Abstract
Substance P (SP) signaling facilitates nociceptive sensitization in various inflammatory and chronic pain models and we postulated that SP signaling might also contribute to the development of post-incisional hyperalgesia. These studies used mice with a deletion of the pre-protachykinin A gene (ppt-A −/−) which codes for SP to determine the role of SP signaling in post-incisional pain and in the increased cytokine and nerve growth factor (NGF) expression observed in the incised skin. SP deficient ppt-A(−/−) mice displayed reduced mechanical allodynia and heat hyperalgesia compared to the wild type (wt) mice at all post-incision time points, despite similar baseline values (p<0.001). Furthermore, the NK-1 receptor antagonist LY303870 attenuated mechanical allodynia produced by incision in the wt mice (p<0.001). Incision also up-regulated IL-6, TNF-α and KC levels but not IL-1β after 2 hours in the wt mice skin. However, ppt-A(−/−) mice had more skin NGF levels 2 hours post incision. Subcutaneous hind paw SP injection produced acute and transient elevations of IL-1β, IL-6, and KC but modest elevations in TNF-α levels in the wt mice. Systemic LY303870 reversed the SP induced elevations of these cytokines. Hind paw injection of IL-6 and NGF dose dependently produced less mechanical allodynia in the ppt-A(−/−) compared to wt mice. Additionally, SP produced mechanical allodynia in a dose dependent fashion in wt mice. Therefore, SP supports nociceptive sensitization after hind paw incision and potentially participates directly in modulating the intensity of inflammatory response in peri-incisional tissue.
Keywords: Cytokines, Nerve Growth Factor, Substance P, Neurokinin Receptor, Mechanical Allodynia, Heat Hyperalgesia, Skin, Mice
1. Introduction
The pre-protachykinin-A gene (ppt-A) codes for primary afferent neurotransmitters substance P (SP) and neurokinin A (NKA) [11; 20], with the former being better studied. SP is a neuromodulator with a well-described role in pain signaling, possessing the unique feature of only being released upon strong nociceptive stimulation. For instance, spinal cord internalization of the NK-1 receptor, an index of SP release, occurs only to a minor extent in lamina I neurons during threshold-level heat stimulation in normal animals [11]. However, same level of heat stimulation leads to both a greater percentage of NK-1 internalization in lamina I neurons and internalization of receptors in deeper spinal cord laminae after inflammation [1]. Interestingly, SP does not seem to be required for normal heat intensity coding or peak firing of these neurons across a range of temperatures, rather it seems to prolong heat stimulus responses [32]. It is these curious properties which may underlie the normal responses ppt-A (−/−) mice display to low intensity heat, mechanical and chemical stimuli with deficits only seen in paradigms involving intense or prolonged stimulation [11]. Sensitization in chronic pain models like nerve injury (neuropathic) and CFA-induced (chronic inflammatory) are normal in the ppt-A(−/−) mice [11]. This knockout model displays the favorable feature of having intact NK-1 receptor expression, with normal levels of expression of several primary afferent neurotransmitters like calcitonin-gene-related peptide, dynorphin, galanin, neuropeptide Y and somatostatin [11].
Incision represents an acute, relatively intense and persistent nociceptive stimulus. The role of ppt-A gene products signaling in supporting nociceptive sensitization after incision has not been explored. Aside from the neurotransmitter function of SP and NKA in the spinal cord, both ppt-A gene products have significant peripheral actions which might impact their roles in controlling nociceptive sensitization and other characteristics of incisional wounds. For example, SP is an important participant in neurogenic extravasation. Limited neurogenic extravasation is seen in the ppt-A (−/−) mice or after administration of NK-1 antagonists in rats [11; 26; 33]. Neurogenic inflammation mediated by SP has been linked to scar formation and several chronic skin conditions [2]. Additionally, SP has been shown to control the production of cytokines in skin and skin cells after injury or inflammation [20; 38; 39]. Moreover, SP mediated increase in cytokine production in coordination with inflammatory cellular infiltration at the injury site appears to be necessary for adequate wound healing and the ability to resist infection [21; 22; 24; 43] . Many of the cytokines modulated in skin by SP have also been linked to nociceptive sensitization including IL-1β, IL-6 and TNF-α [17–19]. Local and systemic administration of NGF, the cutaneous level of which is also modulated by SP, is also strong nociceptive sensitizer [4; 5]. Limited information concerning NKA suggests it can interact with cytokines in enhancing airway hyperreactivity [49].
Our hypothesis in designing this work was that ppt-A gene product SP enhances incisional mechanical allodynia via two distinct mechanisms. First is that SP directly participates in nociceptive signaling acting as a neurotransmitter during peripheral sensitization following incision. Secondly, that this neurokinin supports pro-nociceptive cytokine production in skin thus indirectly enhancing nociceptive sensitization.
2. Materials and Methods
2.1. Animal use
All experimental protocols were reviewed and approved by Veterans Affairs Palo Alto Healthcare System Institutional Animal Care and Use Committee prior to beginning the work. Male mice 12–14 weeks old of the C57Bl/6J strain obtained from Jackson Laboratories (Bar Harbor, MA) were kept in our facility a minimum of 1 week prior to initiating the experiments. Breeding pairs of ppt-A(+/−) mice congenic in the C57BL/6J background were acquired from Jackson Labs and a breeding colony was established and each mouse was genotyped according to standard procedures. These mice were derived as previously described [11]. All mice were kept under standard conditions with a 12 h light/dark cycle and an ambient temperature of 22±1°C and were allowed food and water ad libitum.
2.2. Hind paw incision
The hind paw incision model was used as modified for mice [47]. We have used this model previously in order to study cytokine levels and analgesic effects following incision [15; 16; 36]. Briefly, mice were anesthetized using isoflurane 2–3% delivered through a nose cone. After sterile preparation with alcohol, a 5mm longitudinal incision was made with a number 11 scalpel on the plantar surface of the right hind paw. This incision was sufficiently deep to divide deep tissues including the plantaris muscle longitudinally. After controlling bleeding, a single 6–0 nylon suture was placed through the midpoint of the wound and antibiotic ointment was applied. Nociceptive testing and tissue harvest took place at time points up to 48 hours after incision.
2.3. Drug administration
For some groups of mice nociceptive mediators or vehicle were injected subcutaneously into the plantar skin (i.pl.) of the hind paws of non-incised mice. For these injections mice were gently restrained. The injection volume was 15 µl administered through a 30 gauge needle which raised a bleb similar to the length of the incisional wounds and approximately 1mm of surrounding tissue. For these experiments, substance P (SP), intrleukin-6 (IL-6) and nerve growth factor (NGF) were obtained from Sigma Chemicals, St. Louis, MO. The selective NK-1 antagonist LY303870 was obtained from Lilly Pharmaceuticals. Cytokines and NGF were prepared in sterile 0.9% PBS which was the vehicle used for control injections. SP and LY303870 were prepared in sterile 0.9% saline.
