Abstract
Pain normally subserves a vital role in the survival of the organism, prompting the avoidance of situations associated with tissue damage. However, the sensation of pain can become dissociated from its normal physiological role. In conditions of neuropathic pain, spontaneous or hypersensitive pain behavior occurs in the absence of the appropriate stimuli. Our incomplete understanding of the mechanisms underlying chronic pain hypersensitivity accounts for the general ineffectiveness of currently available options for the treatment of chronic pain syndromes. Despite its complex pathophysiological nature, it is clear that neuropathic pain is associated with short- and long-term changes in the excitability of sensory neurons in the dorsal root ganglia (DRG) as well as their central connections. Recent evidence suggests that the upregulated expression of inflammatory cytokines in association with tissue damage or infection triggers the observed hyperexcitability of pain sensory neurons. The actions of inflammatory cytokines synthesized by DRG neurons and associated glial cells, as well as by astrocytes and microglia in the spinal cord, can produce changes in the excitability of nociceptive sensory neurons. These changes include rapid alterations in the properties of ion channels expressed by these neurons, as well as longer-term changes resulting from new gene transcription. In this chapter we review the diverse changes produced by inflammatory cytokines in the behavior of sensory neurons in the context of chronic pain syndromes.
Keywords: Cytokine, Chemokine, DRG, Pain, Inflammation
1 Introduction
Primary afferent sensory neurons are responsible for processing important sensory information, including temperature, touch, proprioception, and pain. The cell bodies of these pseudounipolar neurons are found in the dorsal root ganglia (DRG), which are situated outside the central nervous system. DRG neurons exhibit a wide range of sizes and degrees of myelination. Neurons that transmit afferent information about potentially damaging stimuli that lead to the perception of pain are known as “nociceptors” (noci- is derived from the Latin for “hurt”). These nociceptive sensory neurons are subdivided into two groups on the basis of nerve fiber types: (1) fast conducting myelinated Aδ-fibers, which convey the initial stimulus of nociception (mechanosensitive or mechanothermal), and (2) slowly conducting, unmyelinated C-fibers, which transmit a less intense nociceptive sensation. Nociceptors have both a peripheral connection innervating potentially diseased or traumatized nerves, muscles, tendons, organs, and epithelia, and a centrally projecting axon that enters the central nervous system. This central axon conveys “nociceptive” information to second-order neurons in the dorsal horn of the spinal cord. Neural connections from the dorsal horn to the thalamus and from there to the cortex relay this noxious information to higher centers of conscious and emotional experience. The central axons of primary afferent nociceptive neurons also provide information to polysynaptic spinal cord interneurons, which are essential for the initiation of the nociceptive withdrawal reflex. These neurons trigger motor reflexes that are important for the avoidance of potentially harmful painful stimuli. Descending pathways originating in the cortex and/or midbrain provide modulatory feedback signals at the level of the spinal cord that also regulate the nociceptive experience, thereby providing a closed loop feedback control of this behavior. Additionally, impulses can travel back along the peripheral axon of the nociceptive sensory neuron toward the distal nerve endings, resulting in the local release of neuropeptides in the injury environment. This neuropeptide release produces vasodilatation, venule permeability, plasma extravasation, edema, and leukocyte influx – a process termed “neurogenic inflammation” (Fig. 1).
Fig. 1.
Nociceptive pathways in the dorsal root ganglia (DRG) and central nervous system. Axons of nociceptors transmit information from the periphery to second-order neurons in the dorsal horn of the spinal cord. Neural connections from the dorsal horn to the thalamus and from there to the cortex relay this noxious information to higher centers of the central nervous system. The central axons of primary afferent nociceptive neurons also provide information to polysynaptic spinal cord interneurons, which are essential for the withdrawal reflex. Descending pathways originating in the cortex and/or midbrain provide modulatory feedback signals at the level of the spinal cord. Impulses can also travel back along the peripheral axon toward the distal nerve endings, resulting in neurogenic inflammation
Although pain clearly plays an important survival role in safeguarding the individual from potential sources of tissue destruction, the perception of pain can also be the result of a dysfunctional nervous system. Typically, the local response to various types of injury or infection involves the release of peripheral chemical mediators. These injury-associated factors produce two effects. One role is to attract leukocytes to the point of injury as part of the inflammatory response (Charo and Ransohoff 2006), and the other is to sensitize nociceptors, enhancing their responses to painful stimuli (Zimmermann 2001). The increased excitatory activity of nociceptors produces increased transmitter release in the spinal cord, enhancing neuronal activity in pain pathways in the central nervous system, a phenomenon known as spinal sensitization (Woolf 1983). Under some circumstances, nociceptor-driven electrical activity in the spinal cord becomes divorced from normal physiological function and pathological condition, so pain is produced in the absence of any appropriate stimulus (Campbell et al. 1988; Torebjork et al. 1992). This is now known as pathological, or “neuropathic,” pain.
Neuropathic pain is experienced in association with many types of injury to the nervous system or as a consequence of diabetes, cancer, infectious agents (e.g., HIV-1), or the toxic side effects of diverse drug regimens. From the behavioral point of view, neuropathic pain is associated with different types of painful responses to a mechanical stimulus, including allodynia (pain evoked by a normally innocuous stimulus) and hyperalgesia (enhanced pain evoked by a noxious stimulus).
From the therapeutic point of view, neuropathic pain is an extremely intractable problem. Once established, pain of this type is not readily susceptible to treatment with nonsteroidal anti-inflammatory drugs. Moreover, although opiates may be employed acutely or for chronic pain states (e.g., terminal cancer), alleviation of neuropathic pain is more problematic as high doses are often required, narrowing the therapeutic index (Hempenstall et al. 2005). The remaining available drugs used to treat these syndromes (tricyclic antidepressants, antiepileptics) are not particularly effective and are also associated with a number of negative side effects (Watson 2000). Hence, a complete understanding of the cellular and molecular processes involved in the development of neuropathic pain is essential for the development of novel therapies.
In general, neuropathic pain is the result of abnormal activity of nociceptive neurons. This activity is thought to initially result from the increased neuronal expression and activation of ion channels and receptors that mediate the abnormal generation of action potentials and synaptic transmission in primary afferent nociceptive neurons and/or other parts of the pain pathway. But what causes these changes to occur? It is presumed that some peripheral event provokes primary afferent nociceptive neurons to express different sets of genes, resulting in a new and abnormal chronically hyperexcitable “pain” phenotype.
It has been shown that peripheral nerve injury (trauma-, disease-, or drug-induced) can trigger a wide variety of cellular changes in sensory neurons and, as we have discussed, neuropathic pain following peripheral nerve injury is a consequence of enhanced excitability associated with the chronic sensitization of nociceptive neurons in the peripheral and central nervous systems. Interestingly, following a peripheral nerve injury, not only a subset of injured (Wall and Devor 1983; Kajander et al. 1992; Kim et al. 1993; Amir et al. 1999), but also neighboring noninjured peripheral sensory neurons exhibit spontaneous, ectopic discharges (Tal and Devor 1992; Sheth et al. 2002; Ma et al. 2003; Obata et al. 2003; Liu and Eisenach 2005; Xie et al. 2005). Abnormal excitability of pain neurons may even extend to the spinal cord dorsal horn contralateral to the nerve injury (Sluka et al. 2001, 2007; Raghavendra et al. 2004; Tanaka et al. 2004; Twining et al. 2004; Romero-Sandoval et al. 2005; Bhangoo et al. 2007a; Jung et al. 2007). Although it is clear that molecular changes in the sensory ganglia and spinal cord dorsal horn are responsible for chronic pain, it remains a mystery as to what event(s) are critical for its development and maintenance.
2 Peripheral Nerve Injury and Inflammation
One important development in our understanding of the cellular and molecular processes that produce neuropathic pain concerns the role of the immune system. Immunity can be dissociated into two different phases – innate and acquired. Acquired immunity involves the phenomenon of immunological memory and includes the antibody and lymphocyte responses to specific antigens. The forerunner to acquired immunity is the innate immune response. This more basic type of immunity involves a generalized immune cell response to a variety of toxic or pathological intrusions into physiological homeostasis. Molecules such as Toll-like receptors (TLRs), Nod-like receptors, and RIG-like receptors expressed by numerous types of cells, including leukocytes, Schwann cells, neurons, astrocytes, and microglia, can recognize shared molecular patterns expressed by infectious agents, cell debris, or other cellular detritus initiating a cascade of cytokine synthesis that orchestrates a general cellular response to these potential problems (Tanga et al. 2005; Creagh and O'Neill 2006; Kim et al. 2007; Tawfik et al. 2007; Watkins et al. 2007b). As noted before, this response is inflammatory in nature and involves the recruitment of leukocytes to areas of tissue damage. The activation of innate immune inflammatory responses is also frequently linked to the development of disease. In the present context, it is believed that the innate immune response to injury plays a prominent role in the establishment of chronic pain states, extending beyond its role in promoting the influx and activation of leukocytes. Although inflammatory and neuropathic pain syndromes are often considered distinct entities, emerging evidence suggests that proinflammatory cytokines produced in association with the innate immune response are clearly implicated in the actual development and maintenance of neuropathic pain, and are a necessary prelude to its development. As such, both neuroinflammatory and associated immune responses following nerve damage may contribute as much to the development and maintenance of neuropathic pain as the initial nerve damage itself.
The traditional view of the post-nerve-trauma environment has been that the influx of leukocytes associated with inflammation was responsible for secreting the chemical mediators that produced pain. However, as we shall discuss, current evidence suggests that the role of the inflammatory response in the generation of pain is not limited to effects produced by the influx of leukocytes per se. Thus, it is currently believed that the proinflammatory cytokines that drive chronic pain behavior may be derived from the cellular elements of the nervous system itself, and that these molecules can act directly on receptors expressed by neurons and other cells of the nervous system (White et al. 2005a). The effects produced by these factors may lead to chronic hyperexcitability and alterations in gene expression by nociceptors, abnormal processing of pain signals, and enhanced pain states. In this way, signaling pathways designed to facilitate a protective response to tissue injury become sources of chronic pathological pain. Generally speaking, the development of chronic pain behavior seems to require the participation of cells in both the peripheral nerve and the dorsal horn of the spinal cord. For example, at various points in time following the initial nerve injury, cytokine synthesis is upregulated in the peripheral nerve, including by DRG satellite cells and nerve-associated Schwann cells, as well as in central elements in the dorsal horn, including microglia and astrocytes (McMahon et al. 2005). Leukocyte influx may also be a participating event. It is clear that complex interactive signaling occurs between various cell types that ultimately results in long-term changes in the excitability of neurons in the pain pathway. Thus, activated sensory neurons can signal to microglia, microglia can signal to neurons, Schwann cells and satellite glial cells can signal to DRG neurons, and vice versa. Ultimately, nociceptive neurons become hyperexcitable and their communication with neurons in the dorsal horn becomes “sensitized.” The molecular signatures of this increased nerve activity involve changes in the complement of receptors and ion channels expressed by neurons as well as the neurotransmitters they use. The molecules that orchestrate these changes are inflammatory cytokines.
The question therefore arises as to exactly which inflammatory cytokines are concerned with the development and maintenance of pain states across time and what molecular signaling processes underlie the development of pain hypersensitivity? Furthermore, which cellular elements in the peripheral nerve or dorsal horn of the spinal cord are responsible for the elaboration of cytokine synthesis and subsequently how do these molecules produce their effects? The innate immune response is associated with the development of a complex cascade of cytokine expression in which many inflammatory mediators are synthesized in a mutually dependent manner. What is the precise order and cellular localization of the molecules involved in such cascades? It is likely that several important cytokines are concerned in the establishment of the phenotype that characterizes neuropathic pain. As we shall now discuss, considerable progress has been made on the identification and mechanism of action of proalgesic cytokines. It is now clear that in response to injury or infection, cytokines can be produced by both neurons and glia and this can occur both peripherally and centrally. Cytokines can also be produced by immune cells that participate in the response to injury, infection, or toxicity. Once synthesized by these different types of cells, cytokines produce both short-term and long-term effects on the excitability of sensory neurons. Some of these effects are produced by the cytokines themselves and some by the upregulated synthesis and release of downstream mediators under their control. Thus, the cytokine response is a complex interlocking series of events that ultimately results in long-term changes in nociceptor behavior.
3 Early Events in Sensory Nerve Cytokine Signaling
As we have discussed, it is clear that many of the events that ultimately give rise to chronic pain hypersensitivity initiate a “cascade” of cytokine production, which in turn produces the observed alterations in sensory neuron behavior. These cytokine cascades appear to start with the production of certain key multifunctional cytokines that initiate and orchestrate the subsequent production of further downstream cytokines and numerous other proalgesic mediators. The first cytokines linked to inflammatory hypernociception have frequently been shown to be interleukin-1β (IL-1β) (Ferreira et al. 1988) and tumor necrosis factor α (TNF-α) (Cunha et al. 1992). These cytokines may produce direct effects on sensory neurons and may give rise to further downstream mediators, including other cytokines, chemokines, prostanoids, neurotrophins, NO, kinins, lipids, ATP, and members of the complement pathway (Park and Vasko 2005; Ma and Quirion 2006; Pezet and McMahon 2006; Levin et al. 2008; White et al. 2007b; Donnelly-Roberts et al. 2008; Ting et al. 2008). Upregulation of IL-1β and TNF-α represents one of the earliest events observed in sensory nerves in response to trauma or infection. For example, after chronic constriction injury to the sciatic nerve, levels of both TNF-α and IL-1β in the injured nerve increased over tenfold within 1 h (Uceyler et al. 2007). Both of these cytokines are capable of upregulating the synthesis of numerous downstream mediators and can produce pain hypersensitivity behaviors when administered locally to the skin, systemically, or into the spinal cord (Opree and Kress 2000; Schafers et al. 2003a, b; McMahon et al. 2005). Indeed, elaboration of local cytokine synthesis appears to be sufficient to produce all of the subsequent molecular changes that underlie chronic pain (White et al. 2007a). On the other hand, inhibition of TNF-α or IL-1β action, using neutralizing antibodies or similar strategies, inhibits the development of chronic pain behavior in a variety of models (Schafers and Sommer 2007). For example, TNF-α antibodies attenuate the development of thermal hyperalgesia and mechanical allodynia in several models of neuropathic pain (Cunha et al. 2007; Sasaki et al. 2007; Zanella et al. 2008). Results such as these have encouraged the view that manipulation of cytokine synthesis at an early point in the development of chronic pain behavior with reagents of this type may have a therapeutic role to play in the treatment of chronic pain syndromes.
