete medium for the growth of Neisseria gonorrhoeae. J Clin Microbiol. 1977 Jan;5(1):9–14. doi: 10.1128/jcm.5.1.9-14.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maruyama H., Easterday R. L., Chang H. C., Lane M. D. The enzymatic carboxylation of phosphoenolpyruvate. I. Purification and properties of phosphoenolpyruvate carboxylase. J Biol Chem. 1966 May 25;241(10):2405–2412. [PubMed] [Google Scholar]
- Morris G. K., DeWitt W. E., Gangarosa E. J., McCormack W. M. Enhancement by sodium chloride of the selectivity of thiosulfate citrate bile salts sucrose agar for isolating Vibrio cholerae biotype El Tor. J Clin Microbiol. 1976 Aug;4(2):133–136. doi: 10.1128/jcm.4.2.133-136.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THEODORE T. S., ENGLESBERG E. MUTANT OF SALMONELLA TYPHIMURIUM DEFICIENT IN THE CARBON DIOXIDE-FIXING ENZYME PHOSPHOENOLPYRUVIC CARBOXYLASE. J Bacteriol. 1964 Oct;88:946–955. doi: 10.1128/jb.88.4.946-955.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TUTTLE D. M., SCHERP H. W. Studies on the carbon dioxide requirement of Neisseria meningitidis. J Bacteriol. 1952 Aug;64(2):171–182. doi: 10.1128/jb.64.2.171-182.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley R. S., Baugh C. L. Effects of bicarbonate on growth of Neisseria gonorrhoeae: replacement of gaseous CO2 atmosphere. Appl Microbiol. 1975 Apr;29(4):469–471. doi: 10.1128/am.29.4.469-471.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


