Abstract
Tetramethylpentadecane (TMPD, or commonly known as pristane)-induced lupus is a murine model of systemic lupus erythematosus (SLE). Renal disease and autoantibody production strictly depend on signaling through the interferon (IFN)-I receptor. The major source of IFN-I is immature monocytes bearing high levels of the surface marker Ly6C. Interferon production is mediated exclusively by signaling through TLR7 and the adapter protein MyD88. It is likely that endogenous TLR7 ligands such as components of small nuclear ribonucleoprotein complexes are involved in triggering disease. Lupus autoantibodies are produced in ectopic lymphoid tissue developing in response to TMPD. This model is well suited for examining links between dysregulated IFN-I production and the pathogenesis of human SLE, which like TMPD-lupus, is associated with high levels of IFN-I.
The immunological effects of TMPD (pristane]
The naturally occurring hydrocarbon oil TMPD (2,6,10,14-tetramethylpentadecane), more commonly known as pristane, induces chronic inflammation when introduced into the peritoneal cavity. Over the past 15 years, it has been found that the inflammatory response to TMPD causes a lupus-like disease in mice. The mechanisms involved in TMPD-lupus are coming into clearer focus and may be highly relevant to human systemic lupus erythematosus (SLE), an immune disorder increasingly linked to the overproduction of the type 1 interferons (IFN) α and β.
Inflammatory and carcinogenic properties of medium-length alkanes
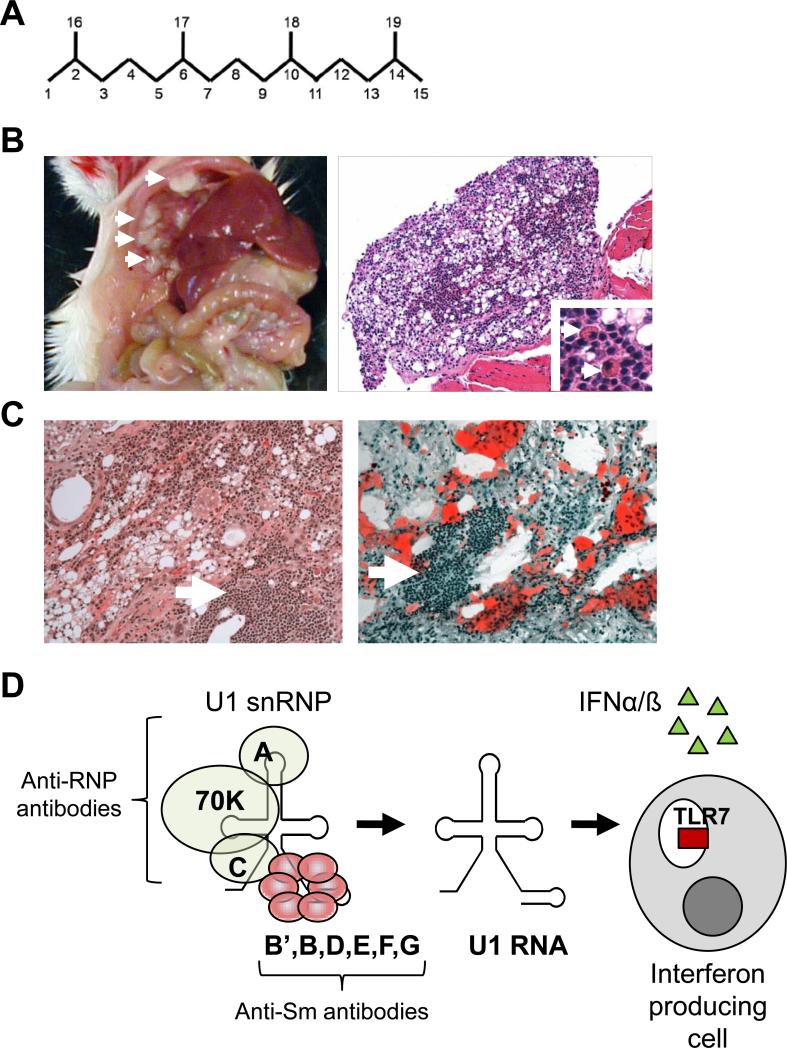
Pristane (TMPD) is an isoprenoid alkane found in small quantities in many plants and thought to be derived primarily from the metabolism of phytol, a ubiquitous ester of chlorophyll. Relatively high levels are also found in various marine organisms, including algae and zooplanktonic copepods, which can convert phytol from their diet to pristane [1]. It is concentrated to remarkable levels in the livers of various planktivorous sharks. TMPD also occurs in crude oils and is a common constituent of mineral oil, a byproduct of the fractional distillation of petroleum containing straight- and branched-chain paraffinic, naphthenic, and aromatic hydrocarbons with 15 or more carbons and boiling points between 300−600° C. Medicinal (pharmaceutical or food grade) mineral oils, which are free of aromatic and unsaturated compounds, are used as laxatives, protective coatings for foods, and in cosmetics. For instance, canned sardines contain up to 370 mg/kg and white bread up to 550 mg/kg of mineral oil [2,3]. Dietary exposure to mineral oil is estimated at 9−45 grams per year, some of which is absorbed through the intestine [4]. Intestinal absorption of dietary mineral oil is thought to be responsible for the formation of “lipogranulomas” (follicular lipidosis) seen in the liver, spleen, lymph nodes, and other organs of most individuals living in developed countries [5-7]. In 1962, Potter reported that mineral oil induces plasmacytomas in BALB/c mice following intraperitoneal injection [8]. The most active component of mineral oil is TMPD [9] (Figure 1A). Plasma cell tumors arise in structures termed “lipogranulomas”, which represent a chronic inflammatory response to the hydrocarbon (Figure 1B). TMPD-induced plasmacytomas have been studied extensively as a model of multiple myeloma [10].
Figure 1. Pathological and immunological abnormalities induced by TMPD.
A. Chemical structure of 2,6,10,14-tetramethylpentadecane (TMPD, C19H40) more commonly known as pristane. Numbers indicate carbon molecules. B. Gross pathology (left, arrows indicate individual lipogranulomas) and microscopic pathology (formalin fixation, hematoxylin & eosin staining, right) of ectopic lymphoid tissue in a mouse given an intraperitoneal injection of TMPD. Low power (200x) shows numerous oil droplets and clusters of infiltrating lymphocytes and plasma cells (indicated by arrows, inset, 400x). C. Microscopic pathology of the lung of a patient with exogenous lipoid pneumonia due to the aspiration of mineral oil. Left, formalin-fixed tissue with hematoxylin & eosin staining; Right, unfixed tissue with oil red staining. Note the presence of lymphocytic infiltrates closely resembling those in TMPD treated mice (arrows) and the numerous oil droplets (left), which in unfixed tissue stain intensely with oil red. D. Structure of the U1 snRNP, an autoantigen containing immunostimulatory RNA. The U1 snRNP consists of a single molecule of U1 small nuclear RNA. Anti-RNP and anti-Sm antibodies recognize different subsets of the proteins bound to this RNA. Anti-RNP antibodies bind to proteins U1-A, U1−70K, and U1-C, whereas anti-Sm antibodies bind to the Sm B’, B, D1, D2, D3, E, F, and G proteins. The purified U1 RNA is a ligand for TLR7, capable of associating with TLR7 inside of endosomes and stimulating the production of the Type I interferons IFNα and IFNß.
Adjuvant properties
Hydrocarbons can induce inflammation and enhance immune responsiveness. Medium-length straight chain hydrocarbons (optimum ∼12 carbons) are the most potent adjuvants [11]. In humans, inadvertent cutaneous injection of these hydrocarbon oils leads to an intense inflammatory reaction, often with skin necrosis, permanent loss of hand function, or the need for amputation of affected digits [12]. Similarly, aspiration of mineral oil causes “lipoid pneumonia” [13], an inflammatory lesion of the lung closely resembling murine lipogranulomas (Figure 1C), and ingestion of mineral oil induces similar lesions in lymph nodes or along the walls of hepatic venules in human livers [14]. At present, the clinical significance of these lesions, which can raise suspicion of an infection or neoplasm, is unknown. However, epidemiological studies suggest that occupational exposure to mineral oil or petroleum waste is associated with rheumatoid arthritis and possibly lupus [15,16]. In addition, the production of antinuclear antibodies is common in farmed salmon receiving vaccines containing mineral oil adjuvants [17]. Thus, there is evidence that the adjuvant properties of certain hydrocarbons can precipitate inflammatory or autoimmune disease in humans and animals.