2.3. Nociceptive testing
Mechanical allodynia was assayed using nylon von Frey filaments according to the “up-down” algorithm described by Chaplan et al. [14] as used previously to detect allodynia in mice after incision [15; 35; 37]. In these experiments, mice were placed on wire mesh platforms in clear cylindrical plastic enclosures 10cm in diameter and 40cm in height. After 15 minutes of acclimation, fibers of sequentially increasing stiffness were applied 1mm lateral to the central wound edge, pressed upward to cause a slight bend in the fiber and left in place 5 sec. Withdrawal of the hind paw from the fiber was scored as a response. When no response was obtained the next stiffest fiber in the series was applied to the same paw; if a response was obtained a less stiff fiber was applied. Testing proceeded in this manner until 4 fibers had been applied after the first one causing a withdrawal response. Estimation of the mechanical withdrawal threshold by data fitting algorithm permitted the use of parametric statistics for analysis [48].
Response latencies to noxious heat stimulation were measured using the method of Hargreaves [29] modified for use with mice [34]. In this assay mice were placed on a temperature controlled glass platform (23.5–24.0 °C) in a plastic enclosure as described above. After 15 minutes of acclimation, a beam of focused light was directed towards the same area of the hind paw as described for the von Frey assay. The time to withdraw the foot from the beam of light was measured. A 15-second cutoff was used to prevent tissue damage. Two measurements were made per animal per test session. For experiments involving extended time course measurements the animals were returned to their cages between nociceptive testing sessions.
2.4. Cytokine analysis
Cytokines and nerve growth factor (NGF) present in the skin surrounding the wounds or injection sites were assessed in a manner similar to that described previously [15; 16; 36]. To obtain skin samples for cytokine quantification animals were first sacrificed by CO2 asphyxiation and an ovular patch of full-thickness skin providing 1–1.5mm margins surrounding the hind paw incisions was collected rapidly. These samples containing approximately 12 mg tissue per paw were placed immediately into ice cold 0.9% NaCl containing a cocktail of protease inhibitors (Complete™, Roche Applied Science, Indianapolis, IN). Approximately 750 µl inhibitor containing saline was used per 25 mg tissue. The samples were homogenized using a Polytron device (Brinkman Instruments Inc., Westbury, NY), then centrifuged for 10 min at 12,000 times gravity at 4°C to remove large particles. Supernatant fractions were kept frozen at −80°C until use. An aliquot was subjected to protein assay (DC Protein Assay, Bio-Rad Laboratories, Hercules, CA) to normalize mediator levels.
For the cytokine assays, custom Bio-Rad (Bio-Rad laboratories, Hercules, CA) Bio-Plex cytokine analysis kits were used in conjunction with the Bio-Plex system array reader according to the manufacturer’s directions as described previously [15]. The specific cytokines were chosen based on our previously reported results and included IL-1β, IL-6, KC and TNF-α [15; 16]. Samples were diluted 1:2 prior to analysis in the buffer supplied, and all samples were run in duplicate for each assay. We demonstrated previously that the dynamic range of sensitivity of this assay was sufficient to measure both baseline and incision-stimulated levels of the chosen cytokines [15]. Standard curves for each of the analyzed substances were included in each run, and sample concentrations were calculated using Bio-Plex Manager software. NGF assays were done with the ChemiKine ELISA kit (Chemicon, Billerica, MA). Sample preparation was identical to that described for the cytokines.
2.5. Statistical analysis
Data from the mechanical allodynia and heat hyperalgesia experiments were analyzed by Repeated Measures one-way analysis of variance (ANOVA) followed by post-hoc Dunnett’s Multiple Comparison Test. Cytokine analysis data were analyzed by a two-way ANOVA followed by post-hoc Bonferroni Multiple Comparison Tests. A value of p<0.05 was taken to be significant. All data are presented as Mean±S.E.M. unless otherwise noted.
3. Results
3.1. Mechanical allodynia and heat hypersensitivity after hind paw incision
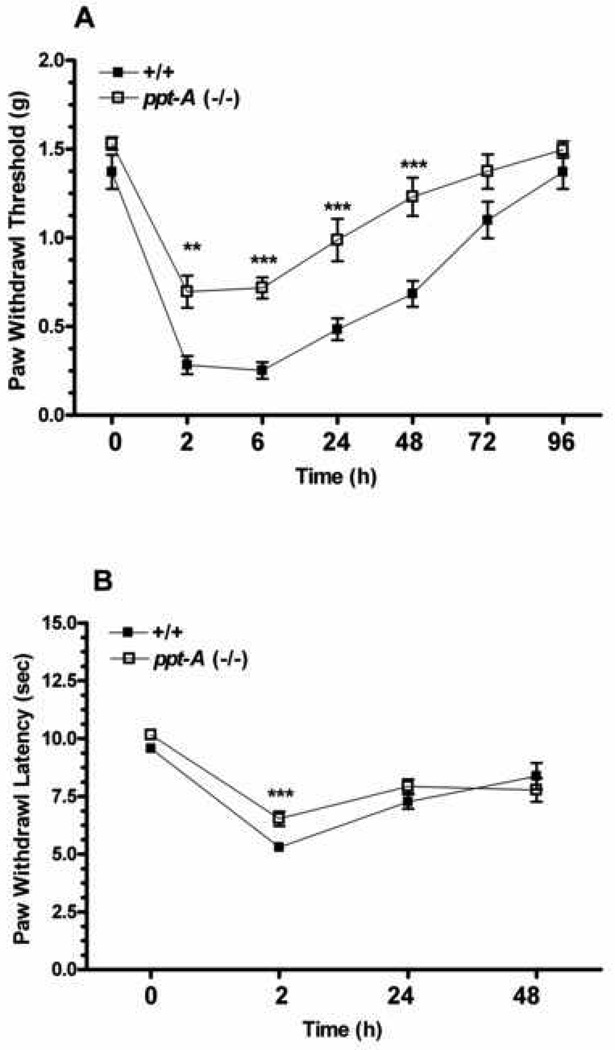
The first step in our analyses was to determine the degree of heat hyperalgesia and mechanical allodynia generated in both wild type ppt-A(+/+) and ppt-A(−/−) mice congenic in the C57BL/6 background after incision. The ppt-A(−/−) mice displayed reduced mechanical allodynia compared to the wild type mice at all time points measured after incision despite having comparable baseline values (F1,98=66.10, p<0.001; Figure 1A). Likewise, the wild type mice developed a more robust heat hyperalgesia after incision compared to the ppt-A(−/−) mice (F1,66=20.43, p<0.001; Figure 1B). However, significant differences between the two groups was observed for the 2 hours post incision time point only, despite the wild type mice having significantly lower paw withdrawal latencies up to 48 hours (compared to baseline values). Therefore, only assessment of mechanical sensitization was carried out for the rest of the experiments.
Figure 1.
Assessment of mechanical allodynia and heat hyperalgesia after hind paw incision. A-Mechanical allodynia was measured in the wild type (n=8) and ppt-A(−/−) (n=8) mice using calibrated von Frey filaments before and at different time points after incision. B- Paw withdrawal latencies to heat stimuli was measured in the wild type (n=12) and ppt-A(−/−) (n=12) mice using the Hargreaves method. Data are presented as Mean ± S.E.M and were analyzed by two way ANOVA with post hoc Bonferroni tests comparing strains at corresponding time points. *p<0.05, **p<0.01, ***p<0.001.