Despite an enormous amount of work on inflammatory stimulus-induced cytokine cascades and the development of pain hypersensitivity, very few groups have investigated the mechanisms of maintenance of chronic pain. One recent investigation has provided evidence that there are two distinct mechanisms contributing to the development of chronic pain states. The early mechanistic state is dependent on calcium as the use of a calpain inhibitor can diminish both IL-1β and TNF-α levels 1 h after injury, whereas inhibitors of excitatory synaptic transmission (e.g., with the NMDA receptor blocker MK801) did not affect the cytokine levels. In sharp contrast, MK801 successfully diminished IL-1β and TNF-α levels at 3 days, while calpain inhibitors had no effect (Uceyler et al. 2007). Thus, it is possible that one element of chronic pain maintenance is dependent on the activity of the sensory neurons.
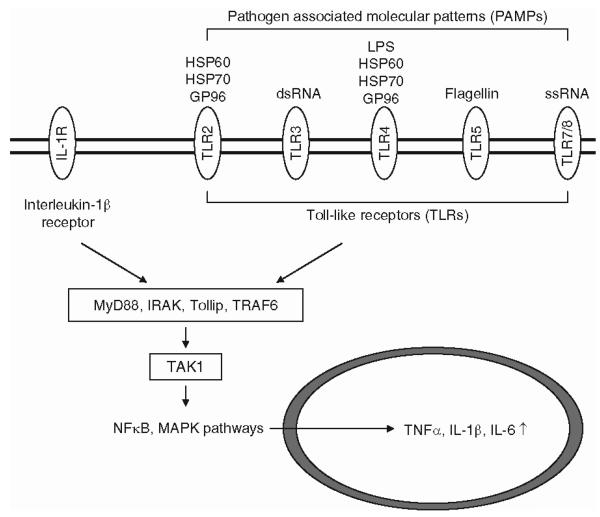
Elucidating the exact sequence of cellular and molecular events that leads to the initiation of pain-related cytokine cascades is clearly an important task. It is reasonable to ask what kinds of molecular mechanisms are directly proximal to the original insult and serve as the initiators of all of these subsequent events. Although the answer to this question in not completely clear, there is good evidence that the earliest events are the same as those identified as upstream initiating signals that trigger the innate immune response. For example, the activation of TLRs is one possible entry point into the cytokine pathway that results in the upregulated synthesis of master pleiotropic cytokines such as TNF-α, IL-1β, and frequently also interleukin-6 (IL-6) (Fig. 2). This makes sense because as we discussed earlier, TLRs are initiators of the innate immune response in numerous other cases as well (Martin and Wesche 2002; Guo and Schluesener 2007). The ligands that activate TLRs are pathogen-associated epitopes, or “pathogen-associated molecular patterns.” These elements include molecular components of viruses such as nucleic acids which can activate TLRs 7/8 (single-stranded RNA) or TLR3 (double-stranded RNA). Similarly, molecules from bacteria, including cell wall components such as lipopolysaccharide from Gram-negative bacteria, can activate TLR4. TLR5 mediates the immune response to bacterial flagellins. In addition, molecules released from cells undergoing stress or degradation following injury may also act as signals (Dalpke and Heeg 2002). These include toxicity- or injury-induced release of the heat shock proteins HSP60, HSP70 and/or GP96, which, in turn, activate TLR2 and TLR4 (Vabulas et al. 2002).
Fig. 2.
The Toll-like receptor (TLR) pathway. TLR activation initiates the innate immune response, which is thought to lead to the detrimental cytokine cascade in chronic pain. Activation of TLR signaling proceeds via a family of adaptor proteins which includes the protein MyD88, the interleukin-1 receptor associated kinase, Toll interacting protein, and the adapter protein TRAF6. These proteins activate the kinase TAK1, which ultimately leads to the activation of signaling cascades including nuclear factor κB and mitogen activated protein kinase (MAPK) pathways. The activation of these pathways can then direct the synthesis of cytokines such as tumor necrosis factor α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6). Both TLRs and receptors for IL-1β have similar structures and share a cytoplasmic motif. Activation of both types of receptors recruits a scaffolding complex that leads to upregulation of the production of similar cytokines
Thirteen functional TLRs are known to exist in mammals and several of these forms are expressed by different types of cells known to be important in generating chronic pain behavior (Martin and Wesche 2002; Guo and Schluesener 2007). These include DRG neurons, microglia, astrocytes, Schwann cells, different types of leukocytes, and different peripheral target tissues such as skin (keratinocytes). TLRs are single-pass transmembrane proteins that exist as preassembled homodimers or heterodimers depending on the situation. In some cases, further ancillary membrane proteins are also required for the binding of pathogen-associated molecular patterns and resulting TLR activation. This includes the requirement for the proteins MD-2 and CD14 in binding of lipopolysaccharide by TLR4. Activation of TLR signaling proceeds via a family of adaptor proteins that produce activation of protein kinases and ultimately of transcription factors such as nuclear factor κB. These transcription factors can then direct the synthesis of cytokines such as TNF-α at the gene transcriptional level. Interestingly, elements of TLR “signalosome” are shared with signaling intermediates produced by activation of the IL-1β receptor (IL-1R) (Boraschi and Tagliabue 2006). IL-1βR is also a dimer consisting of two chains, the IL-1R type 1 (IL-1R1) and the IL-1R accessory protein (IL-1RacP). Both TLRs and receptors for IL-1β have similar structures and share a cytoplasmic motif, the Toll/IL-r receptor (TIR) domain. Activation of both types of receptors recruits a scaffolding complex that includes the protein MyD88, the interleukin-1 receptor associated kinase (IRAK), the Toll interacting protein (Tollip) and the adapter protein TRAF6. Recruitment and activation of this signaling complex then leads to activation of the kinase TAK1 that produces phosphorylation and activation of regulatory kinases in different important downstream signaling pathways such as those involved in nuclear factor κB or mitogen activated protein kinase (MAPK) activity. Activation of such signaling pathways ultimately leads to upregulation of the production of cytokines such as TNF-α or IL-1β itself. The fact that TLRs and IL-1Rs share downstream signaling motifs means that activation of TLRs produces a great deal of amplification of their own signaling consequences. The involvement of TLR function in the generation of neuropathic pain is highlighted by recent observations demonstrating reduced pain behavior and inflammatory cytokine upregulation in the spinal cords of TLR2 and four knockout mice (Tanga et al. 2005; Kim et al. 2007). These data imply that damaged, infected, or poisoned neurons or glia release factors that activate TLRs, leading to the synthesis and release of TNF-α and other important cytokines. TLR2 and TLR4, which have been particularly implicated in these events, are expressed by microglia (Tanga et al. 2005; Kim et al. 2007). This interaction implies that cytokine production by these cells in particular may be very early events in the pathway leading from injury to aberrant pain behavior.
If the early production of cytokines is important in the generation of pain we must then ask exactly how such molecules produce the observed phenotypic changes in peripheral nerves and the central connections that underlie pain. It is clear that both sensory neurons and the peripheral and central glial cells associated with them are capable of both elaborating and responding to TNF-α, suggesting a complex network of interdependent signaling occurs between these various cellular elements of sensory nerves (Schafers et al. 2003a, b; Ohtori et al. 2004; Takeda et al. 2007). As we shall discuss further, cytokines such as TNF-α clearly produce changes in the behavior of sensory neurons at a variety of levels. Some of the long-term changes in behavior require alterations in gene transcription and protein expression. However, it is also clear that TNF-α and other upstream cytokines can produce very rapid changes in neuronal excitability which do not seem to require alterations in gene transcription. These changes in excitability probably arise from direct effects of cytokine signaling on the properties of important ion channels, including voltage-dependent sodium channels and transient receptor potential (TRP) channels expressed by sensory nerves (Fig. 3). Presumably, these kinds of effects are the earliest influences on nociceptor excitability produced by cytokines once they have been synthesized and released. For example, perfusion of DRG in vitro with TNF-α produces a rapid increase in A- and C-fiber discharge and also a rapid increase of calcitonin gene-related peptide (CGRP) release from the terminals of nociceptors in the spinal cord (Opree and Kress 2000). How might such rapid effects on neuronal excitability be produced? TNF-α produces its effects via the activation of two TNF-α receptor subtypes, TNFR1 and TNFR2 (MacEwan 2002). TNFR1 is expressed exclusively on neuronal cells and the TNFR2 is mostly expressed on macrophages and/or monocytes in the DRG under inflammatory conditions (Li et al. 2004). Actions via TNFR1 r appear to be the most relevant to the development of pain behavior because (1) mechanical hyperalgesia induced by exogenous TNF-α or by inflammation is reduced in TNFR1 but not in TNFR2 knockout mice and (2) TNFR1 but not TNFR2 neutralizing antibodies as well as antisense RNA against TNFR1 can reduce experimentally induced hyperalgesia (Sommer et al. 1998; Parada et al. 2003). How can the action of TNF-α on sensory neurons produce rapid changes in excitability? Interestingly, the ability of TNF-α to produce thermal, but not mechanical sensitization was reduced in TRP vanilloid 1 (TRPV1) knockout mice, suggesting that another conductance was the target underlying TNF-α induced mechanical pain hypersensitivity (Jin and Gereau 2006). Consistent with this idea, it was also observed that application of TNF-α to DRG neurons in culture produced a rapid (within 1 min) enhancement of the amplitude of the tetrodotoxin (TTX)-resistant sodium current in these cells (Jin and Gereau 2006). As with TLRs and IL-1R discussed above, activation of TNF-α receptors produces a wide array of signaling options, including activation of the MAPK pathway. In the present context, it was observed that inhibitors of the p38 MAPK could selectively abolish TNF-α induced mechanical pain hypersensitivity and enhancement of the DRG TTX-resistant sodium current. These data suggest a model in which the rapid effects of TNF-α might involve activation of TNFR1 expressed by nociceptors leading to enhanced mechanical hypersensitivity mediated by p38-induced phosphorylation of TTX-resistant sodium current subunits together with thermal hypersensitivity produced by actions on TRPV1 (Jin and Gereau 2006).
Fig. 3.
Possible molecular mechanisms of TNF-α action. TNF-α produces changes in the behavior of sensory neurons at a variety of levels. While some long-term changes in behavior require alterations in gene transcription and protein expression, TNF-α and other upstream cytokines can produce very rapid changes in neuronal excitability as well. These changes in excitability probably arise from direct effects of cytokine signaling on the properties of important ion channels, including voltage-dependent sodium channels and transient receptor potential (TRP) channels expressed by sensory nerves. Activation of TNF-α receptors (TNFRs) produces a wide array of signaling options beginning with recruitment of TNFR-associated death domain protein, receptor-interacting protein, and TNFR-associated factor 2. These proteins go on to activate extracellular-signal-related kinase/MAPK, p38/MAPK, and NFκB pathways
In keeping with this hypothesis, it is clear that treatment of DRG neurons with TNF-α also produces rapid upregulation of TRPV1 function and expression. While addition of TNF-α to cultured DRG neurons alone did not directly lead to the release of CGRP, the addition of a thermal stimulus enhanced the release of this neuropeptide, implying that rapid transactivation of TRPV1 by TNF-α can also occur (Jin and Gereau 2006; Hensellek et al. 2007). In addition, TNF-α was also able to produce subsequent upregulation of TRPV1 protein expression when applied to cultured DRG neurons employing a pathway involving extracellular-signal-related kinase rather than p38 signaling. However, this effect required chronic treatment of the cells (more than 8 h) (Jin and Gereau 2006; Hensellek et al. 2007). Hence, it is clear that TNF-α can produce both rapid and long-term excitatory effects on DRG neurons through a variety of molecular mechanisms. The observation that TNF-α expression in DRG neurons, as well as by microglia (Ohtori et al. 2004; Jin and Gereau 2006), is an early event following tissue injury suggests that rapid autocrine excitation of DRG nociceptors by TNF-α may be of importance in the initiation of the cytokine-mediated cascade that eventually results in pain hypersensitivity. The multiple cellular sources of TNF-α together with the multiple effects it can produce on DRG excitability over a broad time course illustrate the complex nature of the impact of inflammatory cytokines on the function of pain sensory neurons.