Autoantibodies in TMPD-treated mice
Consistent with the evidence that exposure to adjuvant hydrocarbons may provoke inflammatory or autoimmune responses in humans, BALB/c mice injected intraperitoneally (i.p.) with TMPD develop a local inflammatory response (lipogranulomas) and an erosive arthritis resembling rheumatoid arthritis (RA) [18] and TMPD was subsequently found to induce autoantibodies and the clinical manifestations of SLE [19].
Susceptibility to TMPD-lupus among non-autoimmune prone mice is widespread [20]. The primarily IgG autoantibodies induced by TMPD, all of which are associated with SLE, are targeted to a variety nuclear components including double-stranded (ds) DNA, single-stranded (ss) DNA, chromatin, Sm, RNP, Su, and ribosomal P. TMPD also causes polyclonal hypergammaglobulinemia, another immunological feature of SLE, which is in part a response to sustained cytokine production stimulated by TMPD.
Although TMPD induces autoantibodies against type II collagen and rheumatoid factor [21], lupus autoantibodies (anti-Sm, RNP, dsDNA, chromatin, ribosomal P, and Su (argonaute 2) are produced at much higher levels [19] (Table 1). Although there are strain differences, autoantibody responses are largely restricted to this limited repertoire of specificities. A single i.p. dose of TMPD leads to autoantibodies against the U1 small nuclear ribonucleoprotein (U1 snRNP, RNP, and-or Sm antigens), and Su autoantigen in 50−90% of BALB/c mice over 4−6 months and against dsDNA at 6−10 months [19,22]. Titers of anti-U1 snRNP autoantibodies are as high as 1 to 1 million . Incomplete Freund's adjuvant, hexadecane (a component of tobacco smoke, diesel fumes, gasoline, and jet fuel to which human exposure is common), and squalene (a metabolite of the cholesterol biosynthetic pathway found at high levels in shark liver and used as an adjuvant for human vaccines) induce a similar spectrum of autoantibodies, but less efficiently than TMPD [23]. In contrast, medicinal mineral oil does not induce these autoantibodies [24]. Autoantibody production is independent of exogenous microbes, as germ-free mice are susceptible [25]. Thus, the ability of TMPD to induce autoimmunity does not rely on microbial “danger signals” recognized by pattern receptors of the innate immune system, such as Toll-like receptors (TLRs), suggesting that the ability of these substances to break tolerance is truly endogenous.
Table 1.
Some autoantibodies produced by TMPD-treated mice
| Autoantibody | Autoantigen | Nucleic acid component | Frequency in BALB/c mice | Frequency in B6 mice |
|---|---|---|---|---|
| Anti-Sm* | U1, U2, U4-U6, and U5 snRNPs (proteins B', B, D, E, F, G) | U1, U2, U4, U6, U5 small nuclear RNAs | 20−40% | 10% |
| Anti-RNP | U1 snRNP (proteins A, C, 70K) | U1 small nuclear RNA | 50−90% | 25% |
| Anti-ribosomal P* | Ribosomal P0, P1, P2 proteins | Ribosomal RNAs | 0%1 | 20% |
| Anti-Su | Argonaute 2 protein | Micro-RNAs | 50−70% | 25% |
| Anti-dsDNA* | Native DNA | Native DNA | 40% | 0% |
| Anti-chromatin | DNA-histone complexes | DNA | 60% | 0% |
specific for the diagnosis of SLE
BALB/cByJ 0%; BALB/cJ 5−10%
Autoantibodies against the U1, U2, U4−6, and U5 small nuclear ribonucleoproteins (snRNPs) are strongly linked to SLE, and anti-Sm antibodies (recognizing the Sm core proteins of U1-U6 snRNPs) are pathognomonic (Figure 1D) [26]. Anti-nRNP antibodies, which are less lupus-specific than anti-Sm, recognize the U1-A, U1-C, and U1−70K components of U1 snRNPs. Anti-dsDNA and anti-ribosomal P0, P1, or P2 autoantibodies, like anti-Sm, are lupus-specific in humans. Autoantibodies to the Su antigen (identified as the micro-RNA associated protein argonaute 2), occur in SLE and other systemic autoimmune disorders [27]. These IgG autoantibodies do not develop in BALB/c nu/nu (nude) mice or T-cell receptor deficient (C57BL6 TcRβ−/−, TcRδ −/−) mice following TMPD treatment [28,29], but IgM rheumatoid factor production is T-cell independent [30]. Thus, the lupus-specific, but not RA-associated, autoantibodies induced by TMPD are derived primarily from class-switched (IgG2a) producing B cells requiring T cell help for their generation. In view of the striking predominance of IgG2a autoantibodies, the cytokines IFNγ (produced by T helper 1 cells), IFNα, and IFNβ are strongly implicated in their pathogenesis.
Effects of cytokines
Along with the Type I interferons (IFN) α and ß (see below), IL-6, IFNγ, and IL-12 promote autoantibody production in TMPD-lupus. IL-6 deficiency abrogates the induction of IgG anti-DNA and chromatin, but not anti-Sm, RNP, or Su autoantibodies by TMPD [31]. IFN. deficient mice are resistant to the induction of IgG anti-chromatin autoantibodies, but still produce IgG2a anti-Sm or RNP. In addition to IFN., IFNα, and ß signaling is essential for anti-Sm or RNP autoantibodies [32] (see below) and also promotes switching to IgG2a. Furthermore there is cross-talk between the IFNγ, ß, γ, and IL-6 signaling pathways [33,34]. Since mice deficient in the Type I interferon receptor (IFNAR) do not exhibit increased IL-12 levels following TMPD treatment [32], IL-12 and IFNγ is probably downstream of IFNβ and ß signaling. As noted above, other hydrocarbons such as hexadecane, squalene, and Freund.s incomplete adjuvant can induce a similar spectrum of autoantibodies [23,35]. Their ability to promote autoantibody production correlates with the ability to stimulate release of IFN-I producing cells from the bone marrow (Figure 2A).
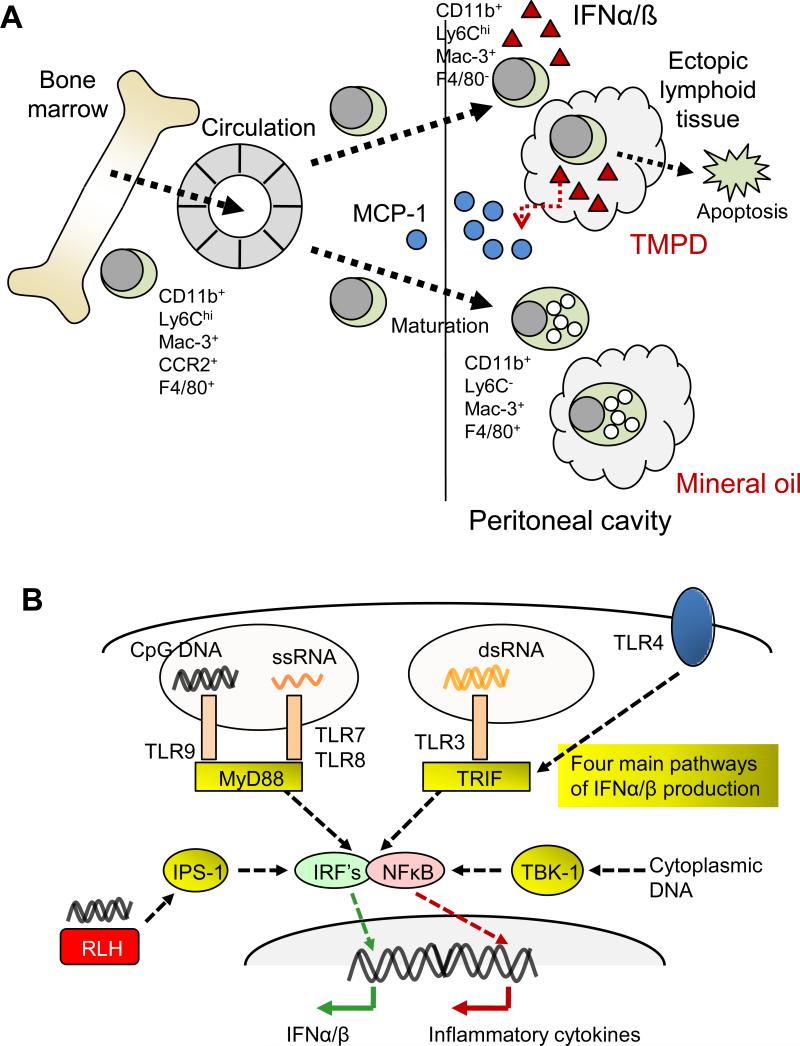
Figure 2. Pathogenesis of autoimmunity in TMPD-treated mice.