3.2. Effect of NK-1 receptor blockade on incision induced mechanical allodynia
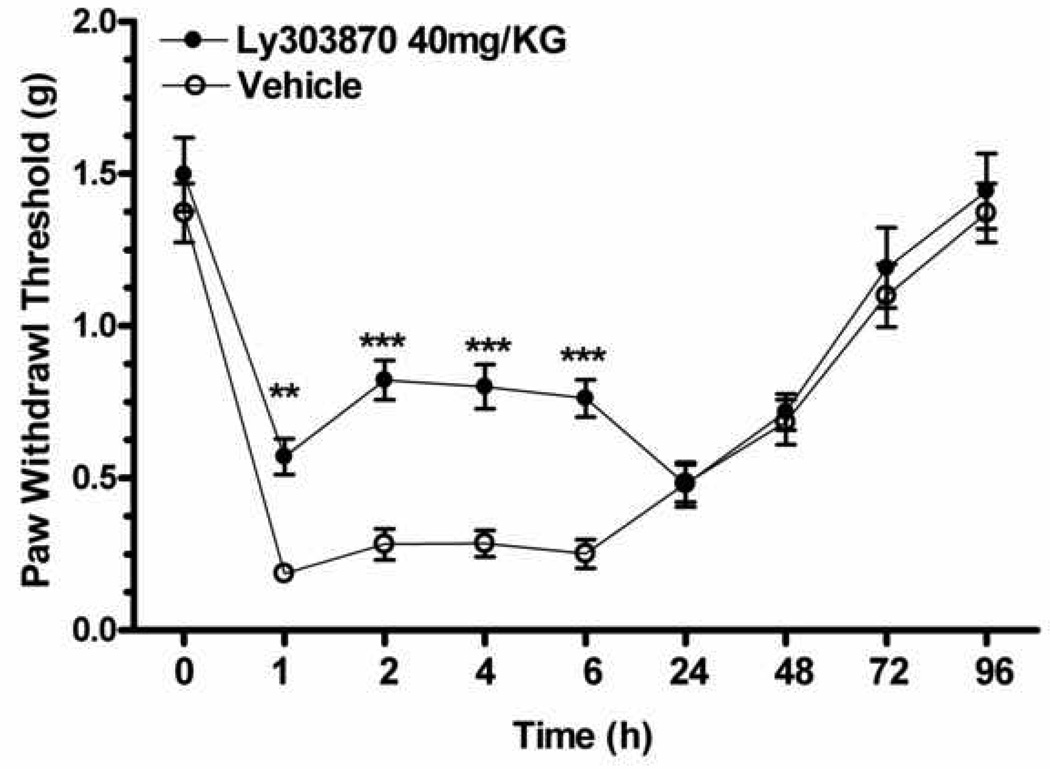
The specific question of SP participation in post-incisional sensitization was addressed by administering the NK-1 selective antagonist LY303870 (40 mg/kg. i.p.) 30 min prior to incision. As can be seen in Figure 2, selective blockade of this receptor produced a significant attenuation of incision induced mechanical sensitivity for approximately the first 12 hours after incision consistent with the half life of the drug in mice(F1,126=43.41, p<0.001).
Figure 2.
Assessment of selective NK-1 receptor antagonist effects on incision induced mechanical allodynia. Mechanical hypersensitivity was measured in two different groups of wild type mice: vehicle pretreatment/incision, LY303870 (40 mg/kg. i.p.) pretreatment/incision (n=8 each group). Mean ± S.E.M values of each group were analyzed by two way ANOVA with post hoc Bonferroni tests comparing treatment groups at each time point. *p<0.05, **p<0.01, ***p<0.001.
3.3. Effects of hind paw incision on peripheral cytokines and NGF levels
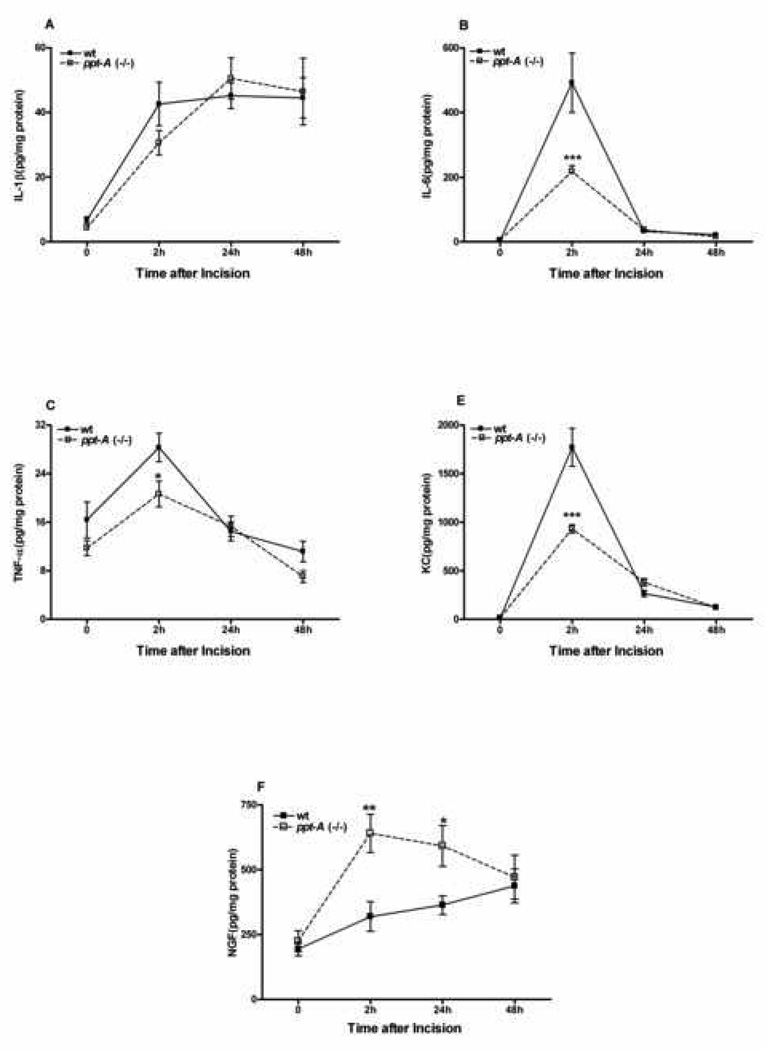
The demonstrated roles of cytokines in nociceptive sensitization including the sensitization of skin surrounding incisions as well as the ability of primary afferent nerve fibers to control inflammation in tissues surrounding wounds lead us to determine if the production of incision area cytokines and NGF were altered in ppt-A(−/−) mice. The cytokines chosen for assay were demonstrated in previous experiments to be modulated after hind paw incision [15]. The time course of cytokine generation and the magnitude of changes in wild type animals were similar to that seen in previous studies. Figure 3A–E demonstrates that 3 of the 4 cytokines were generated in lesser quantities in ppt-A(−/−) mice, though differences from the wild type mice were significant only at the 2 hour time point.
Figure 3.
Effects of hind paw incision on peripheral cytokines and NGF levels. The levels of cytokines and NGF were measured at baseline and at the 2–48 hours time points after incision. The selected time points were based on the behavior data presented in Figure 1. Different groups of mice of both wild type and ppt-A(−/−) were used for each time point (n=6 per group). Data are presented as Mean ± S.E.M and were analyzed by two way ANOVA with post hoc Bonferroni tests comparing strains at corresponding time points. *p<0.05, **p<0.01, ***p<0.001.