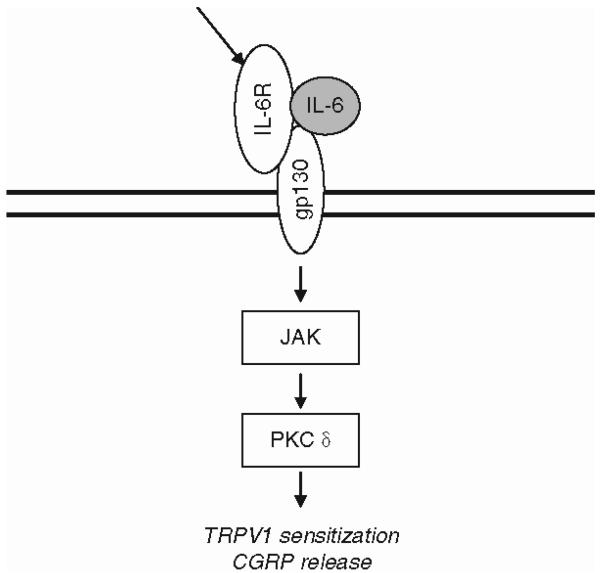
It is possible that other important upstream cytokines can also produce rapid excitatory signaling in DRG neurons. For example, it is known that DRG neurons can express IL-1β and IL-6 under some circumstances, as well as components of the IL-1R and IL-6 receptor complexes, suggesting that both of these cytokines may also produce direct effects on DRG neuron excitability (Gadient and Otten 1996; Inoue et al. 1999; Gardiner et al. 2002; Lee et al. 2004; Li et al. 2005; Nilsson et al. 2005) (Fig. 4). In the case of IL-6, DRG neurons have been shown to express the glycoprotein 130 (gp130) cytokine receptor subunit, a common feature of all cytokine receptors in the IL-6 family (Gadient and Otten 1996; Thompson et al. 1998; Gardiner et al. 2002; Summer et al. 2008). DRG neurons also express the gp130 binding subunit for the IL-6 related cytokine leukemia inhibitory factor, although the binding component for IL-6 itself (glycoprotein 80) has not been detected (Opree and Kress 2000; Gardiner et al. 2002). Addition of IL-6 to cultured DRG neurons was not effective by itself, but was able to rapidly (minutes) sensitize TRPV1 conductances to heat as well as to stimulate CGRP release, provided that the IL-6 was first primed with a soluble fragment of its receptor (Obreja et al. 2005). Appropriately, for a gp130-linked receptor, Janus kinase and protein kinase C inhibitors inhibited the enhancement of TRPV1 sensitivity. The fact that IL-6 only functions when added together with a soluble form of its binding subunit suggests that it may be activated in trans by soluble IL-6 receptors secreted from other cell types in the vicinity.
Fig. 4.
Rapid effects of the IL-6 pathway. DRG neurons can express IL-6 under some circumstances, as well as components of the IL-6 receptor complexes, suggesting that it can also produce direct effects on DRG neuron excitability. DRG neurons have been shown to express the glycoprotein 130 cytokine receptor subunit, a common feature of all cytokine receptors in the IL-6 family. IL-6 is able to rapidly sensitize vanilloid 1 (TRPV1) conductances to heat as well as to stimulate calcitonin gene-related peptide release via Janus kinase and protein kinase Cd pathways. It is likely that the binding portion of the IL-6 receptor can be provided in trans
IL-1β was also unable to increase DRG excitability by itself, but as with IL-6 and TNF-α, produced rapid increases in the sensitivity of TRPV1 and heat-activated CGRP release, implying that IL-1β can also transactivate TRPV1 expressed by DRG neurons (Obreja et al. 2002). It appears that DRG neurons express all the molecular components required for IL-1β signaling and these can be upregulated in inflammatory pain states (Inoue et al. 1999; Li et al. 2005). In the related trigeminal ganglia, it was observed that following the induction of inflammation with complete Freund's adjuvant, IL-1β was highly expressed by satellite glial cells, whereas IL-1R was expressed in the cell bodies of trigeminal neurons. Addition of IL-1β produced rapid excitation of these neurons. Moreover, an IL-1β antagonist reduced complete Freund's adjuvant induced neuronal hyperexcitability, again suggesting a role for cytokine signaling in the development of hyperexcitability of pain sensory neurons (Takeda et al. 2008). In summary, the major upstream cytokines that are rapidly induced in association with the innate immune response can excite DRG neurons by a variety of mechanisms. Some of these effects are too rapid to involve effects on gene transcription and are likely to involve kinase regulation of important conductances such as sodium currents and TRP channels. Molecules such as TNF-α, IL-1β, and IL-6 can be rapidly upregulated by microglia in the spinal cord and frequently by peripheral elements such as the sensory neurons themselves or their associated glial cells. Whatever the cellular source of the cytokines produced in response to injury, increases in sensory neuron excitability are likely to be one of the first cytokine-induced effects that lead to changes in neuronal phenotypes underlying chronic pain. Moreover, these same cytokines may also have rapid electrophysiological effects on second-order neurons in the dorsal horn, so effects on neuronal excitability induced by cytokines may be an early feature of the cytokine response in pain at numerous points in the neuraxis (Kawasaki et al. 2008).
4 Chemokines, Glia, and Chronic Pain
The previous discussion focused on the role of upstream cytokines and their receptors expressed by neurons in particular. It is clear that these molecules are expressed by other types of cells in the DRG and central nervous system, which may also participate in the development and maintenance of neuropathic pain. Some cytokine/receptor signaling events following peripheral injury or infection appear to be primarily mediated by molecular and/or morphological remodeling of glial cells that in turn become a source of inflammatory mediators. It has been proposed that such “activated” Schwann cells, DRG satellite cells, astrocytes, and microglia also play an essential role in the development of chronic pain hypersensitivity (Watkins and Maier 2003). Indeed, drugs that inhibit the activation of these cells have also been reported to interfere with the development of chronic pain behavior, presumably by suppressing the release of inflammatory mediators such as cytokines associated with their activation.
A real “paradigm shift” in pain research has been the recognition that reciprocal communication between neurons and microglia is important in regulating the quiescent and reactive states of glial cells. Glial receptors for inflammatory cytokines, ATP, neuropeptides, neurotransmitters, neurotrophic factors, and chemokines appear to contribute to these events. A clear example of signaling between DRG neurons and microglia in the spinal cord involves the chemokine fractalkine/CX3CL1 and its receptor CX3CR1(Verge et al. 2004; Zhuang et al. 2007). Fractalkine has an unusual structure for a chemokine in that it is tethered to the membrane by means of a transmembrane mucin-like stalk. Normally fractalkine is expressed by neurons and its receptor is particularly highly expressed by microglia (Verge et al. 2004). Fractalkine can signal to its receptor on target microglia in a “tethered” state or it can be released following proteolytic cleavage producing a soluble form of the chemokine that can act at a distance (Milligan et al. 2004). Injection of fractalkine into the spinal cord produces pain hypersensitivity. Moreover, production of soluble fractalkine has been observed in some chronic pain models (Milligan et al. 2004, 2005; Lindia et al. 2005; Zhuang et al. 2007). Recent studies have revealed how fractalkine may act in vivo. It has been demonstrated that the enzyme cathepsin S, which can cleave tethered membrane-bound fractalkine to its soluble form, can itself be released from activated microglia (Clark et al. 2007). The released fractalkine can then act upon microglia to upregulate the release of proallodynic inflammatory mediators such as those discussed above. Thus, fractalkine may act as a neuron to a microglia messenger that amplifies ongoing pain-producing mechanisms. This model also illustrates the fact that neurons and glia can interact in a variety of complex ways to elaborate ongoing pain stimuli producing the mediators that may then initiate transcriptional and other changes resulting in chronic neuronal hyperexcitability and pain. In addition to the example of fractalkine, activated spinal microglia also express C-C chemokine receptor 2 (CCR2) and C-X-C chemokine receptor 3 (CXCR3), making them potential targets for the chemokines monocyte chemotactic protein 1 (MCP-1) or interferon-g inducing protein 10 (IP-10) upregulated and released from DRG neurons, (Abbadie et al. 2003; Flynn et al. 2003; Tanuma et al. 2006), as we shall now discuss.
5 Downstream Cytokine Signaling
A relatively novel family of cytokines that has now been linked to the induction and maintenance of chronic pain are the chemotactic cytokines (chemokines). Chemokines are small, secreted proteins that exert all of their known effects through the activation of G protein coupled receptors. Chemokines were originally identified as migrational effectors for the attraction of different classes of leukocytes in association with the development of inflammation (Charo and Ransohoff 2006). Several subfamilies of chemokines and their receptors are known to exist. Most chemokines are not constitutively expressed at high levels, their production and secretion being normally associated with activation of the inflammatory response. Thus, the synthesis of most chemokines is usually greatly stimulated through the action of one of the major upstream inflammatory cytokines discussed earlier. An exception to this rule is the chemokine stromal cell derived factor 1 (SDF-1/CXCL12). SDF-1 is the most evolutionarily ancient member of the chemokine family and existed phylo-genetically prior to the development of an immune system, indicating that chemokine signaling originally played a role other than the regulation of leukocyte chemotaxis (Huising et al. 2003; Knaut et al. 2003). Still, chemotaxis appears to be one ancient function of this chemokine as well. In mammals, SDF-1 signaling through its major receptor, C-X-C chemokine receptor 4 (CXCR4), has been shown to be important for the development of the embryo where SDF-1 regulates the migration of the stem/progenitor cells that form numerous tissues (Tachibana et al. 1998; Lu et al. 2002). Postnatally, this signaling system is still used in this way to retain hematopoietic stem cells in the bone marrow (Wright et al. 2002).
Although chemokines clearly have a central role in orchestrating the normal inflammatory response, the pathogenesis of many chronic inflammatory conditions such as atherosclerosis, arthritis, and inflammatory bowel disease has been shown to be mediated in large part by the actions of chemokines (Charo and Ransohoff 2006). In addition, numerous neurological conditions which are accompanied by activation of the innate immune response during their onset or progression appear to involve the action of chemokines in their pathogenesis. These include autoimmune disorders (e.g., multiple sclerosis), neurodegenerative disorders (e.g., cerebral ischemic injury, Parkinson's, Huntington's, and Alzheimer's diseases) as well as virus-based diseases (e.g., HIV-1 and herpes simplex) (Streit et al. 2001; Cartier et al. 2005; Ubogu et al. 2006). This chemokine-mediated component is also likely to extend to the pathogenesis and maintenance of chronic pain in both disease-related conditions (e.g., multiple sclerosis, HIV-1, and herpes simplex) and following trauma, all of which are associated with innate immune responses and prolonged expression of chemokines and their receptors by the cellular elements of the nervous system (White et al. 2005a). This being the case, interference with chemokine function represents a promising approach for the development of both novel anti-inflammatory medication and the treatment of chronic pain conditions.
6 Chemokines and Their Receptors in Acute and Chronic Pain
There is now a large amount of data indicating that chemokines and their receptors can influence both the acute and chronic phases of pain. However, why is chemokine function of particular interest in this regard? It has become apparent that the cellular elements of the nervous system (e.g., neurons, glia, and microglia) are able to both synthesize and respond to chemokines, something that is quite independent of their traditional role in the regulation of leukocyte chemotaxis and function. Oh et al. (2001) first demonstrated that the simple injection of the chemokines SDF-1, regulated upon activation, normal T cell expressed, and secreted (RANTES/CCL5), or macrophage inflammatory protein 1α (MIP-1α/CCL3) into the adult rat hind paw produced dose-dependent tactile allodynia. These authors also demonstrated that cultured DRG neurons expressed numerous types of chemokine receptors, indicating that the observed pain behavior might result from a direct action of chemokines on these neurons. In support of this possibility, chemokines were found to strongly excite DRG neurons in culture (White et al. 2005b; Sun et al. 2006) and chemokine-induced excitation was associated with the release of pain related neurotransmitters such as substance P and CGRP (Qin et al. 2005; Jung et al. 2008). The cellular mechanism underlying chemokine-induced excitation of sensory neurons in culture has been shown to have at least two components. The first of these is the transactivation of TRP cation channels, such as TRPV1 and TRP ankyrin 1 (TRPA1), which are also expressed by populations of nociceptive neurons (Bandell et al. 2004; Ruparel et al. 2008; Jung et al. 2008), and the second of these is inhibition of K+ conductances that normally regulate neuronal excitability. MIP-1α, for example, can enhance the thermal sensitivity of TRPV1 (Zhang et al. 2005). The receptor for MIP-1α, C-C chemokine receptor 1 (CCR1), is expressed by more than 85% of cultured DRG neurons which also express TRPV1 (Zhang et al. 2005). Activation of other chemokine receptors such as CCR2 expressed by cultured DRG neurons (see below) also produces excitation through transactivation of both TRPV1 and TRPA1 (Jung et al. 2008). In the former instance, the mechanism of activation appears to be due to phospholipase C induced removal of tonic phosphatidylinositol 4,5-bisphosphate mediated channel block (Chuang et al. 2001), whereas in the second instance the transactivation appears to involve a protein kinase C mediated event (Cesare and McNaughton 1996; Premkumar and Ahern 2000; Sugiura et al. 2002). Importantly, TRPA1 activation is central to acute pain, neuropeptide release, and neurogenic inflammation (McNamara et al. 2007; Trevisani et al. 2007). These data suggest that chemokine-induced excitation involving TRP channel activation may be of key importance to driving increased excitation observed in chronic pain states.