A. TMPD stimulates IFNα and IFNß production by immature (Ly6Chi) monocytes. The IFN-I inducible chemokine MCP-1 (CCL2) is produced following intraperitoneal injection of TMPD and promotes the egress of immature monocytes bearing the markers CD11b, Ly6Chi, Mac-3, F4/80, and CCR2 (the receptor for MCP-1) from the bone marrow. These cells enter the circulation and are recruited to the inflamed peritoneal cavity. In mice treated with TMPD, the Ly6Chi monocytes down-that forms in response to the hydrocarbon. These cells persist for ∼3 days before undergoing programmed cell death (apoptosis). In mineral oil treated mice, the Ly6Chi monocytes rapidly mature in the peritoneum to CD11b+, Ly6C−, Mac-3+, F4/80+ cells with numerous endocytic vacuoles. In contrast to TMPD elicited monocytes, these cells do not produce IFNα or ß. B. IFN-I and pro-inflammatory cytokine production can be stimulated through four main cellular pathways employing different adaptor proteins or signaling intermediates: i.e. via TRIF (TLRs 3 and 4), MyD88 (TLRs 7, 8, and 9), IPS-1 (Rig-I like helicases, RLH)), and TBK1 (receptor not yet clearly defined). The endosomal recognition of unmethylated CpG motifs in DNA by TLR9 or of single stranded RNA by TLR7 or TLR8 leads to the activation of IFNα and ß gene expression via a pathway involving the adapter protein MyD88, several kinases (not shown), and the transcription factor interferon regulatory factor (IRF) 7. In contrast, the endosomal recognition of dsRNA by TLR3 or cell surface recognition of lipopolysaccharide (endotoxin) by TLR4 leads to IFNα and ß gene expression via a pathway involving the adapter protein TRIF, kinases, and the transcription factor IRF-3. In addition to the endosomal pathways, the recognition of cytoplasmic (viral) dsRNA by the RLH Rig-I or Mda5 leads to the activation of IFNα and ß gene expression via the adapter protein IPS-1 and the transcription factors IRF3 and IRF7. Cytoplasmic DNA is detected by receptors that remain to be fully elucidated and signal via the kinase TBK-1, resulting in IFNα and ß gene expression. All these pathways converge on nuclear factor kappa B (NFκB). The induction of IFNα and ß by TMPD relies exclusively on the TLR7-MyD88-IRF7 pathway, whereas the other three pathways are dispensable.
Autoimmune disease in TMPD-treated mice
TMPD treated mice develop clinical manifestations of lupus, including arthritis, immune complex-mediated glomerulonephritis, and pulmonary capillaritis as well as autoantibodies. Inflammation of the pericardium and pleura also occurs, but it is unclear whether this is autoimmune in origin. SLE is a human syndrome classified using a set of 11 criteria and TMPD-treated mice meet these criteria (Table 2). As in TMPD lupus [36], human SLE is primarily a disease of females (female : male ratio ∼9 : 1).
Table 2.
Comparison of (NZB × NZW) F1 and TMPD lupus models
| |
(NZB × NZW)F1 |
TMPD (BALB/c) |
TMPD (C57BL/6) |
|---|---|---|---|
| Lupus criteria* met | |||
| Immune complex nephritis | +++ | ++ | + |
| Arthritis | (−) | ++ | (−) |
| Serositis | (−) | ++ (?) | ++ (?) |
| Antinuclear antibodies | +++ | +++ | +++ |
| Anti-dsDNA | +++ | ++ (late) | (−) |
| Anti-Sm | (−) | +++ | ++ |
| Anti-RNP | (−) | +++ | ++ |
| Anti-ribosomal P |
(−) |
(−) |
++ |
| Other manifestations |
Hemolytic anemia in NZB mice |
None |
Pulmonary vasculitis |
| Lupus criteria not met |
skin, photosensitivity, mucocutaneous ulcers, serositis, arthritis, nervous system, cytopenias |
skin, photosensitivity, mucocutaneous ulcers, nervous system, cytopenias |
skin, photosensitivity, mucocutaneous ulcers, arthritis, nervous system, cytopenias |
| Role of IFN-I | Disease is accelerated by IFN-I | IFN signature is present (peripheral blood) | IFN signature is present (peripheral blood) |
Criteria for the classification of SLE include malar rash, discoid rash, photosensitivity, mucocutaneous ulcers, arthritis, pleuritis/pericarditis, glomerulonephritis, seizures or psychosis, hematological abnormalities (leukopenia, lymphopenia, thrombocytopenia), anti-dsDNA, anti-Sm, or anti-phospholipid autoantibodies, and antinuclear antibodies.
Arthritis
TMPD and other hydrocarbons cause arthritis in mice [21]. BALB/cJ and DBA/1 mice develop synovial hyperplasia, periostitis, and marginal erosions reminiscent of RA following intraperitoneal TMPD injection [18,21]. TMPD, hexadecane, squalene, and mineral oil also induce arthritis in Lewis and Dark Agouti rats. Arthritogenicity correlates with adjuvanticity with unbranched alkanes ≥12 carbons generally being more arthritogenic [11]. The arthritis might be TNFα-mediated, since disease is ameliorated by TNF inhibitors [37]. In general, lupus arthritis is not erosive, although in some cases an erosive arthritis similar to RA is seen. These individuals are thought to have an overlap syndrome with features of both disorders. The nature of the joint disease in TMPD-treated mice suggests that the autoimmune disorder could be something akin to this so-called “rhupus” syndrome.
Glomerulonephritis
TMPD induces immune complex-mediated glomerulonephritis in BALB/c and SJL mice, which develop glomerular IgG and complement deposits, cellular proliferation, and proteinuria; C57BL/6 mice develop milder (mesangial, Class II) disease [22,38,39]. In BALB/c and SJL mice, mesangial immune complex deposition is followed by subendothelial lesions consistent with diffuse proliferative (Class IV) lupus nephritis [22]. IL-6 [31], IFNγ[40], and IL12p35 [41] deficient mice are highly resistant to the induction of renal disease. IFNα/β receptor deficient (IFNAR−/−) mice also have greatly decreased proteinuria but unchanged glomerular immune complex deposition following TMPD injection [32]. Although IFNAR−/− mice produce antinuclear antibodies, the major lupus specificities, including anti-DNA (implicated in the pathogenesis of lupus nephritis), are absent. A similar picture is seen in NZB/W mice deficient in the common γ-chain of the stimulatory Fcγ receptors [42]. These mice developed anti-DNA antibodies and also had extensive immune complex deposits, but were protected from severe nephritis, probably due to suppression of the renal inflammatory response to immune complexes. Interestingly, in TMPD-treated IFNAR−/− mice glomerular cellularity is reduced compared to untreated control levels, suggesting that IFNAR signaling may drive renal inflammation. Consistent with this possibility, increased glomerular expression of IFN-I has been noted in active human lupus nephritis [43]. An influx of monocytes is thought to play a significant role in the pathogenesis of human and murine lupus nephritis [44]. As several of the key chemokines involved in monocyte recruitment are products of IFNα and ß-inducible e.g. CCL2 (MCP-1) and/or IFNγ-inducible (e.g. IP-10) genes, decreased production by glomerular cells in response to immune complexes could modulate the severity of renal disease in IFNAR−/− and IFNγ−/− mice.