The neurotrophin nerve growth factor (NGF) has also been linked to incisional sensitization [8], and may be under the control of SP in skin [4]. Therefore, we performed experiments in which NGF levels in the skin surrounding wounds was measured both before and at various time points after incision in wild type and ppt-A(−/−) mice. In the wild type samples NGF levels slowly increased after incision whereas in the ppt-A(−/−) mice NGF levels were increased more rapidly and to a greater extent (Figure 3F). The differences from the wild type mice were statistically significant at the 2 and 24 hour time points. Thus while ppt-A deficiency leads to a transient deficit in cytokine production, it leads to an over-expression of NGF in peri-incisional skin.
3.4. Effects of peripheral substance-P administration and subsequent NK-1 blockade on cytokines and NGF levels
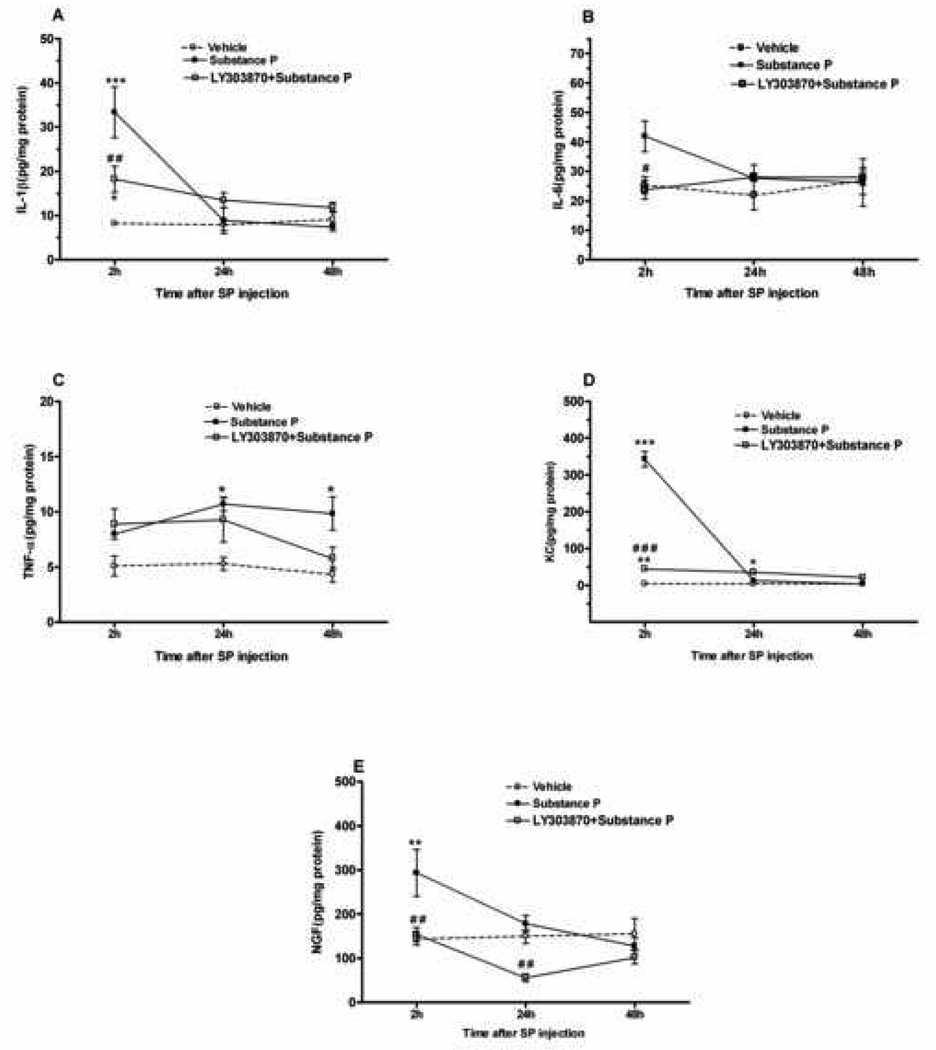
In order to test the hypothesis that SP itself can promote cytokine production in skin, 1µg SP was injected (i.pl.) in wt mice, followed by tissue harvest from 2 to 48 hours after administration. Figure 4 demonstrates significant elevations of IL-1β, IL-6, KC and NGF levels at the 2 hour time point compared to vehicle, with the levels returning back to baseline values by 24 hours post SP administration. However, local SP injection had a modest effect on TNF-α production. As demonstrated here, the effects of peripheral SP administration were acute and transient. The selective NK-1 receptor antagonist LY303870 (40 mg/kg. i.p.) 30 min prior to SP (lµg/15µl; i.pl.) in wt mice was able to reduce the acute and transient elevations of IL-1β, IL-6, KC and NGF levels (2 hour), while having no effect on production of these cytokines at later time points (Fig. 4). In order to examine the 24 and 48 hours effect of LY303870 blockade of SP cytokine production, the NK-1 receptor antagonist had to be re-administered every 12 hours.
Figure 4.
Effects of intra-plantar substance P (SP:1 µg/15 µL), LY303870 (40 mg/kg. i.p.) pretreatment/ intra-plantar substance P (SP:1 µg/15 µL) and vehicle (15 µL Saline) on peripheral cytokines and NGF levels. The levels of cytokines and NGF were measured 2–48 hours time points after SP administration. Different groups of mice of both wild type and ppt-A(−/−) were used for each time point (n=4–6 per group). Data are presented as Mean ± S.E.M and were analyzed by two way ANOVA with post hoc Bonferroni tests comparing strains at corresponding time points. Significant difference from vehicle:*p<0.05, **p<0.01, ***p<0.001. Significant difference from SP: #p<0.05, ##p<0.01, ###p<0.001.
3.5. Effects of peripheral administration of substance-P, IL-6 and NGF on mechanical sensitivity
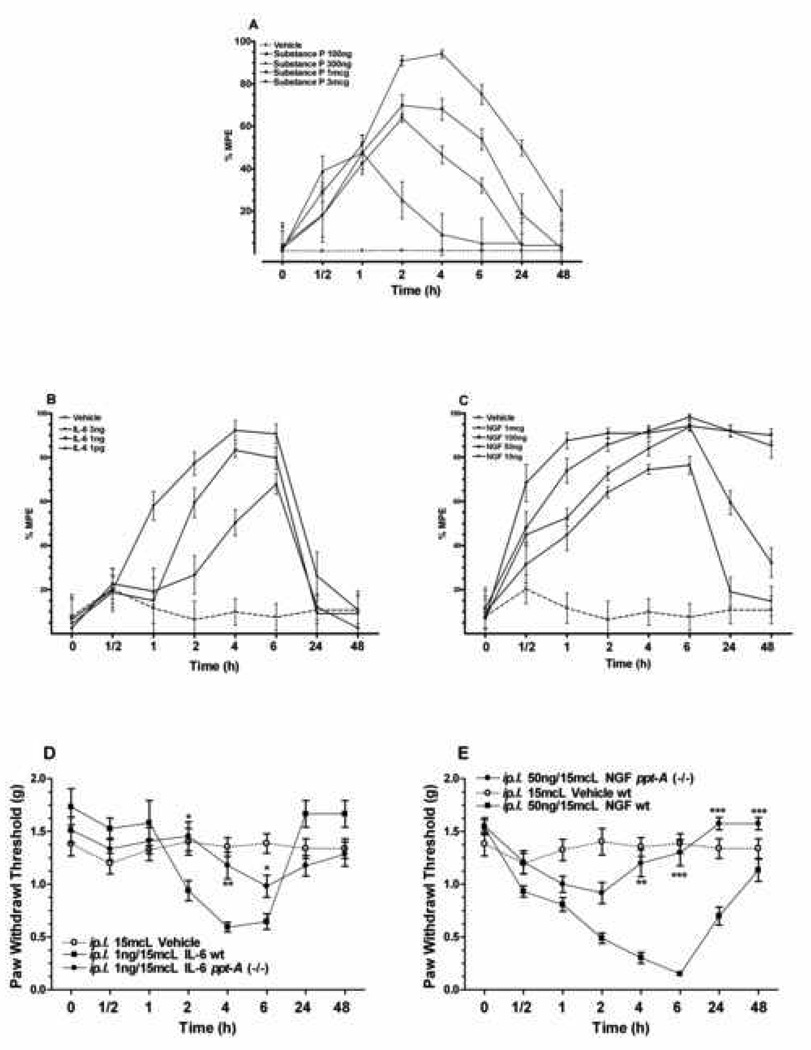
While the data obtained so far suggest possible roles for SP, several cytokines and NGF in incisional sensitization, the direct effects of these mediators on nociception needed to be determined. We therefore selected SP, IL-6 (one of the most robustly modulated cytokines) and NGF for injection into hind paw skin using the robust mechanical assay to follow nociceptive sensitization. Figure 5A shows the dose dependant response of peripheral SP administration on the wild type mice, while vehicle administration had no effect in this assay.