A significant question is whether such data, mostly obtained in cell culture studies, have relevance to the situation prevailing in chronic pain states in vivo. A key role for chemokines and their receptors in chronic pain has come from the results of experiments using several accepted models of neuropathic pain in rodents. These models include sciatic nerve transaction (Taskinen and Roytta 2000; Subang and Richardson 2001), partial ligation of the sciatic nerve (Abbadie et al. 2003; Tanaka et al. 2004; Lindia et al. 2005), chronic constriction injury of the sciatic nerve (Milligan et al. 2004; Kleinschnitz et al. 2005; Zhang and De Koninck 2006), chronic compression of the L4L5 DRG, a rodent model of spinal stenosis (White et al. 2005b; Sun et al. 2006), lysophosphatidylcholine-induced focal nerve demyelination (Bhangoo et al. 2007a; Jung et al. 2008), bone cancer pain (Vit et al. 2006; Khasabova et al. 2007), and zymosan-induced inflammatory pain (Milligan et al. 2004; Verge et al. 2004; Xie et al. 2006). Each of these models resulted in upregulation of one or more chemokine receptors by DRG neurons associated with, or in close proximity to, the injury. Moreover, in several instances it has also been demonstrated that sensory neurons will actually upregulate the synthesis of chemokines in addition to their cognate receptors (White et al. 2005b; Sun et al. 2006; Bhangoo et al. 2007a; Jung et al. 2007, 2008). Thus, in association with chronic pain the same DRG neuron may upregulate both a chemokine and its receptor, suggesting some form of cell autologous regulation of DRG excitability by these molecules may occur. For example, it might be imagined that under these circumstances DRG neurons could release chemokines that would then activate receptors expressed by the same neuron or by others in the vicinity. As chemokines can excite DRG neurons, this process might contribute to the neuronal hyperexcitability observed under these circumstances (White et al. 2005b; Sun et al. 2006). As chemokines are also of central importance in the recruitment of leukocytes, they would have a unique role in simultaneously coordinating inflammation and neuronal excitability.
One good example of the validity of this type of model concerns the potential role of the chemokine MCP-1 and its receptor CCR2 in the genesis of neuropathic pain. The role of MCP-1/CCR2 signaling in neuropathic pain states was suggested following peripheral nerve injury in genetically engineered mice lacking CCR2 receptors (Abbadie et al. 2003). These receptor knockout mice failed to display mechanical hyperalgesia following partial ligation of the sciatic nerve without a detectable change in acute pain behavior, while transgenic mice overexpressing glial MCP-1 production exhibited enhanced nociceptive responses (Menetski et al. 2007).
In keeping with these results, both CCR2 and its preferred ligand, MCP-1, are extensively upregulated in sensory neurons following chronic compression of the DRG (White et al. 2005b) and focal demyelination of the sciatic nerve (Bhangoo et al. 2007a). Functionally, many of these injured neurons respond to the exogenous administration of MCP-1 with membrane threshold depolarization, action potentials, and Ca2+ mobilization. Appropriately, these MCP-1-induced excitatory events were not observed in control animals and the use of a CCR2 receptor antagonist effectively reversed hypernociception (Bhangoo et al. 2007a). Subsequent investigations have revealed that two ionic mechanisms contribute to the excitatory effects of MCP-1; a non-voltage-dependent, depolarizing current with the properties of a nonselective cation conductance, quite possibly a TRP channel (Jung et al. 2008), and activation of another nonv-oltage-dependent depolarizing current with characteristics similar to those of a nonselective cation conductance (Sun et al. 2006).
Such chemokine-induced excitatory effects on sensory neurons may further facilitate the axonal transport and the release of excitatory neuropeptides, such as CGRP (Qin et al. 2005) and substance P from the terminals of DRG neurons in the spinal cord. Zhang and De Koninick (2006) recently demonstrated that MCP-1 is also present in central afferent fibers in the spinal cord. Thus, electrical activity due to peripheral nerve injury may also stimulate central afferent release of MCP-1 into the spinal cord dorsal horn, further activating CCR2-expressing microglial cells or central neurons (Abbadie et al. 2003; Bursztajn et al. 2004; Zhang and De Koninck 2006). Neurons from the dorsal horn express CCR2 receptors and MCP-1/CCR2 signaling reduces the inhibitory effects of GABA on these cells. Hence, release of MCP-1 may mediate excitatory effects at the level of both the DRG and the spinal cord.
Overall, these results suggest that injury-induced expression of MCP-1 may, in effect, function as a neurotransmitter in DRG neurons and may be central to the maintenance of chronic neuropathic pain states. Moreover, examination of the distribution of MCP-1 at a subcellular level following its synthesis in cultured DRG neurons has revealed that it is initially processed via the trans-Golgi network and packaged into the same synaptic vesicles as the peptide neurotransmitter CGRP (Jung et al. 2008). Vesicles that contain both proteins can be observed in the neuronal soma and following transport to nerve terminals. Depolarization of these neurons results in calcium-dependent release of MCP-1 either from the soma or from the nerve terminals (Jung et al. 2008). Presumably release of the chemokine from the cell soma within the DRG would have the effect of depolarizing neighboring CCR2-expressing neurons, eliciting excitation and promoting further MCP-1 release within the DRG or within the dorsal horn or the spinal cord, where it could interact with CCR2-expressing neurons and glia. In this way upregulation of MCP-1 and CCR2 might be an important component of DRG hyperexcitability and maintenance of chronic pain. It is interesting to note that in the brain the chemokine CCL21/exodus has also been shown to be upregulated by neurons following excitotoxic stimulation and to be packaged into secretory vesicles and released upon neuronal depolarization (de Jong et al. 2005). Thus, it appears that when chemokines are expressed in neurons under different circumstances they may generally play a novel role as neurotransmitters. MCP-1 and CCR2, as well as certain other chemokines and chemokine receptors, exhibit an exceptionally prolonged upregulation in the injury-associated DRG (White et al. 2005b; Zhang and De Koninck 2006; Bhangoo et al. 2007a, b), and the trigeminal ganglion following peripheral nerve injury (White et al. 2006) or herpes simplex virus infection (Theil et al. 2003; Cook et al. 2004; Wickham et al. 2005), supporting the possibility that this type of signaling could contribute to the chronic nature of neuropathic pain.
Overall, the evidence suggests that prolonged chemokine and chemokine receptor expression in sensory ganglia may well be a significant contributor to many injury-induced and virus-associated neuropathic pain syndromes (Fig. 5). This being the case, it is also of interest to define the signaling pathways in DRG neurons that result in the upregulation of chemokine and chemokine receptor expression as they may represent novel targets for intervention in the treatment of chronic pain. In the case of CCR2 receptors, some information on this issue has been obtained (Jung and Miller 2008). Analysis of the structure of the mouse and human CCR2 genes revealed several upstream regulatory elements that might potentially mediate the action of different transcription factors. Included in these is a conserved binding site for the transcription factor nuclear factor of activated T cells (NFAT) (Jung and Miller 2008). Members of the NFAT family of proteins are expressed by DRG neurons and expression of constitutively active NFAT derivatives produced upregulation of CCR2 receptors in these neurons (Jung and Miller 2008). NFAT is activated by being dephosphorylated by the calcium-dependent phophatase calcineurin. When the intracellular calcium concentration is increased in DRG neurons following depolarization via voltage-dependent calcium channels, calcineurin/NFAT activation effectively modulates increases in CCR2 expression (Jung and Miller 2008). This then provides a possible pathway for the induction of CCR2 in the context of neuropathic pain. Some upstream mediator is envisaged as initially depolarizing DRG neurons, leading to calcium influx and CCR2 upregulation. Once CCR2 has been upregulated, signaling via CCR2 could increase DRG excitation further and potentiate ongoing excitability. Interestingly, although MCP-1 is upregulated by DRG neurons together with CCR2 in chronic pain (White et al. 2005b), MCP-1 is not a target gene for NFAT regulation. On the other hand, we have observed that MCP-1 is upregulated in DRG neurons by the action of the cytokine TNF-α (Jung and Miller 2008). Indeed, as discussed earlier, TNF-α is known to increase the excitability of DRG neurons by a variety of mechanisms (Nicol et al. 1997; Sorkin and Doom 2000). In addition, TNF-α may also act as an upstream regulator of chemokine signaling in these cells and the chemokines produced may help to maintain the hyperexcitability of nociceptors. Such a possibility would also help to explain why increased DRG excitability extends to uninjured neurons that are both ipsilateral and contralateral to the nerve injury, observations that suggest an important role for diffusible mediators in triggering these events.
Fig. 5.
Injury-induced chemokine expression in DRG. Evidence suggests that prolonged chemokine and chemokine receptor expression in sensory ganglia may be a significant contributor to neuropathic pain syndromes. It is likely that chemokines affect neuronal hyperexcitability by transactivating TRP channels. While proinflammatory cytokines such as TNF-α, IL-6 and prostaglandin E are expressed early on and contribute to the genesis of chronic pain, evidence suggests that chemokines are expressed at later time points and may act as the trigger to convert the acute pain to one that is chronic in nature
Although most of the data obtained on chemokine signaling in the DRG in the context of neuropathic pain concern the role of MCP-1/CCR2, it is clear that other types of chemokine signaling may also be dynamically regulated under similar conditions. Upregulated expression of the CXCR3, CXCR4, and C-C chemokine receptor 5 (CCR5) receptors as well as their chemokine ligands has also been observed in populations of DRG neurons in chronic pain models (Bhangoo et al. 2007a, b). As with CCR2, upregulation of some of these chemokine receptors appears to be NFAT-dependent (e.g., CCR5), whereas upregulation of others is not (e.g., CXCR4) (Jung and Miller 2008). The precise expression patterns and time course of upregulation of these diverse chemokine signaling systems differ in each case, and so the details of how they each participates in pain behavior may vary according to the circumstances and will require further clarification. Nevertheless, the fact that chemokines are packaged into neurotransmitter secretory vesicles in DRG neurons indicates that they may play a neuromodulatory role in chronic pain (de Jong et al. 2005; Jung and Miller 2008). It is interesting to note that in the case of SDF-1 and MCP-1 these vesicle populations are clearly different (unpublished observations), indicating that even when two chemokines are secreted by the same neuron they may subserve somewhat different functions.
As an example of the role of chemokine signaling in chronic pain one might consider peripheral nervous system and central nervous system inflammatory demyelinating diseases such as Guillain-Barre syndrome, Charcot-Marie-Tooth disease types 1 and 4, and multiple sclerosis, which are frequently accompanied by a neuropathic pain syndrome (Carter et al. 1998; Boukhris et al. 2007). Epidemiological studies suggest that chronic pain syndromes afflict 50–80% of patients with multiple sclerosis and 70–90% of individuals with Guillain-Barre syndrome (Moulin 1998). Disease-related components that may be central to this overall pattern of symptoms of neuropathic pain include axon and Wallerian degeneration (Bruck 2005), which may act as a trigger for the cytokine cascades that result in the upregulation and chronic expression of chemokines and their cognate receptors (Mahad et al. 2002; Charo and Ransohoff 2006).
Studies of several rodent models of demyelinating diseases known to elicit neuropathic pain behavior, including late-developing peripheral axon demyelination in periaxin knockout mice (Gillespie et al. 2000), lysophosphatidylcholine-induced transient focal demyelination of the sciatic nerve in mice and rats (Wallace et al. 2003; Bhangoo et al. 2007a; Jung et al. 2008) and the late, acute clinical phase of experimental autoimmune neuritis (Moalem-Taylor et al. 2007), have indicated a possible role for chemokine-mediated signaling in these models. Recent studies on rats and mice subjected to transient focal demyelination of the sciatic nerve revealed chronic upregulation of MCP-1, IP-10/CXCL10, and the chemokine receptors CCR2, CCR5, and CXCR4 in primary sensory neurons (Bhangoo et al. 2007a). Application of these same chemokines to neurons isolated from DRG of animals following demyelination produced an increase in excitation. Thus, upregulation of these chemokines and their receptors may effectively drive the chronic excitability and pain behavior in demyelinating diseases of this type. It is also of interest that administration of small-molecule CCR2 receptor antagonists to these animals afforded some relief from ongoing pain, further indicating the role of chemokine signaling and the potential therapeutic effectiveness of inhibiting these events (Bhangoo et al. 2007a).
As in the case of upstream inflammatory cytokines such as TNF-α, glia in the DRG and peripheral nerve may also represent a source of, and a target for, the action of chemokines. For example, in response to nerve injury MCP-1 is upregulated in Schwann cells (Toews et al. 1998; Taskinen and Roytta 2000; Orlikowski et al. 2003). Thus, these cells might also represent a source of chemokine release for the activation of CCR2 chemokine receptors upregulated in adjacent DRG neurons.
The key involvement of chemokine signaling between glia and DRG neurons appears to be likely in certain chronic pain states such as those experienced in association with HIV-1 infection. This has particular relevance for the present discussion, as the cellular receptors for glycoprotein 120 (gp120), the HIV-1 coat protein, are the CXCR4 and CCR5 chemokine receptors. One example of an HIV-1 associated pain syndrome is distal symmetrical polyneuropathy (DSP), which affects as many as one third of all HIV-1 infected individuals (Skopelitis et al. 2006). This painful sensory neuropathy frequently begins with paresthesias in the fingers and toes, progressing over weeks to months, followed by the development of pain, often of a burning and lancinating nature, which can make walking very difficult. Measurements of pain hypersensitivity have demonstrated allodynia and hyperalgesia in HIV-1 infected individuals. Interestingly, as is the case of HIV-1 associated effects on the central nervous system, there is no productive infection of peripheral neurons by the virus. Thus, indirect effects of HIV-1 must lead to the development of this pain state.