Pulmonary vasculitis
TMPD-treated C57BL/6 and C57BL/10 mice often develop pulmonary hemorrhage resembling that seen in SLE; 0−50% die, depending on housing or other factors [39,45]. All TMPD-treated C57BL/10 mice develop pulmonary capillaritis with perivascular infiltrates of macrophages, neutrophils, lymphocytes, and eosinophils and there is endothelial activation, but not immune complex deposition [39]. Vasculitis is restricted to the lung and anti-neutrophil cytoplasmic antibodies (ANCA) are not produced [39].
TMPD induction of SLE: relevance to human disease
Animal models are useful if they reproduce all or some of the clinical features of a disease. SLE is a syndrome (not a disease) and is defined clinically as a constellation of ≥4 of 11 classification criteria. NZB/W mice, a standard lupus model, meet three criteria: glomerulonephritis, anti-nuclear antibodies (ANA), and anti-dsDNA antibodies (Table 2). TMPD-treated BALB/c mice have less severe glomerulonephritis (3−4+ proteinuria, proliferative changes) [22], arthritis [18,21], ANA, and diverse lupus autoantibodies, including anti-dsDNA and anti-Sm [19]. Thus, in terms of the lupus criteria, the TMPD-lupus is as good as or better than the NZB/W model (Table 2). Moreover, TMPD-lupus is associated with abnormal production of IFNα and ß, which appears to have a central role in SLE (see below).
Like human SLE, NZB/W lupus is genetically-mediated. In contrast, TMPD induces lupus in mice that are not genetically prone to the disease. Thus, although genetic factors mediate susceptibility to TMPD-induced disease [21,46], TMPD-lupus is not a suitable model for identifying the genetic abnormalities involved in spontaneous lupus. However, if IFN-I overproduction is central to the pathogenesis of the disease, as suggested by the induction of lupus following IFNα therapy in humans [47,48] and the elevated interferon levels in SLE patients, it is reasonable to suppose that the pathways mediating TMPD-lupus might be relevant to a subset of human SLE. . TMPD-lupus is advantageous since the role of complex signaling pathways regulating IFN-I production can be examined readily using gene targeting and gene expression techniques. Thus, neither the NZB/W nor the TMPD model completely reproduces human lupus, but both exhibit important similarities to SLE.
The role of Type I interferon (IFN-I) in SLE
IFN-I is increasingly recognized as a key mediator of SLE. The IFN-I family includes multiple IFNα subtypes and IFNβ, all binding the same IFNAR (receptor) complex [49]. Initially described for its anti-viral effects, IFN-I links innate and adaptive immunity. Details of IFN-I biology and functions have been reviewed [49] and will not be discussed further. Elevated serum IFN-I was noted in SLE patients 30 years ago [50] and interest in the antiviral cytokine family has revived with recognition that an “interferon signature” is associated with lupus. Microarray and quantitative PCR studies have identified numerous interferon-stimulated genes (ISGs) over-expressed in the peripheral blood of many SLE patients [51,52]. This gene expression program is associated with disease severity, nephritis, and autoantibodies against dsDNA and Sm or RNP [53-55]. In NZB/W mice, glomerulonephritis, ANA and anti-dsDNA antibody production are accelerated by exogenous IFNα[56], whereas deletion of IFNAR in NZB and B6.Nba2 mice slows disease progression and enhances survival [57,58]. TMPD-lupus uniquely exhibits the interferon signature and might therefore be the most suitable animal model for examining the IFN-I dysregulation seen in SLE [reviewed in [59]].
IFN-I production in TMPD-lupus
Ectopic lymphoid tissue forming in TMPD-lupus (see below) displays increased expression of many ISGs including Mx-1, IRF7, ISG15, and IP-10 [60]. This is not observed in ectopic lymphoid tissue from mice treated with medicinal mineral oil, which does not induce lupus. The IFN signature is also seen in the peripheral blood of TMPD-treated wild type, but not IFNAR−/− mice [32]. Although IFN-I has been implicated in spontaneous murine lupus [56-58], TMPD-lupus is the first model shown to recapitulate the IFN signature found in more than half of SLE patients.
IFN-I has a profound effect on the pathogenesis of TMPD-lupus. In IFNAR−/− mice, autoantibodies against DNA, chromatin, RNP, Sm, and Su are not produced and the severity of glomerulonephritis is reduced markedly [32]. Similar findings were reported recently in mice deficient in IRF9 or STAT1, two key signaling molecules downstream of the IFNAR [61]. Curiously, TMPD-treated IFNAR−/− mice develop low titer ANA of unknown specificity [32]. These IFN-I-independent ANA might be analogous to the ANA in a subset of healthy humans who have neither IFN-I dysregulation nor manifestations of lupus [55]. Interestingly, IFNAR−/−mice develop glomerular immune deposits but not proteinuria, perhaps because inflammatory monocytes are recruited to the kidney by IFN-I-inducible chemokines (e.g. CCL2).
Although autoantibodies develop 3−4 months after TMPD injection, IFN-I production is established within two weeks [62], at which time circulating lymphocytes (in C57BL/6 and 129/Sv strains) express high levels of the ISG Sca-1 on their surface, consistent with a systemic increase in IFN-I [63]. In contrast, Sca-1 is absent in mice treated with mineral oil or squalene.
Immature monocytes are a major source of IFN-I in TMPD-lupus
Although plasmacytoid dendritic cells (PDCs) are thought to be the primary source of IFN-I in both healthy individuals and in SLE [64], their role might be limited in TMPD-lupus as their depletion has little effect on IFN-I or ISG expression [62]. Instead, inflammatory monocytes expressing high levels of Ly6C are a major source of IFN-I (Figure 2A). The function of Ly6C, which is expressed by mice but not humans, is unclear. High levels of Ly6C expression characterize monocytes that are newly released from the bone marrow, and expression is normally down-regulated as these cells transit through the blood [65]. However, Ly6Chi monocytes accumulate in the inflamed peritoneum [66] and in inflammatory atherosclerotic plaques [67,68], and they also transport Listeria monocytogenes bacteria to the brain during systemic infection in mice [69]. However, Ly6Chi monocytes do not appear to produce IFN-I constitutively, and it is not known whether they produce it in inflammatory conditions other than the TMPD-inflamed peritoneum.
Normally absent in the peritoneal cavity, Ly6Chi monocytes are attracted by CCL2, and comprise ∼30% of peritoneal exudate cells (PECs) two weeks after TMPD injection. Their depletion with clodronate-containing liposomes rapidly eliminates the IFN-I signature [62]. They also accumulate in ectopic lymphoid tissue induced by TMPD (but not mineral oil or squalene) (Figure 2A). In contrast, medicinal mineral oil stimulates an influx of Ly6Chi monocytes into the peritoneal cavity, which rapidly mature into Ly6C−F4/80+ monocyte/macrophages with abundant cytoplasm and prominent phagosomes [62]. These more mature cells are nearly absent in TMPD-treated mice. Other populations of peritoneal cells (e.g. mesothelial cells) also can express IFN-I, but whether it suffices to induce lupus remains to be determined.
Mechanism of IFN-I production
The mechanism(s) of IFN-I over-production in SLE is a topic of ongoing research. Mammalian cells utilize several innate receptors to initiate IFN-I production in response to pathogen-associated molecular patterns [70] (Figure 2B). TLR7 and -8, which recognize viral ssRNA, and TLR9, a sensor for unmethylated CpG DNA, have received considerable attention due to their ability to recognize endogenous nucleic acids [71-73]. These endosomal TLRs trigger IFN-I secretion via the adaptor molecule MyD88 [70]. In contrast, TLR3 and TLR4 mediate IFN-I production through the adapter protein TRIF upon encountering dsRNA or lipopolysaccharide, respectively [70]. In the cytoplasm, viral RNA binds to the intracellular pathogen sensors RIG-I or MDA-5 to trigger IFN-I activation via the adapter protein IPS-1, whereas cytoplasmic DNA activates a TBK-1-dependent pathway [70].