Figure 5.
Effects of peripheral administration of substance-P, IL-6 and NGF on mechanical sensetivity. A: Intra-plantar substance P and vehicle were given after baseline mechanical paw withdrawal threshold determination. B–C: Intra-plantar IL-6, NGF and vehicle given after baseline measurements of paw withdrawal threshed. Data of the effect of different doses of SP, IL-6 and NGF are presented as mean percent effect, with baseline values as being zero effect. Higher doses of NGF produced ongoing mechanical hyperalgesia for several days (Data not shown). D–E: Comparison of the paw withdrawal thresholds of wild type and ppt-A(−/−) mice at various time points given 1ng/15 µL of IL-6 and 50ng/15 µL of NGF by intra-plantar injection. Data are presented as Mean ± S.E.M and were analyzed by two way ANOVA with post hoc Bonferroni tests comparing strains at corresponding time points. *p<0.05, **p<0.01, ***p<0.001.
Subsequently, we addressed the question of whether a given dose of nociceptive mediator caused as much sensitization in the ppt-A (−/−) as in the wild type mice. Both IL-6 and NGF dose-dependently lowered mechanical thresholds, and the thresholds remained depressed for extended periods after injection (Figure 5B–C). Finally, Figure 5C–D demonstrates that ppt-A gene deletion reduces the degree and duration of mechanical allodynia after the subcutaneous administration of IL-6 and NGF. The doses utilized in these experiments were chosen as the lowest dose that produced near maximal effect from the previous set of experiments.
4. Discussion
The ppt-A gene product SP has been implicated in modulating relatively high intensity nociceptive signaling occurring with the application of strong heat, mechanical and chemical stimuli. It was unclear, however, how SP participates in models of incisional pain. Earlier pharmacological testing has implicated the NK-1 SP receptor in post-incisional sensitization [25]; [62]. Consistent with those reports the present study demonstrated that the ppt-A(−/−) mice exhibit deficits in both heat and mechanical nociceptive signaling in the incisional model. Subsequently, we demonstrated that SP has at least two potential mechanisms in supporting nociception after incision thus furthering our understanding of SP function in this model. The first is the well established role SP has as a neurotransmitter in activating the spinal cord dorsal horn neurons. Local injection of the classic pro-nociceptive cytokine (IL-6) and neurotrophin NGF sensitized the wild type mice to a greater degree than the ppt-A(−/−) animals. These data are consistent with others showing that chemical stimulation of peripheral primary afferent fibers leads to less nociceptive behavior in these knockout mice [11]. Our studies, however, used endogenously produced mediators present in humans wounds [13]. Interestingly, we observed that cytokine and neurotrophin production in the knock out mice incised skin were dysregulated when compared to wild-type mice; the production of several cytokines (IL-6, KC and TNF-α) was partially inhibited. Moreover, we demonstrated that increased production of skin cytokines and NGF after local SP injection could be reversed by NK1 receptor antagonism. Thus, disruption of SP signaling lowers the responses to key nociceptive mediators and reduces the production of some of those same mediators.
Our observations of sharply reduced mechanical allodynia in ppt-A(−/−) mice after hind paw incision seem to fit with our current understanding of peripheral nociceptive signal transmission. Substance P is expressed predominantly by C-fibers. Detailed electrophysiological studies using the hind paw incision model have demonstrated sensitization and expansion of the receptive fields for both C- and A-delta afferent fibers [28; 47]. Additional studies have provided evidence of spontaneous C-fiber activation in incisional wounds specifically [6; 7]. Furthermore, capsaicin stimulation of small afferent C-fibers is attenuated in ppt-A(−/−) mice [11]. Finally, the unusual mammal Heterocephalus glaber (mole-rat) fails to produce detectable levels of SP in cutaneous c-fibers, thought to be responsible for it’s the lack of sensitization after capsaicin injection [46]. Thus the existing literature is consistent with a role for SP expressing C-fibers supporting mechanical allodynia after incision.
Much less clear prior to undertaking these studies was whether endogenously produced substances involved in inflammatory and incisional pain, e.g. NGF and cytokines, rely on SP to support nociceptive signaling. Several cytokines and recombinant NGF have been demonstrated to cause hyperalgesia when injected intrademally in rats and mice [3; 4; 17–19; 53; 58–60]. Our own studies demonstrated that intradermal IL-6 and NGF as well as SP dose-dependently cause profound mechanical allodynia in treated paws of wild type mice. Thus the reductions in skin cytokine abundance seen at an early post-incisional time point in the ppt-A(−/−) mice, might be sufficient to explain in part the longer period of reduced mechanical allodynia observed here.
Direct evidence for peripheral cytokine sensitization of C-nociceptive afferent nerve fibers is somewhat less abundant. Fu and Longhurst were able to show the sensitization of visceral C-fibers after intra-arterial administration of IL-1β [9]. Local perfusion of dorsal root ganglia (DRGs) with IL-1β, IL-6 and TNF-α also causes enhanced responsiveness of C-fibers [45; 63]. Using a model of joint pain, Brenn et al documented enhanced C-fiber activity after the application of IL-6 [10]. Other investigators failed to observe sensitization of sural nerve C-fibers after TNF-α, IL-1β and IL-6 application [42]. Thus it is not clear if all C-fiber populations are responsive to cytokine sensitization under all circumstances. Even less clear is whether the cytokines are interacting directly with cytokine receptors on peripheral fibers to cause sensitization as has been postulated by Summer at al for IL-6, or whether complex local signaling cascades involving cytokines and prostanoids in addition to the recruitment of inflammatory neutrophils to the area is the underlying relevant pathway [53]; [18; 19]. Much additional work needs to be completed before conclusions concerning the mechanism(s) of action of cytokines in supporting nociception in incisional wounds can be reached. One mechanism not addressed in our work involves SP-induced activation of glial cells and subsequent release of cytokines as has been implicated in the genesis of inflammatory and neuropathic pain states [27; 44; 54; 55; 61].