In addition to the effects of inflammatory mediators (including chemokines) released by virally infected leukocytes, there are at least two ways in which HIV-1 induced DSP may involve the direct effects of HIV-1 gp120 on chemokine receptors in the DRG: (1) viral protein shedding in the peripheral nervous system might enable gp120 to produce painful neuropathy via glial to neuronal signaling in the DRG and/or spinal cord (Milligan et al. 2000; Keswani et al. 2003) or (2) by the direct activation of CCR5/CXCR4-bearing sensory neurons by gp120 (Herzberg and Sagen 2001; Oh et al. 2001; Wallace et al. 2007). Indeed, Keswani et al. (2003, 2006) have presented a model in which gp120 can act in both these ways. In the first instance, these authors demonstrated that binding of gp120 to CXCR4 receptors expressed by DRG satellite glial cells upregulates the release of the chemokine RANTES, which can then activate CCR5 receptors expressed by DRG neurons. In the second instance, gp120 can directly bind to and activate CXCR4 receptors expressed by DRG neurons (Oh et al. 2001). Moreover, this initial excitation of DRG neurons by gp120 and/or glial mediators might produce Ca2+-dependent upregulation of CCR2 expression by these neurons by the mechanisms discussed above (Jung and Miller 2008), leading to a second level of chemokine-mediated excitation. In support of such a model we have observed that treatment of the sciatic nerve with a T-tropic gp120 subsequently leads to MCP-1 and CCR2 upregulation (unpublished observations), which would also be expected to promote excitation of DRG neurons.
Complicating matters further, AIDS patients who are treated by highly active, antiretroviral therapeutical (HAART) agents can also develop a painful sensory neuropathy. Intriguingly, the symptoms of this syndrome are clinically indistinguishable from those of HIV-1 induced DSP, including a burning sensation in the hands and feet and hypersensitivity to pain (Snider et al. 1983; Pardo et al. 2001; Skopelitis et al. 2006). The fact that the two syndromes are usually seen in association with one another makes diagnosis more difficult.
Recent studies have shed new light on the mechanisms of HAART-induced DSP and the role of chemokine signaling in particular. It was observed that the drug 2′,3′-dideoxycytidine (zalcitabine, or ddC) produced neuropathic pain behavior together with upregulated expression of both SDF-1 and CXCR4 in DRG satellite glial cells and some neurons. This suggests that SDF-1 release from DRG glia might be involved in the autologous regulation of excitatory substances from these same cells and that released SDF-1 might directly excite DRG neurons. Significantly, zalcitabine-induced pain was completely blocked by the CXCR4 antagonist AMD3100, illustrating the key role of CXCR4 signaling in this behavior. (Bhangoo et al. 2007b). Hence, the proallodynic actions of both HIV-1 and zalcitabine are dependent on chemokine signaling between DRG glia and neurons.
7 Chemokine Interactions with Other Neurotransmitters
As we have discussed, chemokines and their receptors expressed by DRG neurons in chronic pain conditions may contribute to nociceptor hyperexcitability in many instances by directly exciting these neurons. However, other consequences of chemokine signaling may also indirectly contribute to pain behavior. One important example of this concerns chemokine interactions with the endogenous opioid system. Generally speaking, activation of μ-opioid receptors in the DRG and dorsal horn reduces neuronal excitability and synaptic transmission at synapses between nociceptors and first-order neurons in the spinal cord. This is believed to be one of the mechanisms by which opiates reduce pain behavior. However, it has been demonstrated that upregulation of chemokine signaling routinely modulates μ-opioid receptor function (Szabo et al. 2002; Zhang et al. 2005). One mechanism through which this may occur is by heterologous desensitization resulting from the effects of chemokine receptor activation on μ-receptor function (Grimm et al. 1998; Chen et al. 2007). In addition, fluorescence resonance energy transfer studies have demonstrated that some chemokine receptors can directly dimerize with μ-opioid receptors, raising the possibility that this interaction may also alter μ-receptor signaling (Toth et al. 2004). Interestingly, the opposite may also be true as the μ-opiate receptor agonist DAMGO can downregulate chemokine activation of chemokine receptors, something that may account for opiate-drug-induced immune suppression (Patel et al. 2006).
A particularly interesting interaction between cytokine/chemokine and opioid signaling seems to occur in the context of chronic opioid use. As has now been well documented, chronic opioid use or opioid withdrawal may result in significant hyperalgesia and this may present itself as a clinically significant problem (DeLeo et al. 2004; Watkins et al. 2007c). Although mechanisms such as those discussed above suggest that some chemokine/opioid interactions may be responsible for the downregulation of opioid effects in pain, they do not explain the development of pain hypersensitivity. However, several hypotheses do try to accommodate this phenomenon. According to one view, the normal opioid-induced analgesic effects that are observed are always concomitantly accompanied by opposing opioid-mediated hyperalgesic effects (Hutchinson et al. 2007). These latter effects are not mediated by the actions of opioids on neurons, but on glia (Watkins et al. 2007a). Thus, it is proposed that the activation of microglia by drugs such as morphine normally results in the upregulation of inflammatory cytokines by these cells as well as a reduction in the synthesis of anti-inflammatory cytokines such as interleukin-10 (Johnston et al. 2004; Milligan et al. 2006; Hutchinson et al. 2007; Ledeboer et al. 2007; Liang et al. 2008). The triggering of this cytokine cascade eventually results in pain hypersensitivity as described above. Viewed in this way, the proalgesic effects of chronic opioid use are another version of the “neuroinflammatory” response that is associated with pain hypersensitivity syndromes. This is even more the case given that it is also proposed that the opioid receptors that are expressed by microglia are none other than the TLR4 receptors (Hutchinson et al. 2007). It has been shown that unlike the effects of morphine on μ-receptors that are stereospecifically blocked by the opioid antagonist naloxone (only the – isomer being effective), the ability of morphine to activate TLR4 receptors is inhibited by both (+)-naloxone and (−)-naloxone. It is suggested that these proalgesic effects eventually overwhelm the antinociceptive effects of morphine to shift the balance to pain-producing rather than pain-preventing behavior. Recently, we have observed that chronic morphine treatment produces an upregulation of SDF-1 expression by DRG sensory neurons and that morphine-induced pain hypersensitivity is blocked by the CXCR4 antagonist AMD3100 (Wilson et al. 2008). As we have discussed, chemokines are often among the proteins synthesized downstream of cytokine cascades and can act directly on DRG neurons to enhance their excitability (White et al. 2005b; Sun et al. 2006). Hence, it is possible that this represents a further example of this phenomenon.
As we have discussed, chemokines expressed by DRG neurons may also be responsible for chemoattractant effects resulting in leukocyte influx into the ganglia. Some leukocytes can actively secrete opioid peptides, an action which is potentially analgesic (Labuz et al. 2006; Rittner et al. 2006). Thus, chemokines may be potentially proalgesic by directly exciting DRG neurons and downregulating opioid signaling as well as potentially analgesic owing to their effects on endorphin release from leukocytes. How these diverse effects play out in the context of chronic pain behavior is incompletely understood, and may have different degrees of importance depending on the precise type of pain syndrome under consideration. Clearly, numerous cellular mechanisms through which upregulated chemokine signaling might occur result in pain hypersensitivity or related phenomena.
8 Conclusions
Recent research has made it clear that inflammatory processes are critical for the development of states of chronic pain and for the changes in behavior of pain neurons that accompany these syndromes. The development of such behavior may involve reciprocal signaling interactions between the different cellular elements of the central and peripheral nervous systems. As we have discussed here, inflammatory cytokines and chemokines seem to be one set of molecules that play a key role in coordinating injury-associated nociceptive events as they serve to regulate inflammatory responses and can simultaneously act upon elements of the nervous system (Fig. 6). Importantly, chemokines in DRG neurons seem to act as upregulatable neurotransmitters that produce excitatory effects in the DRG and spinal cord through a variety of mechanisms. The ability of small-molecule antagonists of CCR2 and CXCR4 receptors to ameliorate ongoing pain hypersensitivity in animal models clearly indicates the importance of chemokine signaling in this behavior. Furthermore, antibodies to cytokines such as TNF-α also prevent the development of chronic pain. We therefore conclude that targeting inflammatory cytokine and chemokine signaling may provide a novel form of therapeutic intervention into states of chronic pain.
Fig. 6.
Injury-induced changes in the nociceptive pathway. In neuropathic pain, nociceptive pathways are altered at all levels of the central and peripheral nervous systems. Inflammatory cytokines and chemokines may play a key role in coordinating injury-associated nociceptive events as they regulate the inflammatory response and can simultaneously act upon elements of the nervous system, including the peripheral nerve (a), the DRG (b), and the dorsal horn of the spinal cord (c). Rapid effects of these molecules can alter the excitability of neurons, and the sensitization of ion channels involved in the neuropathic pain mechanism. Slower effects of cytokines include altered gene expression, which could the result in the subsequent upregulation of other proinflammatory cytokines, and ion channel expression. These events, which occur in many different cell types, when taken together result in an altered state of excitability that contributes to the chronic pain state. NGF nerve growth factor, NO nitric oxide, TNF-α tumor necrosis factor α, IL-1β interleukin-1β, TLR Toll-like receptor, ATP adenosine triphosphate, TRPV1 transient receptor potential vanilloid 1, CGRP calcitonin gene-related peptide, IL-6 interleukin-6, CCR2 C-C chemokine receptor 2, CXCR3 C-X-C chemokine receptor 3, CXCR4 C-X-C chemokine receptor 4, MCP-1 monocyte chemotactic protein 1, SDF-1 stromal cell derived factor 1, RANTES regulated upon activation, normal T cell expressed and secreted
Acknowledgements
F.A.W acknowledges NIH grants NS049136, National Multiple Sclerosis Society Pilot Award, and Illinois Excellence in Medicine, State of Illinois. R.J.M. acknowledges NIH grants NS043095, DA013141, and MH040165.
References
- Abbadie C, Lindia JA, Cumiskey AM, Peterson LB, Mudgett JS, Bayne EK, DeMartino JA, MacIntyre DE, Forrest MJ. Impaired neuropathic pain responses in mice lacking the chemokine receptor CCR2. Proc Natl Acad Sci USA. 2003;100:7947–7952. doi: 10.1073/pnas.1331358100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir R, Michaelis M, Devor M. Membrane potential oscillations in dorsal root ganglion neurons: role in normal electrogenesis and neuropathic pain. J Neurosci. 1999;19:8589–8596. doi: 10.1523/JNEUROSCI.19-19-08589.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bhangoo S, Ren D, Miller RJ, Henry KJ, Lineswala J, Hamdouchi C, Li B, Monahan PE, Chan DM, Ripsch MS, White FA. Delayed functional expression of neuronal chemokine receptors following focal nerve demyelination in the rat: a mechanism for the development of chronic sensitization of peripheral nociceptors. Mol Pain. 2007a;3:38. doi: 10.1186/1744-8069-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhangoo SK, Ren D, Miller RJ, Chan DM, Ripsch MS, Weiss C, McGinnis C, White FA. CXCR4 chemokine receptor signaling mediates pain hypersensitivity in association with antiretroviral toxic neuropathy. Brain Behav Immun. 2007b;21:581–591. doi: 10.1016/j.bbi.2006.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boraschi D, Tagliabue A. The interleukin-1 receptor family. Vitam Horm. 2006;74:229–254. doi: 10.1016/S0083-6729(06)74009-2. [DOI] [PubMed] [Google Scholar]
- Boukhris S, Magy L, Khalil M, Sindou P, Vallat JM. Pain as the presenting symptom of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) J Neurol Sci. 2007;254:33–38. doi: 10.1016/j.jns.2006.12.012. [DOI] [PubMed] [Google Scholar]