Using mice deficient in components of these four pathways, TMPD was found to elicit IFNI production exclusively through the TLR7-MyD88 pathway [63]. Accumulation of Ly6Chi monocytes and anti-RNP, -Sm, or -Su autoantibody production are abolished in the absence of MyD88 or TLR7. Similar to their IFNAR−/− counterparts, TLR7−/− mice are protected from glomerulonephritis [74]. Peritoneal Ly6Chi monocytes express high levels of TLR7, consistent with their role as major IFN-I producing cells. The effects of TMPD are augmented further by TLR7 gene duplication in Y-linked autoimmune accelerated (Yaa) mutant mice [75], making these male mice (with two active copies of TLR7) more sensitive to the effects of TMPD than female mice (with a single active copy) [63]. Mortality from renal disease in Yaa mutant mice is greatly accelerated when they are treated with TMPD. Other TLRs and cytoplasmic nucleic acid sensors are dispensable for IFN-I production, as deficiency of TRIF, IPS-1, or TBK-1 has no effect on TMPD-lupus.
It has been hypothesized that aberrant clearance of apoptotic or necrotic cells in SLE results in formation of immune complexes consisting of autoantibodies and RNA- or DNA- containing autoantigens [76]. In vitro, Fcγ receptors (FcγR) on PDCs mediate the transport of DNA- or RNA-containing immune complexes into endosomes, allowing the activation of TLR7, -8, or -9 by internalized endogenous nucleic acids [77,78]. This implies that generation of autoantibodies against RNA-containing autoantigens (such as the U1 snRNP) is a prerequisite for chronic IFN-I production. In TMPD-lupus, however, IFN-I production occurs long before the appearance of anti-dsDNA or anti-nRNP or Sm autoantibodies. Moreover, intact production of IFN-I and autoantibodies in FcγR-deficient animals excludes a major role of immune complexes in the initial generation of interferon responses [63,79]. Together with the absence of autoantibody production in IFNAR−/− mice, these data indicate an upstream effect of IFN-I on autoantibody production, a view also supported by the development of autoantibodies in patients treated with recombinant IFNα[48]. Dependence of lupus autoantibodies on IFN-I signaling also has been shown in other lupus models [57,58].
The exact mechanism of TMPD-induced TLR7 activation is undefined. The chemical structure of TMPD (Figure 1A) is distinct from known TLR7 ligands and TMPD is not a TLR7 ligand [63]. Instead, TMPD (but not mineral oil or squalene) enhances the stimulatory effects of TLR7 ligands. TMPD might augment the response to endogenous TLR7 ligands such as the U1 RNA component of Sm or RNP antigen (Figure 1D). Alternatively, incorporation of TMPD into the cell membrane might disturb the endosomal location of TLR7, providing access to endogenous ligands [80]. TMPD might also interfere with degradation of cellular debris, increasing the availability of endogenous nucleic acids. However, neither TLR7 localization nor cellular endocytosis or phagocytosis is affected by TMPD treatment [63]. Moreover, TMPD does not up-regulate TLR7 expression. Interestingly, its ability to promote autoantibody production requires Fas-Fas ligand signaling [45]. Although C57BL/6 (B6) mice are susceptible to TMPD-lupus, both B6-lpr/lpr (Fas deficient) and B6-gld/gld (Fas ligand deficient) mice are highly resistant, raising the possibility that Fas-mediated apoptosis might generate endogenous TLR7 ligands.
In summary, TMPD-lupus uniquely recapitulates the “interferon signature” human SLE. IFN-I is essential to disease development and is elicited through a TLR7-MyD88-dependent, but FcγR-independent pathway. Thus, although not suitable for defining lupus genetics, TMPD-lupus allows temporal assessment of the upstream and downstream effects of IFN-I dysregulation and is well-suited for evaluating therapies targeting components of the TLR7-MyD88-IFN-I pathway.
Lymphoid neogenesis
Association of lymphoid neogenesis with autoimmunity
Although originally termed “lipogranulomas”, the inflammatory lesions arising in response to the presence of TMPD within the peritoneal cavity actually represent a striking example of lymphoid neogenesis [60]. Lymphoid neogenesis, the formation of ectopic lymphoid tissue at sites of inflammation [81], is strongly associated with autoantibody production [82]. Ectopic lymphoid tissue closely resembles secondary lymphoid tissue and arises by a similar developmental pathway. It frequently exhibits compartmentalization into discrete B-cell zones and T-cell and dendritic cell areas vascularized by high endothelial venules (HEV). Organization of this tissue is directed by the lymphoid chemokines CCL19 (ELC), CCL21 (SLC), CXCL12 (SDF-1), and CXCL13 (BLC) [82]. Ectopic lymphoid tissue forms when the immune response fails to eliminate pathogens, such as hepatitis C or Helicobacter pylori and also is common in autoimmune diseases such as Hashimoto's thyroiditis, Sjögren's syndrome, and RA [82].
An important issue is whether ectopic lymphoid tissue is a site of class switching, somatic hypermutation, and autoantibody generation. Lymphocytic foci in rheumatoid synovium exhibit restricted κ-light chain rearrangements and evidence of somatic hypermutation [83,84]. Removal of self-reactive B-cells might be inefficient in ectopic lymphoid tissue [85], and B-cells specific for the Ro (SS-A) and La (SS-B) ribonucleoprotein autoantigens can be detected in the salivary glands of patients with Sjögren's syndrome, rheumatoid factor-specific B-cells in rheumatoid synovium [86,87], and anti-nRNP specific B-cells in TMPD lipogranulomas (see below). Cytokines produced in the ectopic lymphoid tissue [60] might play a role in autoantibody production, since IFNα or β and IL-6 promote plasma cell development [88].
Antigen specific B-cell responses in TMPD-induced ectopic lymphoid tissue
Ectopic lymphoid tissue in the peritoneum of TMPD-treated mice is a site of substantial IFN-I production [60] (Figures 2A). Pathological analysis of TMPD-induced “lipogranulomas” [89] reveals the development of B, T, and dendritic cell zones, HEVs and the expression of CXCL13, CCL19, and CCL21 consistent with lymphoid neogenesis [60]. Lipogranulomas afford an opportunity to explore whether antibody responses develop within the ectopic lymphoid tissue or if B-cells only migrate there secondarily.
Following primary immunization with the hapten-carrier NP-KLH, NP-specific B-cells expressing the B cell receptor heavy-chain V186.2 and related heavy chains as well as λ-light chains accumulate in lipogranulomas [90]. In contrast to the diverse heavy-chains found in lipogranulomas from un-immunized mice, heavy-chain sequences from individual lipogranulomas isolated 12 days after primary immunization are derived from unique oligo- or monoclonal populations of NP-specific B-cells [90]. Heavy-chain complementarity determining region sequences from lipogranulomas have numerous replacement mutations, suggestive of an antigen-driven, T-cell-mediated immune response. Consistent with this possibility, lipogranulomas from TMPD-treated mice adoptively transferred with OT-II or DO11.10 (ovalbumin-specific, OVA) transgenic T-cells accumulate transgenic T-cells after subcutaneous immunization with OVA [90]. The selective co-localization of proliferating, antigen-specific T- and B-cells in lipogranulomas implicates ectopic lymphoid tissue as a potential site of antigen-specific cognate T-B cell interactions.
Germinal center formation is associated with T-cell dependent expansion of antigen-specific B-cells [91]. Lipogranulomas resemble germinal centers: they contain proliferating T and B lymphocytes, express activation-induced cytidine deaminase (AID), and have class switched B-cells [29]. Circular DNA intermediates, a hallmark of active class switch recombination, are present, consistent with local class switching. After immunization, lipogranuloma T-cells secrete IL-21, a mediator of plasma cell differentiation and class switching [92].