It is clear, however, that the ppt-A gene product SP can stimulate the production of cytokines in skin. In fact, SP is found in significant levels in human wound fluids [13]. Evidence supporting the hypothesis that SP can stimulate cytokine production includes the observations of Dallos et al. who demonstrated that SP applied to keratinocyte cultures increased IL-1α, IL-8 and TNF-α mRNA levels [20]. Liu et al. observed similar results when SP was applied to HaCaT cells (human epidermal keratinocyte cell line) [38]. The subcutaneous injection of SP causes nociceptive sensitization which can be blocked by NK-1 receptor antagonists, though the direct effects of SP on afferent neurons versus indirect activating effects through the stimulation of cytokine production has not been explored [12]. Our own results show that the skin administration of SP in mice causes rapid, dose dependant but transient sensitization, It has been proposed that SP signaling via the NK1 receptor is involved in the induction but not maintenance of nociception [40; 56]. Yet again, the very long time course of mechanical allodynia after direct cytokine administration suggests that the deficiency in production of multiple cytokines in ppt-A(−/−) mice early after incision might explain some of the diminished mechanical sensitization observed at later time points.
Our results concerning NGF levels in the skin of wild type versus ppt-A (−/−) mice after incision were somewhat unexpected. While baseline levels of this neurotrophin were indistinguishable between the genotypes, incision lead to greater NGF production in the skin of knockout mice compared with wild-type controls. However, as demonstrated here, NGF is a pronociceptive molecule when injected into skin. To understand this apparent conflict it should be kept in mind that in the ppt-A(−/−) responses to many types of stimuli ranging from cytokines to formalin to capsaicin are diminished. This suggests that despite presence of an increased level, NGF may have an impaired ability to cause maximal sensitization in ppt-A (−/−) mice after incision, potentially providing an explanation for our observations. Another compelling explanation for the enhanced post incisional NGF accumulation in the skin of the ppt-A(−/−) mice is that denervation of the skin leads to an up-regulation of NGF expression [30; 41]. This increase in NGF may be responsible both for nerve regeneration to the area and, perhaps, for enhanced nociceptive sensitivity in nerve injury models [23; 50; 51]. Deletion of the ppt-A gene might be viewed as a functional partial denervation of the skin thus explaining the enhanced abundance of NGF after incision. Arguing against this simple view, however, is the observation that direct treatment of keratinocyte cultures with SP leads to an increase in NGF [20] and our own observation that skin SP injection increases NGF levels via a NK-1 receptor dependent mechanism. Perhaps the mechanisms linking SP release to NGF production are more complex in the setting of incision than when SP is injected in purified form to normal tissue.
The (ppt-A) gene codes for both SP and NKA, the contribution of the latter to cytokine and neurotrophin production, as well as, the phenotype observed in the incisional model has not been investigated. The scope of the present study has been limited to one component of the (ppt-A) gene product (SP) and therefore the effect of a selective NK1 receptor antagonist has been studied. At this juncture, it is noteworthy to mention that the efficacy of SP receptor blockade in human pain conditions has not lived up to the results of animal studies [31; 52]. Several mechanisms has been proposed for this lack of agreement: the role of the stress component of pain perception, species variations in NK1 receptors and the choice of clinical paradigms influencing the analgesic efficacy of the antagonists [31; 57]. Therefore, further studies to address the role of NKA signaling in the incisional model, as well as, investigating the effects of selective NK1 and NK2 receptor antagonists on cytokine production in incision induced hyperalgesia are required.
In summary our data are consistent with the notion that the ppt-A gene product SP supports nociceptive sensitization after hind paw incision. In addition to the relatively well established roles this neuropeptide has as a neurotransmitter released into the dorsal horn of the spinal cord, it is possible that lack of this substance reduces the intensity of the inflammatory reaction surrounding incisions thereby providing a second mechanism for the reduced mechanical and heat sensitization observed in ppt-A(−/−) mice. Future studies might be directed at further defining the likely impact reduced cytokine production could have on pain or other parameters of wound healing. Additional experimentation is required to define direct versus indirect effects of cytokines on afferent neurons in incision.
Acknowledgements
We thank Dr. De-Yong Liang for valuable discussions throughout the course of this work. The authors do not have financial or other relationships that might lead to a conflict of interest. This work was supported by NIH grants DA021332 and GM079126.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17(20):8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Akaishi S, Ogawa R, Hyakusoku H. Keloid and hypertrophic scar: neurogenic inflammation hypotheses. Med Hypotheses. 2008;71(1):32–38. doi: 10.1016/j.mehy.2008.01.032. [DOI] [PubMed] [Google Scholar]
- 3.Amann R, Schuligoi R. Beta adrenergic inhibition of capsaicin-induced, NK1 receptor-mediated nerve growth factor biosynthesis in rat skin. Pain. 2004;112(1–2):76–82. doi: 10.1016/j.pain.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Amann R, Schuligoi R, Herzeg G, Donnerer J. Intraplantar injection of nerve growth factor into the rat hind paw: local edema and effects on thermal nociceptive threshold. Pain. 1996;64(2):323–329. doi: 10.1016/0304-3959(95)00120-4. [DOI] [PubMed] [Google Scholar]
- 5.Andreev N, Dimitrieva N, Koltzenburg M, McMahon SB. Peripheral administration of nerve growth factor in the adult rat produces a thermal hyperalgesia that requires the presence of sympathetic post-ganglionic neurones. Pain. 1995;63(1):109–115. doi: 10.1016/0304-3959(95)00024-M. [DOI] [PubMed] [Google Scholar]
- 6.Banik RK, Brennan TJ. Spontaneous discharge and increased heat sensitivity of rat C-fiber nociceptors are present in vitro after plantar incision. Pain. 2004;112(1–2):204–213. doi: 10.1016/j.pain.2004.08.026. [DOI] [PubMed] [Google Scholar]