- Bruck W. The pathology of multiple sclerosis is the result of focal inflammatory demyelination with axonal damage. J Neurol. 2005;252(Suppl 5):v3–v9. doi: 10.1007/s00415-005-5002-7. [DOI] [PubMed] [Google Scholar]
- Bursztajn S, Rutkowski MD, Deleo JA. The role of the N-methyl-d-aspartate receptor NR1 subunit in peripheral nerve injury-induced mechanical allodynia, glial activation and chemokine expression in the mouse. Neuroscience. 2004;125:269–275. doi: 10.1016/j.neuroscience.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Raja SN, Meyer RA, Mackinnon SE. Myelinated afferents signal the hyperalgesia associated with nerve injury. Pain. 1988;32:89–94. doi: 10.1016/0304-3959(88)90027-9. [DOI] [PubMed] [Google Scholar]
- Carter GT, Jensen MP, Galer BS, Kraft GH, Crabtree LD, Beardsley RM, Abresch RT, Bird TD. Neuropathic pain in Charcot–Marie–Tooth disease. Arch Phys Med Rehabil. 1998;79:1560–1564. doi: 10.1016/s0003-9993(98)90421-x. [DOI] [PubMed] [Google Scholar]
- Cartier L, Hartley O, Dubois-Dauphin M, Krause K-H. Chemokine receptors in the central nervous system: role in brain inflammation and neurodegenerative diseases. Brain Res Rev. 2005;48:16–42. doi: 10.1016/j.brainresrev.2004.07.021. [DOI] [PubMed] [Google Scholar]
- Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charo IF, Ransohoff RM. The many roles of chemokines and chemokine receptors in inflammation. N Engl J Med. 2006;354:610–621. doi: 10.1056/NEJMra052723. [DOI] [PubMed] [Google Scholar]
- Chen X, Geller EB, Rogers TJ, Adler MW. Rapid heterologous desensitization of anti-nociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend. 2007;88:36–41. doi: 10.1016/j.drugalcdep.2006.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang H-h, Prescott ED, Kong H, Shields S, Jordt S-E, Basbaum AI, Chao MV, Julius D. Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature. 2001;411:957–962. doi: 10.1038/35082088. [DOI] [PubMed] [Google Scholar]
- Clark AK, Yip PK, Grist J, Gentry C, Staniland AA, Marchand F, Dehvari M, Wotherspoon G, Winter J, Ullah J, Bevan S, Malcangio M. Inhibition of spinal microglial cathepsin S for the reversal of neuropathic pain. Proc Natl Acad Sci USA. 2007;104:10655–10660. doi: 10.1073/pnas.0610811104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook WJ, Kramer MF, Walker RM, Burwell TJ, Holman HA, Coen DM, Knipe DM. Persistent expression of chemokine and chemokine receptor RNAs at primary and latent sites of herpes simplex virus 1 infection. Virol J. 2004;1:5. doi: 10.1186/1743-422X-1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creagh EM, O'Neill LA. TLRs, NLRs and RLRs: a trinity of pathogen sensors that cooperate in innate immunity. Trends Immunol. 2006;27:352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor alpha in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha TM, Verri WA, Jr, Fukada SY, Guerrero AT, Santodomingo-Garzon T, Poole S, Parada CA, Ferreira SH, Cunha FQ. TNF-alpha and IL-1beta mediate inflammatory hypernociception in mice triggered by B1 but not B2 kinin receptor. Eur J Pharmacol. 2007;573:221–229. doi: 10.1016/j.ejphar.2007.07.007. [DOI] [PubMed] [Google Scholar]
- Dalpke A, Heeg K. Signal integration following Toll-like receptor triggering. Crit Rev Immunol. 2002;22:217–250. [PubMed] [Google Scholar]
- de Jong EK, Dijkstra IM, Hensens M, Brouwer N, van Amerongen M, Liem RS, Boddeke HW, Biber K. Vesicle-mediated transport and release of CCL21 in endangered neurons: a possible explanation for microglia activation remote from a primary lesion. J Neurosci. 2005;25:7548–7557. doi: 10.1523/JNEUROSCI.1019-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLeo JA, Tanga FY, Tawfik VL. Neuroimmune activation and neuroinflammation in chronic pain and opioid tolerance/hyperalgesia. Neuroscientist. 2004;10:40–52. doi: 10.1177/1073858403259950. [DOI] [PubMed] [Google Scholar]
- Donnelly-Roberts D, McGaraughty S, Shieh CC, Honore P, Jarvis MF. Painful purinergic receptors. J Pharmacol Exp Ther. 2008;324:409–415. doi: 10.1124/jpet.106.105890. [DOI] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Bristow AF, Poole S. Interleukin-1 beta as a potent hyper-algesic agent antagonized by a tripeptide analogue. Nature. 1988;334:698–700. doi: 10.1038/334698a0. [DOI] [PubMed] [Google Scholar]
- Flynn G, Maru S, Loughlin J, Romero IA, Male D. Regulation of chemokine receptor expression in human microglia and astrocytes. J Neuroimmunol. 2003;136:84–93. doi: 10.1016/s0165-5728(03)00009-2. [DOI] [PubMed] [Google Scholar]
- Gadient RA, Otten U. Postnatal expression of interleukin-6 (IL-6) and IL-6 receptor (IL-6R) mRNAs in rat sympathetic and sensory ganglia. Brain Res. 1996;724:41–46. doi: 10.1016/0006-8993(96)00264-8. [DOI] [PubMed] [Google Scholar]
- Gardiner NJ, Cafferty WB, Slack SE, Thompson SW. Expression of gp130 and leukaemia inhibitory factor receptor subunits in adult rat sensory neurones: regulation by nerve injury. J Neurochem. 2002;83:100–109. doi: 10.1046/j.1471-4159.2002.01101.x. [DOI] [PubMed] [Google Scholar]
- Gillespie CS, Sherman DL, Fleetwood-Walker SM, Cottrell DF, Tait S, Garry EM, Wallace VC, Ure J, Griffiths IR, Smith A, Brophy PJ. Peripheral demyelination and neuropathic pain behavior in periaxin-deficient mice. Neuron. 2000;26:523–531. doi: 10.1016/s0896-6273(00)81184-8. [DOI] [PubMed] [Google Scholar]
- Grimm MC, Ben-Baruch A, Taub DD, Howard OM, Resau JH, Wang JM, Ali H, Richardson R, Snyderman R, Oppenheim JJ. Opiates transdeactivate chemokine receptors: delta and mu opiate receptor-mediated heterologous desensitization. J Exp Med. 1998;188:317–325. doi: 10.1084/jem.188.2.317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo LH, Schluesener HJ. The innate immunity of the central nervous system in chronic pain: the role of Toll-like receptors. Cell Mol Life Sci. 2007;64:1128–1136. doi: 10.1007/s00018-007-6494-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hempenstall K, Nurmikko TJ, Johnson RW, A'Hern RP, Rice AS. Analgesic therapy in postherpetic neuralgia: a quantitative systematic review. PLoS Med. 2005;2:e164. doi: 10.1371/journal.pmed.0020164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hensellek S, Brell P, Schaible HG, Brauer R, Segond von Banchet G. The cytokine TNF-alpha increases the proportion of DRG neurones expressing the TRPV1 receptor via the TNFR1 receptor and ERK activation. Mol Cell Neurosci. 2007;36:381–391. doi: 10.1016/j.mcn.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Herzberg U, Sagen J. Peripheral nerve exposure to HIV viral envelope protein gp120 induces neuropathic pain and spinal gliosis. J Neuroimmunol. 2001;116:29–39. doi: 10.1016/s0165-5728(01)00288-0. [DOI] [PubMed] [Google Scholar]
- Huising MO, Stet RJ, Kruiswijk CP, Savelkoul HF, Lidy Verburg-van Kemenade BM. Molecular evolution of CXC chemokines: extant CXC chemokines originate from the CNS. Trends Immunol. 2003;24:307–313. doi: 10.1016/s1471-4906(03)00120-0. [DOI] [PubMed] [Google Scholar]
- Hutchinson MR, Bland ST, Johnson KW, Rice KC, Maier SF, Watkins LR. Opioid-induced glial activation: mechanisms of activation and implications for opioid analgesia, dependence, and reward. Sci World J. 2007;7:98–111. doi: 10.1100/tsw.2007.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue A, Ikoma K, Morioka N, Kumagai K, Hashimoto T, Hide I, Nakata Y. Interleukin-1beta induces substance P release from primary afferent neurons through the cyclooxygenase-2 system. J Neurochem. 1999;73:2206–2213. [PubMed] [Google Scholar]
- Jin X, Gereau RWt. Acute p38-mediated modulation of tetrodotoxin-resistant sodium channels in mouse sensory neurons by tumor necrosis factor-alpha. J Neurosci. 2006;26:246–255. doi: 10.1523/JNEUROSCI.3858-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston IN, Milligan ED, Wieseler-Frank J, Frank MG, Zapata V, Campisi J, Langer S, Martin D, Green P, Fleshner M, Leinwand L, Maier SF, Watkins LR. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J Neurosci. 2004;24:7353–7365. doi: 10.1523/JNEUROSCI.1850-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung H, Miller RJ. Activation of the nuclear factor of activated T cells (NFAT) mediates upregulation of CCR2 chemokine receptors in dorsal root ganglion (DRG) neurons: A possible mechanism for activity-dependent transcription in DRG neurons in association with neuropathic pain. Mol Cell Neurosci. 2008;37:170–177. doi: 10.1016/j.mcn.2007.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung J, Bhangoo SK, Fitzgerald MP, Miller RJ, White FA. 2007 neuroscience meeting planner. Society for Neuroscience; San Diego: 2007. Expression of functional chemokine receptors in bladder-associated sensory neurons following focal demyelination of sciatic nerve. (Program no. 185.188). [Google Scholar]
- Jung H, Toth PT, White FA, Miller RJ. Monocyte chemoattractant protein-1 functions as a neuromodulator in dorsal root ganglia neurons. J Neurochem. 2008;104:254–263. doi: 10.1111/j.1471-4159.2007.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajander KC, Wakisaka S, Bennett GJ. Spontaneous discharge originates in the dorsal root ganglion at the onset of a painful peripheral neuropathy in the rat. Neurosci Lett. 1992;138:225–228. doi: 10.1016/0304-3940(92)90920-3. [DOI] [PubMed] [Google Scholar]
- Kawasaki Y, Zhang L, Cheng JK, Ji RR. Cytokine mechanisms of central sensitization: distinct and overlapping role of interleukin-1beta, interleukin-6, and tumor necrosis factor-alpha in regulating synaptic and neuronal activity in the superficial spinal cord. J Neurosci. 2008;28:5189–5194. doi: 10.1523/JNEUROSCI.3338-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keswani SC, Polley M, Pardo CA, Griffin JW, McArthur JC, Hoke A. Schwann cell chemokine receptors mediate HIV-1 gp120 toxicity to sensory neurons. Ann Neurol. 2003;54:287–296. doi: 10.1002/ana.10645. [DOI] [PubMed] [Google Scholar]
- Keswani SC, Jack C, Zhou C, Hoke A. Establishment of a rodent model of HIV-associated sensory neuropathy. J Neurosci. 2006;26:10299–10304. doi: 10.1523/JNEUROSCI.3135-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Stucky CL, Harding-Rose C, Eikmeier L, Beitz AJ, Coicou LG, Hanson AE, Simone DA, Seybold VS. Chemical interactions between fibrosarcoma cancer cells and sensory neurons contribute to cancer pain. J Neurosci. 2007;27:10289–10298. doi: 10.1523/JNEUROSCI.2851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Na HS, Sheen K, Chung JM. Effects of sympathectomy on a rat model of peripheral neuropathy. Pain. 1993;55:85–92. doi: 10.1016/0304-3959(93)90187-T. [DOI] [PubMed] [Google Scholar]
- Kim D, Kim MA, Cho IH, Kim MS, Lee S, Jo EK, Choi SY, Park K, Kim JS, Akira S, Na HS, Oh SB, Lee SJ. A critical role of Toll-like receptor 2 in nerve injury-induced spinal cord glial cell activation and pain hypersensitivity. J Biol Chem. 2007;282:14975–14983. doi: 10.1074/jbc.M607277200. [DOI] [PubMed] [Google Scholar]
- Kleinschnitz C, Brinkhoff J, Sommer C, Stoll G. Contralateral cytokine gene induction after peripheral nerve lesions: dependence on the mode of injury and NMDA receptor signaling. Brain Res Mol Brain Res. 2005;136:23–28. doi: 10.1016/j.molbrainres.2004.12.015. [DOI] [PubMed] [Google Scholar]
- Knaut H, Werz C, Geisler R, Nusslein-Volhard C. A zebrafish homologue of the chemokine receptor Cxcr4 is a germ-cell guidance receptor. Nature. 2003;421:279–282. doi: 10.1038/nature01338. [DOI] [PubMed] [Google Scholar]
- Labuz D, Berger S, Mousa SA, Zollner C, Rittner HL, Shaqura MA, Segovia-Silvestre T, Przewlocka B, Stein C, Machelska H. Peripheral antinociceptive effects of exogenous and immune cell-derived endomorphins in prolonged inflammatory pain. J Neurosci. 2006;26:4350–4358. doi: 10.1523/JNEUROSCI.4349-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledeboer A, Jekich BM, Sloane EM, Mahoney JH, Langer SJ, Milligan ED, Martin D, Maier SF, Johnson KW, Leinwand LA, Chavez RA, Watkins LR. Intrathecal interleukin-10 gene therapy attenuates paclitaxel-induced mechanical allodynia and proinflammatory cytokine expression in dorsal root ganglia in rats. Brain Behav Immun. 2007;21:686–698. doi: 10.1016/j.bbi.2006.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HL, Lee KM, Son SJ, Hwang SH, Cho HJ. Temporal expression of cytokines and their receptors mRNAs in a neuropathic pain model. Neuroreport. 2004;15:2807–2811. [PubMed] [Google Scholar]
- Levin ME, Jin JG, Ji RR, Tong J, Pomonis JD, Lavery DJ, Miller SW, Chiang LW. Complement activation in the peripheral nervous system following the spinal nerve ligation model of neuropathic pain. Pain. 2008;137(1):182–201. doi: 10.1016/j.pain.2007.11.005. [DOI] [PubMed] [Google Scholar]