Anti-RNP autoantibody production in ectopic lymphoid tissue
The striking association between ectopic lymphoid tissue formation and autoimmunity raises the question of whether autoantibodies are produced in the lipogranulomas. IgM and IgG antibody forming cells (AFCs) producing autoantibodies to U1-A protein, a component of U1 snRNPs (Figure 1D), are more abundant in the ectopic lymphoid tissue than the spleen [29]. The large numbers of IgG anti-U1-A AFCs and T-cell dependence of anti-Sm and RNP responses [28,29] suggest that post-germinal center U1-A-specific B-cells might be stimulated to undergo plasma cell differentiation within the lipogranulomas themselves, either by autoantigen-specific T-cells or by TLR7 ligation with the RNA components of the Sm and RNP antigen (Figure 1D). Another possibility is that the IgG anti-U1-A AFCs represent long-lived plasma cells, which accumulate at sites of chronic inflammation, such as the nephritic kidneys and spleens of NZB/W mice [93].
Summary and conclusions
TMPD-lupus is a model of SLE associated with IFN-I dysregulation. The pathogenesis of lupus autoantibodies and glomerulonephritis in this model strictly requires IFNAR signaling. Most of the IFN-I is produced by immature monocytes instead of PDCs, and its production is mediated exclusively by the TLR7-MyD88 pathway. It is likely that endogenous TLR7 ligands such as U1RNA are involved, as germ-free mice are susceptible to disease induction. However, immune complex uptake via Fc receptors is dispensable. Autoantibody production is concentrated in ectopic lymphoid tissue induced by TMPD. This chronic inflammatory tissue may be a site of cognate T-B interactions, but the precise role of TLR ligand(s) and the mechanisms responsible for dysregulation of the autoreactive B-cells remain areas of active investigation. In light of the strong association between lymphoid neogenesis and autoantibody formation, elucidating the pathogenesis of TMPD-lupus might have broader implications for other autoimmune disorders, including RA, Sjögren's syndrome, and chronic hepatitis C-induced autoimmunity.
Acknowledgements
This work was supported by research grants AR44731 and AR51766 from the US Public Health Service and by the Lupus Foundation of America. P.Y.L and J.S.W are NIH T32 trainees (DK07518 and AR007603).
Glossary
- ANA
antinuclear antibodies
- AFC
antibody forming cell
- dsDNA
double-stranded DNA
- FcγR
Fcγ receptor
- IFN
interferon
- IFN-I
type-I interferons
- IFNAR
Interferon alpha/beta receptor
- i.p.
intraperitoneal
- IPS-1
interferon-β promoter stimulator −1
- ISG
Interferon stimulated gene
- MCP
monocyte chemoattractant protein
- MyD88
myeloid differentiation factor 88
- NZB/W
(New Zealand Black × New Zealand White) F1 hybrid mice
- NP-KLH
4-hydroxy-3-nitrophenyl acetyl-conjugated keyhole limpet hemocyanin
- OVA
ovalbumin
- PECs
peritoneal exudate cells
- PDCs
Plasmacytoid dendritic cells
- RA
Rheumatoid arthritis
- SLE
systemic lupus erythematosus
- snRNP
small nuclear ribonucleoprotein
- TMPD
tetramethylpentadecane
- TLR
Toll-like receptor
- TRIF
TIR domain-containing adaptor inducing IFN-β
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Avigan J, Blumer M. On the origin of pristane in marine organisms. J. Lipid Res. 1968;9:350–352. [PubMed] [Google Scholar]
- 2.Grob K, et al. Mineral oil material in canned foods. Food Additives & Contaminants. 1997;14:83–88. doi: 10.1080/02652039709374500. [DOI] [PubMed] [Google Scholar]
- 3.Castle L, et al. Migration of mineral hydrocarbons into foods. 4. Waxed paper for packaging dry goods including bread, confectionary and for domestic use including microwave cooking. Food Additives and Contaminants. 1994;11:79–89. doi: 10.1080/02652039409374204. [DOI] [PubMed] [Google Scholar]
- 4.Heimbach JT, et al. Dietary exposures to mineral hydrocarbons from food-use applications in the United States. Food Chem. Toxicol. 2002;40:555–571. doi: 10.1016/s0278-6915(02)00063-7. [DOI] [PubMed] [Google Scholar]
- 5.Cruickshank B. Follicular (mineral oil) lipidosis: I. Epidemiologic studies of involvement of the spleen. Hum. Pathol. 1984;15:724–730. doi: 10.1016/s0046-8177(84)80162-8. [DOI] [PubMed] [Google Scholar]
- 6.Cruickshank B, Thomas MJ. Mineral oil (follicular) lipidosis: II. histologic studies of spleen, liver, lymph nodes, and bone marrow. Hum. Pathol. 1984;15:731–737. doi: 10.1016/s0046-8177(84)80163-x. [DOI] [PubMed] [Google Scholar]
- 7.Wanless IR, Geddie WR. Mineral oil lipogranulomata in liver and spleen: a study of 465 autopsies. Arch. Pathol. Lab. Med. 1985;109:283–286. [PubMed] [Google Scholar]
- 8.Potter M, Boyce CR. Induction of plasma-cell neoplasms in strain BALB/c mice with mineral oil and mineral oil adjuvants. Nature (Lond. ) 1962;193:1086–1087. doi: 10.1038/1931086a0. [DOI] [PubMed] [Google Scholar]
- 9.Anderson PN, Potter M. Induction of plasma cell tumours in BALB/c mice with 2,6,10,14-tetramethylpentadecane (pristane). Nature (Lond. ) 1969;222:994–995. doi: 10.1038/222994a0. [DOI] [PubMed] [Google Scholar]
- 10.Potter M, Wiener F. Plasmacytomagenesis in mice: model of neoplastic development dependent upon chromosomal translocations. Carcinogenesis. 1992;13:1681–1697. doi: 10.1093/carcin/13.10.1681. [DOI] [PubMed] [Google Scholar]
- 11.Whitehouse MW, et al. Freund's adjuvants: relationship of arthritogenicity and adjuvanticity in rats to vehicle composition. Immunology. 1974;27:311–330. [PMC free article] [PubMed] [Google Scholar]
- 12.Valentino M, et al. Hand injuries due to high-pressure injection devices for painting in shipyards: circumstances, management, and outcome in twelve patients. Am. J. Ind. Med. 2003;43:539–542. doi: 10.1002/ajim.10218. [DOI] [PubMed] [Google Scholar]
- 13.Spickard A. Exogenous lipoid pneumonia. Arch. Intern. Med. 1994;154:686–692. [PubMed] [Google Scholar]
- 14.Dincsoy HP, et al. Lipogranulomas in non-fatty human livers: a mineral oil induced environmental disease. Am. J. Clin. Pathol. 1982;78:35–41. doi: 10.1093/ajcp/78.1.35. [DOI] [PubMed] [Google Scholar]
- 15.Sverdrup B, et al. Association between occupational exposure to mineral oil and rheumatoid arthritis: results from the Swedish EIRA case-control study. Arthritis Res. Ther. 2005;7:R1296–R1303. doi: 10.1186/ar1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dahlgren J, et al. Cluster of systemic lupus erythematosus (SLE) associated with an oil field waste site: a cross sectional study. Environ. Health. 2007;6:8. doi: 10.1186/1476-069X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Koppang EO, et al. Vaccination-induced systemic autoimmunity in farmed Atlantic salmon. J. Immunol. 2008;181:4807–4814. doi: 10.4049/jimmunol.181.7.4807. [DOI] [PubMed] [Google Scholar]
- 18.Potter M, Wax JS. Genetics of susceptibility to pristane-induced plasmacytomas in BALB/cAn: Reduced susceptibility in BALB/cJ with a brief description of pristane-induced arthritis. J. Immunol. 1981;127:1591–1595. [PubMed] [Google Scholar]
- 19.Satoh M, Reeves WH. Induction of lupus-associated autoantibodies in BALB/c mice by intraperitoneal injection of pristane. J. Exp. Med. 1994;180:2341–2346. doi: 10.1084/jem.180.6.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Satoh M, et al. Widespread susceptibility among inbred mouse strains to the induction of lupus autoantibodies by pristane. Clin. Exp. Immunol. 2000;121:399–405. doi: 10.1046/j.1365-2249.2000.01276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wooley PH, et al. Pristane-induced arthritis. The immunologic and genetic features of an experimental murine model of autoimmune disease. Arthritis Rheum. 1989;32:1022–1030. doi: 10.1002/anr.1780320812. [DOI] [PubMed] [Google Scholar]