- 7.Banik RK, Brennan TJ. Sensitization of primary afferents to mechanical and heat stimuli after incision in a novel in vitro mouse glabrous skin-nerve preparation. Pain. 2008 doi: 10.1016/j.pain.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Banik RK, Subieta AR, Wu C, Brennan TJ. Increased nerve growth factor after rat plantar incision contributes to guarding behavior and heat hyperalgesia. Pain. 2005;117(1–2):68–76. doi: 10.1016/j.pain.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 9.Binder R, Kress A, Kirschfink M. Modulation of C5a-mediated effector functions of human polymorphonuclear leukocytes by tumor necrosis factor alpha and granulocyte macrophage colony-stimulating factor. Exp Clin Immunogenet. 1999;16(4):212–225. doi: 10.1159/000019113. [DOI] [PubMed] [Google Scholar]
- 10.Brenn D, Richter F, Schaible HG. Sensitization of unmyelinated sensory fibers of the joint nerve to mechanical stimuli by interleukin-6 in the rat: an inflammatory mechanism of joint pain. Arthritis Rheum. 2007;56(1):351–359. doi: 10.1002/art.22282. [DOI] [PubMed] [Google Scholar]
- 11.Cao YQ, Mantyh PW, Carlson EJ, Gillespie AM, Epstein CJ, Basbaum AI. Primary afferent tachykinins are required to experience moderate to intense pain. Nature. 1998;392(6674):390–394. doi: 10.1038/32897. [DOI] [PubMed] [Google Scholar]
- 12.Carlton SM, Zhou S, Coggeshall RE. Localization and activation of substance P receptors in unmyelinated axons of rat glabrous skin. Brain Res. 1996;734(1–2):103–108. [PubMed] [Google Scholar]
- 13.Carvalho B, Clark DJ, Angst MS. Local and systemic release of cytokines, nerve growth factor, prostaglandin E2, and substance P in incisional wounds and serum following cesarean delivery. J Pain. 2008;9(7):650–657. doi: 10.1016/j.jpain.2008.02.004. [DOI] [PubMed] [Google Scholar]
- 14.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 15.Clark JD, Qiao Y, Li X, Shi X, Angst MS, Yeomans DC. Blockade of the complement C5a receptor reduces incisional allodynia, edema, and cytokine expression. Anesthesiology. 2006;104(6):1274–1282. doi: 10.1097/00000542-200606000-00024. [DOI] [PubMed] [Google Scholar]
- 16.Clark JD, Shi X, Li X, Qiao Y, Liang D, Angst MS, Yeomans DC. Morphine reduces local cytokine expression and neutrophil infiltration after incision. Mol Pain. 2007;3:28. doi: 10.1186/1744-8069-3-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cunha JM, Cunha FQ, Poole S, Ferreira SH. Cytokine-mediated inflammatory hyperalgesia limited by interleukin-1 receptor antagonist. Br J Pharmacol. 2000;130(6):1418–1424. doi: 10.1038/sj.bjp.0703434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cunha TM, Verri WA, Jr, Schivo IR, Napimoga MH, Parada CA, Poole S, Teixeira MM, Ferreira SH, Cunha FQ. Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol. 2008;83(4):824–832. doi: 10.1189/jlb.0907654. [DOI] [PubMed] [Google Scholar]
- 19.Cunha TM, Verri WA, Jr, Silva JS, Poole S, Cunha FQ, Ferreira SH. A cascade of cytokines mediates mechanical inflammatory hypernociception in mice. Proc Natl Acad Sci U S A. 2005;102(5):1755–1760. doi: 10.1073/pnas.0409225102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dallos A, Kiss M, Polyanka H, Dobozy A, Kemeny L, Husz S. Effects of the neuropeptides substance P, calcitonin gene-related peptide, vasoactive intestinal polypeptide and galanin on the production of nerve growth factor and inflammatory cytokines in cultured human keratinocytes. Neuropeptides. 2006;40(4):251–263. doi: 10.1016/j.npep.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 21.Delgado AV, McManus AT, Chambers JP. Production of tumor necrosis factor-alpha, interleukin 1- beta, interleukin 2, and interleukin 6 by rat leukocyte subpopulations after exposure to substance P. Neuropeptides. 2003;37(6):355–361. doi: 10.1016/j.npep.2003.09.005. [DOI] [PubMed] [Google Scholar]
- 22.Delgado AV, McManus AT, Chambers JP. Exogenous administration of Substance P enhances wound healing in a novel skin-injury model. Exp Biol Med (Maywood) 2005;230(4):271–280. doi: 10.1177/153537020523000407. [DOI] [PubMed] [Google Scholar]
- 23.Doubleday B, Robinson PP. The role of nerve growth factor in collateral reinnervation by cutaneous C-fibres in the rat. Brain Res. 1992;593(2):179–184. doi: 10.1016/0006-8993(92)91306-y. [DOI] [PubMed] [Google Scholar]
- 24.Gibran NS, Jang YC, Isik FF, Greenhalgh DG, Muffley LA, Underwood RA, Usui ML, Larsen J, Smith DG, Bunnett N, Ansel JC, Olerud JE. Diminished neuropeptide levels contribute to the impaired cutaneous healing response associated with diabetes mellitus. J Surg Res. 2002;108(1):122–128. doi: 10.1006/jsre.2002.6525. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez MI, Field MJ, Holloman EF, Hughes J, Oles RJ, Singh L. Evaluation of PD 154075, a tachykinin NK1 receptor antagonist, in a rat model of postoperative pain. Eur J Pharmacol. 1998;344(2–3):115–120. doi: 10.1016/s0014-2999(97)01581-1. [DOI] [PubMed] [Google Scholar]
- 26.Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108(1–2):95–107. doi: 10.1016/j.pain.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 27.Guo W, Wang H, Watanabe M, Shimizu K, Zou S, LaGraize SC, Wei F, Dubner R, Ren K. Glial-cytokine-neuronal interactions underlying the mechanisms of persistent pain. J Neurosci. 2007;27(22):6006–6018. doi: 10.1523/JNEUROSCI.0176-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamalainen MM, Gebhart GF, Brennan TJ. Acute effect of an incision on mechanosensitive afferents in the plantar rat hindpaw. J Neurophysiol. 2002;87(2):712–720. doi: 10.1152/jn.00207.2001. [DOI] [PubMed] [Google Scholar]
- 29.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32(1):77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 30.Harper SJ, Buchman VL, Owen D. Denervation of the skin following section of the inferior alveolar nerve leads to increased NGF accumulation without change in NGF mRNA expression. Exp Neurol. 1999;155(2):327–330. doi: 10.1006/exnr.1998.7000. [DOI] [PubMed] [Google Scholar]
- 31.Hill R. NK1 (substance P) receptor antagonists--why are they not analgesic in humans? Trends Pharmacol Sci. 2000;21(7):244–246. doi: 10.1016/s0165-6147(00)01502-9. [DOI] [PubMed] [Google Scholar]
- 32.Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24(33):7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kingery WS, Davies MF, Clark JD. A substance P receptor (NK1) antagonist can reverse vascular and nociceptive abnormalities in a rat model of complex regional pain syndrome type II. Pain. 2003;104(1–2):75–84. doi: 10.1016/s0304-3959(02)00467-0. [DOI] [PubMed] [Google Scholar]
- 34.Li X, Angst MS, Clark JD. A murine model of opioid-induced hyperalgesia. Brain Res Mol Brain Res. 2001;86(1–2):56–62. doi: 10.1016/s0169-328x(00)00260-6. [DOI] [PubMed] [Google Scholar]
- 35.Liang D, Li X, Lighthall G, Clark JD. Heme oxygenase type 2 modulates behavioral and molecular changes during chronic exposure to morphine. Neuroscience. 2003;121(4):999–1005. doi: 10.1016/s0306-4522(03)00483-4. [DOI] [PubMed] [Google Scholar]