- Li Y, Ji A, Weihe E, Schafer MK-H. Cell-specific expression and lipopolysaccharide-induced regulation of tumor necrosis factor {alpha} (TNF{alpha}) and TNF receptors in rat dorsal root ganglion. J Neurosci. 2004;24:9623–9631. doi: 10.1523/JNEUROSCI.2392-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Shi J, Tang JR, Chen D, Ai B, Chen J, Wang LN, Cao FY, Li LL, Lin CY, Guan XM. Effects of complete Freund's adjuvant on immunohistochemical distribution of IL-1 beta and IL-1R I in neurons and glia cells of dorsal root ganglion. Acta Pharmacol Sin. 2005;26:192–198. doi: 10.1111/j.1745-7254.2005.00522.x. [DOI] [PubMed] [Google Scholar]
- Liang DY, Shi X, Qiao Y, Angst MS, Yeomans DC, Clark JD. Chronic morphine administration enhances nociceptive sensitivity and local cytokine production after incision. Mol Pain. 2008;4:7. doi: 10.1186/1744-8069-4-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindia JA, McGowan E, Jochnowitz N, Abbadie C. Induction of CX3CL1 expression in astrocytes and CX3CR1 in microglia in the spinal cord of a rat model of neuropathic pain. J Pain. 2005;6:434–438. doi: 10.1016/j.jpain.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Liu B, Eisenach JC. Hyperexcitability of axotomized and neighboring unaxotomized sensory neurons is reduced days after perineural clonidine at the site of injury. J Neurophysiol. 2005;94:3159–3167. doi: 10.1152/jn.00623.2005. [DOI] [PubMed] [Google Scholar]
- Lu M, Grove EA, Miller RJ. Abnormal development of the hippocampal dentate gyrus in mice lacking the CXCR4 chemokine receptor. Proc Natl Acad Sci USA. 2002;99:7090–7095. doi: 10.1073/pnas.092013799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma C, Shu Y, Zheng Z, Chen Y, Yao H, Greenquist KW, White FA, LaMotte RH. Similar electrophysiological changes in axotomized and neighboring intact dorsal root ganglion neurons. J Neurophysiol. 2003;89:1588–1602. doi: 10.1152/jn.00855.2002. [DOI] [PubMed] [Google Scholar]
- Ma W, Quirion R. Targeting invading macrophage-derived PGE2, IL-6 and calcitonin gene-related peptide in injured nerve to treat neuropathic pain. Expert Opin Ther Targets. 2006;10:533–546. doi: 10.1517/14728222.10.4.533. [DOI] [PubMed] [Google Scholar]
- MacEwan DJ. TNF receptor subtype signalling: differences and cellular consequences. Cell Signal. 2002;14:477–492. doi: 10.1016/s0898-6568(01)00262-5. [DOI] [PubMed] [Google Scholar]
- Mahad DJ, Howell SJL, Woodroofe MN. Expression of chemokines in the CSF and correlation with clinical disease activity in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;72:498–502. doi: 10.1136/jnnp.72.4.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin MU, Wesche H. Summary and comparison of the signaling mechanisms of the Toll/interleukin-1 receptor family. Biochim Biophys Acta. 2002;1592:265–280. doi: 10.1016/s0167-4889(02)00320-8. [DOI] [PubMed] [Google Scholar]
- McMahon SB, Cafferty WB, Marchand F. Immune and glial cell factors as pain mediators and modulators. Exp Neurol. 2005;192:444–462. doi: 10.1016/j.expneurol.2004.11.001. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci USA. 2007;104(33):13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menetski J, Mistry S, Lu M, Mudgett JS, Ransohoff RM, Demartino JA, Macintyre DE, Abbadie C. Mice overexpressing chemokine ligand 2 (CCL2) in astrocytes display enhanced nociceptive responses. Neuroscience. 2007;149:706–714. doi: 10.1016/j.neuroscience.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Mehmert KK, Hinde JL, Harvey LO, Jr, Martin D, Tracey KJ, Maier SF, Watkins LR. Thermal hyperalgesia and mechanical allodynia produced by intrathecal administration of the human immunodeficiency virus-1 (HIV-1) envelope glycoprotein, gp120. Brain Res Mol Brain Res. 2000;861:105–116. doi: 10.1016/s0006-8993(00)02050-3. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Zapata V, Chacur M, Schoeniger D, Biedenkapp J, O'Connor KA, Verge GM, Chapman G, Green P, Foster AC, Naeve GS, Maier SF, Watkins LR. Evidence that exogenous and endogenous fractalkine can induce spinal nociceptive facilitation in rats. Eur J Neurosci. 2004;20:2294–2302. doi: 10.1111/j.1460-9568.2004.03709.x. [DOI] [PubMed] [Google Scholar]
- Milligan E, Zapata V, Schoeniger D, Chacur M, Green P, Poole S, Martin D, Maier SF, Watkins LR. An initial investigation of spinal mechanisms underlying pain enhancement induced by fractalkine, a neuronally released chemokine. Eur J Neurosci. 2005;22:2775–2782. doi: 10.1111/j.1460-9568.2005.04470.x. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Soderquist RG, Malone SM, Mahoney JH, Hughes TS, Langer SJ, Sloane EM, Maier SF, Leinwand LA, Watkins LR, Mahoney MJ. Intrathecal polymer-based interleukin-10 gene delivery for neuropathic pain. Neuron Glia Biol. 2006;2:293–308. doi: 10.1017/S1740925X07000488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moalem-Taylor G, Allbutt HN, Iordanova MD, Tracey DJ. Pain hypersensitivity in rats with experimental autoimmune neuritis, an animal model of human inflammatory demyelinating neuropathy. Brain Behav Immun. 2007;21:699–710. doi: 10.1016/j.bbi.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Moulin DE. Pain in central and peripheral demyelinating disorders. Neurol Clin. 1998;16:889–898. doi: 10.1016/s0733-8619(05)70103-1. [DOI] [PubMed] [Google Scholar]
- Nicol GD, Lopshire JC, Pafford CM. Tumor necrosis factor enhances the capsaicin sensitivity of rat sensory neurons. J Neurosci. 1997;17:975–982. doi: 10.1523/JNEUROSCI.17-03-00975.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson A, Moller K, Dahlin L, Lundborg G, Kanje M. Early changes in gene expression in the dorsal root ganglia after transection of the sciatic nerve; effects of amphiregulin and PAI-1 on regeneration. Brain Res Mol Brain Res. 2005;136:65–74. doi: 10.1016/j.molbrainres.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguchi K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:65–77. doi: 10.1016/s0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- Obreja O, Rathee PK, Lips KS, Distler C, Kress M. IL-1 beta potentiates heat-activated currents in rat sensory neurons: involvement of IL-1RI, tyrosine kinase, and protein kinase C. FASEB J. 2002;16:1497–1503. doi: 10.1096/fj.02-0101com. [DOI] [PubMed] [Google Scholar]
- Obreja O, Biasio W, Andratsch M, Lips KS, Rathee PK, Ludwig A, Rose-John S, Kress M. Fast modulation of heat-activated ionic current by proinflammatory interleukin-6 in rat sensory neurons. Brain. 2005;128:1634–1641. doi: 10.1093/brain/awh490. [DOI] [PubMed] [Google Scholar]
- Oh SB, Tran PB, Gillard SE, Hurley RW, Hammond DL, Miller RJ. Chemokines and glycoprotein120 produce pain hypersensitivity by directly exciting primary nociceptive neurons. J Neurosci. 2001;21:5027–5035. doi: 10.1523/JNEUROSCI.21-14-05027.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtori S, Takahashi K, Moriya H, Myers RR. TNF-alpha and TNF-alpha receptor type 1 upregulation in glia and neurons after peripheral nerve injury: studies in murine DRG and spinal cord. Spine. 2004;29:1082–1088. doi: 10.1097/00007632-200405150-00006. [DOI] [PubMed] [Google Scholar]
- Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–6293. doi: 10.1523/JNEUROSCI.20-16-06289.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlikowski D, Chazaud B, Plonquet A, Poron F, Sharshar T, Maison P, Raphael J-C, Gherardi RK, Creange A. Monocyte chemoattractant protein 1 and chemokine receptor CCR2 productions in Guillain-Barre syndrome and experimental autoimmune neuritis. J Neuroimmunol. 2003;134:118–127. doi: 10.1016/s0165-5728(02)00393-4. [DOI] [PubMed] [Google Scholar]
- Parada CA, Yeh JJ, Joseph EK, Levine JD. Tumor necrosis factor receptor type-1 in sensory neurons contributes to induction of chronic enhancement of inflammatory hyperalgesia in rat. Eur J Neurosci. 2003;17:1847–1852. doi: 10.1046/j.1460-9568.2003.02626.x. [DOI] [PubMed] [Google Scholar]
- Pardo CA, McArthur JC, Griffin JW. HIV neuropathy: insights in the pathology of HIV peripheral nerve disease. J Peripher Nerv Syst. 2001;6:21–27. doi: 10.1046/j.1529-8027.2001.006001021.x. [DOI] [PubMed] [Google Scholar]
- Park KA, Vasko MR. Lipid mediators of sensitivity in sensory neurons. Trends Pharmacol Sci. 2005;26:571–577. doi: 10.1016/j.tips.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Patel JP, Sengupta R, Bardi G, Khan MZ, Mullen-Przeworski A, Meucci O. Modulation of neuronal CXCR4 by the micro-opioid agonist DAMGO. J Neurovirol. 2006;12:492–500. doi: 10.1080/13550280601064798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet S, McMahon SB. Neurotrophins: mediators and modulators of pain. Annu Rev Neurosci. 2006;29:507–538. doi: 10.1146/annurev.neuro.29.051605.112929. [DOI] [PubMed] [Google Scholar]
- Premkumar LS, Ahern GP. Induction of vanilloid receptor channel activity by protein kinase C. Nature. 2000;408:985–990. doi: 10.1038/35050121. [DOI] [PubMed] [Google Scholar]
- Qin X, Wan Y, Wang X. CCL2 and CXCL1 trigger calcitonin gene-related peptide release by exciting primary nociceptive neurons. J Neurosci Res. 2005 doi: 10.1002/jnr.20612. doi:10.1002/jnr.20612. [DOI] [PubMed] [Google Scholar]
- Raghavendra V, Tanga FY, DeLeo JA. Complete Freunds adjuvant-induced peripheral inflammation evokes glial activation and proinflammatory cytokine expression in the CNS. Eur J Neurosci. 2004;20:467–473. doi: 10.1111/j.1460-9568.2004.03514.x. [DOI] [PubMed] [Google Scholar]
- Rittner HL, Labuz D, Schaefer M, Mousa SA, Schulz S, Schafer M, Stein C, Brack A. Pain control by CXCR2 ligands through Ca2+-regulated release of opioid peptides from polymorphonuclear cells. FASEB J. 2006;20(14):2627–2629. doi: 10.1096/fj.06-6077fje. [DOI] [PubMed] [Google Scholar]
- Romero-Sandoval EA, McCall C, Eisenach JC. Alpha2-adrenoceptor stimulation transforms immune responses in neuritis and blocks neuritis-induced pain. J Neurosci. 2005;25:8988–8994. doi: 10.1523/JNEUROSCI.2995-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruparel NB, Patwardhan AM, Akopian AN, Hargreaves KM. Homologous and heterologous desensitization of capsaicin and mustard oil responses utilize different cellular pathways in nociceptors. Pain. 2008;135(3):271–279. doi: 10.1016/j.pain.2007.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki N, Kikuchi S, Konno S, Sekiguchi M, Watanabe K. Anti-TNF-alpha antibody reduces pain-behavioral changes induced by epidural application of nucleus pulposus in a rat model depending on the timing of administration. Spine. 2007;32:413–416. doi: 10.1097/01.brs.0000255097.18246.bc. [DOI] [PubMed] [Google Scholar]
- Schafers M, Sommer C. Anticytokine therapy in neuropathic pain management. Expert Rev Neurother. 2007;7:1613–1627. doi: 10.1586/14737175.7.11.1613. [DOI] [PubMed] [Google Scholar]
- Schafers M, Svensson CI, Sommer C, Sorkin LS. Tumor necrosis factor-alpha induces mechanical allodynia after spinal nerve ligation by activation of p38 MAPK in primary sensory neurons. J Neurosci. 2003a;23:2517–2521. doi: 10.1523/JNEUROSCI.23-07-02517.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafers M, Lee DH, Brors D, Yaksh TL, Sorkin LS. Increased sensitivity of injured and adjacent uninjured rat primary sensory neurons to exogenous tumor necrosis factor-alpha after spinal nerve ligation. J Neurosci. 2003b;23:3028–3038. doi: 10.1523/JNEUROSCI.23-07-03028.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth RN, Dorsi MJ, Li Y, Murinson BB, Belzberg AJ, Griffin JW, Meyer RA. Mechanical hyperalgesia after an L5 ventral rhizotomy or an L5 ganglionectomy in the rat. Pain. 2002;96:63–72. doi: 10.1016/s0304-3959(01)00429-8. [DOI] [PubMed] [Google Scholar]
- Skopelitis EE, Kokotis PI, Kontos AN, Panayiotakopoulos GD, Konstantinou K, Kordossis T, Karandreas N. Distal sensory polyneuropathy in HIV-positive patients in the HAART era: an entity underestimated by clinical examination. Int J STD AIDS. 2006;17:467–472. doi: 10.1258/095646206777689062. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Kalra A, Moore SA. Unilateral intramuscular injections of acidic saline produce a bilateral, long-lasting hyperalgesia. Muscle Nerve. 2001;24:37–46. doi: 10.1002/1097-4598(200101)24:1<37::aid-mus4>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Sluka KA, Radhakrishnan R, Benson CJ, Eshcol JO, Price MP, Babinski K, Audette KM, Yeomans DC, Wilson SP. ASIC3 in muscle mediates mechanical, but not heat, hyper-algesia associated with muscle inflammation. Pain. 2007;129:102–112. doi: 10.1016/j.pain.2006.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider WD, Simpson DM, Nielsen S, Gold JW, Metroka CE, Posner JB. Neurological complications of acquired immune deficiency syndrome: analysis of 50 patients. Ann Neurol. 1983;14:403–418. doi: 10.1002/ana.410140404. [DOI] [PubMed] [Google Scholar]
- Sommer C, Schmidt C, George A. Hyperalgesia in experimental neuropathy is dependent on the TNF receptor 1. Exp Neurol. 1998;151:138–142. doi: 10.1006/exnr.1998.6797. [DOI] [PubMed] [Google Scholar]
- Sorkin LS, Doom CM. Epineurial application of TNF elicits an acute mechanical hyper-algesia in the awake rat. J Peripher Nerv Syst. 2000;5:96–100. doi: 10.1046/j.1529-8027.2000.00012.x. [DOI] [PubMed] [Google Scholar]