- 22.Satoh M, et al. Antinuclear antibody production and immune complex glomerulonephritis in BALB/c mice treated with pristane. Proc. Natl. Acad. Sci. USA. 1995;92:10934–10938. doi: 10.1073/pnas.92.24.10934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Satoh M, et al. Induction of lupus autoantibodies by adjuvants. J. Autoimm. 2003;21:1–9. doi: 10.1016/s0896-8411(03)00083-0. [DOI] [PubMed] [Google Scholar]
- 24.Kuroda Y, et al. Distinctive patterns of autoimmune response induced by different types of mineral oil. Toxicol. Sci. 2004;78:222–228. doi: 10.1093/toxsci/kfh063. [DOI] [PubMed] [Google Scholar]
- 25.Mizutani A, et al. Pristane-induced autoimmunity in germ-free mice. Clin. Immunol. 2005;114:110–118. doi: 10.1016/j.clim.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 26.Craft J. Tolerance to self antigens in normal and lupus mice: the role of autoantigen specific B and T cell responses in autoantibody production. Rheumatology in Europe Supplement. 1995;2:11–20. [Google Scholar]
- 27.Jakymiw A, et al. Autoimmune targeting of key components of RNA interference. Arthritis Res. Ther. 2006;8:R87. doi: 10.1186/ar1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards HB, et al. Disparate T cell requirements of two subsets of lupus-specific autoantibodies in pristane-treated mice. Clin. Exp. Immunol. 1999;115:547–553. doi: 10.1046/j.1365-2249.1999.00825.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nacionales DC, et al. B cell proliferation, somatic hypermutation, and class switching in ectopic lymphoid tissue in murine lupus. J. Immunol. 2009 doi: 10.4049/jimmunol.0800771. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Levitt NG, et al. Pristane induced arthritis in mice. IV. Immunotherapy with monoclonal antibodies directed against lymphocyte subsets. J. Rheumatol. 1992;19:1342–1347. [PubMed] [Google Scholar]
- 31.Richards HB, et al. IL-6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J. Exp. Med. 1998;188:985–990. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nacionales DC, et al. Deficiency of the Type I interferon receptor protects mice from experimental lupus. Arthritis Rheum. 2007;56:3770–3783. doi: 10.1002/art.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mitani Y, et al. Cross talk of the interferon-alpha/beta signalling complex with gp130 for effective interleukin-6 signalling. Genes Cells. 2001;6:631–640. doi: 10.1046/j.1365-2443.2001.00448.x. [DOI] [PubMed] [Google Scholar]
- 34.Levy DE, et al. Synergistic interaction between interferon-alpha and interferon-gamma through induced synthesis of one subunit of the transcription factor ISGF3. EMBO J. 1990;9:1105–1111. doi: 10.1002/j.1460-2075.1990.tb08216.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kuroda Y, et al. Induction of lupus-related specific autoantibodies by non-specific inflammation caused by an intraperitoneal injection of n-hexadecane in BALB/c mice. Toxicology. 2006;218:186–196. doi: 10.1016/j.tox.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 36.Smith DL, et al. A female preponderance for chemically induced lupus in SJL/J mice. Clin. Immunol. 2007;122:101–107. doi: 10.1016/j.clim.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beech JT, Thompson SJ. Anti-tumour necrosis factor therapy ameliorates joint disease in a chronic model of inflammatory arthritis. Br. J. Rheumatol. 1997;36:1129. doi: 10.1093/rheumatology/36.10.1129. [DOI] [PubMed] [Google Scholar]
- 38.Satoh M, et al. Autoantibodies to ribosomal P antigens with immune complex glomerulonephritis in SJL mice treated with pristane. J. Immunol. 1996;157:3200–3206. [PubMed] [Google Scholar]
- 39.Chowdhary VR, et al. Characterization of haemorrhagic pulmonary capillaritis: another manifestation of Pristane-induced lupus. Rheumatology (Oxford) 2007;46:1405–1410. doi: 10.1093/rheumatology/kem117. [DOI] [PubMed] [Google Scholar]
- 40.Richards HB, et al. Interferon-gamma promotes lupus nephritis in mice treated with the hydrocarbon oil pristane. Kidney Int. 2001;60:2173–2180. doi: 10.1046/j.1523-1755.2001.00045.x. [DOI] [PubMed] [Google Scholar]
- 41.Calvani N, et al. Nephritogenic autoantibodies but absence of nephritis in IL-12p35-deficient mice with pristane-induced lupus. Kidney Int. 2003;64:897–905. doi: 10.1046/j.1523-1755.2003.00178.x. [DOI] [PubMed] [Google Scholar]
- 42.Clynes R, et al. Uncoupling of immune complex formation and kidney damage in autoimmune glomerulonephritis. Science (Wash. D. C. ) 1998;279:1052–1054. doi: 10.1126/science.279.5353.1052. [DOI] [PubMed] [Google Scholar]
- 43.Peterson KS, et al. Characterization of heterogeneity in the molecular pathogenesis of lupus nephritis from transcriptional profiles of laser-captured glomeruli. J. Clin. Invest. 2004;113:1722–1733. doi: 10.1172/JCI19139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kulkarni O, et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J. Am. Soc. Nephrol. 2007;18:2350–2358. doi: 10.1681/ASN.2006121348. [DOI] [PubMed] [Google Scholar]
- 45.Satoh M, et al. Fas and Fas ligand mutations inhibit autoantibody production in pristane-induced lupus. J. Immunol. 2000;165:1036–1043. doi: 10.4049/jimmunol.165.2.1036. [DOI] [PubMed] [Google Scholar]
- 46.Vingsbo-Lundberg C, et al. Genetic control of arthritis onset, severity and chronicity in a model for rheumatoid arthritis in rats. Nat. Genet. 1998;20:401–404. doi: 10.1038/3887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ioannou Y, Isenberg DA. Current evidence for the induction of autoimmune rheumatic manifestations by cytokine therapy. Arthritis Rheum. 2000;43:1431–1442. doi: 10.1002/1529-0131(200007)43:7<1431::AID-ANR3>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 48.Ronnblom LE, et al. Autoimmunity after alpha-interferon therapy for malignant carcinoid tumors. Ann. Intern. Med. 1991;115:178–183. doi: 10.7326/0003-4819-115-3-178. [DOI] [PubMed] [Google Scholar]
- 49.Pestka S, et al. Interferons, interferon-like cytokines, and their receptors. Immunol. Rev. 2004;202:8–32. doi: 10.1111/j.0105-2896.2004.00204.x. [DOI] [PubMed] [Google Scholar]
- 50.Hooks JJ, et al. Immune interferon in the circulation of patients with autoimmune disease. N. Engl. J. Med. 1979;301:5–8. doi: 10.1056/NEJM197907053010102. [DOI] [PubMed] [Google Scholar]
- 51.Baechler EC, et al. Interferon-inducible gene expression signature in peripheral blood cells of patients with severe lupus. Proc. Natl. Acad. Sci. U. S. A. 2003;100:2610–2615. doi: 10.1073/pnas.0337679100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bennett L, et al. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J. Exp. Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Feng X, et al. Association of increased interferon-inducible gene expression with disease activity and lupus nephritis in patients with systemic lupus erythematosus. Arthritis Rheum. 2006;54:2951–2962. doi: 10.1002/art.22044. [DOI] [PubMed] [Google Scholar]
- 54.Kirou KA, et al. Activation of the interferon-alpha pathway identifies a subgroup of systemic lupus erythematosus patients with distinct serologic features and active disease. Arthritis Rheum. 2005;52:1491–1503. doi: 10.1002/art.21031. [DOI] [PubMed] [Google Scholar]
- 55.Zhuang H, et al. Association of anti-nucleoprotein autoantibodies with upregulation of Type I interferon-inducible gene transcripts and dendritic cell maturation in systemic lupus erythematosus. Clin. Immunol. 2005;117:238–250. doi: 10.1016/j.clim.2005.07.009. [DOI] [PubMed] [Google Scholar]