- 36.Liang D, Shi X, Qiao Y, Angst MS, Yeomans DC, Clark JD. Chronic morphine administration enhances nociceptive sensitivity and local cytokine production after incision. Mol Pain. 2008;4:7. doi: 10.1186/1744-8069-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liang DY, Li X, Clark JD. Formalin-induced spinal cord calcium/calmodulin-dependent protein kinase II alpha expression is modulated by heme oxygenase in mice. Neurosci Lett. 2004;360(1–2):61–64. doi: 10.1016/j.neulet.2004.02.050. [DOI] [PubMed] [Google Scholar]
- 38.Liu JY, Hu JH, Zhu QG, Li FQ, Wang J, Sun HJ. Effect of matrine on the expression of substance P receptor and inflammatory cytokines production in human skin keratinocytes and fibroblasts. Int Immunopharmacol. 2007;7(6):816–823. doi: 10.1016/j.intimp.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 39.Luger TA. Neuromediators--a crucial component of the skin immune system. J Dermatol Sci. 2002;30(2):87–93. doi: 10.1016/s0923-1811(02)00103-2. [DOI] [PubMed] [Google Scholar]
- 40.Ma QP, Woolf CJ. Involvement of neurokinin receptors in the induction but not the maintenance of mechanical allodynia in rat flexor motoneurones. J Physiol. 1995;486(Pt 3):769–777. doi: 10.1113/jphysiol.1995.sp020852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mearow KM, Kril Y, Diamond J. Increased NGF mRNA expression in denervated rat skin. Neuroreport. 1993;4(4):351–354. doi: 10.1097/00001756-199304000-00002. [DOI] [PubMed] [Google Scholar]
- 42.Moalem G, Grafe P, Tracey DJ. Chemical mediators enhance the excitability of unmyelinated sensory axons in normal and injured peripheral nerve of the rat. Neuroscience. 2005;134(4):1399–1411. doi: 10.1016/j.neuroscience.2005.05.046. [DOI] [PubMed] [Google Scholar]
- 43.Muangman P, Tamura RN, Muffley LA, Isik FF, Scott JR, Xie C, Kegel G, Sullivan SR, Liang Z, Gibran NS. Substance P enhances wound closure in nitric oxide synthase knockout mice. J Surg Res. 2009;153(2):201–209. doi: 10.1016/j.jss.2008.03.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Obata K, Noguchi K. [Contribution of primary sensory neurons and spinal glial cells to pathomechanisms of neuropathic pain] Brain Nerve. 2008;60(5):483–492. [PubMed] [Google Scholar]
- 45.Ozaktay AC, Kallakuri S, Takebayashi T, Cavanaugh JM, Asik I, DeLeo JA, Weinstein JN. Effects of interleukin-1 beta, interleukin-6, and tumor necrosis factor on sensitivity of dorsal root ganglion and peripheral receptive fields in rats. Eur Spine J. 2006;15(10):1529–1537. doi: 10.1007/s00586-005-0058-8. [DOI] [PubMed] [Google Scholar]
- 46.Park TJ, Lu Y, Juttner R, Smith ES, Hu J, Brand A, Wetzel C, Milenkovic N, Erdmann B, Heppenstall PA, Laurito CE, Wilson SP, Lewin GR. Selective inflammatory pain insensitivity in the African naked mole-rat (Heterocephalus glaber) PLoS Biol. 2008;6(1):e13. doi: 10.1371/journal.pbio.0060013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pogatzki EM, Gebhart GF, Brennan TJ. Characterization of Adelta- and C-fibers innervating the plantar rat hindpaw one day after an incision. J Neurophysiol. 2002;87(2):721–731. doi: 10.1152/jn.00208.2001. [DOI] [PubMed] [Google Scholar]
- 48.Poree LR, Guo TZ, Kingery WS, Maze M. The analgesic potency of dexmedetomidine is enhanced after nerve injury: a possible role for peripheral alpha2-adrenoceptors. Anesth Analg. 1998;87(4):941–948. doi: 10.1097/00000539-199810000-00037. [DOI] [PubMed] [Google Scholar]
- 49.Reynolds AM, Holmes MD, Scicchitano R. Cytokines enhance airway smooth muscle contractility in response to acetylcholine and neurokinin A. Respirology. 2000;5(2):153–160. doi: 10.1046/j.1440-1843.2000.00240.x. [DOI] [PubMed] [Google Scholar]
- 50.Sabsovich I, Wei T, Guo TZ, Zhao R, Shi X, Li X, Yeomans DC, Klyukinov M, Kingery WS, Clark JD. Effect of anti-NGF antibodies in a rat tibia fracture model of complex regional pain syndrome type I. Pain. 2008;138(1):47–60. doi: 10.1016/j.pain.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schicho R, Skofitsch G, Donnerer J. Regenerative effect of human recombinant NGF on capsaicin- lesioned sensory neurons in the adult rat. Brain Res. 1999;815(1):60–69. doi: 10.1016/s0006-8993(98)01094-4. [DOI] [PubMed] [Google Scholar]
- 52.Sindrup SH, Graf A, Sfikas N. The NK1-receptor antagonist TKA731 in painful diabetic neuropathy: a randomised, controlled trial. Eur J Pain. 2006;10(6):567–571. doi: 10.1016/j.ejpain.2005.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135(1–2):98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 54.Svensson CI, Hua XY, Protter AA, Powell HC, Yaksh TL. Spinal p38 MAP kinase is necessary for NMDA-induced spinal PGE(2) release and thermal hyperalgesia. Neuroreport. 2003;14(8):1153–1157. doi: 10.1097/00001756-200306110-00010. [DOI] [PubMed] [Google Scholar]
- 55.Svensson CI, Marsala M, Westerlund A, Calcutt NA, Campana WM, Freshwater JD, Catalano R, Feng Y, Protter AA, Scott B, Yaksh TL. Activation of p38 mitogen-activated protein kinase in spinal microglia is a critical link in inflammation-induced spinal pain processing. J Neurochem. 2003;86(6):1534–1544. doi: 10.1046/j.1471-4159.2003.01969.x. [DOI] [PubMed] [Google Scholar]
- 56.Traub RJ. The spinal contribution of substance P to the generation and maintenance of inflammatory hyperalgesia in the rat. Pain. 1996;67(1):151–161. doi: 10.1016/0304-3959(96)03076-X. [DOI] [PubMed] [Google Scholar]
- 57.Urban LA, Fox AJ. NK1 receptor antagonists--are they really without effect in the pain clinic? Trends Pharmacol Sci. 2000;21(12):462–464. doi: 10.1016/s0165-6147(00)01578-9. author reply 465. [DOI] [PubMed] [Google Scholar]
- 58.Verri WA, Jr, Cunha TM, Magro DA, Domingues AC, Vieira SM, Souza GR, Liew FY, Ferreira SH, Cunha FQ. Role of IL-18 in overt pain-like behaviour in mice. Eur J Pharmacol. 2008 doi: 10.1016/j.ejphar.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 59.Verri WA, Jr, Cunha TM, Parada CA, Wei XQ, Ferreira SH, Liew FY, Cunha FQ. IL-15 mediates immune inflammatory hypernociception by triggering a sequential release of IFN-gamma, endothelin, and prostaglandin. Proc Natl Acad Sci U S A. 2006;103(25):9721–9725. doi: 10.1073/pnas.0603286103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Verri WA, Jr, Molina RO, Schivo IR, Cunha TM, Parada CA, Poole S, Ferreira SH, Cunha FQ. Nociceptive effect of subcutaneously injected interleukin-12 is mediated by endothelin (ET) acting on ETB receptors in rats. J Pharmacol Exp Ther. 2005;315(2):609–615. doi: 10.1124/jpet.105.089409. [DOI] [PubMed] [Google Scholar]
- 61.Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2(12):973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- 62.Yamamoto T, Sakashita Y. The role of the spinal opioid receptor like1 receptor, the NK-1 receptor, and cyclooxygenase-2 in maintaining postoperative pain in the rat. Anesth Analg. 1999;89(5):1203–1208. [PubMed] [Google Scholar]
- 63.Zhang JM, Li H, Liu B, Brull SJ. Acute topical application of tumor necrosis factor alpha evokes protein kinase A-dependent responses in rat sensory neurons. J Neurophysiol. 2002;88(3):1387–1392. doi: 10.1152/jn.2002.88.3.1387. [DOI] [PubMed] [Google Scholar]