- Streit WJ, Conde JR, Harrison JK. Chemokines and alzheimer's disease. Neurobiol Aging. 2001;22:909–913. doi: 10.1016/s0197-4580(01)00290-1. [DOI] [PubMed] [Google Scholar]
- Subang MC, Richardson PM. Influence of injury and cytokines on synthesis of monocyte chemoattractant protein-1 mRNA in peripheral nervous tissue. Eur J Neurosci. 2001;13:521–528. doi: 10.1046/j.1460-9568.2001.01425.x. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Tominaga M, Katsuya H, Mizumura K. Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J Neurophysiol. 2002;88:544–548. doi: 10.1152/jn.2002.88.1.544. [DOI] [PubMed] [Google Scholar]
- Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Sun JH, Yang B, Donnelly DF, Ma C, LaMotte RH. MCP-1 enhances excitability of nociceptive neurons in chronically compressed dorsal root ganglia. J Neurophysiol. 2006;96:2189–2199. doi: 10.1152/jn.00222.2006. [DOI] [PubMed] [Google Scholar]
- Szabo I, Chen XH, Xin L, Adler MW, Howard OM, Oppenheim JJ, Rogers TJ. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc Natl Acad Sci USA. 2002;99:10276–10281. doi: 10.1073/pnas.102327699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tachibana K, Hirota S, Iizasa H, Yoshida H, Kawabata K, Kataoka Y, Kitamura Y, Matsushima K, Yoshida N, Nishikawa S, Kishimoto T, Nagasawa T. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- Takeda M, Tanimoto T, Kadoi J, Nasu M, Takahashi M, Kitagawa J, Matsumoto S. Enhanced excitability of nociceptive trigeminal ganglion neurons by satellite glial cytokine following peripheral inflammation. Pain. 2007;129:155–166. doi: 10.1016/j.pain.2006.10.007. [DOI] [PubMed] [Google Scholar]
- Takeda M, Takahashi M, Matsumoto S. Contribution of activated interleukin receptors in trigeminal ganglion neurons to hyperalgesia via satellite glial interleukin-1beta paracrine mechanism. Brain Behav Immun. 2008;22(7):1016–1023. doi: 10.1016/j.bbi.2008.03.004. [DOI] [PubMed] [Google Scholar]
- Tal M, Devor M. Ectopic discharge in injured nerves: comparison of trigeminal and somatic afferents. Brain Res. 1992;579:148–151. doi: 10.1016/0006-8993(92)90753-v. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Minami M, Nakagawa T, Satoh M. Enhanced production of monocyte chemoattractant protein-1 in the dorsal root ganglia in a rat model of neuropathic pain: possible involvement in the development of neuropathic pain. Neurosci Res. 2004;48:463–469. doi: 10.1016/j.neures.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Tanga FY, Nutile-McMenemy N, DeLeo JA. The CNS role of Toll-like receptor 4 in innate neuroimmunity and painful neuropathy. Proc Natl Acad Sci USA. 2005;102:5856–5861. doi: 10.1073/pnas.0501634102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanuma N, Sakuma H, Sasaki A, Matsumoto Y. Chemokine expression by astrocytes plays a role in microglia/macrophage activation and subsequent neurodegeneration in secondary progressive multiple sclerosis. Acta Neuropathol (Berl) 2006;112:195–204. doi: 10.1007/s00401-006-0083-7. [DOI] [PubMed] [Google Scholar]
- Taskinen HS, Roytta M. Increased expression of chemokines (MCP-1, MIP-1alpha, RANTES) after peripheral nerve transection. J Peripher Nerv Syst. 2000;5:75–81. doi: 10.1046/j.1529-8027.2000.00009.x. [DOI] [PubMed] [Google Scholar]
- Tawfik VL, Nutile-McMenemy N, Lacroix-Fralish ML, Deleo JA. Efficacy of propentofylline, a glial modulating agent, on existing mechanical allodynia following peripheral nerve injury. Brain Behav Immun. 2007;21:238–246. doi: 10.1016/j.bbi.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Theil D, Derfuss T, Paripovic I, Herberger S, Meinl E, Schueler O, Strupp M, Arbusow V, Brandt T. Latent herpesvirus infection in human trigeminal ganglia causes chronic immune response. Am J Pathol. 2003;163:2179–2184. doi: 10.1016/S0002-9440(10)63575-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson SW, Priestley JV, Southall A. gp130 Cytokines, leukemia inhibitory factor and interleukin-6, induce neuropeptide expression in intact adult rat sensory neurons in vivo: time-course, specificity and comparison with sciatic nerve axotomy. Neuroscience. 1998;84:1247–1255. doi: 10.1016/s0306-4522(97)00553-8. [DOI] [PubMed] [Google Scholar]
- Ting E, Guerrero AT, Cunha TM, Verri WA, Jr, Taylor SM, Woodruff TM, Cunha FQ, Ferreira SH. Role of complement C5a in mechanical inflammatory hypernociception: potential use of C5a receptor antagonists to control inflammatory pain. Br J Pharmacol. 2008;153:1043–1053. doi: 10.1038/sj.bjp.0707640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toews AD, Barrett C, Morell P. Monocyte chemoattractant protein 1 is responsible for macrophage recruitment following injury to sciatic nerve. J Neurosci Res. 1998;53:260–267. doi: 10.1002/(SICI)1097-4547(19980715)53:2<260::AID-JNR15>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Torebjork HE, Lundberg LE, LaMotte RH. Central changes in processing of mechanoreceptive input in capsaicin-induced secondary hyperalgesia in humans. J Physiol. 1992;448:765–780. doi: 10.1113/jphysiol.1992.sp019069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth PT, Ren D, Miller RJ. Regulation of CXCR4 receptor dimerization by the chemokine SDF-1alpha and the HIV-1 coat protein gp120: a fluorescence resonance energy transfer (FRET) study. J Pharmacol Exp Ther. 2004;310:8–17. doi: 10.1124/jpet.103.064956. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci USA. 2007;104(33):13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twining CM, Sloane EM, Milligan ED, Chacur M, Martin D, Poole S, Marsh H, Maier SF, Watkins LR. Peri-sciatic proinflammatory cytokines, reactive oxygen species, and complement induce mirror-image neuropathic pain in rats. Pain. 2004;110:299–309. doi: 10.1016/j.pain.2004.04.008. [DOI] [PubMed] [Google Scholar]
- Ubogu EE, Cossoy MB, Ransohoff RM. The expression and function of chemokines involved in CNS inflammation. Trends Pharmacol Sci. 2006;27:48–55. doi: 10.1016/j.tips.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Uceyler N, Tscharke A, Sommer C. Early cytokine expression in mouse sciatic nerve after chronic constriction nerve injury depends on calpain. Brain Behav Immun. 2007;21:553–560. doi: 10.1016/j.bbi.2006.10.003. [DOI] [PubMed] [Google Scholar]
- Vabulas RM, Wagner H, Schild H. Heat shock proteins as ligands of Toll-like receptors. Curr Top Microbiol Immunol. 2002;270:169–184. doi: 10.1007/978-3-642-59430-4_11. [DOI] [PubMed] [Google Scholar]
- Verge GM, Milligan ED, Maier SF, Watkins LR, Naeve GS, Foster AC. Fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) distribution in spinal cord and dorsal root ganglia under basal and neuropathic pain conditions. Eur J Neurosci. 2004;20:1150–1160. doi: 10.1111/j.1460-9568.2004.03593.x. [DOI] [PubMed] [Google Scholar]
- Vit JP, Ohara PT, Tien DA, Fike JR, Eikmeier L, Beitz A, Wilcox GL, Jasmin L. The analgesic effect of low dose focal irradiation in a mouse model of bone cancer is associated with spinal changes in neuro-mediators of nociception. Pain. 2006;120:188–201. doi: 10.1016/j.pain.2005.10.033. [DOI] [PubMed] [Google Scholar]
- Wall PD, Devor M. Sensory afferent impulses originate from dorsal root ganglia as well as from the periphery in normal and nerve injured rats. Pain. 1983;17:321–339. doi: 10.1016/0304-3959(83)90164-1. [DOI] [PubMed] [Google Scholar]
- Wallace VCJ, Cottrell DF, Brophy PJ, Fleetwood-Walker SM. Focal lysolecithin-induced demyelination of peripheral afferents results in neuropathic pain behavior that is attenuated by cannabinoids. J Neurosci. 2003;23:3221–3233. doi: 10.1523/JNEUROSCI.23-08-03221.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace VC, Blackbeard J, Segerdahl AR, Hasnie F, Pheby T, McMahon SB, Rice AS. Characterization of rodent models of HIV-gp120 and anti-retroviral-associated neuropathic pain. Brain. 2007;130(10):2688–2702. doi: 10.1093/brain/awm195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Maier SF. Glia: a novel drug discovery target for clinical pain. Nat Rev Drug Discov. 2003;2:973–985. doi: 10.1038/nrd1251. [DOI] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Milligan ED, Maier SF. “Listening” and “talking” to neurons: implications of immune activation for pain control and increasing the efficacy of opioids. Brain Res Rev. 2007a;56:148–169. doi: 10.1016/j.brainresrev.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Glia as the “bad guys”: Implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007b;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins LR, Hutchinson MR, Ledeboer A, Wieseler-Frank J, Milligan ED, Maier SF. Norman Cousins Lecture. Glia as the “bad guys”: implications for improving clinical pain control and the clinical utility of opioids. Brain Behav Immun. 2007c;21:131–146. doi: 10.1016/j.bbi.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson CP. The treatment of neuropathic pain: antidepressants and opioids. Clin J Pain. 2000;16:S49–S55. doi: 10.1097/00002508-200006001-00009. [DOI] [PubMed] [Google Scholar]
- White FA, Bhangoo SK, Miller RJ. Chemokines: integrators of pain and inflammation. Nat Rev Drug Discov. 2005a;4:834–844. doi: 10.1038/nrd1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Sun J, Waters SM, Ma C, Ren D, Ripsch M, Steflik J, Cortright DN, Lamotte RH, Miller RJ. Excitatory monocyte chemoattractant protein-1 signaling is up-regulated in sensory neurons after chronic compression of the dorsal root ganglion. Proc Natl Acad Sci USA. 2005b;102:14092–14097. doi: 10.1073/pnas.0503496102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White FA, Bauer WR, Jellish WS, Chan DM, LaMotte RH, Miller RJ. MCP-1/CCL2 and CCR2 upregulation following partial ligation of the infraorbital nerve: possible involvement in the development and maintenance of trigeminal neuralgia. J Pain. 2006;7:628. [Google Scholar]
- White FA, Monahan P, LaMotte RH. Chemokines and their receptors in the nervous system: a link to neuropathic pain. In: Watkins LA, DeLeo JA, Sorkin L, editors. Immune and glial activation in pain. IASP; Seattle: 2007a. [Google Scholar]
- White FA, Jung H, Miller RJ. Chemokines and the pathophysiology of neuropathic pain. Proc Natl Acad Sci USA. 2007b;104:20151–20158. doi: 10.1073/pnas.0709250104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham S, Lu B, Ash J, Carr DJ. Chemokine receptor deficiency is associated with increased chemokine expression in the peripheral and central nervous systems and increased resistance to herpetic encephalitis. J Neuroimmunol. 2005;162:51–59. doi: 10.1016/j.jneuroim.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Wilson N, Ripsch MS, Miller RJ, White FA. Morphine-induced hypernociception is reversed with the CXCR4 receptor antagonist, AMD3100; Society for Neuroscience annual meeting; Washington: 2008. [Google Scholar]
- Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–688. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- Wright DE, Bowman EP, Wagers AJ, Butcher EC, Weissman IL. Hematopoietic stem cells are uniquely selective in their migratory response to chemokines. J Exp Med. 2002;195:1145–1154. doi: 10.1084/jem.20011284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W, Strong JA, Meij JT, Zhang JM, Yu L. Neuropathic pain: early spontaneous afferent activity is the trigger. Pain. 2005;116:243–256. doi: 10.1016/j.pain.2005.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie WR, Deng H, Li H, Bowen TL, Strong JA, Zhang JM. Robust increase of cutaneous sensitivity, cytokine production and sympathetic sprouting in rats with localized inflammatory irritation of the spinal ganglia. Neuroscience. 2006;142(3):809–822. doi: 10.1016/j.neuroscience.2006.06.045. 809-822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanella JM, Burright EN, Hildebrand K, Hobot C, Cox M, Christoferson L, McKay WF. Effect of etanercept, a tumor necrosis factor-alpha inhibitor, on neuropathic pain in the rat chronic constriction injury model. Spine. 2008;33:227–234. doi: 10.1097/BRS.0b013e318162340a. [DOI] [PubMed] [Google Scholar]
- Zhang J, De Koninck Y. Spatial and temporal relationship between monocyte chemoattractant protein-1 expression and spinal glial activation following peripheral nerve injury. J Neurochem. 2006;97:772–783. doi: 10.1111/j.1471-4159.2006.03746.x. [DOI] [PubMed] [Google Scholar]
- Zhang N, Inan S, Cowan A, Sun R, Wang JM, Rogers TJ, Caterina M, Oppenheim JJ. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc Natl Acad Sci USA. 2005;102:4536–4541. doi: 10.1073/pnas.0406030102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang ZY, Kawasaki Y, Tan PH, Wen YR, Huang J, Ji RR. Role of the CX3CR1/p38 MAPK pathway in spinal microglia for the development of neuropathic pain following nerve injury-induced cleavage of fractalkine. Brain Behav Immun. 2007;21:642–651. doi: 10.1016/j.bbi.2006.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann M. Pathobiology of neuropathic pain. Eur J Pharmacol. 2001;429:23–37. doi: 10.1016/s0014-2999(01)01303-6. [DOI] [PubMed] [Google Scholar]