- 56.Mathian A, et al. IFN-alpha induces early lethal lupus in preautoimmune (New Zealand Black × New Zealand White) F1 but not in BALB/c mice. J. Immunol. 2005;174:2499–2506. doi: 10.4049/jimmunol.174.5.2499. [DOI] [PubMed] [Google Scholar]
- 57.Santiago-Raber ML, et al. Type-I interferon receptor deficiency reduces lupus-like disease in NZB mice. J. Exp. Med. 2003;197:777–788. doi: 10.1084/jem.20021996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jorgensen TN, et al. Type I interferon signaling is involved in the spontaneous development of lupus-like disease in B6.Nba2 and (B6.Nba2 × NZW)F(1) mice. Genes Immun. 2007;8:653–662. doi: 10.1038/sj.gene.6364430. [DOI] [PubMed] [Google Scholar]
- 59.Banchereau J, Pascual V. Type I interferon in systemic lupus erythematosus and other autoimmune diseases. Immunity. 2006;25:383–392. doi: 10.1016/j.immuni.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 60.Nacionales DC, et al. Type I interferon production by tertiary lymphoid tissue developing in response to 2, 6, 10, 14 tetramethylpentadecane (pristane). Am. J. Pathol. 2006;168:1227–1240. doi: 10.2353/ajpath.2006.050125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thibault DL, et al. IRF9 and STAT1 are required for IgG autoantibody production and B cell expression of TLR7 in mice. J. Clin. Invest. 2008;118:1417–1426. doi: 10.1172/JCI30065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lee PY, et al. A novel type I IFN-producing cell subset in murine lupus. J. Immunol. 2008;180:5101–5108. doi: 10.4049/jimmunol.180.7.5101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lee PY, et al. TLR7-dependent and FcgammaR-independent production of type I interferon in experimental mouse lupus. J. Exp. Med. 2008;205:2995–3006. doi: 10.1084/jem.20080462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bave U, et al. Fc gamma RIIa is expressed on natural IFN-alpha-producing cells (plasmacytoid dendritic cells) and is required for the IFN-alpha production induced by apoptotic cells combined with lupus IgG. J. Immunol. 2003;171:3296–3302. doi: 10.4049/jimmunol.171.6.3296. [DOI] [PubMed] [Google Scholar]
- 65.Sunderkotter C, et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 2004;172:4410–4417. doi: 10.4049/jimmunol.172.7.4410. [DOI] [PubMed] [Google Scholar]
- 66.Benech P, et al. 3′ end structure of the human (2′−5′) oligo A synthetase gene: prediction of two distinct proteins with cell type-specific expression. Nucleic Acids Res. 1985;13:1267–1281. doi: 10.1093/nar/13.4.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J. Clin. Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Swirski FK, et al. Ly-6C monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J. Clin. Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Drevets DA, et al. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J. Immunol. 2004;172:4418–4424. doi: 10.4049/jimmunol.172.7.4418. [DOI] [PubMed] [Google Scholar]
- 70.Kawai T, Akira S. Innate immune recognition of viral infection. Nat. Immunol. 2006;7:131–137. doi: 10.1038/ni1303. [DOI] [PubMed] [Google Scholar]
- 71.Means TK, et al. Human lupus autoantibody-DNA complexes activate DCs through cooperation of CD32 and TLR9. J. Clin. Invest. 2005;115:407–417. doi: 10.1172/JCI23025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Savarese E, et al. U1 small nuclear ribonucleoprotein immune complexes induce type I interferon in plasmacytoid dendritic cells through TLR7. Blood. 2006;107:3229–3234. doi: 10.1182/blood-2005-07-2650. [DOI] [PubMed] [Google Scholar]
- 73.Vollmer J, et al. Immune stimulation mediated by autoantigen binding sites within small nuclear RNAs involves Toll-like receptors 7 and 8. J. Exp. Med. 2005;202:1575–1585. doi: 10.1084/jem.20051696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Savarese E, et al. Requirement of Toll-like receptor 7 for pristane-induced production of autoantibodies and development of murine lupus nephritis. Arthritis Rheum. 2008;58:1107–1115. doi: 10.1002/art.23407. [DOI] [PubMed] [Google Scholar]
- 75.Pisitkun P, et al. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science (Wash. D. C. ) 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 76.Herrmann M, et al. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 77.Vallin H, et al. Anti-double-stranded DNA antibodies and immunostimulatory plasmid DNA in combination mimic the endogenous IFN-alpha inducer in systemic lupus erythematosus. J. Immunol. 1999;163:6306–6313. [PubMed] [Google Scholar]
- 78.Barrat FJ, et al. Nucleic acids of mammalian origin can act as endogenous ligands for Toll-like receptors and may promote systemic lupus erythematosus. J. Exp. Med. 2005;202:1131–1139. doi: 10.1084/jem.20050914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Clynes R, et al. Modulation of the immune response in pristane-induced lupus by expression of activation and inhibitory Fc receptors. Clin. Exp. Immunol. 2005;141:230–237. doi: 10.1111/j.1365-2249.2005.02847.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bly JE, et al. Pristane induced changes in rat lymphocyte membrane fluidity. Cancer Biochem. Biophys. 1990;11:145–154. [PubMed] [Google Scholar]
- 81.Kratz A, et al. Chronic inflammation caused by lymphotoxin is lymphoid neogenesis. J. Exp. Med. 1996;183:1461–1472. doi: 10.1084/jem.183.4.1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat. Rev. Immunol. 2006;6:205–217. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 83.Schroder AE, et al. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc. Natl. Acad. Sci. U. S. A. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gause A, et al. The B lymphocyte in rheumatoid arthritis: analysis of rearranged V kappa genes from B cells infiltrating the synovial membrane. Eur. J. Immunol. 1995;25:2775–2782. doi: 10.1002/eji.1830251010. [DOI] [PubMed] [Google Scholar]
- 85.William J, et al. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science (Wash. D. C. ) 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 86.Horsfall AC, et al. Autoantibody synthesis in salivary glands of Sjogren's syndrome patients. J. Autoimmun. 1989;2:559–568. doi: 10.1016/0896-8411(89)90189-3. [DOI] [PubMed] [Google Scholar]
- 87.Randen I, et al. The identification of germinal centres and follicular dendritic cell networks in rheumatoid synovial tissue. Scand. J. Immunol. 1995;41:481–486. doi: 10.1111/j.1365-3083.1995.tb03596.x. [DOI] [PubMed] [Google Scholar]
- 88.Jego G, et al. Plasmacytoid dendritic cells induce plasma cell differentiation through type I interferon and interleukin 6. Immunity. 2003;19:225–234. doi: 10.1016/s1074-7613(03)00208-5. [DOI] [PubMed] [Google Scholar]
- 89.Leak LV, et al. Response of the peritoneal mesothelium to the mineral oil, pristane. Curr. Top. Microbiol. Immunol. 1985;122:221–233. doi: 10.1007/978-3-642-70740-7_32. [DOI] [PubMed] [Google Scholar]
- 90.Weinstein JS, et al. Colocalization of antigen-specific B and T cells within ectopic lymphoid tissue following immunization with exogenous antigen. J. Immunol. 2008;181:3259–3267. doi: 10.4049/jimmunol.181.5.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat. Rev. Immunol. 2008;8:22–33. doi: 10.1038/nri2217. [DOI] [PubMed] [Google Scholar]
- 92.Ettinger R, et al. IL-21 induces differentiation of human naive and memory B cells into antibody-secreting plasma cells. J. Immunol. 2005;175:7867–7879. doi: 10.4049/jimmunol.175.12.7867. [DOI] [PubMed] [Google Scholar]
- 93.Cassese G, et al. Inflamed kidneys of NZB / W mice are a major site for the homeostasis of plasma cells. Eur. J. Immunol. 2001;31:2726–2732. doi: 10.1002/1521-4141(200109)31:9<2726::aid-immu2726>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]